Abstract
Purpose
Internalizing symptoms have been associated with both higher and blunted cortisol responses in adolescents. Little attention has been paid to subjective experiences of distress in conjunction with internalizing symptoms in hypothalamic-pituitary-adrenal (HPA) axis responses to laboratory stressors. This report examines whether adolescents’ internalizing symptoms moderate the association between cortisol responses and distress in response to a common stressor in adolescence: family conflict. Differences are also examined between adolescents with current, past only, and no history of internalizing symptoms.
Methods
Adolescents (N = 70) discussed areas of conflict with their parents and subsequently reported on distress experienced during the discussion. Baseline and 5 post-stressor saliva samples were collected. Adolescents’ internalizing symptoms were assessed concurrently with the discussion and at three previous time-points.
Results
Internalizing symptoms moderated the association between adolescents’ reported distress and cortisol reactivity in response to family conflict. Adolescents with current and past internalizing symptoms had a blunted cortisol response, whereas adolescents with no history of internalizing symptoms showed greater cortisol reactivity when reporting greater distress.
Conclusions
This study expands the understanding of how current and remitted internalizing symptoms are related to adolescents’ responses to everyday family conflicts. Adolescents with current and past internalizing symptoms demonstrated a lack of correspondence between psychological and physiological stress, whereas adolescents with no history of internalizing symptoms showed the anticipated correspondence. This study has important implications for understanding the link between internalizing symptoms and adolescents’ HPA functioning in response to common social stressors.
Introduction
Extensive research illustrates the importance of salivary cortisol as an index of the hypothalamic-pituitary-adrenal (HPA) axis for understanding arousal and stress reactivity. Similar to physical stressors, acute psychological stress typically activates HPA responses; however, stress responses are related to the nature of the stressor and are subject to individual differences in the way threat is perceived [1,2,3,4]. The connection between psychological stress and short-term HPA activation is studied primarily via responses to laboratory-induced stressors, e.g. the Trier Social Stress Test (TSST) [5], as well as through monitoring stressful events in youths’ daily activities [1,6]. The present study examines adolescents’ HPA activity surrounding the social stress of conflictual parent-child discussions.
We also examine adolescents’ internalizing symptoms and subjective distress as putative influences on HPA activity. Because internalizing disorders include symptoms of social rejection and anxiety in social situations, persons with internalizing symptoms are anticipated to show heightened cortisol reactivity to tasks involving social evaluation. Studies examining HPA reactivity to short-term stressors show that adults and adolescents with internalizing symptoms demonstrate higher cortisol reactivity to stressors [7,8]. However, studies wherein parent-child discussions are the social stressor show mixed results. Klimes-Dougan and colleagues [9] reported that internalizing symptoms were associated with reductions in cortisol activity; Granger et al. (1996) reported that children’s internalizing symptoms were associated with HPA reactivity, but the direction of that reactivity was not clear [10]. Overlooked in previous studies is adolescents’ subjective experience of stress associated with family conflict and how this relates to and interacts with internalizing symptoms. Youth are likely to experience varying levels of distress in response to any stressor task, but particularly in response to parent-child conflict discussions. In adults, trait anxiety amplified the association between the subjective experience of short-term stressors and HPA axis reactions [11]. Examining whether symptoms of anxiety and depression amplify participants’ perceived situational stress may explain discrepant findings for cortisol reactivity.
Diurnal patterns of cortisol activity are another consideration in understanding HPA reactivity to short-term stressors in adolescents with internalizing disorders. The characteristic diurnal pattern for cortisol tends to be different in persons with internalizing disorders compared to those without, although the results vary [1,12,13]. The most consistent feature associated with internalizing symptoms is a flattening of the typical diurnal rise and fall, e.g., less decline overall [9], flatter evening patterns, [14] higher cortisol around sleep onset [15], and sluggish nocturnal rise [16]. Moreover, in youth with major depressive disorder, follow-up data show higher evening cortisol in youth with recurring, chronic depression, but not in youth without further episodes [17,18]. These findings highlight the need to consider time of day and whether internalizing symptoms are current or have remitted.
The present study examines youth’s experience of stress in conflictual family discussions with their parents and whether internalizing symptoms moderate their psychological and cortisol stress responses. Stress is examined in two ways: adolescents’ self-reports of distress following the discussion (subjective), and cortisol samples collected before and following the discussion (objective). The primary question is: Does subjective distress relate to cortisol activity in the same way for adolescents currently experiencing internalizing symptoms as those without such symptoms? We hypothesized an interaction between internalizing symptoms and adolescents’ reports of subjective distress related to the conflict discussions; we did not hypothesize whether internalizing symptoms will lessen or increase the anticipated association between subjective distress and cortisol activity given prior mixed findings. Second, we compare adolescents with current, past only, and no internalizing symptoms. Both current and past internalizing groups were expected to show different HPA responses from the group without internalizing symptoms, with greater differences for current symptoms. In all analyses, we controlled for time of day, pubertal status [19], use of medications [20], and gender [21] given reported associations with alterations in cortisol patterns.
