Abstract
Introduction
Outcomes are suboptimal in ulcerative colitis (UC). Telemedicine for UC is feasible and improves outcomes. Our goals were to evaluate a home telemanagement system for UC (UC HAT) on disease activity, quality of life (QoL), and adherence compared to best available care (BAC) in a randomized, controlled trial.
Materials and Methods
Adults with UC were randomly assigned to receive UC HAT or BAC for 12 months. UC HAT recruits answered questions regarding disease activity, adherence, side effects, and measured their weight weekly. An educational curriculum was delivered after each session. Alerts and action plans were generated based on the results. BAC underwent routine follow up, received written action plans and were given educational fact sheets. Seo Index scores, IBDQ scores, and adherence rates were compared between UC HAT and BAC at one year.
Results
25 patients were randomized to UC HAT and 22 to BAC. After 12 months, 11 withdrew in UC HAT compared to 5 in BAC. Disease activity, QoL, and adherence were not different between groups at any time point post baseline. Adjusted analyses of trial completers using all available data, demonstrated decreased Seo index (11.9 in UC HAT (p=0.08) vs. 1.2 in BAC (p=0.84) and increased IBDQ scores (12.5 in UC HAT (p=0.04) vs. to −3.8 in BAC (p=0.47) from baseline in UC HAT compared to BAC.
Discussion
UC HAT did not improve disease activity, QoL or adherence compared to BAC after 1 year. After adjustment for baseline disease knowledge, UC HAT trial completers experienced significant gains in disease-specific quality of life from baseline compared to BAC trial completers. Our results suggest a potential benefit of UC HAT. Further research is indicated to determine if telemedicine improves outcomes in patients with IBD.
Keywords: Ulcerative colitis, telemedicine, quality of life, adherence
INTRODUCTION
Inflammatory bowel disease (IBD), comprising ulcerative colitis (UC) and Crohn’s disease, is a chronic inflammatory condition of the intestines affecting over 1,000,000 Americans1. Effective medical therapies exist to treat the disease; however chronic therapy is required to prevent relapses. Poor adherence to medications is one barrier to successful treatment as only 40% of patients are adherent to therapy; nonadherent patients are five times more likely to experience a flare of disease2. Inadequate monitoring of symptoms is another important factor in poor outcomes. Improved methods to monitor patients with UC are needed to improve outcomes.
Although not routinely applied to chronic gastrointestinal illnesses such as IBD, telemedicine has been applied to chronic conditions similar to IBD. A home telemanagement system in asthma patients, similar to the one used in this study, resulted in greater adherence with self-action plans3, improved quality of life and patient knowledge, and decreased urgent care visits4. In a follow up study, significant improvements were noted in asthma symptoms, spirometry, and in decreased use of quick relief inhalers4. In diabetes, several studies demonstrated reduced glycosylated hemoglobin levels after use of telemedicine systems5–10. In congestive heart failure, telemedicine improved quality of life, decreased hospitalizations, and decreased hospitalization costs in the telemedicine group11–13.
Few published studies have evaluated the impact of telemedicine in IBD. Twenty one patients with mild to moderate UC reported the ability to initiate a self-care plan and experienced improvements in knowledge after interaction with a web-based treatment program and patient education center14. Preliminary studies by our group of a telemanagement system were viewed positively by participants15,16. Furthermore, when our group evaluated a telemanagement system in a 6-month trial in patients with IBD, adherence to self-testing was over 90% and patient acceptance of the telemanagement system was high. In addition, improvements were noted in disease activity, quality of life, and patient knowledge compared to baseline17,18. A randomized, controlled trial of patients with mild to moderate UC from Europe showed that a self-administered, web-based e-health treatment program improved adherence, quality of life, decreased duration of relapse, and decreased the number of outpatient visits compared to standard care19.
In this study we evaluate the clinical impact of home telemanagement in UC (UC HAT) using a randomized controlled trial design. We hypothesized that UC HAT would improve disease activity and disease-specific quality of life scores compared to best available care (BAC) through improved monitoring, medical adherence, and participant knowledge. Our objectives were to evaluate the impact of UC HAT on disease activity, disease-specific quality of life, and medical adherence compared to BAC over a one year time period.
MATERIALS AND METHODS
Study Design
The study was a randomized, controlled trial conducted to assess the effectiveness of UC HAT compared to BAC from 11/28/2007 until 2/3/2010.
