Abstract
Human blood outgrowth endothelial cells (HBOECs) are expanded from circulating endothelial progenitor cells in peripheral blood and thus could provide a source of autologous endothelial cells for tissue-engineered vascular grafts. To examine the suitability of adult HBOECs for use in vascular tissue engineering, the shear stress responsiveness of these cells was examined on bioartificial tissue formed from dermal fibroblasts entrapped in tubular fibrin gels. HBOECs adhered to this surface, deposited collagen IV and laminin, and remained adherent when exposed to 15 dyn/cm2 shear stress for 24 h. The shear stress responses of HBOECs were compared to human umbilical vein endothelial cells (HUVECs). As with HUVECs, HBOECs upregulated vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 when exposed to tumor necrosis factor (TNF)-α and shear stress decreased the expression of these adhesion molecules on TNF-α-activated monolayers. Nitric oxide production was elevated by shear stress, but did not vary between cell types. Both cell types decreased platelet adhesion to the bioartificial tissue, whereas pre-exposing the cells to flow decreased platelet adhesion further. These results illustrate the potential utility for HBOECs in vascular tissue engineering, as not only do the cells adhere to bioartificial tissue and remain adherent under physiological shear stress, they are also responsive to shear stress signaling.
Introduction
Human blood outgrowth endothelial cells (HBOECs) are a promising cell source for vascular tissue engineering. These cells can be isolated by outgrowth of circulating endothelial progenitor cells (EPCs) from a patient blood sample1–3 and thus could provide a convenient source of autologous endothelial cells for seeding on tissue-engineered vessels before implantation. HBOECs, also called endothelial colony forming cells and late outgrowth EPCs, have been shown to uniformly express endothelial cell markers, form tubular structures de novo, and have typical endothelial cell morphology and a robust proliferative capacity, expanding from 20 cells to 1019 cells in 9 weeks.1 HBOECs are negative for hematopoietic cell markers CD45, CD14, and CD115 and do not phagocytize bacteria.1,3–5 This is in contrast to other putative EPCs, termed early outgrowth endothelial cells or colony forming unit endothelial cells (CFU-ECs), which do not form vessels in vivo, will ingest bacteria, and express hematopoietic, as well as endothelial, cell markers.4 These CFU-ECs are likely of hematopoietic origin,4,6–9 but may express endothelial cell markers due to the uptake of platelet microparticles by mononuclear cells.10 This distinction between various cells termed EPCs in the literature is an important one, as unlike CFU-ECs, HBOECs have the potential to provide a highly proliferative, autologous endothelial cell source for vascular grafts.
Rigorous assessment of HBOECs is required to ensure successful use of these cells for vascular grafts. The endothelial layer needs to be confluent and remain adherent under normal physiological shear stresses, acting as a barrier between the blood and the procoagulant, sub-endothelial surface. Further, these cells need to be in a nonactivated, quiescent state, producing protective molecules that suppress platelet adhesion and activation, such as prostacyclin (PGI2) and nitric oxide (NO), anticoagulation factors, such as thrombomodulin (TM), and fibrinolytic factors. In conjunction with this phenotype, HBOECs should show limited expression of proinflammatory molecules, such as intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), P-Selectin, and E-Selectin.11,12
In vitro and in vivo studies have examined EPCs derived by a variety of methods for seeding on synthetic grafts,13–16 decellularized grafts,17 and tissue-engineered blood vessels.18,19 Fewer studies have examined the specific HBOEC population for this same purpose.20,21 However, these initial reports are promising as good HBOEC retention is reported on synthetic materials. Eighty percent of ovine BOECs were retained on cholesterol-modified polyurethane after 2 h of flow exposure at 75 dyn/cm2.21 BOECs derived from cord blood have been shown to adhere on fibronectin or smooth muscle cell (SMC) monolayers at supraphysiological shear stresses (up to 300 dyn/cm2) for short durations and align with flow after 48 h of shear stress exposure at a physiological shear stress (15 dyn/cm2).22 Importantly, HBOECs have been successfully isolated from patients with coronary artery disease,23 indicating that isolation of adult HBOECs may be a viable option for seeding of vascular grafts for bypass surgery.
To examine the suitability of adult HBOECs for use in vascular tissue engineering, the shear stress responsiveness of these cells was examined on bioartificial tissue formed from neonatal human dermal fibroblasts (HDFs) entrapped in tubular fibrin gels. During 4 weeks in culture, the HDFs degrade the initial fibrin scaffold and deposit new extracellular matrix (ECM), producing bioartificial tissue. The tissue strengthens and stiffens over time, producing a matrix that more closely mimics native vascular tissue.24–26 Immunohistochemistry was used to identify predominate ECM on the surface of the bioartificial tissue, as well as ECM deposited by the HBOECs postseeding. Cell density, elongation, alignment, NO production, and expression of the proinflammatory molecules ICAM-1 and VCAM-1 by HBOECs under shear stress were compared to human umbilical vein endothelial cells (HUVECs) to determine if HBOEC flow responses were similar to mature endothelial cells. A whole blood assay was used to examine platelet adhesion to HBOEC- and HUVEC-seeded constructs pre-exposed to shear stress.
Materials and Methods
Cell source
HBOECs were isolated and expanded from the peripheral blood of healthy, human volunteers, as previously described.1 The protocol for blood collection was approved by the University of Minnesota Institutional Review Board. The cells were maintained in endothelial growth medium (EGM; EBM-2 medium with EGM Singlequot supplement kit; Clonetics) supplemented with 8% additional fetal bovine serum (FBS; Hyclone), and 1% penicillin/streptomycin (Invitrogen). HBOECs were plated onto rat tail collagen I (BD Biosciences) and subcultured at 80%–90% confluence using 0.05% trypsin EDTA (Gibco Invitrogen Co.). Flow cytometry of HBOEC isolations at passage 2 ensured positive staining for endothelial cell markers, including VE-Cadherin and P1H12, and negative staining for CD14 and CD45, as described previously.1 These cells also displayed the characteristic cobblestone morphology of ECs, formed tubules in Matrigel, and took up acetylated low-density lipoprotein (Biomedical Technologies, Inc.). HBOECs were used from passage 8–12 for all experiments. For comparison, HUVECs (Clonetics) were maintained in the same culture medium and used from passage 4–6.
Neonatal HDFs (Clonetics) were maintained in 50:50 Dulbecco's modified Eagle's medium (DMEM)/F12 medium (Invitrogen) supplemented with 10% FBS (Hyclone), 100 U/mL penicillin (Invitrogen), 100 μg/mL streptomycin (Invitrogen), and 2.5 μg/mL amphotericin-β (Invitrogen). HDFs were plated at 8500 cells/cm2, passaged at 100% confluence, and used at passage 9.
