Abstract
Background
Intestinal infection with Shiga toxin (Stx)-producing E.coli is a leading cause of hemolytic uremic syndrome and acute renal injury in otherwise healthy children in the US. Antibiotics are contraindicated and a therapeutic priority is agents that act intracellularly against the bacterial toxins which drive kidney injury. Our aim was to evaluate whether intravenous administration of a cell-permeable peptide (TVP) that binds to Stx2 will reduce disease severity and rescue juvenile baboons from a lethal Stx2 dose (50ng/kg).
Methods
TVP (5mg/kg) was delivered i.v. simultaneously with toxin (prevention protocol) or at 6 or 24 hours after toxin with daily 1mg/kg supplements up to Day 4 (rescue protocols). Biomarkers were monitored in blood and urine up to 28 days.
Results and Conclusions
TVP therapy resulted in either absence of clinical signs of acute kidney injury and normal urine output (prevention), or delayed and reduced BUN and creatinine levels (rescue) with concomitant survival. Delayed peptide administration significantly reduced thrombocytopenia, but surprisingly did not alter anemia even when monitored for 28 days in rescued survivors. This is the first successful cell-permeable therapeutic that counteracts Stx2 lethality in an animal model which recapitulates many of the human responses to enteric infection.
Keywords: Shiga toxins, enterohemorrhagic E.coli, hemolytic uremic syndrome, nonhuman primate, acute renal injury
Introduction
Development of hemolytic uremic syndrome (HUS) as a consequence of intestinal infection with Shiga toxin-producing enterohemorrhagic Escherichia coli (STEC) occurs in 5–15% of patients within the first 1–2 weeks after prodromal diarrhea. Children are particularly vulnerable and in some patients HUS develops, characterized by thrombocytopenia, hemolytic anemia and thrombotic microangiopathy. This is often accompanied by acute renal injury, necessitating hospitalization and frequently dialysis, and a mortality rate of 3–5% [1]. The most common STEC serotype globally is O157:H7, which came to U.S. national attention in 1992 when over 500 restaurant customers consumed contaminated, undercooked hamburgers [2]. A massive 1996 outbreak in Japanese school children [3, 4] and the 2006 US recall of contaminated spinach [5] illustrate the continuing public health and economic impact of STEC strains.
Antibiotic treatment is not recommended for suspected or confirmed infection because it does not reduce the risk of HUS [6, 7] and may increase risk [8]. The latter is related to mechanisms that regulate expression of bacterial toxins Shiga toxin type-1 and -2 (Stx1, Stx2), the primary bacterial virulence factors that cause coagulopathy and organ damage [9]. STEC strains that secrete Stx2 are more frequently associated with more severe disease and development of HUS [10–12].
Stx1 and Stx2 are AB-type ribosome-inactivating holotoxins, similar to Shiga toxin from Shigella dysenteriae serotype-1 and ricin from castor beans. The enzymatic A subunit and a cell binding B subunit that organizes into pentamers recognize a globotriaosylceramide membrane receptor (Gb3, CD77) [13]. Internalized toxin-receptor complexes undergo retrograde transport to the endoplasmic reticulum via the Golgi apparatus where the A subunit N-glycosidase removes an adenine base from 28S ribosomal RNA to inhibit protein synthesis [14]. Other effects may arise from toxin activities, such as inducement of inflammatory responses in animal models [15] and patients [16, 17], sub-lethal endothelial dysfunction [18] and stress responses in susceptible cells [19, 20].
Since antibiotics are contraindicated and little toxin antigen is detected in the circulation or stool [21], agents that act intracellularly are a priority. This is underscored by the observation that toxins are detectable in patient intestinal epithelial cells, even in the absence of overt bacterial colonization [22]. In a novel approach, Nishikawa and colleagues synthesized a multivalent peptide library and identified cell-permeable tetravalent peptides that bind to site 3 of the Stx2 B subunit [23]. The peptides had a 3-lysine core bifurcating at each end with peptide arms made from proline, lysine and/or arginine. The peptides redirected internalized toxin to the lysosomal degradation pathway, thereby protecting cells from toxicity. When administered intragastrically on day 2 or 3, an acetylated tetravalent peptide [TVP; Ac-(PPPRRRR-AU)4-Lys3] rescued mice from a lethal dose of E.coli O157:H7 [23]. This mode of administration inactivates Stx2 in the intestinal epithelium by inducing aberrant intracellular transport, preventing toxin distribution into the circulation [24]. Although TVP is cell permeable, intravenous administration of the peptide does not rescue mice from a lethal enteric STEC challenge, and is effective only with oral administration [23, 24], which may be a limitation in patients with hemorrhagic diarrhea.
Our nonhuman primate models of Stx1 and Stx2 toxemia develop a pathophysiology similar to that of patients. Unlike murine or porcine models, the baboons develop HUS with thrombocytopenia and hemolytic anemia, and acute kidney injury [25–27]. Because of these differences in animal models, we decided to evaluate the efficacy of the acetylated tetravalent peptide (TVP) with an intravenous administration using both prevention and rescue protocols. We monitored a comprehensive array of clinical and inflammatory indicators up to 28 days after challenge. We found that even delayed intravenous administration of TVP could rescue baboons from lethal Stx2 challenge, with resolution of hematology, physiologic, and inflammatory profiles and reduced acute kidney injury in the treated animals.
Methods
Reagents
Recombinant Stx2 was from BEI Resources (Manassas, VA). Recombinant Stx1 was from Dr. Cheleste Thorpe (Tufts University School of Medicine, Boston, MA). Prepared toxins contained <0.1 ng endotoxin/ml, as determined by Limulus amoebocyte lysate assay (Associates of CapeCod, Inc., East Falmouth, MA). Tetravalent peptide [TVP; Ac-MAPPPRRRRA-Ahx]4-MAP[Lys3]-amide] and biotinylated-TVP [Ac-MAPPPRRRRA-Ahx-4-MAP[Lys3]-G-[C-biotin]-amide] were synthesized by Fmoc chemistry, HPLC purified and verified by mass spectrometry (21st Century Biochemicals, Inc, Marlboro, MA). Unlabeled TVP is structurally identical to that administered orally in murine studies [23]. Lyophilized peptide was solubilized in sterile isotonic saline before administration.
Vero Cell Viability
Monkey kidney epithelial Vero cells (CRL-2983; ATCC, Rockville, MD) were maintained in EMEM supplemented with 10% fetal bovine serum, penicillin (50U/ml), streptomycin (50 µg/ml) at 37°C in humidified 5% CO2. Vero cells (104 cells/well) were incubated overnight, then for 48 hours in triplicate with Stx1 or Stx2 (400 ng/ml) with media (control) or TVP (0.1–10µM). Live cells were detected with an MTT assay (Cell Titer 96 Non-Radioactive Cell Proliferation Assay, Promega, Madison, WI) and viability calculated relative to untreated cells (100%).
Immunofluorescence
Vero cells were seeded onto cover slips and cultured overnight as described above. Cells were rinsed and then incubated with biotinylated-TVP (1uM final concentration) for 30 minutes at 37°C in media. Media was removed; cells were rinsed, then fixed in 4% formaldehyde for 10 minutes at room temperature, permealized with 0.1% Triton X-100 in Hanks balanced salt solution (HBSS), and blocked overnight with 3% bovine serum albumin in HBSS at room temperature. Samples were incubated with 0.5 µg/ml Texas Red-conjugated streptavidin (Invitrogen, Carlsbad, CA) in HBSS for 30 minutes at 4°C, then washed 3x with HBSS. Slowfade with DAPI (Invitrogen, Carlsbad, CA) was added before each slide was covered and sealed. Stained cells were viewed with a Nikon deconvolution wide-field Epifluorescence microscope (Nikon ECLIPSE TE-2000E).