Setting
Adult patients with UC from the University of Maryland, Baltimore and the gastroenterology clinic of the VAMHCS, Baltimore were eligible to participate. All participants underwent visits every 4 months for one year. All study visits took place in the University of Maryland, Baltimore General Clinical Research Center. Study questionnaires and blood draws for measurement of albumin, sedimentation rate, and hemoglobin were done at each study visit.
Patients
All eligible patients from the University of Maryland, Baltimore and the VAMHCS, Baltimore were sent a letter inviting them to participate in the study. Patients were also invited to participate at the time of clinic visits. Assignment to the experimental intervention was made using a random permuted block design with randomly varied block sizes; randomization was stratified within baseline disease activity strata (disease in remission vs. active disease). Group assignment was concealed and was not revealed to the patient or the research team members until after all baseline data were collected. Research staff at study visits was blinded to treatment allocation of research participants for subsequent visits.
Interventions
Experimental intervention
The design, technical features, and capabilities of UC HAT have been described previously16. UC HAT is comprised of a home unit, a decision support server and a web-based clinician portal (Figure 1). The UC HAT home unit consists of a netbook computer and an electronic weight scale. Participants answer questions regarding symptoms, side effects, adherence, and receive disease-specific education using the home unit. The home unit automatically transmits the results to the decision support server after each self-testing session through an active telephone line; for participants without an active telephone line, a cell phone is provided to transmit self-testing results over a secure wireless network in a similar manner. Data transmitted from the participant’s home are de-identified and encrypted. The web portal provides an interface for the collected participant data. The web-based care management portal is used to set up customized clinical alerts and action plans. Updated action plans are automatically transmitted to participant home units. If certain clinical conditions are met, email alerts are sent to the nurse coordinator. The coordinator reviews the information and if necessary consults the medical provider and the participant for management changes.
Figure 1.
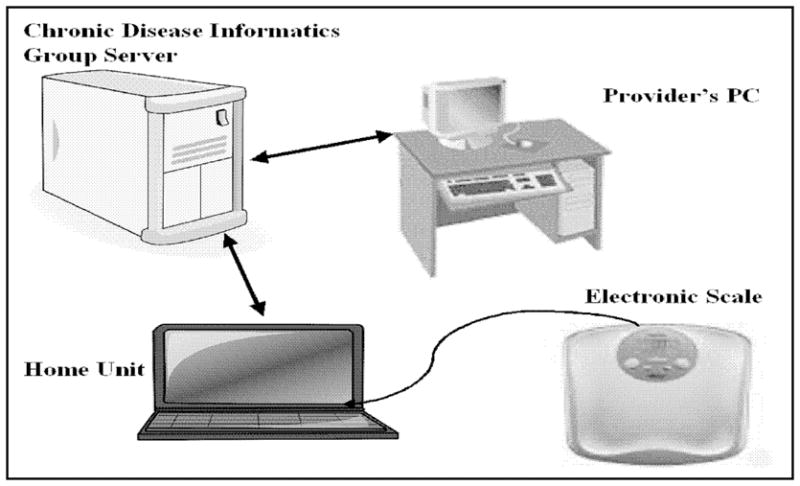
The Home Telemanagement System for Patients with Ulcerative Colitis (UC HAT). UC HAT is comprised of a home unit (laptop computer and electronic weight scale) and web portal.
UC HAT Home Unit
After turning on the laptop, participants are prompted to respond to a series of simple questions about their UC symptoms over the past week (UC Symptom Diary). The symptom diary consists of 15 questions that assess the participant’s overall well-being, bowel symptoms, systemic symptoms, and extraintestinal manifestations. After completing the symptom diary, participants are asked a series of questions about side effects and the number of doses of medications that they took over the past week. Participants receive an audio prompt with instructions on how to use the electronic scale. An educational curriculum was developed for patients from materials provided by the Crohn’s and Colitis Foundation of America. Participants can send an electronic message to the nurse coordinator at any time by accessing the messaging menu of the home unit. At the completion of self-testing, the participant receives a customized action plan based on responses to the symptom diary (See Table 1).
Table 1.
Example of YELLOW zone action plan for participants with ulcerative colitis in the home telemanagement for patients with ulcerative colitis trial
| Yellow Zone | Symptoms | Actions |
|---|---|---|
| Moderate Symptoms | Overall health poor | Continue your current medications; it can take a few weeks to take effect |
| 4–6 BM’s/day | Take one Canasa suppository nightly | |
| 1–3 nocturnal awakenings | Take one Rowasa enema nightly | |
| More than trace blood in stool | Double the dose of your mesalamine | |
| Start prednisone 20 mg daily | ||
| Call our office to schedule Remicade | ||
| Call our office |
Participants randomized to the experimental intervention arm were required to complete self-testing with the UC HAT weekly.
