Abstract
Purpose
Real-time intraoperative near-infrared fluorescence (NIRF) imaging is a promising technique for lymphatic mapping and sentinel lymph node (SLN) detection. The purpose of this technical feasibility pilot study was to evaluate the applicability of NIRF imaging with indocyanin green (ICG) for the detection of the SLN in cervical cancer.
Procedures
In ten patients with early stage cervical cancer, a mixture of patent blue and ICG was injected into the cervix uteri during surgery. Real-time color and fluorescence videos and images were acquired using a custom-made multispectral fluorescence camera system.
Results
Real-time fluorescence lymphatic mapping was observed in vivo in six patients; a total of nine SLNs were detected, of which one (11%) contained metastases. Ex vivo fluorescence imaging revealed the remaining fluorescent signal in 11 of 197 non-sentinel LNs (5%), of which one contained metastatic tumor tissue. None of the non-fluorescent LNs contained metastases.
Conclusions
We conclude that lymphatic mapping and detection of the SLN in cervical cancer using intraoperative NIRF imaging is technically feasible. However, the technique needs to be refined for full applicability in cervical cancer in terms of sensitivity and specificity.
Electronic supplementary material
The online version of this article (doi:10.1007/s11307-010-0425-7) contains supplementary material, which is available to authorized users.
Key words: Cervical cancer, Near-infrared fluorescence, Sentinel lymph node, Multispectral intraoperative imaging
Introduction
Over the last decades, the sentinel lymph node (SLN) concept has proven feasible and safe in selected types of cancer such as vulvar cancer, breast cancer, early gastric cancer, and melanoma [1–5]. Recently, intraoperative near-infrared fluorescence (NIRF) imaging was developed as a novel technique for SLN detection. By using a NIR fluorescent dye such as indocyanin green (ICG) and a sensitive fluorescence camera system, intraoperative lymph node mapping has been proven feasible in breast cancer and skin cancer patients, with comparable to or slightly better detection rates than conventional techniques [6–9]. Advantages of this technique are the real-time feature for lymphatic mapping and the avoidance of radioactivity. Furthermore, the one-step procedure of NIRF imaging takes place entirely during surgery, thus improving patient comfort. To date, NIRF imaging has not been applied in cervical cancer.
The prognosis of early stage cervical cancer is substantially influenced by lymph node (LN) status, with positive LNs adding to an unfavorable prognosis [10, 11]. Standard surgical treatment therefore consists of radical hysterectomy combined with bilateral pelvic lymphadenectomy. Metastatic involvement is found in no more than 13–27% of the LNs [1, 11], implying that radical pelvic lymphadenectomy can be regarded as overtreatment without clinical benefit in more than three quarters of the patients. Moreover, LN dissection may have the unfavorable consequence of lymphedema of the legs, occurring in 14–32% of patients [12, 13]. The SLN procedure could lead to more selective LN dissection, but its definitive value in cervical cancer is still a matter of debate and subject of ongoing investigation. The reliability in terms of sensitivity has improved markedly in the last two decades, mainly due to the combination of a radioactive tracer combined with a blue dye rather than using blue dye alone [14]. This bimodal approach yields false negative rates of 0% in tumors <2 cm [15, 16]. Although these data are encouraging, SLN detection in the pelvic region remains complicated due the bilateral and not fully predictable lymphatic routing originating from the uterine cervix. About 80% of SLNs are found in the area of internal and external iliac nodes and in the obturator fossa, but involvement of lymph nodes in the parametrium and para-aortal regions has also been reported (Fig. 1) [17–20]. Bilateral SLNs are found in 59–66% of patients [15, 16]. In particular, the blue dye can be difficult to trace in extrapelvic areas because of overlying muscle tissue or fat.
Fig. 1.
