Abstract
Relatively, little is known about the relationship between biodiversity and ecosystem functioning in forests, especially in the tropics. We describe the Sabah Biodiversity Experiment: a large-scale, long-term field study on the island of Borneo. The project aims at understanding the relationship between tree species diversity and the functioning of lowland dipterocarp rainforest during restoration following selective logging. The experiment is planned to run for several decades (from seed to adult tree), so here we focus on introducing the project and its experimental design and on assessing initial conditions and the potential for restoration of the structure and functioning of the study system, the Malua Forest Reserve. We estimate residual impacts 22 years after selective logging by comparison with an appropriate neighbouring area of primary forest in Danum Valley of similar conditions. There was no difference in the alpha or beta species diversity of transect plots in the two forest types, probably owing to the selective nature of the logging and potential effects of competitive release. However, despite equal total stem density, forest structure differed as expected with a deficit of large trees and a surfeit of saplings in selectively logged areas. These impacts on structure have the potential to influence ecosystem functioning. In particular, above-ground biomass and carbon pools in selectively logged areas were only 60 per cent of those in the primary forest even after 22 years of recovery. Our results establish the initial conditions for the Sabah Biodiversity Experiment and confirm the potential to accelerate restoration by using enrichment planting of dipterocarps to overcome recruitment limitation. What role dipterocarp diversity plays in restoration only will become clear with long-term results.
Keywords: enrichment planting, species richness, ecosystem functioning, selectively logged forest, Dipterocarpaceae, Sabah Biodiversity Experiment
1. Introduction
‘We have done the easy stuff, working experimentally with herbaceous communities, and have learned a great deal about the diversity/functioning/stability relationship. However, we now must move on to address those ecosystems that control a good portion of the carbon, nutrient and water balances of the earth—the forests’.
Harold Mooney [1, p. VI]
(a). Biodiversity and the functioning and stability of ecosystems
As well as being renowned for their biodiversity, tropical forests also provide multiple local, regional and global ecosystem services [2]. For example, at the global scale, they contribute to climate regulation, whereas at the regional scale they provide water-storage capacity and at the local scale they can support pest regulation, pollination, seed dispersal and soil fertility [3]. However, both the biodiversity and functioning of tropical forest ecosystems are under threat from human activities including over-harvesting of selectively logged trees and forest habitat fragmentation through conversion to agriculture and other land-use changes.
Recent research [4–6] has established that loss of biodiversity can have negative impacts on the functioning and stability of ecosystems. Indeed, Darwin laid out the rationale for a link between biodiversity and ecosystem functioning during the formative years of evolution and ecology [7,8]. Darwin's principle of divergence proposed that the evolution of species into different, complementary niches leads to an ecological ‘division of labour’, such that an ecosystem with a diverse community of species functions more effectively in terms of resource capture and cycling, and higher levels of productivity [9]. Consequently, sudden loss of biodiversity can have a negative effect on functioning by leaving niches vacant or under-used [10].
Meta-analysis has shown that, all else being equal, more diverse communities do indeed lead to more complete resource capture and retention and to higher levels of productivity [4,11–13]. Because different sets of species influence different ecosystem processes, higher levels of biodiversity are required to support full ecosystem multi-functionality than to underpin any single process [14–16]. Diversity also increases stability whenever species in a mixed community differ in their response to perturbation and when diversity increases mean levels of an ecosystem process relative to the variability in that process [17,18]. Ecosystem functioning refers to all the transfers of energy and matter owing to biogeochemical processes (or ecosystem processes) even though (for practical reasons) much research to date has focused on biomass production.
(b). Does biodiversity loss impact ecosystem functioning in tropical forests?
Relatively little is known about the relationship between biodiversity and ecosystem functioning in forests, particularly in the tropics. Indeed, one of the most significant recent conceptual advances in tropical forest ecology (and in community ecology in general), the unified neutral theory of biodiversity and biogeography [19], appears to imply no link between diversity and functioning. In neutral theory, species are identical in terms of the small number of traits that play a role in community dynamics, namely birth and death rates and immigration and speciation rates. Species are therefore identical and interchangeable and populations follow the random walk of ecological drift. Because species are identical, they co-occur entirely owing to the so-called equalizing forces that slow time to extinction while density- or frequency-dependent stabilizing forces are absent [20]. The neutral theory is supported by its unexpected early success in recreating realistic community characteristics such as relative abundance distributions. Despite this success, the plausibility of key assumptions of the neutral theory has come under increasing question [21,22]. The debate has now moved on to the relative strength of equalizing and stabilizing in ecological communities, with tropical forests as one system suggested to have relatively strong equalizing forces (species fitnesses are similar) and weak stabilizing forces. If this were the case, then it would appear to suggest only weak effects of biodiversity loss on functioning in forest ecosystems. On the other hand, simulation studies predict impacts of species loss on carbon storage under some extinction scenarios [23].
