Abstract
Rationale
Most individuals can accurately assess the risks and rewards associated with choice alternatives and decide accordingly; however, drug users often display maladaptive decision-making, such that choices are biased toward excessively risky options.
Objective
The purpose of this study was to investigate the effects of a range of drugs of abuse on risky decision-making.
Methods
Male Long–Evans rats were trained in the Risky Decision-Making Task, in which they chose between two levers, one which produced a small, “safe” food reward and the other which produced a large, “risky” food reward. The large reward was accompanied by the risk of a mild footshock, the probability of which increased over the course of each test session (0%, 25%, 50%, 75%, and 100%).
Results
Nicotine (0.6 mg/kg) and amphetamine (1.5 mg/kg) caused a significant decrease in choice of the large risky reward (decreased risk taking). Diazepam (1.0 mg/kg) caused a significant increase in choice of the large risky reward (increased risk taking), whereas morphine (3.0 mg/kg) caused only a trend toward increased choice of the large risky reward. Ethanol had no effect on choice behavior.
Conclusions
These results show that acute administration of drugs of abuse can modulate risk taking in a drug-specific manner, either increasing or decreasing preference for highly rewarding, but risky, options.
Keywords: Decision-making, Risk, Punishment, Amphetamine, Ethanol, Morphine, Nicotine, Diazepam
Individuals are faced with daily decisions among competing alternatives, some of which may be accompanied by adverse consequences (punishment). Most people are able to accurately assess the risks and rewards of such alternatives and decide adaptively. However, maladaptive decision-making, such that choices are overly biased toward risky options (in which the probability of adverse consequences may outweigh the rewards gained), is often evident in psychopathological conditions including attention deficit/hyperactivity disorder, major depressive disorder, bipolar disorder, schizophrenia, and, particularly, addiction (Bechara et al. 2001; Ernst et al. 2003; Ludewig et al. 2003; Taylor Tavares et al. 2007; Drechsler et al. 2008; Heerey et al. 2008). Indeed, maladaptive choices in the face of risks of adverse consequences (such as incarceration, illness, and loss of employment) are a defining feature of addiction (American Psychiatric Association 2000); however, there are few data on how drugs of abuse themselves modulate such decision-making.
Decision-making under conditions of risk of punishment is commonly studied in laboratory tasks in humans such as the Iowa Gambling Task (IGT) and the Cambridge Gambling Task (CGT) in which poor choices result in loss of earnings (Bechara et al. 1997; Rogers et al. 1999). Such tasks correspond well to real-world decisions in humans, but it is difficult to employ such designs in animals due to the apparent infeasibility of the use of such “earnings” systems in animals (although see van den Bos et al. 2006; Rivalan et al. 2009; Zeeb et al. 2009; Jentsch et al. 2010). An alternative approach in animals has been to use explicitly punishing stimuli as the adverse consequence or “cost” in decision-making tasks (Negus 2005). Our laboratory has recently developed such a task (the Risky Decision-Making Task), in which rats choose between small “safe” rewards and large rewards that are accompanied by varying risks of delivery of a mild footshock punishment (this task is similar in design to the “probability discounting task”, in which risk of reward omission is the “cost” associated with choice of the large reward; Cardinal and Howes 2005; Floresco et al. 2008). Previous work in our laboratory showed that amphetamine decreased risk taking in this task, whereas cocaine rendered rats insensitive to changes in the risk of punishment (Simon et al. 2009). The purpose of this study was to determine how acute administration of other drugs of abuse affects risky decision-making, using acute administration of nicotine, morphine, ethanol, and diazepam.
Methods
Subjects
Male Long–Evans rats (n=23, weighing 275–300 g on arrival; Charles River Laboratories, Raleigh, NC, USA) were individually housed and kept on a 12 h light/dark cycle (lights on at 0800 h) with free access to food and water except as noted. During behavioral testing, rats were maintained at 85% of their free-feeding weight, with allowances for growth. All animal procedures were conducted during the light cycle (0900–1100) and were approved by the Texas A&M University Laboratory Animal Care and Use Committee and followed NIH guidelines.
Apparatus
Testing was conducted in standard behavioral test chambers (Coulbourn Instruments, Whitehall, PA, USA) housed within sound-attenuating isolation cubicles. Each chamber was equipped with a recessed food pellet delivery trough fitted with a photobeam to detect head entries and a 1.12-W lamp to illuminate the food trough, which was located 2 cm above the floor in the center of the front wall. Forty-five milligram grain-based food pellets (PJAI, Test Diet, Richmond, IN, USA) could be delivered into the food trough. Two retractable levers were located to the left and right of the food trough, 11 cm above the floor. A 1.12-W house light was mounted on the rear wall of the isolation cubicle. The floor of the test chamber was composed of steel rods connected to a shock generator that delivered scrambled footshocks. Locomotor activity was assessed throughout each session with an infrared activity monitor mounted on the ceiling of the test chamber. This monitor consisted of an array of infrared (body heat) detectors focused over the entire test chamber. Movement in the test chamber (in x, y, or z planes) was defined as a relative change in the infrared energy falling on the different detectors. Test chambers were interfaced with a computer running Graphic State software (Coulbourn Instruments), which controlled task event delivery and data collection.
Shaping
Shaping procedures followed those used previously (Cardinal et al. 2000; Simon et al. 2007, 2009). Following magazine training, rats were trained to press a single lever (either the left or the right, counterbalanced across groups; the other lever was retracted during this phase of training) to receive a single food pellet. After reaching a criterion of 50 lever presses in 30 min, rats were then trained on the opposite lever under the same criterion. This was followed by further shaping sessions in which both levers were retracted and rats were shaped to nose poke into the food trough during simultaneous illumination of the trough and house lights. When a nose poke occurred, a single lever was extended (left or right), and a lever press resulted in immediate delivery of a single food pellet. Immediately following the lever press, the trough light was extinguished and the lever was retracted. Rats were trained to a criterion of 30 presses on each lever within 60 min.
Risky decision-making task
Testing procedures followed Simon et al. (2009). Sessions were 60 min in duration and consisted of five blocks of 18 trials each. Each 40-s trial began with a 10-s illumination of the food trough and house lights. A nose poke into the food trough extinguished the trough light and triggered extension of either a single lever (forced choice trials) or of both levers simultaneously (choice trials). If rats failed to nose poke within the 10-s time window, the lights were extinguished and the trial was scored as an omission.
