Abstract
Background
The effects of maternal subclinical hypothyroidism (M-SCH) on the neuropsychological development of the offspring are not clear. We evaluated the intellectual development of children of mothers who had M-SCH during the pregnancy for these children.
Methods
Sixty-two children were recruited. After excluding those age <4 or age >15, 44 were enrolled. The mothers of these children were part of a sub-pool of 90, of 441 hypothyroid women of reproductive age seen in Tehran endocrine clinics between 1991 and 2003 and who were observed during gestation. Mothers were receiving levothyroxine (LT4) before gestation. Mothers of 19 children (control group) had normal serum thyrotropin (TSH) during the pregnancy that produced these children. Mothers of the other 25 children had increased TSH during the comparable pregnancy. Nineteen mothers had M-SCH (case group) and six had overt hypothyroidism. Serum TSH and free T4 (FT4) and urine iodine were measured, and seven cognitive performance and intelligence quotient (IQ) tests were performed.
Results
Case children were similar to control children with respect to gender, age, parental education, maternal age at time of pregnancy and at the time of their hypothyroidism, percent mothers having thyroid peroxidase antibodies, LT4 dose of mothers during pregnancy, gestational age at delivery, birth weight, and duration of breast feeding. Maternal TSH (mean±standard deviation) in the case group during their mother's pregnancies was 11.3±5.3 and 1.4±1.0 mU/L in the controls (p<0.001). Serum TSH, FT4 and urinary iodine concentrations were similar in the two groups. Total IQ, performance IQ, and verbal IQ were similar, being 120±14, 117±12, and 121±16, respectively, in the case group and 121±11, 120±7, and 117±15 in the control group. Cognitive performance tests were similar in both groups. No relationships were observed between variables and IQ except for education level of the mother and neonatal weight.
Conclusion
IQ level and cognitive performance of children born to LT4-treated hypothyroid mothers is similar in those whose mothers have M-SCH during pregnancy compared with those whose mothers have normal serum TSH concentrations during pregnancy.
Introduction
Subclinical hypothyroidism (SCH), a relatively common abnormality in pregnancy, occurs in approximately 3% of pregnant women (1). Maternal hypothyroidism may be associated with myopathy, congestive heart failure, anemia, hypertension and preeclampsia, and placental abnormalities (2,3).
Neuropsychological deficits in the offspring have been observed in overt maternal hypothyroidism (4) and in infants born to mothers with isolated hypothyroxinemia during the first trimester of pregnancy (5–7).
Autoimmune thyroiditis is present in approximately 55% of women with SCH. Pregnant women with SCH have higher incidence of placental abortion, and prenatal and neonatal morbidity and mortality (8,9), although contradictory results have also been reported (10). However, it is not clear if SCH during pregnancy affects the neuropsychological development of the offspring (11). In one study, even when hypothyroid pregnant women were insufficiently treated with levothyroxine (LT4), the intelligence quotient (IQ) scores of their offspring were not different from those of controls (4). Therefore, this study was designed to evaluate the effect of maternal subclinical hypothyroidism (M-SCH) on possible neuropsychological deficits in the offspring.
Materials and Methods
This was a historical cohort study. From a total of 441 hypothyroid patients of reproductive ages, seen in endocrine clinics in Tehran, between the years 1991 and 2003, 90 women with 106 pregnancies were observed. All patients (cases and controls) were known cases of hypothyroidism before gestation and were adequately treated with LT4 and serum thyrotropin (TSH) in every patient was kept ≤2.5 mU/L before gestation. There were 10 miscarriages. In 2007, 62 (65%) children born to these mothers were recruited for this study. There was no statistical difference in age, and serum concentrations of TSH and thyroid hormones in mothers of 62 children recruited and mothers of other children who were not available for study.
Children under 4 years of age (n=17) and above 15 years of age (n=1) were omitted from the study. The remaining 44 children were divided into two groups, based on the mother's serum TSH values during pregnancy: TSH ≤3 mU/L, at least in two occasions in the first half of pregnancy, as control group (n=19), and TSH >3 mU/L, in at least two occasions in the first half of the pregnancy (n=25, of whom 19 had SCH, as the case group). One serum TSH >3 mU/L had been detected in each mother of case group before the 10th week of gestation. Serum T4≥7.0 μg/dL was considered normal in pregnant mothers.