Animals
Papio c. anubis baboons were purchased from the Oklahoma Baboon Research Resource at the University of Oklahoma Health Sciences Center (Dr. Gary White, Director). Baboons were outbred juvenile males (1.5–3 years; 5–8kg) and free of tuberculosis. Animals were housed and used in accordance with approved IACUC and IBC protocols from Boston University School of Medicine and the University of Oklahoma Health Sciences Center.
Therapeutic Protocol Designs
Two treatment protocols were designed to compare with results obtained from animals challenged with only Stx2 on Day 0. Untreated baboons were challenged i.v. with a lethal dose of Stx2 (50ng/kg; n=6) [2 5]. In the Prevention Protocol (n=3), Stx2 (50ng/kg;) was mixed with TVP peptide (5mg/kg) and injected intravenously at T(0). No additional peptide was given after that time. In Rescue Protocols, Stx2 (50ng/kg) was administered i.v. at T(0), and 5mg/kg peptide was administered i.v. starting either at 6 hours (n=3) or 24 hours (n=4) after toxin challenge. Each day thereafter up to day 4, the baboons received one supplement i.v. dose of peptide (1mg/kg). Blood and timed urine samples were obtained periodically. Animals were weighed daily and saline was infused for hydration based on criteria developed in previous studies [25, 27]. Prevention Protocol animals were euthanized at Day 7 for tissue harvest. Surviving animals from Rescue Protocols (6/7) were monitored until day 28 to observe long-term trends. Survival curves were determined by Kaplan-Meier analysis and statistical differences using log-rank (Mantel-Cox) analyses. In accordance with the ethical use of nonhuman primates, a treatment group was considered complete when survival differences from untreated animals were detected at p≤ 0.05.
Toxin Challenge Procedures
Animal studies were performed essentially as described [25, 28]. Briefly, juvenile baboons were sedated, orally intubated and anesthesia was maintained with sodium pentobarbital (5–10 mg/kg i.v.). A forearm cephalic vein catheter was used for Day 0 infusion of toxin or toxin+TVP. A femoral vein catheter was inserted by enous cutdown and secured subcutaneously by an internal injection cap (Braun) where it remained for the rest of the study period and was used for TVP administration on days 1–4, blood draws, saline infusion, blood pressure monitoring, and anesthesia. Death is not an endpoint; baboons were euthanized according to established criteria if deemed necessary. All animals received prophylactic Baytril (enrofloxacin; 10mg/kg i.m.) on Day 0, and either Levaquin (levofloxacin; 3.5mg/kg i.v.) or Baytril (10mg/kg i.m.) each day over the experimental period. Urine was collected aseptically using a lubricated 6fr silicon Foley catheter (Rusch). Existing urine was allowed to purge from the bladder for 10 minutes; a 20 minute timed sample was then collected (mL/kg/hr).
Sample Analyses and Normal Baboon Values
Urinalysis was done with Siemens Multistix 10 SG dipsticks. Fibrinogen levels (T0=100%) and activated partial thromboplastin clotting times (APTT) were determined using standard methods as described [25]. Complete blood counts (CBCs) were determined with a Horiba ABX Micros 60 hematology analyzer (Horiba, Irvine, CA). Blood smears were stained with Wright’s stain; schistocyte counts were determined as percentages of 200 red blood cells. Plasma chemistry analyses (BUN, creatinine) were performed by IDEXX Laboratories (Westbrook, ME). Urine creatinine was determined with a chromogenic Creatinine Assay kit (Cayman Chemical Co., Ann Arbor, MI). Normal baboon values (mean ± S.D.; from 37 healthy animals) are as follows: platelets, 276.2 ± 52.5 × 104/µl; RBC, 5.1 ± 0.4 × 106/µl; BUN, 14.2 ± 3.0 mg/dL; creatinine, 0.5 ± 0.1 mg/dL; APTT, 40.4 ± 5.8 seconds.
Cytokine Analyses
Plasma and urine cytokine levels were analyzed with xMAP multiplex fluorescent bead-based assays using a Luminex 200 IS system (Millipore, Billerica, MA), Luminex xPONENT software (Luminex, Austin, TX), and nonhuman primate cytokine panel kits (Millipore) as described [25]. Variation from T0 values were considered significantly different at p<0.05 (Student’s t test).
Results
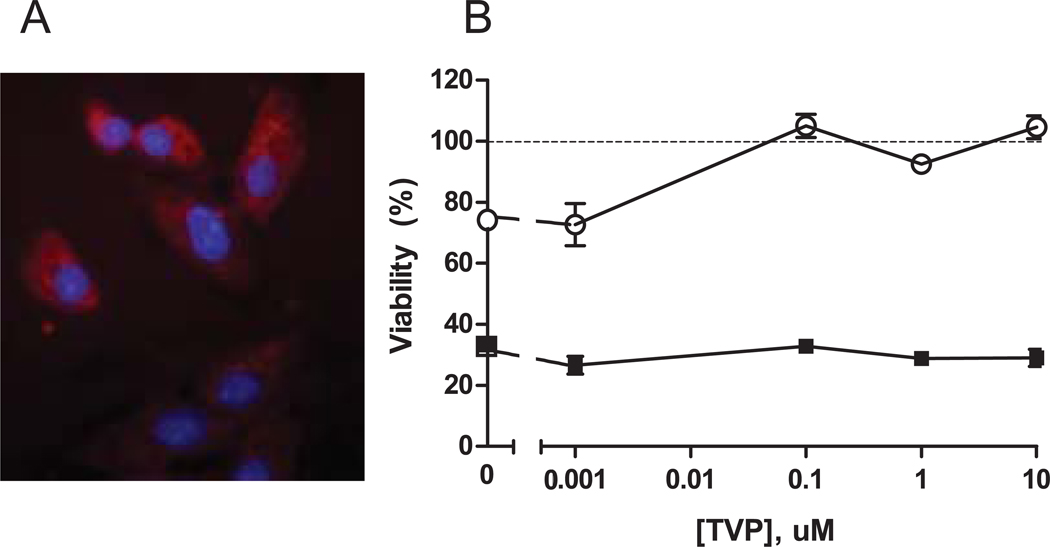
Vero cells (monkey kidney epithelial cells) are sensitive to Shiga toxins and initial in vitro studies confirmed that synthetic TVP was cell permeable and that it protects Vero cells from Stx2. Immunofluorescence staining showed biotin-TVP distribution in the cytoplasm of monkey Vero cells (Fig.1A). Stx2 and Stx1 reduced Vero cell viability as expected and increasing TVP concentrations protected cells from Stx2 cytotoxicity, but had no effect on Stx1-mediated cell death (Fig. 1B). These results are consistent with prior studies detailing toxin specificity and protective mechanism of TVP in Vero cells and intestinal epithelial cells [23, 24].
Figure 1. TVP Cell Permeability and Protection.
A. Intracellular localization of biotin-TVP stained with streptavidin-Texas Red in monkey Vero cells; nucleus, blue (40x). B. Cell viability of Vero cells after incubation with 400 ng/ml Stx1(■) or Stx2 (○) and TVP (0–10µM) for 48hr relative to untreated cells (100%). Data are expressed as mean ± S.D of triplicate wells.
