Abstract
We evaluated an ex-vivo photodepletion technique to selectively deplete graft-versus-host disease (GVHD) alloreacting T cells given to 24 HLA-identical sibling stem cell transplant (SCT) recipients. Donor lymphocytes were activated by 72 hr exposure to irradiated in-vitro expanded recipient T lymphocytes and pulsed with TH9402 photosensitizer. Alloactivated T cells preferentially retaining the photosensitizer were eliminated by light exposure. The photodepletion product showed an inverted CD4+/CD8+ ratio with greatest depletion occurring in the CD4+naïve and central memory populations. In contrast, the CD8+ naive and effector cells were relatively conserved, reflecting the differential extrusion of TH9402 by T cell subsets. CMV reactive T cells were reduced in the photodepletion product and in recipient blood 100 days after SCT when compared with contemporaneous HLA-identical sibling donor T cell depleted SCT recipients. Although PD SCT recipients experienced similar absolute lymphocyte counts during the first 100 days after SCT, they achieved 100% donor T cell chimerism more rapidly and had higher CD8+ naive T cell counts early after SCT. SCT recipients of photodepleted products with the lowest CD4 central memory content had the highest risk of developing chronic GVHD (p = 0.04), and a poorer survival (p = 0.03). While the persistence of CD8+ naive T cells may have contributed to important anti-leukemia responses resulting in a relatively low relapse rate, our findings emphasize the role of donor memory T cells and CD4 cells in establishing immune competence post SCT. Although photodepletion is associated with excellent outcomes in the haploidentical setting, the low frequency of alloactivations in HLA-matched pairs makes the PD approach used by our group for allodepletion in HLA-matched sibling transplantations an inefficient technique.
INTRODUCTION
Hematopoietic stem cell transplantation (SCT) offers a curative potential for many hematological malignancies. However, the success of SCT is limited by the morbidity and mortality associated with severe graft-versus-host disease (GVHD) and delayed immune reconstitution. The GVHD alloresponse can be prevented or reduced in severity by post-transplant immune suppression and T cell depletion (TCD), but with a risk of impairing anti-viral and anti-tumor responses of the incoming donor immune system 1. One approach to selectively reduce the risk of GVHD is to stimulate the donor lymphocyte product prior to transplantation with recipient antigen-presenting cells (APC), and then subsequently target and eliminate host-alloreactive T cells 2-5. We recently conducted a clinical trial using a photodepletion (PD) technique to selectively deplete host-reacting T cells from HLA-matched sibling SCTs with the goal of reducing acute GVHD. This PD technique was designed to target p-glycoprotein differences between activated and non-activated cells, and had been validated in MHC-mismatched donor-recipient pairs in mice and humans 6, 7. After stimulation with recipient APCs alloactivated donor T cells were eliminated based on their preferential retention of the photosensitizer 4, 5-dibromorhodamine 123 (TH9402) and exposure to visible light (Kiadis Pharma, The Netherlands). Pre-clinical studies demonstrated that photodepleted cell products retained third party alloreactivity and CMV reactivity, while losing alloreactivity to the original stimulator. The PD approach was equally applicable to HLA-matched and mismatched donor-recipient pairs 8. After FDA and IRB approval, 24 patients were enrolled in the clinical trial. However, due to recurrent infections and increased rates of chronic GVHD we elected to close the trial early. The clinical results of this study are reported separately 9. Here we describe the characteristics of the PD product and associated abnormalities of immune reconstitution and transplant outcome in this patient group. The data are contrasted with a contemporaneous group of patients receiving an identical preparative regimen and a TCD SCT with 5×104 CD3+ cells/kg.
PATIENTS, MATERIALS AND METHODS
Patients
Between July 2006 and October 2010, 24 patients underwent a PD SCT and 35 patients underwent a TCD SCT from an HLA-identical sibling on the National Heart, Lung and Blood Institute (NHLBI) institutional review board-approved protocols (07-H-0136 and 06-H-0248). Patients and donors provided written informed consent before enrolling in the transplantation protocol.
Reagents for Flow Cytometry
The following monoclonal antibodies (mAb) and fluorescent dyes were used: from Beckman Coulter: α-CD27-Cy5-phyocoerythrin (Cy5PE; clone 1A4CD27); from Becton Dickinson (BD): α-CD3-AmCyan (clone SK7), α-CD4-V500 (clone PRA-T4), α-CD4-Cy7PE (clone SK3), α-CD8-H7-allophycocyanin (H7APC) or -Cy7APC (clone SK1), α-CD107a-fluorescein isothyocyanate (FITC; clone H4A3), α-CD25-FITC (clone 2A3), α-Macrophage inflammatory protein-1β (MIP-1β)-PE (clone D21-1351), α-tumor necrosis factor-α (TNFα) -Cy7PE (clone Mab 11), α-CD154-APC (clone TRAP1), α-FOXP3-APC (clone 229D/C7), α-CD28 (clone L293), and α-CD49d (clone L25); from Invitrogen: α-CD3 Quantum Dot 605 (QD605; clone UCHT1), α-CD14-pacific blue (PB; clone TuK4), α-CD19-PB (clone SJ25-C1), α-CD45RO-PE (clone UCHL1), the violet dead cell-exclusion dye ViViD, and the cell tracker dye 5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester (CFSE).
