Abstract
BACKGROUND
Katanin p60 is a microtubule-severing protein and is involved in microtubule cytoskeleton organization in both mitotic and non-mitotic processes. Its role in cancer metastasis is unknown.
METHODS
Differential protein profiles of bone marrow aspirates were analyzed by chromatography, electropheresis and mass spectrometry. Expression of katanin p60 in primary and metastatic prostate cancer was examined by immunohistochemistry. Cellular function of katanin p60 was further examined in prostate cell lines.
RESULTS
In a proteomic profiling of bone marrow aspirates from men with prostate cancer, we found that katanin p60 was one of the proteins differentially expressed in bone metastasis samples. Immunohistochemical staining showed that katanin p60 was expressed in the basal cells in normal human prostate glands. In prostatic adenocarcinomas, in which the basal cells were absent, katanin p60 was expressed in the prostate cancer cells. In the specimens from bone metastasis, katanin p60 was detectable in the metastatic cancer cells. Strikingly, some of the metastatic cancer cells also co-expressed basal cell biomarkers including the tumor suppressor p53-homologous protein p63 and the high molecular weight cytokeratins, suggesting that the metastatic prostate cancer cells may have a basal cell-like phenotype. Moreover, overexpression of katanin p60 inhibited prostate cancer cell proliferation but enhanced cell migration activity.
CONCLUSIONS
Katanin p60 was aberrantly expressed during prostate cancer progression. Its expression in the metastatic cells in bone was associated with the re-emergence of a basal cell-like phenotype. The elevated katanin p60 expression may contribute to cancer cell metastasis via a stimulatory effect on cell motility.
Keywords: katanin, bone marrow, prostate cancer, metastasis
INTRODUCTION
Androgen refractory prostate cancer metastasis is a major clinical challenge. More than 80% men who died of prostate cancer had evidence of metastasis in bone in autopsies (1,2). However, the mechanism leading to the metastasis of prostate cancer cells to bone is still not clear. Investigation of proteins expressed in the bone metastasis samples may lead to the identification of novel factors involved in the prostate cancer metastasis.
In this study, we used a proteomic approach to identify the proteins that are differentially expressed in the bone marrow aspirates from the prostate cancer patients without or with clinical evidence of bone metastasis. One of the unique proteins that we identified in the pooled bone marrow metastasis samples was katanin p60, an ATPase-containing subunit A of katanin heterodimer. Katanin p60 is a microtubule-severing enzyme and belongs to the AAA (ATPases associated with various cellular activities) protein family (3,4). Katanin p60 has diverse biological functions, including regulating mitosis, meiosis to mitosis switching, deflagellation, and neuronal development. In interphase vertebrate cells, katanin p60 is predominately cytoplasmic with only a fraction present at the centrosome. In mitotic vertebrate cells, katanin p60 is concentrated at the minus ends of microtubules at the spindle poles and is involved in spindle length control (5). In migrating neuronal cells, centrosome-associated katanin p60 regulates microtubule release from the centrosome for transport along the axons and dendrites, while cytoplasmic katanin p60 regulates microtubule number, length and deposition to maintain axonal outgrowth, branching and migration (6–8).
The role of katanin p60 in human cancers is unknown. As a first step to determining the role of katanin p60 in prostate cancer and metastasis, we examined the expression and distribution of katanin p60 in the tissue specimens of normal prostate glands, primary tumors and bone metastases of prostate cancer. We also examined the effect of elevated katanin p60 on cell growth and migration in prostate cancer cell lines. Together, these studies reveal insight into aberrant katanin p60 expression in metastatic prostate cancer cells with basal cell-like phenotype.
MATERIALS AND METHODS
Bone Marrow Specimen Collection
Bone marrow aspirates were drawn from the iliac crest from a single site from each patient according to protocols approved by the University of Texas M. D. Anderson Cancer Center. The heparinized bone marrow was processed immediately after drawing. The supernatants and cell pellets were stored at −85°C.
All patients were diagnosed with adenocarcinoma of the prostate and had received hormonal ablation therapy. Osseous metastases were evaluated by bone scintigraphy using Technetium-99m labeled methylene bisphosphonate. Bone marrow aspirates from six patients (median age 60, collected between 8/25/97 – 10/20/98) who had no evidence of bone metastasis, as determined by bone scintigraphy and histology of bone biopsy, were used as control samples (Ctrl). Bone marrow aspirates from six patients (median age 68, collected between 2/6/96 – 12/10/96) who had extensive bone metastases were used as the metastatic samples (Met). The Gleason scores in initial biopsies of the Ctrl group ranged from 7 – 9 (median 8) and the Met group ranged from 7 – 8 (median 7). At the time of sample collection, the Ctrl and Met group patients have been in hormonal therapy for 9 – 82 months (median 37 months) and 18 – 74 months (median 50 months), respectively. The serum prostate specific antigen levels were: Ctrl samples, <0.1 – 21.6 ng/ml (median 1.5; average 6.8 ± 8.4); Met samples, 47.9 – 1691.2 ng/ml (median 211.6; average 498.6 ± 610.8). The serum bone-specific alkaline phosphatase levels were: Ctrl samples, 54 – 123 IU/L (median 76; average 80.7 ± 23.2); Met samples, 91 – 3534 (median 160; average 855.3 ± 1334.7). About 2.5 ml of bone marrow supernatant from each patient was pooled to generate a total of 15.6 ml each of Ctrl and Met samples.
WGA-Agarose Affinity Chromatography
WGA-Agarose (2 ml) (EY-Laboratories) was transferred to a Poly-Prep Chromatography Column (Bio-Rad Laboratory), washed with 50 mM Hepes, pH 7.4, and equilibrated with 50 mM Hepes containing 5% Triton X-100. The bone marrow samples were adjusted to 5% TritonX-100 in a final volume of 33 ml and passed through the WGA-agarose column. The column was washed with equilibration buffer and eluted with 0.3 M N-acetylglucosamine. Fifteen 1-ml fractions were collected.