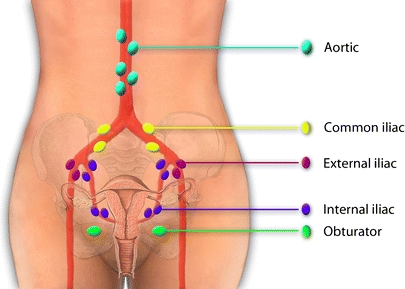
Lymph drainage of the cervix uteri is complex, bilateral, and can affect lymph nodes in several areas. In our pilot study, nine SLNs were found. These were localized in the left obturator fossa (three), right obturator fossa (two), left external iliac (one), right external iliac (one), right common iliac (one), and on the junction of the right internal iliac and obturator fossa (one).
A great advantage of NIRF imaging over existing techniques is the high target-to-background ratio. When applied in cervical cancer, we hypothesized that real-time NIRF intraoperative imaging could be advantageous in the visualization of the drainage pattern and, thus, dynamic detection of the SLN in the pelvic anatomy. We therefore initiated this pilot study to evaluate the technical feasibility and applicability of a clinical prototype intraoperative fluorescence camera system for detection of the SLN in cervical cancer.
Patients and Methods
Study Population
This pilot study was approved by the Institutional Review Board of the University Medical Center Groningen and was performed in accordance with the ethical standards of the Helsinki Declaration of 1975 (Dutch Trial Register no. NTR1981, EudraCT no. 2009-010560-42). Consecutive patients with a cervical cancer stage IA1, IB1, or IIA, eligible for abdominal hysterectomy and pelvic lymphadenectomy aged 21 years and older were included. They were recruited by the gynecologist and/or the research physician in the outpatient clinic. Informed consent was obtained after at least 1 week of reflection time. All patient data were depersonalized. Exclusion criteria included severe renal failure (serum creatinine ≥400 μmol/L), severe cardiac or pulmonary failure (ASA III–IV), past or present hyperthyroidism, pregnancy, or a previous severe allergic or anaphylactic reaction to insect bites or medication.
Imaging System
The prototype intraoperative multispectral fluorescence camera system used in this study (Fig. 2) was developed at the Helmholtz Zentrum of the Technical University Munich, Germany (NJ, GT, AS, VN) in close collaboration with SurgOptix (SurgOptix Inc., Redwood Shores, CA, USA). The system and its components were recently extensively described by Themelis et al. [21] and will be pointed out briefly.
Fig. 2.
Camera system wrapped in sterile drapes in the operating theater.
A white light source illuminates the operating field and a laser diode provides light of a desired wavelength for excitation of the fluorescent probe. Light passes through a system of optics and is separated into visible light (color), light at the emission (fluorescence) wavelength band, and light at the excitation wavelength band (intrinsic). These bundles are detected by sensitive CCD cameras. Multispectral signals from all three cameras are processed and combined in order to correct for artifacts and yield true quantitative fluorochrome biodistribution. Color and fluorescence signals can be displayed as separate images on external monitors or superimposed in one image, allowing for adequate anatomical positioning of the fluorescence signal. Images and real-time videos up to 12 frames per second are generated using customized software. During surgery, both the color image and the fluorescence signal are presented on external monitors, providing the surgeon with an unhindered view. Sterile drapes are used in the operating room to cover the camera system completely, making it applicable and safe for intraoperative use. The technical service of the University Medical Center Groningen performed necessary tests for leakage current and safety of the application of the camera in its current form during surgery.
Imaging Agents
Shortly prior to surgery, a vial of 25 mg ICG (Pulsion Medical Systems AG, Munich, Germany) was dissolved in 50 mL of water for injection (B. Braun Medical), yielding a concentration of 0.5 mg/mL. This concentration has previously been described adequate for SLN imaging [5]. For each patient, a new vial of ICG was used because of degradation of the fluorescence signal of ICG after dissolving. One milliliter of undiluted patent blue (Bleu patenté, Guerbet, France) was mixed with 1 mL of dissolved ICG in a syringe for injection into the cervix uteri and protected from light to prevent bleaching.