Intravenous TVP Administration Improves Survival after Lethal Stx2 Challenge
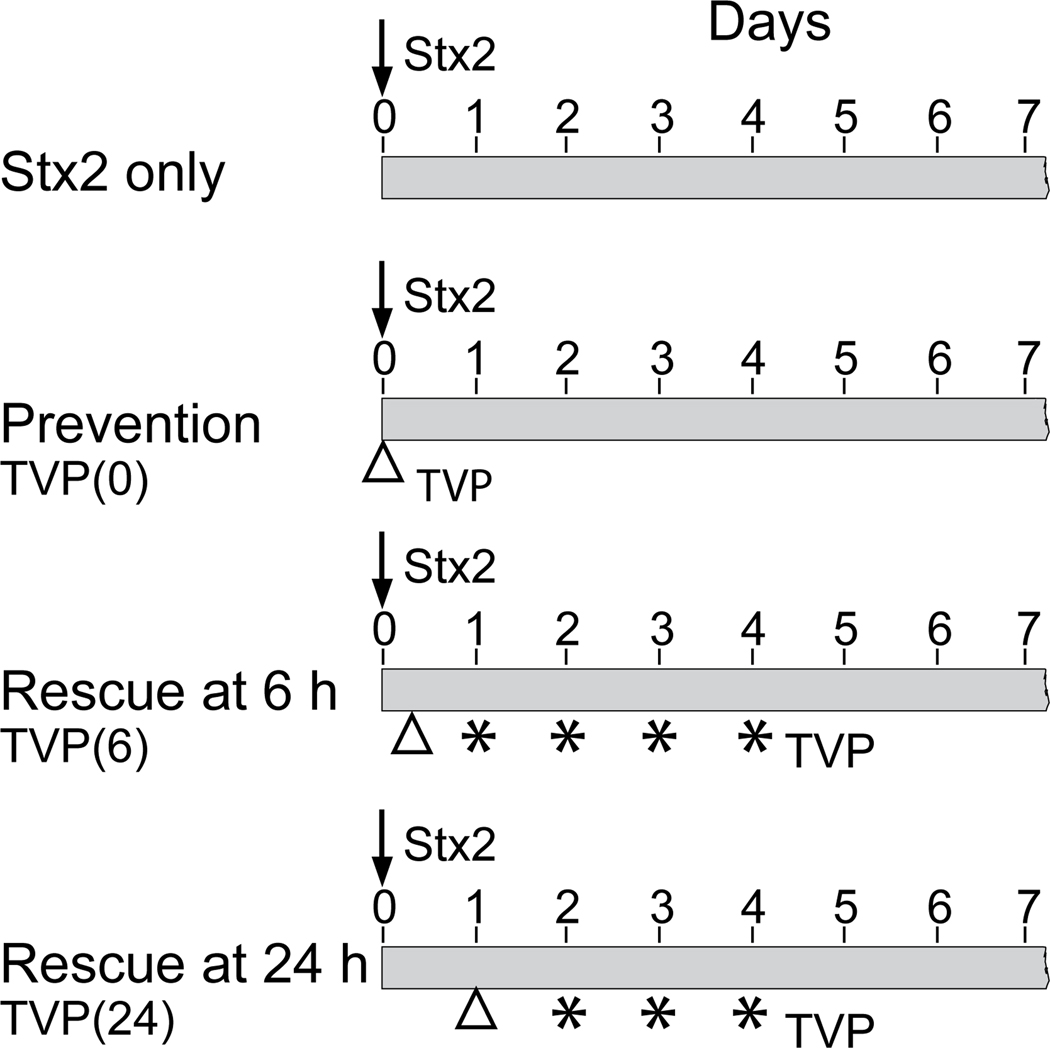
To test the in vivo efficacy of TVP administered intravenously, we designed different treatment protocols to compare with results obtained from animals challenged with only Stx2 (50ng/kg) on Day 0 (Fig. 2). The Prevention Protocol evaluates consequences of a single dose of TVP and Stx2 administered simultaneously. The Rescue Protocols evaluate effects when peptide is administered 6 or 24 hours after the lethal Stx2 dose with supplemental peptide administered up to Day 4. Prevention Protocol animals were euthanized on Day 7 for tissue harvest. Rescue Protocol animals were monitored until Day 28 to observe long-term trends.
Figure 2.
Experimental Designs. A lethal Stx2 toxin dose (50ng/kg) was injected I.v. on Day 0 (arrow) in all animals. Prevention Protocol animals received Stx2 pre-mixed with TVP (5mg/kg; △; TVP(0)). No additional peptide was administered and animals were monitored for 7 days. Rescue Protocol animals received toxin on Day 0 and TVP (5mg/kg i.v., △) at 6 hours (TVP(6)) or 24 hours (TVP(24)) after toxin. A supplement TVP dose (1mg/kg i.v., star) was given daily up to day 4. Rescued animals were monitored up to 28 days post-challenge.
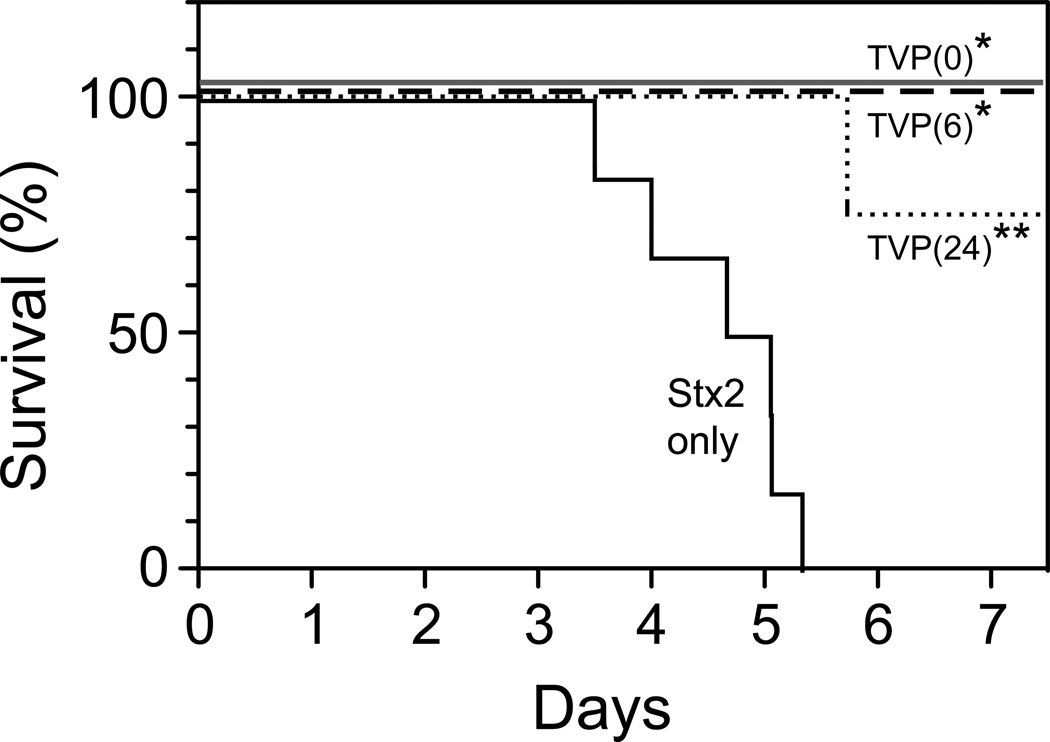
Simultaneous i.v. administration of TVP (5mg/kg) and Stx2 (50ng/kg) at T(0) resulted in 100% survival and prevented lethality (3/3; p<0.01 compared to toxin alone; Fig. 3). When TVP administration was delayed and given at 6 hours after toxin, followed by a lower supplement dose on days 1–4 (1mg/kg), all animals were rescued and survived (3/3; p <0.01). When TVP was administered i.v. at 24 hours after a lethal toxin dose, followed by supplemental lower doses, there was one euthanasia at 137 hours (5.7 days), but all other animals were rescued and survived (3/4; p<0.003).
Figure 3.