Generation of Recipient Stimulator Cells
Details of the technique used to produce the PD product were previously reported 8. Briefly, ex vivo–expanded T lymphocytes from the recipient were used as stimulator cells due the ease of obtaining sufficient numbers of stimulators potentially free of myeloid and B-cell-derived antigens that might later induce a graft-versus-leukemia effect. These stimulator cells were generated from immunomagnetically CD3-selected T cells (Miltenyi Biotech, Bergisch Gladbach, Germany) obtained from a single leukapheresis product (8- to 12-liter blood volume processed). After selection, CD3+ cells were cultured in X-VIVO 20 (Cambrex Bio Science) supplemented with 2% autologous heat-inactivated plasma and 100 IU/mL interleukin-2 (Proleukin; Chiron) in gas-permeable polyolefin tissue culture bags (Lifecell PL732; Baxter Cellular Therapies) precoated with OKT3 antibody (murumonab-CD3, Orthoclone OKT3; Ortho Biotech). Cells were then incubated at 37°C, 7% CO2, and 90% humidity with fresh medium added to maintain the cell concentration between 0.5 × 106 and 1.5 × 106 cells/mL. Stimulator cells were harvested after 10 to 12 days, and then concentrated, washed, and cryopreserved.
Coculture
Cells were thawed, gamma irradiated (25 Gy), resuspended in X-VIVO 20 (Cambrex Bio Science) supplemented with 2% autologous heat-inactivated plasma, at a concentration of 5 × 106 cells/mL, and then combined with responder cells at a ratio of 1:1. Responder and irradiated stimulator cells were cocultured for 72 hours at 37°C, 7% CO2. Cocultures were performed in gas-permeable polyolefin tissue culture bags. Following the 72-hour coculture, cells were concentrated in preparation for PD.
Photodepletion (PD)
Following the coculture, concentrated cells were resuspended at a concentration of 5 × 106 cells/mL and incubated at 37°C with 7.5 μM 4,5-dibromorhodamine 123 (TH9402; Kiadis Pharma, The Netherlands). After 40 minutes of incubation, cells were centrifuged and resuspended in TH9402-free medium (X-VIVO 20 without phenol red, Cambrex Bio Science) supplemented with 2.5% plasma for 90 minutes to allow dye efflux. After the 90 minute dye extrusion period, cells were exposed to visible light using a fluorescent light-scanning device (Theralux device, Model 514; Kiadis) delivering 5 J/cm2 at a wavelength of 514 nm and 180 rotations per minute. Afterward, cells were cryopreserved at a final concentration of 5% DMSO and 6% pentastarch, and then transferred into a liquid nitrogen tank at liquid or vapor phase (−200°C to −150°C) for storage until thaw and distribution.
Transplant Procedure
Patients on both the PD and TCD protocols received a conditioning regimen of fludarabine (125mg/m2 over 5 days), fractionated TBI (12 Gy; 4.0 Gy if over 55y) in eight fractions over 4 days, followed by cyclophosphamide (120 mg/kg over 2 days). Depletion of T cells from the stem cell transplant products was accomplished by positive selection of CD34+ cells using the Miltenyi CliniMacs system (Miltenyi Biotec Inc., Auburn, CA). On the day of SCT all patients received this enriched CD34+ stem cell product with a target dose of 5 × 106 CD34+ cells/kg. Patients undergoing a TCD (06-H-0248) SCT received a predetermined dose of 5×104T cells/kg with the stem cell product that was adjusted by adding back T cells from the selection process if necessary. Patients enrolled in the PD protocol (07-H-0136) received 5 × 106 photodepleted CD3+ cells/kg.
Enumeration of T cell Subsets
Samples of the lymphocyte allograft before and after PD, and peripheral blood mononuclear cell (PBMC) samples were collected at 100 days after SCT and cryopreserved. In preparation for analysis, PBMC were thawed and rested overnight. Cell staining was done essentially as previously described 10, 11. Data were acquired on a CantoII flow cytometer (BD) and analyzed using FACSDiva (BD, version 6.1) or FlowJo (Treestar, version 8.6.6). T cell subsets were defined as follows: Naive CD27+CD45RO−, Central Memory CD27+CD45RO+, Effector Memory CD27−CD45RO+, and Effectors CD27−CD45RO−. Regulatory T cells (Tregs) are defined as CD4+CD25+FOXP3+ T cells.
Measurement of P-glycoprotein (Pgp) Activity
To evaluate Pgp activity, elutriated lymphocytes from 8 volunteers donors were stained with ViViD and mAb to CD3, CD4, CD8, CD14, CD19, CD45RO, and CD27 as previously reported 12, washed, and then resuspended at a concentration of 5 × 106 cells/mL in X-VIVO 10 (Cambrex Bio Science) containing 7.5 μM TH9402 for 40 minutes at 37°C. Cells were than centrifuged and resuspended X-Vivo 10 free of TH9402 in the presence of absence of verapamil (10μM, Sigma). Flow cytometry was then performed on a CantoII flow cytometer. In accordance with standard methods, Pgp activity was determined by the difference in TH9402 median fluorescence intensity in the presence and absence of verapamil 13. Data were analyzed using FACSDiva software.