Control Group
The standard of care for participants in this study is modeled after the standard of care at our institution, and based on current evidence-based guidelines including comprehensive assessment, a guideline-concordant therapy plan, scheduled and as needed clinic visits, scheduled and as needed telephone calls, and administration of educational fact sheets about disease-specific topics when appropriate. We expanded the care received by controls to make the groups more comparable. First, we provided the control group with all currently available educational fact sheets from the Crohn’s and Colitis Foundation at the time of group allocation. Second, we provided the control group with individualized written action plans at the time of group assignment without reinforcement. We termed the care given in the control intervention group as best available care (BAC).
Main Outcome Measures
Assessment of Clinical Disease Activity
Clinical disease activity was assessed using the Seo index20. An activity index <120 represents clinical remission, whereas scores of 121–150, 151–220, and >221 correlate with mild, moderate, and severe disease respectively. The Seo index is sensitive to change, with a decrease in the index of 35 correlating with a clinical response21.
Assessment of Disease-Specific Quality of Life
Disease-specific quality of life was assessed using the IBD questionnaire (IBDQ). Scores for the IBDQ range from 32 to 224 with higher scores being associated with better quality of life22. Score changes of 16 have been found to be significant changes when compared to baseline values23.
Assessment of Medication Adherence
Adherence was assessed using the Morisky Medication Adherence Score24, a 4 item survey in which participants self-report medication-taking behavior. This scale has been validated in patients with hypertension24 and has been used by our group in patients with IBD18. Each question that is answered with a No receives a score of 1. The possible scoring range is therefore 0 to 4. Higher scores correlate with better medical adherence. For the purpose of evaluating percent of participants adherent to therapy, the variable was dichotomized to “Adherent” or “Non-adherent”. Any response of Yes to one of the 4 items was scored as “Non-Adherent”.
Assessment of Intervening and Confounding Variables
The role of various confounding variables on the impact of UC HAT on clinical outcomes was also explored. Variables considered included age, gender, race, extent of disease, duration of disease, site, educational level, and disease knowledge. Disease knowledge was assessed using the Crohn’s and Colitis Knowledge Score25.
Primary Endpoints
The primary outcome variables were the difference in Seo index scores, IBDQ scores and medical adherence rates between the UC HAT and BAC groups at 12 months.
Statistical analyses
Sample size
Sample size calculations were performed for each primary outcome measure. All sample size estimates were performed assuming a Type 1 error rate of 5%, a type 2 error rate of 20%, and an attrition rate of 10%18. Assuming a standard deviation of 39.420 in Seo Index scores, a standard deviation of 3426 in IBDQ scores, and a baseline adherence rate of 50% in the BAC arm, we had sufficient power with a sample size of 84 participants to detect a 35-point difference in Seo scores, a 32-point difference in IBDQ scores, and a 30% difference in adherence rates.
Statistical Methods
To assess the impact of UC HAT on disease activity and disease-specific quality of life, we compared the differences in Seo index and IBDQ scores between the UC HAT and BAC groups at 12 months using a t-test. We also compared the difference in change in Seo index and IBDQ scores from baseline to 12 months between the two groups using a t-test. Comparisons of the UC HAT and BAC groups with all available data across the four time points were made using repeated measures mixed model regression to account for significant differences in baseline clinical characteristics or confounding variables. The difference in the proportion of patients in remission and adherent to therapy at 12 months was compared between the UC HAT and BAC group using the chi square test.
All analyses were performed using the intention-to-treat principle. In sensitivity analyses, missing data were imputed with either the last observation carried forward or the first observation carried forward; results of the imputations were compared with results from the repeated measures mixed model analyses using all available data. All tests of significance were two sided with a p-value < 0.05.
ETHICAL CONSIDERATIONS
The study was approved by the Human Research Protections Office at the University of Maryland School of Medicine and the Research and Development Office of the Veterans Affairs Maryland Health Care System Baltimore. All participants signed informed consent before entry into the study.