Survival. The median time to euthanasia for baboons challenged with 50ng/kg Stx2 was 4.8 days (solid line; n=6). All animals survived when TVP was administered simultaneously with toxin (Prevention Protocol, TVP(0), n=3) or first given i.v. at 6 hours after toxin (Rescue Protocol, TVP(6); n=3). TVP first given i.v. at 24 hours after toxin rescued 3 of 4 baboons (dotted line, TVP(24); n=4). All rescued animals were long-term survivors without clinical signs of acute kidney injury. *p<0.01, **p<0.003 compared to toxin alone.
TVP Reduces Acute Kidney Injury due to Stx2
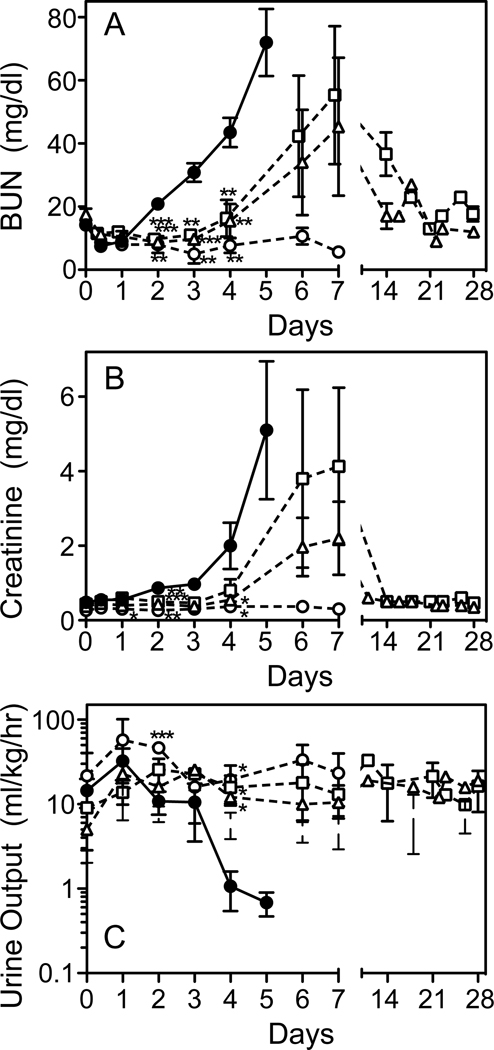
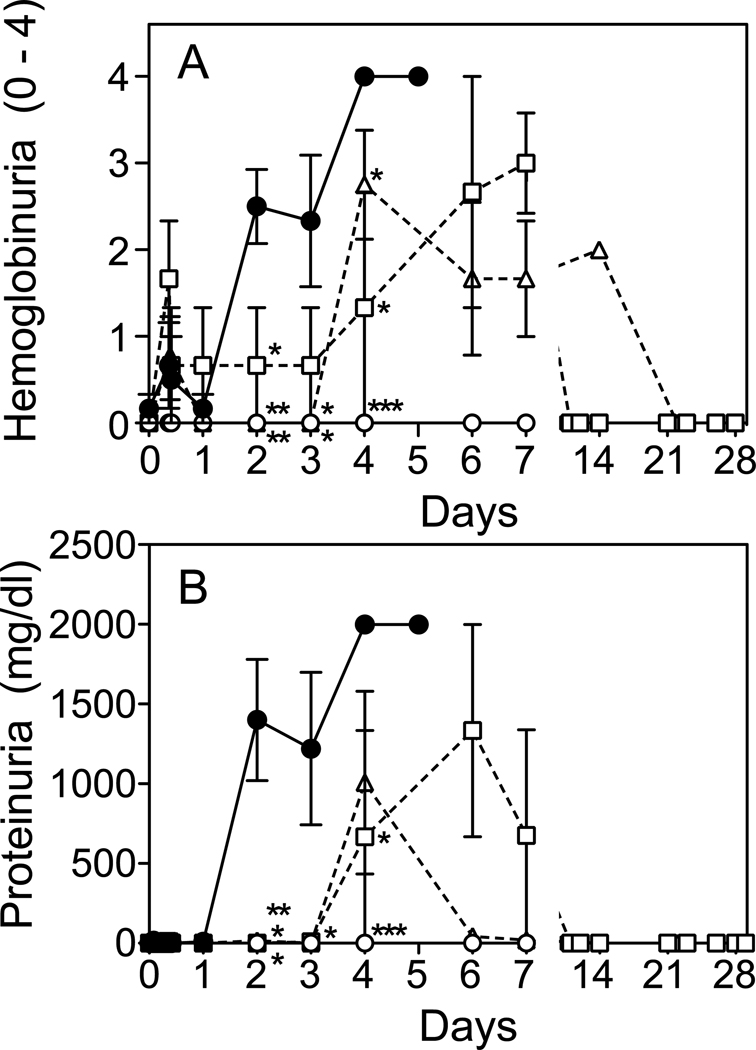
The kidney is a primary target organ during Stx2 toxemia, as judged by elevations in plasma BUN and creatinine, decreased urine output (Fig. 4A–C), and elevated blood (hemoglobin) and protein in the urine (Fig. 5A,B). TVP treatment simultaneously with toxin challenge prevented changes in these clinical signs of renal injury and urine output remained normal. Animals first treated with TVP at 6 or 24 hours after toxin, had significant delays in rising BUN and creatinine levels (p<0.001) and normal urine output. No differences were observed between Rescue Protocol groups. Creatinine levels had normalized by day 14, but BUN values were slower to return to baseline, normalizing by day 14–21. Blood (homogenous pattern) and protein were detected in urine of rescued animals through day 7, although delayed by 1–2 days compared to Stx2 alone, particularly proteinuria (Fig. 5). No urinary protein was detected in the next scheduled urine collection (day 10–14 depending on the animal).
Figure 4.
Acute Renal Injury. Plasma BUN (A), creatinine (B) and urine output (C) were measured at the indicated times after toxin only (●) and in Stx2+TVP treated animals. Urine output was preserved in all TVP-treated animals. BUN and creatinine levels remained normal (○; prevention protocol) or increases delayed when peptide was first given at 6hrs (□) or 24 hrs (△) after toxin (rescue protocols). *p<0.05, **p<0.01, ***p<0.001 compared to toxin only.
Figure 5.
Urinalysis. High levels of blood (hemoglobin) (A) and protein (B) were detected in urine by day 2 after Stx2 only challenge (●), but were not observed in Prevention Protocol animals (○), and were delayed or reduced in Rescue Protocol animals (TVP(6) □ ; TVP(24) △). *p<0.05, **p<0.01, ***p<0.001 compared to toxin only.