Surgical Procedure
After laparotomy, the camera system was prepared for imaging and moved above the operating field. In this setting, the lens of the camera is situated approximately 25 cm above the patient. Real-time video capturing was started simultaneously with or shortly after the injection of the 2 mL mixture of ICG and patent blue in four quadrants of the cervix uteri. Propulsion of the ICG/patent blue mixture through the lymphatics was followed real time. The first appearing LN was denominated as the SLN. The SLN or—in the case of bilateral lymph flow—multiple SLNs were removed and the fluorescence signal was quantified ex vivo. Next, the remaining LNs in the obturator fossa and in the internal, external, and communal iliac regions were dissected and evaluated for fluorescence and blue discoloration ex vivo. From this point on, surgery was carried out following the standard procedure. In case no fluorescent SLN was found in vivo, all LNs were dissected and evaluated ex vivo for a possible fluorescent signal and/or blue discoloration. All LNs were sent to the pathologist for histopathological examination. In case of a fluorescent hot spot in a cluster of LNs, the fluorescent node was excised and examined separately for tumor involvement.
Results
Study Population
Demographic and clinical characteristics are shown in Table 1. Ten patients were included in this pilot study (mean age 51.3, range 39–74; mean BMI 22.7, range 15–30). The majority of patients was diagnosed with stage IB1; five patients had a tumor <2 cm. No side effects were reported following the injection of ICG and patent blue. Histopathological examination revealed three patients with adenocarcinoma and seven with squamous carcinoma of the cervix. This is concordant with incidence rates in the population [22, 23].
Table 1.
Demographics and results
| Study no. | Age | BMI | FIGO stage | Tumor typea | Tumor size |
|---|---|---|---|---|---|
| 1 | 41 | 19 | IB1 | Squamous | No tumor left after LEEP |
| 2 | 70 | 26 | IB1/IIA | Squamous | >2 cm |
| 3 | 48 | 24 | IB1 | Squamous | >2 cm |
| 4 | 40 | 24 | IA2 | Adeno | <2 cm |
| 5 | 40 | 22 | IB1 | Adeno | >2 cm |
| 6 | 40 | 20 | IB1 | Adeno | No tumor left after LEEP |
| 7 | 70 | 23 | IB1 | Squamous | >2 cm |
| 8 | 74 | 24 | IB2 | Squamous | >2 cm |
| 9 | 39 | 15 | IB1 | Squamous | <2 cm |
| 10 | 51 | 30 | IB1 | Squamous | <2 cm |
aHistologic subtype: squamous cell carcinoma (squamous) or adenocarcinoma (adeno)
BMI body mass index, FIGO International Federation of Gynecology and Obstetrics, LEEP loop electrosurgical excision procedure conization
Intraoperative Fluorescence Imaging
The fluorescent signal in the lymphatics became visible within 30 s upon injection of ICG and patent blue, with the SLN generally appearing within 1–2 min (ESM Supplemental Video 1). Lymphatic mapping was clearly discernible in vivo in six patients (nos. 1, 3, 4, 5, 7, and 9). In one of these patients (no. 7), the lymph vessels were visible, but no SLN appeared. In contrast, no lymph vessels were visible in one patient (no. 10); nevertheless, the SLN could easily be detected in vivo. This case is illustrated in Fig. 3. In total, one or more SLNs were detected in six patients (nos. 1, 3, 4, 5, 9, and 10). In these patients, a total number of nine SLNs were detected. A bilateral SLN was detected in three patients. The SLNs were located in the left obturator fossa (three), right obturator fossa (two), left external iliac (one), right external iliac (one), right common iliac (one), and on the junction of the right internal iliac and obturator fossa (one, Fig. 1). Of these nine fluorescent SLNs, six also showed a blue discoloration. Ex vivo fluorescence imaging confirmed the strong fluorescent signal in all excised SLNs. One of the SLNs turned out to contain metastatic disease on histopathological examination. A summary of the results is shown in Table 2.
Fig. 3.