Most of the evidence for the relationship between diversity and ecosystem functioning comes from observational surveys in temperate and boreal forests [24–26], where it is difficult to separate the effects of diversity from other confounding environmental variables. For example, these natural gradients of diversity often include one species that is present along the entire gradient, confounding species diversity with species identity [24]. Inventory data suggest a positive [27,28] or null [29] relationship between tree diversity and biomass production. Tree diversity has been found to positively affect soil cation exchange capacity [30] and nutrient turnover [31], but also decomposer fauna at the local scale [32]. Forest soil communities show contrasting behaviour in the way species richness and overall density respond to tree diversity: true bugs (Hemiptera, Heteroptera) were primarily affected by tree diversity and heterogeneity [33,34] but herbivores [35] and predatory arthropods [36] were correlated more with tree species identity than with species richness.
Some studies in tropical forests have compared the functionality of monocultures and mixtures in plantations [37,38] with results showing that mixed species stands can sometimes outperform monocultures, but that whether or not this occurs depends on having complementary mixtures of species and not just species richness ‘per se’. Some species grew better [39] and had greater canopy development [40] in mixtures, others in monocultures. Overall, a review of monoculture and polyculture plantations found that mixed species stands tend to be more productive [41]. Apart from these reviews comparing mixed- and single-species plantations, the small literature chiefly comprises work from the Neotropics by Ewel and co-worker [42,43] on predicting complementary mixtures of species and a pioneering biodiversity experiment by Potvin and co-workers [44–46].
The work by Ewel & co-worker involved only three species that were very different from one another. Their results emphasize the importance of species identity (traits) and community composition (interactions in mixture). Species mixtures were sometimes complementary but the balance with competitive interactions changed over time primarily driven by competition for light. Predicting the development of subsequent interactions from the initial combination of species' traits in a community was highlighted as the major challenge for designing utilitarian combinations of species, for use in agro-forestry for example.
In their biodiversity experiment in Panama, Potvin & co-workers planted six tree species in monocultures and mixtures, replicated over 24 plots. The first outcomes indicate a positive effect of biodiversity on productivity owing to increased individual growth, but without any significant change in mortality rates [44,45]. Tree species richness was found to positively affect soil respiration [47], nutrient storage [48], and nitrogen and phosphorus pools [49], a result mainly explained by complementarity. Litter production and decomposition were primarily affected by species identity [50], although the lack of consideration for spatio-temporal heterogeneity might have influenced this finding.
Most recently, Ruiz-Jaen & Potvin [46] estimated above-ground carbon stocks in 124 subplots of 20 × 20 m of a 5 ha inventory plot in a 200 year-old tropical forest in Panama and used redundancy analysis to compare the influence of (i) tropical plant diversity (four functional groups given by the combinations of trees versus palms and understory versus canopy), (ii) the effects of five abiotic environmental parameters (topography and soil depth, bulk density, texture and colour), and (iii) spatial heterogeneity. Altogether, the three sources of variation accounted for 41 per cent of the variability in carbon storage, with plant functional group diversity accounting for the largest share of the variation at 20 per cent. The environmental variables and spatial heterogeneity, plus their two- and three-way interactions, accounted for the other half of the explained variation. These effects of biodiversity in a 200 year-old forest differ somewhat from those of the approximately 50 km apart plantation experiment described above and they also differ from the finding from the Barro Colorado Island 50 ha plot that there was no relationship between diversity and above-ground biomass [51]. The reasons for the differences in the results from these three studies all based in Panama are not clear but could include differences in the age of forests, rainfall or physical conditions.
