Abstract
Aims:
Salt sensitivity, a trait characterized by a pressor blood pressure (BP) response to increased dietary salt intake, has been associated with higher rates of cardiovascular target organ damage and cardiovascular disease events. Recent experimental studies have highlighted the potential role of the natriuretic peptides and aldosterone in mediating salt sensitivity.
Methods and Results:
We evaluated 1575 non-hypertensive Framingham Offspring cohort participants (mean age 55±9 years, 58% women) who underwent routine measurements of circulating aldosterone and N-terminal proatrial natriuretic peptide (NT-ANP) and assessment of dietary sodium intake. Participants were categorized as potentially ‘salt-sensitive’ if their serum aldosterone was >sex-specific median but plasma NT-ANP was ≤sex-specific median value. Dietary sodium intake was categorized as lower versus higher (dichotomized at the sex-specific median). We used multivariable linear regression to relate presence of salt sensitivity (as defined above) to longitudinal changes (Δ) in systolic and diastolic BP on follow-up (median 4 years). Participants who were ‘salt-sensitive’ (N=437) experienced significantly greater increases in BP (Δ systolic, +4.4 and +2.3 mmHg; Δ diastolic, +1.9 and −0.3 mmHg; on a higher versus lower sodium diet, respectively) as compared to the other participants (Δ systolic, +2.8 and +1.0 mmHg; Δ diastolic, +0.5 and −0.2 mmHg; on higher versus lower sodium diet, respectively; p=0.033 and p=0.0127 for differences between groups in Δ systolic and Δ diastolic BP, respectively).
Conclusion:
Our observational data suggest that higher circulating aldosterone and lower NT-ANP concentrations may be markers of salt sensitivity in the community. Additional studies are warranted to confirm these observations.
Keywords: salt sensitivity, aldosterone, N-terminal proatrial natriuretic peptide, ANP
Introduction
“Sensitivity“ to salt, characterized by a pressor BP response with greater intake of dietary salt (sodium chloride), has been identified as an important contributing factor to the development of high BP in some individuals. The prevalence of salt sensitivity has been estimated to be as high as 26% in non-hypertensive individuals and 51% in patients with hypertension.1 Salt sensitivity has also been associated with greater cardiovascular target organ damage and an increased risk of cardiovascular disease (CVD) events.2, 3
Identification of salt sensitivity in the community is challenged by varying definitions for the condition,3, 4 the requirement of a saline/salt challenge to identify people1, 3, 5 (which is not practical at a general population level), and the limited reproducibility of pressor responses to salt intake within individuals.6 We hypothesized that serum biomarkers involved in renal sodium homeostasis may be potentially useful for identifying salt sensitive individuals in the community. Aldosterone and atrial natriuretic peptide (ANP) are both involved in renal sodium handling7, 8 and experimental studies strongly support important opposing roles for these hormones in BP regulation.9, 10 For instance, transgenic animals that underexpress or lack ANP manifest salt sensitivity.9 And mice with targeted disruption of the aldosterone synthase gene display low blood pressure and impaired Na+ and water absorption in the kidney.11 We, therefore, postulated that non-hypertensive individuals with lower circulating NT-ANP but higher aldosterone concentrations will be prone to renal sodium retention, and thus, may experience greater increases in BP prospectively, a trait that may be modified by the amount of dietary salt intake. We analyzed longitudinal changes in BP modeled as a continuous variable (as opposed to incident hypertension or progression of BP stage) because changes in dietary salt are associated with modest changes in systolic and diastolic BP.12
Methods
Study sample
Details about the study sample are displayed in the online supplement. Participants free of hypertension (BP≥140/90 mm Hg or on antihypertensive medication) and CVD at the sixth Offspring examination cycle (1995-1998, the baseline examination) with available measurements of circulating aldosterone and NT-ANP, and who attended the follow-up examination cycle 7 (1998-2001) were eligible for the present investigation (n=1575). The study protocol was approved by Boston University Medical Center; all participants provided written informed consent and the study complies with the Declaration of Helsinki.
Blood pressure measurement
During each Heart Study visit, the BP measurements are obtained twice by a physician after the participant had been sitting for about 5 minutes using a mercury column sphygmomanometer and a standardized protocol as detailed in the online supplement. The average of both physician-obtained measurements was considered the examination BP.
Biomarker measurements
Blood was drawn at the baseline examination from fasting and supine participants in the early morning after for 5-10 minutes rest. Blood samples were immediately centrifuged and stored at −80° C until biomarkers were assayed. Plasma NT-ANP and serum aldosterone were measured with a high-sensitivity immunoradiometric assay (Shionogi Inc., Japan) and a radioimmunoassay (Quest Diagnostics, MA), respectively. The average interassay coefficients of variation were 12.7% for NT-ANP, and 4.0% (high concentrations) and 9.8% (low concentrations) for aldosterone.
Statistical analyses
Classification of participants according to their aldosterone and NT-ANP levels and their dietary sodium intake
Participants were classified based on whether their serum aldosterone and plasma NT-ANP levels were above (>) or equal or below (≤) the sex-specific median into one of the two groups:
1. Serum aldosterone > and NT-ANP levels ≤ the sex-specific median (referred to as potentially ‘salt-sensitive’); 2. All others were categorized as the referent group. The median serum aldosterone levels were 10 and 9 ng/dL in women and men, respectively. Median plasma NT-ANP levels were 327 and 250 pmol/L for women and men, respectively.