RESULTS
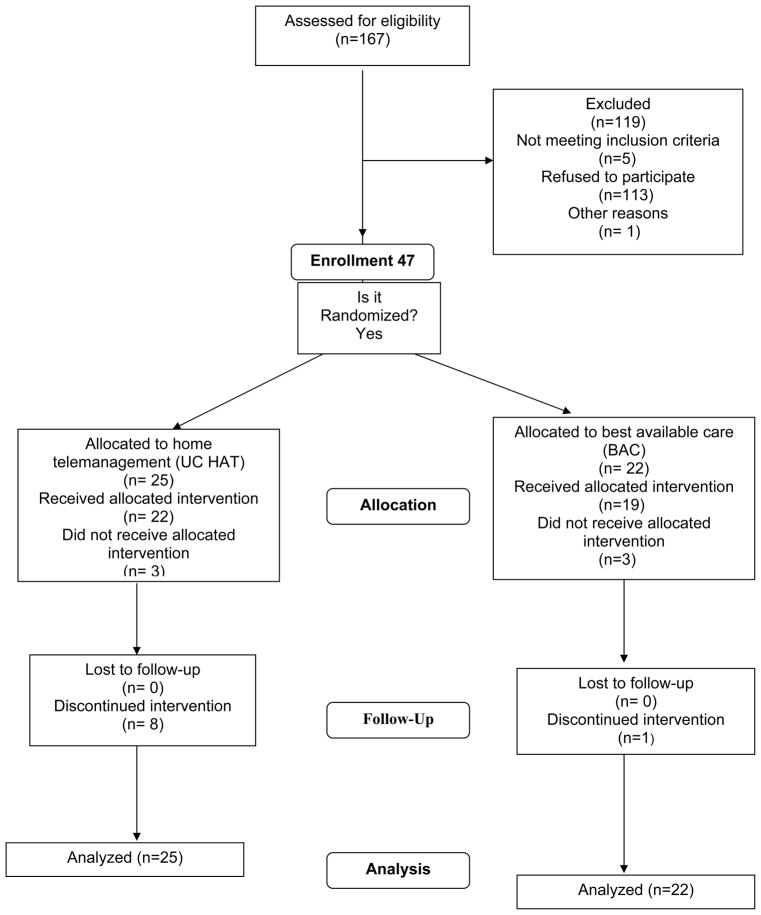
Participant Flow
The flow of participants through the trial is shown in Figure 2. 22 participants were randomized to BAC and 25 to UC HAT. Three participants in each group withdrew after the baseline visit; thus three patients never received the UC HAT intervention. During the course of the trial, 8 participants withdrew in UC HAT compared to 1 in BAC. There were no significant differences between trial completers and participants that withdrew except that trials completers had less extensive colitis (data not shown).
Figure 2.
Participant Flow in the Home Telemanagement for Patients with Ulcerative Colitis Trial
Demographics of Study Population
The demographics of the study population are listed in Table 2. 64% (n=30) of participants were female and 66% (n=31) were Caucasian. 53% (n=25) had pancolitis. There were no significant differences between the study groups, except that 27% (n=6) of participants in BAC used immune suppressants compared to 56% (n=14) in UC HAT (p=0.05). 32% (n=7) of BAC used infliximab compared to 28% (n=7) of UC HAT (p=0.78).
Table 2.
Demographics of Participants of the Home Telemanagement for Patients with Ulcerative Colitis Trial
| Variable | Overall (n=47) | BAC (n=22) | UC HAT (n=25) | p value† |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Age (years+/− SD)* | 41.1+/−14.0 | 40.3+/−14.4 | 41.7+/−13.9 | 0.74 |
| Gender | ||||
| Male | 17 (36) | 7 (32) | 10 (40) | 0.56 |
| Female | 30 (64) | 15 (68) | 15 (60) | |
| Race | ||||
| White | 31 (66) | 15 (68) | 16 (64) | 0.76 |
| Other | 16 (34) | 7 (32) | 9 (36) | |
| Smoking Status | ||||
| Never | 31 (66) | 16 (73) | 15 (60 | |
| Former | 15 (32) | 5 (23) | 10 (40) | |
| Current | 1 (2) | 1 (4) | 0 (0) | 0.28 |
| Disease Extent | ||||
| Proctitis/Left | 22 (47) | 10 (45) | 12 (48) | |
| Sided Pancolitis | 25 (53) | 12 (55) | 13 (52) | |
| Steroid Use | ||||
| Yes | 5 (11) | 2 (9) | 3 (12) | 0.75 |
| No | 42 (89) | 20 (91) | 22 (88) | |
| Immune Suppressant Use | ||||
| Yes | 20 (43) | 6 (27) | 14 (56) | 0.05 |
| No | 27 (57) | 16 (73) | 11 (44) | |
| Infliximab Use | ||||
| Yes | 14 (30) | 7 (32) | 7 (28) | 0.78 |
| No | 33 (70) | 15 (68) | 18 (72) | |
| Disease Knowledge | ||||
| Limited | 7 (15) | 3 (14) | 4 (16) | |
| Good | 30 (64) | 15 (68) | 15 (60) | 0.83 |
| Excellent | 10 (21) | 4 (18) | 6 (24) | |
t-test comparing BAC to UC HAT group
χ2 test of association comparing BAC to UC HAT group
Comparison of Disease Activity between Groups