Proliferation Assays
Measurement of in vitro proliferation was performed by the method of CFSE dilution14. Immunomagnetically -selected T cells were used as responder cells, and the CD3-depleted fraction from the same donor was used for antigen presentation (APC). Isolated CD3+ cells were stained with 0.5 μM CFSE (Invitrogen) and incubated at 37°C for 7 minutes. CFSE was quenched by adding 1mL normal AB serum (NABS, Gemini Bio-Products) for 2 minutes, and the cells were washed twice in RPMI 1640 containing 10% NABS. Stimulator cells were labeled with 0.5 nM Far Red (Invitrogen) following the same procedure. Fully HLA-disparate healthy donor cells were used as targets for the measurement of 3rd party reactivity, and all targets were irradiated with 25 Gy. 5 × 104 Responder cells were stimulated in a 96 round-bottom plates with an equal number of with autologous APC loaded with 1 ug/ml custom-synthesized peptide library, covering the entire open reading frames of either cytomegalovirus immediate early-1 (IE1) or phosphoprotein 65 (pp65)15, both from Princeton Biomolecules, Inc, in the presence of 1 ug/ml α-CD28 and α-CD49d for costimulation. Staphylococcus enterotoxin B (SEB, Toxin Technology, Inc.) was used as a positive control. After 6 days, cells were harvested and stained with ViViD, followed by staining with mAb to CD3, CD4, CD8, CD14, CD19 as outlined above, and acquired on a LSRFortessa (BD). List mode files were analyzed using FlowJo.
T cell Functional Assays
To determine the reactivity of T cells with viral antigens, PBMC were thawed and rested as outlined above, and then stimulated with pretitered amounts of the IE1 and pp65 peptide library, or cell lysates prepared from an adenovirus (Ad)-, cytomegalovirus (CMV)-, influenza-, or varicella zoster virus (VZV)-infected cell line. As a control, a lysate from an uninfected cell line was used. All lysates were from Microbix. Stimulations were carried out in 96-well U bottom plates, and in the presence of the cytokine secretion blockers brefeldin A (Golgiplug; BD), monensin (Golgistop; BD), α-CD107a-FITC, and α-CD28 and α-CD49d. After 6 hours at 37°C/ 5% CO2 the cells were washed and stained with ViViD and mAb to cell surface antigens as described above, followed by intracellular staining for MIP-1β, TNFα, and CD154 using Cytofix/cytoperm and Cytoperm/cytowash kit (BD). The cells were acquired on a LSRFortessa, and data were examined using FlowJo.
Definition of Terms
Limited chronic GvHD was defined as localized skin involvement resembling scleroderma with or without liver involvement; no other organ involvement present. Extensive chronic GvHD was defined as generalized skin or multiple organ involvement 16. Overall survival (OS) was defined as the number of days after SCT of death.
Statistical Analysis
Survival was measured to the last contact date or death. Univariate analysis was performed using Logrank and Cox proportional-hazard regression models. Multivariate analyses were performed using Cox proportional-hazard regression model and included all factors associated with a p-value less than 0.2 by univariate analysis. A stepwise backward procedure was then used with a cut-off significance level of 0.05 to remove factors from the model. P-values are two-sided, with a type I error rate fixed at 0.05. Statistical analyses were performed with SPSS 15.0 and Prism 4 software.
Results
Cellular Characteristics of Photodepleted Product
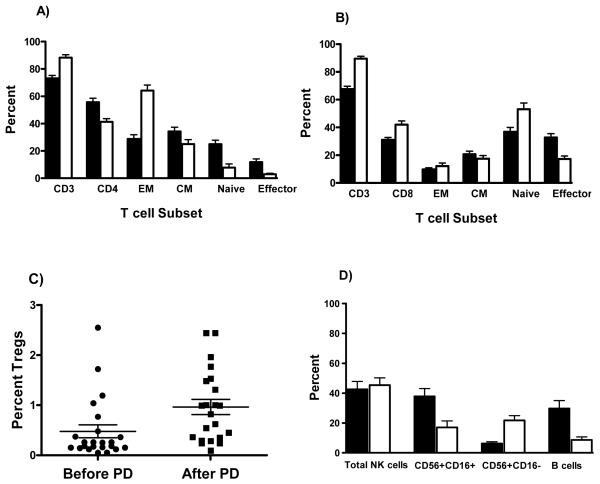
Details of the photodepleted products given are listed in table 1. All PD cell products transfused were adjusted to a final dose of 5 × 106 CD3+ cells/kg viable cells measured within 5 hours following PD (median ± SEM, 90 ± 1.4%.). However, after thawing and resting, the median viability of products fell to 66 ± 2.3%. The relative frequency of the T cell subsets also changed following PD. The CD4+ T cell compartment experienced a greater degree of depletion causing an inversion of the CD4+/CD8+ ratio. Within the CD4+ compartment Tregs were relatively enriched (figure 1C), but there was a significant depletion of naïve, central memory, and effector populations with a relative preponderance of the effector memory subset. Conversely, within the CD8+ T cell compartment the frequency of naïve and effector subsets was conserved with depletion predominantly in the central memory population. Additionally, an 80% reduction in B cells occurred. Cytotoxic (CD56+CD16+) NK cells were reduced by 60%, whereas regulatory (CD56+CD16−) NK cells were well preserved (figure 1).
