Abstract
The concept of “pharmacogenomics” or “pharmacogenetics” promises to offer the ultimate in personalized medicine, and the renin-angiotensin system (RAS) is one of the most plausible candidates for the application of this approach in the area of hypertension. For the past two decades, genetic variants of the RAS have been tested for association with blood pressure response, but the results have been inconsistent. The problems have been attributed to many issues, but the most fundamental concern is thought to be the statistical power of the studies. Therefore, we have tried to put together a new systematic review using a database search including only recent reports with adequate numbers of subjects, and 11 reports were identified. From the results, we were able to draw conclusions with nearly consistent findings that the conventional genetic variants of the system (i.e., the ACE I/D, AGT M235T, AT1 A1166C, and AT2 variant) are not associated with antihypertensive effects by RAS blockade, at least by one individual SNP. By contrast, significant associations have been reported (by one report each) for AGT rs7079, AT1 haplotype, REN, and ACE2. For these variants, further evaluations and confirmation are anticipated.
Keywords: Renin, Renin-angiotensin system, RAS, Genetic variant, Polymorphism, Pharmacogenomics, Pharmacogenetics, Angiotensin-converting enzyme inhibitor, ACEI, Angiotensin receptor blocker, ARB, Direct renin inhibitor, Blood pressure response, Responder
Introduction
One of the most intriguing issues for the next stage of the post-genome era is “pharmacogenomics” or “pharmacogenetics,” a concept that offers the ultimate personalized medicine, by which favorable effects of medications are maximized and adverse effects are minimized, compared with conventional “trial and error” prescribing. This type of “tailored” medicine is coming to be expected. In the area of hypertension, the renin-angiotensin system (RAS) is the most plausible candidate for pharmacogenomics. The RAS plays major roles in blood pressure regulation and electrolyte metabolism [1] and also has pivotal roles in cardiovascular [2], renal [3], and metabolic conditions [4]. During almost two decades, genetic variants of this system have been tested for their association with hypertension and cardiovascular conditions. Conventionally (with controversy), angiotensin-converting enzyme (ACE) insertion/deletion polymorphism has been associated with ischemic heart disease [5] and the development of end-stage renal diseases [6–9]. Angiotensinogen (AGT) M235T has been associated with the development of hypertension [10, 11]. Angiotensin II type 1 receptor (AT1) A1166C has been associated with the development of hypertension and ischemic heart disease [12, 13]. Recently, a number of large-scale prospective studies have proven that RAS blockade has favorable effects on cardiovascular and renal conditions [14–19]. However, the association between genetic variants of the RAS and the effects of angiotensin II receptor blockers (ARBs) and ACE inhibitors was still unclear in a 2007 systematic review by Farahani et al. [20]. The uncertainty has been attributed to many issues, but the most fundamental concern is thought to be lack of statistical power. Therefore, we have tried to produce an updated systematic review, using a database search for the latest reports with adequate numbers of subjects.
Identification of Studies
This review focuses on the genetic variants of the RAS and blood pressure response to RAS blockade. Studies were identified by an initial PubMed search with condition 1 ((“pharmacogenetics”[All Fields] OR “pharmacogenomics”[All Fields]) AND (“renin”[All Fields] OR “ACE”[All Fields] OR “angiotensinogen”[All Fields])) or condition 2 ((“genetic variant”[All Fields] OR “polymorphism”[All Fields]) AND (“blood pressure response”[All Fields] OR (“blood pressure responder”[All Fields]) AND “angiotensin”[All Fields]). Condition 1 identified 136 studies, and condition 2 found 52. From these studies (which included reviews), studies were identified that evaluated the genes for ACE, AGT, AT1, angiotensin II type 2 receptor (AT2), renin (REN), and angiotensin-converting enzyme 2 (ACE2). The evaluated RAS-blocking drugs were ARBs and ACE inhibitors.