At baseline, Seo index scores were 115.1+/−21.5 in BAC compared to 127+/−42.3 in UC HAT (p=0.24). At 12 months, Seo Index scores were 113.6+/−28.0 in BAC compared to 122.0+/−39.3 in UC HAT (p=0.41). There were no differences in Seo index scores at 4 and 8 months between groups. Sixty eight and sixty percent of BAC and UC HAT participants were in remission at baseline (p=0.56); remission rates increased to 77% and 76% respectively at 12 months (p=0.92). Remission rates were not different between the groups at 4 and 8 months. An analysis of all available data did not show significant differences between the groups at any time point. However, after adjustment for baseline quality of life, Seo Index scores at 12 months decreased 11.9+/−6.6 points from baseline (p=0.08) compared to 1.2+/−6.0 in BAC (p=0.84).
Comparison of Disease-Specific Quality of Life between Groups during Trial
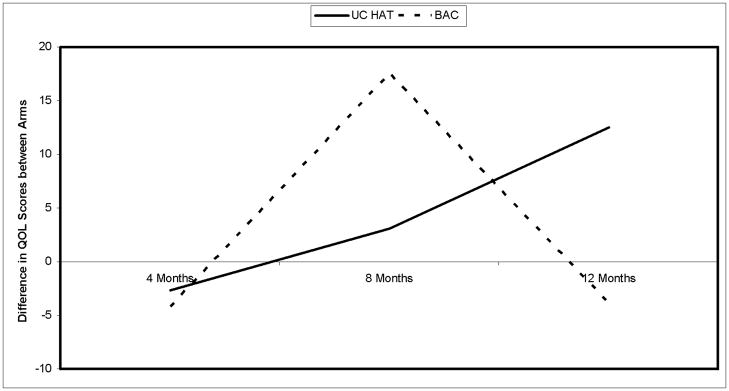
IBDQ scores were significantly higher at baseline in BAC, 190.8+/−24.2 compared to 171.6+/−30.1 in UC HAT (p=0.02). IBDQ scores increased in UC HAT and remained stable in BAC; however the differences were not significant at any time point post baseline between groups (4 months: UC HAT 178.7+/−28.1, BAC 183.1+/−43.7; 8 months: UC HAT 178.8+/−28.1, BAC 186.1+/−42.0; 12 months: UC HAT 178.1+/−32.1, BAC 187.3+/−32.2. Completer analyses demonstrated similar findings (data not shown). When changes from baseline in IBDQ scores were compared, adjusted for baseline disease knowledge using all available data, IBDQ scores improved 12.5+/−5.9 points in UC HAT (p=0.04) compared to −3.8+/−5.3 in BAC (p=0.47). The difference in IBDQ scores at 12 months from baseline between groups was 16.3+/−7.9 (p=0.04) (see Figure 3).
Figure 3.
Difference in IBDQ Scores from baseline to the 12 month visit between the UC HAT and BAC Arms in the Home Telemanagement for Patients with Ulcerative Colitis Trial. UC HAT=home telemanagement, BAC=Best Available Care. Groups were compared using repeated measures linear regression. Analyses shown are per protocol analyses.
Comparison of Adherence Rates between Groups
At baseline, adherence was 45 and 40% for BAC and UC HAT respectively (p=0.71). At 12 months, 68% and 44% of BAC and UC HAT participants were adherent respectively (p=0.10). There was no difference between groups in adherence rates at 4 and 8 months. Completer analyses revealed higher adherence rates in the UC HAT group at 12 months compared to the intention to treat analysis (BAC 67%, UC HAT 57%, p=0.26).
DISCUSSION
Overall, we found a higher rate of attrition in UC HAT compared to BAC. Intention to treat analyses did not demonstrate improved disease activity, disease-specific quality of life, or adherence in UC HAT compared to BAC. However, at baseline immune suppressant use was twice as high and IBDQ scores were lower in UC HAT compared to BAC. After adjustment for baseline IBDQ scores, trial completers in the UC HAT arm had decreased disease activity scores at 12 months compared to baseline. After adjustment for baseline disease knowledge, UC HAT trial completers experienced significant gains in disease-specific quality of life from baseline compared to BAC trial completers.