The importance of study was explained to parents and a signed consent form was obtained. The parents completed questionnaires, and were asked regarding systemic and thyroid diseases, and growth and development of the child. None of the mothers in the two groups smoked cigarettes or consumed alcohol during pregnancy. A blood sample for TSH and free T4 (FT4) and a urine sample for iodine measurements were taken from each child. Serum samples were kept at −70°C till the time of examination.
IQ and cognitive performance tests were done by expert psychologists at the Institute of Cognitive Sciences of Tehran University of Medical Sciences. The examiner was unaware of the result of mothers' thyroid function during pregnancy. IQ and cognitive performance were measured by seven different tests: Wechsler Intelligence Scale for Children, Spatial Working Memory, Pattern Recognition Memory, Stocking of Cambridge, Continuous Performance, Wisconsin Card Sorting, and the Motor Free Visual Perception Test (12).
Serum FT4 and T4 were measured by RIA and serum TSH was measured by IRMA (Izotope, Budapest, Hungary) using the gamma counter Wallac Wizard, Turku, Finland. The inter- and intra-coefficients of variation were 2.8% and 3.7% for T4, 2.7% and 3.0% for FT4, and 3.8% and 4.9% for TSH, respectively. Urinary iodine was assayed by the acid digestion method; inter- and intra-coefficients of variation for urinary iodine were 7% and 8.4%, respectively.
Statistical analysis
The normality of the distribution of dependent variables was examined. To compare the two groups, the t-test was used for comparison of normal variables and the Mann–Whitney test was used for variables with non-normal distribution. Pearson correlation test was used for estimating the correlation between IQ and other variables. SPSS 16 software was used for statistical studies. p-Values<0.05 were considered significant.
The study was approved by the ethics committee of the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences.
Results
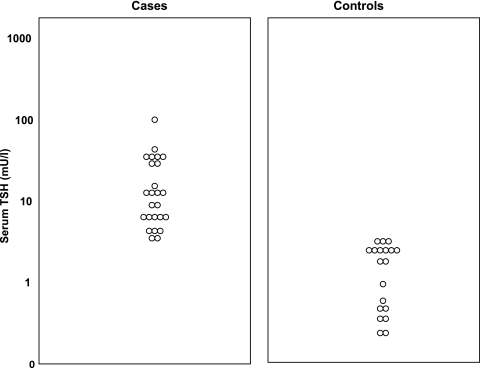
The mean age of children was 7.8, with a range of 4.0–14.5 years. There were 9 children aged 4–6 years and 16 children aged 6–15 years in the group of mothers whose TSH was elevated during the children's pregnancy; in the control group, these numbers were 5 and 14, respectively. In the group of children whose mothers had elevated serum TSH during the children's pregnancy, 19 mothers (76%) had subclinical hypothyroidism and 6 mothers (24%) had overt hypothyroidism during pregnancy. By definition, serum T4 concentrations in mothers with overt hypothyroidism during pregnancy had to be low and serum T4 concentrations in mothers who had SCH had to be normal during pregnancy. The 19 children whose mothers had SCH during their pregnancy made up the case group. The basic characteristics of 19 children in the case group and 19 children in the control group are shown in Table 1. There was no statistically significant difference among the basic characteristics, except for maternal TSH and T4, in two groups. Since maternal serum TSH was the parameter that determined assignment of children to case and control groups it follows that maternal serum TSH concentrations were elevated in mothers of case group children compared with mothers of control group children. Serum TSH concentrations of mothers in the case and control groups are shown in Figure 1. In addition, maternal serum T4 concentrations were slightly but significantly lower in mothers of case children compared with mothers of control children.
Table 1.
