Abstract
Purpose
A multidisciplinary workshop was convened at the National Institutes of Health (NIH) to discuss the management of the orthopedic and other complications of Proteus syndrome (PS), a progressive, disproportionate overgrowth disorder. While PS poses many complex challenges, the focus of the workshop was the management of the asymmetric and disorganized skeletal overgrowth that characterizes this multisystem disorder.
Methods
Workshop participants developed recommendations for clinical research and patient management and surveillance to maximize the benefits and reduce the risks of surgical and other interventions.
Results
Recommendations for clinical care and management included assessments of skeletal overgrowth and its progression with modalities such as X-ray, magnetic resonance imaging (MRI), dual-energy X-ray absorptiometry, and computerized tomography (CT) imaging. The recommendations also cover the assessment of non-orthopedic complications of PS that significantly impact surgical risk, such as pulmonary embolism and lung bullae. Surgical considerations in PS include assessment of the contribution of contractures to deformities and prophylactic soft-tissue release, aggressive and early use of epiphysiodesis and epiphysiostasis, amputation, and spinal bracing.
Conclusion
Decisions on the timing of orthopedic procedures in children with PS are challenging because they entail balancing the risks of intervention in this high-risk and complex population against the increasing morbidity that patients experience with progressive bony overgrowth. If surgery is delayed too long, the condition may become inoperable. We hope that these recommendations will help clinicians gather appropriate data and assist their patients in making timely treatment decisions.
Keywords: Proteus syndrome, Overgrowth, Scoliosis, Limb-length inequality
Introduction
Proteus syndrome (PS) causes asymmetric, disproportionate, and severe postnatal overgrowth, particularly bone, in a mosaic pattern [1]. Although skeletal features predominate, the disease may affect any tissue and lead to the overgrowth of brain, skin, adipose, vasculature, immune, gut, and other tissues (Fig. 1). The cause of PS is unknown, but it is hypothesized that it is caused by a new, mosaic, mutation acquired early in development; cells derived from the mutated cell line carry this mutation and result in affected tissues [2, 3].
Fig. 1.
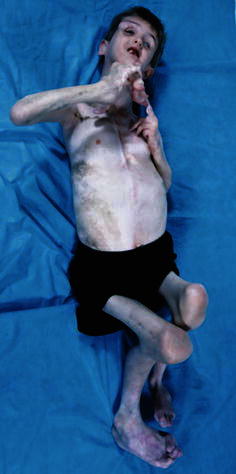
An 8-year-old boy with Proteus syndrome (PS) with many of the hallmark features of the disorder, including linear verrucous epidermal nevi, cerebriform connective tissue nevi, dysregulated adipose tissue, and distortion of body structures due to disproportionate skeletal overgrowth
The primary feature that distinguishes PS from other overgrowth syndromes is rapid, progressive, postnatal distortion of the architecture of the bone. The rate of overgrowth and resultant distortion of skeletal structures can be overwhelming. A key attribute of the overgrowth is that it tends to significantly alter the architecture of the affected bones, commonly affecting periarticular regions (Fig. 2). The disorder causes severe morbidity and early mortality. Many patients manifest severe scoliosis, loss of joint mobility, and cosmetic disfigurement. Life-threatening complications include deep venous thrombosis (DVT) and pulmonary embolism (PE), bullous lung disease, and restrictive lung disease [4, 5].
Fig. 2.