A press on one lever (either left or right, balanced across animals) resulted in one food pellet (the small safe reward) delivered immediately following the lever press. A press on the other lever resulted in immediate delivery of three food pellets (the large, risky reward). However, selection of this lever was also accompanied immediately by a possible 1-s footshock contingent on a preset probability specific to each trial block. The large reward was delivered following every choice of the large reward lever, regardless of whether or not the footshock occurred. The probability of footshock accompanying the large reward was set at 0% during the first 18-trial block. In subsequent 18-trial blocks, the probability of footshock increased to 25%, 50%, 75%, and 100%. The intensity of the footshock varied by experiment (see below). Each 18-trial block began with eight forced choice trials in which only a single lever was extended and which were used to establish the punishment contingencies in effect for that block (four for each lever), followed by 10 choice trials (Cardinal and Howes 2005; Simon et al. 2009; St Onge and Floresco 2009). Once either lever was pressed, both levers were immediately retracted. Food delivery was accompanied by re-illumination of both the food trough and house lights, which were extinguished upon entry to the food trough to collect the food or after 10 s, whichever occurred sooner. On the forced choice trials (in which only one lever was present) the probability of shock following a press on the large reward lever was dependent across the four trials in each block. For example, in the 25% risk block, one and only one of the four forced choice trials (randomly selected) always resulted in shock, and in the 75% risk block, three and only three of the four forced choice trials always resulted in shock. In contrast, the probability of shock on the free choice trials (in which both levers were present) was entirely independent, such that the probability of shock on each trial was the same, irrespective of shock delivery on previous trials in that block.
Over the course of testing, rats displayed some degree of habituation to the shock accompanying the large, risky reward, manifested as a gradually increasing preference for the large, risky reward across multiple sessions. To compensate for this effect (and to maintain the mean levels of baseline, non-drug, choice performance as close to the middle of the parametric space as possible so as to avoid potential floor or ceiling effects), the shock intensity was adjusted upward by increments of 0.05 mA between tests with different drugs. During initial training and nicotine and ethanol administration, the shock intensity was 0.4 mA. This was increased to 0.45 mA prior to morphine testing and to 0.5 mA prior to amphetamine testing. The separate group of rats tested with diazepam received a 0.55-mA shock. Shock intensity was increased for all rats at the same time (i.e., for all tests, all rats received the same footshock intensity). After each shock increase, stable performance (as described below) was established.
Drugs and injections
Injections were administered prior to testing over a period of 8 days using the following schedule: dose 1, no injections, dose 2, no injections, dose 3, no injections, dose 4, no injections. The order in which the doses of each drug (including the vehicle condition) were administered was counterbalanced across subjects. Prior to testing each drug, task performance was checked for stability (defined below in “Data analysis”). Drugs were tested in the following order in the first cohort of rats (n=11): nicotine, ethanol, morphine, and amphetamine. The effects of diazepam were tested in a second cohort of rats (n=12). At least 7 days elapsed between tests with different drugs, during which stable performance was established. The doses of each drug and timing of injections were chosen based on their effects on performance in other decision-making tasks in rats (Shimizu et al. 1992; Evenden 1998; Solinas and Goldberg 2005; Pattij et al. 2009a; Simon et al. 2009; Mendez et al. 2010).
Nicotine hydrogen tartrate (Sigma, St Louis, MO, USA; 0.1, 0.3, 0.6 mg/kg, calculated as the weight of the salt) or PBS vehicle was administered s.c. at a volume of 1 ml/kg 15 min prior to the start of test sessions. Ethanol (10% solution, 0.5 g/kg administered at a volume of 5 ml/kg, 1 g/kg at 10 ml/kg, 1.5 g/kg at 15 ml/kg) or PBS vehicle at a volume of 15 ml/kg was administered i.p. 5 min prior to the start of test sessions. Morphine sulfate (Sigma; 1.0, 3.0, 10.0 mg/kg calculated as the weight of the salt) or PBS vehicle was administered s.c. at a volume of 1 ml/kg 15 min prior to the start of test sessions. d-Amphetamine sulfate (Sigma; 0.3, 1.0, 1.5 mg/kg, calculated as the weight of the salt) or PBS vehicle was administered i.p. at a volume of 1 ml/kg, 10 min prior to the start of test sessions. Diazepam (Butler-Schein, Dublin, OH, USA; 0.3, 1.0, 3.0 mg/kg) or a vehicle solution of 50% sterile water, 40% propylene glycol, and 10% ethanol was administered i.p. at a volume of 1 ml/kg, 15 min prior to the start of test sessions.
Data analysis
Raw data files were exported from Graphic State software and compiled using a custom macro written for Microsoft Excel (Dr. Jonathan Lifshitz, University of Kentucky). Statistical analyses were conducted in SPSS 18.0. Stable behavior was defined by the absence of a main effect of session, the absence of an interaction between session and trial block, and the presence of a main effect of trial block within a repeated measures ANOVA over five consecutive sessions (Winstanley et al. 2006; Simon et al. 2009, 2010a). The effects of drug manipulations in all tasks were assessed using two-way ANOVA, with both drug dose and trial block (i.e., level of risk) as repeated measures variables. Baseline locomotor activity was measured by averaging activity across all ITI segments (in which no lights or levers were present). Shock reactivity was defined as activity (movement units) during the 1-s shock period, averaged across the test session. In all cases, p values less than 0.05 were considered significant.
Results
Rats achieved stable performance prior to each round of drug testing. In addition, there were no main effects or interactions involving the factor of session across the no-injection test sessions interposed between drug testing days with any of the drugs (Fs<0.188 and ps>0.9, i.e., performance remained stable) suggesting that there were no carryover effects of the drugs on choice performance. In addition, the main effect of trial block (risk of punishment) was significant in each drug experiment (Fs<5.4, ps<0.002) and will not be reported further.
Nicotine
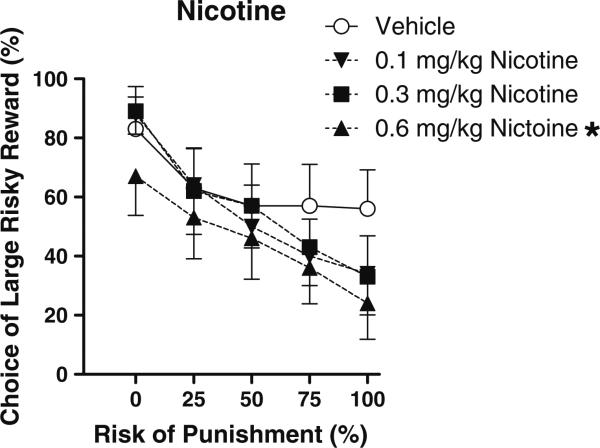
There was a main effect of nicotine dose on preference for the large risky reward (F(3,30)=5.48, p< 0.005) suggesting that rats became more risk averse with increasing doses of nicotine (Fig. 1). Individual pair-wise comparisons between vehicle and each nicotine dose showed that the 0.6 mg/kg dose caused a significant decrease in preference for the large reward (p<0.05). Nicotine also produced a dose-dependent increase in baseline locomotor activity (F(3,30)=9.60, p<0.0001) which was carried by the lowest dose of the drug, but there was no effect on shock reactivity (locomotion during the 1-s shock presentations), indicating that the observed behavioral changes were likely not a result of nicotine-induced alterations in shock sensitivity (see Table 1).