Methods
Participants
Seventy adolescents (32 females; 38 males) and their parents participated in this study as part of a multi-wave project examining family conflict and children’s adjustment and physiology. Inclusion criteria for wave 1 were that the family had a child age 9-10, both parents lived with the child for at least three years, and all three participating family members spoke English. Of the 119 families participating in wave 1, 101 families were invited to participate in wave 4. The families represented here include those who agreed to participate in the discussion and to provide saliva samples (4 refused participation and 13 had scheduling difficulties or could not be located). Although a total of 84 families participated wave 4 procedures, 11 did not provide saliva samples (9 participated from home, and 2 declined participation in saliva collection). Two families had missing youth internalizing symptom data, and one adolescent reported mouth sores—these cases were not included, resulting in 70 adolescents. Adolescents’ mean age in the present study was 15.3 (SD = .8); 12.3 (SD = .7) in wave 3, 11.1 (SD=.7) in wave 2, and 10.0 (SD=.6) in wave 1. Adolescents are 41.4% Hispanic/Latino; race is 15.7% African American, 32.9% Caucasian, 10.0% Asian, and 41.4% multi-ethnic. Mean family income is $69,421 (SD = $36,813); 10% of families had an income < $20,000. Families participating in wave 4 did not differ significantly from non-participating families in terms of wave 1 internalizing and externalizing symptoms, mother’s education, and family income (all p values >.05). Participating wave 4 fathers had slightly more years of education, M=14.48 (SD=2.43) than non-participants, M=13.37 (SD=2.52), t(117) = −2.30, p = .02. Wave 4 boys vs. girls showed no significant differences in internalizing and externalizing symptoms, subjective distress, or cortisol measures.
Procedures
Waves 1-4 involved a 3-4 hour laboratory visit with both parents and the youth. The university IRB approved all procedures and we obtained parents’ consent and adolescents’ assent at each data collection wave. The assessment of internalizing symptoms was consistent across all waves. Wave 4 procedures included a 10-minute relaxation task, a 15-min conflictual family discussion, 6 saliva samples and several questionnaires. When scheduling the wave 4 appointment, we instructed participants not to eat or smoke for 1-hr before their appointment and not to consume alcohol or caffeine for 24-hrs prior to the appointment. Before saliva collection, parents and their adolescents completed a questionnaire on recent eating, drinking, smoking, and other health behaviors (e.g., medications taken) that could affect cortisol measurements. If families had eaten, we postponed cortisol measurements to allow for a 1hr interval before collection. We limited the hours in which we collected data to avoid the morning cortisol peak; the earliest T1 sample was 11:15, M = 13:56 hrs, SD = 2:04, range = 11:15 to 19:17. In general, we scheduled lab appointments at 11:00 and at 14:00; 80% of the families began before 16:00 with several exceptions due to parents’ work schedules.
Conflictual Family Discussion
To maximize the likelihood that the family discussed a topic that truly was conflictual, we administered a questionnaire assessing how much conflict they experienced with one another concerning 33 topics, and how upset these conflicts made them. Experimenters then conducted 5-min individual priming interviews with each family member, identifying areas of most intense and frequent conflict, and encouraging them to express their viewpoints. Based on the questionnaires and interviews, experimenters identified the three topics that were most conflictual and, when the family members were back together, gave the following instructions: “…Our purpose in having you do this discussion is to understand family disagreements and family members’ different points of view. So please make sure that each of you get your points across…” Immediately after the discussion, family members completed a questionnaire assessing reactions to the discussion and emotions they experienced. Twelve of the families engaged in dyadic discussions due to the unavailability of one parent, whereas all other families engaged in triadic discussions with both parents.
Saliva Samples
The 6 saliva samples occurred at the following intervals: baseline (T1), post-discussion (T2 standardized at T1 + 40 min), and four additional post-discussion (T3-T6) intervals: T2 + 10 min, T2 + 20 min, T2 + 40 min, T2 + 60 min. Experimenters set timers to ensure consistent timing of saliva collection. Baseline cortisol measurements (T1) occurred immediately after a standardized procedure in which family members watched a 10-minute video of nature images with relaxing music. T2 occurred directly after the discussion. During the next hour (T3-T6), family members worked on questionnaires in separate rooms with brief interruptions for additional saliva samples. The saliva samples were stored at −20 degrees Celsius and then shipped in dry ice for commercially available assay procedures (Salimetrics, State College PA) using a high-sensitive enzyme immunoassay. The samples were assayed for cortisol in duplicate for reliability, r(497)=.99, p<.0001; the mean of these two values was used for all analyses. Repeated analysis was used for sample pairs that had results differing more than 7%.