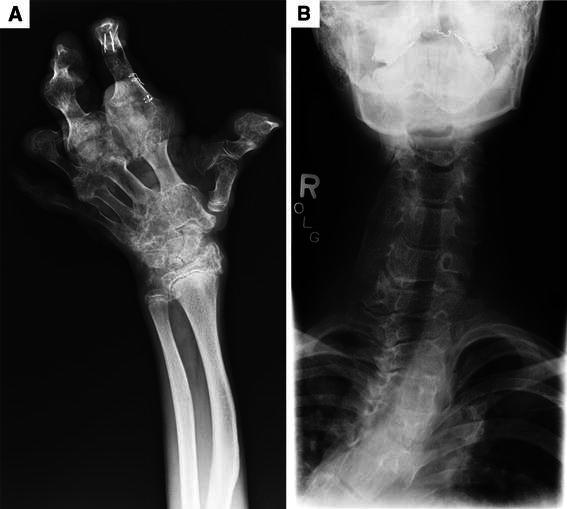
a Hand X-ray showing severe periarticular bony overgrowth in a 10-year-old patient. Note the radial bowing and that the fifth finger is relatively spared. b Anteroposterior (AP) spine imaging showing fusion of the lateral processes and elongation and distortion of the bodies of the cervical vertebrae in a 13-year-old patient
The diagnostic criteria for PS are summarized in Table 1 [1]. Despite these criteria, PS has been confused with a number of other disorders, including hemihyperplasia syndromes, CLOVE syndrome, type 2 segmental Cowden syndrome, and others [3, 6–8].
Table 1.
Diagnostic criteria for Proteus syndrome (PS)
| General criteria |
| Mosaic distribution |
| Progressive course |
| Sporadic occurrence |
| Specific criteria |
| Category A |
| Cerebriform connective tissue nevus |
| Category B |
| Linear epidermal nevus |
| Asymmetric, disproportionate overgrowth of two of: |
| Limbs, skull, external auditory canal, vertebrae, or viscera |
| Specific tumors in the first decade of life: |
| Bilateral ovarian cystadenomas |
| Monomorphic parotid adenomas |
| Category C |
| Dysregulated adipose tissue |
| Vascular malformations: |
| Capillary, venous, and/or lymphatic |
| Lung bullae |
| Facial phenotype: |
| Long face, dolichocephaly, down-slanted palpebral fissures, low nasal bridge, wide or anteverted nares, open mouth at rest |
The diagnosis of Proteus syndrome requires all three general criteria, plus the presence of the criterion from category A, or two criteria from category B, or three criteria from category C [1]
The rapid growth and distortion of bony alignment in PS, commonly causing significant functional loss, suggest a need for an aggressive surgical approach to minimize orthopedic deformity and maintain function. However, this perspective must be temporized by an appreciation of the risk of severe cardiopulmonary complications inherent in the disorder. Clearly, clinicians need a better understanding of the natural history of PS in order to advise patients and families regarding the risks and benefits of interventions.
To address these needs, a workshop was convened at the National Institutes of Health (NIH) on September 27–28, 2007, to: (1) summarize current clinical recommendations, (2) develop a clinical research agenda to improve knowledge of the skeletal manifestations of PS, and (3) propose new approaches to the evaluation and treatment of the orthopedic complications of PS. The workshop participants included individuals with a wide array of expertise, including clinical aspects of PS, orthopedics, and skeletal biology (see Acknowledgments).
Orthopedic manifestations of Proteus syndrome
Patients with PS typically have no apparent congenital skeletal overgrowth prenatally. Overgrowth commonly begins between 6 and 18 months of age, is rapid, and distorts the skeletal macroarchitecture. The overgrowth of PS is typically asymmetrical, but is often bilateral. In addition, in the forearm and lower limb, it sometimes involves only one of two bones, while in the hands and feet, it will frequently involve some but not all fingers or toes. Although the disease is best recognized by the presence of dysplastic or disorganized bone macroarchitecture, patients can also have enlarged bones with a generally normal shape and contour (Fig. 3). It is not unusual for a child with PS to develop a leg-length discrepancy of greater than 10 cm before the age of 10 years. As noted in Table 1, PS is distinguished by the presence of irregular and disorganized bone, including hyperostosis, hyperproliferation of osteoid with variable calcification causing abnormal bony edges, abnormally calcified connective tissue, and invasion of joint spaces, which frequently result in joint immobility (Fig. 4).
Fig. 3.