Table 1. Characteristics of PD Products.
| Patient | SCT CD34/kg (106) |
SCT CD3/kg (102) |
SD CD3/kg (106) |
Viability after processing (%) |
Viability after thaw (%) |
CD4/CD8 ratio prior to PD |
CD4/CD8 ratio after PD |
|---|---|---|---|---|---|---|---|
| 1 | 6.0 | 6.2 | 5.0 | 93 | 48 | 1.63 | 0.56 |
| 2 | 7.9 | 8.1 | 5.0 | 87 | 81 | 0.94 | 0.77 |
| 3 | 6.0 | 25.7 | 5.0 | 93 | 58 | No Sample | 0.93 |
| 4 | 5.4 | 11.3 | 5.0 | 89 | 73 | 0.78 | 0.74 |
| 5 | 9.5 | 0 | 5.0 | 96 | 53 | 0.89 | 0.57 |
| 6 | 6.0 | 0 | 5.0 | 87 | 59 | 1.68 | 1.15 |
| 7 | 4.9 | 5.2 | 5.0 | 86 | 61 | 1.40 | 0.29 |
| 8 | 7.3 | 24.8 | 5.0 | 92 | 71 | 3.13 | 2.11 |
| 9 | 6.7 | 20.3 | 5.0 | 90 | 76 | 2.06 | No Sample |
| 10 | 7.3 | 70.0 | 5.0 | 92 | 82 | 3.11 | 2.23 |
| 11 | 4.4 | 9.1 | 5.0 | 87 | 67 | 1.57 | 2.32 |
| 12 | 3.9 | 19.4 | 5.0 | 73 | 51 | 2.77 | No Sample |
| 13 | 3.1 | 10.9 | 5.0 | 93 | 48 | 3.60 | 1.51 |
| 14 | 9.6 | 29.0 | 5.0 | 70 | 75 | 1.13 | 1.37 |
| 15 | 6.3 | 0 | 5.0 | 92 | 52 | 1.78 | 2.05 |
| 16 | 6.9 | 59.6 | 5.0 | 91 | 73 | 1.01 | 0.68 |
| 17 | 7.9 | 16.2 | 5.0 | 93 | 77 | 3.97 | 0.79 |
| 18 | 4.7 | 9.6 | 5.0 | 87 | 65 | 0.91 | 0.56 |
| 19 | 3.6 | 16.3 | 5.0 | 84 | 65 | 2.21 | 2.57 |
| 20 | 7.3 | 7.5 | 5.0 | 75 | 47 | 1.02 | 0.94 |
| 21 | 6.8 | 27.7 | 5.0 | 85 | 50 | 2.06 | 0.79 |
| 22 | 7.6 | 7.7 | 5.0 | 95 | 83 | 2.03 | 0.76 |
| 23 | 6.9 | 21.2 | 5.0 | 90 | 73 | 2.47 | 0.73 |
| 24 | 5.0 | 10.0 | 5.0 | 96 | 75 | 1.35 | 1.16 |
| Median | 6.5 | 11.1 | 5.0 | 90 | 66 | 1.67 | 0.56 |
Figure 1.
Changes in frequency of T cell subsets after PD. Mean percentage +/− SEM of the A) CD4, B) the CD8 T cell subsets, C) Tregs, and D) NK and B cells before (solid bar) and after PD (white bar). EM, effector memory; CM, central memory.
Dye Efflux Variability across T cell Subsets
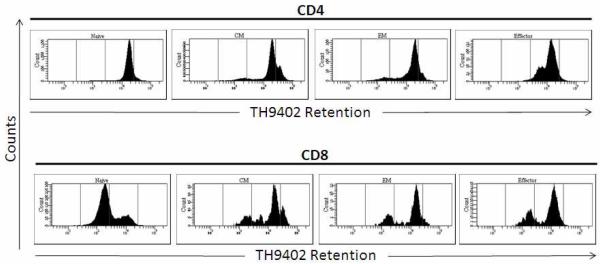
Efflux of TH9402 was determined following a 90 minute extrusion period that was performed after a 40 minute coloration period. Overall, CD8+ T cells displayed a stronger efflux of TH9402 than CD4+ T cells, indicating increased Pgp activity. Among the T cell subsets, TH9402 extrusion varied dramatically (figure 2). Naïve CD8+ T cells eliminated dye most efficiently with a median (±SEM) of 38 ± 7% actively extruding dye after 90 minutes. Central memory CD8+ T cells showed intermediate dye extrusion (20 + 8%), whereas effector memory, central memory, and naïve CD4+ T cell subsets showed the most dye retention; a median of only 13 ± 3% of the total CD4+ cells were actively extruding TH9402 at the end of the 90 minute period (table 2). After 4.5 hours in TH9402-free medium, the difference in extrusion among the subsets was more apparent with 28 ± 5% of the CD4+ and 66 ± 15% of CD8+ populations actively extruding dye. Although the Pgp activity in naïve CD4+ T cells increased to levels comparable to the other CD4+ subsets with a longer extrusion time, the naïve CD8+ T cell subset maintained the greatest extrusion capacity (76 + 9%). The variability in dye extrusion between subsets was consistent with the skewed proportions of CD4+ and CD8+ T cells observed in the photodepleted products transfused.
Figure 2.
Differences in TH9402 extrusion of T cell subsets in representative sample by flow cytometry.