(c). A new biodiversity experiment in the forests of Borneo
The Sabah Biodiversity Experiment (www.sabahbiodiversityexperiment.net) is part of an informal network of tree diversity experiments (www.TreeDivNet.ugent.be) including the BIOTREE (www.biotree.bgc-jena.mpg.de/deutsch/index.html) and BEF China (www.bef-china.de), where it is currently one of only two tropical studies and the sole study based in the palaeotropics [25]. The experiment differs in some important ways from any other biodiversity experiment conducted to date. Before introducing the experimental design and methodology, we highlight some of these key features. The Sabah Biodiversity Experiment is situated in mixed dipterocarp forest of South East Asia, which differs from those in Africa and Central America by being strongly dominated by a single family of trees, the Dipterocarpaceae [52]. Dipterocarp forests also often have higher canopies and higher above-ground biomass compared with the forests in other parts of the tropics [53]. The experiment is an attempt to transfer ideas and methods developed with model systems to a real-world setting. In particular, selectively logged forests in Sabah and the region are restored using enrichment planting. Enrichment planting is used for a number of reasons in different projects including timber production (e.g. the INNOPRISE corporation), carbon storage (e.g. the INFAPRO project), and the restoration of biodiversity and the forest ecosystem structure (INIKEA). Enrichment planting in Sabah is usually performed by planting seedlings of dipterocarps (and a small number of other species) along cleared lines cut into the selectively logged forest vegetation. So, the aspect of diversity that is manipulated in the Sabah Biodiversity Experiment is that of the enrichment-planted seedlings, and these are placed into an existing matrix of vegetation left over from selective logging.
We introduce the Sabah Biodiversity Experiment in detail and present some initial results. As results will take time to emerge, we focus on documenting the initial conditions and the potential for forest restoration. While alpha and beta diversities of plant species in the selectively logged forest are not reduced, we document the expected changes in forest structure including a reduction of large trees and an increase in saplings. These changes in structure are associated with lower above-ground biomass and carbon stores.
2. Methods
(a). Location
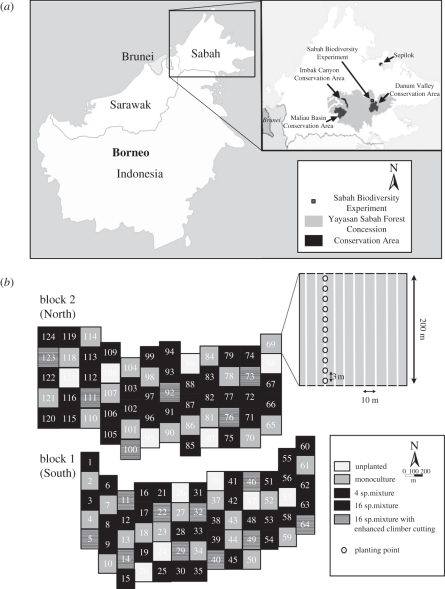
The Sabah Biodiversity Experiment is named after the Malaysian state that forms the northeastern tip of the island of Borneo (figure 1a). The region is relatively aseasonal with an annual rainfall of greater than 3000 mm. In 2000, a suitable experimental location was identified in the southern part of the Malua Forest Reserve (05°05′20′′ N, 117°38′32′′ E, 102 m a.s.l.)—a 35 000 ha area of selectively logged production forest (figure 1a inset). The Sabah Biodiversity Experiment Malua field camp is a satellite of the larger Danum Valley Field Centre that lies to the south (22.6 km air distance). The Malua Forest Reserve is part of a concession of 1 million ha that belongs to the publicly owned Yayasan Sabah (Sabah Foundation), whose purpose is to increase the welfare of the local people of Sabah by exploiting common natural resources, primarily timber [54]. Most of the area has been logged twice, once in the 1980s and once within the last 10 years, but an area of unlogged primary forest—the Danum Valley Conservation Area—was left at the heart of the concession. More specifically, the Malua Forest Reserve as a whole was selectively logged during the early 1980s and the area where the Sabah Biodiversity Experiment is now sited between 1984 and 1986. The area was re-logged in 2007 with the exception of the area used for the Sabah Biodiversity Experiment. The pre-logging timber volume was estimated as 193–221 m3 ha−1 with dipterocarps comprising between 180 and 216 m3 ha−1. These values are comparable with estimates of above-ground tree biomass at Danum Valley, supporting the comparability of the two sites (Yayasan Sabah, unpublished data; see §4). The soil of the area was classified as orthic acrisol, which is moderately acid (pH < 6), highly weathered and low in available nutrients, with a base saturation of 81 per cent (detailed protocols in the study of Majalap & Chu [55]). It has a marked increase of clay content with depth [56] and a low organic carbon content (topsoil: 1.2%, 1 m depth: 0.6% [57]). Bedrock consists of a mixture of mudstone and sandstone areas with other miscellaneous rock types (Sabah Forestry Department 2010, unpublished data).