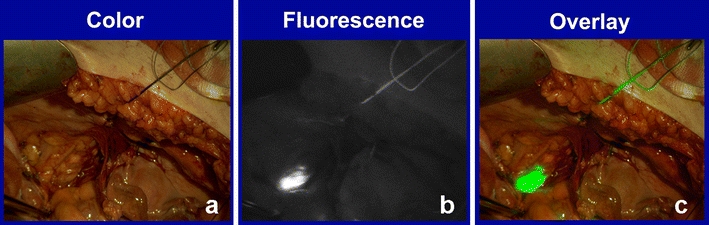
In vivo fluorescence imaging of a lymph node. a Color image. b Fluorescence image. c Overlay of the pseudocolor fluorescent signal on top of the color image.
Table 2.
Summary of in vivo and ex vivo results
| SLN detected in vivo | SLNs fluorescenta | SLNs blueb | SLN metastasisc | LNs dissected | LNs fluorescentd | LNs bluee | LN metastasisf |
|---|---|---|---|---|---|---|---|
| Yes (n = 6) | 9 | 6 | 1 | 116 | 6 | 0 | 0 |
| No (n = 4) | n/a | n/a | n/a | 81 | 5 | 3 | 1 |
| Total (n = 10) | 9 | 5 | 1 (11%) | 197 | 11 | 3 | 1 (0.5%) |
aNumber of SLNs showing fluorescence in vivo
bNumber of SLNs showing a blue discoloration
cNumber of SLNs that were confirmed to contain metastatic tumor tissue
dNumber of non-sentinel LNs showing fluorescence ex vivo
eNumber of LNs showing a blue discoloration ex vivo
fNumber of LNs that were confirmed to contain metastatic tumor tissue
In four patients, no lymphatic mapping could be observed in vivo. Three of these patients had tumors >2 cm. In patient 2, intra-abdominal fat impeded detection of fluorescence. However, upon dissection, a blue LN was discovered in the obturator fossa, also showing a strong fluorescent signal ex vivo. On histopathological examination, this LN turned out to contain metastatic tumor tissue. In patient 6, a smaller abdominal incision prevented maximal exposure of all the LN basins to the camera, thus hampering detection. Leakage of ICG and patent blue into the pelvis as a result of a deep intracervical injection prevented SLN detection in patient 7. The last patient (no. 8) in whom no SLN could be detected in vivo was initially diagnosed with stage IB1 cervical cancer, but was upstaged to IB2 during surgery, a stage that does not strictly apply to the inclusion criteria. The size of the tumor could be the cause of hampered lymph flow and concomitant fluorescent and blue agents through the lymphatics.
Ex vivo Fluorescence Imaging
After LN dissection, all excised LNs were imaged for fluorescence ex vivo (Tables 2 and 3). A total of 197 non-sentinel LNs were taken out, with a mean number of 20 LNs per patient (range 10–27).
Table 3.
Localization of fluorescent sentinel lymph nodes in vivo and fluorescent non-sentinel lymph nodes ex vivo
| Study no. | No. of SLN(s) | Localization SLN(s)a | SLNs with metastasis | No. of fluorescent LN(s) ex vivo | Localization fluorescent LNsb | LNs with metastasisc |
|---|---|---|---|---|---|---|
| 1 | 2 | Obturator left | 0 | 0 | – | 0 |
| Obturator right | ||||||
| 2 | 0 | – | – | 1 | Obturator right | 1 |
| 3 | 2 | External iliac left | 0 | 1 | Junction external/common iliac right | 0 |
| External iliac right | ||||||
| 4 | 2 | Obturator left | 0 | 2 | External iliac right | 0 |
| Obturator right | Obturator right | |||||
| 5 | 1 | Obturator left | 1 | 0 | – | 0 |
| 6 | 0 | – | – | 1 | External iliac left | 0 |
| 7 | 0 | – | – | 3 | External iliac left (2) | 0 |
| External iliac right | ||||||
| 8 | 0 | – | – | 0 | – | 0 |
| 9 | 1 | External iliac right | 0 | 2 | Obturator left | 0 |
| Common iliac right | ||||||
| 10 | 1 | Common iliac right | 0 | 1 | External iliac left | 0 |
aLocalization in vivo where the SLN(s) was found
bInitial localization of LNs that showed fluorescence ex vivo after dissection
cNumber of LNs showing fluorescence ex vivo that were confirmed to contain metastatic tumor tissue
In the six patients in whom SLN(s) were detected in vivo, a total of 116 non-sentinel LNs were excised. None of these contained metastases. In four of these six patients, a total of six LNs were found to show a fluorescent signal on ex vivo imaging; none of these were blue. The localization of these fluorescent LNs was in two cases contralateral from the SLN (patients 9 and 10; Table 3).