On a parallel note, individuals were classified according to whether their dietary sodium intake (using the Food Frequency Questionnaire13 at the baseline examination) was higher (defined as >the sex-specific median) versus lower (defined as ≤the sex-specific median). Median dietary sodium intake was 2047 mg in women, and 2217 mg in men, although this likely reflects an underestimation because of the instrument used. Thus, participants could be categorized into the following 4 groups: 1. Individuals who were potentially salt-sensitive and had higher dietary sodium intake; 2. Individuals who were potentially salt-sensitive but had a lower dietary sodium intake; 3. Individuals in the referent group who had a higher dietary sodium intake; 4. Individuals in the referent group who had a lower dietary sodium intake.
Assessing construct validity of proposed categorization schema
In order to assess the validity of using NT-ANP and aldosterone to categorize salt sensitivity status, and dietary sodium intake to classify lower versus higher salt consumption in the context of the BP outcomes of interest, we used generalized additive models (Using proc GAM in SAS with its default settings: cubic smoothing splines with 4 degrees of freedom) to plot the relations of serum aldosterone, plasma NT-ANP and dietary sodium intake individually (each analyzed as a continuous variable) to Δ systolic and Δ diastolic BP during follow-up adjusting for key covariates and accounting for BP treatment on follow-up (see section below). Specifically, we adjusted for the following variables: sex, age, systolic and diastolic BP, and body mass index (BMI) at baseline, as well as percent weight change on follow-up. We evaluated if these plots suggested non-linearity or were consistent with linearity assumption of relations for each of these 3 variables (serum aldosterone, plasma NT-ANP and dietary sodium intake) with systolic and diastolic BP and if the directionality of association was inverse for NT-ANP but positive for aldosterone and dietary sodium.
Comparison of longitudinal blood pressure changes during follow-up according to presence or absence of potential salt sensitivity and dietary sodium intake
We used linear regression models to evaluate whether there were statistically significant differences in the change (Δ) in systolic and diastolic BP during follow-up (from examination cycle 6 to 7) between the 4 groups defined above based on presence versus absence of salt sensitivity and lower versus higher dietary sodium intake. We adjusted for age, sex, percent weight change on follow-up, and baseline BMI, systolic and diastolic BP. An imputation method previously used by our group was used to estimate BP on follow up among treated individuals, accounting for the use of antihypertensive treatment (5.5% of individuals).14 Separate analyses were conducted for Δ systolic and Δ diastolic BP. In secondary analyses, we additionally adjusted for total dietary caloric intake, dietary potassium intake, fasting serum glucose, heart rate, and estimated glomerular filtration rate (eGFR).15 eGFR was included as a potential confounder, because it has been shown to be correlated with aldosterone levels16 and also affects blood pressure. Likewise, low potassium levels seem to play a role in the pathogenesis of hypertension and might interact with aldosterone.17, 18 We assessed the homogeneity in systolic and diastolic BP changes on follow-up in the 3 groups combined into the referent group (individuals without putative salt sensitivity; thus with aldosterone levels below the sex-specific median and/or NT-ANP levels above the sex-specific median). We also performed sensitivity analyses: defining salt sensitivity based on sex-specific tertiles of aldosterone and NT-ANP levels (a value in the top aldosterone tertile and in the lowest NT-ANP tertile was considered salt sensitive); and defining high dietary sodium intake as a value in the top sex-specific tertile. Furthermore, we tested for effect modification by BMI and by dietary potassium intake by including respective interaction terms in the multivariable-adjusted models evaluating changes in systolic and diastolic BP. Finally, we tested for interaction between potential salt sensitivity and the metabolic syndrome,19 but this interaction was not significant in the age- and sex-adjusted as well as in fully adjusted models predicting change in SBP and DBP (all p-values>0.22). SAS 9.1 was used for all statistical analyses and statistical significance was defined as a 2-sided p-value below 0.05.
Results
The baseline characteristics of our sample are shown in Table 1. About one quarter of the study sample was potentially salt sensitive by definition (using sex-specific median cut points for serum aldosterone and plasma NT-ANP). Potentially salt sensitive participants were slightly younger and had higher baseline levels of diastolic BP and BMI.
Table 1.