There are several possible explanations for our findings. First, our study was powered to detect moderate differences in disease activity, quality of life, and adherence rates. Unfortunately, we were not able to recruit the number of participants needed to detect a moderate effect size in adherence rates between groups. However, we did have sufficient power to detect moderate differences in disease activity and disease-specific quality of life between groups. In addition, we experienced higher than expected attrition rates which further hampered our ability to detect moderate differences for all outcome measures. Furthermore, despite the randomization process, significant differences were present in the groups at baseline. Notably, UC HAT participants had higher use of immune suppressants and had lower baseline IBDQ scores. These results suggest that UC HAT participants had more severe disease at baseline than BAC participants. This may explain why analyses of trial completers adjusted for baseline differences between the groups demonstrated improvement in Seo Index and IBDQ scores from baseline in the UC HAT arm.
Our trial also had higher rates of attrition in the UC HAT arm than expected. There were several reasons for participants withdrawing in the UC HAT arm. Four participants were withdrawn by the research team because they were nonadherent with weekly self-testing. Two participants withdrew because they changed their mind about participating, one patient moved out of the country, and one patient developed severe depression unrelated to the testing and was withdrawn from the study. Participants who continued to use UC HAT experienced significant improvements in quality of life over time. It is not clear if patients will use a system like UC HAT long term. It is plausible that a variation of UC HAT that does not require home installation and that can be accessed via the web may be more successful for increasing recruitment and in retaining participants in the study. A recently published study from Europe reported an attrition rate of only 24% at one year in UC participants that used a web-based telemedicine system; interestingly attrition rates were higher in the telemedicine arm in this study also19. In addition, decreasing the frequency of self-testing may also improve participant retention and adherence with self-testing. Another reason for lack of improvement in clinical outcomes between groups could be that participants seemed to experience little variability in their disease course during the trial. Seo index and IBDQ scores during the trial were consistent with patients in clinical remission. This is likely a result of our inclusion criteria, as we did not require patients to have active disease at baseline. Therefore, it is possible that if we recruited patients with active disease and/or those with shorter periods of remission at baseline, we might have seen more variability in Seo and IBDQ scores and differences in clinical outcomes. Conversely, the low Seo index and high IBDQ scores throughout the study could also be a reflection of care provided by referral centers for IBD. Thus, it is possible that UC HAT is less effective when combined with specialty IBD care as opposed to when it is applied in community practice. It is also possible that UC HAT would be more effective in a homogenous UC population with less severe disease. For example, a web-based telemedicine system in European patients with UC demonstrated improved outcomes. These patients all had mild to moderate disease and were only on aminosalicylates (5-ASA) at baseline. Including UC patients with mild to moderate disease on 5-ASA only allowed providers in this study to utilize a single, rapid treatment approach (high dose 5-ASA) for most flares19. Despite these explanations, it is possible that UC HAT does not improve outcomes in patients with UC.
To our knowledge, our trial is the second randomized, controlled trial to assess the impact of a telemedicine system on clinical outcomes in patients with UC. Our study has several strengths. First, the study was a randomized, controlled trial. Second, we concealed allocation to groups, in that researchers were masked to group assignment at the time of study visits. Third, to make our results generalizable, we included a subset of patients from the VA as they tend to have less severe disease and are more representative of a community practice of UC patients. Fourth, we gave action plans and an education curriculum to controls so that we could evaluate the impact of the interaction between participants and UC HAT and the importance of frequent monitoring and prompting. By creating a BAC arm, we could potentially avoid criticism that the success of the system was solely due to use of action plans or the educational curriculum. There were several weaknesses to our study. First, we were underpowered to detect small to moderate differences in outcomes between groups. Second, we included patients in remission for varying intervals in the study. Including patients with long-term remission may have biased our results to the null as these patients experience little variability over time. Third, participants were not masked to their group assignments. We discussed giving all patients UC HAT but only monitoring the “intervention” group. However, it is likely that participants over time would have recognized that they were not being monitored. It is possible that participants in the UC HAT arm responded to disease activity and quality of life indices differently at study visits because of a bias towards a perceived benefit of UC HAT. If this is true, are results would be biased away from the null hypothesis. Our participants had high rates of immune suppressant and infliximab use consistent with a tertiary referral population. Therefore, our findings are probably not generalizable to the entire community with UC.