Salivary Cortisol
In addition to cortisol scores at baseline, two summary scores were calculated as indices of cortisol total output and reactivity. Cortisol output was measured through the total area under the curve with respect to ground (AUCg), and cortisol reactivity was measured through area under the curve with respect to increase (AUCi) [22]. As per Granger et al. (2006) [23], we followed a common procedure for outlier values on cortisol concentration (11 out of 490 total samples) and rescaled those values to three SDs above the mean for the relevant sampling interval. Cortisol AUCg scores were log-transformed to decrease the skewness of the distribution; AUCi was sufficiently normally distributed and did not require transformation.
Youth-Reported Internalizing and Externalizing Symptoms Collected in Waves 1-4
The Youth Self Report [24], a widely used 112-item questionnaire, measures broadband scales of internalizing (alpha = .89) and externalizing (alpha = .90) symptoms in youth ages 6-18 years. We examined wave 4 T-scores, based on national age norms for males and females, to measure current internalizing and externalizing symptoms. To examine the difference between youth who had current, past, or no internalizing symptoms over the four waves, we categorized adolescents into three groups: (a) those who currently report internalizing symptoms at a T-score ≥ 60 (n = 10); (b) those who had a T-score ≥ 60 in waves 1, 2, or 3, but not currently (n = 23); and (c) those who never had a T-score ≥ 60 (n = 37). Wave 4 internalizing T-scores ranged from 26 to 77, M= 49.0 (SD= 10.4). Externalizing symptoms, tested as a control variable in the analyses, ranged from 32 to 74, M = 51.8 (SD = 9.9).
Youth’s Subjective Ratings of Distress
The Post-Discussion Questionnaire contained 8 negative emotions (e.g., angry, frustrated, sad) reflecting subjective distress. Youth rated the degree to which they experienced each emotion on a scale from 0 (none) to 4 (a lot). Cronbach’s alpha for these items was .84. Scores ranged from 0 to 26 out of a possible 32 (M = 7.1, SD = 6.0). For group analyses, we defined high subjective distress as scores ≥ 10, indicating more than some distress on average across all distress items.
Saliva Sample Information
The saliva information questionnaire, given immediately following consent, included 20 questions regarding time of awakening, medications, and mouth sores (used as covariates and to assess sample validity), as well as most recent food and drink.
Pubertal Status
Parents completed Repetti and colleagues’ [25] non-intrusive estimate of pubertal development that assesses the degree to which growth spurts, skin changes, and body hair growth have begun on a scale from 1 (has not yet begun) to 4 (has been completed). For the three items, scores ranged from 6 to 10 (M = 8.5, SD = .9) for males, and from 6 to 12 (M = 9.2, SD = 1.5) for females, indicating that all adolescents had begun puberty.
Results
Table 1 displays the means and SDs for the total sample and each internalizing group. Other than the current internalizing T-score, which was used for grouping, analyses of covariance (ANCOVA), adjusting for time since awakening, the perception of puberty-related changes, and gender revealed no significant differences between internalizing groups. Partial correlations between the Table 1 variables using the same covariates yielded a positive correlation between subjective reports of distress and current internalizing T-scores, r(70) = .38, p = .002. There were no other significant correlations. In our sample, 41% of adolescents were “responders,” or demonstrated an increase in cortisol after the discussion (i.e., a positive AUCi).
Table 1.
Means (and Standard Deviations) for the Total Sample and for Adolescents with No, Past Only, and Current Internalizing Symptoms
| Variables | Total Sample (n = 70) |
No Internalizing (n = 37) |
Past Only Internalizing (n = 23) |
Current Internalizing (n = 10) |
F (2, 70) |
|---|---|---|---|---|---|
| % Female | 45.7 | 48.6 | 39.1 | 50.0 | --- |
| Mean Age | 15.3 (.8) | 15.3 (.7) | 15.4 (1.0) | 15.4 (.5) | .43 |
| Subjective Distress | 7.1 (6.0) | 6.1 (5.6) | 7.2 (4.7) | 10.7 (8.9) | 1.09 |
| Current Internalizing T | 49.0 (10.4) | 45.0 (8.7) | 48.2 (7.2) | 65.9 (4.4) | 11.62** |
| Cortisol AUCg | 526.9 (311.9) | 528.3 (326.7) | 578.8 (328.9) | 402.2 (174.6) | 1.40 |
| Cortisol AUCi | 14.5 (267.5) | 45.0 (308.5) | −1.5 (242.0) | −61.9 (117.1) | 1.30 |
| Baseline Cortisol | .09 (.06) | .09 (.06) | .10 (.06) | .08 (.03) | .40 |
| Post-discussion Cortisol | .09 (.07) | .09 (.07) | .11 (.08) | .08 (.04) | .39 |
p < .001 Note. Above analyses adjusted for time since awakening, pubertal development, and gender (excluded for ANCOVA with gender as the dependent variable). Cortisol AUCg was log-transformed for ANCOVA. Baseline and post-discussion cortisol concentrations are in ug/dL.