Fibrin-based vascular graft fabrication
Tubular fibrin constructs were made as previously reported.27 Briefly, HDFs suspended in DMEM were mixed into a solution of bovine fibrinogen (Sigma) in 20 mM HEPES-buffered saline. A mixture of bovine thrombin (Sigma) and calcium chloride in DMEM was then added to the suspension, mixed well, and injected into tubular glass molds measuring 8 cm long and 9 mm in inner diameter and containing 2 mm glass rods with stoppers on either end. The glass rods had been coated in 5% Pluronics F127 (Sigma) for 3 hours to reduce adhesion between the fibrin gel and the mandrel. Final gel component concentrations were 3.3 mg/mL fibrin, 500,000 HDF/mL, 0.2 U/mL thrombin, and 1.2 mM CaCl2. The solution was allowed to gel at 37°C for 30 min, at which time the outer mold was removed and the constructs placed in DMEM supplemented with 10% FBS, 10 U/mL penicillin, 100 μg/mL streptomycin, and 2.5 μg/mL amphotericin-β. About 2 μg/mL insulin and 50 μg/mL ascorbic acid were added to the medium to enhance fibrin gel remodeling into tissue. Construct medium was changed 3 times per week until use at 4 weeks. Vascular constructs at 4 weeks were 3–4 cm long and 2 mm in diameter, with a wall thickness of ∼200 μm.
HBOEC and HUVEC seeding on bioartificial tissue
After 4 weeks, remodeled fibrin tissue constructs were slit axially and placed lumenal surface down on a polycarbonate sheet using a method adapted from Kladakis and Nerem.28 A polycarbonate cap with a 3 mm high groove was placed around the piece of tissue and filled with a solution of 4% low gel temperature agarose (A0701; Sigma). The agarose was allowed to gel and then the polycarbonate sheet was removed, exposing the lumenal surface of the construct. A Teflon ring was placed around the cap to form a well for cell culture medium. HBOECs or HUVECs were labeled with 2.5 μM CellTracker Green (Invitrogen) and then were seeded on the lumenal surface of the tissue constructs. The cells were seeded slowly, drop-wise onto the tissue at a concentration of 2×106 cells/mL so as to achieve a seeding of 100,000 cells/cm2. One hour after seeding the tissue was rinsed with Hank's balanced salt solution (HBSS) to remove nonadherent cells and 4 mL of EGM medium was added to each well. For tumor necrosis factor (TNF)-α activation or shear stress experiments, cells were allowed to adhere for 24 h before start of the experiment.
Cell staining
HBOEC and HUVEC expression of TM, tissue factor (TF), endothelial nitric oxide synthase (eNOS), VCAM-1, ICAM-1, and VE-Cadherin was examined by immunocytochemistry. Cells seeded for 48 h in EGM medium on tissue culture plastic (TCP) coated with type I rat tail collagen or on tissue constructs were rinsed with HBSS, fixed in 4% paraformaldehyde for 10 min, and blocked with 5% normal donkey serum for 1 h at room temperature. Samples were incubated with mouse anti-human TM (5 μg/mL; American Diagnostica), goat anti-human TF (10 μg/mL; American Diagnostica), rabbit anti-human eNOS (4 μg/mL; Santa Cruz), mouse anti-human VCAM-1 (5 μg/mL; Chemicon), mouse anti-human ICAM-1 (5 μg/mL; Chemicon), goat anti-human VE-Cadherin (10 μg/mL; Santa Cruz), and isotype-matched controls for 1 h. After rinsing, Dylight 549 conjugated, host-matched secondary antibody (1:400; Jackson Immuno) was applied for 1 h at room temperature. Hoechst 33342 (1 μg/mL; Invitrogen) was used for staining of nuclei. Images were captured using epi-fluorescent microscopy (Olympus IX700) and confocal microscopy (Olympus Fluoview 1000). Measurement of the integrated fluorescent density per image for VCAM-1 and ICAM-1 was obtained with ImageJ software (NIH), as were cell counts per image. The integrated density per cell was calculated for each image and background values of the matched species IgG controls were subtracted.
Vascular graft ECM characterization
Constructs for histology were fixed in 4% paraformaldehyde for 3 h at 4°C, followed by overnight infiltration with 30% sucrose and 5% dimethyl sulfoxide in phosphate-buffered saline (PBS). Samples were frozen in OCT (Tissue-Tek), sectioned into 9-μm-thick cross sections, and then stained with Lillie's trichrome.29
Construct sections were also stained for the presence of various ECM proteins by immunohistochemistry. Sections were incubated with rabbit anti-human collagen I (2 μg/mL; Novus Biologicals), collagen IV (6 μg/mL; Abcam), laminin (10 μg/mL; Abcam), and fibronectin (1 μg/mL; American Diagnostica), goat anti-human fibrinogen (2 μg/mL; American Diagnostica), and isotype-matched controls in 5% normal donkey serum in PBS (Jackson Immuno) overnight. After rinsing, sections were incubated with Dylight 549 donkey anti-rabbit or anti-goat (1:400 dilution; Jackson Immuno) in PBS for 45 min and counterstained for 10 min with Hoechst 33342 (1 μg/mL; Invitrogen).
Shear stress experiments
The polycarbonate caps with the embedded tissue seeded with HBOECs or HUVECs were placed in parallel plate flow chambers (PPFCs) modified from the design of Sakariassen et al.30 The PPFC had a slot height of 0.5 mm, with a tapered inlet and outlet, reaching a final width of 1 cm. The chamber was assembled in a closed loop consisting of a medium reservoir allowing for gas exchange, a peristaltic pump (Cole-Parmer), and a pulse dampener (Cole Parmer). The system was primed with the EGM medium before placing the tissue within the system and then the flow rate was increased 0.5 dyn/cm2 every minute, starting at 3 dyn/cm2 and increasing to 15 dyn/cm2. Steady laminar shear stress was applied for 24 h and then the constructs were harvested for analysis. In the case of TNF-α exposure, 10 U/mL TNF-α was added to static and flow samples at the time flow was started.
For short-duration shear stress experiments, the shear stress was ramped as described; however, at 0, 1, 5, 10, 15, and 25 dyn/cm2 the shear stress was held for 20 min and then the construct surface was imaged. HBOECs were seeded on bioartificial tissue and type I collagen-coated TCP for 24 h before application of shear stress. Twelve images per sample were obtained of the CellTracker Green-labeled HBOECs using an Olympus IX70 inverted microscope. Images were thresholded at a fixed level and analyzed with ImageJ software (NIH) for percent area coverage. Unseeded TCP and bioartificial tissue were used as negative controls.
Cell retention, orientation and alignment
HBOECs and HUVECs were stained and imaged for VE-Cadherin and Hoechst as described above. Confocal images were taken at 20× and 40× to eliminate imaging of Hoechst staining of the HDF nuclei. The numbers of nuclei per image were counted using ImageJ software and normalized to the area of the image to give a cell density. To quantitate cell orientation and alignment, ImageJ software was used to outline each cell, based on the VE-Cadherin staining, and calculate the area, perimeter, major axis, minor axis, and angle of the major axis (cell alignment) with the flow direction. The shape index was calculated using:
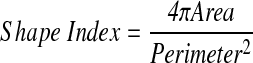 |
The shape index is a measure of the elongation of a cell and is equal to one for a circle and zero for a line. Decreasing values thus indicate that cells are becoming more elongated.
NO production
Medium samples were collected from HUVEC and HBOEC seeding bioartificial tissue after 24 h of static culture of exposure to 15 dyn/cm2 shear stress. Samples were frozen at −80°C, lyophilized, and reconstituted at 2.5× the initial concentration. A commercially available kit was used to convert nitrate to nitrite, followed by the spectrophotometric quantitation of nitrite levels using Griess Reagent (Calbiochem).