Basic Characteristics of Children Born to Mothers with Maternal Subclinical Hypothyroidism (Case) and Control Chiildren
| Variable | Cases (n=19) | Controls (n=19) |
|---|---|---|
| Male/Female (n)* | 8/11 | 10/9 |
| Age of children (year) | 7.9±3.2 | 7.5±2.1 |
| Educational level of father (year) | 13.5±3.0 | 12.9±3 |
| Educational level of mother (year) | 12.5±2.9 | 13.3±2.4 |
| Mother age at time of hypothyroidism (year) | 28.2±4.9 | 27.6±4.3 |
| Mother age at pregnancy (year) | 29.7±5.0 | 29.9±4.9 |
| Positive TPOAb in mother (%)* | 65 | 71 |
| Levothyroxine dose in pregnancy (μg/day) | 118±35 | 109±23 |
| Duration of maternal hypothyroidism (year) | 4.2±3.0 | 4.5±3.5 |
| Maternal TSH (mU/L) during pregnancy | ||
| Mean±SD | 11.3±5.3a | 1.4±0.96 |
| Range* | 3.9–27.0 | 0.1–2.8 |
| Maternal T4 (μg/dL) during pregnancy | ||
| Mean±SD | 9.0±2.1a | 11.7±2.6 |
| Range* | 7.1–12.0 | 7.6–14.3 |
| Gestational age at delivery (week) | 39.3±1.5 | 39.4±1.3 |
| Cesarean/vaginal delivery (n)* | 12/7 | 11/8 |
| Birth weight (g) | 3319±651 | 3341±433 |
| Duration of breastfeeding (month) | 18.4±8.3 | 18.8±6.1 |
Data values are presented as mean±SD, except those indicated by *.
p<0.001.
TPOAb, thyroperoxidase antibody; TSH, thyrotropin; T4, thyroxine; SD, standard deviation.
FIG. 1.
Serum TSH concentration in the mothers of case and control groups during pregnancy. TSH, thyrotropin.
No statistically significant differences were seen in serum TSH, FT4, or urinary iodine between case and control group children (Table 2). Verbal performance and total IQ were not statistically different between the case and control groups (Table 3). None of the children had IQ <85. On the whole, there were no significant differences between case and control groups in any of the cognitive performance tests. No significant difference was seen between two groups in terms of the parameters of cognitive performance (Table 4). The mean difference between IQ of two groups must have been 13.2 to have a significant effect size, with 90% power and 5% type 1 error.
Table 2.
Serum Thyrotropin, Free Thyroxine, and Urinary Iodine Concentrations in Studied Children
| Variable | Cases (n=19) | Controls (n=19) | p-Value |
|---|---|---|---|
| TSH (mU/L) | 3.2±1.9 | 3.3±1.6 | NS |
| FT4 (pmol/L) | 13.8±3.1 | 13.9±2.2 | NS |
| Urine iodine (μg/L) | 162±87 | 140±69 | NS |
Data values are presented as mean±SD.
NS, nonsignificant; FT4, free thyroxine.
Table 3.
Total, Performance, and Verbal Intelligence Quotient of Children by the Wechsler Intelligence Scale in Children of Two Study Groups
| Variable | Cases (n=19) | Controls (n=19) |
|---|---|---|
| Total IQ | 120±14 | 121±11 |
| Performance IQ | 117±12 | 120±7 |
| Verbal IQ | 121±16 | 117±15 |
Data values are presented as mean±SD.
IQ, Intelligence quotient.
Table 4.