Two-Dimensional Gel Electrophoresis (2-DE) and Protein Identification by LC-MS
Samples were dialyzed overnight against rehydration buffer (8M urea, 0.1% CHAPS, 0.0002% bromophenol blue) and adjusted to 2% CHAPS, 0.5% ampholytes pH 4–7, and 20 mM DTT. The first dimension separation was carried out using immobilized pH strips (IPG strips with a linear separation range of pH 4–7) and the isoelectric focusing was performed using the IPGphor system (Amersham). The second dimension was separated on 4–12% gradient gels. Proteins were stained by Coomassie blue or silver stain (Sigma), excised, digested with modified trypsin (Roche), and analyzed by liquid chromatography-mass spectrometry (LCQ MS/MS; Hewlett-Packard HP1100 connected to a Thermo-Finnigan LCQdeca electrospray ionization ion trap mass spectrometer). The MS data were analyzed by SEQUEST software (Thermo-Finnigan) against the NCBI database.
Recombinant Protein Production
His-tagged katanin p60 was produced in Sf9 insect cells by transfection with pVL-KATNA1 cDNA in a baculovirus expression vector, and purified from the cell pellet using Ni2+-NTA (Qiagen) affinity column. GST-tagged katanin p60 was produced in E. coli BL21 by transformation with a pGEX-4T-KATNA1 cDNA construct, and purified by using glutathione agarose beads (Amersham).
Antibody Generation, Purification and Validation
His-tagged katanin p60 was used to generate polyclonal antibodies in rabbit. The anti-katanin p60 antibody serum was affinity purified by binding to GST-katanin p60 that was immobilized on a nitrocellulose membrane, eluted with a glycine buffer (pH 2.0), and immediately neutralized with 1/10 volume of 1M Tris buffer (pH 8.0). Specificity of the anti-katanin p60 antibody was validated by Western blotting. Inducible expression of katanin p60-EGFP in a 293T/Tet-On cell line was achieved using pTRE-KATNA1-EGFP plasmid and induction with 1 µg/ml Doxycycline for 24 hours. KATNA1-specific shRNA (Sigma-Aldrich) was used for knockdown. KATNA1 cDNA in pcDNA3.1D/V5-His vector was used for cell transfection.
Western Blotting
Protein samples were resolved on 4–12% gradient NuPage gels (Invitrogen), transferred to nitrocellulose membrane using Tris-glycine transfer buffer containing 20% methanol, probed with anti-katanin p60 antibody (1:2000), anti-androgen receptor antibody (Upstate), CK-34βE12 (Enzo Life Sciences), p63-4A4 (Thermo Scientific) or anti-V5 antibody (Invitrogen), followed by peroxidase-labeled anti-rabbit IgG antibody, and visualized by chemiluminescence (Pierce).
Immunohistochemical Staining
Bone tissues were decalcified with formic acid, embedded in paraffin, dewaxed with xylene, and rehydrated through graded alcohols. Antigen retrieval was performed by steaming the tissue sections in a citrate buffer (10 mM, pH 6.0). The sections were treated with 3% H2O2 in methanol and blocked with normal goat serum. Primary antibody of katanin p60 (1:200), CK-34βE12 (1:100) (Enzo Life Sciences) or p63-4A4 (1:400) (Thermo Scientific) were added on sections respectively and incubated at 4°C overnight. Antibody binding was detected using the ABC kit (Vector) with 3,3’-diaminobenzidine as the chromogen and sections were counterstained with hematoxylin.
Cell Proliferation Assay
Cells (1×106) cultured in RPMI 1640 supplemented with 10% fetal bovine serum (growth medium) were transfected by Amaxa Nucleofector with 2 µg empty vector or pcDNA3.1-KATNA1 DNA, reseeded after 24 hours at 5×103 cells/well in 96-well plates, and counted daily using a cell counting kit-8 (Dojindo Molecular Technologies).
Cell Migration Assay
Cells were resuspended in RPMI 1640, seeded at 1×104 cells/well in a 96-well FluoroBlok insert system or 2×105 cells/well in a 24-well insert system (BD Falcon), placed into chambers containing growth medium, incubated for 20 h, and stained with 1 µM calcein AM (Invitrogen). The number of cells that had reached the other side of the filter was analyzed by serial photomicroscopy and quantified using the NIH Image-J program.
RESULTS
Katanin p60 was differentially expressed in the bone marrow from men with prostate cancer and bone metastasis
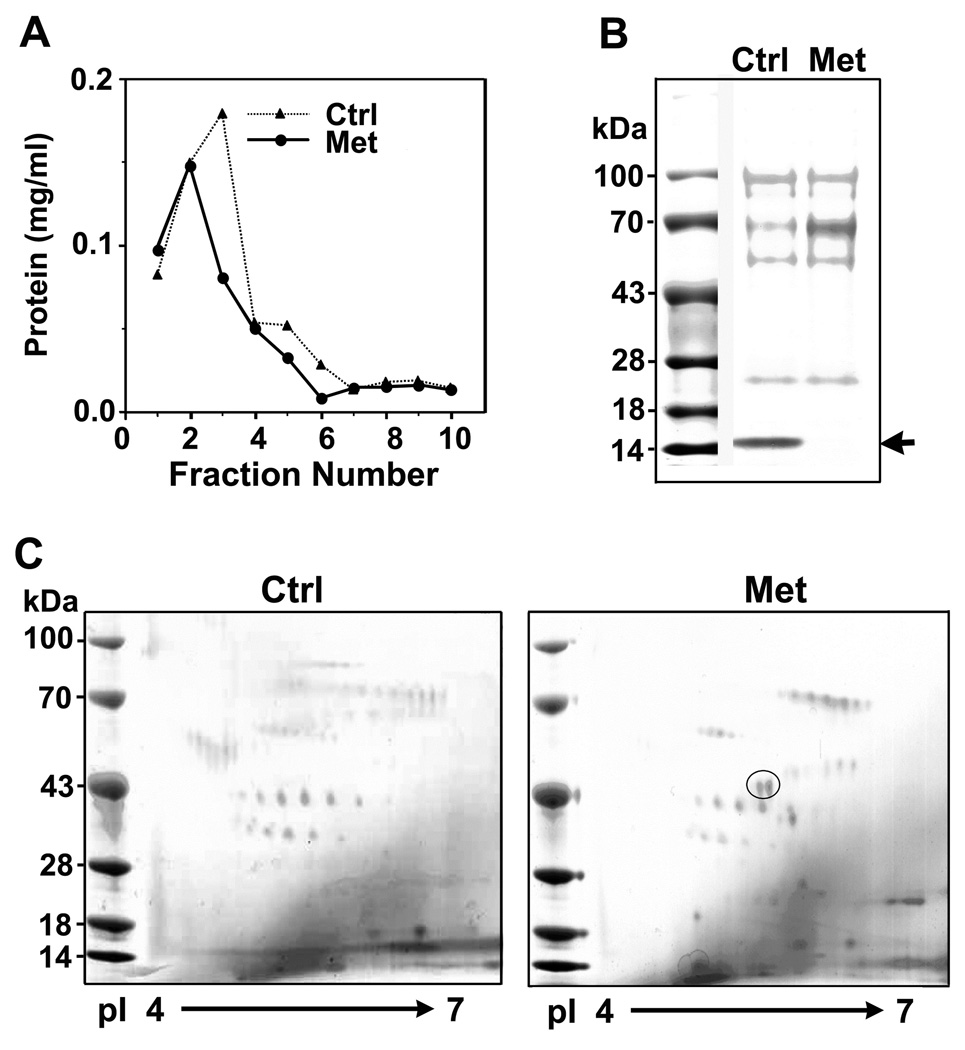
To identify the protein factors involved in prostate cancer metastasis, we first used protein chromatography to enrich for low abundance proteins in bone marrow supernatants. Bone marrow samples pooled from six patients with clinical evidence of bone metastasis (Met) or six without evidence of bone metastasis (Ctrl) were run through a wheat germ agglutinin (WGA)-agarose column in parallel, and showed similar elution profiles (Figure 1A). Each sample yielded a total of 0.4 – 0.5 mg lectin affinity-purified proteins, which represented a 2400-fold enrichment of glycoproteins and their associated proteins from 1200 mg of total bone marrow proteins. After SDS-PAGE resolution, the WGA-enriched fractions showed similar protein patterns, except the presence of a 15 kDa protein in the control samples, which was identified by mass spectrometry to be hemoglobin β-chain. The decrease in hemoglobin levels in the Met sample was not unexpected as patients with bone metastasis usually have a certain degree of anemia.