Fig. 1.
Effects of acute administration of different doses of nicotine on reward choice in the Risky Decision-Making Task. Data points represent mean (±SEM) percent choice of the large, risky reward on choice trials under different risks of punishment. The vehicle condition is represented by open circles, the low dose of nicotine by downward facing arrows, the medium dose by squares, and the high dose by upward facing arrows. *p<0.05, significantly different from vehicle condition
Table 1.
Drug effects on trial omissions, locomotion, and shock reactivity in the Risky Decision-Making Task
| Drug | Percent omitted trials | Locomotion (locomotor units/ITI) | Shock reactivity (locomotor units/shock) |
|---|---|---|---|
| Nicotine | |||
| Vehicle | 2.5 (1.7) | 41.82 (3.4) | 2.84 (0.3) |
| 0.1 mg/kg | 0.2 (0.2) | 53.92 (4.8)* | 2.55 (0.3) |
| 0.3 mg/kg | 2.2 (1.2) | 40.78 (4.5) | 2.34 (0.3) |
| 0.6 mg/kg | 4.0 (2.1) | 39.40 (4.3) | 2.14 (0.3) |
| Amphetamine | |||
| Vehicle | 0.0 (0.0) | 47.08 (2.9) | 2.67 (0.4) |
| 0.3 mg/kg | 0.4 (0.2) | 53.86 (4.3) | 2.97 (0.4) |
| 1.0 mg/kg | 4.0 (2.9) | 65.30 (4.6)* | 2.75 (0.2) |
| 1.5 mg/kg | 1.0 (0.6) | 69.77 (3.0)* | 2.60 (0.4) |
| Morphine | |||
| Vehicle | 0.2 (0.2) | 41.15 (3.3) | 2.57 (0.3) |
| 1.0 mg/kg | 0.0 (0.0) | 42.25 (3.7) | 2.79 (0.2) |
| 3.0 mg/kg | 0.4 (0.4) | 47.47 (3.1) | 2.68 (0.3) |
| Ethanol | |||
| Vehicle | 0.4 (0.2) | 38.66 (3.9) | 2.43 (0.2) |
| 0.5 g/kg | 5.8 (5.2) | 34.36 (3.6)+ | 2.42 (0.3) |
| 1.0 g/kg | 6.0 (5.2) | 33.06 (4.2)* | 2.75 (0.2) |
| 1.5 g/kg | 8.9 (5.3) | 15.51 (2.8)* | 2.37 (0.2) |
| Diazepam | |||
| Vehicle | 1.5 (1.0) | 39.03 (5.6) | 8.75 (1.7) |
| 0.3 mg/kg | 8.7 (8.0) | 39.11 (6.4) | 9.18 (1.5) |
| 1.0 mg/kg | 1.7 (1.7) | 34.85 (5.9) | 9.53 (1.7) |
| 3.0 mg/kg | 3.5 (2.0) | 27.41 (4.4) | 9.74 (1.7)+ |
Asterisks indicate that there is a significant change from vehicle. Plus symbols indicate that there is a near-significant change from vehicle. SEMs are in parentheses
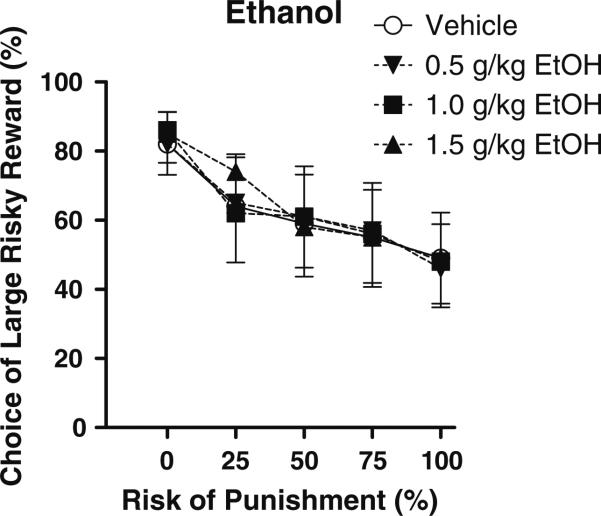
Ethanol
Ethanol had no effect on preference for the large risky reward (Fig. 2) despite the fact that the doses used were clearly effective in that there was a significant decrease in baseline locomotor activity, with the highest dose of ethanol producing the greatest effect (F(3,30)=17.24, p<0.0001). There was a near-significant main effect of ethanol dose (F(3,30)=3.05, p=0.06) on shock reactivity; however, pair-wise comparisons of each dose with the vehicle condition revealed no consistent or significant effects of ethanol on this measure.
Fig. 2.
Effects of acute administration of different doses of ethanol on reward choice in the Risky Decision-Making Task. Conventions as in Fig. 1
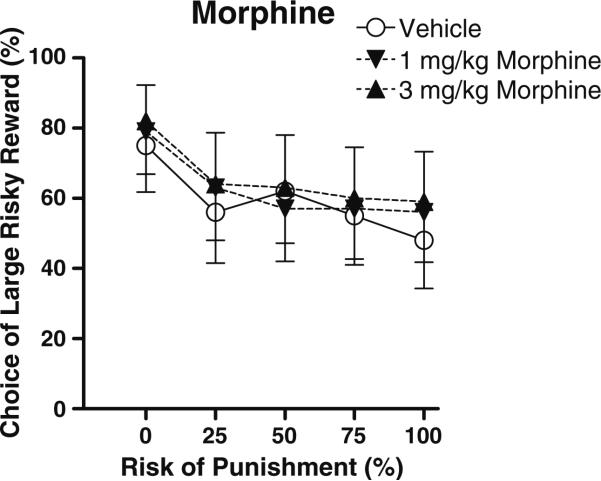
Morphine
The main effect of morphine dose on preference for the large risky reward neared, but did not quite reach, significance (Fig. 3, F(2,20)=3.06, p=0.07; note that a higher dose of morphine, 10 mg/kg, caused 60% of trials to be omitted, and those data were excluded from further analysis). Exploratory two-way ANOVAs were performed to determine which of the morphine doses was contributing to the trend toward increased choice of the large, risky reward. We found that the 3.0 mg/kg dose produced a small but significant increase in preference for the large, risky reward (main effect of dose, F(1,10)=5.75, p<0.05). There were no effects of morphine on baseline locomotor activity or shock reactivity.
Fig. 3.