Baseline characteristics of the study sample according to presence or absence of potential salt sensitivity and lower vs. higher dietary sodium intake (≤ vs. >sex-specific median)
| Potentially salt-sensitive individuals* | Referent individuals* | ||||
|---|---|---|---|---|---|
| Dietary sodium intake* | Dietary sodium intake* | ||||
| All (n=1575) | Lower (n=219) | Higher (n=218 ) | Lower (n=559) | Higher (n=579 ) | |
| Women, % | 58% | 55% | 59% | 60% | 58% |
| Age, years, mean (SD) | 55 (9) | 53 (8) | 52 (8) | 57 (9) | 56 (9) |
| Systolic BP, mm Hg, mean (SD) | 118 (12) | 117 (12) | 118 (12) | 119 (11) | 119 (12) |
| Diastolic BP, mm Hg, mean (SD) | 73 (8) | 74 (8) | 75 (7) | 72 (8) | 73 (8) |
| Body mass index, kg/m2, mean (SD) | 27.0 (4.8) | 27.7 (4.9) | 28.3 (5.3) | 26.4 (4.3) | 26.9 (4.9) |
| Aldosterone, ng/dL, median (Q1, Q3) | 10.0 (7.0,14.0) | 14.0 (12.0,18.0) | 14.5 (12.0,18.0) | 8.0 (6.0,11.0) | 8.0 (6.0,11.0) |
| NT-ANP, pmol/L, median (Q1, Q3) | 295 (207,402) | 203 (153,254) | 196 (143,247) | 353 (269,469) | 338 (246,445) |
| Dietary sodium intake, mg/day, median (Q1, Q3) | 2120 (1610,2644) | 1639 (1326,1904) | 2646 (2388,3247) | 1596 (1309,1848) | 2621 (2345,3095) |
| Total caloric intake, UNIT, median (Q1, Q3) | 1827 (1420,2243) | 1475 (1220,1742) | 2267 (1917,2650) | 1427 (1208,1717) | 2189 (1907,2584) |
|
Dietary potassium intake, mg/day, median (Q1, Q3) |
2888 (2310,3580) | 2332 (1924,2893) | 3420 (2924,3986) | 2447 (1981,2863) | 3476 (2876,4001) |
| Fasting glucose, mg/dL, mean (SD) | 97.4 (16.5) | 99.3 (23.9) | 99.4 (16.9) | 96.1 (13.7) | 97.3 (15.3) |
| Heart rate, bpm, mean (SD) | 63 (9) | 64 (10) | 65 (10) | 62 (9) | 63 (9) |
| eGFR, ml/min/1.73m2), mean (SD) | 92.5 (46.3) | 98.2 (75.5) | 97.3 (34.7) | 91.0 (48.8) | 90.0 (30.1) |
See text (Methods) for definitions of salt sensitivity and lower/higher sodium intake.
BP, blood pressure; bpm, beats per minute; eGFR, estimated glomerular filtration rate; NT-ANP, N-terminal proatrial natriuretic peptide; Q1 and Q3 refer to first and third quartile cutpoints; SD, standard deviation.
Construct Validity of Classification Schema
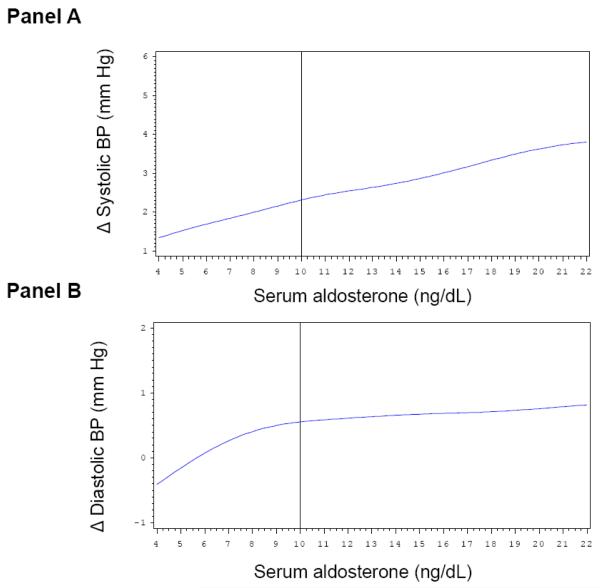
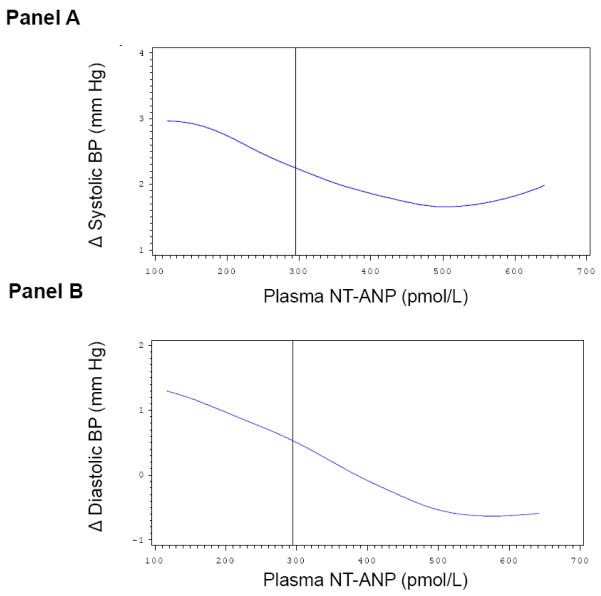
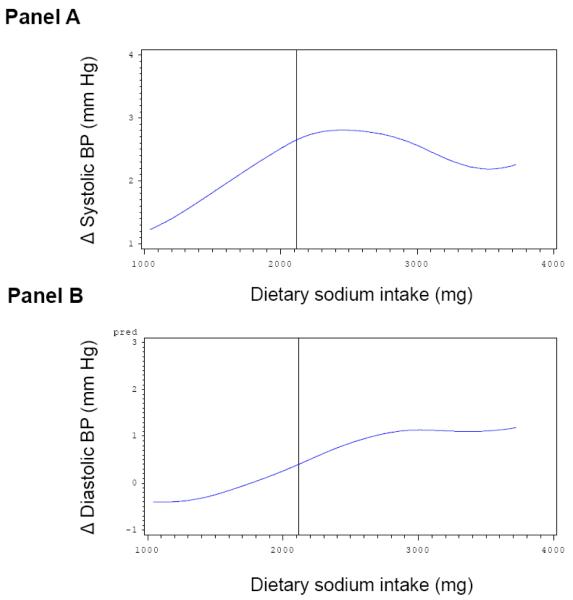
Multivariable-adjusted splines displayed approximately linearly increasing Δ systolic BP (Figure 1, Panel A) and Δ diastolic BP levels (Figure 1, Panel B) on follow-up with increasing baseline serum aldosterone concentrations. However, the magnitude for individual change in BP according to baseline serum aldosterone concentrations was small and did not reach statistical significance. On a parallel note, changes in systolic and diastolic BP during follow-up were inversely and linearly associated with baseline plasma NT-ANP (Figure 2, Panel A and B, respectively). Similarly, higher dietary sodium intake was associated with an increase in systolic and diastolic BP on follow-up (Figure 3, Panel A and B). The solid vertical line in these Figures indicates the pooled-sex median levels.