Figure 1.
Identification of differentially expressed proteins in the bone marrow samples from men with or without bone metastasis from prostate cancer. A. Elution profiles of the pooled bone marrow supernatants with WGA-agarose affinity chromatography. Bone marrow supernatants from prostate cancer patients who did not have clinical evidence of bone metastasis served as control (Ctrl) for those with bone metastasis (Met). B. SDS-PAGE of the WGA-affinity purified fractions. Arrow indicates a decrease in the level of a 16 kDa protein in the Met samples. Mass spectrometry analysis identified it as hemoglobin β-chain. C. Two-dimensional gel electrophoresis of the proteins eluted from WGA-affinity chromatography. Two discernible protein spots in the Met samples are circled. One of them was identified to be katanin p60 by mass spectrometry analysis.
Upon further analysis by two-dimensional gel electrophoresis (2-DE), two clearly discerned protein spots closely positioned at about 43 kDa were found to be unique to the Met samples (Figure 1C, circle). Mass spectrometry analysis identified one of the proteins to be a soluble ErbB3 protein, which has been demonstrated to play an important role in bidirectional interactions between tumor cells and osteoblasts in the bone microenvironment (9,10). The other protein was identified as katanin p60 ATPase-containing subunit A, which is encoded by the gene KATNA1 (GenBank Accession NM_007044) and has a predicted molecular mass of 56 kDa. The smaller molecular mass shown on 2-DE was likely due to partial degradation during purification. Identification of katanin p60 as a protein differentially expressed in the Met sample suggests that it plays a role in the bone metastasis of prostate cancer.
Katanin p60 was detectable in the basal cells in normal prostate glands
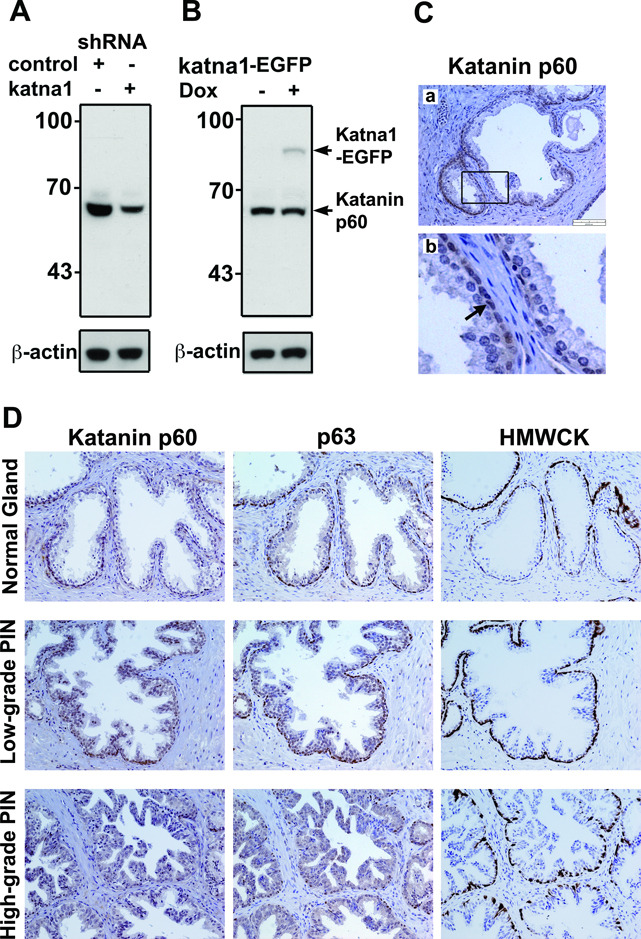
To determine the expression of katanin p60 protein in the clinical tissue samples, we generated an anti-katanin p60 antibody and tested its specificity in Western blotting of two 293T-derived cell lines with increased (by overexpression) or decreased (by knockdown) levels of katanin p60 (Figure 2A). The antibody recognized an endogenous protein with an apparent molecular mass of about 60 kDa. Knockdown of katanin p60 by KATNA1-shRNA reduced the binding of the antibody. The antibody also recognized a recombinant katanin p60-EGFP fusion protein with a molecular mass of 85 kDa (Figure 2B). These results confirmed that the anti-katanin p60 antibody specifically recognized both endogenous and recombinant katanin p60.
Figure 2.