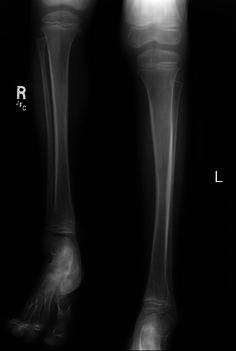
Lower extremity X-ray demonstrating significant leg-length discrepancy in a 7-year-old boy. Note the relatively normal contour of the involved bones on the left
Fig. 4.
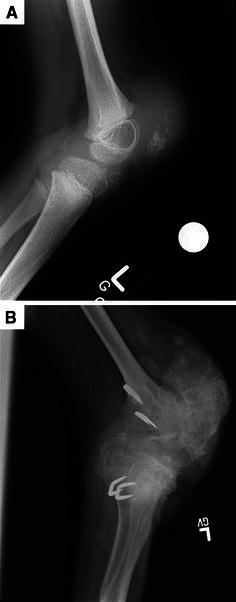
a, b Lateral X-rays demonstrating progressive bony overgrowth resulting in complete knee immobility over a period of 7 years [from age 5.5 years in (a) to 12.5 years in (b)]
As noted above, it is thought that PS is mosaic, and this predicts random effects across tissues. Unpublished data indicate that some musculoskeletal manifestations may be more common in PS than would be predicted by a random model (Biesecker et al., unpublished data). Extraordinary overgrowth of the lower extremities is common, and is typically associated with pronounced valgus, causing marked dysfunction. Several patients have developed iliotibial band contractures, which may contribute to the knee distortion. The hands are also frequently involved, with significant functional compromise (see Fig. 2a). The shoulders and elbows, by contrast, are rarely involved. This pattern of musculoskeletal involvement raises the question of whether trauma plays a role in the development of bony abnormalities, since the hands, feet, and knees are more prone to trauma and iatrogenic injury than other areas.
Scoliosis, a common manifestation of PS, can range from a single long, gentle curve to multiple, severe curves. While the time of onset of scoliosis in PS is similar to that of adolescent idiopathic scoliosis, the severity and rate of progression in PS can be remarkable (Fig. 5). Cervical curves are rare in idiopathic scoliosis, but they are common in PS. The diagnosis of cervical scoliosis in PS is typically accompanied by elongation and vertebral body distortion and severe neck motion compromise. The deformity can compromise both the spinal canal and the trachea.
Fig. 5.
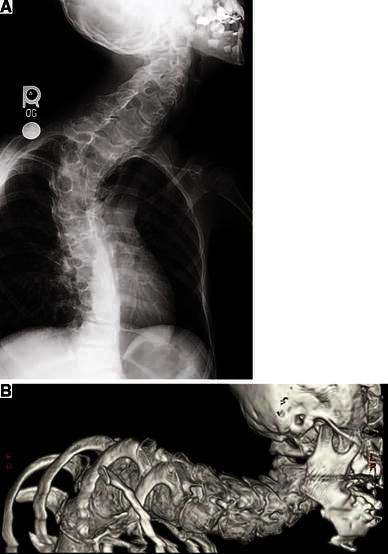
a Plain X-ray of an 8-year-old girl with distorting vertebral overgrowth and scoliosis. b Computerized tomography (CT) with 3D reconstruction of the same patient as in (a) at age 11 years. Scoliosis in patients with PS can progress extremely rapidly and have severe consequences—this patient died at age 15 years as a result of respiratory insufficiency
Craniofacial overgrowth is common in patients with PS. Overgrowth of bones of the external auditory canal can cause conductive hearing loss, infection, and cholesteatomas. The frontal bone is commonly affected, typically unilaterally, which can lead to severe disfigurement (Fig. 6).
Fig. 6.