Figure 1.
Multivariable adjusted splines relating serum aldosterone levels (x-axis) to change in systolic (Panel A) and diastolic (Panel B) BP on follow-up. Δ, change in BP between examination cycle 6 and 7. Solid vertical line corresponds to the sex-pooled median serum aldosterone (10 ng/dL). Adjustment for: sex, age, systolic and diastolic BP, baseline BMI and percent weight change on follow-up.
Figure 2.
Multivariable adjusted splines relating plasma N-terminal proatrial natriuretic peptide (NT-ANP, x-axis) to change in systolic (Panel A) and diastolic (Panel B) BP on follow-up. Δ, change in BP between examination cycle 6 and 7. Solid vertical line corresponds to the sex-pooled median plasma NT-ANP (295 pmol/L). Adjustment for: sex, age, systolic and diastolic BP, baseline BMI and percent weight change on follow-up.
Figure 3.
Multivariable adjusted splines relating dietary sodium intake (x-axis) to change in systolic (Panel A) and diastolic (Panel B) BP on follow-up. Δ, change in BP between examination cycle 6 and 7. Solid vertical line corresponds to the sex-pooled median dietary sodium intake (2120 mg/day). Adjustment for: sex, age, systolic and diastolic BP, baseline BMI and percent weight change on follow-up.
Increase in blood pressure during follow-up according to presence versus absence of salt sensitivity as defined by biomarkers
We observed statistically significant differences in Δ systolic and Δ diastolic BP on follow-up between the 4 groups defined based on potential salt sensitivity (based on higher serum aldosterone and lower plasma NT-ANP concentrations) and dietary salt intake (p-values 0.016 for systolic and <0.0001 for diastolic blood pressure; Table 2). Individuals who were potentially salt sensitive with a salt intake above the sex-specific median had the highest mean increase in both systolic and diastolic blood pressure. On the other hand, individuals who were not salt sensitive and had lower sodium intake had the lowest mean increase in systolic blood pressure and a mean slight decrease in diastolic blood pressure (Table 2). Secondary analyses revealed that the changes in systolic and diastolic BP in individuals collapsed into the referent group (individuals without putative salt sensitivity; thus with either aldosterone levels below the sex-specific median and/or NT-ANP levels above the sex-specific median) was relatively homogeneous (data not shown). Adjusting for total dietary potassium intake, fasting serum glucose, heart rate, and estimated glomerular filtration rate (eGFR) did not alter the findings noted above (results in Table 2 were unchanged). We did not observe effect modification by BMI or by dietary potassium intake (p values for interaction terms exceeded 0.20). Defining putative salt sensitivity as having aldosterone levels above the top sex-specific tertile and NT-ANP levels below the lowest sex-specific tertile revealed comparable results (online Table 1). Varying the threshold defining high sodium intake did not alter our results (online Table 2). Also, excluding individuals on antihypertensive medication upon follow-up did not alter the results.
Table 2.
Change in systolic and diastolic blood pressure (BP) on follow-up in potentially salt sensitive (defined based on serum aldosterone and plasma NT-ANP levels) and referent individuals stratified by higher vs. lower dietary sodium intake at baseline
| Potentially salt sensitive individuals* | Referent indivuals* | ||||
|---|---|---|---|---|---|
| Dietary sodium intake* | Dietary sodium intake* | ||||
| Lower | Higher | Lower | Higher | p-value | |
| Multivariable-adjusted model † | |||||
|
|
|||||
| Δ Systolic BP, mm Hg, mean (95% CI) | 2.7 (1.1,4.3) | 4.3 (2.7,5.9) | 1.2 (0.2,2.2) | 2.5 (1.6,3.5) | 0.016 |
| Δ Diastolic BP, mm Hg mean (95% CI) | −0.3 (−1.2,0.6) | 2.1(1.2,3.0) | −0.2 (−0.8,0.3) | 0.7 (0.1,1.2) | <0.0001 |
|
| |||||
| Multivariable-adjusted model ** | |||||
|
|
|||||
| Δ Systolic BP, mm Hg, mean (95% CI) | 2.3 (0.6,4.1) | 4.4 (2.6,6.1) | 1.0 (−0.1,2.2) | 2.8 (1.7,3.9) | 0.033 |
| Δ Diastolic BP, mm Hg mean (95% CI) | −0.3 (−1.3,0.7) | 1.9 (0.9,2.8) | −0.2 (−0.8,0.5) | 0.5 (−0.1,1.2) | 0.0127 |
Potential salt sensitivity defined as: serum aldosterone > and plasma NT-ANP levels ≤sex-specific median. See text (Methods) for definition of lower/higher sodium intake.