Figure 1.
(a) Location of the Sabah Biodiversity Experiment within Borneo, Sabah and the local area (inset) showing some of the major areas of conserved forest. (b) Plan of the experimental site and enrichment-planting lines (inset).
The most recent development relevant to the Sabah Biodiversity Experiment and the Malua Forest Reserve is the creation in 2008 of the Malua BioBank (www.maluabank.com), comprising 34 000 ha under a Conservation Management Plan that aims to ‘improve wildlife habitat and promote ecosystem service functions including carbon sequestration and storage in above-ground biomass’ in order to sell Biodiversity Conservation Certificates representing 100 m2 of forest. The Sabah Biodiversity Experiment should therefore provide scientific information that could be used to guide and assess the management of the Malua BioBank.
(b). Experimental design
The Sabah Biodiversity Experiment is a field scale forest rehabilitation project and tree biodiversity experiment that covers 500 ha. Seedlings of 16 native canopy tree species (Dipterocarpaceae; table 1) were enrichment planted along lines cut into the pre-existing selectively logged forest (figure 1b). The project comprises 124 4 ha plots (200 × 200 m) that follow a randomized block design. The core of the project is the set of 96 plots that form a gradient in the species richness of enrichment-planted dipterocarps, which includes enrichment planting with one of each of the 16 study species, 16 different four-species mixtures and all 16 species combined. Each diversity level comprises 32 plots divided equally between the two blocks so that each of the enrichment plantings using one or four species occurs once in each of the two blocks with 16 identical replicates of the full 16-species enrichment-planting mixture in each block. Responses in ecosystems properties and processes along this gradient of 96 plots enrichment planted with one, four or 16 species can be compared with those of 12 unplanted control plots (six in each block). The diversity gradient of 96 plots plus unplanted controls sum to 108 plots divided equally between the two blocks. The final 16 plots form a sub-experiment to look at management treatments involving the frequency with which climbing plants (lianas) are removed during restoration. Standard enrichment-planting methods involve cutting of planting lines and of climbing species that would otherwise compete and damage the dipterocarp seedlings [58]. A recent suggestion to speed-up restoration of selectively logged dipterocarp forest is enhanced climber cutting, where the cutting occurs more widely in the intervening areas separating the planting lines that run in parallel at 10 m intervals. The final 16 plots will receive enhanced climber cutting for comparison with matched 16-species plots from the core diversity gradient. The initial plan was to have two identical blocks but for various logistical reasons (erosion of plots, neighbouring streams, etc.) the two blocks have different numbers of plots with 60 in the Northern block and 64 in the South and the replication of the enhanced climber-cutting plots is therefore unequal with six plots in the Northern block and 10 in the South.
Table 1.
The 16 species of the Dipterocarpaceae family planted in the Sabah Biodiversity Experiment and their IUCN Red List status (downloaded on 20 November 2010 from www.iucnredlist.org).
| genus | species | species authority | IUCN status |
|---|---|---|---|
| Shorea | johorensis | Foxw. | critically endangered |
| gibbosa | Brandis. | critically endangered | |
| argentifolia | Sym. | endangered | |
| faguetiana | Heim. | endangered | |
| leprosula | Miq. | endangered | |
| macrophylla | Ashton | vulnerable | |
| macroptera | King | — | |
| ovalis | Korth. | — | |
| parvifolia | Dyer. | — | |
| beccariana | Bruck | not listed | |
| Parashorea | malaanonan | (Blanco) Merr. | critically endangered |
| tomentella | Meijer | not listed | |
| Hopea | sangal | Korth. | critically endangered |
| ferruginea | Parijs | critically endangered | |
| Dryobalanops | lanceolata | Burck | endangered |
| Dipterocarpus | conformis | Slooten | — |
(c). Planting and replanting
The seedlings required for the initial planting were collected throughout the neighbouring Ulu Segama and Malua forest reserves with the exception of Hopea ferruginea (INIKEA nursery at Lawasong) and Dipterocarpus conformis (Tawau Hill area collection). The ages of the seedlings varied but were predominantly from a single fruiting and seedlings were selected to be of as similar size as possible. The planting material was assembled at the Innoprise-FACE Foundation Rainforest Rehabilitation Project (INFAPRO) nursery in 2001, with the first round of planting beginning in July 2002 with block one completed by December and block two completed in September 2003. Seedlings are planted on parallel lines 10 m apart with one seedling planted every 3 m (except where not possible due to rocks, streams, etc.). It is standard practice to have one round of replanting following a period to allow initial mortality to occur. Therefore, a second collection of seedlings was assembled at a purpose-built nursery in the Malua field camp from both local fruiting events and the INFAPRO nursery. Replanting began in January 2009, and the first block was completed in October 2010. Final seedling collections in December 2009 and August 2010 provided the final seedling stock for replanting of the second block, which is underway at the time of writing and planned for completion during 2011. The survival and growth of the enrichment-planting seedlings are being regularly monitored.