In the four patients in whom no SLN was detected in vivo, 81 LNs were taken out. Five LNs showed a fluorescent signal; three of these were also blue. An example of ex vivo fluorescence in a cluster of lymph nodes is shown in Fig. 4. Bilateral fluorescent LNs were found in patient 2. Only one of these LNs, which were both fluorescent and blue, turned out to contain metastases. None of the non-fluorescent LNs contained metastases.
Fig. 4.
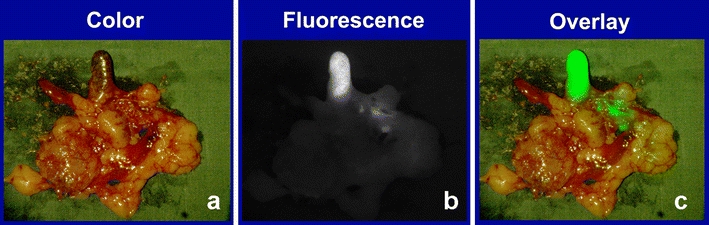
Ex vivo fluorescence imaging of a cluster of lymph nodes. a Color image. b Fluorescence image. c Overlay of the pseudocolor fluorescent signal on top of the color image. This fluorescent lymph node did not contain metastases.
In summary, one of nine SLNs (11%) contained metastases. Eleven of 197 non-sentinel LNs (5.6%) showed a fluorescent signal ex vivo; three of these (1.5%) were also blue. One of these fluorescent non-sentinel LNs contained metastases.
Discussion
This pilot study is, to our knowledge, the first clinical study to assess the technical feasibility of NIRF for the detection of the SLN in cervical cancer. We show that lymphatic mapping and SLN detection using the fluorescent contrast agent ICG is technically feasible. One or more SLNs were found in six out of ten patients; bilateral SLNs were detected in three of these patients. Overall, lymphatic mapping using fluorescence imaging was more straightforward in lean patients in whom the pelvic lymph basins could be more easily exposed and somewhat more difficult in obese patients. Use of the intraoperative camera system in itself did not hinder the surgeon, and the surgical procedure was not extended with more than 30 min due to imaging. However, the limited range of movement of the camera head combined with smaller surgical incisions caused difficulties in the detection of fluorescence in deep areas of the pelvis.
A number of summarized studies report detection of one or more SLNs in early stage cervical cancer in 84–97% of patients using radiocolloid in combination with blue dye [24–27]. The lower detection rate of 60% in our pilot study can be explained by a number of factors, i.e., patient characteristics, the learning curve concerned with the new technique, and technical features of the camera system and the fluorescent agent.
Several studies report that the SLN technique is less applicable in patients with cervical tumors >2 cm, with detection rates decreasing to 67–77% [24, 28]. This could be the result of extensive tumor growth, causing either embolization or mass blocking of the lymph vessels.
Consistent with literature, we observed better detection rates in tumors smaller than 2 cm. SLNs were found in four out of five patients with tumors <2 cm (80%) and in only two out of five patients with tumors >2 cm (40%). Bilateral SLNs were detected in two tumors <2 cm (40%) and in one tumor >2 cm (20%). Furthermore, in one patient who presented with a large tumor (stage IB2, patient 8), neither the fluorescent nor the blue agent could be located in vivo in the pelvis of ex vivo in dissected LNs, indicating that the agents were not carried from the tumor into the lymphatics toward the SLN or LNs. Although these data suggest support for existing literature regarding the impact of tumor size on (bilateral) SLN detection, our numbers are too small to draw definitive conclusions from.
