Abstract
For decades, the predominant hypothesis of schizophrenia centered on dysfunctions of the dopamine system. However, recent evidence now suggests that the dopamine system may be “normal” in its configuration, but instead is regulated abnormally by modulatory processes. Convergent studies in animals and in humans have now focused on the hippocampus as a central component in the generation of psychosis and possibly other symptom states in schizophrenia. Thus, activity in the ventral hippocampus has been shown to regulate dopamine neuron responsivity by controlling the number of dopamine neurons that can be phasically activated by stimuli. In this way, this structure determines the gain of the dopamine signal in response to stimuli. However, in schizophrenia, the hippocampus appears to be hyperactive, possibly due to attenuation of function of inhibitory interneurons. As a result, the dopamine system is driven into an overly responsive state. Current medications have focused on blockade of overstimulated dopamine receptors; however, this now appears to be several synapses downstream from the pathological antecedent. Therapeutic approaches that focus on normalizing hippocampal function may prove to be more effective treatment avenues for the schizophrenia patient.
The Dopamine Hypothesis of Schizophrenia
The high morbidity associated with schizophrenia coupled with the comparatively less effective treatments available compared to other psychiatric disorders has generated a substantial amount of research into the pathophysology of this devastating disorder. Nonetheless, the basic neurobiological constructs that lead to the disruptions associated with schizophrenia are comparatively poorly understood. For a number of years, the dopamine (DA) hypothesis has driven the primary line of inquiry into this disorder, based on strong pharmacological data wherein drugs that augment DA transmission will exacerbate psychosis in schizophrenia patients (Janowsky et al., 1973) and mimic it in controls (Angrist et al., 1974), whereas all drugs currently used for the treatment of schizophrenia act on the DA D2 receptor (Kapur and Remington, 2001; Kapur et al., 2000). Thus, in its simplest form, the DA hypothesis of schizophrenia posits that there are abnormally high levels of DA transmission leading to the pathological state (van Rossum, 1966). Nonetheless, there is little evidence for a substantial deficit in the DA system itself in the schizophrenia patient’s brain (Beuger et al., 1996; Post et al., 1975; van Kammen et al., 1986). This has led to a model in which the DA system itself is likely to be normal, but instead is being driven in an abnormal manner by brain regions in which the primary deficit lies (Grace, 2000). Indeed, studies show that amphetamine-induced dopamine release is significantly greater in schizophrenia patients, and moreover this increase above controls is proportional to the ability of the amphetamine to exacerbate psychosis (Laruelle and Abi-Dargham, 1999). Thus, there appears to be an identity to increased dopamine system responsivity and psychosis in schizophrenia. But, what system in the schizophrenia brain is driving this hyper-responsivity in the dopamine system? One system in particular that has attracted substantial attention is the glutamatergic system. Thus, drugs that alter glutamate function, particularly those that act on NMDA receptors, exhibit potent psychomimetic effects that are often indistinguishable from schizophrenia (Javitt and Zukin, 1991).
There are several regions that have emerged as potential sites for dysregulation in the brain of the schizophrenia patient. One region that rose to early prominence is the prefrontal cortex. The prefrontal cortex is a region that is known to be involved in executive function (Goldman-Rakic, 1996), and it is well- established that these functions are impaired in schizophrenia patients (Weinberger and Gallhofer, 1997). Indeed, imaging studies show that normal individuals activate the prefrontal cortex in working memory tasks, whereas schizophrenia patients fail to activate the prefrontal cortex when they fail to accurately complete the task (Berman and Weinberger, 1990; Weinberger et al., 1986). Such data were interpreted as a deficit in prefrontal cortical activity, or hypofrontality (Ingvar and Franzen, 1974). However, more recent studies suggest that the prefrontal cortex may be hyper-active in schizophrenia, such that it cannot be further activated during a task (Bassett et al., 2009), and this may lead to ineffective task performance.
Another region that has also received substantial attention in schizophrenia is the hippocampus. Several studies have reported anatomical changes in the hippocampus in schizophrenia patients (Conrad et al., 1991; Kovelman and Scheibel, 1984; Suddath et al., 1990). Moreover, studies suggest that the hippocampus may be hyper-active in schizophrenia. Thus, imaging studies show hippocampal hyperactivity in schizophrenia patients (Heckers, 2001; Kegeles et al., 2000; Malaspina et al., 1999; Medoff et al., 2001), and moreover this hyperactivity correlates with psychosis (Silbersweig et al., 1995). This suggests that the dopaminergic psychotic features of schizophrenia may be driven by abnormally heightened hippocampal activity. Indeed, basic science studies show that activation of the hippocampus does indeed lead to a hyperdopaminergic state. To understand the type of hyperactivity induced and its relevance for schizophrenia, the types of dopamine neuron activity states and their regulation is important to outline.