Whole blood assay
A platelet adhesion assay was performed with 1 U/mL heparinized whole blood in the PPFCs described above. Whole blood was collected from healthy, consenting donors under University of Minnesota Institutional Review Board approval and used at 37°C within 2 h of the blood draw. The system was primed with HBSS at 37°C before loading with whole blood. The initial 5–10 mL of blood was discarded to remove blood that had mixed with the HBSS and then the system was run as a closed loop at a shear rate of 400 s−1. After 20 min of whole blood exposure, HBSS was run through the system for 2 min to remove unbound platelets and leukocytes. The samples were removed from the flow chamber and fixed with 4% PFA for 10 min and then rinsed with HBSS. The tissue was simultaneously stained for EC junctions with goat anti-human VE-Cadherin and platelets with mouse anti-human GP-1b (1 μg/mL; Abcam) for 1 h at room temperature. After rinsing with PBS, Dylight 549 donkey anti-mouse and Dylight 649 donkey anti-goat secondary antibody (1:400; Jackson Immuno) were applied for 45 min, followed by staining with Hoechst 33342 for 10 min. Samples were imaged on an Olympus Fluoview 1000 confocal microscope and platelet and leukocyte coverage quantified using ImageJ software. Images were converted to binary by use of a fixed threshold. These images were used to calculate the area coverage of platelets. Values were normalized to the HBOEC static group.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software for Windows (GraphPad Software, Inc.). One-way and multivariate analysis of variance (ANOVA) with Bonferroni post hoc analysis was conducted to evaluate significant differences between groups. A significance level of α=0.05 was used for all tests. All graphs indicate mean±standard error of the mean.
Results
HBOECs formed a confluent monolayer on bioartificial tissue
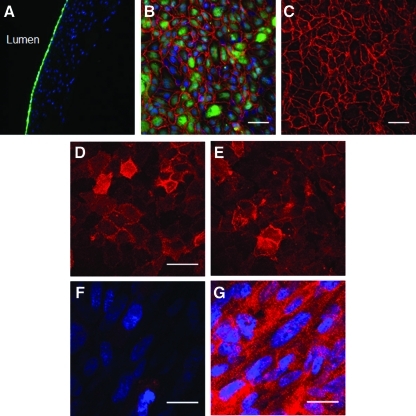
HBOECs adhered to the bioartificial tissue and formed a confluent monolayer (Fig. 1A–C). The cells had typical cobblestone morphology, stained for VE-Cadherin, and maintained cell-to-cell contact (Fig. 1B, C). The HBOECs also expressed TM (Fig. 1D), an important endothelial anticoagulant molecule also expressed by HUVECs (Fig. 1E), and displayed limited TF expression (Fig. 1F) until exposed to the agonist TNF-α (Fig. 1G). Staining throughout the thickness of the tissue showed that TNF-α stimulated TF expression by the HBOECs on the lumenal surface as well as by the underlying fibroblasts.
FIG. 1.
HBOECs seeded on bioartificial tissue. (A) Cross-sectional and (B, C) En face images of CellTracker Green-labeled HBOECs seeding on bioartificial tissue show that the HBOECs form a confluent monolayer on the tissue surface. Nuclei of HBOEC and human dermal fibroblast in the tissue are stained blue, and VE-Cadherin staining is pseudo-colored red. (D) HBOECs and (E) HUVECs on the bioartificial tissue express thrombomodulin. (F) Tissue factor expression by HBOEC on unstimulated tissue and (G) after exposure to TNF-α. (B–E) Scale bar=50 μm. (F, G) Scale bar=25 μm. HBOEC, human blood outgrowth endothelial cell; HUVEC, human umbilical vein endothelial cell; TNF, tumor necrosis factor. Color images available online at www.liebertonline.com/tea
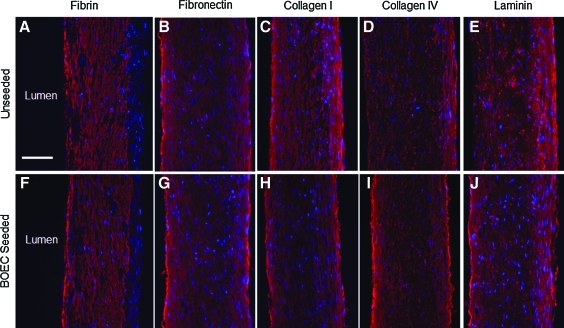
Nonendothelialized and HBOEC-seeded bioartificial tissue was stained for ECM molecules to assess the protein composition at the lumenal surface. The tissue was embedded in agarose with the lumenal surface exposed and cultured with or without HBOECs for 7 days. Immunostaining for fibrin, fibronectin, collagen I, collagen IV, and laminin highlighted the presence of all of these ECM proteins throughout the unseeded (Fig. 2A–E) and HBOEC-seeded tissue (Fig. 2F–J). Staining for IgG controls was negative, whereas staining for ECM components showed positive, diffuse staining through the tissue. Residual fibrin, fibronectin, and collagen I were present on the lumenal surface (Fig. 2A–C); however, collagen IV and laminin staining was limited on the lumenal surface of the unseeded tissue (Fig. 2D, E). This is in contrast to the HBOEC-seeded tissue, where distinct bands of collagen IV and laminin were present on the lumenal surface (Fig. 2I, J). This alteration on the lumenal surface of the HBOEC-seeded tissue is indicative of new ECM deposition by the HBOECs.
FIG. 2.
ECM staining of bioartificial tissue. (A–E) Bioartificial tissue stained for ECM components. (F–J) Bioartificial tissue seeded with HBOECs for 7 days before staining. (A, F) Fibrin staining. (B, G) Fibronectin staining. (C, H) Collagen I staining. (D, I) Collagen IV staining. (E, J) Laminin staining. The lumenal surface is on the left side of each image, nuclei counterstained with Hoechst 33342. Scale bar=100 μm. ECM, extracellular matrix. Color images available online at www.liebertonline.com/tea
HBOECs remained adherent on bioartificial tissue under physiological shear stress and elongated in the flow direction
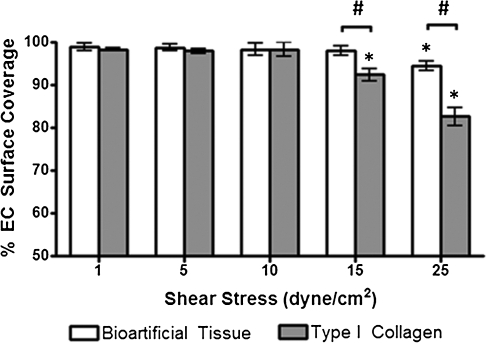
HBOECs were seeded on collagen-coated TCP or bioartificial tissue and allowed to adhere for 24 h. Surfaces were then exposed to increasing shear stress (1, 5, 10, 15, and 25 dyn/cm2) and cell retention quantified after 20 min at each shear stress level. Surface coverage was improved by seeding on bioartificial tissue, with a surface coverage after exposure to 25 dyn/cm2 of 94.4%±0.6% as compared with 82.6%±1.2% on collagen-coated TCP (Fig. 3). The surface coverage was statistically lower for HBOEC on TCP beginning at 15 dyn/cm2, but was not lower for HBOEC on bioartificial tissue until 25 dyn/cm2.
FIG. 3.