In summary, we did not demonstrate significant improvements in disease activity, quality of life, or adherence in participants using UC HAT. However, UC HAT trial completers experienced improvements in Seo index and IBDQ score from baseline compared to BAC trial completers after adjustment for baseline quality of life scores and knowledge scores respectively. Our results are suggestive that telemedicine may improve outcomes in patients with UC. Newer telemedicine systems that do not require home installation and that can be accessed from anywhere via the web need to be developed to enhance ease of use, as well as participant recruitment and retention. Larger studies are needed to demonstrate small to moderate differences in disease outcomes in IBD. Further, consideration of length of time in remission and the mix of community and referral patients will need to be addressed for future trials.
Acknowledgments
This study was sponsored by the Broad Medical Research Program (BRMP-0190), University of Maryland General Clinical Research Center Grant (M01 RR 16500), General Clinical Research Centers Program, National Center for Research Resources (NCRR), NIH, and the Baltimore Education and Research Foundation. The authors would like to thank Ashley Hanahan, Kathryn Lambert, and Allison Steele for their assistance with monitoring UC HAT participants during the study. We would also like to thank Darryn Potosky and Sandra Quezada for their assistance in developing the Educational Curriculum for IBD.
ABBREVIATIONS
- UC
Ulcerative colitis
- IBD
inflammatory bowel disease
- UC HAT
home telemanagement system for ulcerative colitis
- QoL
quality of life
- BAC
best available care
- IBDQ
Inflammatory Bowel Disease Questionnaire
References
- 1.Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004 May;126(6):1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 2.Kane S, Huo D, Aikens J, Hanauer S. Medication nonadherence and the outcomes of patients with quiescent ulcerative colitis. Am J Med. 2003 Jan;114(1):39–43. doi: 10.1016/s0002-9343(02)01383-9. [DOI] [PubMed] [Google Scholar]
- 3.Finkelstein J, O'Connor G, Friedman RH. Development and implementation of the home asthma telemonitoring (HAT) system to facilitate asthma self-care. Medinfo. 2001;10(Pt 1):810–814. [PubMed] [Google Scholar]
- 4.Joshi A, Amelung P, Arora M, Finkelstein J. Clinical impact of home automated telemanagement in asthma. AMIA Annu Symp Proc. 2005:1000. [PMC free article] [PubMed] [Google Scholar]
- 5.Hee-Sung K. Impact of Web-based nurse's education on glycosylated haemoglobin in type 2 diabetic patients. J Clin Nurs. 2007 Jul;16(7):1361–1366. doi: 10.1111/j.1365-2702.2007.01506.x. [DOI] [PubMed] [Google Scholar]
- 6.Montori VM, Helgemoe PK, Guyatt GH, et al. Telecare for patients with type 1 diabetes and inadequate glycemic control: a randomized controlled trial and meta-analysis. Diabetes Care. 2004 May;27(5):1088–1094. doi: 10.2337/diacare.27.5.1088. [DOI] [PubMed] [Google Scholar]
- 7.Shea S, Weinstock RS, Starren J, et al. A randomized trial comparing telemedicine case management with usual care in older, ethnically diverse, medically underserved patients with diabetes mellitus. J Am Med Inform Assoc. 2006 Jan-Feb;13(1):40–51. doi: 10.1197/jamia.M1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dang S, Ma F, Nedd N, Florez H, Aguilar E, Roos BA. Care coordination and telemedicine improves glycaemic control in ethnically diverse veterans with diabetes. J Telemed Telecare. 2007;13(5):263–267. doi: 10.1258/135763307781458958. [DOI] [PubMed] [Google Scholar]
- 9.Quinn CC, Clough SS, Minor JM, Lender D, Okafor MC, Gruber-Baldini A. WellDoc mobile diabetes management randomized controlled trial: change in clinical and behavioral outcomes and patient and physician satisfaction. Diabetes Technol Ther. 2008 Jun;10(3):160–168. doi: 10.1089/dia.2008.0283. [DOI] [PubMed] [Google Scholar]