Δ, delta (change in BP from examination cycle 6 to 7); BP, blood pressure; CI confidence intervals
P-value from ANCOVA test for differences between groups.
Adjustment for sex, age, systolic and diastolic BP, and BMI at baseline, percent weight change on follow-up
Additional adjustment for total caloric intake, dietary potassium, fasting glucose, heart rate and eGFR.
Discussion
We observed significant differences in systolic and diastolic BP changes during short-term (4 years) follow-up between individuals who were grouped at baseline based on presence vs. absence of potential salt sensitivity (serum aldosterone levels >the sex-specific median and plasma NT-ANP levels ≤the sex-specific median) and higher versus lower intake of dietary sodium (> vs. ≤ the sex-specific median). Individuals classified as potentially salt sensitive on a higher dietary salt-intake had greater increases in systolic and diastolic blood pressure as compared to individuals who were not potentially salt sensitive and who had a lower dietary salt intake. Our data also support the concept that higher dietary sodium intake per se is associated with a modest longitudinal increase in BP relative to a lower sodium intake.
In the context of the published literature
Sodium and Blood Pressure
It has been noticed since the middle of the 20th century that dietary salt intake might be an important determinant of high BP in the general population. However, a large observational study (INTERSALT) reported essentially no association between urinary sodium excretion and BP, although a relation of salt-intake and age-related BP slope was observed.20 Meta analyses of interventional trials observed only modest reductions in BP in response to dietary sodium restriction,12 These relatively modest effects of dietary salt on BP may be due to the fact that the BP response to a given dietary salt intake varies considerably across individuals, a phenomenon referred to as ‘salt sensitivity.’
We confirm in the present analyses, including only participants free of hypertension and CVD at baseline, that the change in BP during a 4 year follow-up varies significantly between individuals, and that salt intake and salt sensitivity (defined based on circulating hormone levels) are modulators of this BP change.
In the literature, different definitions for salt sensitivity have been used, based on the observed BP change in response to a given salt-load or salt restriction.1, 3, 4 Importantly, these definitions are usually based on interventional studies that require controlled clinical conditions, including application of saline preparations and diuretics, and are not practical for screening of the general population. These clinical studies suggest that about 26% of non-hypertensive individuals and 51% of all patients with hypertension are salt sensitive,1 indicating that a significant proportion of the population may be more susceptible to the pressor effects of salt. This observation underscores the potential utility of identifying salt sensitive individuals if practical methods were available. The present investigation raises the possibility that circulating aldosterone and NT-ANP may be potential biomarkers that could facilitate the identification of salt sensitive individuals.
Our observation, that individuals without potential salt-sensitivity were younger as compared to participants without salt-sensitivity seems counterintuitive given reports that the prevalence of salt-sensitivity increases with age.21 Further investigations are needed to further elucidate how the biomarker levels track with age.
Hormonal markers of salt sensitivity
Supporting evidence for our findings comes from a recent study reporting that individuals with the metabolic syndrome are salt-sensitive.22 Low ANP23 and high aldosterone levels24 are correlates of the metabolic syndrome and might explain why individuals with the metabolic syndrome are salt-sensitive.
Melander and colleagues25 reported that baseline Nt-proANP levels were related to salt sensitivity (defined as the difference in 24h systolic BP on a high salt diet minus 24h systolic BP on a low salt diet). Individuals with lower Nt-proANP levels displayed a smaller difference in systolic BP (on a high salt vs. a low salt diet) as compared to individuals with higher baseline Nt-proANP levels, indicating that individuals with lower Nt-proANP were less prone to the pressure effects of salt. While these observations are intriguing, they were obtained under highly standardized conditions in a metabolic ward and reflect the dynamic responses to salt loading, while we aimed to identify salt sensitive individuals on an unrestricted diet in the community. Furthermore, experimental studies likewise support the concept that low ANP levels predispose to salt sensitivity (see next section).
Salt sensitivity in hypertension has been reported as comprised of individuals with low-renin essential hypertension and non-modulators.26 Low-renin hypertension is observed more in elderly and black, is characterized by near normal circulating aldosterone but low renin levels, an increased resposiveness of aldosterone levels to angiotensin II infusions, and a better therapeutic BP response to diuretics.27 Nonmodulators, on the other hand, are characterized by a maladaptation (nonmodulation) of renal blood flow and of aldosterone responsiveness to given changes in dietary sodium intake, have normal to high plasma renin levels, are more likely to be male and older, and have a better therapeutic BP response to angiotensin converting enzyme blockers.28 These two subtypes suggest that renin levels may be variable in salt sensitive subsets of hypertension but share an inappropriate aldosterone response to angiotensin II infusions.