Results of Cognitive Performance Tests of Children Whose Mothers Had Subclinical Hypothyroidism During Pregnancy and Control Children
| Cognitive performance test | Cases (n=19) | Controls (n=19) | p-Value |
|---|---|---|---|
| Continuous performance test | |||
| Omission errors | 4.2±4.2 | 6.1±3.5 | 0.2 |
| Commission errors | 8.9 (3–10) | 10.5 (8–38) | 0.04 |
| Mean reaction time (second) | 0.79±0.07 | 0.81±0.15 | 0.6 |
| Wisconsin card sorting test | |||
| Conceptual level responses | 23±14 | 30±12 | 0.19 |
| Number category completed | 1.86±1.24 | 2.21±1.25 | 0.46 |
| Preservative responses | 27.7±16.5 | 17.6±9.4 | 0.06 |
| Corrective responses | 32.1±11.3 | 37.8±9.0 | 0.2 |
| Motor free visual perception test | 31.2±6.7 | 32.5±5.1 | 0.4 |
| Stocking of cambridge test | |||
| Mean initial thinking time(2 moves) (millisecond) | 2694±2811 | 2564±2208 | 0.9 |
| Mean initial thinking time (3 moves) | 7311±4449 | 6667±5293 | 0.7 |
| Mean initial thinking time (4 moves) | 7547±4783 | 1006±1135 | 0.5 |
| Mean initial thinking time (5 moves) | 9054±1110 | 6039±5779 | 0.4 |
| Mean moves (2 moves) | 2 (2–2) | 2 (2–2) | 0.5 |
| Mean moves (3 moves) | 3.32±0.46 | 3.78±0.95 | 0.1 |
| Mean moves (4 moves) | 6.62±1.27 | 5.73±1.03 | 0.05 |
| Mean moves (5 moves) | 7.55±1.8 | 7.53±1.45 | 0.97 |
| Mean subsequent thinking time (2 moves) (millisecond) | 225 (0–1049) | 575 (0–1636) | 0.7 |
| Mean subsequent thinking time (3 moves) | 572 (0–5928) | 1472 (0–6519) | 0.8 |
| Mean subsequent thinking time (4 moves) | 9140±5424 | 6394±6862 | 0.3 |
| Mean subsequent thinking time (5 moves) | 2798±2345 | 2584±2600 | 0.8 |
| Problems solved in minimum moves | 6.6±1.7 | 6.5±2.2 | 0.9 |
| Pattern recognition memory | |||
| Mean correct latency (millisecond) | 3240±873 | 2887±558 | 0.2 |
| Number correct | 20.6±2.2 | 20.5±1.6 | 0.8 |
| Percent correct | 86±9 | 85±7 | 0.8 |
| Spatial working memory test | |||
| Between errors | 46.7±19.3 | 52.8±20.2 | 0.4 |
| Between errors (4 boxes) | 1.2±2.0 | 2.6±2.5 | 0.09 |
| Between errors (6 boxes) | 15.2±12.2 | 16.1±6.9 | 0.8 |
| Between errors (8 boxes) | 30.2±8.77 | 34.1±13.2 | 0.3 |
| Double errors | 2.86±4.29 | 1.85±1.99 | 0.4 |
| Double errors (4 boxes) | 0 | 0 | 0.3 |
| Double errors (6 boxes) | 0 | 0 | 0.7 |
| Double errors (8 boxes) | 1 (0–3) | 0.5 (0–3) | 0.9 |
| Strategy | 37.26±5.13 | 39.14±5.28 | 0.3 |
| Total errors | 48.1±20.5 | 54.2±20.2 | 0.4 |
| Within errors | 4.3±5.8 | 3.2±3.4 | 0.6 |
| Within errors (4 boxes) | 0 (0–1) | 0 (0–0.25) | 0.9 |
| Within errors (6 boxes) | 0 (0–3) | 0 (0–2) | 0.4 |
| Within errors (8 boxes) | 1.8±1.9 | 2.0±2.44 | 0.8 |
Data values are presented as mean±SD or median (interquartile range).
Except for education level of mother and neonatal birth weight, other variables had no correlation with total IQ of offspring (Table 5). Neonatal birth weight and education level of mothers correlated with offspring performance IQ, whereas education level of mothers correlated with offspring verbal IQ.
Table 5.
Correlation Coefficient Between Variables and Total Intelligence Quotient
| Variable | r | p-Value |
|---|---|---|
| Educational level of father | 0.047 | 0.8 |
| Educational level of mother | 0.37 | 0.01 |
| Mother age at time of hypothyroidism | 0.26 | 0.07 |
| Birth weight | 0.37 | 0.01 |
| Gestational age at birth | −0.06 | 0.7 |
| Duration of breastfeeding | 0.23 | 0.1 |
| Levothyroxine dose during pregnancy (μg/day) | −0.18 | 0.2 |
| TPOAb level | −0.12 | 0.6 |
| Duration of mother hypothyroidism | 0.07 | 0.6 |
| Maternal TSH | −0.17 | 0.2 |
| Maternal FT4 | 0.11 | 0.5 |
| Route of delivery(cesarean/vaginal) | −0.1 | 0.7 |
Discussion
This study showed that IQ level and cognitive performance in children born of LT4-treated hypothyroid mothers who had SCH during their pregnancy were similar to those LT4-treated hypothyroid mothers who maintained a normal serum TSH during pregnancy.