Internalizing Symptoms as a Moderator of the Association between Subjective Distress and Cortisol Reactivity
Table 2 presents multiple regression analyses examining the effects of current internalizing symptoms, subjective distress, and their interaction on cortisol AUCg and AUCi. Exploratory analyses controlled for externalizing symptoms because of their co-morbidity with internalizing symptoms [26] and their association with low cortisol [27], but the results were not altered; they were not examined further. Annual family income, days since last period (for females), parents’ years of education, and medications were also examined as covariates. These controls did not significantly affect the results and were thus not included. All analyses presented in this report adjust for time since awakening, the perception of puberty-related changes, and gender. Youth-reported internalizing symptoms and subjective distress were entered into the model, centered on mean scores, followed by the interaction between these two variables. For total cortisol output (AUCg), there was a significant main effect for subjective distress, which was associated with higher cortisol AUCg, and a significant effect for internalizing symptoms, which were associated with lower cortisol AUCg. The interaction between internalizing symptoms and subjective distress for AUCg was not significant.
Table 2.
Regressions Examining Subjective Distress, Current Internalizing Symptoms, and Cortisol AUCg and AUCi
| Cortisol AUCg | Cortisol AUCi | |||||||
|---|---|---|---|---|---|---|---|---|
| β | T | F | Adj R2 | β | T | F | Adj R2 | |
| Step 1 | 2.94* | .12 | 1.46 | .03 | ||||
| Current Internalizing Symptoms | −.35 | −2.80** | −.19 | −1.45 | ||||
| Subjective Distress | .31 | 2.48* | .19 | 1.49 | ||||
| Step 2 | 2.42* | .11 | 2.34* | .10 | ||||
| Current Internalizing Symptoms | −.35 | −2.75** | −.25 | −1.94 | ||||
| Subjective Distress | .31 | 2.27* | .34 | 2.47* | ||||
| Current Internalizing Symptoms X | −.02 | −.13 | −.32 | −2.48* | ||||
| Subjective Distress | ||||||||
p < .05.
p <.01.
Note. Equations adjust for time since awakening, perceived puberty-related changes, and gender. Cortisol AUCg was log-transformed; Cortisol AUCi was sufficiently normally distributed and did not need transformation. YSR T-scores and subjective distress scores were centered on their means.
In contrast, for cortisol AUCi, there was a significant interaction between internalizing symptoms and subjective distress. The interaction indicates that internalizing symptoms moderate the association between subjective distress and cortisol reactivity. The slope for adolescents with low internalizing symptoms was greater than zero, T(4,66)=3.04, p=.003, whereas the slope for internalizing adolescents did not differ from zero T(4,67)=−.32, ns. For youth with few internalizing symptoms, high distress during the discussion relates to high AUCi, and low distress relates to low AUCi. Youth with high internalizing symptoms, in contrast, show low cortisol reactivity, even when reporting high subjective distress. This interaction suggests that current internalizing symptoms are associated with a blunted physiological response to the family discussion.