Practical clinical implications require detection of a difference in diastolic blood pressure between groups of almost 5 mm Hg. Thus, we calculated the necessary sample size of the study, assuming a standard deviation for the diastolic blood pressure of 10 mm Hg by a bilateral paired Student’s t-test with protection against type I error of 5% and 80% of power, and determined that a study required 200 subjects in total to have adequate statistical power. We therefore adopted studies with about 200 or more subjects for assessment, finding the 11 studies shown in Table 1. Three of these studies tested ARBs and eight looked at ACE inhibitors.
Table 1.
Pharmacogenomics in the renin-angiotensin system (RAS): Studies of blood pressure response to RAS blockade among genetic variants
| Study | Year | Area | Ethnicity | Patients, N | Drug class | Drug | Genetic variant of the RAS | Significant difference? |
|---|---|---|---|---|---|---|---|---|
| Nordestgaard et al. [24••] | 2010 | Scandinavia | White (92%) | 1,774 | ARB | Losartan | ACE (I/D) | No |
| AGT (M235T) | No | |||||||
| AT1 (A1166C) | No | |||||||
| Konoshita et al. [25••] | 2009 | Japan | Japanese (100%) | 231 | ARB | Valsartan | ACE (I/D) | No |
| AGT (M235T) | No | |||||||
| AT1 (A1166C) | No | |||||||
| AT2 (C3123A) | No | |||||||
| REN (C-5312T) | Yes | |||||||
| Redon et al. [26] | 2005 | Spain | Spanish (100%)? | 206 | ARB | Telmisartan | ACE (I/D) | No |
| AGT (A-6G) | No | |||||||
| AT1 (A1166C, | No | |||||||
| C573T) | No | |||||||
| Yu et al. [33] | 2009 | China | Chinese (100%) | 509 | ACEI | Imidapril or benazepril | AT1 (A1166C) | No |
| Filigheddu et al. [27] | 2008 | Italy (North Sardinia) | White (100%) | 191 | ACEI | Fosinopril | ACE (I/D) | No |
| AGT (A-6G) | No | |||||||
| AT1 (A1166C) | No | |||||||
| Fan et al. [43] | 2007 | China | Chinese (100%) | 624 | ACEI | Captopril | ACE2 (rs2106809) | Yes |
| Brunner et al. [34] | 2007 | USA | White (35%) | 551 | ACEI | Trandolapril | AT1 (A1166C) | No |
| Hispanic (44%) | ||||||||
| Su et al. [32] | 2007 | China (Shanghai) | Chinese (100%) | 1,447 | ACEI | Benazepril | AGT (multi loci) | Yes |
| AT1 (multi loci) | Yes | |||||||
| AT2 (multi loci) | No | |||||||
| Arnett et al. [28] | 2005 | USA | White (61%) | 7,528 | ACEI | Lisinopril | ACE (I/D) | No |
| Black (35%) | ||||||||
| Yu et al. [31] | 2005 | China | Chinese (100%) | 501 | ACEI | Imidapril or benazepril | AGT (M235T) | No |
| Yu et al. [29] | 2003 | China | Chinese (100%) | 517 | ACEI | Imidapril or benazepril | ACE (I/D) | No |
ARB angiotensin receptor blocker; ACE angiotensin-converting enzyme, ACEI ACE inhibitor; ACE (I/D) insertion and deletion genetic variant of intron 16; AGT angiotensinogen; AT1 angiotensin II type 1 receptor; AT2 angiotensin II type 2 receptor; REN renin
ACE Gene Variant
The most evaluated gene in pharmacogenomics studies for the RAS is ACE, especially the insertion and deletion (I/D) genetic variant of intron 16. The first report, from Rigat et al. [21], indicated that serum ACE concentration differs according to the I/D allele numbers, and this phenomenon has been reconfirmed in many studies. Accordingly, the I/D variant is one of the most plausible candidates for pharmacogenomic RAS blockade intervention. Indeed, early studies showed significant differences of blood pressure reduction among the variants. A greater reduction by enalaprilat in II genotype compared with DD genotype was reported in 23 normotensive men [22], and a greater reduction by irbesartan in D allele compared with I allele was reported in 43 hypertensive patients [23]. However, as shown in Table 1, recent relatively well-powered studies have almost consistently shown no difference in blood pressure reduction in ACE genotypes (mainly the I/D variant) by ARBs [24••, 25••, 26] or ACE inhibitors [27–29]. Thus, we may conclude that the ACE I/D gene variant is not associated with antihypertensive effects from RAS blockade.