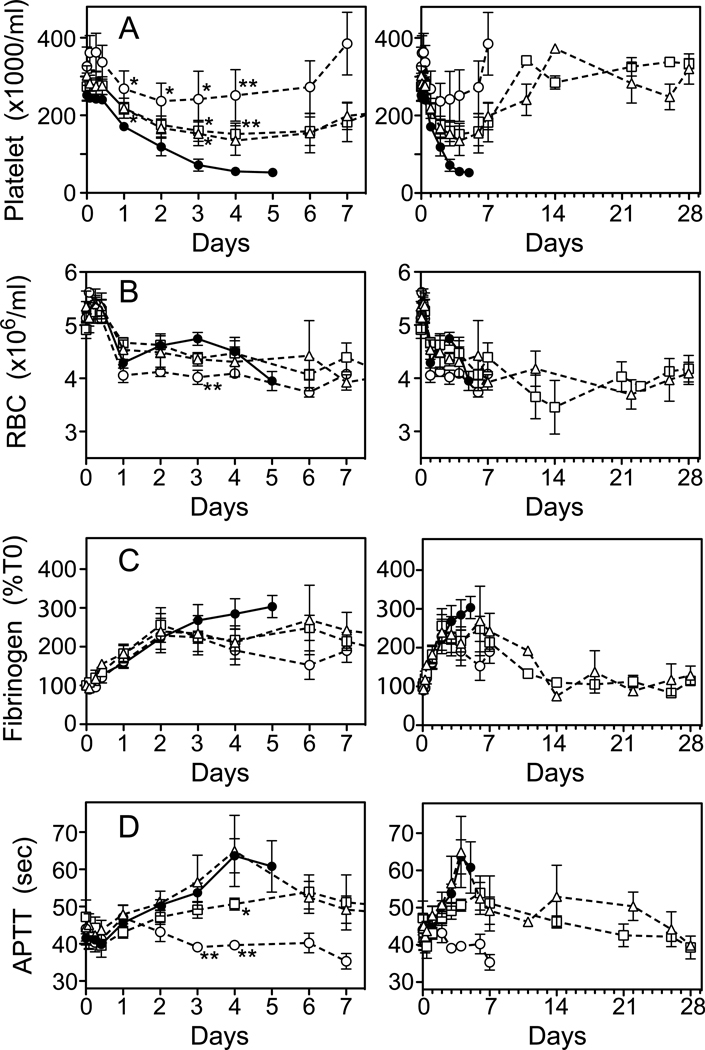
Alteration of Hematologic Changes by TVP
Changes in hematologic parameters are shown during the first 7 days (Fig. 6, left) and for Rescue Protocol survivors up to 28 days to show long-term trends (right). Stx2-induced thrombocytopenia was attenuated by TVP with significant differences due to peptide treatment emerging by 24 hours (Fig. 6A, left). Single dose TVP at T(0) prevented thrombocytopenia, whereas delayed TVP administration in Rescue Protocols reduced the decline in platelet counts. No differences were detectable between Rescue Protocol groups. In rescued survivors, platelets counts returned to pre-challenge levels by day 10–14 and remained steady through day 28.
Figure 6.
Hematology. Changes in hematology parameters are shown for all animals during the first 7 days (left) and in TVP rescued survivors over 28 days (right). Changes in platelets counts (A), red blood cells (B), fibrinogen (C) and APTT clotting times (D) were monitored for animals who received toxin alone (●), prevention protocol TVP (○), rescue protocol TVP(6) (□) and rescue protocol TVP (24) (△). *p<0.05, **p<0.01 compared to toxin alone.
Interestingly, TVP treatment had no effect on the anemia induced by Stx2 (Fig. 6B). Red cell counts declined similarly in all animals, and anemia persisted through day 28 in all rescued survivors (Fig. 6B, right), even though the animals appeared clinically healthy, and were eating, drinking and behaving normally. Schistocyte counts increased modestly to 2.8% at day 4 in untreated Stx2 baboons and TVP treatment had no significant effect (data not shown). Early fibrinogen elevations were similar and plateaued by Day 2–3 in treated animals, but differences from untreated animals did not reach significance (Fig. 6C, left). Fibrinogen levels normalized in Rescue Protocol survivors after the first week (Fig. 6C, right). Prolongation of APTT clotting times was modest, plateaued at day 4–5 and were similar in animals who received only toxin and those who first received TVP at 24 hours (Fig. 6D, left). Earlier treatment with TVP either abrogated (Prevention Protocol) or significantly reduced (T6hrs Rescue Protocol; p<0.001) prolonged clotting times.
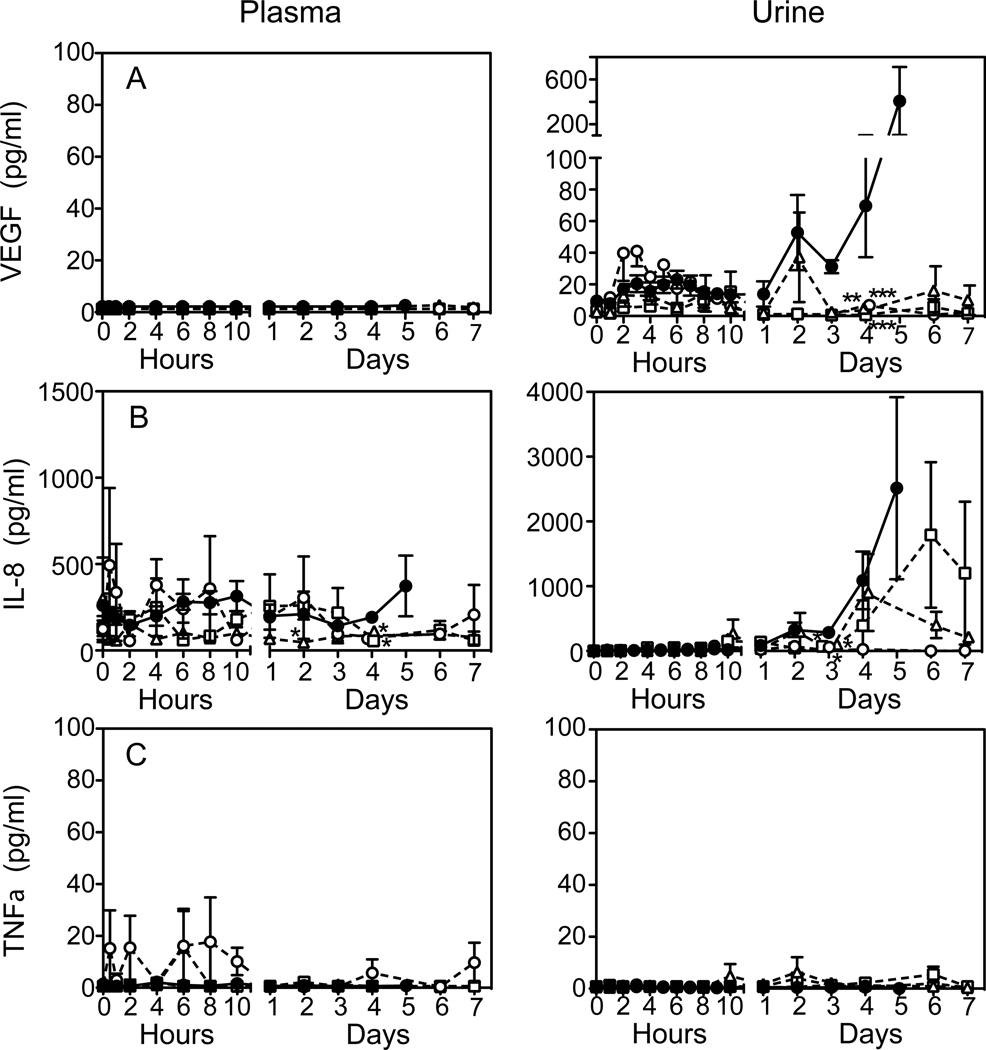
TVP Reduced the Inflammatory Response to Stx2
Inflammation biomarkers were measured in plasma (Fig. 7, left) and urine (right) up to day 7 after toxin challenge. Cytokines were not measured after day 7 in Rescue Protocol survivors. Of 19 biomarkers measured, only VEGF levels were remarkably different between plasma and urine (Fig. 7A). There was no detectable plasma VEGF in any of the animals, but urine VEGF increased in untreated animals from day 2 until euthanasia. TVP either prevented urine VEGF increases (Prevention Protocol) or reduced it by day 4 (Rescue Protocols). Plasma IL-8 chemokine varied inconsistently (Fig. 7B), but urine IL-8 levels were of interest. At day 3, urine IL-8 in all groups declined, then remained undetectable (Prevention) or rising again in the Rescue Protocol animals. The mechanism for this biphasic response is not known. Little TNFα was found in plasma or urine in any of the animals, whether treated or not (Fig.7C).
Figure 7.