Validation of katanin p60 antibody and immunohistochemical staining of normal prostatic glands and prostatic intraepithelial neoplasia. A. Western blot with katanin p60 antibody showed a 60 kDa band that was reduced after knockdown by KATNA1-shRNA but not by control shRNA in 293T cells. B. Katanin p60 antibody recognized the 85 kDa katanin p60-EGFP fusion protein. Expression of katanin p60-EGFP was achieved by transfection with a pTRE-KATNA1-EGFP in a Tet-On 293T cell line and induction with 1 µg/ml Doxycycline (Dox) for 24 hours. C. Immunostaining of normal prostate tissues with katanin p60 antibody showed that katanin p60 protein was weakly detectable in the epithelial cells. A boxed region is enlarged in panel (b). Note that arrow points to katanin p60 staining in the basal cell layer. D. Immunostaining of katanin p60 and basal cell markers (i.e. p63 and HMWCK) in normal prostatic glands, low-grade and high-grade PIN.
We then examined katanin p60 expression in normal human prostate glands by immunohistochemical staining with the anti-katanin p60 antibody. Of 27 independent samples examined, 25 (92%) showed faint staining in normal glands, primarily in the basal cell layer of the epithelial compartment (Figure 2C, arrow in b). To confirm the basal cells, we examined the tissue sections for two well-known prostatic basal cell markers (Figure 2D), the high molecular weight cytokeratins (HMWCK) and the p63 protein, a homologue of the p53 tumor suppressor (11,12). Overall, the staining pattern indicated that katanin p60 is expressed weakly in the normal prostate gland.
Katanin p60 expression was upregulated in prostatic adenocarcinomas
Next, we examined katanin p60 expression in prostatic neoplasia. In prostatic intraepithelial neoplasia (PIN), the lesions still contain basal cells (Figure 2D). The secretory cells on the inner surface of glandular structures transform to low and high grade pre-malignant cells. In low grade PIN, katanin p60 staining was found in the nucleus in both the basal cells and epithelial cells. However, in high grade PIN, katanin p60 was diffusely localized in the cytoplasm of the epithelial cells (Figure 2D).
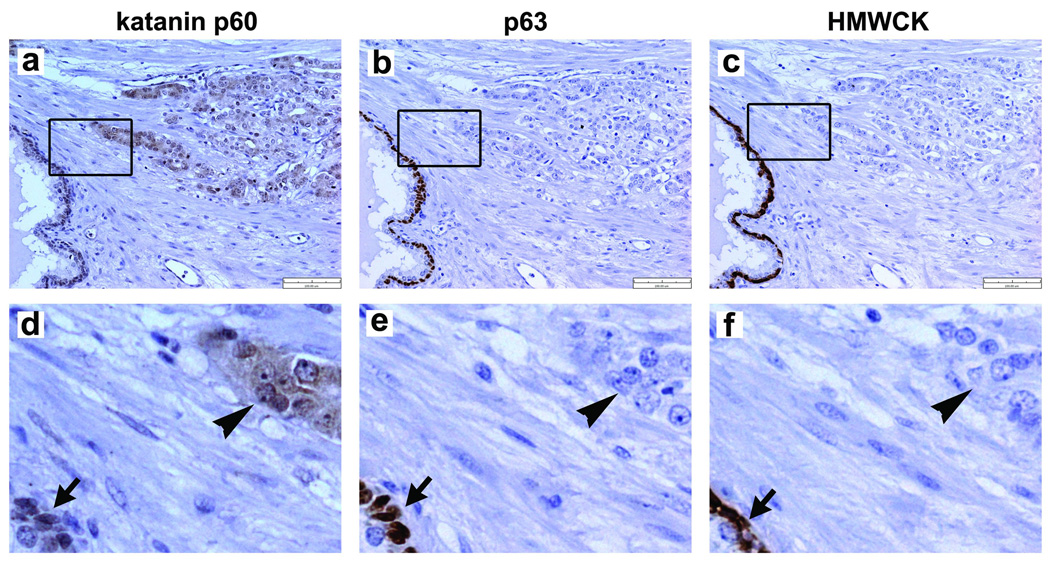
In prostatic adenocarcinoma, which lost the basal cells, katanin p60 staining was increasingly visible in cancerous epithelial cells (Figure 3a, arrowhead in d). Of 18 independent samples of prostatic carcinomas examined, 15 (83%) were positive for katanin p60 staining. The staining was localized primarily in the cytoplasm of carcinoma cells (Figure 3d, arrowhead). Although the positive staining of cancerous epithelial cells was evident, the staining intensities in the carcinoma areas did not significantly correlate with the Gleason grades in these samples.
Figure 3.
Immunostaining of katanin p60 and basal cell biomarkers in prostatic carcinomas. (a) Elevated expression of katanin p60 was detectable in carcinomas. A representative sample (Greason grade 4) is shown. An enlarged area is shown in panel (d). Arrowhead indicates katanin p60 staining in the carcinoma cells. Arrow indicates katanin p60 staining in the basal cells in a neighboring normal gland. (b) An adjacent tissue section was stained with anti-p63 antibody clone 4A4. An enlarged area (e) showed the carcinoma was negative for p63 staining (arrowhead). (c) An additional adjacent tissue section was stained with anti-HMWCK antibody 34βE12. The enlarged image (f) showed the carcinoma was also negative of HMWCK staining (arrowhead), while the basal cells in the neighboring gland were positive for HMWCK staining (arrow).
Immunostaining of basal cell markers showed the positive layer in normal prostatic glands (Figure 3b,c; arrows in e,f) but which was absent in carcinoma regions (Figure 3b,c; arrowhead in e,f). This is consistent with the established diagnostic criteria of the basal cell degeneration in primary prostatic adenocarcinomas (11,12). Comparison of the staining patterns of katanin p60, p63 and HMWCK in adjacent tissue sections showed that their staining overlapped in the normal glands (Figure 3d–f, arrows). In prostatic carcinoma regions, however, only katanin p60 was positively stained (Figure 3d, arrowhead).