Dopamine neuron activity states
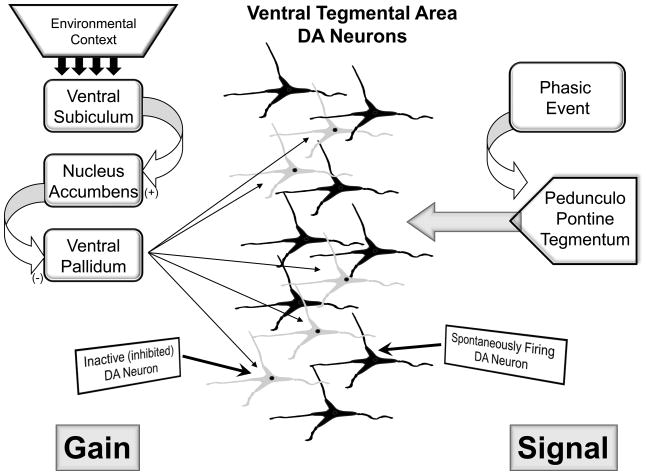
Dopamine neurons are known to exist in several activity states. In the control state, dopamine neurons are either spontaneously firing or are in an inhibited, nonfiring state (Bunney and Grace, 1978; Grace and Bunney, 1984). Approximately half of the dopamine neurons are firing in the control rat; a parameter known as population activity. Since dopamine neurons are driven by a pacemaker conductance, the nonfiring state must be maintained by an active inhibitory input. One of the most potent inhibitory inputs to the dopamine neurons is a GABAergic inhibitory input (Grace and Bunney, 1985) arising from the ventral pallidum. The ventral pallidum is a region that fires continuously at high firing rates (Maslowski-Cobuzzi and Napier, 1994; Mogenson et al., 1993). Moreover, dopamine neurons recorded intracellularly in vivo are constantly bombarded with high amplitude GABAergic ipsps (Grace and Bunney, 1985). Therefore, the ventral pallidum provides a tonic high amplitude GABAergic inhibition that keeps a proportion of dopamine neurons from firing spontaneously. The ventral pallidum, in turn, is controlled by a pathway originating in the ventral hippocampus. The ventral hippocampus projects to the nucleus accumbens, which in turn inhibits the ventral pallidum. Therefore, when the ventral hippocampus is excited, it drives nucleus accumbens inhibition of the ventral pallidum, releasing the dopamine neurons from inhibition (Floresco et al., 2001) (Fig. 1). Indeed, pharmacological activation of the ventral hippocampus was shown to specifically increase the number of dopamine neurons firing spontaneously (i.e., increased dopamine neuron population activity), and this increase could be attenuated by interrupting the circuit at the level of the nucleus accumbens (Floresco et al., 2003) or the ventral pallidum ((Valenti and Grace, 2009).
Figure 1.
The DA neuron population is comprised of neurons that are firing spontaneously, and those that are inhibited from firing due to the influence of the ventral pallidum. The ventral pallidum, in turn, is controlled by a hippocampal ventral subiculum-nucleus accumbens pathway. The state of activity of the subiculum is dependent on the context; with benign contexts the drive is low, but is augmented when the environment warrants a higher level of attention. Whenever the ventral subiculum is activated, it in turn activates the nucleus accumbens, inhibits the ventral pallidum, and removes the inhibitory influence from the DA neurons. Therefore with increased subicular drive, there is an increase in the proportion of DA neurons firing spontaneously. When a phasic, behaviorally relevant event takes place, the pedunculopontine tegmentum provides a rapid, glutamatergic input to the DA neuron population; this is the behaviorally salient signal. However, only DA neurons that are firing spontaneously can respond to the phasic input with burst firing. Therefore, by controlling the number of DA neurons that are firing spontaneously, and therefore capable of being phasically activated, the ventral subiculum controls the gain of the DA signal.
What is the relevance of changes in the number of dopamine neurons firing? This measure is important in determining the amplitude of the dopamine signal. Thus, studies show that when an animal is exposed to a behaviorally activating stimulus, the dopaminergic neurons fire in bursts of action potentials. These bursts appear to be driven by a glutamatergic input, with the most potent of these inputs arising from the brainstem region known as the pedunculopontine tegmentum. Thus, activation of the pedunculopontine tegmentum causes dopamine neurons to fire in phasic bursts, with burst firing being the behaviorally relevant output of the dopamine system (Floresco et al., 2001; Floresco et al., 2003). However, not all of the dopamine neurons are driven into burst firing; only dopamine neurons that are already firing spontaneously respond to pedunculopontine activation with burst firing. Thus, phasic glutamatergic drive from the pedunculopontine tegmentum causes the dopaminergic neurons to emit a burst of spikes. However, the amplitude of this phasic dopaminergic signal depends on the number of dopamine neurons firing. Thus, the greater the ventral hippocampus-modulated population activity, the larger the phasic dopamine signal (Floresco et al., 2003; Lodge and Grace, 2006) (Fig. 1).
Therefore, the ventral hippocampus is positioned to modulate the amplitude of the phasic dopamine signal; i.e., the pedunculopontine tegmentum provides the behaviorally salient phasic drive, but the ventral hippocampus determines the amplitude of the phasic dopamine response. This is consistent with the proposed behavioral function of the ventral hippocampus. Thus, studies have suggested that the ventral hippocampus, and the ventral subiculum of the hippocampus in particular, is involved in context-related behaviors (Fanselow, 2000; Jarrard, 1995; Maren, 1999; Sharp, 1999). This is consistent with the anatomy of the hippocampus, which in the rat is organized in a dorsal-ventral gradient. The most dorsal portions are the regions involved in location in space, or place cells (O’Keefe, 1979). However, as one moves ventrally, the location information is overlayed with limbic, emotion-related inputs. Indeed, primarily the ventralmost portions of the hippocampus receive potent amygdala inputs (French et al., 2003). Thus, in the ventralmost regions, cells would be expected to respond to the affective significance of location, which in general terms would be context (Grace, 2010a). This would be consistent with the impact on the dopamine system, since the amplitude of the phasic dopamine response should be dependent on the contextual relevance of the situation. As an example, if one is in a benign, safe environment and a stimulus is received, the reaction to the stimulus would be blunted due to the low ventral hippocampal drive of the dopamine system. However, if one is in an environment where threat/reward is high, such as hunting, competing for a mate, etc., the charged context would cause the ventral hippocampus to increase dopamine neuron population activity, engendering a large dopamine response to a behaviorally relevant stimulus.