Cortisol Reactivity and Subjective Distress in Adolescents with No, Past Only, and Current Internalizing Symptoms
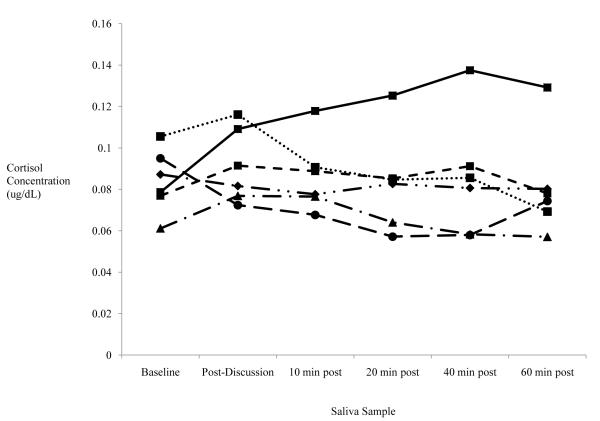
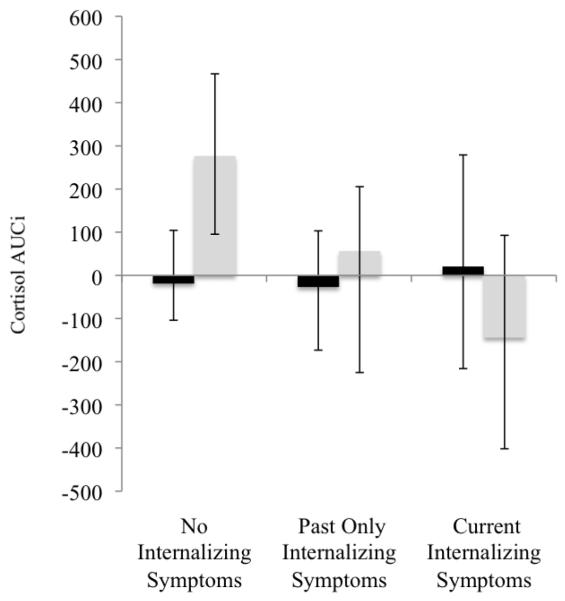
Figure 1 displays mean cortisol concentrations over time by internalizing symptom group and level of subjective distress. To clarify whether HPA activation is differentially related to current, past, or no history of internalizing symptoms, we conducted 3 (group: no, past only, and current internalizing symptoms) by 2 (high versus low subjective distress) ANCOVAs on AUCg and AUCi. There were no significant findings for cortisol AUCg. However, we found a significant main effect for group with cortisol AUCi, F(2,70) = 3.28, p = .04, and for the interaction between group and subjective distress, F(2,70) = 3.50, p = .04. Figure 2 displays AUCi means for high and low subjective distress in the three internalizing symptom groups. Simple contrasts demonstrate that cortisol AUCi is greater in adolescents with no internalizing symptoms than in adolescents with past only, p ≤ .05, and current internalizing symptoms, p ≤ .05. No significant difference in cortisol AUCi was found between past only and current internalizing symptom groups. Furthermore, planned comparisons show significantly higher cortisol AUCi at high versus low reports of subjective distress for adolescents with no internalizing symptoms, F(1,37) = 3.28, p = .02, but not in the internalizing groups. In addition, there is a significant correlation between distress and AUCi for adolescents with no history of internalizing symptoms, r(37) = .37, p < .05, but not for those with current or past internalizing symptoms, r(33) = −.05, ns.
Figure 1.
Cortisol Concentrations Across Time by Internalizing and Distress Group
 .No Internalizing Symptoms-Low Distress
.No Internalizing Symptoms-Low Distress
 Past Only Internalizing Symptoms-Low Distress
Past Only Internalizing Symptoms-Low Distress
 Current Internalizing Symptoms-Low Distress
Current Internalizing Symptoms-Low Distress
 No Internalizing Symptoms-High Distress
No Internalizing Symptoms-High Distress
 Past Only Internalizing Symptoms-High Distress
Past Only Internalizing Symptoms-High Distress
 Current Internalizing Symptoms-High Distress
Current Internalizing Symptoms-High Distress
Figure 2.
Cortisol AUCi Across Internalizing Symptom Groups and Subjective Distress Level
■ Low Subjective Distress
 High Subjective Distress
High Subjective Distress
Note. AUCi = Area under the curve with respect to increase.
Discussion
The present study demonstrates that internalizing symptoms are linked to a lower HPA response in adolescents, even after accounting for time since awakening, pubertal development, gender, and other potentially influential factors. Specifically, there is a correspondence between subjective distress and HPA reactivity in adolescents without internalizing symptoms. However, those with internalizing symptoms do not show a correspondence between subjective distress and HPA reactivity. To assess whether a lower HPA response persists after internalizing symptoms have remitted, we also examined whether the presence of current, previous, or no history of internalizing symptoms differentially affects HPA reactivity to family conflict. A history of internalizing symptoms related to lower cortisol reactivity, even if symptoms were not currently present.
Increased HPA activity focuses attention, improves cognitive functioning, and regulates behavioral and emotional responses [28]. Thus, HPA activity generally increases when individuals face emotionally or physically challenging circumstances. We designed the present study to create an ecologically-valid, stress-evoking situation for adolescents through conflictual family discussions. Adolescents’ subjective ratings show that some youth actually experienced the conversations as distressing whereas others did not. Those who reported subjective distress also displayed anticipated cortisol increases—but only if they did not report internalizing symptoms. Thus, in the group of adolescents without internalizing symptoms, there was a correspondence between reported emotional distress and physiological activity. These adolescents displayed what might be characterized as emotionally and physiologically attuned responses to a situation experienced as personally distressing.