HBOEC retention under physiological shear stress for short durations. HBOECs were seeded on type I collagen-coated TCP or bioartificial tissue and incrementally exposed to 1, 5, 10, 15, or 25 dyn/cm2 for 20 min at which time HBOEC surface coverage was calculated based on the CellTracker Green staining. Plot shows mean±SE for n=3 independent experiments. *Significant difference compared to 1 dyn/cm2 samples on the same substrate (p<0.05). #Significant difference between HBOEC on TCP and bioartificial at the same shear stress (p<0.05). TCP, tissue culture plastic; SE, standard error of the mean.
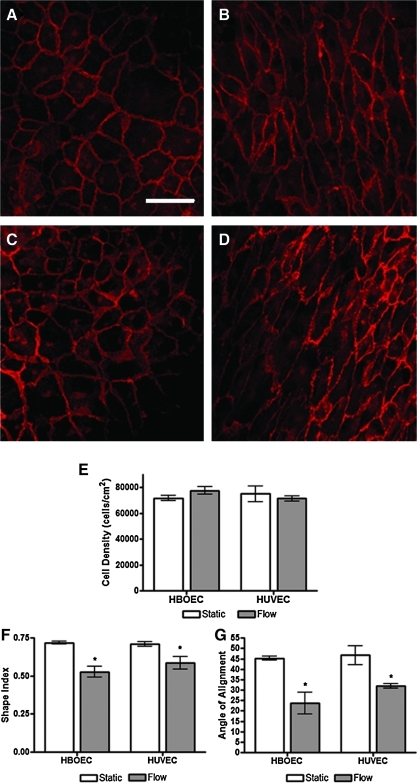
HBOECs and HUVECs were seeded on the bioartificial tissue and allowed to adhere for 24 h. After 24 h, seeded tissue was either maintained in static culture for another 24 h (Fig. 4A, C) or placed in a PPFC and laminar, steady shear stress applied at 15 dyn/cm2 (Fig. 4B, D). After 24 h under flow, HBOECs and HUVECs had begun to elongate in the flow direction, as illustrated with VE-Cadherin staining. VE-Cadherin staining of the cell junctions was visible in all samples and confirmed that HBOEC and HUVEC under flow still maintained a confluent monolayer. Although the HBOECs and HUVECs exposed to flow showed a marked change in cell morphology, the cell density was not significantly altered by application of shear stress (Fig. 4E). HBOEC and HUVEC elongation and alignment were quantified for comparison by calculation of the cell shape index and alignment angle. These measurements confirmed that by 24 h, HBOECs and HUVECs were elongating and aligning in the flow direction (Fig. 4F, G). There was no significant difference between HBOECs and HUVECs in shape index or the strength of alignment with flow by 24 h.
FIG. 4.
Retention, elongation, and alignment of HBOECs and HUVECs seeded on bioartificial tissue and exposed to shear stress. VE-Cadherin staining of (A, B) HBOECs and (C, D) HUVECs. (A, C) Statically cultured cells. (B, D) Cells cultured under 15 dyn/cm2 for 24 h. (E) Cell density, (F) elongation, and (G) alignment of HBOECs and HUVECs after exposure to 15 dyn/cm2 shear stress or static culture for 24 h. The elongation is given as a shape index where 0 is a line and 1 is a circle. Cell alignment is given on a scale from perfectly aligned (0°) to no alignment (45°). *p<0.05 compared to static conditions. All plots show mean±SE for n=3 independent experiments. Scale bar=50 μm and applies to all images. Color images available online at www.liebertonline.com/tea
Expression of proinflammatory molecules VCAM-1 and ICAM-1 was modulated with shear stress and TNF-α stimulation
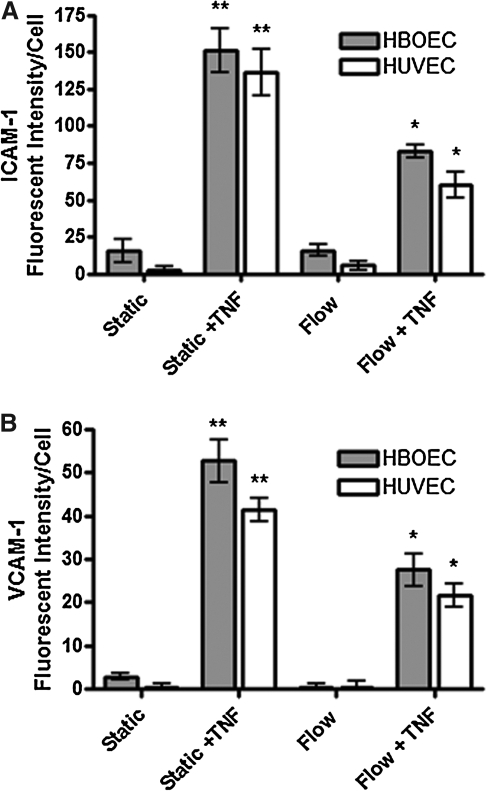
Application of 15 dyn/cm2 shear stress for 24 h did not significantly alter VCAM-1 or ICAM-1 expression by HBOECs or HUVECs compared to static culture (Fig. 5). Background due to nonspecific binding of the VCAM-1 and ICAM-1 antibodies was subtracted from adhesion molecule expression during quantification by subtraction of mouse IgG control values; thus, the values near zero indicate minimal expression of either VCAM-1 or ICAM-1, under static or shear stress culture, as these groups could not be distinguished statistically from the IgG controls.
FIG. 5.
VCAM-1 and ICAM-1 expression by HBOECs and HUVECs seeded on bioartificial tissue and cultured under static or flow conditions for 24 h, in the presence or absence of TNF-α. (A) VCAM-1 expression. (B) ICAM-1 expression. Plots show mean±SE for n=3 tissue samples. *Significant increase over static and flow groups of the same cell type (p<0.05). **Significant increase over all other groups of the same cell type (p<0.05). ICAM-1, intercellular adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1.
To examine the responsiveness of the HBOECs to an inflammatory stimulus, 10 U/mL TNF-α was added to the cultures for 24 h, during static culture or shear stress exposure. Addition of TNF-α increased VCAM-1 and ICAM-1 expression for both cell types, whereas shear stress was able to modulate this effect; shear stress conditioning combined with TNF-α stimulation decreased VCAM-1 and ICAM-1 expression compared to the static, TNF-α-treated cells. VCAM-1 and ICAM-1 expression in the flow-conditioned, TNF-α-stimulated cells remained higher than the static or flow-cultured, nonstimulated HBOECs and HUVECs. This indicates that HBOECs, like HUVECs, are responsive to biochemical inflammatory stimuli and modulate this response under steady laminar shear stress of physiological value.