Katanin p60 expression in metastatic cells and tumor foci in bone marrow
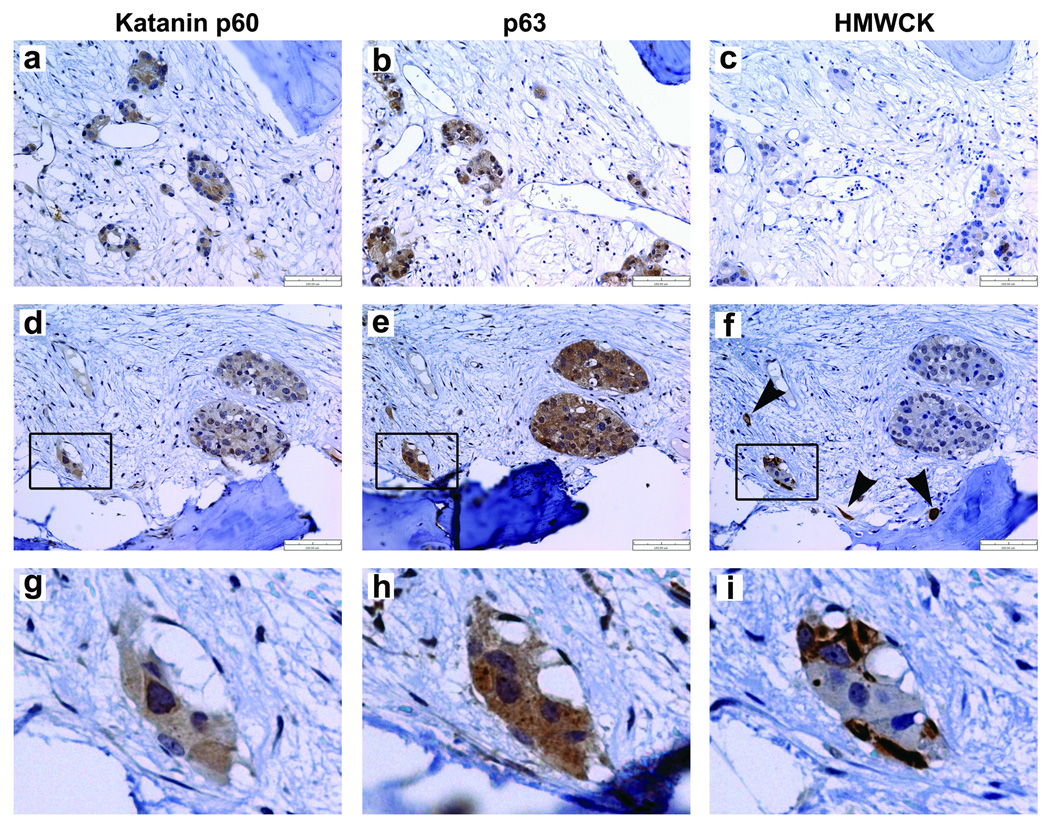
We then investigated the expression of katanin p60 in the metastatic prostate cancer in bone. Katanin p60 expression was readily detected in many metastatic cells and tumor foci in the bone marrow (Figure 4a, d, g). In 11 independent patient bone specimens examined, seven (64%) were positive for katanin p60 staining. This observation is consistent with the proteomic analyses that showed increased levels of katanin p60 in the bone marrow samples from men with bone metastasis.
Figure 4.
Immunostaining of katanin p60 and the basal cell biomarkers in the bone metastasis of prostate cancer. (a and d) Expression of katanin p60 was detectable in the scattering metastatic cells and tumor foci in bone marrow cavities. Two independent samples were shown. The boxed area from (d) is enlarged in panel (g), in which katanin p60 was localized primarily in the cytoplasm. (b and e) In the adjacent tissue sections, the basal cell marker p63 was detected in the cytoplasm of metastatic cells and tumor foci. An enlarged area from (e) is shown in panel (h). (c and f) High molecule weight cytokeratins (HMWCK) were detected in a minor population of metastatic cells and small tumor foci in the additional adjacent tissue sections. Note the arrowheads point to scattered single prostate cancer cells (f) and the basal-like cells at the edge of a small tumor focus (i).
We also examined the expression of basal cell markers in the bone metastasis specimens. Unexpectedly, the metastatic cells and tumor foci displayed detectable basal cell markers in serial sections of the bone biopsies. The staining of p63 (Figure 4b,e,h) matched well to that of katanin p60 in most of the tumor foci. Noticeably, p63 in the metastatic cancer cells showed diffuse staining in the cytoplasm, an obvious difference from its nuclear localization in the normal basal cell (Figure 3b,e). The mislocalized cytoplasmic p63 in tumor cells has been reported previously for association with reduced apoptosis, higher proliferative activity and poor patient survival (13,14). HMWCK staining was limited to a minor population of scattering tumor cells and small tumor foci (Figure 4c,f, i) and was often negative or weakly positive in the large tumor foci. Because p63 and HMWCK were not detectable in the primary prostatic adenocarcinomas (Figure 3), their re-expression in the metastatic cancer cells suggests that the cellular properties of the metastatic cancer cells in bone are different from those in the primary sites. Clearly, some of the metastatic prostate cancer cells in bone have acquired certain basal cell-like properties.
Overexpression of katanin p60 in prostate cancer cells inhibited cell proliferation but enhanced cell migration
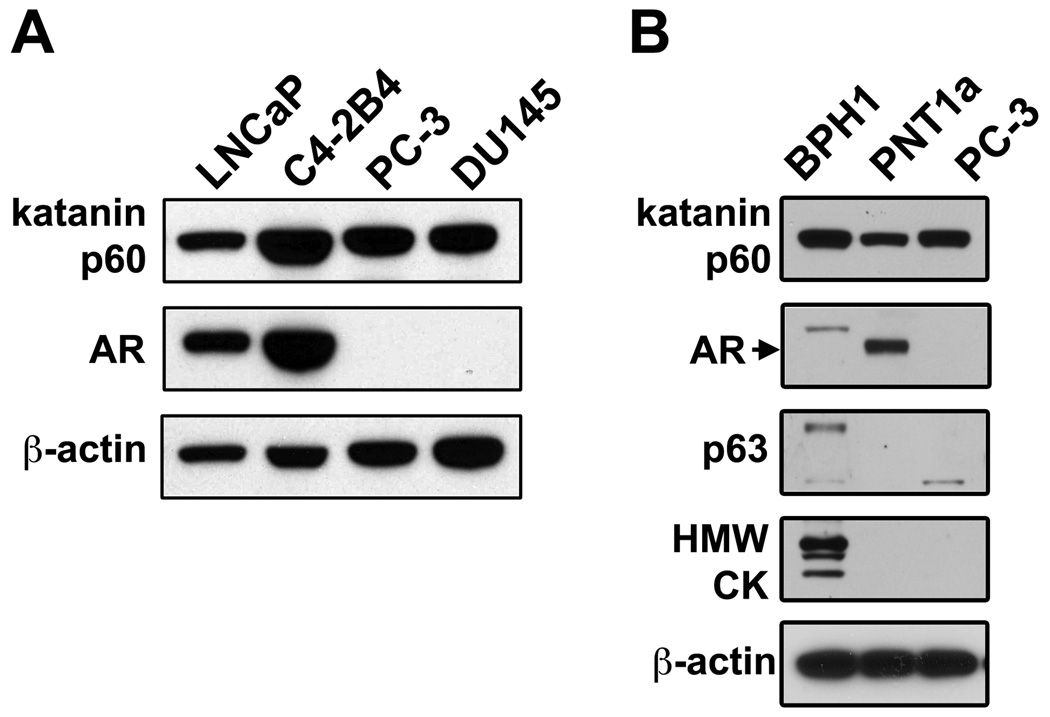
To determine the function of katanin p60 in prostate cancer cells, we examined katanin p60 expression in a series of human prostate cell lines. LNCaP and C4-2B4 are syngeneic lines; C4-2B4 has bone metastatic potential (15). PC-3 and DU145 were derived respectively from bone metastasis and brain metastasis (16,17); whereas PNT1a and BPH1 were derived from benign prostate epithelial cells by immortalization with SV40 large-T antigen (18). BPH1 has basal cell-like phenotype (19). In Western blotting, anti-katanin p60 antibody recognized a protein band of approximate 60 kDa in all prostate cell lines examined (Figure 5A, B), indicating that katanin p60 is expressed in human prostate cell lines from various origins. Expression of katanin p60 in prostate cancer cells is not influenced by their androgen receptor expression status. The bone metastatic cell line C4-2B4 expressed a higher level of katanin p60 than the parental line LNCaP (Figure 5A). However, the relationship between elevated katanin p60 expression and the tendency for bone metastasis was not apparent among non-syngeneic cell lines, as the highly metastatic PC-3 cells expressed a similar level of katanin p60 as LNCaP cells. In addition, BPH1 cells expressed both p63 and HMWCK basal cell markers (Figure 5B).