The adolescents with current and past internalizing symptoms, in contrast, did not show the anticipated correspondence between subjective distress and HPA activity. Adolescents with internalizing symptoms reported comparable rates of subjective distress to the group without internalizing symptoms; we cannot attribute this low HPA activity to lack of subjective distress. Moreover, there was a modest positive correlation between internalizing symptoms and subjective distress, suggesting that adolescents with internalizing symptoms might be expected to show greater, rather than less, cortisol reactivity. The low HPA axis activity for adolescents with internalizing symptoms thus reflects a lack of correspondence between subjective distress and physiological reactivity—not a lack of emotional distress.
The lack of concordance between reported distress and physiological reactivity has several possible explanations. On the one hand, the absence of HPA reactivity to stressors may reflect disruptions in the neurobiological stress response and may be a marker for psychological problems in adolescents [13]. Without the benefit of HPA reactivity, which typically prepares individuals to handle the external environment, some adolescents may be more prone to show the poor coping that accompanies internalizing symptoms [29]. On the other hand, reduced cortisol reactivity to distress may be a functional pattern for adolescents with internalizing symptoms. That is, those experiencing chronic stress may have physiologically habituated to common emotionally distressing stimuli. Whereas some research on internalizing adolescents supports HPA hyper-arousal in response to a performance task, a few studies show the opposite pattern of lower cortisol activity in response to psychosocial stressors [9,30], which is the pattern demonstrated here. Adolescents with internalizing symptoms may become accustomed to recurring psychosocial stressors, family conflict being one such example. Thus, for these adolescents, low HPA activity may be adaptive.
Our findings of lowered cortisol reactivity in adolescents with remitted internalizing symptoms adds to a growing literature on longitudinal associations between HPA functioning and internalizing symptoms. Those who have remitted major depression demonstrate blunted HPA reactivity to psychosocial stressors [31]. Although sometimes viewed as a consequence of depression, alterations in HPA activity also appear to be biological risk factors for longer recovery and relapse of internalizing symptoms in adolescents [13,32,33,34,35]. Despite increasing evidence supporting links between HPA activity and internalizing problems, particularly in the context of high social stress [33], the direction of effects and implications of HPA reactivity for adaptation are unknown.
Limitations and future directions warrant consideration. Due to the sample size, the number of participants in the current and past only internalizing groups was small, potentially obscuring differences between these two groups that we were unable to detect. Moreover, although we differentiated adolescents with current versus past internalizing symptoms, there is complexity to the trajectories of internalizing symptoms beyond what we could examine here. For example, future research should address questions related to symptom onset, duration, time elapsed following symptom remission, and differences in those with both past and current versus current only internalizing symptoms. Examining depression or anxiety alone, as contrasted with internalizing symptoms, could further explain certain results as different emotions have been linked to different HPA responses [36]. In addition, measuring cortisol over several days would provide an assessment of intra-individual differences in situations of high versus low interpersonal stress. Other clinically relevant future research suggested here includes whether interventions would lead to increased HPA reactivity in adolescents after internalizing symptoms lessen [37], and whether, by incorporating observational measures, we would find concordance between behavioral measures of stress, self-reported stress, and systems of physiological stress [38, 39].
Despite some limitations, this study demonstrated that family conflict, an experience common to adolescents [40], elicited a cortisol response in some adolescents but led to decreased cortisol in others. By examining subjective distress and HPA reactivity, this study reveals patterns of concordance between perceived distress and physiological reactivity and thus expands upon previous findings of internalizing youth and HPA activity where the emotional impact of the stressor was unknown. These results, however, highlight the need for future research identifying under what circumstances lack of correspondence between cortisol activity and perceived distress ultimately is protective or problematic for adolescents.