Effects of acute administration of different doses of morphine on reward choice in the Risky Decision-Making Task. Conventions as in Fig. 1
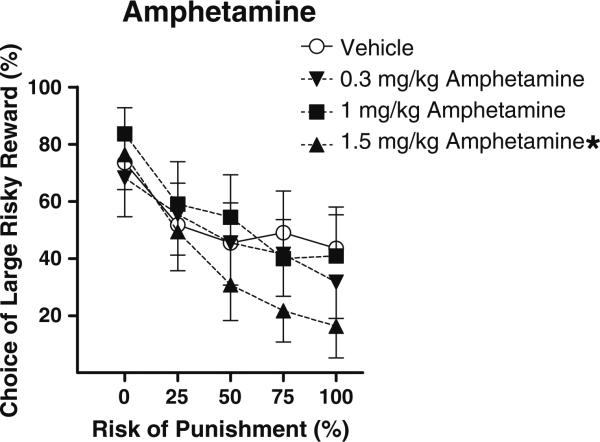
Amphetamine
There was a main effect of amphetamine on preference for the large risky reward (F(3,30)=4.55, p<0.05), as well as an interaction between dose and block (F(12,120)=1.89, p<0.05), such that rats became more risk-averse with increasing doses of amphetamine, particularly in high-risk blocks (Fig. 4). Individual pair-wise comparisons between vehicle and each amphetamine dose revealed that the 1.5 mg/kg dose caused a near significant decrease in preference for the large reward (main effect, p=0.05), as well as a significant dose×block interaction (p<0.005), but no significant effects at the other doses. In addition to its effects on choice behavior, amphetamine caused a dose-dependent increase in baseline locomotor activity (F(3,30)=6.73, p<0.05); however, there was no effect of amphetamine on shock reactivity, indicating that effects on choice preference were likely not a result of amphetamine-induced alterations in shock sensitivity.
Fig. 4.
Effects of acute administration of different doses of amphetamine on reward choice in the Risky Decision-Making Task. Conventions as in Fig. 1
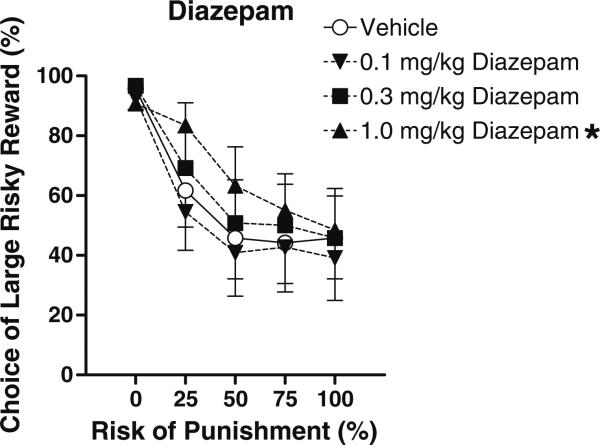
Diazepam
There was a main effect of diazepam dose on preference for the large risky reward (F(3,30)=4.07, p<0.05), as well as a significant interaction between dose and block (F(12, 120) =2.54, p<0.05) such that diazepam dose-dependently increased rats’ preference for the large risky reward, particularly under moderate risk of punishment (Fig. 5). Individual pair-wise comparisons between vehicle and individual diazepam doses revealed that the highest (3.0 mg/kg) dose caused a near-significant increase in preference for the large, risky reward (main effect, p=0.06), as well as a significant drug×block interaction (p<0.05), but no significant effects at other doses. There was no effect of diazepam on baseline locomotion; however, there was a near significant increase in shock reactivity (F(3,15)=3.15, p=0.05) that was carried by the highest dose of diazepam.
Fig. 5.
Effects of acute administration of different doses of diazepam on reward choice in the Risky Decision-Making Task. Conventions as in Fig. 1
Discussion
There is current interest in development of novel animal decision-making tasks in which subjects must balance reward against the potential for adverse consequences (i.e., risky decision-making) in order to model the decision-making deficits prominent in a range of psychopathologies including addictions. Our laboratory has recently created such a task, in which rats choose between small “safe” rewards and large “risky” rewards that are accompanied by various probabilities of punishment. We investigated the influence of acute administration of several drugs of abuse on task performance and found that both nicotine and amphetamine decreased preference for the large “risky” reward, diazepam increased this preference, morphine produced a trend toward increased preference, and ethanol had no effect. Importantly, these results were unlikely to have been due to drug-induced alterations in food motivation, locomotor activity, or shock reactivity. We have shown previously that preference for the large risky reward is not affected by either 1 or 24 h of pre-feeding prior to testing (Simon et al. 2009), and the effects of the drugs on locomotion and shock reactivity in the current experiments did not track their effects on reward preference in any obvious way, suggesting that these factors did not play a significant role in the drugs’ effects on reward preference. Note that it is possible that the observed “habituation” to the shock across tests (see “Methods”) was due to long-lasting effects of the drugs; however, this seems unlikely, as we have observed similar effects in previous experiments both with different drugs and in the absence of any drug administration. More importantly, any such effects would be compensated for by the increase in shock intensity between tests with different drugs.
Nicotine use (smoking) in humans is associated with an increase in risky decision-making in some studies (Mitchell 1999; Reynolds et al. 2004; Yu and Dayan 2005; Xiao et al. 2008), although these effects are unlikely to reflect solely acute effects of the drug. In contrast, acute nicotine administration in the present study caused a dose-dependent decrease in preference for the large risky reward (risk aversion). There are several possible explanations for this outcome. Nicotine has been shown to produce a number of pro-cognitive effects such as enhanced attention and memory (Semenova et al. 2007) and reduced deficits in young adults with ADHD on the Stop Signal Task, Choice Delay task, and High-Low Imagery Task (Potter and Newhouse 2008). Thus, its effects in the Risky Decision-Making Task could reflect such actions. Acute nicotine can also have anxiogenic effects (Zarrindast et al. 2010, although see Szyndler et al. 2001) that could account for the decrease in preference for the large risky reward. The Risky Decision-Making Task shares some similarities with punished responding tasks such as the Geller–Seifter task (Geller et al. 1962), which measures willingness to perform a reward-related action that is accompanied by punishment (Simon et al. 2009). Anxiogenic treatments tend to decrease responding for punished rewards in a manner similar to that produced here by nicotine (Geller et al. 1962; Howard et al. 1982; Lerner et al. 1986; Dalterio et al. 1989; Wiley et al. 1993); therefore, such effects could account for the observed results (although notably, we have failed to observe relationships between several measures of anxiety and choice preference in the Risky Decision-Making Task; Simon et al. 2010b and below for further discussion of this point).
Consistent with our previous findings (Simon et al. 2009), acute amphetamine caused a dose-dependent decrease in preference for the large risky reward. Although there were some similarities between the effects of amphetamine and nicotine (perhaps reflective of the potent dopamine-releasing effects of nicotine through actions on pre-synaptic terminals; Fung and Lau 1986; Mifsud et al. 1989; Rapier et al. 1990; Turner 2004; Alsene et al. 2005; Tsukada et al. 2005), the pattern of the effects of the two drugs differed. In addition to a main effect of dose, amphetamine also produced a robust dose×block interaction (such that it reduced preference for the large reward only in blocks in which there was a risk of shock), whereas nicotine produced only a main effect of dose (in which it reduced preference for the large reward even in block 1 in which there was no risk of punishment). This difference could be reflective of effects of the two drugs on within-session vs. across-session variables, respectively, and it will be of interest in future work to conduct direct comparisons between them.