(d). Diversity and composition
Four identical transect plots of 10 × 250 m were established in the primary forest of Danum Valley Conservation Area and in the Sabah Biodiversity Experiment (surveying only the background vegetation between the planting lines). All trees greater than 10 cm diameter at breast height (d.b.h. at 130 cm) were identified by a local tree expert to genus level, or to species level where possible. In 1 ha, we identified 104 species in unlogged forest of Danum Valley and 107 species in selectively logged forest of the Sabah Biodiversity Experiment (electronic supplementary material, table S1). For comparison, an independent survey of the same area of selectively logged forest identified up to 180 species for a total area of 5.25 ha (Sabah Forestry Department 2010, unpublished data). Further details are available in the study of Saner [57]. Forest community composition was analysed with non-metric multi-dimensional scaling (NMDS) ordination to identify the effects of disturbance history on plant community assemblage. The proportion of each species in a transect plot and the Bray–Curtis dissimilarity function were used to calculate the distance matrix [59]. Alpha and beta diversities were calculated with Shannon's diversity index and Whittacker's measure of beta diversity, respectively [60]. This was implemented using the diversity function in the vegan package in R [61]. Values were calculated per transect plot within each forest type and means and standard errors were calculated from these values.
(e). Structure and functioning
Above-ground tree biomass was calculated for unlogged forest and for selectively logged forest based on d.b.h. measurements. Total stand basal area was calculated from d.b.h. and height and volume were predicted from d.b.h. using established allometric equations from Pinard [62]. Above-ground stem (trunk) biomass for individual trees was then calculated by multiplying volume with wood density. Total above-ground biomass was then calculated by multiplying above-ground stem biomass by the standard factor of 1.9 [63]. Carbon stocks were estimated only for the logged forest following the standard assumption that half of above-ground biomass is carbon [63,64].
(f). Baseline carbon details
Apart from above-ground tree biomass, several other components of the baseline carbon estimation were estimated or measured along the transect plots of the selectively logged forest. Below-ground coarse roots were estimated from existing root shoot ratios (17% of above-ground tree biomass [65]). Dead standing trees were surveyed and their contribution to total carbon stocks was calculated based on d.b.h. measurements and a mean wood density of 500 kg m−3 [66]. Furthermore, litterfall traps (1 m2, n = 40) were randomly allocated along the transect plots, and fine litterfall was collected every other week over one year (n = 25). At the same 40 sites, soil respiration rates were estimated using an infrared gas analyser CARBOCAP GMP343 (Vaisala, Finland) and a self-made chamber [67]. Over two months, nine measurements were performed (seven day-time (08.00–12.00) and two night-time (20.00–04.00) measurements) over 5 min intervals. Along the transect plots, quadrats (5 × 5 m, n = 24) were randomly selected and all saplings and seedlings were harvested. Subquadrats (0.5 × 0.5 m) were established within quadrats for collecting the standing litter, including leaf litter and woody debris. Within each subquadrat, vertical cores (100 cm3) were taken from the top soil (0–5 cm) to determine fine root biomass (≥2 mm diameter). All collected samples were dried in a glasshouse (7 days, 60°C) prior to measurement. A carbon content of 50 per cent of total biomass was used for harvesting saplings, seedlings and fine root biomass and 42 per cent for fine litter fall, leaf litter and woody debris.
Thirteen random sites were selected across the Sabah Biodiversity Experiment for a soil organic carbon profile down to 1 m depth. Soil pits were excavated and soil cores were taken from layers of 0.1 m depth (n = 396). Carbon content was determined by the Walkley–Black method, a wet chemical analysis. For further details on any of these components of the baseline carbon estimation, see Saner [57].