Dopamine System Overdrive and Schizophrenia
What about in the case of schizophrenia? As reviewed above, there is substantial evidence that the dopamine system is hyper-responsive in schizophrenia patients; in particular, there is abnormally high amphetamine-induced dopamine release that correlates with exacerbation of psychosis (Laruelle and Abi-Dargham, 1999). Moreover, there is also increased baseline D2 stimulation in terms of D2 occupancy in the schizophrenia patient. This is consistent with what has been observed in a developmental animal model of schizophrenia. Specifically, one animal model that has proven effective in mimicking schizophrenia in rodents involves developmental disruption. A number of types of disruption, ranging from neonatal ventral hippocampal lesions (Lipska et al., 1993) to immune system activation (Meyer et al., 2005) also produce a state in which the rat is hyper-sensitive to amphetamine administration. A model that we have used that shows a number of parallels with schizophrenia is the prenatal injection of the mitotoxin methyl-azoxymethanol acetate (MAM). This model was developed in our laboratory (Grace and Moore, 1998; Moore et al., 2006) and extensively characterized (Flagstad et al., 2004; Gourevitch et al., 2004; Le Pen et al., 2006). In this case, the DNA methylating agent is injected into the pregnant dam during gestational day 17 and the offspring examined as adults. These rats display a number of features consistent with a model of schizophrenia, including thinning of limbic cortices without neuronal loss, alterations in sensory gating (prepulse inhibition of startle), disrupted learning (latent inhibition) disrupted executive function, social interactions, and hyper-responsivity to psychotomimetic drugs such as phencyclidine and PCP; all states consistent with schizophrenia (Flagstad et al., 2004; Moore et al., 2006; Talamini et al., 1999) (Gourevitch et al., 2004; Lodge et al., 2009). Furthermore, electrophysiological studies show that the dopamine system is also overdriven, in that the population activity of dopamine neurons is more than double that found in control rats, and moreover this corresponds with ventral hippocampal hyperactivity (Lodge and Grace, 2007). Indeed, this is consistent with the hyperactivity in the hippocampus of schizophrenia patients. What is the source of this hippocampal hyperactivity? In both the MAM rat and in the human schizophrenia patient, the limbic hippocampal region has shown to exhibit a significant reduction in staining for a particular class of GABAergic interneurons; i.e., those containing the marker parvalbumin (Lewis et al., 2001; Lodge et al., 2009; Zhang and Reynolds, 2002). Thus, a common loss of an important class of GABAergic interneurons may be responsible for the increased limbic hippocampal activity and consequently increased dopamine neuron population activity, rendering the dopamine system hyper-responsive to stimuli (Lodge et al., 2009).
Under the condition of abnormally high hippocampal drive of dopamine neuron population activity, the dopaminergic system would be rendered hyper-responsive to phasic stimuli. Thus, the level of amplification of the dopamine system is always turned to maximal. Under such a state, all stimuli, whether threatening, rewarding, or benign, would cause a maximal phasic activation of the dopamine system (Grace, 2010a, b). Therefore, there would be a mismatch between the actual behavioral salience of the object, and the much greater salience that is attributed by the dopaminergic response. As a result, all stimuli would be treated as one that requires maximal attention and reaction; a state that has been termed aberrant salience (Kapur, 2003).
Stress and the Pathophysiology of Schizophrenia
Taking together data from animal studies and human imaging, an explanation for the pathophysiology of schizophrenia points to a dysfunction of hippocampal interneurons leading to overdrive of tonic dopamine neuron population activity; this results in an abnormally amplified dopamine response to stimuli. However, what is the origin of the hippocampal dysfunction that leads to the delayed emergence of psychosis? Although direct evidence for this model does not exist, evidence gleaned from other studies provide the basis for a model integrating early stress exposure and hippocampal pathology. Thus, the fact that it appears to depend on the hippocampus is intriguing, since the hippocampus is a brain region that is known to be sensitive to stress. Maintained stressors are known to lead to hippocampal damage (Magarinos and McEwen, 1995; McEwen, 2000; Sapolsky, 2000), and high levels of the stress hormone cortisol are associated with a smaller hippocampus in first-episode schizophrenia patients (Mondelli et al., 2010). Stress-activated regions such as the amygdala send excitatory afferents to the hippocampus (French et al., 2003), and studies show that acute activation of the amygdala-hippocampal pathway by injection of picrotoxin into the amygdala also leads to parvalbumin interneuron damage in this region (Berretta et al., 2004). We have preliminary evidence (Lipski and Grace, in preparation) that acute stressors, such as restraint stress, increases c-fos in ventral hippocampal pyramidal neurons that project to the accumbens, whereas chronic stressors such as chronic cold stress increases excitability in the amygdala (Buffalari and Grace, 2009), which sends excitatory afferents to the hippocampus (French et al., 2003). There is also evidence of increased HPA activation people at risk for schizophrenia, which is consistent with stress effects (Garner et al., 2005). Indeed, another condition that leads to sustained activation of the hippocampus, i.e. temporal lobe epilepsy, is also associated with psychosis (Mendez et al., 1993), and is also known to lead to loss in hippocampal parvalbumin interneuron staining (Knopp et al., 2005). As a consequence of increased hippocampal activity, our model suggests that there would be increased dopamine neuron population activity, which we propose underlies the hyper-responsivity to amphetamine (Cifelli & Grace, submitted). Therefore, stress may be a factor in the etiology of schizophrenia via a deleterious action in the hippocampus. Whether this proposed stress-induced damage is due to the direct effects of chronic stress or instead reflect a failure of the HPA axis to respond appropriately to acute stressors is unknown. Indeed, it may be that the etiology may be different in different individuals.