Table 2. TH9402 Extrusion of T cell Subsets.
| Subset | TH9402 extrusion after 90minutes median +/− SEM |
TH9402 extrusion after 4.5 hours median +/−SEM |
|---|---|---|
| CD4 | 13% +/− 3 | 28% +/− 3 |
| Naïve | 8% +/− 3 | 34% +/− 7 |
| Central Memory | 14% +/− 4 | 32% +/− 12 |
| Effector Memory | 14 % +/− 4 | 28% +/− 7 |
| Effector | 20% +/− 4 | 23% +/− 3 |
| CD8 | 23% +/− 7 | 66% +/− 15 |
| Naïve | 38% +/− 7 | 76% +/− 9 |
| Central Memory | 20% +/− 8 | 47% +/− 10 |
| Effector Memory | 28% +/− 8 | 44% +/− 9 |
| Effector | 31% +/− 7 | 51% +/− 16 |
CMV Reactivity in Photodepleted Products
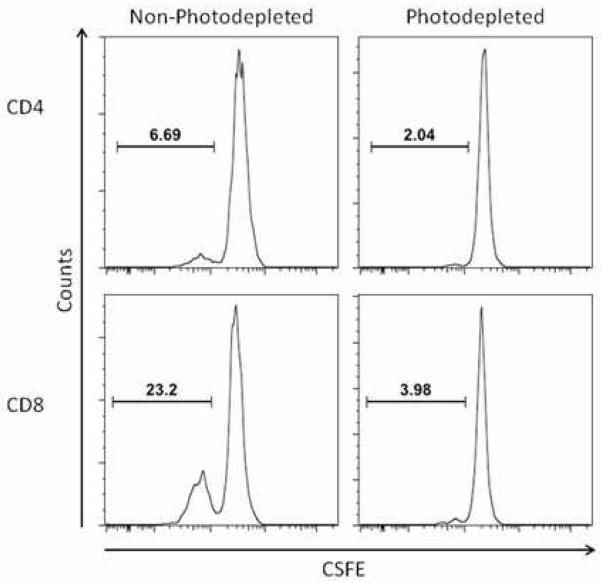
CD4+ and CD8+ T cell reactivity with the immunodominant CMV antigens IE1 and pp65 was assessed in cellular products from 12 CMV+ donors prior to and following PD using a proliferation-based assay. CMV immunoreactivity was clearly detectable prior to PD. However, all donor products demonstrated a loss of anti-CMV activity in T cells after PD that was not restored with the addition of the non-PD APC fraction. Proliferative activity to SEB and 3rd party lymphocytes was retained (figure 3).
Figure 3.
T cell proliferation against CMV antigens before and after PD.
Immune Reconstitution Post transplant of PD Samples
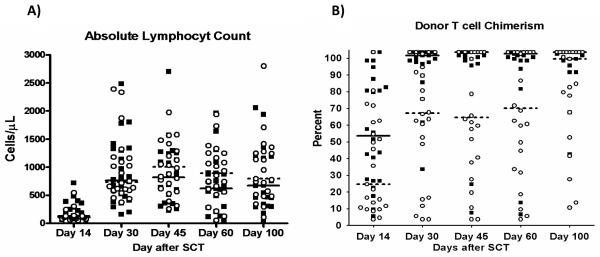
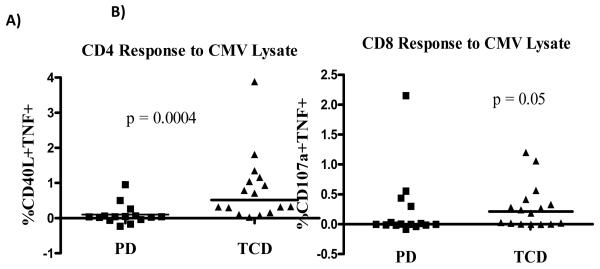
The peripheral blood lymphocyte counts were compared to a group of 24 patients receiving the same conditioning regimen but given a transplant depleted of T cells (final dose 5 × 104 CD3+ /kg). Lymphocyte recovery over the first 100 days is shown in figure 4. For patients that received a photodepleted product, absolute lymphocyte counts rose to a median of 761/μL +/− 120 and 680/μL +/− 264 by day 30 and 100, respectively, after SCT. Similarly, for patients that received a TCD SCT, absolute lymphocyte counts rose to a median of 733/μL +/− 199 by day 30 and 740/μL +/− 131 by day 100. T cell phenotyping and functional analysis was performed on day 100 peripheral blood samples in 16 patients of CMV+ donors from the PD protocol and compared with results of 16 patients of CMV+ donors that received a TCD SCT. Although the frequency and absolute concentrations of T cells, B cells, and NK cells subsets were similar in both protocols, patients receiving a photodepleted product had a higher frequency of naïve CD8+ T cells on day 100 after SCT (p = 0.025, supplemental material table 1). Of all antigens tested, a decreased T cell response to in vitro viral antigen stimulation was noted in patients receiving a photodepleted product. Specifically, no anti-CMV or anti-influenza reactivity was detected in CD4+ T cells, and a lack of anti-CMV and anti-Ad reactivity was observed in the CD8+ T cell compartment (figure 5). Clinically, this resulted in a higher incidence of CMV reactivation that required treatment within the first 100 days after PD SCT than observed after TCD SCT (median 2 vs. 1 episode, p < 0.05). The loss of anti-Ad and anti-influenza reactivity noted in samples of PD SCT recipients collected 90 days after SCT could not be evaluated for clinical impact due to the low incidence of these viral infections occurring after SCT (4 adenoviral infections in TCD patients; 3 adenoviral infections in PD patients; and 1 influenza infection in PD patient).
Figure 4.
A) Absolute lymphocyte counts during the first 100 days for patient receiving either a T cell depleted (open circles) or photodepleted (dark squares) SCT. B) T cell chimerism during the first 100 days after SCT.
Figure 5.
Ex vivo anti-CMV immune response in samples collected 100 days after PD and TCD SCT in the A) CD4 and B) CD8 T cells. PD, photodepletion; TCD, T cell depletion; TNF, tumor necrosis factor.