(g). Species functional traits
Wood density was estimated with a random intercept linear mixed effects model using all the data on Dipterocarpaceae from www.worldagroforestrycentre.org and Burgess [68]. Site-specific estimates for a subset of species were also made following the water-displacement protocols of Chave et al. [69], which correlated strongly with the previously published data (data not shown). Seed volume was estimated using measurements from the literature (calculated as a spheroid based on mean nut length and width estimates obtained from Newman et al. [70,71]). Survival, a key demographic process, was estimated for each species as the proportion of the first round of enrichment-planted tree seedlings surviving after seven years. Further details can be found in the study of Dzulkifli [72].
3. Results
(a). Diversity and composition
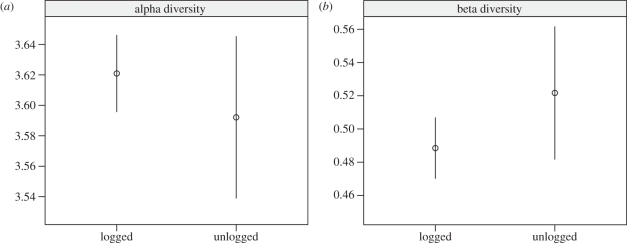
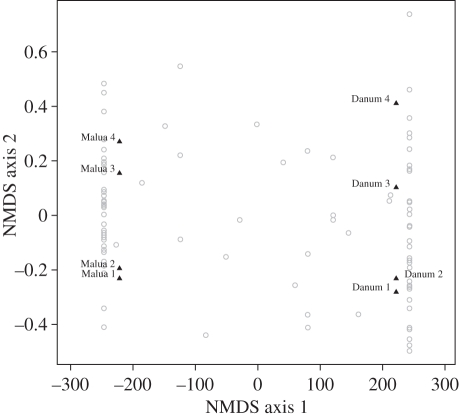
Logging had no effect on average alpha or beta diversities of transect plots within each forest type (figure 2). This was not an effect of differences in individual tree density (see below and tables 2 and 3). However, the NMDS multi-variate analysis suggests that logging had impacts on species composition, with Malua and Danum transect plots clustering on opposing sides of NMDS axis 1 (figure 3). The NMDS shows a large dissimilarity in species composition between unlogged and selectively logged forest transect plots as well as identifying only a handful (<25) of shared species.
Figure 2.
(a) Alpha and (b) beta diversities in selectively logged and unlogged forests (mean ± s.e.m.; n = 4). Shannon's diversity index estimates the number of species per transect lines within each forest type and Whittacker's beta diversity estimates the similarity/turnover in species between transect lines within each forest type.
Table 2.
Unlogged forest: overview of the nine most important tree families and the 10 most important species (>10 cm d.b.h.). BA, mean (±s.e.m.) basal area.
| family | species | BA (m2 ha−1) | BA (%) | d.b.h. range (cm) | tree density (ha−1) |
|---|---|---|---|---|---|
| Dipterocarpaceae | 18.24 (±0.66) | 61.0 | 10.1–170.3 | 87 | |
| Shorea johorensis | 9.09 (±0.67) | 30.4 | 10.5–170.3 | 21 | |
| Shorea parvifolia | 6.22 (±0.09) | 20.8 | 11.7–116.3 | 28 | |
| Parashorea malaanonan | 1.61 (±0.26) | 5.4 | 10.6–67.2 | 12 | |
| Hopea nervosa | 0.99 (±0.11) | 3.3 | 10.1–47.5 | 17 | |
| Meliaceae | 1.91 (±0.11) | 6.4 | 10.0–32.3 | 61 | |
| Chisocheton sarawakensis | 0.55 (±0.04) | 1.8 | 10.0–30.0 | 17 | |
| Aglaia elliptica | 0.45 (±0.02) | 1.5 | 10.1–31.8 | 15 | |
| Aglaia macrocarpa | 0.39 (±0.03) | 1.3 | 11.4–31.0 | 10 | |
| Leguminosae | 1.89 (±0.40) | 6.3 | 10.0–144.8 | 6 | |
| Koompassia excelsa | 1.65 (±0.41) | 5.6 | 144.8 | 1 | |
| Lauraceae | 1.33 (±0.09) | 4.4 | 10.0–45.6 | 44 | |
| Euphorbiaceae | 1.13 (±0.08) | 3.8 | 20.0–36.1 | 61 | |
| Myrtaceae | 0.98 (±0.13) | 3.3 | 10.7–57.1 | 19 | |
| Syzygium fastigiatum | 0.63 (±0.12) | 2.1 | 14.1–57.1 | 5 | |
| Tiliaceae | 0.76 (±0.08) | 2.5 | 10.0–43.1 | 21 | |
| Pentace laxiflora | 0.65 (±0.08) | 2.2 | 11.4–43.1 | 15 | |
| Fagaceae | 0.62 (±0.06) | 2.1 | 11.7–50.5 | 10 | |
| Burseraceae | 0.38 (±0.03) | 1.3 | 10.6–41.4 | 11 | |
| others | 2.67 (±0.04) | 8.9 | 10.0–52.0 | 99 | |
| total | 29.91 (±0.66) | 100 | 10.0–170.3 | 410 |
Table 3.