It is unclear how the construct of salt sensitivity that we have defined in non-hypertensive people herein relates to the two hypertensive subtypes described in the literature. We based our construct on steady state circulating aldosterone and NT-ANP levels in ambulatory nonhypertensive people on a random salt diet. Additional investigations are warranted to further evaluate our proposed classification schema using dynamic salt loading studies in both hypertensive and nonhypertensive individuals with the measurement of a larger panel of neurohormonal markers.
Possible mechanisms for the observed association
It is widely accepted that renal pressure-natriuresis is the predominant mechanism regulating BP. This system has an ‘infinite gain’, which means that BP responses to modest increases in sodium are tempered immediately by renal mechanisms that mediate increased sodium excretion.29 Salt-sensitive individuals have a relative flattening of the pressure-natriuresis relationship30 so that greater increments in BP can occur upon being subjected to a higher sodium load than one would expect from a normal pressure-natriuretic set point in referent individuals who are not salt sensitive. The tight autoregulation of total renal blood flow and glomerular filtration rate means that tubular sodium reabsorption is the likely site where salt sensitivity may operate.
Pathophysiological evidence strongly supports circulating aldosterone and NT-ANP as biomarkers for salt sensitivity. Both hormones are involved in renal sodium handling. Aldosterone stimulates sodium re-absorption through an apical/epithelial sodium channel (ENaC).8 Patients with homozygous mutations in the aldosterone-synthase encoding CYP11B2 gene display salt-wasting hypotension and common genetic variation in the CYP11B2 gene has been linked to salt sensitivity in the general population.31
Experimental studies also support a role for aldosterone in mediating salt sensitivity. Mice overexpressing the aldosterone synthase gene displayed significantly higher BP on a high-salt diet than wild-type mice,32 whereas homozygous disruption of the aldosterone synthase gene resulted in low BP that could not be normalized upon salt supplementation.11
NT-ANP promotes sodium excretion in the distal convoluted tubules of the kidney via the ENaC receptors.33 Mice with targeted disruption of the ANP encoding gene displayed elevated BP levels on a standard (0.5 percent sodium chloride) as well as an intermediate (2 percent sodium chloride) salt diet. Genetic variants at the NPPA and the NPPB (natriuretic peptide precursor A and B, respectively) locus have been associated with circulating natriuretic peptide concentrations and BP levels in the general population.34 It is conceivable that participants with higher aldosterone but lower NT-ANP levels are prone to retaining sodium, which could explain the observed increases in BP, especially when dietary salt intake is high.
Strengths and Limitations
The large sample, the prospective design and the standardized assessment of BP and covariates strengthen our investigation. The following limitations merit consideration. The generalizability of our findings, which were obtained in a middle-aged sample of white individuals of European ancestry, to other ethnicities and to other age groups is unclear. We used the Food Frequency Questionnaire (FFQ) to assess dietary sodium intake, which has been shown to yield reproducible results that correlate well with dietary records35 and urinary sodium excretion36 although this instrument may underestimate actual sodium intake.35 In our sample, no other information about salt intake (urine sodium or repeat FFQ) were available. Circulating aldosterone and NT-ANP were measured only once in each participant, which is problematic, given the substantial variability of NT-proANP.37 Also, these measurements were obtained on a ‘usual’ sodium diet. Additionally, levels of NT-ANP and aldosterone are influenced by multiple other factors including posture, obesity, diet, medications, etc. Therefore, ANP and aldosterone could be secondary indicators of other conditions, beyond being influenced by inherent (genetically determined) salt sensitivity.
All these factors likely led to random misclassification of salt sensitivity, which could have reduced our ability to detect associations of biomarker levels and changes in BP. In addition, we cannot exclude residual confounding by factors that affect biomarker and BP levels. Furthermore, the follow-up period (4 years) was rather short. In addition, the cut-off points (sex-specific medians) to define putative salt sensitivity based on circulating aldosterone and NT-ANP levels were arbitrary, although analyses based on a classification scheme using sex-specific tertiles revealed comparable results. Like most biological phenomena, salt sensitivity is a continuous rather than a binary trait. Furthermore, no information about total water intake or cortisol levels were available. Due to insufficient power, we did not perform subgroups analyses stratified by sex or intake of drugs potentially affecting aldosterone or NT-ANP.
Conclusion
Although our findings require confirmation/replication, the present analyses provide initial evidence that higher serum aldosterone and lower plasma NT-ANP concentrations may be markers of salt sensitivity in the general population. Additional studies are required to compare our observations made on a random sodium diet with the gold standard strategy for defining salt sensitivity that requires salt challenge using standardized protocols1, 3, 5 and controlled conditions.
Supplementary Material
Acknowledgement
none
Sources of Funding: This work was supported by NHLBI Contract N01-HC-25195 and 2 K24 HL04334 (to RSV); and the U.S. Department of Agriculture, Agricultural Research Service, agreement No. 58-1950-7-707.