The detection rate of 60% observed in this pilot study may furthermore be influenced by the learning curve of working with the intraoperative camera system and correct injection of the fluorescent agent. As gynecologic oncologists got more acquainted with the technique and the injection, the procedure became more standardized. For example, in patient 2, no SLN was detected in vivo; however, when exposing the obturator lymph basin better, a blue LN was found, which turned out to be fluorescent as well. In retrospect, we believe that this LN would have been detected as the SLN had we better exposed the lymph basin. In two of three patients with a unilateral SLN, we observed fluorescence ex vivo in a contralaterally located LN. These findings indicate that in these patients, although the fluorescent agent was transported bilaterally from the uterine cervix; not all SLNs were detected. More experience with the technique may lead to a higher detection rate of SLNs in vivo.
Apart from patient selection and learning curve, we encountered several technical aspects influencing our results. Deep-lying LNs were in some cases difficult to visualize due to the limited range of movement of the camera head. A smaller, more flexible, preferably handheld camera system or even a laparoscopic system for intraoperative fluorescence imaging could overcome this problem. Technical progress will undoubtedly lead to more ergonomic and clinically suited camera systems.
The most important limitation, however, appeared to be the penetration depth of ICG which lies around 1 cm. This is sufficient for superficial LNs, but generally not enough to visualize lymph nodes covered by adipose tissue or located deep in the pelvis hidden by the iliac vessels. In the current, still limited range of FDA-approved fluorescent agents, none has a larger penetration depth than ICG. In order to improve fluorescence imaging in the pelvis, future developments should be aimed at designing fluorescent agents with stronger tissue-penetrating properties.
Apart from these pitfalls, fluorescence imaging also bears several advantages. The most important benefit for patients is that the fluorescent agent is injected in a one-step sequence during surgery, when the patient is already anesthetized, thus preventing four injections in the cervix several hours or even a day prior to surgery, as is necessary when using a radiocolloid. Furthermore, we observed that the fluorescence signal is well discernable in the pelvic anatomy as long as the desired region of interest is projected within the field of view of the camera system. As mentioned earlier, more flexible camera systems with a greater range of movement will most certainly yield more optimized detection in deeper seated areas. Although the penetration depth of NIR fluorescent agents will not exceed 1 cm, fluorescence is easy to locate in the pelvic region when compared to detection of the blue dye by visual inspection. Additionally, the real-time video properties aid the gynecologic oncologist in dynamic tracing of lymph flow toward the SLN within minutes after injection into the cervix. This unique feature of NIRF imaging provides a real-time image of the location and flow of the fluorescent agent in the lymphatic system, in close relation to the adjacent anatomical structures. This would, to our opinion, be the major advantage of NIRF imaging over conventional SLN detection methods. Future NIR fluorescent agents with better penetration properties are needed to improve detection rates.
Conclusion
We show that NIRF imaging using ICG for the detection of the SLN in early stage cervical cancer is technically feasible. Advantages of this approach are the real-time tracing of lymph flow and the one-step procedure. However, technical improvements and fluorescent dyes with larger penetration strength are needed to refine the technique.
Electronic Supplementary Materials
Below is the link to the electronic supplementary material.
This fluorescence video shows the propulsion of indocyanine green (ICG) through the lymphatic vessels within 1 min of injection into the uterine cervix (patient 4). The fluorescence signal is clearly visible, both in the lymphatic vessels and in the sentinel lymph node (first appearing fluorescent lymph node). (WMV 7774 kb)
Acknowledgments
The authors wish to thank Dr. M. van Oosten and Dr. K.T. Buddingh for assistance in data collection.