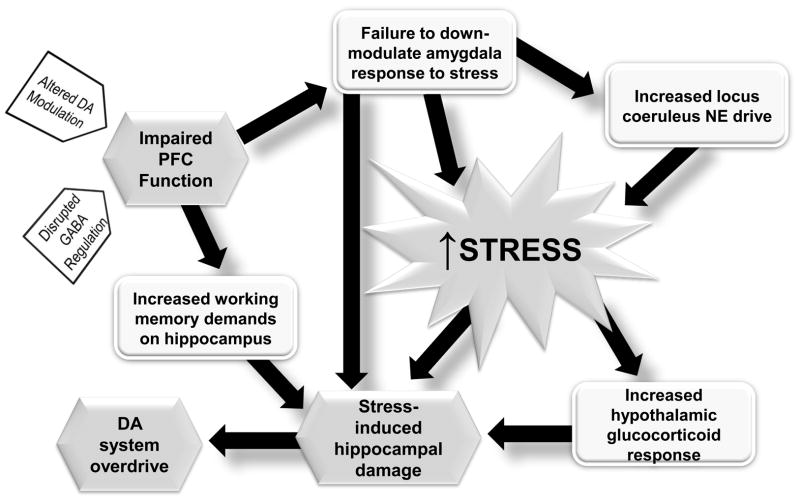
What is different about the schizophrenia patient that allows stressors to exert damage to the brain? One region that is known to play a substantial role in the regulation of stress is the medial prefrontal cortex. Thus, the impact of stressful stimuli on the amygdala is attenuated via activation of the prefrontal cortex (Rosenkranz et al., 2003). There is evidence that the prefrontal cortex may be dysfunctional early in schizophrenia, given evidence for executive dysfunction in the premorbid state (Silverstein et al., 2003). Moreover, data suggests that the increase in D1 receptors in the prefrontal cortex of schizophrenia is due to diminished dopamine action (Abi-Dargham and Moore, 2003), and dopamine is required for functional activation of the prefrontal cortex (Sawaguchi and Goldman-Rakic, 1994). Indeed, loss of prefrontal cortical dopamine will increase subcortical dopamine responses to stressors (Deutch et al., 1990; King and Finlay, 1997). Finally, studies of children that are at risk for schizophrenia show that the individuals that show abnormally high responsivity to stressors tend to be the people that convert to schizophrenia (Johnstone et al., 2002). Taken together, a picture emerges by which a dysfunction of the prefrontal cortex or its dopamine innervations early in life prevents effective regulation of stress responses. As a result, stressors would have a larger, less regulated impact on the brain of predisposed individuals, eventually leading to amygdala activation and hippocampal parvalbumin interneuron dysfunction, hippocampal hyperactivity, and psychosis (Grace, 2004; Thompson et al., 2004) (Figure 2).
Figure 2.
The medial prefrontal cortex (PFC) has a multitude of functions; among them being regulation of stress responses. Therefore, under stressful conditions DA is released into the PFC, which in turn can down-regulate stress responses subcortically. However, in schizophrenia, a premorbid dysfunction in the PFC, possibly due to disrupted GABAergic regulation, altered DA modulation, or other factors, interferes with these actions. As a result, the amygdala is overactivated by stressors, increasing the stress response. This will also lead to increased locus coeruleus drive which will further augment stress responses. Stress itself will activate the glucocorticoid system. In addition, the dysfunction of the PFC will necessitate the hippocampus to assume greater information processing responsibility. All of these effects will then act in concert to lead to hippocampal damage, parvalbumin interneuron dysfunction, and an overdrive of the dopamine system, leading to psychosis. Therefore, in the impaired system, unchecked stress responses will initiate a cascade of events in late adolescence that can culminate in hippocampal damage, dopamine system overdrive, and the emergence of psychosis.
Treatment
Schizophrenia is modeled above as a hippocampal pathology leading to an overdrive of the DA system. The treatment of schizophrenia for the past half century has focused almost exclusively on blocking overactivity within the DA system by blocking DA D2 receptors. However, whether simple receptor blockade is and adequate explanation for the efficacy of antipsychotic drugs remains in doubt. Thus, simple D2 blockade would be expected to induce several compensatory processes, such as increased DA release from increased DA neuron firing and DA release, as well as the development of D2 supersensitivity (Grace et al., 1997). Therefore, one would predict that this would enable an initial short-duration benefit followed by longer term compensation. However, it seems that the opposite has been observed in treating schizophrenia; i.e., an initial benefit that increases over weeks of treatment (Johnstone et al., 1978; Pickar et al., 1986; Pickar et al., 1984). One factor that was advanced to explain such a phenomenon is the development of DA neuron depolarization block (Grace et al., 1997). Thus, in normal rats, 3 or more weeks of repeated daily administration of antipsychotic drugs lead to an excitation-induced inactivation of DA neuron spike firing (Bunney and Grace, 1978; Grace et al., 1997). Moreover, the effect was region- and drug-specific, in that drugs that were effective at treating schizophrenia caused depolarization block in the limbic ventral tegmental area DA neurons, whereas drugs that induced extrapyramidal side-effects caused depolarization block in the substantia nigra DA neurons (Chiodo and Bunney, 1983; White and Wang, 1983). The decrease in number of DA neurons firing would, in effect reverse the increase in DA neuron population activity that the MAM model predicts would be present in schizophrenia.
On the other hand, more recent data has shown that, even though antipsychotic drugs do not exhibit tolerance, their onset of action can be comparatively rapid (Agid et al., 2003). At first approximation, this did not appear to be consistent with the findings of antipsychotic drug action in normal animals. However, schizophrenia patients are not “normal,” and if the DA system is in a state such as that observed in MAM-treated rats, the DA system would not be in the same state. Thus, although antipsychotic drugs cause an increase in DA neuron population activity in normal animals, in the MAM-treated rats the DA neurons are already near maximal population activity. In this condition, the DA system could not compensate for D2 blockade by increasing the number of DA neurons firing. How do antipsychotic drugs affect DA neuron firing in the MAM-treated rat? Consistent with that observed in schizophrenia patients, single doses of antipsychotic drugs can induce a rapid onset of depolarization block in about 50% of DA neurons in MAM-treated animals, bringing the population activity back down to control levels (Valenti et al., 2009). Therefore, in the case of an overactive DA system as is proposed to be present in the DA neurons in schizophrenia animals, antipsychotic drug action would have a more rapid onset due to the absence of a major means of compensation for antipsychotic drug blockade of D2 receptors.