Hypothyroidism is associated with increased risks of adverse pregnancy outcomes and neurocognitive deficits in the developing fetus (2–4). However, in comparison with overt hypothyroidism data on neuropsychological development of the offspring in M-SCH are scanty, variable, and inconsistent (13,14).
Retrospective studies had shown psychoneurological deficits in the progeny of mothers having maternal hypothyroidism. Data from a large case–control study found a 7-point reduction in IQ and delay in motor, language, and attention among 7–9-year-old children born to untreated hypothyroid pregnant women when compared with euthyroid controls (4). The severity of hypothyroidism varied from overt to SCH. In this study, however, children of mothers who were treated with LT4, despite having elevated TSH during pregnancy, had neuropsychological results similar to the control group. Another study investigated children born to women inadequately treated with LT4 during pregnancy and found that some components of intelligence were affected whereas others were not (15).
A preliminary report from the Controlled Antenatal Thyroid screening (CATS) showed that IQs of 3.5-year-old children of mothers who were treated for SCH during pregnancy were not significantly different from children of mothers with untreated M-SCH (16). The findings of the present study are in agreement with preliminary data of CATS and those of Haddow et al. (4). Using seven different neuropsychological tests, we found no differences in various parameters of neurodevelopment between the children of mothers who were optimally treated for hypothyroidism during pregnancy and the children of treated hypothyroid mothers who had thyroid function tests consistent with SCH during their pregnancy.
Other independent factors can affect child development. For example, it has been reported that children of women with SCH and elevated thyroperoxidase antibody had IQ score 8.88 points lower than those of the control group (17). Some of these confounding factors were considered in this study. Parental demographic characteristics such as education level, mother's lifestyle (alcohol and cigarette use), mothers' age at the time of hypothyroidism, birth weight, gestational age at birth time, gestational age at hypothyroidism, duration of breastfeeding, mothers' thyroperoxidase antibody level, and route of delivery were similar in the two groups. Although we did not know the amount of mothers' daily iodine intake during pregnancy, Tehran has been known as an area of iodine sufficiency since 1990 and throughout the years of this study (18).
The consequences of maternal hypothyroidism on the offspring are the result of various factors acting in combination (10). Decreased availability of maternal thyroid hormones at crucial stages of brain development, obstetric events associated with maternal thyroid dysfunction, and prolonged undisclosed hypothyroidism may play a role in this event. The lack of data during pregnancy in this historical cohort study is one of the few limitations. Low sample size, lack of evaluating other confounding factors such as postpartum depression, and family economic status are other constraints of this study.
In conclusion, we have found that no major neuropsychological deficit occurs in children born to mothers with SCH during pregnancy. Prospective studies with larger sample size may shed more light on this important issue.
Disclosure Statement
All authors have nothing to declare.
References
- 1.Vaidya B. Anthony S. Bilous M. Shields B. Drury J. Hutchison S. Bilous R. Detection of thyroid dysfunction in early pregnancy: universal screening or targeted high-risk case finding? J Clin Endocrinol Metab. 2007;92:203–207. doi: 10.1210/jc.2006-1748. [DOI] [PubMed] [Google Scholar]
- 2.Allan WC. Haddow JE. Palomaki GE. Williams JR. Mitchell ML. Hermos RJ. Faix JD. Klein RZ. Maternal thyroid deficiency and pregnancy complications: implications for population screening. J Med Screen. 2000;7:127–130. doi: 10.1136/jms.7.3.127. [DOI] [PubMed] [Google Scholar]