Based on multivariate ANOVA, the major source of variation in VCAM-1 and ICAM-1 expression was accounted for by the application of shear stress or TNF-α; however, cell type was a significant source of variability for VCAM-1 expression as well (p<0.05). VCAM-1 expression by HBOECs was elevated compared to HUVECs when TNF-α was applied to statically cultured cells. Cell type was not a significant source of variability for ICAM expression.
eNOS expression and NOx production were elevated by exposure to shear stress
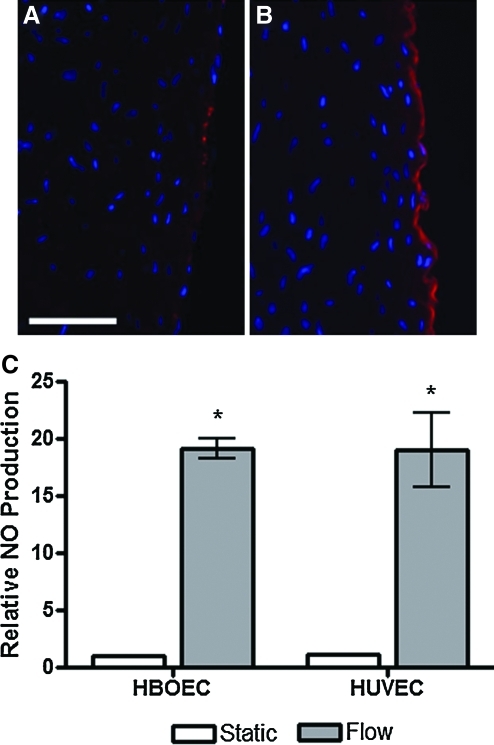
After 24 h of static culture or shear stress exposure at 15 dyn/cm2, HBOECs seeded on the bioartificial tissue were stained for eNOS (Fig. 6A, B). eNOS was localized to the HBOEC layer on the lumenal surface of the tissue. While eNOS staining of the statically cultured HBOECs was sporadic throughout the HBOEC layer (Fig. 6A), flow conditioning increased the eNOS intensity and created a continuous band of eNOS throughout the HBOEC monolayer (Fig. 6B).
FIG. 6.
eNOS expression and NOx production by HBOECs and HUVECs seeded on bioartificial tissue and cultured under static and flow conditions for 24 h. eNOS staining of HBOEC cross sections after (A) static culture or (B) flow exposure. (C) NO production was quantified by measurement of total nitrate and nitrite in the HBOEC and HUVEC culture medium. Values are normalized to the statically cultured HBOEC value. Plot shows mean±SE for n=3 independent experiments. *NO production by HBOECs and HUVECs cultured under flow conditions increased compared to HBOEC static culture (p<0.01). Scale bar=100 μm. eNOs, endothelial nitric oxide synthase. Color images available online at www.liebertonline.com/tea
To assess the effect of higher eNOS expression by HBOEC or HUVEC, NO production was measured for these same samples. NO production was measured via the quantitation of NO byproducts, nitrate and nitrite, in the cell culture medium after 24 h of static culture or flow conditioning at 15 dyn/cm2 (Fig. 6C). Similar basal levels of NO were seen in HUVEC and HBOEC static culture. NO production was elevated ∼19-fold in HBOEC and HUVEC cultures when shear stress was applied for 24 h.
Platelet adhesion was lower after exposure to shear stress
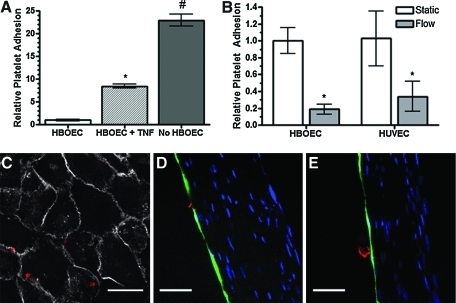
As an in vitro method for examining the functional effect of HBOEC and HUVEC seeding on the bioartificial tissue, endothelialized and nonseeded tissue was exposed to 1 U/mL heparinized whole blood in PPFCs for 20 min and platelet adhesion to the exposed surfaces was then quantified (Fig. 7A, B). Activation of HBOECs with TNF-α for 4 h before application of the whole blood increased platelet adhesion to the surface (Fig. 7A). Platelet surface coverage was higher on nonseeded tissue (11.0%±2.8%) than on HBOEC-seeded tissue (0.5%±0.1%) or HUVEC-seeded tissue (0.5%±0.1%). Thus, HBOECs, like HUVECs, show decreased platelet adhesion compared to unseeded tissue.
FIG. 7.
Platelet adhesion on HBOEC- and HUVEC-seeded bioartificial tissue preconditioned with 15 dyn/cm2 shear stress for 24 h. (A) Normalized platelet coverage. HBOEC-seeded tissue was compared to HBOEC-seeded tissue that was pre-exposed to TNF-α for 4 h or nonseeded tissue. All groups were normalized to the statically cultured HBOEC group. *p<0.001 compared to unstimulated HBOEC. #p<0.001 compared to stimulated and nonstimulated HBOEC-seeded tissue. (B) HBOEC- and HUVEC-seeded tissue was statically cultured or exposed to 15 dyn/cm2 shear stress for 24 h before whole blood flow exposure. *p<0.05 compared to the statically cultured cells of the same EC type. (C) En face image of platelet localization. Scale bar=25 μm. Cross-sectional views of (D) platelet localization and (E) platelet aggregation on the surface of statically cultured HBOECs. Scale bar=50 μm. Plots show mean±SE for one representative experiment with n=3 tissue samples. Color images available online at www.liebertonline.com/tea
A subset of the HBOEC- and HUVEC-seeded tissue was exposed to 15 dyn/cm2 shear stress for 24 h before whole blood exposure. Preconditioning of the HBOECs and HUVECs with shear stress decreased platelet adhesion compared to the statically cultured cells. Higher platelet adhesion on the static samples could be due to application of shear stress for the first time during the whole blood study, which causes an initial loss of ECs that had not been pre-exposed to shear stress and allows platelet adhesion to the exposed ECM. To examine the possibility that monolayer integrity was lost during whole blood exposure to statically cultured HBOECs or HUVECs, tissue samples were stained en face or after sectioning for VE-Cadherin and the platelet marker, GP-1b (Fig. 7C–E). Images indicated HBOEC and HUVEC monolayer coverage after whole blood exposure for samples that were statically cultured or shear stress-conditioned before the whole blood assay. Platelet binding most often appeared co-localized with the VE-Cadherin stain (Fig. 7C). Platelets adhered near the junctions of cells, primarily as individual platelets (Fig. 7C, D) and occasionally as small aggregates (Fig. 7E).
An assessment of leukocyte binding to the HBOECs using a CD45 antibody, performed in parallel with these platelet binding studies under whole blood flow, revealed no leukocyte binding unless the HBOECs were TNF-α stimulated (data not shown).
Discussion
The fabrication of a small-diameter vascular graft will require the incorporation of a nonthrombogenic lumenal surface, most likely through the formation of a functional neoendothelium, to obviate the need for anti-coagulation therapy. HBOECs are a promising cell source for this application, as they can be easily and noninvasively isolated, are highly proliferative, and do not suffer from SMCs contamination issues. The successful use of HBOECs for vascular tissue engineering will require (1) formation of a confluent monolayer on the tissue surface, (2) retention of the HBOECs under physiological shear stress, and (3) presentation of a nonthrombogenic and anti-inflammatory phenotype. As with native endothelium, responsiveness of the HBOECs to physiological shear stress will likely be necessary for the maintenance of a thromboresistant phenotype.