Figure 5.
Expression of katanin p60, androgen receptor and basal cell markers in human prostate cell lines. A. Katanin p60 was detected by Western blotting in human prostate cancer cell lines. Androgen receptor was detected by Upstate PG-21 antibody. B. Expression of prostatic basal cell markers p63 and HMWCKs in BPH1, PNT1a and PC-3 cells were examined with p63-4A4 and CK-34βE12 antibodies, respectively.
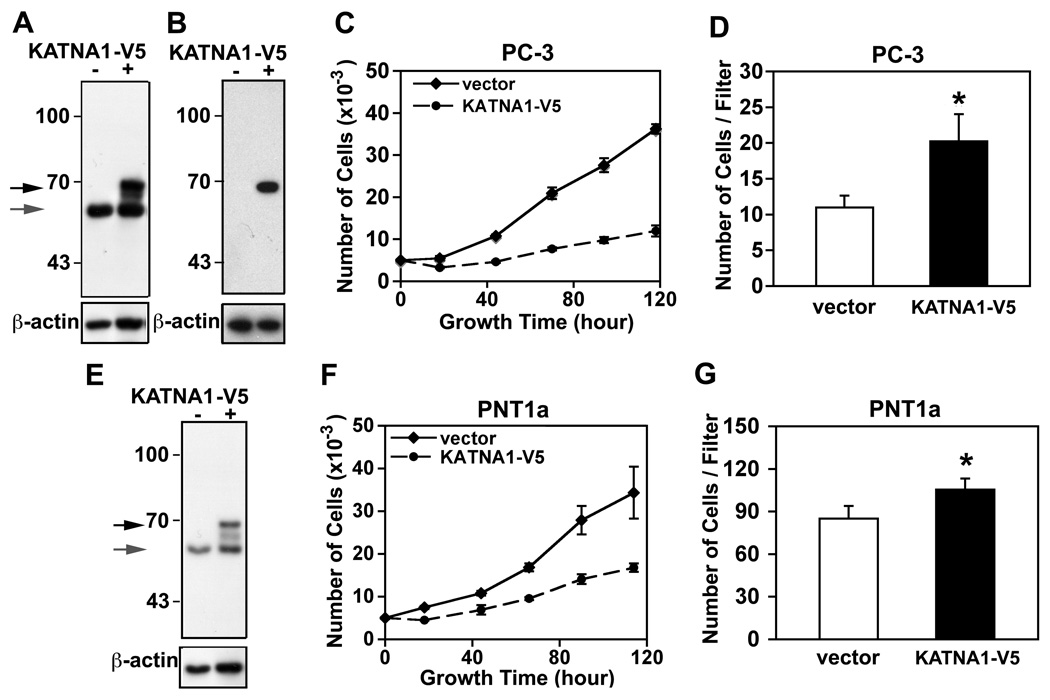
To assess the effect of increased expression of katanin p60 on cancer cell properties, we utilize KATNA1-V5 cDNA transient transfection to overexpress katanin p60 in PNT1a and PC-3 cells (Figure 6A, E). Overexpression of katanin p60 significantly inhibited proliferation of both PC-3 and PNT1a cells (Figure 6C, F). However in transwell migration assays, PC-3 and PNT1a cells overexpressing katanin p60 showed 1.8-fold (1.84 ± 0.18) and 1.2-fold (1.21 ± 0.14) increases, respectively, in cell migration (Figure 6D, G), suggesting that an increase in katanin p60 expression favors cell migration over cell proliferation in human prostate cells.
Figure 6.
Effect of katanin p60 overexpression on PC-3 and PNT1a cell growth and migration. A. Overexpression of V5-tagged katanin p60 in PC-3 cells by transient transfection of a pcDNA3.1D-KATNA1-V5-6xHis (KATNA1-V5). Recombinant protein and endogenous katanin p60 were detected by immunoblotting with anti-katanin p60 antibody. B. The recombinant protein was confirmed by anti-V5 epitope antibody. C. Overexpression of katanin p60 inhibits cell proliferation in PC-3 cells. Growth curves were recorded from the PC-3 cells transfected with an empty vector or a KATNA1-V5 expression construct. D. Cell migration properties of the transfected PC-3 cells were determined in transwell assays. Three independent experiments for each cell line were performed and a representative experiment with PC-3 cells is shown. *, P < 0.05. E. Recombinant and endogenous katanin p60 in PNT1a cells were detected by immunoblotting with anti-katanin p60 antibody. F. Overexpression of katanin p60 inhibited cell proliferation in PNT1a cells. G. Elevated katanin p60 levels induced an increase in cell migration in PNT1a cells. *, P < 0.05.