Conflict of Interest
Dr. G.M. van Dam and V. Ntziachristos are members of the Scientific Advisory Board of SurgOptix Inc. All other authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- ICG
Indocyanin green
- LN
Lymph node
- NIR
Near-infrared
- NIRF
Near-infrared fluorescence
- SLN
Sentinel lymph node
Footnotes
An erratum to this article can be found at http://dx.doi.org/10.1007/s11307-010-0443-5
References
- 1.Loar PV, III, Reynolds RK. Sentinel lymph node mapping in gynecologic malignancies. Int J Gynaecol Obstet. 2007;99:69–74. doi: 10.1016/j.ijgo.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Veronesi U, Paganelli G, Galimberti V, et al. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet. 1997;349:1864–1867. doi: 10.1016/S0140-6736(97)01004-0. [DOI] [PubMed] [Google Scholar]
- 3.Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392–399. doi: 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- 4.van der Zee AG, Oonk MH, De Hullu JA, et al. Sentinel node dissection is safe in the treatment of early-stage vulvar cancer. J Clin Oncol. 2008;26:884–889. doi: 10.1200/JCO.2007.14.0566. [DOI] [PubMed] [Google Scholar]
- 5.Kelder W, Nimura H, Takahashi N, et al. Sentinel node mapping with indocyanine green (ICG) and infrared ray detection in early gastric cancer: an accurate method that enables a limited lymphadenectomy. Eur J Surg Oncol. 2010;36:552–558. doi: 10.1016/j.ejso.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Troyan SL, Kianzad V, Gibbs-Strauss SL, et al. The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann Surg Oncol. 2009;16:2943–2952. doi: 10.1245/s10434-009-0594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirche C, Murawa D, Mohr Z, et al. ICG fluorescence-guided sentinel node biopsy for axillary nodal staging in breast cancer. Breast Cancer Res Treat. 2010;121:373–378. doi: 10.1007/s10549-010-0760-z. [DOI] [PubMed] [Google Scholar]
- 8.Murawa D, Hirche C, Dresel S, et al. Sentinel lymph node biopsy in breast cancer guided by indocyanine green fluorescence. Br J Surg. 2009;96:1289–1294. doi: 10.1002/bjs.6721. [DOI] [PubMed] [Google Scholar]
- 9.Fujiwara M, Mizukami T, Suzuki A, et al. Sentinel lymph node detection in skin cancer patients using real-time fluorescence navigation with indocyanine green: preliminary experience. J Plast Reconstr Aesthet Surg. 2009;62:e373–e378. doi: 10.1016/j.bjps.2007.12.074. [DOI] [PubMed] [Google Scholar]
- 10.Tinga DJ, Bouma J, Aalders JG. Patients with squamous cell versus adeno(squamous) carcinoma of the cervix, what factors determine the prognosis? Int J Gynecol Cancer. 1992;2:83–91. doi: 10.1046/j.1525-1438.1992.02020083.x. [DOI] [PubMed] [Google Scholar]
- 11.Horn LC, Hentschel B, Fischer U, et al. Detection of micrometastases in pelvic lymph nodes in patients with carcinoma of the cervix uteri using step sectioning: frequency, topographic distribution and prognostic impact. Gynecol Oncol. 2008;111:276–281. doi: 10.1016/j.ygyno.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Matsuura Y, Kawagoe T, Toki N, et al. Long-standing complications after treatment for cancer of the uterine cervix—clinical significance of medical examination at 5 years after treatment. Int J Gynecol Cancer. 2006;16:294–297. doi: 10.1111/j.1525-1438.2006.00354.x. [DOI] [PubMed] [Google Scholar]
- 13.Beesley V, Janda M, Eakin E, et al. Lymphedema after gynecological cancer treatment: prevalence, correlates, and supportive care needs. Cancer. 2007;109:2607–2614. doi: 10.1002/cncr.22684. [DOI] [PubMed] [Google Scholar]
- 14.Rasty G (2010) Sentinel lymph node examination for cancer of the uterine cervix. J Clin Pathol 62(12):1062–1065 [DOI] [PubMed]