Inflammatory Responses. Cytokines were measured in plasma (left) and urine (right) at the indicated times up to 7 days after toxin challenge. Urine cytokines were normalized to urine creatinine levels. VEGF levels (A) were markedly different in plasma and urine. Compared to toxin only (●), TVP prevented (○) or reduced changes in urinary VEGF levels (TVP(6) □ ; TVP(24) △). Plasma IL-8 levels (B) were inconsistent, but urine IL-8 levels increased after Day 2–3 except for Prevention protocol animals. TNFα (C) was essentially undetectable in plasma and urine of all animals. *p<0.05 compared to toxin only.
Discussion
In this study we show that intravenous administration of a synthetic tetravalent peptide (TVP) designed to counteract Stx2 activity within susceptible cells is capable of rescuing baboons from an otherwise lethal dose of toxin. Even when TVP administration was delayed by 24 hours, the survival rate and protection of organ function was substantial and significant. In other STEC animal models, delayed treatment with anti-toxins, such as antibodies [29, 30] or Gb3 receptor mimetics [31] are beneficial and improve survival. However, unlike most other anti-toxin compounds, TVP is cell-permeable even in the absence of toxin (Fig. 1 and [23]) and can complex with Stx2 within living cells [24], so it has the potential to be effective long after toxin delivery.
The kidneys were primary beneficiaries of TVP treatment as judged by complete absence of clinical signs of acute kidney injury in Prevention Protocol animals and significantly reduced injury in Rescue Protocol animals. Urine output remained normal and BUN, creatinine, and urinalysis measures remained normal or considerably reduced despite challenge with a lethal Stx2 dose. Even years after hospitalization with diarrhea + HUS, 25–36% of children have one or more renal sequelae which may be predicted by duration of oliguria (>10 days) or anuria (>5 days) [32]. Although we did not evaluate TVP efficacy beyond 24 hours post-toxin challenge or monitor animals beyond 28 days, the observation that TVP effectively protected urine output suggests that this treatment may have long-term benefits.
Most patients have a fall in platelet counts during the first illness week and monitoring platelet counts is recommended to follow the clinical course [1]. Thrombocytopenia developed rapidly in the baboons after Stx2 whereas platelet counts in Prevention Protocol animals remained within normal ranges. Platelet loss was greater when TVP treatment was delayed, but recovered after the first week post-challenge. When compared to historical dose-response data in this baboon model, platelet counts in Rescue Protocol animals who received 50ng/kg Stx2 essentially overlay those from baboons who received 10ng/kg Stx2, which is a mild, non-lethal dose [25]. The thrombocytopenia is not due to a disseminated consumptive coagulopathy, because prolongation of clotting times was modest, indicating sufficient levels of coagulation factors, and fibrinogen levels did not decrease. There is a pro-coagulant environment, and patients with diarrhea + HUS may have elevated D-dimer and F1+2 levels [33, 34]. Stx induces activation of platelet sub-populations [35], and expression of adhesion [36] and coagulant molecules [37] on glomerular endothelial cells. However, although pro-coagulant activity occurs early in HUS patients [34], there is little evidence to suggest anti-thrombotics are helpful [33, 38] and there is concern of increased bleeding risk.
We observed a moderate and slowly progressive anemia in all the baboons, which persisted even in rescued survivors through day 28. HUS patients can become profoundly anemic and ~80% need erythrocyte transfusions [39]. We do not know the mechanism for prolonged anemia in the baboons, but RBC losses may be due to renal thrombotic microangiopathy which is characteristic of typical HUS. Consistent with this is the observation that the urine dipstick test for blood invariably showed a homogenous pattern, indicating hemoglobinuria. However, schistocyte increases are modest in our Stx2 model, only 2.8–3.5% by 72–96 hours. These observations and the lack of TVP effect suggest an early kidney injury at the cellular level that is not related to survival, but compromises red cell counts. If renal cortical interstitial cells are a target, then low erythropoietin production is possible. Argentina has a high incidence of STEC infection and pediatric HUS, and one study found erythropoietin levels in children with acute diarrhea + HUS to be similar to low levels observed in patients with chronic renal diseases [40]. One clinical study described fewer RBC transfusions in five diarrhea + HUS pediatric patients treated early with erythropoietin [41]. Low erythropoietin levels may explain the anemia in our baboons, but we cannot yet quantify baboon erythropoietin and are pursuing other approaches to evaluate potential Stx2 impact on epo transcription. In the current study, we set the Stx2 dose to be lethal and this probably does not represent a typical toxin load in children with HUS (3–5% mortality), but the baboon toxemia model may be useful for testing efficacy and side effects of therapeutics intended to normalize the hematologic abnormalities caused by Stx.
TVP treatment moderated the systemic inflammation induced by Stx2, but the overall host response was relatively mild. Lipopolysaccharide (endotoxin) is a well known pro-inflammatory mediator from Gram negative bacteria, inducing systemic production of TNFα and other cytokines in humans [42] and baboons [43] through activation of Toll-like receptor 4. The lack of plasma or urinary TNFα in the current study is consistent with essentially undetectable levels of endotoxin in the Stx2 preparations used. It also argues that TNFα is not a required component for the kidney damage inflicted by Stx2. However, most STEC patients do have a robust systemic cytokine response [16, 44], even though the disease is considered to be a toxemia and patients are not bacteremic. It is very possible that intestinal colonization with bacteria that inflicts tissue injury produces damage associated molecular pattern molecules (DAMPs) that contribute to patient inflammation as occurs in other inflammatory bowel conditions [45].
Notably, VEGF levels were markedly different between plasma and urine. Plasma levels remained undetectable, whereas urine VEGF increased to 400pg/ml after toxin, which were reduced to baseline by TVP. These observations suggest local influences contribute to urinary VEGF. Human glomerular epithelia and collecting duct cells have VEGF protein [46] and glomerular capillaries express VEGF receptors [47]. Hypoxia is a well-established VEGF stimulant and local ischemia due to microthrombi may contribute to elevated urinary VEGF. Conceivably, increased urine VEGF could be an early marker of kidney injury, but levels did not increase until two days after challenge, well after platelet counts started to decline. Alternatively, bedside measurement of urinary VEGF levels may provide a non-invasive way to monitor treatment efficacy and clinical course.
While informative, this baboon model is a model of Stx toxemia and will not completely replicate the consequences of intestinal infection with STEC bacteria. Although bacteremia is rare and the toxins drive organ injury, we would expect differences in the magnitude and timing of toxin delivery as bacteria colonize the intestine. In addition, neurologic abnormalities are a frequent complication of STEC infection in patients [48]. We occasionally observe seizures or ataxia in the Stx2-challenged baboons, but they are not observed in all animals. Because of the infrequency and lack of quantifiable neural tissue injury marker(s), we do not know if TVP had any effect on the central nervous system. Finally, while Stx1 and Stx2 are primary virulence factors, other bacterial products likely contribute to host responses [49, 50]. Thus, while intravenous TVP administration was effective, it was effective with respect to the Stx2 challenge and not necessarily a bacterial infection. In ongoing studies, we are developing enteric infection models designed to recapitulate responses observed in patients and test therapeutics for dose, timing and route of administration.
In summary, we show that a small peptide delivered intravenously can protect and rescue nonhuman primates from a lethal dose of Stx2. Even with delayed therapeutic delivery, urine output is preserved, kidney function is protected, and platelet loss is minimized, all of which contributed to a better clinical outcome and improved survival.
Acknowledgements
This work is supported by NIH/NIAID U01AI075386 (S.K.). Baboons were purchased from the Oklahoma Baboon Research Resource at the University of Oklahoma Health Sciences Center supported by NIH P40RR012317 (Gary White, Director).
We gratefully acknowledge Drs. Gary White, Roman Wolf (Comparative Medicine), and Gary Kinasewitz (Pulmonary and Critical Care Medicine) at the University of Oklahoma Health Sciences Center for veterinary support and discussions. We thank Danielle Day for technical support and Lyndianne Joseph for administrative assistance.