DISCUSSION
We used a proteomic approach to identify the differential protein factors in the bone marrow samples from men with prostate cancer, in which the samples with or without bone metastasis were compared. This approach was based on the hypothesis that the bone marrow cavities represent a new tumor microenvironment for the disseminated cancer cells. An adaption process through the interactions between the cancer cells and bone cells is likely involved in the colonization of tumor cells in this microenvironment. Therefore, comparing the protein factors in the bone marrow aspirates from patients with bone metastasis to those without bone metastasis could identify the unique factors relevant to the metastasis in bone.
However, the major challenge with this approach is the technical difficulty of effectively fractionating the tumor-specific proteins from abundant albumin and marrow proteins. We tested various combinations of chromatographic materials and elution conditions (data not shown) and found that only the WGA-agarose purification procedure gave sufficient enrichment (~2400 fold) of a subgroup of albumin-free proteins. Among them, katanin p60 was one of the most discernible proteins on 2-DE separation of the bone metastasis samples of prostate cancer. Its peptide identity was confirmed by mass spectrometry. Katanin p60 is known as an intracellular protein and its presence in the bone marrow supernatant is likely due to tumor cell lysis during sample processing.
Identification of katanin p60 as a differentially expressed protein in the bone marrow samples of metastatic prostate cancer raises the possibility that katanin p60 plays a role in the prostate cancer metastasis. Our study indicates that katanin p60 is expressed at a very low level in the luminal epithelia in normal prostatic glands, but is relatively enriched in the basal cell layer. Elevated expression of katanin p60 in the cytoplasm was observed in some adenocarcinoma cells, and its expression was further found in the metastatic prostate cancer cells in bone metastasis, suggesting that elevation of katanin p60 expression in carcinoma cells was accompanying with disease progression. In this process, the prostate basal cells, expressing HMWCK and p63 (11,12), are degenerated so that the carcinoma cells directly interact with stromal matrix and escape from the primary tumor sites. However, we were surprised to find that basal cell-like phenotypes reemerged in some metastatic prostate cancer cells and tumor foci in bone specimens, as indicated by the expression of not only katanin p60 but also basal cell markers p63 and HMWCK. To our knowledge, this has not been reported previously. Also interestingly, p63 in the bone metastatic cells is localized in the cytoplasm (Figure 4), which is clearly different from the nuclear pattern in normal prostate basal cells (Figure 3). As the cytoplasmic p63 in primary tumors has been associated with poor patient survival (13,14), its mechanism is still unknown. We speculate that co-expression of katanin p60 and basal cell-like phenotype in the metastatic cells leads to an aggressive and widespread metastatic disease.
In the prostate cancer cell lines, we observed that cell proliferation was inhibited by increased katanin p60 expression. However, cell migration was slightly enhanced. This observation agrees with a role of katanin p60 in regulating dynamic microtubule organization during cell division as well as cell migration. Regulation of katanin p60 is a complex process with many cellular factors. For example, katanin p60 protein turnover in mammalian cells is mediated by ubiquitin E3 ligase complexes Cul3/KLHDC5 (20) or DYRK2/EDVP (21). Knockdown of DYRK2 or transient overexpression of katanin p60 in HeLa cells causes a G2/M arrest and the accumulation of polyploid cells (21), suggesting that the proper regulation of katanin p60 level is critical for normal mitosis and for prevention of neoplastic transformation. In migrating cells, microtubules are released from the centrosome and targeted to the leading edge of the cell (22). At the centrosomal localization, katanin p80 is a regulatory subunit and binding partner of katanin p60 (23). A putative tumor suppressor LAPSER1/LZTS2 co-localizes with katanin p80 (24) and exerts an inhibitory function on the katanin p60-mediated microtubule-severing activity (25). Knockdown of LAPSER1 in Saos-2 cells not only increases the mitotic index but also significantly enhances cell migration, suggesting that microtubule severing at centrosomes is a novel tumor-associated molecular subcircuit in cells (25). The genomic locus of human LAPSER1 at chromosome 10q24.3, which neighbors PTEN locus, has been reported to be deleted frequently in various cancers including prostate cancer (26).
CONCLUSIONS
Collectively, our study showed that katanin p60 was aberrantly expressed during prostate cancer progression to bone. Its expression in the metastatic cells in bone was associated with the re-emergence of a basal cell-like phenotype. Functionally, the elevated katanin p60 expression may contribute to cancer cell metastasis via a stimulatory effect on cell migration. The implications of katanin p60 in the reorganization of microtubule cytoskeleton and in promoting cell motility in bone metastasis of prostate cancer warrant further investigation.
ACKNOWLEDGMENTS
The authors would like to thank Dr. M.A. Bilen, Dr. F. Chu, K. Earley, and Hyojin Cho for assistance. This work was supported by grants from the U.S. Department of Defense (PC061279), the National Institutes of Health (CA111479, P50-CA140388, and DK53176), the Charlotte Geyer Foundation, and the Prostate Cancer Foundation.