T cell Subset Allograft Content and Clinical Outcomes
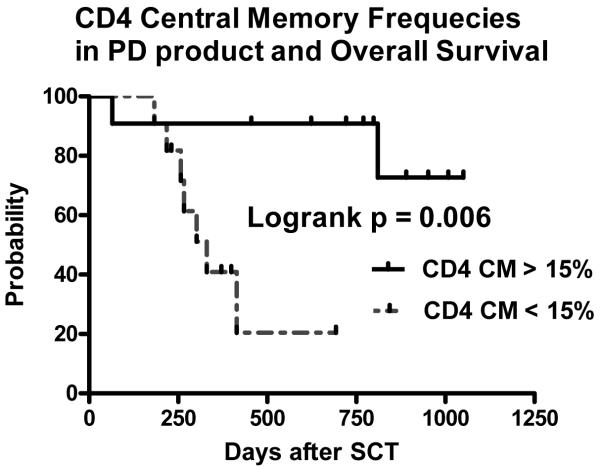
Variability of CD4+ subset conservation within PD products existed. Patients receiving a PD product with low central memory CD4+ T cell content had lower absolute counts of CD4+ central memory cells on day 100 after SCT, increased rates of extensive chronic GvHD and lower overall survival by univariate analysis (Logrank HR 7.7, p = 0.006, figure 6). On multivariate analysis comparing the influence of recipient age, donor sex match (female into male recipient vs. other combinations), disease status (high vs. standard risk hematological malignancy), occurrence of acute GvHD, and the use of a donor lymphocyte infusion, only a low CD4+ CM frequency in the PD product was associated with chronic GvHD (HR 0.931, p = 0.038) and a worse overall survival (HR 0.92, p = 0.028, figure 6). However, no associated was seen between any of the other T cell subset frequencies in the PD allograft and the incidence of transplant related mortality or relapse. The clinical outcomes of the PD SCT recipients are detailed separately 9.
Figure 6.
Overall survival of patients receiving a PD SCT. Median frequency of CD4 central memory cells of 15% was used for survival curve generation (Logrank HR 7.7, p = 0.006). PD, photodepletion; CM, central memory.
Discussion
The possibility of preventing GVHD by selectively removing alloreactive T cells from the SCT product has for more than a decade stimulated us and numerous other investigators to develop selective allodepletion strategies 2, 4, 17. Our most recent attempt using PD in the HLA-identical sibling transplant setting resulted in encouragingly low rates of acute GVHD and relapse, but was complicated by a high incidence of viral reactivation and high transplant related mortality, prompting early closure of the study 9. Here we report the characteristics of the transplanted T cell products and the subsequent immune recovery in the recipient. We found the PD process developed for HLA-identical sibling SCT resulted in a skewed lymphocyte product with a relative depletion of CD4+ T cells, especially naïve and central memory CD4+ T cells. In contrast, CD8+ T cells were relatively well preserved with an enrichment of the naïve subset. On further investigation we found that the changes in T cell subset frequencies following PD were explained by subset differences in TH9402 retention. Dye retention was clearly greater in CD4+ and central memory cells, leading to their preferential elimination by PD (table 2). These results are consistent with earlier studies indicating CD4+ and central memory T cells consistently have lower Pgp activity 18.
These observations raise questions about the impact of our PD process on immune recovery and transplant outcome. One factor that could have affected immune recovery was the viability of the PD product due to the continuing in vitro attrition of dye treated cells. Although the CD3+ T cell dose of the PD product was adjusted for viability, the possibility exists that a reduced number transplanted cells persisted in the recipient. However, some viable cells did persist after transplant that promoting a more rapid conversion to full donor T cell chimerism in PD SCT recipients when compared to TCD recipients who received 2 logs fewer T cells at transplant, suggesting that lymphocyte recovery was not compromised by reduced viability of the photodepleted product. Additionally, the non-selective preferential depletion of some critical T cell subsets that occurred during the PD process could have contributed to a defective capacity of PD recipients to handle infection, in particular CMV reactivation, and may have affected the tendency to develop chronic GVHD. Indeed proliferative responses to CMV were significantly reduced in the PD product, and reactivity to CMV remained much lower than in TCD transplant recipients 100 days post SCT. Intuitively, it might be assumed that the addition of two logs more T cells at transplant would provide greater immunity in the early post SCT period than that contained within a TCD graft. However, we found that the CMV reactivation observed clinically within the first 100 days after PD SCT was twice as frequent as that observed after TCD SCT, indicating that the immune repertoire rather than cell dose was a critical factor in determining viral immune competence. In a multivariate analysis comparing the influence of recipient age, donor sex match (female into male recipient vs. other combinations), disease status (high vs. standard risk hematological malignancy), occurrence of acute GvHD, and the use of a donor lymphocyte infusion, a low CD4+ central memory frequency in the photodepleted product was associated with chronic GvHD and a worse overall survival. In fact, of all the subsets studied, only CD4+ central memory frequency predicted clinical outcomes.
The importance of the various T cell subsets in immune reconstitution has recently become more clearly defined. CD8+ central memory cells are critical to the transfer of intact viral immunity and CD4+ central memory cells are required for sustained antiviral memory 12, 19, both of which were preferentially depleted by PD. Also, CD4+ T cell reconstitution may be essential for development of immune tolerance and the consequent control of c-GvHD 20. In accordance with this observation, the preferential loss of CD4+ T cells in PD may have contributed to chronic GVHD development. In fact because CD4+ loss varied between products, we were able to evaluate the CD4+ dose with incidence of chronic GVHD and found patients that received a higher dose of CD4+ central memory in the photodepleted allograft had significantly lower rates of extensive chronic GVHD. This relationship was also observed when comparing CD4+ central memory frequencies in the donors of TCD transplantation (data not shown).