Although D2 blockade is effective at partially alleviating the psychotic symptoms of schizophrenia, given the model stated above, this would not effectively treat the proposed cause of the disorder. Thus, blocking D2 receptors is at least 4 synapses downstream from the proposed pathology within the ventral hippocampus. Moreover, since depolarization block is not the normal state of the system, producing DA neuron depolarization block would not be directly addressing the pathology, but instead acting to produce an offsetting deficit with its own deleterious consequences (Grace et al., 1997). A more effective approach would be to restore GABAergic function within the ventral hippocampus. Of course, directly manipulating GABA would be problematic, given that GABAergic synapses account for more than a third of the synapses in the brain. However, the hippocampus is known to preferentially exhibit a unique subtype of the GABAA receptor; i.e., one that contains the alpha-5 subunit(Heldt and Ressler, 2007; Ramos et al., 2004; Serwanski et al., 2006). Since the alpha subunit is the region that binds benzodiazepines, specifically targeting this subunit with a benzodiazepine-like drug should selectively increase GABA tone in the hippocampus. Indeed, our studies show that using a GABAA positive allosteric modulator SH-053-2′F-R-CH3 (Savic et al., 2010) will selectively decrease ventral hippocampal excitability, as well as restore normal DA neuron population activity and reverse the hyper-responsivity to amphetamine (Gill et al., 2011).
Summary
Schizophrenia is a disorder that has been resistant to precise definition and treatment. Part of this may be due to the somewhat inappropriate focus on the DA system. However, recent studies suggest that this disorder may result from a pathology within the ventral hippocampus that leads to an overdrive of the DA system. By targeting treatments at the site of pathology rather than at the DA receptor, more effective pharmacotherapeutic approaches may be developed. There is also a major concern regarding treatment as it relates to the cognitive deficits in schizophrenia. Although direct evidence is forthcoming, one would expect that a disruption in hippocampal rhythmicity could also affect cognitive function. Thus, it is known that neocortical neuron gamma oscillatory activity is entrained by theta rhythms arising in the hippocampus (Sirota et al., 2008). This suggests that alteration of hippocampal rhythmic activity could lead to disruption in frontal cortical information processing, potentially leading to cognitive deficits in the schizophrenia patient. The fact that cognitive deficits worsen substantially at first break without substantial accompanying cortical damage is consistent with a neurophysiological origin of such deficits.
Highlights.
Animal and human studies identified the hippocampus as a site of pathology in schizophrenia
Parvalbumin interneuron loss in the hippocampus leads to dopamine system dysfunction
Restoring GABA transmission in the hippocampus can be achieved by an alpha 5 GABA drug
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abi-Dargham A, Moore H. Prefrontal DA transmission at D1 receptors and the pathology of schizophrenia. Neuroscientist. 2003;9:404–416. doi: 10.1177/1073858403252674. [DOI] [PubMed] [Google Scholar]
- Agid O, Kapur S, Arenovich T, Zipursky RB. Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Archives of General Psychiatry. 2003;60:1228–1235. doi: 10.1001/archpsyc.60.12.1228. [DOI] [PubMed] [Google Scholar]
- Angrist B, Sathananthan G, Wilk S, Gershon S. Amphetamine psychosis: behavioral and biochemical aspects. Journal of Psychiatric Research. 1974;11:13–23. doi: 10.1016/0022-3956(74)90064-8. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Bullmore ET, Meyer-Lindenberg A, Apud JA, Weinberger DR, Coppola R. Cognitive fitness of cost-efficient brain functional networks. Proc Natl Acad Sci U S A. 2009;106:11747–11752. doi: 10.1073/pnas.0903641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman KF, Weinberger DR. The prefrontal cortex in schizophrenia and other neuropsychiatric diseases: in vivo physiological correlates of cognitive deficits. Progress in Brain Research. 1990;85:521–536. doi: 10.1016/s0079-6123(08)62698-9. discussion 536-527. [DOI] [PubMed] [Google Scholar]
- Berretta S, Lange N, Bhattacharyya S, Sebro R, Garces J, Benes FM. Long-term effects of amygdala GABA receptor blockade on specific subpopulations of hippocampal interneurons. Hippocampus. 2004;14:876–894. doi: 10.1002/hipo.20002. [DOI] [PubMed] [Google Scholar]
- Beuger M, van Kammen DP, Kelley ME, Yao J. Dopamine turnover in schizophrenia before and after haloperidol withdrawal. CSF, plasma, and urine studies. Neuropsychopharmacology. 1996;15:75–86. doi: 10.1016/0893-133X(95)00158-A. [DOI] [PubMed] [Google Scholar]
- Buffalari DM, Grace AA. Chronic cold stress increases excitatory effects of norepinephrine on spontaneous and evoked activity of basolateral amygdala neurons. Int J Neuropsychopharmacol. 2009;12:95–107. doi: 10.1017/S1461145708009140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunney BS, Grace AA. Acute and chronic haloperidol treatment: comparison of effects on nigral dopaminergic cell activity. Life Sciences. 1978;23:1715–1727. doi: 10.1016/0024-3205(78)90471-x. [DOI] [PubMed] [Google Scholar]