Footnotes
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
REFERENCES
- 1.Bubendorf L, Schopfer A, Wagner U, Sauter G, Moch H, Willi N, Gasser TC, Mihatsch MJ. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31(5):578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 2.Shah RB, Mehra R, Chinnaiyan AM, Shen R, Ghosh D, Zhou M, Macvicar GR, Varambally S, Harwood J, Bismar TA, Kim R, Rubin MA, Pienta KJ. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res. 2004;64(24):9209–9216. doi: 10.1158/0008-5472.CAN-04-2442. [DOI] [PubMed] [Google Scholar]
- 3.McNally FJ, Vale RD. Identification of katanin, an ATPase that severs and disassembles stable microtubules. Cell. 1993;75(3):419–429. doi: 10.1016/0092-8674(93)90377-3. [DOI] [PubMed] [Google Scholar]
- 4.Roll-Mecak A, McNally FJ. Microtubule-severing enzymes. Curr Opin Cell Biol. 22(1):96–103. doi: 10.1016/j.ceb.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McNally K, Audhya A, Oegema K, McNally FJ. Katanin controls mitotic and meiotic spindle length. J Cell Biol. 2006;175(6):881–891. doi: 10.1083/jcb.200608117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmad FJ, Yu W, McNally FJ, Baas PW. An essential role for katanin in severing microtubules in the neuron. J Cell Biol. 1999;145:305–315. doi: 10.1083/jcb.145.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu W, Qiang L, Solowska JM, Karabay A, Korulu S, Baas PW. The microtubule-severing proteins spastin and katanin participate differently in the formation of axonal branches. Mol Biol Cell. 2008;19(4):1485–1498. doi: 10.1091/mbc.E07-09-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toyo-Oka K, Sasaki S, Yano Y, Mori D, Kobayashi T, Toyoshima YY, Tokuoka SM, Ishii S, Shimizu T, Muramatsu M, Hiraiwa N, Yoshiki A, Wynshaw-Boris A, Hirotsune S. Recruitment of katanin p60 by phosphorylated NDEL1, an LIS1 interacting protein, is essential for mitotic cell division and neuronal migration. Hum Mol Genet. 2005;14(21):3113–3128. doi: 10.1093/hmg/ddi339. [DOI] [PubMed] [Google Scholar]
- 9.Vakar-Lopez F, Cheng CJ, Kim J, Shi GG, Troncoso P, Tu SM, Yu-Lee LY, Lin SH. Up-regulation of MDA-BF-1, a secreted isoform of ErbB3, in metastatic prostate cancer cells and activated osteoblasts in bone marrow. J Pathol. 2004;203(2):688–695. doi: 10.1002/path.1568. [DOI] [PubMed] [Google Scholar]
- 10.Lin SH, Cheng CJ, Lee YC, Ye X, Tsai WW, Kim J, Pasqualini R, Arap W, Navone NM, Tu SM, Hu M, Yu-Lee LY, Logothetis CJ. A 45-kDa ErbB3 secreted by prostate cancer cells promotes bone formation. Oncogene. 2008;27(39):5195–5203. doi: 10.1038/onc.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wojno KJ, Epstein JI. The utility of basal cell-specific anti-cytokeratin antibody (34 beta E12) in the diagnosis of prostate cancer. A review of 228 cases. Am J Surg Pathol. 1995;19(3):251–260. doi: 10.1097/00000478-199503000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Shah RB, Zhou M, LeBlanc M, Snyder M, Rubin MA. Comparison of the basal cell-specific markers, 34betaE12 and p63, in the diagnosis of prostate cancer. Am J Surg Pathol. 2002;26(9):1161–1168. doi: 10.1097/00000478-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Dhillon PK, Barry M, Stampfer MJ, Perner S, Fiorentino M, Fornari A, Ma J, Fleet J, Kurth T, Rubin MA, Mucci LA. Aberrant cytoplasmic expression of p63 and prostate cancer mortality. Cancer Epidemiol Biomarkers Prev. 2009;18(2):595–600. doi: 10.1158/1055-9965.EPI-08-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narahashi T, Niki T, Wang T, Goto A, Matsubara D, Funata N, Fukayama M. Cytoplasmic localization of p63 is associated with poor patient survival in lung adenocarcinoma. Histopathology. 2006;49(4):349–357. doi: 10.1111/j.1365-2559.2006.02507.x. [DOI] [PubMed] [Google Scholar]
- 15.Thalmann GN, Anezinis PE, Chang S, Zhau HE, Kim E, Hopwood VL, Pathak S, von Eschenbach AC, Chung LWK. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res. 1994;54:2577–2581. [PubMed] [Google Scholar]
- 16.Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3) Invest Urol. 1979;17(1):16–23. [PubMed] [Google Scholar]
- 17.Stone KR, Mickey DD, Wunderli H, Mickey GH, Paulson DF. Isolation of a human prostate carcinoma cell line (DU145) Int J Cancer. 1978;21:274–281. doi: 10.1002/ijc.2910210305. [DOI] [PubMed] [Google Scholar]
- 18.Degeorges A, Hoffschir F, Cussenot O, Gauville C, Le Duc A, Dutrillaux B, Calvo F. Recurrent cytogenetic alterations of prostate carcinoma and amplification of c-myc or epidermal growth factor receptor in subclones of immortalized PNT1 human prostate epithelial cell line. Int J Cancer. 1995;62(6):724–731. doi: 10.1002/ijc.2910620613. [DOI] [PubMed] [Google Scholar]
- 19.Hayward SW, Dahiya R, Cunha GR, Bartek J, Deshpande N, Narayan P. Establishment and characterization of an immortalized but non-transformed human prostate epithelial cell line: BPH-1. In Vitro Cell Dev Biol Anim. 1995;31(1):14–24. doi: 10.1007/BF02631333. [DOI] [PubMed] [Google Scholar]
- 20.Cummings CM, Bentley CA, Perdue SA, Baas PW, Singer JD. The Cul3/Klhdc5 E3 ligase regulates p60/katanin and is required for normal mitosis in mammalian cells. J Biol Chem. 2009;284(17):11663–11675. doi: 10.1074/jbc.M809374200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maddika S, Chen J. Protein kinase DYRK2 is a scaffold that facilitates assembly of an E3 ligase. Nat Cell Biol. 2009;11(4):409–419. doi: 10.1038/ncb1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abal M, Piel M, Bouckson-Castaing V, Mogensen M, Sibarita JB, Bornens M. Microtubule release from the centrosome in migrating cells. J Cell Biol. 2002;159(5):731–737. doi: 10.1083/jcb.200207076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartman JJ, Mahr J, McNally K, Okawa K, Iwamatsu A, Thomas S, Cheesman S, Heuser J, Vale RD, McNally FJ. Katanin, a microtubule-severing protein, is a novel AAA ATPase that targets to the centrosome using a WD40-containing subunit. Cell. 1998;93:277–287. doi: 10.1016/s0092-8674(00)81578-0. [DOI] [PubMed] [Google Scholar]
- 24.Sudo H, Maru Y. LAPSER1 is a putative cytokinetic tumor suppressor that shows the same centrosome and midbody subcellular localization pattern as p80 katanin. FASEB J. 2007;21(9):2086–2100. doi: 10.1096/fj.06-7254com. [DOI] [PubMed] [Google Scholar]
- 25.Sudo H, Maru Y. LAPSER1/LZTS2: a pluripotent tumor suppressor linked to the inhibition of katanin-mediated microtubule severing. Hum Mol Genet. 2008;17(16):2524–2540. doi: 10.1093/hmg/ddn153. [DOI] [PubMed] [Google Scholar]
- 26.Cabeza-Arvelaiz Y, Thompson TC, Sepulveda JL, Chinault AC. LAPSER1: a novel candidate tumor suppressor gene from 10q24.3. Oncogene. 2001;20(46):6707–6717. doi: 10.1038/sj.onc.1204866. [DOI] [PubMed] [Google Scholar]