- 3.Casey BM. Dashe JS. Wells CE. McIntire DD. Leveno KJ. Cunningham FG. Subclinical hyperthyroidism and pregnancy outcomes. Obstet Gynecol. 2006;107:337–341. doi: 10.1097/01.AOG.0000197991.64246.9a. [DOI] [PubMed] [Google Scholar]
- 4.Haddow JE. Palomaki GE. Allan WC. Williams JR. Knight GJ. Gagnon J. O'Heir CE. Mitchell ML. Hermos RJ. Waisbren SE. Faix JD. Klein RZ. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549–555. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- 5.de Escobar GM. Obregón MJ. del Rey FE. Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract Res Clin Endocrinol Metab. 2004;18:225–248. doi: 10.1016/j.beem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Pop VJ. Brouwers EP. Vader HL. Vulsma T. van Baar AL. de Vijlder JJ. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin Endocrinol (Oxf) 2003;59:282–288. doi: 10.1046/j.1365-2265.2003.01822.x. [DOI] [PubMed] [Google Scholar]
- 7.Henrichs J. Bongers-Schokking JJ. Schenk JJ. Ghassabian A. Schmidt HG. Visser TJ. Hooijkaas H. de Muinck Keizer-Schrama SM. Hofman A. Jaddoe VV. Visser W. Steegers EA. Verhulst FC. de Rijke YB. Tiemeier H. Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: the generation R study. J Clin Endocrinol Metab. 2010;95:4227–4234. doi: 10.1210/jc.2010-0415. [DOI] [PubMed] [Google Scholar]
- 8.Rashid M. Rashid MH. Obstetric management of thyroid disease. Obstet Gynecol Surv. 2007;62:680–688. doi: 10.1097/01.ogx.0000281558.59184.b5. [DOI] [PubMed] [Google Scholar]
- 9.Abalovich M. Gutierrez S. Alcaraz G. Maccallini G. Garcia A. Levalle O. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid. 2002;12:63–68. doi: 10.1089/105072502753451986. [DOI] [PubMed] [Google Scholar]
- 10.Männistö T. Vääräsmäki M. Pouta A. Hartikainen AL. Ruokonen A. Surcel HM. Bloigu A. Järvelin MR. Suvanto-Luukkonen E. Perinatal outcome of children born to mothers with thyroid dysfunction or antibodies: a prospective population-based cohort study. J Clin Endocrinol Metab. 2009;94:772–779. doi: 10.1210/jc.2008-1520. [DOI] [PubMed] [Google Scholar]
- 11.Glinoer D. Abalovich M. Unresolved questions in managing hypothyroidism during pregnancy. BMJ. 2007;335:300–302. doi: 10.1136/bmj.39189.513935.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lezak MD, editor; Howieson DB, editor; Loring DW, editor; Julia Hannay H, editor; Fisher JS, editor. Neuropsychological Assessment. 4th. Oxford University Press; New York: 2004. [Google Scholar]
- 13.Man EB. Jones WS. Holden RH. Mellits ED. Thyroid function in human pregnancy. 8. Retardation of progeny aged 7 years; relationships to maternal age and maternal thyroid function. Am J Obstet Gynecol. 1971;111:905–916. [PubMed] [Google Scholar]
- 14.Man EB. Brown JF. Serunian SA. Maternal hypothyroxinemia: psychoneurological deficits of progeny. Ann Clin Lab Sci. 1991;21:227–239. [PubMed] [Google Scholar]
- 15.Rovet JF. Neurodevelopmental consequences of maternal hypothyroidism during pregnancy. Thyroid; Proceedings of the 76th annual meeting of the American Thyroid Association; Sep 30 3;Oct 30 3;2004 ; Vancouver, Canada. 2004. p. 710. [Google Scholar]
- 16.Lazarus J. Outcome of the CATS study in pregnant women. Presented at the 14th International Thyroid Congress; Paris, France. Sep 11–16;2010 .2010. [Google Scholar]
- 17.Li Y. Shan Z. Teng W. Yu X. Li Y. Fan C. Teng X. Guo R. Wang H. Li J. Chen Y. Wang W. Chawinga M. Zhang L. Yang L. Zhao Y. Hua T. Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25–30 months. Clin Endocrinol (Oxf) 2010;72:825–829. doi: 10.1111/j.1365-2265.2009.03743.x. [DOI] [PubMed] [Google Scholar]
- 18.Azizi F. Mehran L. Sheikholeslam R. Ordookhani A. Naghavi M. Hedayati M. Padyab M. Mirmiran P. Sustainability of a well-monitored salt iodization program in Iran: marked reduction in goiter prevalence and eventual normalization of urinary iodine concentrations without alteration in iodine content of salt. J Endocrinol Invest. 2008;31:422–431. doi: 10.1007/BF03346386. [DOI] [PubMed] [Google Scholar]