The reduction in preference for the large risky reward induced by amphetamine in the Risky Decision-Making Task is consistent with its effects (and the effects of other stimulant drugs) in tests of risk taking in some populations of humans, particularly in conditions such as attention deficit-hyperactivity disorder (ADHD) in which risk taking is elevated (Rahman et al. 2006; White et al. 2007; DeVito et al. 2008). However, these effects stand in contrast to the increase in risk-taking behavior produced by acute amphetamine in other rodent tests of risk taking, particularly probability discounting, in which reward omission (rather than punishment) is used as the “cost” that influences reward value (the probability discounting task is also of interest as the task design, with the exception of the identity of the cost associated with the large reward, is essentially identical to that of Risky Decision-Making Task). This difference in the effects of amphetamine likely reflects in part the nature of the costs associated with the large rewards in these tasks. Although both reward omission and punishment have similar effects on choice behavior (reducing choice of the rewards with which they are associated), it is likely that punishment (even a mild footshock) is a more salient cost than reward omission. As amphetamine and other psychomotor stimulants can enhance control over behavior by both rewards and punishments (Robbins 1976; Thiebot et al. 1991; Killcross et al. 1997; Evenden and Ko 2005), it is likely that the opposite effects of amphetamine in the Risky Decision-Making and probability discounting tasks reflect the different degrees to which choice behavior in these tasks is controlled by punishment vs. reward, respectively (see also Zeeb et al. 2009). It will be of interest in future studies to determine whether the different effects of amphetamine in other decision-making tasks (such as delay discounting, in which it may differentially affect reward choice when delays are signaled vs. unsignaled, or even in high-impulsive vs. low-impulsive sub-populations of subjects) are related to the degree to which appetitive vs. aversive features of the tasks exert greater control over behavior (Cardinal et al. 2000; Stanis et al. 2008; Slezak and Anderson 2009).
There has been little investigation of morphine's effects on decision-making in human subjects, although chronic opioid use is associated with risk taking and poor decision-making (Mintzer and Stitzer 2002; Rotheram-Fuller et al. 2004; Ersche et al. 2005; see also Zacny and de Wit 2009, in which acute oxycodone had no effect on delay or probability discounting). In animal studies, acute morphine increases impulsive choice (i.e., increases preference for small immediate over large delayed rewards) in a delay discounting task (Kieres et al. 2004; Pattij et al. 2009b), but its acute effects on other forms of decision-making have not been examined. Given its analgesic properties, as well as the fact that it can enhance hedonic reactions to food (Berridge 2009), it was predicted that morphine would increase preference for the large risky reward. However, although the results trended in this direction, they did not reach statistical significance, suggesting that, at least under the conditions tested, opioids do not strongly modulate risky decision-making.
There were no effects of ethanol on preference for the large risky reward despite the obvious effectiveness of the drug on locomotion at the highest dose. Although such a negative effect may be intuitively surprising, it matches data from human subjects showing that acute ethanol administration does not alter decision-making on assessments of risk taking such as the Balloon Analogue Risk Task, Iowa Gambling Task, and probability discounting task (Richards et al. 1999; Balodis et al. 2006; Reynolds et al. 2006, although chronic alcoholics do show increased risk taking in the Iowa Gambling Task; Richards et al. 1999; Salgado et al. 2009). The discrepancy between these negative results and intuitive perceptions of ethanol's effects on behavior likely arises from the multi-dimensional nature of risk taking and the fact that each of these tasks likely captures only one or a few of its features.
Systemic diazepam administration produced a robust dose-dependent increase in preference for the large risky reward. While there are some reports of diazepam having analgesic properties (Zambotti et al. 1991; Jimenez-Velazquez et al. 2008, although see Gatch 1999), such effects did not likely play a role in the shift in decision-making, as the highest dose of diazepam actually produced a near-significant increase in shock reactivity. As alluded to above, these findings resemble those in the Geller–Seifter (punished responding) task, in which diazepam causes an increase in punished responding thought to be related to its anxiolytic properties (Shimizu et al. 1992; Yasumatsu et al. 1994; File et al. 2004). The Risky Decision-Making Task differs from such tasks in that it gives animals a “safe” option to avoid punishment, and the probability of punishment changes over the course of the test session (File et al. 2004; Simon et al. 2009). Furthermore, despite the similarities in the effects of diazepam (and amphetamine) on the two tasks, there is also considerable divergence in the pharmacological data (Howard et al. 1982; Lerner et al. 1986). In the Geller–Seifter task, both a D2-like dopamine receptor agonist and ethanol were reported to increase punished responding (Dalterio et al. 1989; Millan et al. 2004), whereas in the Risky Decision-Making Task, the D2-like agonist bromocriptine had the opposite effect (Simon et al. 2010b) and ethanol had no effects whatsoever. Moreover, whereas morphine has been shown to decrease punished responding in the Geller–Seifter task (Howard et al. 1982), its effects trended in the opposite direction in the present study. Thus, despite similarities between the two tasks, it appears that the Risky Decision-Making Task may assess a behavioral construct dissociable from Geller–Seifter tasks.
In conclusion, we found that both nicotine and d-amphetamine dose-dependently decreased preference for the large, risky reward. Ethanol had no effects on this measure of risk taking, whereas morphine and diazepam tended to increase preference for the large risky reward. These data are consistent with available data in human subjects on laboratory tests of risky decision-making, and suggest that the Risky Decision-Making Task may be a useful model for human decision-making under risk of adverse consequences (and perhaps that drugs targeting dopaminergic and/or nicotinic cholinergic receptors may prove useful in reducing risky behavior in pathological conditions in which excessive risk taking is present, such as ADHD and addiction). Despite these consistencies, however, there are a number of differences between the effects of these drugs on the Risky Decision-Making Task and their effects on similarly designed rodent tasks that are designed to parse different types of “costs” of decision-making, including delay to reward delivery and probability of reward delivery (Dallery and Locey 2005; Cardinal 2006; Floresco et al. 2008; Pattij et al. 2009b; Setlow et al. 2009; Mendez et al. 2010). The complexity of these effects (e.g., the fact that the same drug may either increase or decrease choice of a large vs. small reward, depending on the type of cost associated with the large reward) highlights the importance of a thorough consideration of all of the decision-making factors involved when making predictions about the effects of acute drug intoxication on real-world decision-making in humans.