AGT Gene Variant
Another of the most evaluated genes in pharmacogenomics studies for the RAS is AGT, especially the M235T genetic variant. Evidence of genetic linkage between the AGT gene and hypertension, as well as association of AGT with the disease, has been observed, and significant differences in plasma concentrations of angiotensinogen were found among hypertensive subjects with different AGT genotypes [10]. Thus, the AGT variant is one of the most plausible candidates for pharmacogenomic RAS blockade intervention. Although one previous study of 125 cases reported that M235T was associated with lowered blood pressure in response to ACE inhibitors [30], the recent relatively well-powered studies in Table 1 almost consistently observed no difference in blood pressure reduction with M235T and A-6G (which is thought to be in linkage disequilibrium with M235T) by ARBs [24••, 25••, 26] or ACE inhibitors [27, 31]. Thus, it seems that we can conclude that the conventional AGT gene variant, M235T, is not associated with antihypertensive effects of RAS blockade.
Su et al. have reported that M235T is not associated with the antihypertensive effects of benazepril, but a new single nucleotide polymorphism (SNP), rs7079 (C11537A), is associated with blood pressure reduction in response to benazepril [32]. As this is the only report regarding rs7079 (C11537A) of AGT, further evaluations are expected.
AT1 Gene Variant
The AT1 genetic variant, especially A1166C, also has been widely evaluated as a candidate for pharmacogenomic study for the RAS. This variant originally was associated with hypertension and ischemic heart disease [12, 13]. Unlike ACE and AGT, it has no evident intermediate phenotype, such as blood concentrations, but the AT1 variant is nevertheless one of the most plausible pharmacogenomic candidates for RAS blockade intervention, as a major component of the system. As shown in the Table 1, recent relatively well-powered studies have consistently found no difference for A1166C in blood pressure reduction by ARBs [24••, 25••, 26] and ACE inhibitors [27, 33, 34]. Thus, we may conclude that the conventional AT1 gene variant, A1166C, is not associated with the antihypertensive effects of RAS blockade.
Again, however, Su et al. reported that individual SNPs including A1166C are not associated with the antihypertensive effects of benazepril but two haplotypes of AT1 (H2, H3) are associated with blood pressure reduction in response to benazepril [32]. As this is the only report regarding the haplotypes of AT1, further evaluations are expected.
AT2 Gene Variant
AT2, a subtype of the angiotensin II receptor, which is thought to be expressed considerably less in adult tissues than AT1, functions as a vasodilator and has some antiproliferative effects against the AT1 receptor. From database research, we identified two studies about AT2: one is ours, using an ARB [25••], and the other is haplotype analysis with an ACE inhibitor [32]. As the AT2 gene is located on the X chromosome, the evaluation is a little complicated. In our study, we grouped the genotype by the presence or the absence of the A allele, and the other study evaluated haplotypes separately for males and females. No significant difference in blood pressure reduction was observed in these two studies. As these are the only two reports regarding AT2, further evaluations are expected.
REN Gene Variant
In the whole body of the RAS, renin is the first step and the rate-limiting enzyme. The gene is supposed to play a major role in blood pressure regulation and in the response to RAS blockade interventions. However, REN has not been examined as a candidate for pharmacogenomics. To our knowledge, our study [25••] is the only one to report on the relationship between the genetic variants and blood pressure effects of RAS blockade with an adequate sample size. This study comprised 231 hypertensive patients, who were given 40 to 160 mg per day of valsartan as monotherapy for 3 months. Changes in diastolic blood pressure differed significantly between genotypes of REN C-5312T: the reduction was 10.7 mm Hg (from 95.9 ± 12.9 to 85.2 ± 11.4) in CC versus 7.0 mm Hg reduction (from 94.7 ± 14.0 to 87.7 ± 12.6) in CT/TT (P = 0.02 for interactive effects of valsartan and genotype). Responder rates also differed between the genotypes: 72.8% in CC versus 58.0% in CT/TT (P = 0.03). Multiple logistic regression analysis revealed that CC homozygotes of REN C-5312T were an independent predictor for response, with an odds ratio of 2.03 (95% CI, 1.10–3.74).