Acknowledgements
This research was supported by NIH-NICHD Grant R01 HD046807 awarded to Margolin, Gordis, and Oliver, and from the David & Lucile Packard Foundation Grant 00-12802 awarded to Margolin. Work also was supported by K23 HD041428 awarded to Gordis. We are grateful to the families who participated in the study. We are also very appreciative of other members of the USC Family Studies Project, including Diana Bennett, Elyse Guran, Esti Iturralde, Michelle Ramos, Aubrey Rodriguez, Sarah Duman Serrano, and Katrina Vickerman.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Adam EK. Transactions among adolescent train and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31:664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- [2].Gunnar M, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- [3].Kudielka BM, Hellhammer DH, Wüst S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology. 2009;34:2–18. doi: 10.1016/j.psyneuen.2008.10.004. [DOI] [PubMed] [Google Scholar]
- [4].Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the Hypothalamic-Pituitary-Adrenocortical axis in humans. Psychol Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- [5].Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- [6].Flinn MV, England BG. Childhood stress and family environment. Curr Anthropol. 1995;36:854–866. [Google Scholar]
- [7].Burke HM, Davis MC, Otte C, et al. Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology. 2005;30:84–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- [8].Dockray S, Susman EJ, Dorn LD. Depression, cortisol reactivity, and obesity in childhood and adolescence. J Adolesc Health. 2009;45(4):344–350. doi: 10.1016/j.jadohealth.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Klimes-Dougan B, Hastings PD, Granger DA, et al. Adrenocortical activity in at-risk and normally developing adolescents: Individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Dev Psychopathol. 2001;13:695–719. doi: 10.1017/s0954579401003157. [DOI] [PubMed] [Google Scholar]
- [10].Granger DA, Weisz JR, McCracken JT, et al. Reciprocal influences among adrenocortical activation, psychosocial processes, and the behavioral adjustment of clinic-referred children. Child Dev. 1996;67(6):3250–3262. [PubMed] [Google Scholar]
- [11].Schlotz W, Schulz P, Hellhammer J, et al. Trait anxiety moderates the impact of performance pressure on salivary cortisol in everyday life. Psychoneuroendocrinology. 2006;31:459–472. doi: 10.1016/j.psyneuen.2005.11.003. [DOI] [PubMed] [Google Scholar]
- [12].Haglund ME, Nestadt PS, Cooper NS, et al. Psychobiological mechanisms of resilience: Relevance to prevention and treatment of stress-related psychopathology. Dev Psychopathol. 2007;19:889–920. doi: 10.1017/S0954579407000430. [DOI] [PubMed] [Google Scholar]
- [13].Shirtcliff EA, Essex MJ. Concurrent and longitudinal association of basal and diurnal cortisol with mental health symptoms in early adolescence. Dev Psychobiol. 2008;50(7):690–703. doi: 10.1002/dev.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Van den Bergh BR, Calster B, Puissant S, et al. Self-reported symptoms of depressed mood, trait anxiety and aggressive behavior in post-pubertal adolescents: Associations with diurnal cortisol profiles. Horm Behav. 2008;54:253–257. doi: 10.1016/j.yhbeh.2008.03.015. [DOI] [PubMed] [Google Scholar]
- [15].Forbers E, Willamson DE, Ryan ND, et al. Peri-sleep-onset cortisol levels in children and adolescents with affective disorders. Biol Psychiatry. 2006;59:24–30. doi: 10.1016/j.biopsych.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Feder A, Coplan JD, Goetz RR, et al. Twenty-four-hour cortisol secretion patterns in prepubertal children with anxiety or depressive disorders. Biol Psychiatry. 2004;56:198–204. doi: 10.1016/j.biopsych.2004.05.005. [DOI] [PubMed] [Google Scholar]
- [17].Goodyer IM, Park RJ, Herbert J. Psychosocial and endocrine features of chronic first-episode major depression in 8-16 year olds. Biol Psychiatry. 2001;50:351–357. doi: 10.1016/s0006-3223(01)01120-9. [DOI] [PubMed] [Google Scholar]
- [18].Rao U, Dahl RE, Ryan ND, et al. The relationship between longitudinal clinical course and sleep and cortisol changes in adolescent depression. Biol Psychiatry. 1996;40:474–484. doi: 10.1016/0006-3223(95)00481-5. [DOI] [PubMed] [Google Scholar]
- [19].Matchock RL, Dorn LD, Susman EJ. Diurnal and seasonal cortisol, testosterone, and DHEA rhythms in boys and girls during puberty. Chronobiol Int. 2007;24(5):969–990. doi: 10.1080/07420520701649471. [DOI] [PubMed] [Google Scholar]