Acknowledgments
We thank Dr. Ian Mendez and Ms. Rebecca Simmons for their assistance in completing these experiments, and Dr. James Grau for the morphine. Supported by NIH DA024671 (BS) and F31DA0233312 (NWS).
Footnotes
No authors have any conflicts of interest. All experiments were conducted in accordance with applicable animal welfare laws and regulations in the USA.
Contributor Information
Marci R. Mitchell, Department of Psychiatry, McKnight Brain Institute, University of Florida College of Medicine, Gainesville, FL 32610-0256, USA Department of Neuroscience, University of Florida College of Medicine, Gainesville, FL 32610, USA.
Colin M. Vokes, Department of Psychology, Texas A&M University, College Station, TX 77843, USA
Amy L. Blankenship, Department of Psychology, Texas A&M University, College Station, TX 77843, USA
Nicholas W. Simon, Department of Neuroscience, University of Pittsburgh, Pittsburgh, PA 15260, USA
Barry Setlow, Department of Psychiatry, McKnight Brain Institute, University of Florida College of Medicine, Gainesville, FL 32610-0256, USA; Department of Neuroscience, University of Florida College of Medicine, Gainesville, FL 32610, USA.
References
- Alsene KM, Mahler SV, de Wit H. Effects of d-amphetamine and smoking abstinence on cue-induced cigarette craving. Exp Clin Psychopharmacol. 2005;13:209–218. doi: 10.1037/1064-1297.13.3.209. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association DSM-IV-TR Balodis IM, MacDonald TK, Olmstead MC (2006) Instructional cues modify performance on the Iowa Gambling Task. Brain Cogn. 2000;60:109–117. doi: 10.1016/j.bandc.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275:1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Berridge KC. ‘Liking’ and ‘wanting’ food rewards: brain substrates and roles in eating disorders. Physiol Behav. 2009;97:537–550. doi: 10.1016/j.physbeh.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN. Neural systems implicated in delayed and probabilistic reinforcement. Neural Netw. 2006;19:1277–1301. doi: 10.1016/j.neunet.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Howes NJ. Effects of lesions of the nucleus accumbens core on choice between small certain rewards and large uncertain rewards in rats. BMC Neurosci. 2005;6:37. doi: 10.1186/1471-2202-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Robbins TW, Everitt BJ. The effects of d-amphetamine, chlordiazepoxide, alpha-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology (Berl) 2000;152:362–375. doi: 10.1007/s002130000536. [DOI] [PubMed] [Google Scholar]
- Dallery J, Locey ML. Effects of acute and chronic nicotine on impulsive choice in rats. Behav Pharmacol. 2005;16:15–23. doi: 10.1097/00008877-200502000-00002. [DOI] [PubMed] [Google Scholar]
- Dalterio SL, Wayner MJ, Geller I, Hartmann RJ. Ethanol and chronic diazepam interactions on conflict behavior in rats. Alcohol. 1989;6:409–414. doi: 10.1016/0741-8329(89)90012-8. [DOI] [PubMed] [Google Scholar]
- DeVito EE, Blackwell AD, Kent L, Ersche KD, Clark L, Salmond CH, Dezsery AM, Sahakian BJ. The effects of methylphenidate on decision making in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;64:636–639. doi: 10.1016/j.biopsych.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsler R, Rizzo P, Steinhausen HC. Decision-making on an explicit risk-taking task in preadolescents with attention-deficit/hyperactivity disorder. J Neural Transm. 2008;115:201–209. doi: 10.1007/s00702-007-0814-5. [DOI] [PubMed] [Google Scholar]
- Ernst M, Kimes AS, London ED, Matochik JA, Eldreth D, Tata S, Contoreggi C, Leff M, Bolla K. Neural substrates of decision making in adults with attention deficit hyperactivity disorder. Am J Psychiatry. 2003;160:1061–1070. doi: 10.1176/appi.ajp.160.6.1061. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Roiser JP, Clark L, London M, Robbins TW, Sahakian BJ. Punishment induces risky decision-making in methadone-maintained opiate users but not in heroin users or healthy volunteers. Neuropsychopharmacology. 2005;30:2115–2124. doi: 10.1038/sj.npp.1300812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL. The pharmacology of impulsive behaviour in rats II: the effects of amphetamine, haloperidol, imipramine, chlordiazepoxide and other drugs on fixed consecutive number schedules (FCN 8 and FCN 32). Psychopharmacology (Berl) 1998;138:283–294. doi: 10.1007/s002130050673. [DOI] [PubMed] [Google Scholar]
- Evenden J, Ko T. The psychopharmacology of impulsive behaviour in rats VIII: effects of amphetamine, methylphenidate, and other drugs on responding maintained by a fixed consecutive number avoidance schedule. Psychopharmacology (Berl) 2005;180:294–305. doi: 10.1007/s00213-005-2163-0. [DOI] [PubMed] [Google Scholar]
- File SE, Lippa AS, Beer B, Lippa MT. Animal tests of anxiety. Curr Protoc Neurosci. 2004 doi: 10.1002/0471142301.ns0803s26. Chapter 8: Unit 8 3. [DOI] [PubMed] [Google Scholar]
- Floresco SB, St Onge JR, Ghods-Sharifi S, Winstanley CA. Cortico-limbic-striatal circuits subserving different forms of cost–benefit decision making. Cogn Affect Behav Neurosci. 2008;8:375–389. doi: 10.3758/CABN.8.4.375. [DOI] [PubMed] [Google Scholar]
- Fung YK, Lau YS. Acute effect of nicotine on the striatal dopaminergic system in the rat. J Pharm Pharmacol. 1986;38:920–922. doi: 10.1111/j.2042-7158.1986.tb03384.x. [DOI] [PubMed] [Google Scholar]
- Gatch MB. Effects of benzodiazepines on acute and chronic ethanol-induced nociception in rats. Alcohol Clin Exp Res. 1999;23:1736–1743. [PubMed] [Google Scholar]
- Geller I, Kulak JT, Jr, Seifter J. The effects of chlordiazepoxide and chlorpromazine on a punishment discrimination. Psycho-pharmacologia. 1962;3:374–385. doi: 10.1007/BF00408322. [DOI] [PubMed] [Google Scholar]
- Heerey EA, Bell-Warren KR, Gold JM. Decision-making impairments in the context of intact reward sensitivity in schizophrenia. Biol Psychiatry. 2008;64:62–69. doi: 10.1016/j.biopsych.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard JL, Rohrbach KW, Pollard GT. Cumulative dose–effect curves in a conflict test with incremental shock. Psychopharmacology (Berl) 1982;78:195–196. doi: 10.1007/BF00432263. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Woods JA, Groman SM, Seu E. Behavioral characteristics and neural mechanisms mediating performance in a rodent version of the Balloon Analog Risk Task. Neuropsychopharmacology. 2010;35:1797–1806. doi: 10.1038/npp.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Velazquez G, Lopez-Munoz FJ, Fernandez-Guasti A. Participation of the GABA/benzodiazepine receptor and the NO-cyclicGMP pathway in the “antinociceptive-like effects” of diazepam. Pharmacol Biochem Behav. 2008;91:128–133. doi: 10.1016/j.pbb.2008.06.021. [DOI] [PubMed] [Google Scholar]