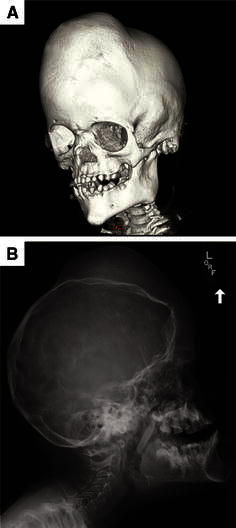
Cranial CT with 3D reconstruction (a) and plain X-ray (b) showing significant craniofacial overgrowth in a 5.5-year-old girl with PS. This patient has undergone several surgical procedures to reduce recurrent external auditory canal stenosis
Workshop recommendations for clinical care and research
On the basis of their discussions and experience, workshop participants made the following recommendations for the care and management of patients with PS and for a clinical research agenda for the disease. Each recommendation is followed by a parenthetic note indicating whether it pertains primarily to clinical care (C) or to clinical research (R).
Annual physical examination and radiography
A thorough skeletal examination should be performed at the time of diagnosis and at least annually thereafter.
Annual physical examinations assess growth, distortion, and mobility of the skeletal elements. Yearly growth should be plotted on standard growth curves. (C)
Many workshop participants believed that the widespread, pleiotropic nature of the manifestations argues for annual skeletal X-rays. Others recommended an initial full survey, followed by annual assessment of body parts that manifest ongoing overgrowth. (C)
Spinal and lower-extremity imaging should be performed by extra-long film imaging at diagnosis. Follow-up studies should be performed annually if the patient has evidence of scoliosis or leg-length discrepancy. Spine films should be performed with the patient sitting in order to avoid distortion caused by leg-length discrepancies. Such studies provide data on the progression of deformity and can be valuable for surgical decision-making. (C)
A bone-by-bone scoring system that quantifies which bones are affected should be developed. (R)
Bone scan
Bone scanning may provide evidence of excess or abnormal bone growth earlier than plain radiographs and could lead to earlier interventions to preserve function. Workshop participants suggested the need for a pilot study to determine the feasibility and utility of 99Tc bone scanning as a potential marker of regions of increased bone turnover in patients with PS. These data could also be useful to understand the pathology of abnormal bone growth in PS. (R)
Magnetic resonance imaging (MRI) with contrast
Pilot studies of magnetic resonance imaging (MRI) with contrast should be considered for patients with overgrowth in the hands and lower extremities. Participants felt that such studies may elucidate differences in affected versus unaffected areas and test the hypothesis of increased vascularity as a cause of overgrowth in patients with PS. (R)
Spinal imaging
High-resolution computerized tomography (CT) with 3D reconstruction should be an option among patients in whom spinal surgery is being considered. In many patients with PS, scoliosis is accompanied by severe distortion of the vertebrae. This can complicate surgical planning, and detailed data on bone structure could be useful. The scoliosis in patients with PS may have a neurologic component. A spinal MRI is recommended at the time that scoliosis is diagnosed. MRI also offers possibilities for 3D reconstruction without the concern of radiation exposure. Early MRI imaging of the cervical spine may help with the decision surrounding surgical intervention for scoliosis. (C)
Bone quality imaging techniques
It is unclear whether low bone density (poor bone quality), is a primary manifestation of PS or if it is secondary to disuse atrophy. Workshop participants recommended that a pilot study be performed in order to assess bone density in patients with PS before they develop significant skeletal deformity, particularly joint contracture, which can make DEXA impractical. (R)
Positron emission tomography (PET)/functional study of bones
Positron emission tomography (PET) scanning may be more sensitive than bone scans and has been used to assess skeletal function and status in cancer patients, but its potential utility in PS is speculative. (R)
Nerve conduction velocities (NCV), electromyography (EMG), serum creatinine phosphokinase (CPK), and muscle biopsy
It is possible that nerve involvement in PS is under-recognized. The association of atrophy and inactivity of muscles in patients with PS is compatible with two hypotheses: (1) that neurological abnormalities may contribute to deformities or (2) that joint restriction leads to atrophy. NCV, EMG, CPK monitoring, and muscle biopsies should be considered and their utility evaluated by a neurologic consultant. (R)
Baseline hand and foot X-ray of same-sex parent