To address these requirements, bioartificial tissue was fabricated for HBOEC seeding. Tubular fibrin gels with entrapped HDF were allowed to remodel for 4 weeks as the HDFs degraded the fibrin and deposited new ECM, including fibronectin, laminin, and collagen I and IV, as shown in this study. The lumenal surface of the tissue stained positively for fibrin, fibronectin, and collagen I; however, collagen IV and laminin were primarily localized on the ablumenal surface. Fibronectin,31–34 laminin,31,35 collagen I,31,32 and fibrin36,37 are all adhesion proteins that have been utilized to improve retention of ECs on vascular grafts, suggesting that a cell-produced matrix rich with these components would be conducive to EC adhesion and retention. HBOECs were seeded on the lumenal surface of the HDF-remodeled tissue, where the HBOECs deposited new ECM, which appeared as localized bands of the basement membrane proteins collagen IV and laminin. These bands were not present in the nonendothelialized tissue. The deposition of laminin and collagen IV was shown after 7 days of HBOEC culture since increased fluorescent intensity at the lumenal surface was not obvious at the 2-day time point. While all of the flow studies were started after only 24 h of seeding, these staining results do show that HBOECs were depositing collagen IV and laminin on bioartificial tissue over the course of the first week. The delayed expression could be a confounding issue with immunohistochemistry. We can visually detect an increase in ECM deposition by 7 days; however, ECM was likely being deposited earlier. A high concentration of ECM deposition may be required before an obvious difference in the immunostained tissue is apparent. Alternatively, the cells may need to adhere and form a complete monolayer with tight junctions before increasing their ECM deposition.
HBOEC adhesion and morphology was assessed on the bioartificial tissue. By 24 h postseeding, HBOECs formed a confluent monolayer of cells, with cell-to-cell junction formation, as assessed by VE-Cadherin staining. In short-duration shear stress experiments, the HBOEC monolayer was better retained on the bioartificial tissue than on collagen-coated TCP. To assess HBOEC retention and alignment under flow conditions, a shear stress of 15 dyn/cm2 was applied to mimic physiological arterial flow. When exposed to 15 dyn/cm2 shear stress for 24 h, HBOECs remained adherent, maintained a similar cell density and VE-Cadherin staining, and elongated and aligned with flow. These results compared well with the HUVEC control cells used in these experiments, as well as with cell retention reported for cord blood-derived HBOECs22 and short-duration, high-shear-stress experiments with HBOECs.20,21,23
While HBOECs grown as monolayers on collagen-coated TCP show limited expression of the proinflammatory markers ICAM-1 and VCAM-1,1 expression of these markers may be altered depending on the underlying ECM as well as the presence of secondary cell types. SMC-EC co-culture has been shown to increase gene and protein expression of activation markers such as VCAM-1 and ICAM-1, whereas shear stress decreases this expression.38 Shear stress also alters activation marker expression in TNF-α-stimulated and unstimulated EC monolayers.39–44 Like mature ECs, HBOECs upregulated VCAM-1 and ICAM-1 when exposed to TNF-α, a proinflammatory cytokine.45 Similar to results found with HBOECs seeded on type I collagen,1 HBOECs and HUVECs seeded on bioartificial tissue expressed low levels of VCAM-1 and ICAM-1 until exposed to TNF-α. TNF-α-stimulated ICAM-1 and VCAM-1 expression was diminished by shear stress when TNF-α was administered during steady laminar shear flow. These studies demonstrate that shear stress conditioning may be useful in decreasing surface expression of various procoagulation molecules on HBOECs, thereby promoting an anti-inflammatory phenotype, as reported for mature ECs.
Shear stress can also be used to increase eNOS expression and NO production in mature ECs.46–48 NO promotes blood vessel vasodilation, inhibits platelet adhesion and aggregation, and is constitutively expressed in native ECs, primarily through eNOS activity.49 Thus, production of NO is a necessary phenotype for functional HBOECs seeded on bioartificial tissue. Similar basal levels of NO were produced by HUVECs and HBOECs cultured statically. HBOECs increased total NO production when cultured with steady laminar shear stress for 24 h, as did the HUVEC control cells. This correlated with increased staining for eNOS in tissue cross sections.
Although assessment of HBOEC retention and function in vivo will be necessary for a full consideration of HBOEC function, in vitro studies allow us to compare HUVECs and HBOECs for a number of quantifiable parameters. As a functional test, we developed a whole blood study to examine platelet adhesion under flow. Bioartificial tissue, either seeded with HBOECs or HUVECs or left unseeded, was exposed to whole blood at a shear rate of 400 s−1 to mimic blood flow in human arteries. This equated to a shear stress of ∼15 dyn/cm2. Platelet coverage was dramatically reduced on HBOEC-seeded bioartificial tissue as compared to nonseeded tissue. HBOECs and HUVECs had similar levels of platelet coverage, both when statically cultured and when preconditioned with shear stress for 24 h before whole blood exposure.
Preconditioning of the HBOECs or HUVECs with 15 dyn/cm2 shear stress for 24 h in EGM medium reduced platelet coverage compared to statically cultured samples. Preconditioning may have improved monolayer integrity, whereas blood flow over statically cultured HBOECs and HUVECs may have disrupted the endothelium, exposing the subendothelial matrix and increasing platelet adhesion on these substrates. Although a limited loss of statically cultured HBOECs or HUVECs during exposure to whole blood flow could not be fully ruled out, VE-Cadherin staining of cross sections indicated that monolayer coverage by the HBOECs and HUVECs was preserved. Increased thromboresistance of ECs pre-exposed to shear stress may also be responsible for decreased platelet adhesion. We have shown that HBOECs and HUVECs exposed to shear stress express higher levels of NO, whereas other groups have reported increased EC expression of PGI2, NO, TM, and tissue plasminogen activator, and decreased expression of plasminogen activator inhibitor type-1 with shear stress.50 Lund et al. showed that HBOECs have lower TF and higher eNOS gene expression when exposed to shear stress.51 This switch to a more thromboresistant phenotype with shear stress exposure could account for the decreased platelet adhesion on the shear stress preconditioned surfaces. Either of these possibilities, improved monolayer integrity or increased EC thromboresistance, leads to the same conclusion: preconditioning with shear stress has the potential to improve the functional outcome with an HBOEC or HUVEC neoendothelium.
Methods to improve HBOEC function before implantation will be important in developing a functional tissue-engineered vascular graft. Our results with HBOECs seeded on bioartificial tissue indicate that (1) HBOECs are shear stress responsive and (2) HBOECs can be preconditioned with shear stress in vitro to increase NO production and decrease platelet adhesion. These results illustrate the potential utility for HBOECs in vascular tissue engineering, as not only do the cells adhere to the bioartificial tissue and remain adherent under physiological shear stress, but they also exhibit low expression of activation markers and reduced platelet binding compared to unseeded tissue.
Acknowledgments
The authors would like to thank Mark Roney and Sethu Nair for help with BOEC culture, Lee Meier for HBOEC and HUVEC culture and seeding, Naomi Ferguson for HDF culture, and Debra Cocking-Johnson for support with the whole blood studies. Funding was from NIH R01 HL083880 (to R.T.T.).
Disclosure Statement
No competing financial interests exist.