- 15.Darlin L, Persson J, Bossmar T, et al. The sentinel node concept in early cervical cancer performs well in tumors smaller than 2 cm. Gynecol Oncol. 2010;117:266–269. doi: 10.1016/j.ygyno.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa S, Kobayashi H, Amada S, et al. Sentinel node detection with (99m)Tc phytate alone is satisfactory for cervical cancer patients undergoing radical hysterectomy and pelvic lymphadenectomy. Int J Clin Oncol. 2010;15:52–58. doi: 10.1007/s10147-009-0010-8. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Xu H, Li Y, et al. The outcome of laparoscopic radical hysterectomy and lymphadenectomy for cervical cancer: a prospective analysis of 295 patients. Ann Surg Oncol. 2008;15:2847–2855. doi: 10.1245/s10434-008-0063-3. [DOI] [PubMed] [Google Scholar]
- 18.Marnitz S, Kohler C, Bongardt S, et al. Topographic distribution of sentinel lymph nodes in patients with cervical cancer. Gynecol Oncol. 2006;103:35–44. doi: 10.1016/j.ygyno.2006.01.061. [DOI] [PubMed] [Google Scholar]
- 19.Bader AA, Winter R, Haas J, et al. Where to look for the sentinel lymph node in cervical cancer. Am J Obstet Gynecol. 2007;197:678–7. doi: 10.1016/j.ajog.2007.09.053. [DOI] [PubMed] [Google Scholar]
- 20.Levenback C, Coleman RL, Burke TW, et al. Lymphatic mapping and sentinel node identification in patients with cervix cancer undergoing radical hysterectomy and pelvic lymphadenectomy. J Clin Oncol. 2002;20:688–693. doi: 10.1200/JCO.20.3.688. [DOI] [PubMed] [Google Scholar]
- 21.Themelis G, Yoo JS, Soh KS, et al. Real-time intraoperative fluorescence imaging system using light-absorption correction. J Biomed Opt. 2009;14:064012. doi: 10.1117/1.3259362. [DOI] [PubMed] [Google Scholar]
- 22.Smith HO, Tiffany MF, Qualls CR, et al. The rising incidence of adenocarcinoma relative to squamous cell carcinoma of the uterine cervix in the United States—a 24-year population-based study. Gynecol Oncol. 2000;78:97–105. doi: 10.1006/gyno.2000.5826. [DOI] [PubMed] [Google Scholar]
- 23.Reimers LL, Anderson WF, Rosenberg PS, et al. Etiologic heterogeneity for cervical carcinoma by histopathologic type, using comparative age-period-cohort models. Cancer Epidemiol Biomark Prev. 2009;18:792–800. doi: 10.1158/1055-9965.EPI-08-0965. [DOI] [PubMed] [Google Scholar]
- 24.Yamashita T, Katayama H, Kato Y, et al. Management of pelvic lymph nodes by sentinel node navigation surgery in the treatment of invasive cervical cancer. Int J Gynecol Cancer. 2009;19:1113–1118. doi: 10.1111/IGC.0b013e3181a83d65. [DOI] [PubMed] [Google Scholar]
- 25.Frumovitz M, Ramirez PT, Levenback CF. Lymphatic mapping and sentinel lymph node detection in women with cervical cancer. Gynecol Oncol. 2008;110:S17–S20. doi: 10.1016/j.ygyno.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 26.van de Lande J, Torrenga B, Raijmakers PG, et al. Sentinel lymph node detection in early stage uterine cervix carcinoma: a systematic review. Gynecol Oncol. 2007;106:604–613. doi: 10.1016/j.ygyno.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Ayhan A, Celik H, Dursun P. Lymphatic mapping and sentinel node biopsy in gynecological cancers: a critical review of the literature. World J Surg Oncol. 2008;6:53. doi: 10.1186/1477-7819-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cibula D, Kuzel D, Slama J, et al. Sentinel node (SLN) biopsy in the management of locally advanced cervical cancer. Gynecol Oncol. 2009;115:46–50. doi: 10.1016/j.ygyno.2009.06.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This fluorescence video shows the propulsion of indocyanine green (ICG) through the lymphatic vessels within 1 min of injection into the uterine cervix (patient 4). The fluorescence signal is clearly visible, both in the lymphatic vessels and in the sentinel lymph node (first appearing fluorescent lymph node). (WMV 7774 kb)