- Kieres AK, Hausknecht KA, Farrar AM, Acheson A, de Wit H, Richards JB. Effects of morphine and naltrexone on impulsive decision making in rats. Psychopharmacology (Berl) 2004;173:167–174. doi: 10.1007/s00213-003-1697-2. [DOI] [PubMed] [Google Scholar]
- Killcross AS, Everitt BJ, Robins TW. Symmetrical effects of amphetamine and alpha-flupenthixol on conditioned punishment and conditioned reinforcement: contrasts with midazolam. Psychopharmacology (Berl) 1997;129:141–152. doi: 10.1007/s002130050174. [DOI] [PubMed] [Google Scholar]
- Lerner T, Feldon J, Myslobodsky MS. Amphetamine potentiation of anti-conflict action of chlordiazepoxide. Pharmacol Biochem Behav. 1986;24:241–246. doi: 10.1016/0091-3057(86)90345-x. [DOI] [PubMed] [Google Scholar]
- Ludewig K, Paulus MP, Vollenweider FX. Behavioural dysregulation of decision-making in deficit but not nondeficit schizophrenia patients. Psychiatry Res. 2003;119:293–306. doi: 10.1016/s0165-1781(03)00103-3. [DOI] [PubMed] [Google Scholar]
- Mendez IA, Damborsky J, Bizon JL, Winzer-Serhan U, Setlow B. The roles of nicotinic and muscarinic cholinergic receptors in cost–benefit decision making Society for Neuroscience Annual Meeting; San Diego, CA. 2010. [Google Scholar]
- Mifsud JC, Hernandez L, Hoebel BG. Nicotine infused into the nucleus accumbens increases synaptic dopamine as measured by in vivo microdialysis. Brain Res. 1989;478:365–367. doi: 10.1016/0006-8993(89)91518-7. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Brocco M, Papp M, Serres F, La Rochelle CD, Sharp T, Peglion JL, Dekeyne A. S32504, a novel naphtoxazine agonist at dopamine D3/D2 receptors: III. Actions in models of potential antidepressive and anxiolytic activity in comparison with ropinirole. J Pharmacol Exp Ther. 2004;309:936–950. doi: 10.1124/jpet.103.062463. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Stitzer ML. Cognitive impairment in methadone maintenance patients. Drug Alcohol Depend. 2002;67:41–51. doi: 10.1016/s0376-8716(02)00013-3. [DOI] [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology (Berl) 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Negus SS. Effects of punishment on choice between cocaine and food in rhesus monkeys. Psychopharmacology (Berl) 2005;181:244–252. doi: 10.1007/s00213-005-2266-7. [DOI] [PubMed] [Google Scholar]
- Pattij T, Schetters D, Janssen M, Wiskerke J, Schoffelmeer A. Acute effects of morphine on distinct forms of impulsive behavior in rats. Psychopharmacology. 2009a;205:489–502. doi: 10.1007/s00213-009-1558-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattij T, Schetters D, Janssen MC, Wiskerke J, Schoffelmeer AN. Acute effects of morphine on distinct forms of impulsive behavior in rats. Psychopharmacology (Berl) 2009b;205:489–502. doi: 10.1007/s00213-009-1558-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA. Acute nicotine improves cognitive deficits in young adults with attention-deficit/hyperactivity disorder. Pharmacol Biochem Behav. 2008;88:407–417. doi: 10.1016/j.pbb.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Rahman S, Robbins TW, Hodges JR, Mehta MA, Nestor PJ, Clark L, Sahakian BJ. Methylphenidate (‘Ritalin’) can ameliorate abnormal risk-taking behavior in the frontal variant of frontotemporal dementia. Neuropsychopharmacology. 2006;31:651–658. doi: 10.1038/sj.npp.1300886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapier C, Lunt GG, Wonnacott S. Nicotinic modulation of [3H] dopamine release from striatal synaptosomes: pharmacological characterisation. J Neurochem. 1990;54:937–945. doi: 10.1111/j.1471-4159.1990.tb02341.x. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Richards JB, Horn K, Karraker K. Delay discounting and probability discounting as related to cigarette smoking status in adults. Behav Process. 2004;65:35–42. doi: 10.1016/s0376-6357(03)00109-8. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Richards JB, de Wit H. Acute-alcohol effects on the Experiential Discounting Task (EDT) and a question-based measure of delay discounting. Pharmacol Biochem Behav. 2006;83:194–202. doi: 10.1016/j.pbb.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Richards JB, Zhang L, Mitchell SH, de Wit H. Delay or probability discounting in a model of impulsive behavior: effect of alcohol. J Exp Anal Behav. 1999;71:121–143. doi: 10.1901/jeab.1999.71-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivalan M, Ahmed SH, Dellu-Hagedorn F. Risk-prone individuals prefer the wrong options on a rat version of the Iowa Gambling Task. Biol Psychiatry. 2009;66:743–749. doi: 10.1016/j.biopsych.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Relationship between reward-enhancing and stereotypical effects of psychomotor stimulant drugs. Nature. 1976;264:57–59. doi: 10.1038/264057a0. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Owen AM, Middleton HC, Williams EJ, Pickard JD, Sahakian BJ, Robbins TW. Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. J Neurosci. 1999;19:9029–9038. doi: 10.1523/JNEUROSCI.19-20-09029.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotheram-Fuller E, Shoptaw S, Berman SM, London ED. Impaired performance in a test of decision-making by opiate-dependent tobacco smokers. Drug Alcohol Depend. 2004;73:79–86. doi: 10.1016/j.drugalcdep.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Salgado JV, Malloy-Diniz LF, Campos VR, Abrantes SS, Fuentes D, Bechara A, Correa H. Neuropsychological assessment of impulsive behavior in abstinent alcohol-dependent subjects. Rev Bras Psiquiatr. 2009;31:4–9. doi: 10.1590/s1516-44462009000100003. [DOI] [PubMed] [Google Scholar]