Footnotes
Conflicts of Interest/Disclosure: none declared
References
- 1.Weinberger MH, Miller JZ, Luft FC, Grim CE, Fineberg NS. Definitions and characteristics of sodium sensitivity and blood pressure resistance. 8 ed. 1986. pp. II-127–II-134. [DOI] [PubMed] [Google Scholar]
- 2.Coca A, De la SA. Salt sensitivity and left ventricular hypertrophy. Adv Exp Med Biol. 1997;432:91–101. doi: 10.1007/978-1-4615-5385-4_10. [DOI] [PubMed] [Google Scholar]
- 3.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37:429–432. doi: 10.1161/01.hyp.37.2.429. [DOI] [PubMed] [Google Scholar]
- 4.Sowers JR, Zemel MB, Zemel P, Beck FW, Walsh MF, Zawada ET. Salt sensitivity in blacks. Salt intake and natriuretic substances. Hypertension. 1988;12:485–490. doi: 10.1161/01.hyp.12.5.485. [DOI] [PubMed] [Google Scholar]
- 5.Kato N, Kanda T, Sagara M, Bos A, Moriguchi EH, Moriguchi Y, Yamori Y. Proposition of a feasible protocol to evaluate salt sensitivity in a population-based setting. Hypertens Res. 2002;25:801–809. doi: 10.1291/hypres.25.801. [DOI] [PubMed] [Google Scholar]
- 6.Obarzanek E, Proschan MA, Vollmer WM, Moore TJ, Sacks FM, Appel LJ, Svetkey LP, Most-Windhauser MM, Cutler JA. Individual blood pressure responses to changes in salt intake: results from the DASH-Sodium trial. Hypertension. 2003;42:459–467. doi: 10.1161/01.HYP.0000091267.39066.72. [DOI] [PubMed] [Google Scholar]
- 7.de Zeeuw D, Janssen WM, de Jong PE. Atrial natriuretic factor: its (patho)physiological significance in humans. Kidney Int. 1992;41:1115–1133. doi: 10.1038/ki.1992.172. [DOI] [PubMed] [Google Scholar]
- 8.O’Shaughnessy KM, Karet FE. Salt handling and hypertension. J Clin Invest. 2004;113:1075–1081. doi: 10.1172/JCI21560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.John SW, Krege JH, Oliver PM, Hagaman JR, Hodgin JB, Pang SC, Flynn TG, Smithies O. Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science. 1995;267:679–681. doi: 10.1126/science.7839143. [DOI] [PubMed] [Google Scholar]
- 10.Makhanova N, Sequeira-Lopez ML, Gomez RA, Kim HS, Smithies O. Disturbed homeostasis in sodium-restricted mice heterozygous and homozygous for aldosterone synthase gene disruption. Hypertension. 2006;48:1151–1159. doi: 10.1161/01.HYP.0000249902.09036.e7. [DOI] [PubMed] [Google Scholar]
- 11.Makhanova N, Lee G, Takahashi N, Sequeira Lopez ML, Gomez RA, Kim HS, Smithies O. Kidney function in mice lacking aldosterone. Am J Physiol Renal Physiol. 2006;290:F61–F69. doi: 10.1152/ajprenal.00257.2005. [DOI] [PubMed] [Google Scholar]
- 12.Hooper L, Bartlett C, Davey SG, Ebrahim S. Systematic review of long term effects of advice to reduce dietary salt in adults. BMJ. 2002;325:628. doi: 10.1136/bmj.325.7365.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willett WC, Hu FB. The food frequency questionnaire. Cancer Epidemiol Biomarkers Prev. 2007;16:182–183. doi: 10.1158/1055-9965.EPI-06-0843. [DOI] [PubMed] [Google Scholar]
- 14.Levy D, DeStefano AL, Larson MG, O’Donnell CJ, Lifton RP, Gavras H, Cupples LA, Myers RH. Evidence for a gene influencing blood pressure on chromosome 17. Genome scan linkage results for longitudinal blood pressure phenotypes in subjects from the framingham heart study. Hypertension. 2000;36:477–483. doi: 10.1161/01.hyp.36.4.477. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 16.Roldan J, Morillas P, Castillo J, Andrade H, Guillen S, Nunez D, Quiles J, Bertomeu V. Plasma aldosterone and glomerular filtration in hypertensive patients with preserved renal function. Rev Esp Cardiol. 2010;63:103–106. doi: 10.1016/s1885-5857(10)70015-3. [DOI] [PubMed] [Google Scholar]
- 17.Krishna GG, Miller E, Kapoor S. Increased blood pressure during potassium depletion in normotensive men. N Engl J Med. 1989;320:1177–1182. doi: 10.1056/NEJM198905043201804. [DOI] [PubMed] [Google Scholar]