- 10.Quinn CC, Gruber-Baldini AL, Shardell M, et al. Mobile diabetes intervention study: testing a personalized treatment/behavioral communication intervention for blood glucose control. Contemp Clin Trials. 2009 Jul;30(4):334–346. doi: 10.1016/j.cct.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Benatar D, Bondmass M, Ghitelman J, Avitall B. Outcomes of chronic heart failure. Arch Intern Med. 2003 Feb 10;163(3):347–352. doi: 10.1001/archinte.163.3.347. [DOI] [PubMed] [Google Scholar]
- 12.Roth A, Kajiloti I, Elkayam I, Sander J, Kehati M, Golovner M. Telecardiology for patients with chronic heart failure: the 'SHL' experience in Israel. Int J Cardiol. 2004 Oct;97(1):49–55. doi: 10.1016/j.ijcard.2003.07.030. [DOI] [PubMed] [Google Scholar]
- 13.Schofield RS, Kline SE, Schmalfuss CM, et al. Early outcomes of a care coordination-enhanced telehome care program for elderly veterans with chronic heart failure. Telemed J E Health. 2005 Feb;11(1):20–27. doi: 10.1089/tmj.2005.11.20. [DOI] [PubMed] [Google Scholar]
- 14.Elkjaer M, Burisch J, Avnstrom S, Lynge E, Munkholm P. Development of a Web-based concept for patients with ulcerative colitis and 5-aminosalicylic acid treatment. Eur J Gastroenterol Hepatol. 2009 Jun 18; doi: 10.1097/MEG.0b013e32832e0a18. [DOI] [PubMed] [Google Scholar]
- 15.Cross RK, Arora M, Finkelstein J. Acceptance of telemanagement is high in patients with inflammatory bowel disease. J Clin Gastroenterol. 2006 Mar;40(3):200–208. doi: 10.1097/00004836-200603000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Cross RK, Cheevers N, Finkelstein J. Home telemanagement for patients with ulcerative colitis (UC HAT) Dig Dis Sci. 2009 Nov;54(11):2463–2472. doi: 10.1007/s10620-008-0640-0. [DOI] [PubMed] [Google Scholar]
- 17.Castro HK, Cross RK, Finkelstein J. Using a Home Automated Telemanagement (HAT) System: Experiences and Perceptions of Patients with Inflammatory Bowel Disease. AMIA Annu Symp Proc. 2006:872. [PMC free article] [PubMed] [Google Scholar]
- 18.Cross RK, Finkelstein J. Feasibility and acceptance of a home telemanagement system in patients with inflammatory bowel disease: a 6-month pilot study. Dig Dis Sci. 2007 Feb;52(2):357–364. doi: 10.1007/s10620-006-9523-4. [DOI] [PubMed] [Google Scholar]
- 19.Elkjaer M, Shuhaibar M, Burisch J, et al. E-health empowers patients with ulcerative colitis: a randomised controlled trial of the web-guided 'Constant-care' approach. Gut. 2010 Dec;59(12):1652–1661. doi: 10.1136/gut.2010.220160. [DOI] [PubMed] [Google Scholar]
- 20.Seo M, Okada M, Yao T, Ueki M, Arima S, Okumura M. An index of disease activity in patients with ulcerative colitis. Am J Gastroenterol. 1992 Aug;87(8):971–976. [PubMed] [Google Scholar]
- 21.Seo M, Okada M, Yao T, Okabe N, Maeda K, Oh K. Evaluation of disease activity in patients with moderately active ulcerative colitis: comparisons between a new activity index and Truelove and Witts' classification. Am J Gastroenterol. 1995 Oct;90(10):1759–1763. [PubMed] [Google Scholar]
- 22.Guyatt G, Mitchell A, Irvine EJ, et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology. 1989 Mar;96(3):804–810. [PubMed] [Google Scholar]
- 23.Powell-Tuck J, Bown RL, Lennard-Jones JE. A comparison of oral prednisolone given as single or multiple daily doses for active proctocolitis. Scand J Gastroenterol. 1978;13(7):833–837. doi: 10.3109/00365527809182199. [DOI] [PubMed] [Google Scholar]
- 24.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986 Jan;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Eaden JA, Abrams K, Mayberry JF. The Crohn's and Colitis Knowledge Score: a test for measuring patient knowledge in inflammatory bowel disease. Am J Gastroenterol. 1999 Dec;94(12):3560–3566. doi: 10.1111/j.1572-0241.1999.01536.x. [DOI] [PubMed] [Google Scholar]
- 26.Feagan BG, Reinisch W, Rutgeerts P, et al. The Effects of Infliximab Therapy on Health-Related Quality of Life in Ulcerative Colitis Patients. Am J Gastroenterol. 2007 Feb 22; doi: 10.1111/j.1572-0241.2007.01094.x. [DOI] [PubMed] [Google Scholar]