- Semenova S, Stolerman IP, Markou A. Chronic nicotine administration improves attention while nicotine withdrawal induces performance deficits in the 5-choice serial reaction time task in rats. Pharmacol Biochem Behav. 2007;87:360–368. doi: 10.1016/j.pbb.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B, Mendez IA, Mitchell MR, Simon NW. Effects of chronic administration of drugs of abuse on impulsive choice (delay discounting) in animal models. Behav Pharmacol. 2009;20:380–389. doi: 10.1097/FBP.0b013e3283305eb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H, Kumasaka Y, Tanaka H, Hirose A, Nakamura M. Anticonflict action of tandospirone in a modified Geller–Seifter conflict test in rats. Jpn J Pharmacol. 1992;58:283–289. doi: 10.1254/jjp.58.283. [DOI] [PubMed] [Google Scholar]
- Simon NW, Mendez IA, Setlow B. Cocaine exposure causes long term increases in impulsive choice. Behav Neurosci. 2007;121:543–549. doi: 10.1037/0735-7044.121.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Gilbert RJ, Mayse JD, Bizon JL, Setlow B. Balancing risk and reward: a rat model of risky decision making. Neuropsychopharmacology. 2009;34:2208–2217. doi: 10.1038/npp.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, LaSarge CL, Montgomery KS, Williams MT, Mendez IA, Setlow B, Bizon JL. Good things come to those who wait: attenuated discounting of delayed rewards in aged Fischer 344 rats. Neurobiol Aging. 2010a;31:853–862. doi: 10.1016/j.neurobiolaging.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Montgomery KS, Beas BS, Mitchell MR, LaSarge CL, Haberman RP, Bizon JL, Setlow B. Dopamine receptor modulation of risky decision-making Society for Neuroscience Annual Meeting; San Diego, CA. 2010b. [Google Scholar]
- Slezak JM, Anderson KG. Effects of variable training, signaled and unsignaled delays, and d-amphetamine on delay-discounting functions. Behav Pharmacol. 2009;20:424–436. doi: 10.1097/FBP.0b013e3283305ef9. [DOI] [PubMed] [Google Scholar]
- Solinas M, Goldberg SR. Motivational effects of cannabinoids and opioids on food reinforcement depend on simultaneous activation of cannabinoid and opioid systems. Neuropsychopharmacology. 2005;30:2035–2045. doi: 10.1038/sj.npp.1300720. [DOI] [PubMed] [Google Scholar]
- St Onge JR, Floresco SB. Dopaminergic modulation of risk-based decision making. Neuropsychopharmacology. 2009;34:681–697. doi: 10.1038/npp.2008.121. [DOI] [PubMed] [Google Scholar]
- Stanis JJ, Burns RM, Sherrill LK, Gulley JM. Disparate cocaine-induced locomotion as a predictor of choice behavior in rats trained in a delay-discounting task. Drug Alcohol Depend. 2008;98:54–62. doi: 10.1016/j.drugalcdep.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyndler J, Sienkiewicz-Jarosz H, Maciejak P, Siemiatkowski M, Rokicki D, Czlonkowska AI, Plaznik A. The anxiolytic-like effect of nicotine undergoes rapid tolerance in a model of contextual fear conditioning in rats. Pharmacol Biochem Behav. 2001;69:511–518. doi: 10.1016/s0091-3057(01)00548-2. [DOI] [PubMed] [Google Scholar]
- Taylor Tavares JV, Clark L, Cannon DM, Erickson K, Drevets WC, Sahakian BJ. Distinct profiles of neurocognitive function in unmedicated unipolar depression and bipolar II depression. Biol Psychiatry. 2007;62:917–924. doi: 10.1016/j.biopsych.2007.05.034. [DOI] [PubMed] [Google Scholar]
- Thiebot MH, Dangoumau L, Richard G, Puech AJ. Safety signal withdrawal: a behavioural paradigm sensitive to both “anxiolytic” and “anxiogenic” drugs under identical experimental conditions. Psychopharmacology (Berl) 1991;103:415–424. doi: 10.1007/BF02244298. [DOI] [PubMed] [Google Scholar]
- Tsukada H, Miyasato K, Harada N, Nishiyama S, Fukumoto D, Kakiuchi T. Nicotine modulates dopamine synthesis rate as determined by L-[beta-11C]DOPA: PET studies compared with [11C]raclopride binding in the conscious monkey brain. Synapse. 2005;57:120–122. doi: 10.1002/syn.20157. [DOI] [PubMed] [Google Scholar]
- Turner TJ. Nicotine enhancement of dopamine release by a calcium-dependent increase in the size of the readily releasable pool of synaptic vesicles. J Neurosci. 2004;24:11328–11336. doi: 10.1523/JNEUROSCI.1559-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos R, Lasthuis W, den Heijer E, van der Harst J, Spruijt B. Toward a rodent model of the Iowa gambling task. Behav Res Methods. 2006;38:470–478. doi: 10.3758/bf03192801. [DOI] [PubMed] [Google Scholar]
- White TL, Lejuez CW, de Wit H. Personality and gender differences in effects of d-amphetamine on risk taking. Exp Clin Psychopharmacol. 2007;15:599–609. doi: 10.1037/1064-1297.15.6.599. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Compton AD, Porter JH. Effects of four anti-psychotics on punished responding in rats. Pharmacol Biochem Behav. 1993;45:263–267. doi: 10.1016/0091-3057(93)90237-n. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev. 2006;26:379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Bechara A, Cen S, Grenard JL, Stacy AW, Gallaher P, Wei Y, Jia Y, Anderson Johnson C. Affective decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in 10th-grade Chinese adolescent smokers. Nicotine Tob Res. 2008;10:1085–1097. doi: 10.1080/14622200802097530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumatsu H, Morimoto Y, Yamamoto Y, Takehara S, Fukuda T, Nakao T, Setoguchi M. The pharmacological properties of Y-23684, a benzodiazepine receptor partial agonist. Br J Pharmacol. 1994;111:1170–1178. doi: 10.1111/j.1476-5381.1994.tb14868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AJ, Dayan P. Uncertainty, neuromodulation, and attention. Neuron. 2005;46:681–692. doi: 10.1016/j.neuron.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Zacny JP, de Wit H. The prescription opioid, oxycodone, does not alter behavioral measures of impulsivity in healthy volunteers. Pharmacol Biochem Behav. 2009;94:108–113. doi: 10.1016/j.pbb.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambotti F, Zonta N, Tammiso R, Conci F, Hafner B, Zecca L, Ferrario P, Mantegazza P. Effects of diazepam on nociception in rats. Naunyn Schmiedebergs Arch Pharmacol. 1991;344:84–89. doi: 10.1007/BF00167386. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Naghdi-Sedeh N, Nasehi M, Sahraei H, Bahrami F, Asadi F. The effects of dopaminergic drugs in the ventral hippocampus of rats in the nicotine-induced anxiogenic-like response. Neurosci Lett. 2010;475:156–160. doi: 10.1016/j.neulet.2010.03.069. [DOI] [PubMed] [Google Scholar]
- Zeeb FD, Robbins TW, Winstanley CA. Serotonergic and dopaminergic modulation of gambling behavior as assessed using a novel rat gambling task. Neuropsychopharmacology. 2009;34:2329–2343. doi: 10.1038/npp.2009.62. [DOI] [PubMed] [Google Scholar]