- 18.Bussemaker E, Hillebrand U, Hausberg M, Pavenstadt H, Oberleithner H. Pathogenesis of Hypertension: Interactions Among Sodium, Potassium, and Aldosterone. Am J Kidney Dis. 2010 doi: 10.1053/j.ajkd.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 19.Grundy SM, Brewer HB, Jr., Cleeman JI, Smith SC, Jr., Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 20.Intersalt Cooperative Research Group Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. BMJ. 1988;297:319–328. doi: 10.1136/bmj.297.6644.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campese VM. Salt sensitivity in hypertension. Renal and cardiovascular implications. Hypertension. 1994;23:531–550. doi: 10.1161/01.hyp.23.4.531. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Gu D, Huang J, Rao DC, Jaquish CE, Hixson JE, Chen CS, Chen J, Lu F, Hu D, Rice T, Kelly TN, Hamm LL, Whelton PK, He J. Metabolic syndrome and salt sensitivity of blood pressure in non-diabetic people in China: a dietary intervention study. Lancet. 2009;373:829–835. doi: 10.1016/S0140-6736(09)60144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang TJ, Larson MG, Keyes MJ, Levy D, Benjamin EJ, Vasan RS. Association of plasma natriuretic peptide levels with metabolic risk factors in ambulatory individuals. Circulation. 2007;115:1345–1353. doi: 10.1161/CIRCULATIONAHA.106.655142. [DOI] [PubMed] [Google Scholar]
- 24.Ingelsson E, Pencina MJ, Tofler GH, Benjamin EJ, Lanier KJ, Jacques PF, Fox CS, Meigs JB, Levy D, Larson MG, Selhub J, D’Agostino RB, Sr., Wang TJ, Vasan RS. Multimarker approach to evaluate the incidence of the metabolic syndrome and longitudinal changes in metabolic risk factors: the Framingham Offspring Study. Circulation. 2007;116:984–992. doi: 10.1161/CIRCULATIONAHA.107.708537. [DOI] [PubMed] [Google Scholar]
- 25.Melander O, von Wowern F, Frandsen E, Burri P, Willsteen G, Aurell M, Hulthen UL. Moderate salt restriction effectively lowers blood pressure and degree of salt sensitivity is related to baseline concentration of renin and N-terminal atrial natriuretic peptide in plasma. J Hypertens. 2007;25:619–627. doi: 10.1097/HJH.0b013e328013cd50. [DOI] [PubMed] [Google Scholar]
- 26.Hurwitz S, Fisher ND, Ferri C, Hopkins PN, Williams GH, Hollenberg NK. Controlled analysis of blood pressure sensitivity to sodium intake: interactions with hypertension type. J Hypertens. 2003;21:951–959. doi: 10.1097/00004872-200305000-00020. [DOI] [PubMed] [Google Scholar]
- 27.Fisher ND, Hurwitz S, Jeunemaitre X, Hopkins PN, Hollenberg NK, Williams GH. Familial aggregation of low-renin hypertension. Hypertension. 2002;39:914–918. doi: 10.1161/01.hyp.0000013784.18175.51. [DOI] [PubMed] [Google Scholar]
- 28.Hollenberg NK. Sodium and the kidney: the non-modulator concept. Nephrol Dial Transplant. 2001;16(Suppl 6):38–39. doi: 10.1093/ndt/16.suppl_6.38. [DOI] [PubMed] [Google Scholar]
- 29.Luft FC, Rankin LI, Bloch R, Weyman AE, Willis LR, Murray RH, Grim CE, Weinberger MH. Cardiovascular and humoral responses to extremes of sodium intake in normal black and white men. Circulation. 1979;60:697–706. doi: 10.1161/01.cir.60.3.697. [DOI] [PubMed] [Google Scholar]
- 30.Hall JE, Guyton AC, Brands MW. Pressure-volume regulation in hypertension. Kidney Int Suppl. 1996;55:S35–S41. [PubMed] [Google Scholar]
- 31.Iwai N, Kajimoto K, Tomoike H, Takashima N. Polymorphism of CYP11B2 determines salt sensitivity in Japanese. Hypertension. 2007;49:825–831. doi: 10.1161/01.HYP.0000258796.52134.26. [DOI] [PubMed] [Google Scholar]
- 32.Makhanova N, Hagaman J, Kim HS, Smithies O. Salt-sensitive blood pressure in mice with increased expression of aldosterone synthase. Hypertension. 2008;51:134–140. doi: 10.1161/HYPERTENSIONAHA.107.098897. [DOI] [PubMed] [Google Scholar]
- 33.Maack T. Role of atrial natriuretic factor in volume control. Kidney Int. 1996;49:1732–1737. doi: 10.1038/ki.1996.257. [DOI] [PubMed] [Google Scholar]
- 34.Newton-Cheh C, Larson MG, Vasan RS, Levy D, Bloch KD, Surti A, Guiducci C, Kathiresan S, Benjamin EJ, Struck J, Morgenthaler NG, Bergmann A, Blankenberg S, Kee F, Nilsson P, Yin X, Peltonen L, Vartiainen E, Salomaa V, Hirschhorn JN, Melander O, Wang TJ. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat Genet. 2009;41:348–353. doi: 10.1038/ng.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 36.Willett W, Lenart E. Reproducibility and validity of food-frequency questionnaires. In: Willet W, editor. Nutritional Epidemiology. 2nd ed. Oxford University Press; New York: 1998. pp. 101–147. [Google Scholar]
- 37.Frankenstein L, Remppis A, Frankenstein J, Hess G, Zdunek D, Gut S, Slottje K, Katus HA, Zugck C. Reference change values and determinants of variability of NT-proANP and GDF15 in stable chronic heart failure. Basic Res Cardiol. 2009;104:731–738. doi: 10.1007/s00395-009-0027-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.