Parental hand size can be an indicator of when to intervene to arrest the growth of a child’s overgrowing phalanges. X-rays of the hands and feet of the mothers of girls and the fathers of boys with PS could provide useful information on when to intervene with physiodesis to arrest the growth plates. For long bones, a simple inseam measurement can provide guidance regarding the timing of epiphysiodesis. (C)
Rehabilitation
Patients with PS can benefit from regular physical medicine evaluations and interventions. Assessment of the patient’s functional limitations and their etiology is essential when considering the treatment options. Non-surgical modifications, such as shoe lifts and ring splints for finger deformities, can improve function. These should be considered in developing an overall surgical plan, especially in patients with multifocal disease. The multiple manifestations of PS can interact to amplify their negative consequence (cerebriform connective tissue nevus [CCTN], lower-extremity overgrowth, and scoliosis, for example, can present complex challenges). For this reason, a team approach that includes physical and occupational therapists, pedorthists, and physiatrists is warranted. (C)
Bone age
Bone age in both affected and unaffected bones is a useful measure for surgical planning and should be performed annually. Dysharmonic bone maturation is present in a number of other overgrowth conditions and it is appropriate to determine if this finding is part of PS. (C)
Venous ultrasound in lower extremities in patients older than 6 years
Since DVT and PE pose significant risks to individuals with PS, prophylactic measures are recommended. Doppler studies and routine measures of coagulation (e.g., fibrin split products, D-dimer fibrin degradation assays) could help determine the risk for DVT. Prophylactic anticoagulation is routine in patients undergoing surgery at the NIH; however, its efficacy has not been proven, and a trial to test this therapy is probably not practical. Chronic anticoagulation presents challenges in patients with vascular malformations, and doses have not been quantified. The use of an umbrella filter poses a high mortality risk (about 1% per year), and its use in the young PS population may be difficult to justify, except in extreme cases. (C)
Psychological counseling, assessment, and support
The progressive and unpredictable nature of PS places an enormous psychological burden on affected individuals and their families. Adding to the uncertainty is the threat of serious complications, such as DVT/PE and severe lung disease. Affected individuals and their families report that the social stigma they encounter as a result of the disfiguring nature of the condition can be difficult [8]. Interventions to assess and help families cope with feelings of loss and management of uncertainty is a critical aspect in counseling those with PS. Families affected by PS should undergo a genetic counseling assessment and may benefit from individual and group counseling and participation in support groups such as those supported by the Proteus Syndrome Foundations (US and UK). (C)
Cognitive evaluation is indicated for any patient with problems with school or hemimegalencephaly. Psychological assessments are important when considering amputation (see below). Performing amputation sooner rather than later (i.e., before body image issues have become difficult) may reduce psychological problems and improve function. (C)
Pulmonary function tests (PFTs) and chest CT
Pulmonary function tests (PFTs) should be performed when patients can cooperate and annually if forced vital capacity/forced expired volume (FVC/FEV) is <75% of the predicted value or if there are new pulmonary symptoms. Such tests are recommended because of the high rates of intrinsic and extrinsic lung disease in PS [1, 5]. PS-associated bullous pulmonary degeneration can compress pulmonary segments, increase dead space, and cause other complications. A common extrinsic complication is restrictive disease from spinal and rib overgrowth, distortion, and restricted mobility. In addition to PFTs, high-resolution chest CT should be performed in any patient with PS who has abnormal PFTs, or known or suspected pulmonary embolism or pneumonia. (C)
Nutritional/basic metabolic rate assessment
Many patients with PS manifest subcutaneous and muscular tissues wasting—a surprising finding, given the concomitant overgrowth of fatty tissue in other areas of the body. A nutritional assessment could help the clinician adjust the caloric intake in order to compensate for these abnormalities. (C)
Pain management consultation