A plausible explanation is as follows: As shown in Fig. 1, our recent studies of the transcription mechanism of the human renin gene revealed that multiple trans-factors and cis-elements are involved in the transcriptional regulation [35–39]. A distal enhancer, a 225-bp region located −5,777/−5,552 base upstream of the human renin gene, gave about 57-fold higher transcription rates [38]. REN C-5312T of the human renin gene was discovered as a functional new polymorphism in this distal enhancer region [40]. The levels of transcription were 45% greater with the 5312T variant than with the 5312C variant. Thus, the variant was validated as a functional SNP. The results of the study demonstrated that the homozygote of the C allele shows greater blood pressure reduction from ARBs than the T allele carrier and can be a predictor of response. One explanation may be that homozygotes of the C allele may have a lower level of tissue renin concentration and are more affected by the drug than T allele carriers. As this is the only report regarding REN, further evaluations are expected, especially to explore the RAS blockade response with different therapeutic combinations. The distinct effects on the RAS by target channel subtype, as shown for calcium channel blockers [41•], should be well considered.
Fig. 1.
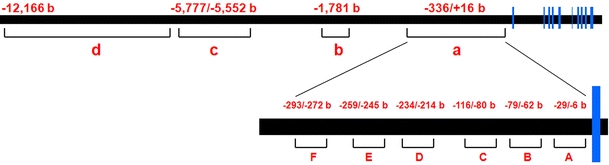
Structure of transcriptional regulatory elements for the human renin gene. The horizontal bars represent alignment of the gene, and the vertical bars represent exons. Shown are a the proximal promoter; b the negative calcium response element (nCaRE); c the distal enhancer; d the tissue-specific expression-determining element; a Ets motif; b HOX-PBX motif; c no known consensus; d cAMP response element (CRE); e direct repeat (DR) motif; f AGE3-like site. (From Konoshita et al. [37], with permission of John Wiley and Sons.)
ACE2 Gene Variant
ACE2, a newly emerging component of the renin-angiotensin system (RAS), is presumed to be a counterregulator against ACE in degrading angiotensin II to angiotensin 1–7, which is thought to act as a vasodilator and to be involved in apoptosis and growth arrest [42]. Accordingly, ACE2 is also a plausible candidate for pharmacogenomic study for the RAS. To our knowledge, only the study by Fan et al. [43] has yet reported a relationship between genetic variants of ACE2 and blood pressure effects with RAS blockade. These authors reported that the adjusted diastolic blood pressure response to captopril in women was 3.3 mm Hg lower in ACE2 rs2106809 T allele carriers than in CC genotype carriers (P = 0.019). As this is the only report regarding ACE2, further evaluations are expected.
Conclusions
A previous well-documented review of this issue in 2007 [20] identified no consistent conclusions, but problems have been attributed to methodologic limitations such as race, patient selection, behavioral factors such as salt intake, combining of different therapies, drug dose, administration term and drug selection, statistical power, combining of different alleles, and end points such as definition of responders. We thought that one of the most important and fundamental concerns should be statistical power estimation. Considering practical clinical implications, the significant and adequate difference is assumed to be about 5 mm Hg in diastolic blood pressure. Thus, the needed minimum sample size was calculated to be about 200 subjects in total. By selecting studies with about 200 or more subjects, we tried to produce an updated statistical review, and we obtained relatively consistent results, concluding that the conventional genetic variants of the system—the ACE I/D, AGT M235T, AT1 A1166C, and AT2 variant—are not associated with the antihypertensive effects of RAS blockade, at least by individual SNPs. In contrast, significant associations have been reported with AGT rs7079, AT1 haplotype, REN, and ACE2, each in one report. For these variants, further evaluations are expected.