- Chiodo LA, Bunney BS. Typical and atypical neuroleptics: differential effects of chronic administration on the activity of A9 and A10 midbrain dopaminergic neurons. J Neurosci. 1983;3:1607–1619. doi: 10.1523/JNEUROSCI.03-08-01607.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad AJ, Abebe T, Austin R, Forsythe S, Scheibel AB. Hippocampal pyramidal cell disarray in schizophrenia as a bilateral phenomenon. Arch Gen Psychiatry. 1991;48:413–417. doi: 10.1001/archpsyc.1991.01810290025003. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Clark WA, Roth RH. Prefrontal cortical dopamine depletion enhances the responsiveness of mesolimbic dopamine neurons to stress. Brain Res. 1990;521:311–315. doi: 10.1016/0006-8993(90)91557-w. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res. 2000;110:73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- Flagstad P, Mork A, Glenthoj BY, van Beek J, Michael-Titus AT, Didriksen M. Disruption of neurogenesis on gestational day 17 in the rat causes behavioral changes relevant to positive and negative schizophrenia symptoms and alters amphetamine-induced dopamine release in nucleus accumbens. Neuropsychopharmacology. 2004;29:2052–2064. doi: 10.1038/sj.npp.1300516. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. Journal of Neuroscience. 2001;21:4915–4922. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nature Neuroscience. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- French SJ, Hailstone JC, Totterdell S. Basolateral amygdala efferents to the ventral subiculum preferentially innervate pyramidal cell dendritic spines. Brain Res. 2003;981:160–167. doi: 10.1016/s0006-8993(03)03017-8. [DOI] [PubMed] [Google Scholar]
- Garner B, Pariante CM, Wood SJ, Velakoulis D, Phillips L, Soulsby B, Brewer WJ, Smith DJ, Dazzan P, Berger GE, Yung AR, van den Buuse M, Murray R, McGorry PD, Pantelis C. Pituitary volume predicts future transition to psychosis in individuals at ultra-high risk of developing psychosis. Biol Psychiatry. 2005;58:417–423. doi: 10.1016/j.biopsych.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Gill KM, Lodge DJ, Cook JM, Aras S, Grace AA. A novel α5GABAAR positive allosteric modulator reverses hyperactivation of the dopamine system in the MAM model of schizophrenia. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.76. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Philos Trans R Soc Lond B Biol Sci. 1996;351:1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- Gourevitch R, Rocher C, Le Pen G, Krebs MO, Jay TM. Working memory deficits in adult rats after prenatal disruption of neurogenesis. Behavioral Pharmacology. 2004;15:287–292. doi: 10.1097/01.fbp.0000135703.48799.71. [DOI] [PubMed] [Google Scholar]
- Grace AA. Gating of information flow within the limbic system and the pathophysiology of schizophrenia. Brain Research - Brain Research Reviews. 2000;31:330–341. doi: 10.1016/s0165-0173(99)00049-1. [DOI] [PubMed] [Google Scholar]
- Grace AA. Developmental dysregulation of the dopamine system and the pathophysiology of schizophrenia. In: Keshavan MS, Kennedy JL, Murray RM, editors. Neurodevelopment and Schizophrenia. Cambridge University Press; Cambridge, UK: 2004. pp. 273–294. [Google Scholar]
- Grace AA. Dopamine system dysregulation by the ventral subiculum as the common pathophysiological basis for schizophrenia psychosis, psychostimulant abuse, and stress. Neurotox Res. 2010a;18:367–376. doi: 10.1007/s12640-010-9154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA. Ventral hippocampus, interneurons and schizophrenia: A new understanding of the pathophysiology of schizophrenia and its implications for treatment and prevention. Current Directions in Psychological Science. 2010b;19:232–237. [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: single spike firing. Journal of Neuroscience. 1984;4:2866–2876. doi: 10.1523/JNEUROSCI.04-11-02866.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Opposing effects of striatonigral feedback pathways on midbrain dopamine cell activity. Brain Research. 1985;333:271–284. doi: 10.1016/0006-8993(85)91581-1. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS, Moore H, Todd CL. Dopamine-cell depolarization block as a model for the therapeutic actions of antipsychotic drugs. Trends in Neurosciences. 1997;20:31–37. doi: 10.1016/S0166-2236(96)10064-3. [DOI] [PubMed] [Google Scholar]
- Grace AA, Moore H. Regulation of information flow in the nucleus accumbens: A model for the pathophysiology of schizophrenia. In: Lenzenweger MF, Dworkin RH, editors. Origins and development of schizophrenia: Advances in experimental psychopathology. American Psychological Association Press; Washington D.C: 1998. pp. 123–157. [Google Scholar]
- Heckers S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus. 2001;11:520–528. doi: 10.1002/hipo.1068. [DOI] [PubMed] [Google Scholar]
- Heldt SA, Ressler KJ. Forebrain and midbrain distribution of major benzodiazepine-sensitive GABAA receptor subunits in the adult C57 mouse as assessed with in situ hybridization. Neuroscience. 2007;150:370–385. doi: 10.1016/j.neuroscience.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingvar DH, Franzen G. Distribution of cerebral activity in chronic schizophrenia. Lancet. 1974;2:1484–1486. doi: 10.1016/s0140-6736(74)90221-9. [DOI] [PubMed] [Google Scholar]
- Janowsky DS, el-Yousel MK, Davis JM, Sekerke HJ. Provocation of schizophrenic symptoms by intravenous administration of methylphenidate. Arch Gen Psychiatry. 1973;28:185–191. doi: 10.1001/archpsyc.1973.01750320023004. [DOI] [PubMed] [Google Scholar]
- Jarrard LE. What does the hippocampus really do? Behavioural Brain Research. 1995;71:1–10. doi: 10.1016/0166-4328(95)00034-8. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Johnstone EC, Crow TJ, Frith CD, Carney MWP, Price JS. Mechanism of the antipsychotic effect in the treatment of acute schizophrenia. Lancet April. 1978;22:848–851. doi: 10.1016/s0140-6736(78)90193-9. [DOI] [PubMed] [Google Scholar]