Bone and joint pain is significant for some individuals with PS. There is no standard therapy for pain management in patients with PS, but appropriate pain care is an important part of treatment. Non-narcotic analgesics have been helpful in the treatment of pain in children and adults with cerebral palsy and bisphosphonates have been reported to reduce pain in patients with fibrosis dysplasia; these treatments may prove successful in patients with PS as well [9, 10]. (C)
Endocrine manipulation
The rate of PS-associated skeletal overgrowth is commonly reduced with the end of puberty. For this reason, some thought has been given to interrupting puberty with exogenous hormones in these patients. Long-term endocrine manipulation, however, poses many risks, including loss of ovarian function. Some have hypothesized that corticosteroids would slow progression of the syndrome, but this has not been demonstrated. Bisphosphonates to suppress bony overgrowth, antimetabolites to slow bone growth, and tetracycline analogs to arrest cartilage growth are other interventions to consider; however, the risks and benefits are unknown. (R)
Workshop recommendations for orthopedic management
Mortality rates in children with PS are higher than for non-PS children with an analogous degree of abnormality. Thus, all orthopedic procedures on patients with PS should be considered to be of high risk and performed by a surgeon familiar with the syndrome and with access to state-of-the-art equipment, facilities, and supportive care, whenever possible. At the same time, while the need for surgery is great in many PS patients, some surgeons and anesthesiologists may be reluctant to operate because of the risk of massive thrombi and sudden death. Unfortunately, delaying surgery may cause the patient to be declared inoperable because of the progression of non-orthopedic PS manifestations, particularly decreasing pulmonary function. This leads to a paradox: the longer surgery is postponed for safety considerations, the more compromised the patient’s condition becomes, thereby, further increasing surgical risk.
Although an increased risk of DVT and PE in PS is well documented, it is unknown whether the risk of these complications correlates with the overall severity of PS manifestations. In addition, this clotting propensity is not well understood. Published [4] and unpublished data show no abnormalities of the circulating clotting factors. A general recommendation has been that patients with PS should be considered for routine anticoagulation prophylaxis, with careful monitoring for DVT and PE during and after surgery [1, 4].
Assess/release contractures
Some of the skeletal deformities in patients with PS appear to be caused by soft-tissue contractures. Early prophylactic soft-tissue releases from contracture may preserve function and relieve pain. (C)
Early use of epiphysiostasis and epiphysiodesis surgical techniques
Early treatment with epiphysiostasis, a form of temporary growth arrest, reduces projected limb length inequality in children who are many years away from puberty (Tosi et al., unpublished data). It is essential to recognize that the short limb is the normal limb and that surgery should only be attempted on the overgrown limb. Lengthening the normal limb risks exceeding the bone’s capacity to expand and, worse yet, may reduce function by compromising the patient’s functional limb. As the children enter their teens, epiphysiodesis is typically preferred. Both epiphysiostasis and epiphysiodesis have the advantage that children can be mobilized quickly postoperatively, which may reduce the risk of thrombosis. The optimal time to operate is unknown, but it is probably when the functional limitations or growth rate become concerning. In some patients with PS, a disadvantage of waiting until overgrowth and deformity are severe is the surgical challenge of finding the growth plate in a mass of dysplastic bone, particularly at the knee. The bones of patients with PS appear to hold surgical implants well. (C)
Amputation
Parents and older children often question the value of preserving a hand or foot or leg that has severe deformity and limited function. Although an extreme step, amputation or removing a non-functional body part and replacing it with a prosthesis may improve the quality of life. Amputation may also reduce pain and the psychological distress of living with a disfiguring condition. Prosthesis technology has advanced rapidly in recent years, although hand prostheses are not as advanced as those for legs. Overgrowth of the amputation stump is a postoperative challenge in some cases. (C)
Patellectomy