References
- 1.Lin Y. Weisdorf D.J. Solovey A. Hebbel R.P. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ingram D.A. Mead L.E. Tanaka H. Meade V. Fenoglio A. Mortell K. Pollok K. Ferkowicz M.J. Gilley D. Yoder M.C. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 3.Ingram D.A. Caplice N.M. Yoder M.C. Unresolved questions, changing definitions, and novel paradigms for defining endothelial progenitor cells. Blood. 2005;106:1525. doi: 10.1182/blood-2005-04-1509. [DOI] [PubMed] [Google Scholar]
- 4.Yoder M.C. Mead L.E. Prater D. Krier T.R. Mroueh K.N. Li F. Krasich R. Temm C.J. Prchal J.T. Ingram D.A. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirschi K.K. Ingram D.A. Yoder M.C. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1584. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rehman J. Li J. Orschell C.M. March K.L. Peripheral blood “Endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 7.Zhang S.J. Zhang H. Wei Y.J. Su W.J. Liao Z.K. Hou M. Zhou J.Y. Hu S.S. Adult endothelial progenitor cells from human peripheral blood maintain monocyte/macrophage function throughout in vitro culture. Cell Res. 2006;16:577. doi: 10.1038/sj.cr.7310075. [DOI] [PubMed] [Google Scholar]
- 8.Harraz M. Jiao C. Hanlon H.D. Hartley R.S. Schatteman G.C. Cd34- blood-derived human endothelial cell progenitors. Stem Cells. 2001;19:304. doi: 10.1634/stemcells.19-4-304. [DOI] [PubMed] [Google Scholar]
- 9.Rohde E. Malischnik C. Thaler D. Maierhofer T. Linkesch W. Lanzer G. Guelly C. Strunk D. Blood monocytes mimic endothelial progenitor cells. Stem Cells. 2006;24:357. doi: 10.1634/stemcells.2005-0072. [DOI] [PubMed] [Google Scholar]
- 10.Prokopi M. Pula G. Mayr U. Devue C. Gallagher J. Xiao Q. Boulanger C.M. Westwood N. Urbich C. Willeit J. Steiner M. Breuss J. Xu Q. Kiechl S. Mayr M. Proteomic analysis reveals presence of platelet microparticles in endothelial progenitor cell cultures. Blood. 2009;114:723. doi: 10.1182/blood-2009-02-205930. [DOI] [PubMed] [Google Scholar]
- 11.Wu K.K. Thiagarajan P. Role of endothelium in thrombosis and hemostasis. Annu Rev Med. 1996;47:315. doi: 10.1146/annurev.med.47.1.315. [DOI] [PubMed] [Google Scholar]
- 12.McGuigan A.P. Sefton M.V. The influence of biomaterials on endothelial cell thrombogenicity. Biomaterials. 2007;28:2547. doi: 10.1016/j.biomaterials.2007.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He H. Shirota T. Yasui H. Matsuda T. Canine endothelial progenitor cell-lined hybrid vascular graft with nonthrombogenic potential. J Thorac Cardiovasc Surg. 2003;126:455. doi: 10.1016/s0022-5223(02)73264-9. [DOI] [PubMed] [Google Scholar]
- 14.Shirota T. He H. Yasui H. Matsuda T. Human endothelial progenitor cell-seeded hybrid graft: proliferative and antithrombogenic potentials in vitro and fabrication processing. Tissue Eng. 2003;9:127. doi: 10.1089/107632703762687609. [DOI] [PubMed] [Google Scholar]
- 15.Griese D.P. Ehsan A. Melo L.G. Kong D. Zhang L. Mann M.J. Pratt R.E. Mulligan R.C. Dzau V.J. Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts: implications for cell-based vascular therapy. Circulation. 2003;108:2710. doi: 10.1161/01.CIR.0000096490.16596.A6. [DOI] [PubMed] [Google Scholar]
- 16.Allen J.B. Khan S. Lapidos K.A. Ameer G.A. Toward engineering a human neoendothelium with circulating progenitor cells. Stem Cells. 2010;28:318. doi: 10.1002/stem.275. [DOI] [PubMed] [Google Scholar]
- 17.Kaushal S. Amiel G.E. Guleserian K.J. Shapira O.M. Perry T. Sutherland F.W. Rabkin E. Moran A.M. Schoen F.J. Atala A. Soker S. Bischoff J. Mayer J.E., Jr Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med. 2001;7:1035. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt D. Asmis L.M. Odermatt B. Kelm J. Breymann C. Gossi M. Genoni M. Zund G. Hoerstrup S.P. Engineered living blood vessels: functional endothelia generated from human umbilical cord-derived progenitors. Ann Thorac Surg. 2006;82:1465. doi: 10.1016/j.athoracsur.2006.05.066. [DOI] [PubMed] [Google Scholar]
- 19.Aper T. Schmidt A. Duchrow M. Bruch H.P. Autologous blood vessels engineered from peripheral blood sample. Eur J Vasc Endovasc Surg. 2007;33:33. doi: 10.1016/j.ejvs.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Thebaud N.B. Bareille R. Remy M. Bourget C. Daculsi R. Bordenave L. Human progenitor-derived endothelial cells vs. venous endothelial cells for vascular tissue engineering: an in vitro study. J Tissue Eng Regen Med. 2010;4:473. doi: 10.1002/term.261. [DOI] [PubMed] [Google Scholar]
- 21.Stachelek S.J. Alferiev I. Connolly J.M. Sacks M. Hebbel R.P. Bianco R. Levy R.J. Cholesterol-modified polyurethane valve cusps demonstrate blood outgrowth endothelial cell adhesion post-seeding in vitro and in vivo. Ann Thorac Surg. 2006;81:47. doi: 10.1016/j.athoracsur.2005.07.061. [DOI] [PubMed] [Google Scholar]
- 22.Brown M.A. Wallace C.S. Angelos M. Truskey G.A. Characterization of umbilical cord blood-derived late outgrowth endothelial progenitor cells exposed to laminar shear stress. Tissue Eng Part A. 2009;15:3575. doi: 10.1089/ten.tea.2008.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stroncek J.D. Grant B.S. Brown M.A. Povsic T.J. Truskey G.A. Reichert W.M. Comparison of endothelial cell phenotypic markers of late-outgrowth endothelial progenitor cells isolated from patients with coronary artery disease and healthy volunteers. Tissue Eng Part A. 2009;15:3473. doi: 10.1089/ten.tea.2008.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grassl E.D. Oegema T.R. Tranquillo R.T. A fibrin-based arterial media equivalent. J Biomed Mater Res. 2003;66:550. doi: 10.1002/jbm.a.10589. [DOI] [PubMed] [Google Scholar]
- 25.Syedain Z.H. Weinberg J.S. Tranquillo R.T. Cyclic distension of fibrin-based tissue constructs: evidence of adaptation during growth of engineered connective tissue. Proc Natl Acad Sci USA. 2008;105:6537. doi: 10.1073/pnas.0711217105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmann K.A. Weinbaum J.S. Johnson S.L. Tranquillo R.T. Fibrin degradation enhances vascular smooth muscle cell proliferation and matrix deposition in fibrin-based tissue constructs fabricated in vitro. Tissue Eng Part A. 2010;16:3261. doi: 10.1089/ten.tea.2009.0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isenberg B.C. Williams C. Tranquillo R.T. Endothelialization and flow conditioning of fibrin-based media-equivalents. Ann Biomed Eng. 2006;34:971. doi: 10.1007/s10439-006-9101-0. [DOI] [PubMed] [Google Scholar]