Footnotes
Disclosure of Conflicts of Interest: The authors declare they have no conflict of interest.
REFERENCES
- 1.Tarr PI, Gordon CA, Chandler WL. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. The Lancet. 2005;365:1073–1086. doi: 10.1016/S0140-6736(05)71144-2. [DOI] [PubMed] [Google Scholar]
- 2.Bell BP, Goldoft M, Griffin PM, et al. A multistate outbreak of Escherichia coli O157: H7-associated bloody diarrhea and hemolytic uremic syndrome from hamburgers. The Washington experience. JAMA. 1994;272:1349–1353. [PubMed] [Google Scholar]
- 3.Fukushima H, Hashizume T, Morita Y, et al. Clinical experiences in Sakai City Hospital during the massive outbreak of enterohemorrhagic Escherichia coli O157 infections in Sakai City, 1996. Pediatr Int. 1999;41:213–217. doi: 10.1046/j.1442-200x.1999.4121041.x. [DOI] [PubMed] [Google Scholar]
- 4.Yoshioka K, Yagi K, Moriguchi N. Clinical features and treatment of children with hemolytic uremic syndrome caused by enterohemorrhagic Escherichia coli O157: H7 infection: experience of an outbreak in Sakai City, 1996. Pediatr Int. 1999;41:223–227. doi: 10.1046/j.1442-200x.1999.4121039.x. [DOI] [PubMed] [Google Scholar]
- 5.Cooley M, Carychao D, Crawford-Miksza L, et al. Incidence and Tracking of Escherichia coli O157: H7 in a Major Produce Production Region in California. PLoS ONE. 2007;2:e1159. doi: 10.1371/journal.pone.0001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell BP, Griffin PM, Lozano P, et al. Predictors of Hemolytic Uremic Syndrome in Children During a Large Outbreak of Escherichia coli O157: H7 Infections. Pediatrics. 1997;100:e12. doi: 10.1542/peds.100.1.e12. [DOI] [PubMed] [Google Scholar]
- 7.Panos GZ, Betsi GI, Falagas ME. Systematic review: are antibiotics detrimental or beneficial for the treatment of patients with Escherichia coli O157: H7 infection? AlimentPharmacol Ther. 2006;24:731–742. doi: 10.1111/j.1365-2036.2006.03036.x. [DOI] [PubMed] [Google Scholar]
- 8.Wong CS, Jelacic S, Habeeb RL, et al. The Risk of the Hemolytic-Uremic Syndrome after Antibiotic Treatment of Escherichia coli O157: H7 Infections. N Engl J Med. 2000;342:1930–1936. doi: 10.1056/NEJM200006293422601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimmitt PT, Harwood CR, Barer MR. Toxin gene expression by shiga toxinproducing Escherichia coli: the role of antibiotics and the bacterial SOS response. EmergInfect Dis. 2000;6:458–465. doi: 10.3201/eid0605.000503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ostroff SM, Tarr PI, Neill MA, et al. Toxin genotypes and plasmid profiles as determinants of systemic sequelae in Escherichia coli O157: H7 infections. The Journal of Infectious Diseases. 1989;160:994–998. doi: 10.1093/infdis/160.6.994. [DOI] [PubMed] [Google Scholar]
- 11.Donohue-Rolfe A, Kondova I, Oswald S, et al. Escherichia coli 0157: H7 Strains That Express Shiga Toxin (Stx) 2 Alone Are More Neurotropic for Gnotobiotic Piglets Than Are Isotypes Producing Only Stx1 or Both Stx1 and Stx2. The Journal of Infectious Diseases. 2000;181:1825–1829. doi: 10.1086/315421. [DOI] [PubMed] [Google Scholar]
- 12.Siegler RL, Obrig TG, Pysher TJ, et al. Response to Shiga toxin 1 and 2 in a baboon model of hemolytic uremic syndrome. PediatrNephrol. 2003;18:92–96. doi: 10.1007/s00467-002-1035-7. [DOI] [PubMed] [Google Scholar]
- 13.Okuda T, Tokuda N, Numata Si, et al. Targeted Disruption of Gb3/CD77 Synthase Gene Resulted in the Complete Deletion of Globo-series Glycosphingolipids and Loss of Sensitivity to Verotoxins. Journal of Biological Chemistry. 2006;281:10230–10235. doi: 10.1074/jbc.M600057200. [DOI] [PubMed] [Google Scholar]
- 14.Saxena S, O'Brien A, Ackerman E. Shiga toxin, Shiga-like toxin II variant, and ricin are all single-site RNA N-glycosidases of 28 S RNA when microinjected into Xenopus oocytes. J Biol Chem. 1989;264:596–601. [PubMed] [Google Scholar]
- 15.Keepers TR, Psotka MA, Gross LK, et al. A murine model of HUS: Shiga toxin with lipopolysaccharide mimics the renal damage and physiologic response of human disease. J Am Soc Nephrol. 2006;17:3404–3414. doi: 10.1681/ASN.2006050419. [DOI] [PubMed] [Google Scholar]
- 16.Proulx F, Turgeon JP, Litalien C, et al. Inflammatory mediators in Escherichia coli O157: H7 hemorrhagic colitis and hemolytic-uremic syndrome. Pediatr Infect Dis J. 1998;17:899–904. doi: 10.1097/00006454-199810000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Inward CD, Varagunam M, Adu D, et al. Cytokines in haemolytic uraemic syndrome associated with verocytotoxin-producing Escherichia coli infection. ArchDis Child. 1997;77:145–147. doi: 10.1136/adc.77.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bitzan MM, Wang Y, Lin J, et al. Verotoxin and ricin have novel effects on preproendothelin-1 expression but fail to modify nitric oxide synthase (ecNOS) expression and NO production in vascular endothelium. J Clin Invest. 1998;101:372–382. doi: 10.1172/JCI522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S-Y, Lee M-S, Cherla RP, et al. Shiga toxin 1 induces apoptosis through the endoplasmic reticulum stress response in human monocytic cells. Cellular Microbiology. 2008;10:770–780. doi: 10.1111/j.1462-5822.2007.01083.x. [DOI] [PubMed] [Google Scholar]
- 20.Smith WE, Kane AV, Campbell ST, et al. Shiga Toxin 1 Triggers a Ribotoxic Stress Response Leading to p38 and JNK Activation and Induction of Apoptosis in Intestinal Epithelial Cells. Infection and Immunity. 2003;71:1497–1504. doi: 10.1128/IAI.71.3.1497-1504.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cornick NA, Jelacic S, Ciol MA, et al. Escherichia coli O157: H7 infections: discordance between filterable fecal shiga toxin and disease outcome. J Infect Dis. 2002;186:57–63. doi: 10.1086/341295. [DOI] [PubMed] [Google Scholar]
- 22.Malyukova I, Murray KF, Zhu C, et al. Macropinocytosis in Shiga toxin 1 uptake by human intestinal epithelial cells and transcellular transcytosis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G78–G92. doi: 10.1152/ajpgi.90347.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishikawa K, Watanabe M, Kita E, et al. A multivalent peptide library approach identifies a novel Shiga toxin inhibitor that induces aberrant cellular transport of the toxin. FASEB J. 2006;20:2597–2599. doi: 10.1096/fj.06-6572fje. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe-Takahashi M, Sato T, Dohi T, et al. An orally applicable Shiga toxin neutralizer functions in the intestine to inhibit the intracellular transport of the toxin. Infect Immun. 2010;78:177–183. doi: 10.1128/IAI.01022-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stearns-Kurosawa DJ, Collins V, Freeman S, et al. Distinct physiologic and inflammatory responses elicited in baboons after challenge with Shiga toxin type 1 or 2 from enterohemorrhagic Escherichia coli. Infect Immun. 2010;78:2497–2504. doi: 10.1128/IAI.01435-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siegler R, Obrig T, Pysher T, et al. Response to Shiga toxin 1 and 2 in a baboon model of hemolytic uremic syndrome. Pediatr Nephrol. 2003;18:92–96. doi: 10.1007/s00467-002-1035-7. [DOI] [PubMed] [Google Scholar]