Acknowledgments
Disclosure
No potential conflicts of interest relevant to this article were reported.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
Recently published papers of interest have been highlighted as • Of importance •• Of major importance
- 1.Corvol P, Soubrier F, Jeunemaitre X. Molecular genetics of the renin-angiotensin-aldosterone system in human hypertension. Pathol Biol (Paris) 1997;45(3):229–39. [PubMed] [Google Scholar]
- 2.Dzau V. The cardiovascular continuum and renin-angiotensin-aldosterone system blockade. J Hypertens Suppl. 2005;23(1):S9–17. doi: 10.1097/01.hjh.0000165623.72310.dd. [DOI] [PubMed] [Google Scholar]
- 3.Wakahara S, Konoshita T, Mizuno S, et al. Synergistic expression of angiotensin-converting enzyme (ACE) and ACE2 in human renal tissue and confounding effects of hypertension on the ACE to ACE2 ratio. Endocrinology. 2007;148(5):2453–7. doi: 10.1210/en.2006-1287. [DOI] [PubMed] [Google Scholar]
- 4.Konoshita T, Wakahara S, Mizuno S, et al. Tissue gene expression of renin-angiotensin system in human type 2 diabetic nephropathy. Diabetes Care. 2006;29(4):848–52. doi: 10.2337/diacare.29.04.06.dc05-1873. [DOI] [PubMed] [Google Scholar]
- 5.Cambien F, Poirier O, Lecerf L, et al. Deletion polymorphism in the gene for angiotensin-converting enzyme is a potent risk factor for myocardial infarction. Nature. 1992;359(6396):641–4. doi: 10.1038/359641a0. [DOI] [PubMed] [Google Scholar]
- 6.Baboolal K, Ravine D, Daniels J, et al. Association of the angiotensin I converting enzyme gene deletion polymorphism with early onset of ESRF in PKD1 adult polycystic kidney disease. Kidney Int. 1997;52(3):607–13. doi: 10.1038/ki.1997.373. [DOI] [PubMed] [Google Scholar]
- 7.Perez-Oller L, Torra R, Badenas C, et al. Influence of the ACE gene polymorphism in the progression of renal failure in autosomal dominant polycystic kidney disease. Am J Kidney Dis. 1999;34(2):273–8. doi: 10.1016/S0272-6386(99)70355-0. [DOI] [PubMed] [Google Scholar]
- 8.Konoshita T, Miyagi K, Onoe T, et al. Effect of ACE gene polymorphism on age at renal death in polycystic kidney disease in Japan. Am J Kidney Dis. 2001;37(1):113–8. doi: 10.1053/ajkd.2001.20595. [DOI] [PubMed] [Google Scholar]
- 9.Parving HH, de Zeeuw D, Cooper ME, et al. ACE gene polymorphism and losartan treatment in type 2 diabetic patients with nephropathy. J Am Soc Nephrol. 2008;19(4):771–9. doi: 10.1681/ASN.2007050582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeunemaitre X, Soubrier F, Kotelevtsev YV, et al. Molecular basis of human hypertension: role of angiotensinogen. Cell. 1992;71(1):169–80. doi: 10.1016/0092-8674(92)90275-H. [DOI] [PubMed] [Google Scholar]
- 11.Caulfield M, Lavender P, Farrall M, et al. Linkage of the angiotensinogen gene to essential hypertension [see comments] N Engl J Med. 1994;330(23):1629–33. doi: 10.1056/NEJM199406093302301. [DOI] [PubMed] [Google Scholar]
- 12.Bonnardeaux A, Davies E, Jeunemaitre X, et al. Angiotensin II type 1 receptor gene polymorphisms in human essential hypertension. Hypertension. 1994;24(1):63–9. doi: 10.1161/01.hyp.24.1.63. [DOI] [PubMed] [Google Scholar]
- 13.Tiret L, Bonnardeaux A, Poirier O, et al. Synergistic effects of angiotensin-converting enzyme and angiotensin-II type 1 receptor gene polymorphisms on risk of myocardial infarction [see comments] Lancet. 1994;344(8927):910–3. doi: 10.1016/S0140-6736(94)92268-3. [DOI] [PubMed] [Google Scholar]