Patellar overgrowth, frequently with a severe knee flexion contracture, is a hallmark of PS. Patellectomy may be considered, but there is little evidence that it is beneficial. There is also some concern that the procedure could trigger increased heterotopic bone formation. However, patellectomy may be worth considering in a young child with an enlarging patella who is ambulatory but beginning to lose function. (C)
Spinal bracing
Early identification and frequent monitoring of scoliosis and vertebral overgrowth is crucial in patients with PS, as both of these issues are common and can be rapidly progressive. While most patients do not have spinal cord anomalies, at least one patient in the NIH cohort was found to have a small cervical syrinx (Biesecker, unpublished data), and a patient with multiple spinal meningiomas has been reported [11]. The contribution that vertebral distortion makes to the severity and rate of progression of scoliosis in patients with PS is uncertain; patients with severe curves do not always have significant vertebral overgrowth. Because scoliosis in PS can progress rapidly with life-threatening consequences (see Fig. 5), the consensus among the workshop participants was that spinal bracing for children with PS should be considered when they have a curve as small as 10°. Time and further experience will indicate which bracing system is the best or most effective; randomized trials in such a small patient cohort are impossible. Although there are no reports of their use in patients with PS, growing rods, which can be initiated early in curve development and long before spine fusion is feasible, may be beneficial, particularly given the pulmonary complications common in patients with PS. (C)
Joint replacement
Total joint replacement is a possible alternative to amputation, but there is no clinical experience with this and it may present an elevated DVT risk. Also, joint replacements wear out, thus, repeated surgical interventions may be necessary. (C)
Foot care
The CCTN in PS is a progressive skin lesion found most commonly on the soles of the feet. An advanced CCTN often contributes to the social stigma encountered by patients as the deep sulci of this lesion harbor microorganisms, resulting in a strong odor [12]. Also, large plantar CCTNs can significantly affect function, altering foot shape and biomechanics, necessitating custom orthotics or shoes. The authors have found that once- or twice-daily foot soaks in 0.25% Dakin’s solution can be extremely helpful in preventing infection and combating odor; alternating this regimen with dilute acetic acid solution soaks and incorporating the daily application of topical emollients can reduce skin dryness. (C)
Conclusions
The management of patients with Proteus syndrome (PS) is challenging. The orthopedic complications are severe and progressive; moreover, many other body systems can be affected. The purpose of these recommendations is to encourage clinicians to aggressively gather clinical data and to enable them to make timely and effective decisions for their patients. No one should harbor the misconception that the management of these patients is straightforward, or that all patients will have full mobility and function if these recommendations are adopted; however, it is certain that patients will do poorly if nothing is done. The data demonstrate that, left untreated, the skeletal manifestations of PS alone can lead to severe loss of function. We encourage our colleagues to evaluate their patients, intervene when appropriate, and share their experiences so that all of us can optimize the care and management of these challenging patients.
Acknowledgments
The authors thank the Proteus Syndrome Foundations of the United States and United Kingdom for their advocacy on behalf of patients with this disease and for their encouragement and major financial support of this workshop. We also are grateful to the National Organization for Rare Diseases (NORD) and the American Society for Bone and Mineral Research (ASBMR) for their support of this workshop LGB and JCS are supported by the intramural research program of the National Human Genome Research Institute. Linda R. Harteker provided expert editorial assistance. Randi Henderson transcribed and organized the workshop proceedings. We are deeply grateful to the workshop participants: Benjamin Alman, James Aronson, Tracy Ballock, Randal Betz, Peter Choyke, M. Micheal Cohen Jr., Michael T. Collins, Benoit de Crombugghe, Eli Hatchwell, Kimberly Hoag, Brendan Lee, Gayle Lester, David Ornitz, Francesco Ramirez, Pamela Robey, Vicki Rosen, Mary Timmerman, Rocky Tuan, Matthew Warman, Tracey Whitewood-Neal, and Yingzi Yang. We also thank Jordan Whitewood-Neal and Charles Alvarado and their families for their participation at the workshop.
Conflict of interest
None.