- 28.Kladakis S.M. Nerem R.M. Endothelial cell monolayer formation: effect of substrate and fluid shear stress. Endothelium. 2004;11:29. doi: 10.1080/10623320490432461. [DOI] [PubMed] [Google Scholar]
- 29.Kiernan J.A. Oxford, United Kingdom, Boston: Butterworth-Heinemann; 1999. Histological and Histochemical Methods: Theory and Practice. [Google Scholar]
- 30.Sakariassen K.S. Aarts P.A. de Groot P.G. Houdijk W.P. Sixma J.J. A perfusion chamber developed to investigate platelet interaction in flowing blood with human vessel wall cells, their extracellular matrix, and purified components. J Lab Clin Med. 1983;102:522. [PubMed] [Google Scholar]
- 31.Anderson J.S. Price T.M. Hanson S.R. Harker L.A. In vitro endothelialization of small-caliber vascular grafts. Surgery. 1987;101:577. [PubMed] [Google Scholar]
- 32.Kaehler J. Zilla P. Fasol R. Deutsch M. Kadletz M. Precoating substrate and surface configuration determine adherence and spreading of seeded endothelial cells on polytetrafluoroethylene grafts. J Vasc Surg. 1989;9:535. [PubMed] [Google Scholar]
- 33.Kesler K.A. Herring M.B. Arnold M.P. Glover J.L. Park H.M. Helmus M.N. Bendick P.J. Enhanced strength of endothelial attachment on polyester elastomer and polytetrafluoroethylene graft surfaces with fibronectin substrate. J Vasc Surg. 1986;3:58. doi: 10.1067/mva.1986.avs0030058. [DOI] [PubMed] [Google Scholar]
- 34.Seeger J.M. Klingman N. Improved in vivo endothelialization of prosthetic grafts by surface modification with fibronectin. J Vasc Surg. 1988;8:476. doi: 10.1067/mva.1988.avs0080476. [DOI] [PubMed] [Google Scholar]
- 35.Koveker G.B. Graham L.M. Burkel W.E. Sell R. Wakefield T.W. Dietrich K. Stanley J.C. Extracellular matrix preparation of expanded polytetrafluoroethylene grafts seeded with endothelial cells: influence on early platelet deposition, cellular growth, and luminal prostacyclin release. Surgery. 1991;109:313. [PubMed] [Google Scholar]
- 36.Gosselin C. Vorp D.A. Warty V. Severyn D.A. Dick E.K. Borovetz H.S. Greisler H.P. Eptfe coating with fibrin glue, fgf-1, and heparin: effect on retention of seeded endothelial cells. J Surg Res. 1996;60:327. doi: 10.1006/jsre.1996.0052. [DOI] [PubMed] [Google Scholar]
- 37.Zilla P. Fasol R. Preiss P. Kadletz M. Deutsch M. Schima H. Tsangaris S. Groscurth P. Use of fibrin glue as a substrate for in vitro endothelialization of ptfe vascular grafts. Surgery. 1989;105:515. [PubMed] [Google Scholar]
- 38.Chiu J.J. Chen L.J. Lee P.L. Lee C.I. Lo L.W. Usami S. Chien S. Shear stress inhibits adhesion molecule expression in vascular endothelial cells induced by coculture with smooth muscle cells. Blood. 2003;101:2667. doi: 10.1182/blood-2002-08-2560. [DOI] [PubMed] [Google Scholar]
- 39.Ohtsuka A. Ando J. Korenaga R. Kamiya A. Toyama-Sorimachi N. Miyasaka M. The effect of flow on the expression of vascular adhesion molecule-1 by cultured mouse endothelial cells. Biochem Biophys Res Commun. 1993;193:303. doi: 10.1006/bbrc.1993.1624. [DOI] [PubMed] [Google Scholar]
- 40.Morigi M. Zoja C. Figliuzzi M. Foppolo M. Micheletti G. Bontempelli M. Saronni M. Remuzzi G. Remuzzi A. Fluid shear stress modulates surface expression of adhesion molecules by endothelial cells. Blood. 1995;85:1696. [PubMed] [Google Scholar]
- 41.Levesque M.J. Nerem R.M. The elongation and orientation of cultured endothelial cells in response to shear stress. J Biomech Eng. 1985;107:341. doi: 10.1115/1.3138567. [DOI] [PubMed] [Google Scholar]
- 42.Chiu J.J. Lee P.L. Chen C.N. Lee C.I. Chang S.F. Chen L.J. Lien S.C. Ko Y.C. Usami S. Chien S. Shear stress increases ICAM-1 and decreases VCAM-1 and e-selectin expressions induced by tumor necrosis factor-[alpha] in endothelial cells. Arterioscler Thromb Vasc Biol. 2004;24:73. doi: 10.1161/01.ATV.0000106321.63667.24. [DOI] [PubMed] [Google Scholar]
- 43.Chiu J.J. Lee P.L. Chang S.F. Chen L.J. Lee C.I. Lin K.M. Usami S. Chien S. Shear stress regulates gene expression in vascular endothelial cells in response to tumor necrosis factor-alpha: a study of the transcription profile with complementary DNA microarray. J Biomed Sci. 2005;12:481. doi: 10.1007/s11373-005-4338-4. [DOI] [PubMed] [Google Scholar]
- 44.Sheikh S. Rainger G.E. Gale Z. Rahman M. Nash G.B. Exposure to fluid shear stress modulates the ability of endothelial cells to recruit neutrophils in response to tumor necrosis factor-alpha: a basis for local variations in vascular sensitivity to inflammation. Blood. 2003;102:2828. doi: 10.1182/blood-2003-01-0080. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y. Ingram D.A. Murphy M.P. Saadatzadeh M.R. Mead L.E. Prater D.N. Rehman J. Release of proinflammatory mediators and expression of proinflammatory adhesion molecules by endothelial progenitor cells. Am J Physiol. 2009;296:H1675. doi: 10.1152/ajpheart.00665.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuchan M.J. Frangos J.A. Role of calcium and calmodulin in flow-induced nitric oxide production in endothelial cells. Am J Physiol. 1994;266:C628. doi: 10.1152/ajpcell.1994.266.3.C628. [DOI] [PubMed] [Google Scholar]
- 47.Kuchan M.J. Jo H. Frangos J.A. Role of g proteins in shear stress-mediated nitric oxide production by endothelial cells. Am J Physiol. 1994;267:C753. doi: 10.1152/ajpcell.1994.267.3.C753. [DOI] [PubMed] [Google Scholar]
- 48.Yee A. Bosworth K.A. Conway D.E. Eskin S.G. McIntire L.V. Gene expression of endothelial cells under pulsatile non-reversing vs. steady shear stress; comparison of nitric oxide production. Ann Biomed Eng. 2008;36:571. doi: 10.1007/s10439-008-9452-9. [DOI] [PubMed] [Google Scholar]
- 49.Harrison D.G. Widder J. Grumbach I. Chen W. Weber M. Searles C. Endothelial mechanotransduction, nitric oxide and vascular inflammation. J Intern Med. 2006;259:351. doi: 10.1111/j.1365-2796.2006.01621.x. [DOI] [PubMed] [Google Scholar]
- 50.Traub O. Berk B.C. Laminar shear stress: mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vasc Biol. 1998;18:677. doi: 10.1161/01.atv.18.5.677. [DOI] [PubMed] [Google Scholar]
- 51.Lund T. Hermansen S.E. Andreasen T.V. Olsen J.O. Osterud B. Myrmel T. Ytrehus K. Shear stress regulates inflammatory and thrombogenic gene transcripts in cultured human endothelial progenitor cells. Thromb Haemostasis. 2010;104:582. doi: 10.1160/TH09-12-0854. [DOI] [PubMed] [Google Scholar]