- 14.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–60. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 15.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–9. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 16.Parving HH, Lehnert H, Brochner-Mortensen J, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345(12):870–8. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 17.Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 18.Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363(9426):2022–31. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 19.Julius S, Nesbitt SD, Egan BM, et al. Feasibility of treating prehypertension with an angiotensin-receptor blocker. N Engl J Med. 2006;354(16):1685–97. doi: 10.1056/NEJMoa060838. [DOI] [PubMed] [Google Scholar]
- 20.Farahani P, Dolovich L, Levine M. Exploring design-related bias in clinical studies on receptor genetic polymorphism of hypertension. J Clin Epidemiol. 2007;60(1):1–7. doi: 10.1016/j.jclinepi.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Rigat B, Hubert C, Alhenc-Gelas F, et al. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86(4):1343–6. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ueda S, Meredith PA, Morton JJ, et al. ACE (I/D) genotype as a predictor of the magnitude and duration of the response to an ACE inhibitor drug (enalaprilat) in humans. Circulation. 1998;98(20):2148–53. doi: 10.1161/01.cir.98.20.2148. [DOI] [PubMed] [Google Scholar]
- 23.Kurland L, Melhus H, Karlsson J, et al. Angiotensin converting enzyme gene polymorphism predicts blood pressure response to angiotensin II receptor type 1 antagonist treatment in hypertensive patients. J Hypertens. 2001;19(10):1783–7. doi: 10.1097/00004872-200110000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Nordestgaard BG, Kontula K, Benn M, et al. Effect of ACE insertion/deletion and 12 other polymorphisms on clinical outcomes and response to treatment in the LIFE study. Pharmacogenet Genomics. 2010;20(2):77–85. doi: 10.1097/FPC.0b013e328333f70b. [DOI] [PubMed] [Google Scholar]
- 25.Konoshita T, Kato N, Fuchs S, et al. Genetic variant of the renin-angiotensin system and diabetes influences blood pressure response to angiotensin receptor blockers. Diabetes Care. 2009;32(8):1485–90. doi: 10.2337/dc09-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Redon J, Luque-Otero M, Martell N, Chaves FJ. Renin-angiotensin system gene polymorphisms: relationship with blood pressure and microalbuminuria in telmisartan-treated hypertensive patients. Pharmacogenomics J. 2005;5(1):14–20. doi: 10.1038/sj.tpj.6500280. [DOI] [PubMed] [Google Scholar]
- 27.Filigheddu F, Argiolas G, Bulla E, et al. Clinical variables, not RAAS polymorphisms, predict blood pressure response to ACE inhibitors in Sardinians. Pharmacogenomics. 2008;9(10):1419–27. doi: 10.2217/14622416.9.10.1419. [DOI] [PubMed] [Google Scholar]
- 28.Arnett DK, Davis BR, Ford CE, et al. Pharmacogenetic association of the angiotensin-converting enzyme insertion/deletion polymorphism on blood pressure and cardiovascular risk in relation to antihypertensive treatment: the Genetics of Hypertension-Associated Treatment (GenHAT) study. Circulation. 2005;111(25):3374–83. doi: 10.1161/CIRCULATIONAHA.104.504639. [DOI] [PubMed] [Google Scholar]
- 29.Yu H, Zhang Y, Liu G. Relationship between polymorphism of the angiotensin-converting enzyme gene and the response to angiotensin-converting enzyme inhibition in hypertensive patients. Hypertens Res. 2003;26(11):881–6. doi: 10.1291/hypres.26.881. [DOI] [PubMed] [Google Scholar]