The probability of relapse for the PD and the TCD groups was comparable (27% vs 35%, p = 0.61), suggesting that GvL was maintained in the PD process. Although PD depletion as performed in our study appears to have preferentially depleted the CD4+ cells that may be important for the development of immune tolerance after SCT, the persistence of naïve CD8+ T cells may have contributed to important anti-leukemia responses that resulted in a relatively low relapse rate for this high risk patient group. However, the study was not designed to evaluate the impact of PD on disease relapse, and leukemic samples from patients were not available to perform in vitro functional analysis, prohibiting a definitive conclusion.
Finally, the impact of PD on Tregs deserves consideration. Since effector Tregs mainly express a CD4+ central memory phenotype, the frequency of central memory CD4+ T cells may represent a surrogate for Treg content of the graft and potential levels after SCT 20, 21. In our analysis, we found that the frequency of Tregs within the CD4+ compartment was relatively enriched. However, the median frequency of Tregs within the total T cell compartment was only minimally increased compared to the enrichment of CD8+ T cells (median increase of 0.5% vs 9.6% of all CD3+ cells, p < 0.01). Although no association was seen between Treg frequencies and SCT outcomes, the relative decrease of all CD4+ T cell subsets would suggest that the photodepleted products may be more prone to cause GvHD.
Our data contrast with the robust immune reconstitution reported for patients receiving haploidentical SCT depleted of alloreacting cells by the same PD technique reported by Roy et al22. A possible explanation for the difference in the immune recovery and clinical outcome between our study and that of the Montreal group is the differences in the strength of the in vitro alloresponse between HLA-identical sibling and haploidentical donor-recipient pairs. Alloreactivity in HLA-identical pairs is hard to elicit and detect in vitro and insufficient to generate a mixed lymphocyte reaction because of the very low frequencies of alloreacting cells. Furthermore, we have shown that stochastic factors determine which alloreacting T cell clones are elicited in HLA-matched pairs. It is therefore possible that the low frequency and variable alloactivations in HLA-matched pairs are confounded with background non-specific activation, making PD an inefficient technique for allodepletion in this setting 23. In contrast haploidentical alloreactions induce strong in vitro proliferation and T cell activation which likely facilitates selective depletion of alloactivated cells with this technique. Another difference in the PD technique used by our group that differs from the Montreal study is the use of expanded T cells rather than monocyte containing APC. These nonconventional APC may be less effective stimulators or have a different spectrum of activation of T cells subsets. These differences merit further investigation if PD is to be used in the HLA-identical sibling setting.
In conclusion, we have shown that not only T cell dose but T cell subset distribution can affect major outcomes after SCT. The PD process produced a naïve CD8+ enriched lymphocyte product that was relatively deficient in CD4+ T cells. As a result, patients that received a PD SCT experienced a deficiency in viral immunity. Although the persistence of naïve CD8+ T cells may have contributed to important anti-leukemia responses that resulted in a relatively low relapse rate for this high risk patient group, the non-specific depletion that occurred warrants caution. While PD is a promising allodepletion technique for haploidentical SCT, further pre-clinical development to eliminate unwanted lymphocyte subset depletion is indicated before applying PD to HLA-matched pairs.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict-of-interest disclosure: The authors declare no conflicts of interests.
REFERENCES
- 1.Dazzi F, Fozza C. Disease relapse after haematopoietic stem cell transplantation: risk factors and treatment. Best Pract Res Clin Haematol. 2007 Jun;20(2):311–27. doi: 10.1016/j.beha.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Fujiwara H, Sconocchia G, Melenhorst J, Eniafe R, Nakamura R, Hensel N, et al. Tissue-restricted T cell alloresponses across HLA barriers: selection and identification of leukemia-restricted CTL in HLA-mismatched stimulator-responder pairs. Bone Marrow Transplant. 2003 Aug;32(4):371–8. doi: 10.1038/sj.bmt.1704142. [DOI] [PubMed] [Google Scholar]
- 3.Michalek J, Collins RH, Durrani HP, Vaclavkova P, Ruff LE, Douek DC, et al. Definitive separation of graft-versus-leukemia- and graft-versus-host-specific CD4+ T cells by virtue of their receptor beta loci sequences. Proc Natl Acad Sci U S A. 2003 Feb 4;100(3):1180–4. doi: 10.1073/pnas.0337543100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mavroudis DA, Dermime S, Molldrem J, Jiang YZ, Raptis A, van Rhee F, et al. Specific depletion of alloreactive T cells in HLA-identical siblings: a method for separating graft-versus-host and graft-versus-leukaemia reactions. Br J Haematol. 1998 Jun;101(3):565–70. doi: 10.1046/j.1365-2141.1998.00748.x. [DOI] [PubMed] [Google Scholar]
- 5.Mielke S, Solomon SR, Barrett AJ. Selective depletion strategies in allogeneic stem cell transplantation. Cytotherapy. 2005;7(2):109–15. doi: 10.1080/14653240510018172. [DOI] [PubMed] [Google Scholar]