- Johnstone EC, Lawrie SM, Cosway R. What does the Edinburgh High-Risk Study tell us about schizophrenia? American Journal of Medical Genetics (Neuropsychiatric Genetics) 2002;114:906–912. doi: 10.1002/ajmg.b.10304. [DOI] [PubMed] [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. American Journal of Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Kapur S, Remington G. Dopamine D(2) receptors and their role in atypical antipsychotic action: still necessary and may even be sufficient. Biological Psychiatry. 2001;50:873–883. doi: 10.1016/s0006-3223(01)01251-3. [DOI] [PubMed] [Google Scholar]
- Kapur S, Zipursky R, Jones C, Remington G, Houle S. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. American Journal of Psychiatry. 2000;157:514–520. doi: 10.1176/appi.ajp.157.4.514. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Shungu DC, Anjilvel S, Chan S, Ellis SP, Xanthopoulos E, Malaspina D, Gorman JM, Mann JJ, Laruelle M, Kaufmann CA. Hippocampal pathology in schizophrenia: magnetic resonance imaging and spectroscopy studies. Psychiatry Res. 2000;98:163–175. doi: 10.1016/s0925-4927(00)00044-5. [DOI] [PubMed] [Google Scholar]
- King D, Finlay JM. Loss of dopamine terminals in the medial prefrontal cortex increased the ratio of DOPAC to DA in tissue of the nucleus accumbens shell: role of stress. Brain Res. 1997;767:192–200. doi: 10.1016/s0006-8993(97)00534-9. [DOI] [PubMed] [Google Scholar]
- Knopp A, Kivi A, Wozny C, Heinemann U, Behr J. Cellular and network properties of the subiculum in the pilocarpine model of temporal lobe epilepsy. J Comp Neurol. 2005;483:476–488. doi: 10.1002/cne.20460. [DOI] [PubMed] [Google Scholar]
- Kovelman JA, Scheibel AB. A neurohistological correlate of schizophrenia. Biol Psychiatry. 1984;19:1601–1621. [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A. Dopamine as the wind of psychotic fire: new evidence from brain imaging studies. Journal of Psychopharmacology. 1999;13:358–371. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]
- Le Pen G, Gourevitch R, Hazane F, Hoareau C, Jay TM, Krebs MO. Peri-pubertal maturation after developmental disturbance: a model for psychosis onset in the rat. Neuroscience. 2006;143:395–405. doi: 10.1016/j.neuroscience.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Cruz DA, Melchitzky DS, Pierri JN. Lamina-specific deficits in parvalbumin-immunoreactive varicosities in the prefrontal cortex of subjects with schizophrenia: evidence for fewer projections from the thalamus. Am J Psychiatry. 2001;158:1411–1422. doi: 10.1176/appi.ajp.158.9.1411. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Jaskiw GE, Weinberger DR. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacology. 1993;9:67–75. doi: 10.1038/npp.1993.44. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. Journal of Neuroscience. 2009;29:2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology. 2006;31:1356–1361. doi: 10.1038/sj.npp.1300963. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27:11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Storer S, Furman V, Esser P, Printz D, Berman A, Lignelli A, Gorman J, Van Heertum R. SPECT study of visual fixation in schizophrenia and comparison subjects. Biol Psychiatry. 1999;46:89–93. doi: 10.1016/s0006-3223(98)00306-0. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurotoxic or electrolytic lesions of the ventral subiculum produce deficits in the acquisition and expression of Pavlovian fear conditioning in rats. Behav Neurosci. 1999;113:283–290. doi: 10.1037//0735-7044.113.2.283. [DOI] [PubMed] [Google Scholar]
- Maslowski-Cobuzzi RJ, Napier TC. Activation of dopaminergic neurons modulates ventral pallidal responses evoked by amygdala stimulation. Neuroscience. 1994;62:1103–1119. doi: 10.1016/0306-4522(94)90347-6. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- Medoff DR, Holcomb HH, Lahti AC, Tamminga CA. Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus. 2001;11:543–550. doi: 10.1002/hipo.1070. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Grau R, Doss RC, Taylor JL. Schizophrenia in epilepsy: seizure and psychosis variables. Neurology. 1993;43:1073–1077. doi: 10.1212/wnl.43.6.1073. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schedlowski M, Yee BK. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev. 2005;29:913–947. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Brudzynski SM, Wu M, Yang CR, Yim CCY. From motivation to action: a review of dopaminergic regulation of limbic -> nucleus accumbens -> ventral pallidum -> pedunculopontine nucleus circuitries involved in limbic-motor integration. In: Kalivas PW, Barnes CW, editors. Limbic motor circuits and neuropsychiatry. CRC; Boca Raton: 1993. pp. 193–236. [Google Scholar]
- Mondelli V, Pariante CM, Navari S, Aas M, D’Albenzio A, Di Forti M, Handley R, Hepgul N, Marques TR, Taylor H, Papadopoulos AS, Aitchison KJ, Murray RM, Dazzan P. Higher cortisol levels are associated with smaller left hippocampal volume in first-episode psychosis. Schizophr Res. 2010;119:75–78. doi: 10.1016/j.schres.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Grace AA. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biol Psychiatry. 2006;60:253–264. doi: 10.1016/j.biopsych.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J. A review of the hippocampal place cells. Prog Neurobiol. 1979;13:419–439. doi: 10.1016/0301-0082(79)90005-4. [DOI] [PubMed] [Google Scholar]