- 30.Hingorani AD, Jia H, Stevens PA, et al. Renin-angiotensin system gene polymorphisms influence blood pressure and the response to angiotensin converting enzyme inhibition. J Hypertens. 1995;13(12 Pt 2):1602–9. [PubMed] [Google Scholar]
- 31.Yu H, Lin S, Liu G, et al. T1198C polymorphism of the angiotensinogen gene and antihypertensive response to angiotensin-converting enzyme inhibitors. Hypertens Res. 2005;28(12):981–6. doi: 10.1291/hypres.28.981. [DOI] [PubMed] [Google Scholar]
- 32.Su X, Lee L, Li X, et al. Association between angiotensinogen, angiotensin II receptor genes, and blood pressure response to an angiotensin-converting enzyme inhibitor. Circulation. 2007;115(6):725–32. doi: 10.1161/CIRCULATIONAHA.106.642058. [DOI] [PubMed] [Google Scholar]
- 33.Yu H, Lin S, Jin L, et al. Adenine/cytosine(1166) polymorphism of the angiotensin II type 1 receptor gene and the antihypertensive response to angiotensin-converting enzyme inhibitors. J Hypertens. 2009;27(11):2278–82. doi: 10.1097/HJH.0b013e328330b654. [DOI] [PubMed] [Google Scholar]
- 34.Brunner M, Cooper-DeHoff RM, Gong Y, et al. Factors influencing blood pressure response to trandolapril add-on therapy in patients taking verapamil SR (from the International Verapamil SR/Trandolapril [INVEST] Study) Am J Cardiol. 2007;99(11):1549–54. doi: 10.1016/j.amjcard.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konoshita T, Germain S, Philippe J, et al. Evidence that renal and chorionic tissues contain similar nuclear binding proteins that recognize the human renin promoter. Kidney Int. 1996;50(5):1515–24. doi: 10.1038/ki.1996.466. [DOI] [PubMed] [Google Scholar]
- 36.Konoshita T, Makino Y, Wakahara S, et al. Candidate cis-elements for human renin gene expression in the promoter region. J Cell Biochem. 2004;93(2):327–36. doi: 10.1002/jcb.20151. [DOI] [PubMed] [Google Scholar]
- 37.Konoshita T, Fuchs S, Makino Y, et al. A proximal direct repeat motif characterized as a negative regulatory element in the human renin gene. J Cell Biochem. 2007;102(4):1043–50. doi: 10.1002/jcb.21341. [DOI] [PubMed] [Google Scholar]
- 38.Germain S, Bonnet F, Philippe J, et al. A novel distal enhancer confers chorionic expression on the human renin gene. J Biol Chem. 1998;273(39):25292–300. doi: 10.1074/jbc.273.39.25292. [DOI] [PubMed] [Google Scholar]
- 39.Fuchs S, Germain S, Philippe J, et al. Expression of renin in large arteries outside the kidney revealed by human renin promoter/LacZ transgenic mouse. Am J Pathol. 2002;161(2):717–25. doi: 10.1016/S0002-9440(10)64227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fuchs S, Philippe J, Germain S, et al. Functionality of two new polymorphisms in the human renin gene enhancer region. J Hypertens. 2002;20(12):2391–8. doi: 10.1097/00004872-200212000-00018. [DOI] [PubMed] [Google Scholar]
- 41.Konoshita T, Makino Y, Kimura T, et al. A new-generation N/L-type calcium channel blocker leads to less activation of the renin-angiotensin system compared with conventional L type calcium channel blocker. J Hypertens. 2010;28(10):2156–60. doi: 10.1097/HJH.0b013e32833d01dd. [DOI] [PubMed] [Google Scholar]
- 42.Boehm M, Nabel EG. Angiotensin-converting enzyme 2—a new cardiac regulator. N Engl J Med. 2002;347(22):1795–7. doi: 10.1056/NEJMcibr022472. [DOI] [PubMed] [Google Scholar]
- 43.Fan X, Wang Y, Sun K, et al. Polymorphisms of ACE2 gene are associated with essential hypertension and antihypertensive effects of Captopril in women. Clin Pharmacol Ther. 2007;82(2):187–96. doi: 10.1038/sj.clpt.6100214. [DOI] [PubMed] [Google Scholar]