- 6.Chen BJ, Cui X, Liu C, Chao NJ. Prevention of graft-versus-host disease while preserving graft-versus-leukemia effect after selective depletion of host-reactive T cells by photodynamic cell purging process. Blood. 2002 May 1;99(9):3083–8. doi: 10.1182/blood.v99.9.3083. [DOI] [PubMed] [Google Scholar]
- 7.Guimond M, Balassy A, Barrette M, Brochu S, Perreault C, Roy DC. P-glycoprotein targeting: a unique strategy to selectively eliminate immunoreactive T cells. Blood. 2002 Jul 15;100(2):375–82. doi: 10.1182/blood-2001-12-0353. [DOI] [PubMed] [Google Scholar]
- 8.Mielke S, Nunes R, Rezvani K, Fellowes VS, Venne A, Solomon SR, et al. A clinical-scale selective allodepletion approach for the treatment of HLA-mismatched and matched donor-recipient pairs using expanded T lymphocytes as antigen-presenting cells and a TH9402-based photodepletion technique. Blood. 2008 Apr 15;111(8):4392–402. doi: 10.1182/blood-2007-08-104471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mielke S, McIver Z, Shenoy A, Fellowes V, Khuu H, Stroncek DF, Leitman SF, Childs R, Battiwalla M, Koklanaris E, Haggerty J, Savani BN, Rezvani K, Barrett AJ. Selectively T cell depleted allografts from HLA-matched sibling donors followed by low-dose post transplant immunosuppression to improve transplant outcome in patients with hematological malignancies. Biol Blood Marrow Transplant. 2011 doi: 10.1016/j.bbmt.2011.05.019. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melenhorst JJ, Scheinberg P, Chattopadhyay PK, Lissina A, Gostick E, Cole DK, et al. Detection of low avidity CD8(+) T cell populations with coreceptor-enhanced peptide-major histocompatibility complex class I tetramers. J Immunol Methods. 2008 Sep 30;338(1-2):31–9. doi: 10.1016/j.jim.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melenhorst JJ, Scheinberg P, Chattopadhyay PK, Gostick E, Ladell K, Roederer M, et al. High avidity myeloid leukemia-associated antigen-specific CD8+ T cells preferentially reside in the bone marrow. Blood. 2009 Mar 5;113(10):2238–44. doi: 10.1182/blood-2008-04-151969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheinberg P, Melenhorst JJ, Brenchley JM, Hill BJ, Hensel NF, Chattopadhyay PK, et al. The transfer of adaptive immunity to CMV during hematopoietic stem cell transplantation is dependent on the specificity and phenotype of CMV-specific T cells in the donor. Blood. 2009 Dec 3;114(24):5071–80. doi: 10.1182/blood-2009-04-214684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huet S, Marie JP, Gualde N, Robert J. Reference method for detection of Pgp mediated multidrug resistance in human hematological malignancies: a method validated by the laboratories of the French Drug Resistance Network. Cytometry. 1998 Dec 15;34(6):248–56. doi: 10.1002/(sici)1097-0320(19981215)34:6<248::aid-cyto2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 14.Brenchley JM, Douek DC. Flow cytometric analysis of human antigen-specific T-cell proliferation. Methods Cell Biol. 2004;75:481–96. doi: 10.1016/s0091-679x(04)75019-0. [DOI] [PubMed] [Google Scholar]
- 15.Melenhorst JJ, Solomon SR, Shenoy A, Hensel NF, McCoy JP, Jr., Keyvanfar K, et al. Robust expansion of viral antigen-specific CD4+ and CD8+ T cells for adoptive T cell therapy using gene-modified activated T cells as antigen presenting cells. J Immunother. 2006 Jul-Aug;29(4):436–43. doi: 10.1097/01.cji.0000211302.52503.93. discussion 365-6. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan KM, Shulman HM, Storb R, Weiden PL, Witherspoon RP, McDonald GB, et al. Chronic graft-versus-host disease in 52 patients: adverse natural course and successful treatment with combination immunosuppression. Blood. 1981 Feb;57(2):267–76. [PubMed] [Google Scholar]
- 17.Solomon SR, Mielke S, Savani BN, Montero A, Wisch L, Childs R, et al. Selective depletion of alloreactive donor lymphocytes: a novel method to reduce the severity of graft-versus-host disease in older patients undergoing matched sibling donor stem cell transplantation. Blood. 2005 Aug 1;106(3):1123–9. doi: 10.1182/blood-2005-01-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ludescher C, Pall G, Irschick EU, Gastl G. Differential activity of P-glycoprotein in normal blood lymphocyte subsets. Br J Haematol. 1998 Jun;101(4):722–7. doi: 10.1046/j.1365-2141.1998.00751.x. [DOI] [PubMed] [Google Scholar]
- 19.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008 Jan;118(1):294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuoka K, Kim HT, McDonough S, Bascug G, Warshauer B, Koreth J, et al. Altered regulatory T cell homeostasis in patients with CD4+ lymphopenia following allogeneic hematopoietic stem cell transplantation. J Clin Invest. 2010 May 3;120(5):1479–93. doi: 10.1172/JCI41072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010 Jul;10(7):490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 22.Roy DC, Lachance S, Kiss T, Cohen S, Busque L, Fish D, et al. Haploidentical Stem Cell Transplantation: High Doses of Alloreactive-T cell Depleted Donor Lymphocytes Administered Post-Transplant Decreases Infections and Improve Survival without Causing Severe GVHD. Blood. 2009;112(22):212. [Google Scholar]
- 23.Scheinberg P, Price DA, Ambrozak DR, Barrett AJ, Douek DC. Alloreactive T cell clonotype recruitment in a mixed lymphocyte reaction: implications for graft engineering. Exp Hematol. 2006 Jun;34(6):788–95. doi: 10.1016/j.exphem.2006.03.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.