- [20].Granger DA, Hibel LC, Fortunato CK, et al. Medication effects of salivary cortisol: Tactics and strategy to minimize the impact in behavioral and developmental science. Psychoneuroendocrinology. 2009;34:1437–1448. doi: 10.1016/j.psyneuen.2009.06.017. [DOI] [PubMed] [Google Scholar]
- [21].Kirschbaum C, Kudielka BM, Gaab J, et al. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- [22].Pruessner JC, Kirschbaum C, Meinlschmid G, et al. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- [23].Granger DA, Kivlighan KT, Blair C, et al. Integrating the measurement of salivary α-amylase into studies of child health, development, and social relationships. J Soc Pers Relat. 2006;23:267–290. [Google Scholar]
- [24].Achenbach T. Manual for the Youth Self-Report. University of Vermont, Department of Psychiatry; Burlington: 1991. [Google Scholar]
- [25].Saxbe DE, Repetti RL. Brief report: Fathers’ and mothers’ marital relationship predicts daughters’ pubertal development two years later. J Adolesc. 2009;32(2):415–423. doi: 10.1016/j.adolescence.2008.06.009. [DOI] [PubMed] [Google Scholar]
- [26].Angold A, Costello EJ, Erkanli A. Comorbidity. J Child Psychol Psychiatry. 1999;40(1):57–87. [PubMed] [Google Scholar]
- [27].Susman EJ. Psychobiology of persistent antisocial behavior: Stress, early vulnerabilities and the attenuation hypothesis. Neurosci Biobehav Rev. 2006;30:376–389. doi: 10.1016/j.neubiorev.2005.08.002. [DOI] [PubMed] [Google Scholar]
- [28].Ellis BJ, Boyce WT. Biological sensitivity to context. Current Directions in Psychol Sci. 2008;17:183–187. [Google Scholar]
- [29].Lonigan CJ, Vasey MW. Negative affectivity, effortful control, and attention to threat-relevant stimuli. J Abnorm Child Psychol. 2009;37:387–399. doi: 10.1007/s10802-008-9284-y. [DOI] [PubMed] [Google Scholar]
- [30].Cicchetti D, Rogosch FA, Gunnar MR, et al. The differential impacts of early abuse on internalizing problems and diurnal cortisol activity in school-aged children. Child Dev. doi: 10.1111/j.1467-8624.2009.01393.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ahrens T, Deuschle M, Krum B, et al. Pituitary-adrenal and sympathetic nervous system responses to stress in women remitted from recurrent major depression. Psychosom Med. 2008;70:461–467. doi: 10.1097/PSY.0b013e31816b1aaa. [DOI] [PubMed] [Google Scholar]
- [32].Goodyer IM, Jerbert J, Tamplin A, et al. Recent life events, cortisol, dehydroepiandrosterone and the onset of major depression in high-risk adolescents. Br J Psychiatry. 2000;177:499–504. doi: 10.1192/bjp.177.6.499. [DOI] [PubMed] [Google Scholar]
- [33].Rao U, Hammen CL, Poland RE. Depression: Neuroendocrine and psychosocial predictors. J Am Acad Child Adolesc Psychiatry. 2010;49(2):141–151. doi: 10.1097/00004583-201002000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zobel AW, Yassouridis A, Frieboes R, et al. Prediction of medium-term outcome by cortisol response to the combined dexamethasone-CRH test in patients with remitted depression. Am J Psychiatry. 1999;156:949–951. doi: 10.1176/ajp.156.6.949. [DOI] [PubMed] [Google Scholar]
- [35].Chen E, Cohen S, Miller GE. How low socioeconomic status affects 2-year hormonal trajectories in children. Psychol Sci. 2010;21:31–37. doi: 10.1177/0956797609355566. [DOI] [PubMed] [Google Scholar]
- [36].Denson TF, Spanovic M, Miller N. Cognitive Appraisals and Emotions Predict Cortisol and Immune Responses: A Meta-Analysis of Acute Laboratory Social Stressors and Emotion Inductions. Psychol Bull. 2009;135(6):823–853. doi: 10.1037/a0016909. [DOI] [PubMed] [Google Scholar]
- [37].Carlson LE, Speca M, Patel KD, et al. Mindfulness-based stress reduction in relation to quality of life, mood, symptoms of stress and levels of cortisol, dehydroepiandrosterone sulfate (DHEAS) and melatonin in breast and prostate cancer outpatients. Psychoneuroendocrinology. 2004;29(4):448–474. doi: 10.1016/s0306-4530(03)00054-4. [DOI] [PubMed] [Google Scholar]
- [38].Gordis EB, Margolin G, Spies LA, et al. Interparental aggression and parent-adolescent salivary alpha amylase symmetry. Physiol Behav. 2010;100(3):225–33. doi: 10.1016/j.physbeh.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gordis E, Granger DA, Susman EJ, Trickett PK. Asymmetry between salivary cortisol and α-amylase reactivity to stress: Relation to aggressive behavior in adolescents. Psychoneuroendocrinology. 2006;31:976–987. doi: 10.1016/j.psyneuen.2006.05.010. [DOI] [PubMed] [Google Scholar]
- [40].Laursen B. Conflict and social interaction in adolescent relationships. J Res Adoles. 1995;5(1):55–70. [Google Scholar]