- Pickar D, Labarca R, Doran AR, Wolkowitz OM, Roy A, Breier A, Linnoila M, Paul SM. Longitudinal measurement of plasma homovanillic acid levels in schizophrenic patients. Archives of General Psychiatry. 1986;43:669–676. doi: 10.1001/archpsyc.1986.01800070059008. [DOI] [PubMed] [Google Scholar]
- Pickar D, Labarca R, Linnoila M, Roy A, Hommer D, Everett D, Paul SM. Neuroleptic-induced decrease in plasma homovanillic acid and antipsychotic activity in schizophrenic patients. Science. 1984;225:954–957. doi: 10.1126/science.6474162. [DOI] [PubMed] [Google Scholar]
- Post RM, Fink E, Carpenter WT, Jr, Goodwin FK. Cerebrospinal fluid amine metabolites in acute schizophrenia. Arch Gen Psychiatry. 1975;32:1063–1069. doi: 10.1001/archpsyc.1975.01760260127011. [DOI] [PubMed] [Google Scholar]
- Ramos B, Lopez-Tellez JF, Vela J, Baglietto-Vargas D, del Rio JC, Ruano D, Gutierrez A, Vitorica J. Expression of alpha 5 GABAA receptor subunit in developing rat hippocampus. Brain Res Dev Brain Res. 2004;151:87–98. doi: 10.1016/j.devbrainres.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. Journal of Neuroscience. 2003;23:11054–11064. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Savic MM, Majumder S, Huang S, Edwankar RV, Furtmuller R, Joksimovic S, Clayton T, Sr, Ramerstorfer J, Milinkovic MM, Roth BL, Sieghart W, Cook JM. Novel positive allosteric modulators of GABAA receptors: do subtle differences in activity at alpha1 plus alpha5 versus alpha2 plus alpha3 subunits account for dissimilarities in behavioral effects in rats? Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:376–386. doi: 10.1016/j.pnpbp.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. The role of D1-dopamine receptor in working memory: local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed-response task. J Neurophysiol. 1994;71:515–528. doi: 10.1152/jn.1994.71.2.515. [DOI] [PubMed] [Google Scholar]
- Serwanski DR, Miralles CP, Christie SB, Mehta AK, Li X, De Blas AL. Synaptic and nonsynaptic localization of GABAA receptors containing the alpha5 subunit in the rat brain. J Comp Neurol. 2006;499:458–470. doi: 10.1002/cne.21115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp PE. Complimentary roles for hippocampal versus subicular/entorhinal place cells in coding place, context, and events. Hippocampus. 1999;9:432–443. doi: 10.1002/(SICI)1098-1063(1999)9:4<432::AID-HIPO9>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Silbersweig DA, Stern E, Frith C, Cahill C, Holmes A, Grootoonk S, Seaward J, McKenna P, Chua SE, Schnorr L, et al. A functional neuroanatomy of hallucinations in schizophrenia. Nature. 1995;378:176–179. doi: 10.1038/378176a0. [DOI] [PubMed] [Google Scholar]
- Silverstein ML, Mavrolefteros G, Turnbull A. Premorbid factors in relation to motor, memory, and executive functions deficits in adult schizophrenia. Schizophr Res. 2003;61:271–280. doi: 10.1016/s0920-9964(02)00312-2. [DOI] [PubMed] [Google Scholar]
- Sirota A, Montgomery S, Fujisawa S, Isomura Y, Zugaro M, Buzsaki G. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron. 2008;60:683–697. doi: 10.1016/j.neuron.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suddath RL, Christison GW, Torrey EF, Casanova MF, Weinberger DR. Anatomical abnormalities in the brains of monozygotic twins discordant for schizophrenia. The New England Journal of Medicine. 1990;322:789–794. doi: 10.1056/NEJM199003223221201. [DOI] [PubMed] [Google Scholar]
- Talamini LM, Koch T, Luiten PG, Koolhaas JM, Korf J. Interruptions of early cortical development affect limbic association areas and social behaviour in rats; possible relevance for neurodevelopmental disorders. Brain Res. 1999;847:105–120. doi: 10.1016/s0006-8993(99)02067-3. [DOI] [PubMed] [Google Scholar]
- Thompson JL, Pogue-Geile MF, Grace AA. The interactions among developmental pathology, dopamine, and stress as a model for the age of onset of schizophrenia symptomatology. Schizophr Bull. 2004;30:875–900. doi: 10.1093/oxfordjournals.schbul.a007139. [DOI] [PubMed] [Google Scholar]
- Valenti O, Cifelli p, Grace AA. Program No. 646.10 Neuroscience Meeting Planner. Chicago, Il: Society for Neuroscience; 2009. Antipsychotic drugs elicit a rapid depolarization inactivation of VTA DA neuron firing in rat model of schizophrenia via the nucleus accumbens-ventral pallidal pathway. Online. [Google Scholar]
- Valenti O, Grace AA. Antipsychotic drug-induced increases in ventral tegmental area dopamine neuron population activity via activation of the nucleus accumbens-ventral pallidum pathway. Int J Neuropsychopharmacol. 2009:1–16. doi: 10.1017/S1461145709990599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kammen DP, van Kammen WB, Mann LS, Seppala T, Linnoila M. Dopamine metabolism in the cerebrospinal fluid of drug-free schizophrenic patients with and without cortical atrophy. Arch Gen Psychiatry. 1986;43:978–983. doi: 10.1001/archpsyc.1986.01800100072010. [DOI] [PubMed] [Google Scholar]
- van Rossum JM. The significance of dopamine-receptor blockade for the mechanism of action of neuroleptic drugs. Arch Int Pharmacodyn Ther. 1966;160:492–494. [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Gallhofer B. Cognitive function in schizophrenia. International Clinical Psychopharmacology. 1997;12:S29–36. doi: 10.1097/00004850-199709004-00006. [DOI] [PubMed] [Google Scholar]
- White FJ, Wang RY. Differential effects of classical and atypical antipsychotic drugs on A9 and A10 dopamine neurons. Science. 1983;221:1054–1057. doi: 10.1126/science.6136093. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Reynolds GP. A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr Res. 2002;55:1–10. doi: 10.1016/s0920-9964(01)00188-8. [DOI] [PubMed] [Google Scholar]