- 27.Taylor FB, Jr., Tesh VL, DeBault L, et al. Characterization of the baboon responses to Shiga-like toxin: descriptive study of a new primate model of toxic responses to Stx-1. American Journal of Pathology. 1999;154:1285–1299. doi: 10.1016/S0002-9440(10)65380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stearns-Kurosawa DJ, Lupu F, Taylor FB, Jr., et al. Sepsis and pathophysiology of anthrax in a nonhuman primate model. American Journal of Pathology. 2006;169:433–444. doi: 10.2353/ajpath.2006.051330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krautz-Peterson G, Chapman-Bonofiglio S, Boisvert K, et al. Intracellular Neutralization of Shiga Toxin 2 by an A Subunit-Specific Human Monoclonal Antibody. Infect Immun. 2008;76:1931–1939. doi: 10.1128/IAI.01282-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Q, Donohue-Rolfe A, Krautz-Peterson G, et al. Gnotobiotic Piglet Infection Model for Evaluating the Safe Use of Antibiotics against Escherichia coli O157: H7 Infection. The Journal of Infectious Diseases. 2009;199:486–493. doi: 10.1086/596509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watanabe M, Matsuoka K, Kita E, et al. Oral therapeutic agents with highly clustered globotriose for treatment of Shiga toxigenic Escherichia coli infections. The Journal of Infectious Diseases. 2004;189:360–368. doi: 10.1086/381124. [DOI] [PubMed] [Google Scholar]
- 32.Oakes RS, Kirkham JK, Nelson RD, et al. Duration of oliguria and anuria as predictors of chronic renal-related sequelae in post-diarrheal hemolytic uremic syndrome. Pediatr Nephrol. 2008;23:1303–1308. doi: 10.1007/s00467-008-0799-9. [DOI] [PubMed] [Google Scholar]
- 33.Van Geet C, Proesmans W, Arnout J, et al. Activation of both coagulation and fibrinolysis in childhood hemolytic uremic syndrome. Kidney Int. 1998;54:1324–1330. doi: 10.1046/j.1523-1755.1998.00103.x. [DOI] [PubMed] [Google Scholar]
- 34.Chandler WL, Jelacic S, Boster DR, et al. Prothrombotic coagulation abnormalities preceding the hemolytic-uremic syndrome. The New England Journal of Medicine. 2002;346:23–32. doi: 10.1056/NEJMoa011033. [DOI] [PubMed] [Google Scholar]
- 35.Karpman D, Papadopoulou D, Nilsson K, et al. Platelet activation by Shiga toxin and circulatory factors as a pathogenetic mechanism in the hemolytic uremic syndrome. Blood. 2001;97:3100–3108. doi: 10.1182/blood.v97.10.3100. [DOI] [PubMed] [Google Scholar]
- 36.Zoja C, Angioletti S, Donadelli R, et al. Shiga toxin-2 triggers endothelial leukocyte adhesion and transmigration via NF-kappaB dependent up-regulation of IL-8 and MCP-1. Kidney Int. 2002;62:846–856. doi: 10.1046/j.1523-1755.2002.00503.x. [DOI] [PubMed] [Google Scholar]
- 37.Nestoridi E, Tsukurov O, Kushak RI, et al. Shiga toxin enhances functional tissue factor on human glomerular endothelial cells: implications for the pathophysiology of hemolytic uremic syndrome. J Thromb Haemost. 2005;3:752–762. doi: 10.1111/j.1538-7836.2005.01205.x. [DOI] [PubMed] [Google Scholar]
- 38.Siegler RL, Pysher TJ, Tesh VL, et al. Prophylactic heparinization is ineffective in a primate model of hemolytic uremic syndrome. PediatrNephrol. 2002;17:1053–1058. doi: 10.1007/s00467-002-1002-3. [DOI] [PubMed] [Google Scholar]
- 39.Brandt JR, Fouser LS, Watkins SL, et al. Escherichia coli O 157: H7-associated hemolytic-uremic syndrome after ingestion of contaminated hamburgers. J Pediatr. 1994;125:519–526. doi: 10.1016/s0022-3476(94)70002-8. [DOI] [PubMed] [Google Scholar]
- 40.Exeni R, Donato H, Rendo P, et al. Low levels of serum erythropoietin in children with endemic hemolytic uremic syndrome. Pediatr Nephrol. 1998;12:226–230. doi: 10.1007/s004670050443. [DOI] [PubMed] [Google Scholar]
- 41.Pape L, Ahlenstiel T, Kreuzer M, et al. Early erythropoietin reduced the need for red blood cell transfusion in childhood hemolytic uremic syndrome—a randomized prospective pilot trial. Pediatric Nephrology. 2009;24:1061–1064. doi: 10.1007/s00467-008-1087-4. [DOI] [PubMed] [Google Scholar]
- 42.van 't Veer C, van den Pangaart PS, van Zoelen MA, et al. Induction of IRAK-M is associated with lipopolysaccharide tolerance in a human endotoxemia model. J Immunol. 2007;179:7110–7120. doi: 10.4049/jimmunol.179.10.7110. [DOI] [PubMed] [Google Scholar]
- 43.Kinasewitz GT, Chang AC, Peer GT, et al. Peritonitis in the baboon: a primate model which stimulates human sepsis. Shock. 2000;13:100–109. doi: 10.1097/00024382-200013020-00003. [DOI] [PubMed] [Google Scholar]
- 44.Lopez EL, Contrini MM, Devoto S, et al. Tumor necrosis factor concentrations in hemolytic uremic syndrome patients and children with bloody diarrhea in Argentina. Pediatr Infect Dis J. 1995;14:594–598. doi: 10.1097/00006454-199507000-00008. [DOI] [PubMed] [Google Scholar]
- 45.Rodriguez-Fandino O, Hernandez-Ruiz J, Schmulson M. From cytokines to toll-like receptors and beyond - current knowledge and future research needs in irritable bowel syndrome. J Neurogastroenterol Motil. 16:363–373. doi: 10.5056/jnm.2010.16.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simon M, Grone HJ, Johren O, et al. Expression of vascular endothelial growth factor and its receptors in human renal ontogenesis and in adult kidney. Am J Physiol. 1995;268:F240–F250. doi: 10.1152/ajprenal.1995.268.2.F240. [DOI] [PubMed] [Google Scholar]
- 47.Witmer AN, Dai J, Weich HA, et al. Expression of vascular endothelial growth factor receptors 1, 2, and 3 in quiescent endothelia. J Histochem Cytochem. 2002;50:767–777. doi: 10.1177/002215540205000603. [DOI] [PubMed] [Google Scholar]
- 48.Oakes RS, Siegler RL, McReynolds MA, et al. Predictors of fatality in postdiarrheal hemolytic uremic syndrome. Pediatrics. 2006;117:1656–1662. doi: 10.1542/peds.2005-0785. [DOI] [PubMed] [Google Scholar]
- 49.Paton AW, Srimanote P, Talbot UM, et al. A new family of potent AB(5) cytotoxins produced by Shiga toxigenic Escherichia coli. J Exp Med. 2004;200:35–46. doi: 10.1084/jem.20040392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmidt H, Scheef J, Huppertz HI, et al. Escherichia coli O157: H7 and O157: H(-) strains that do not produce Shiga toxin: phenotypic and genetic characterization of isolates associated with diarrhea and hemolytic-uremic syndrome. J Clin Microbiol. 1999;37:3491–3496. doi: 10.1128/jcm.37.11.3491-3496.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]