Footnotes
L. L. Tosi and J. C. Sapp contributed equally to this work.
References:
- 1.Biesecker LG. The challenges of Proteus syndrome: diagnosis and management. Eur J Hum Genet. 2006;14(11):1151–1157. doi: 10.1038/sj.ejhg.5201638. [DOI] [PubMed] [Google Scholar]
- 2.Happle R. Lethal genes surviving by mosaicism: a possible explanation for sporadic birth defects involving the skin. J Am Acad Dermatol. 1987;16(4):899–906. doi: 10.1016/S0190-9622(87)80249-9. [DOI] [PubMed] [Google Scholar]
- 3.Happle R. Type 2 segmental Cowden disease vs. Proteus syndrome. Br J Dermatol. 2007;156(5):1089–1090. doi: 10.1111/j.1365-2133.2007.07818.x. [DOI] [PubMed] [Google Scholar]
- 4.Slavotinek AM, Vacha SJ, Peters KF, Biesecker LG. Sudden death caused by pulmonary thromboembolism in Proteus syndrome. Clin Genet. 2000;58(5):386–389. doi: 10.1034/j.1399-0004.2000.580509.x. [DOI] [PubMed] [Google Scholar]
- 5.Zusan E, Smith JM, Parker T. Proteus syndrome: a case report. Am Surg. 2009;75(9):853–856. [PubMed] [Google Scholar]
- 6.Biesecker LG, Peters KF, Darling TN, Choyke P, Hill S, Schimke N, Cunningham M, Meltzer P, Cohen MM., Jr Clinical differentiation between Proteus syndrome and hemihyperplasia: description of a distinct form of hemihyperplasia. Am J Med Genet. 1998;79(4):311–318. doi: 10.1002/(SICI)1096-8628(19981002)79:4<311::AID-AJMG14>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 7.Sapp JC, Turner JT, van de Kamp JM, van Dijk FS, Lowry RB, Biesecker LG. Newly delineated syndrome of congenital lipomatous overgrowth, vascular malformations, and epidermal nevi (CLOVE syndrome) in seven patients. Am J Med Genet A. 2007;143A(24):2944–2958. doi: 10.1002/ajmg.a.32023. [DOI] [PubMed] [Google Scholar]
- 8.Turner J, Biesecker B, Leib J, Biesecker L, Peters KF. Parenting children with Proteus syndrome: experiences with, and adaptation to, courtesy stigma. Am J Med Genet A. 2007;143A(18):2089–2097. doi: 10.1002/ajmg.a.31904. [DOI] [PubMed] [Google Scholar]
- 9.Vogtle LK. Pain in adults with cerebral palsy: impact and solutions. Dev Med Child Neurol. 2009;51(Suppl 4):113–121. doi: 10.1111/j.1469-8749.2009.03423.x. [DOI] [PubMed] [Google Scholar]
- 10.Lala R, Matarazzo P, Andreo M, Marzari D, Bellone J, Corrias A, de Sanctis C, Study Group for Gs alpha Protein Related Diseases of the Italian Society for Pediatric Endocrinology and Diabetes Bisphosphonate treatment of bone fibrous dysplasia in McCune-Albright syndrome. J Pediatr Endocrinol Metab. 2006;19(Suppl 2):583–593. doi: 10.1515/jpem.2006.19.s2.583. [DOI] [PubMed] [Google Scholar]
- 11.Asahina A, Fujita H, Omori T, Kai H, Yamamoto M, Mii K. Proteus syndrome complicated by multiple spinal meningiomas. Clin Exp Dermatol. 2008;33(6):729–732. doi: 10.1111/j.1365-2230.2008.02846.x. [DOI] [PubMed] [Google Scholar]
- 12.Beachkofsky TM, Sapp JC, Biesecker LG, Darling TN. Progressive overgrowth of the cerebriform connective tissue nevus in patients with Proteus syndrome. J Am Acad Dermatol. 2010;63(5):799–804. doi: 10.1016/j.jaad.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]