Logged forest: overview of the nine most important tree families and the 10 most important species (>10 cm d.b.h.). BA, mean (± s.e.m.) basal area.
| family | species | BA (m2 ha−1) | BA (%) | d.b.h. range (cm) | tree density (ha−1) |
|---|---|---|---|---|---|
| Dipterocarpaceae | 6.88 (±0.17) | 27.6 | 10.0–84.3 | 69 | |
| Shorea johorensis | 1.61 (±0.17) | 6.4 | 13.8–84.3 | 7 | |
| Shorea gibbosa | 1.54 (±0.14) | 6.2 | 10.6–72.5 | 13 | |
| Dryobalanops lanceolata | 0.86 (±0.08) | 3.4 | 12.8–72.1 | 7 | |
| Shorea fallax | 0.67 (±0.17) | 2.7 | 13.4–71.0 | 3 | |
| Dipterocarpus caudiferus | 0.59 (±0.10) | 2.4 | 9.8–60.5 | 10 | |
| Euphorbiaceae | 5.42 (±0.24) | 21.7 | 10.0–64.0 | 107 | |
| Macaranga pearsonii | 2.75 (±0.21) | 11.0 | 17.0–64.0 | 24 | |
| Macaranga gigantea | 1.29 (±0.05) | 5.2 | 13.0–38.8 | 23 | |
| Rubiaceae | 3.79 (±0.16) | 15.2 | 10.0–48.0 | 74 | |
| Neolamarckia cadamba | 3.11 (±0.13) | 12.4 | 10.2–48.0 | 33 | |
| Leguminosae | 0.84 (±0.10) | 3.4 | 10.3–72.8 | 14 | |
| Datiscaceae | 0.79 (±0.16) | 3.2 | 23.8–80.9 | 4 | |
| Octomeles sumatrana | 0.79 (±0.16) | 3.2 | 23.8–80.9 | 4 | |
| Lauraceae | 0.75 (±0.07) | 3.0 | 11.1–59.8 | 12 | |
| Sonneratiaceae | 0.71 (±0.14) | 2.8 | 14.2–77.2 | 5 | |
| Duabanga moluccana | 0.71 (±0.14) | 2.8 | 14.2–77.2 | 5 | |
| Sapindaceae | 0.55 (±0.06) | 2.2 | 16.5–51.2 | 8 | |
| Tiliaceae | 0.54 (±0.07) | 2.2 | 11.5–62.1 | 8 | |
| others | 4.69 (±0.13) | 18.8 | 10.0–59.1 | 116 | |
| total | 24.96 (±0.83) | 100 | 10.0–84.3 | 417 |
Figure 3.
Differences in species composition of transects (solid triangles) and in individual species occurrence (open circles) in the Sabah Biodiversity Experiment area of the selectively logged forest of the Malua Forest Reserve (Malua 1–4) and the primary forest of Danum Valley Conservation Area (Danum 1–4) as shown by the first two axes from a multi-variate NMDS analysis.
(b). Structure
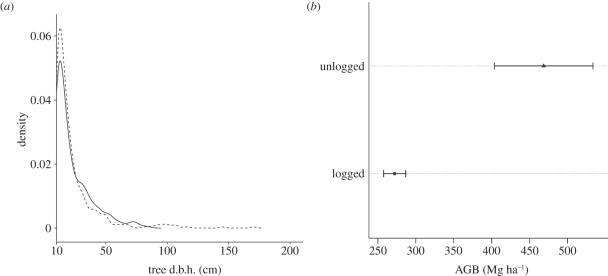
Logging had the expected impacts on forest structure, even 22 years after disturbance. The total stand basal area for unlogged forest was 29.9 ± 0.7 versus 25.0 ± 0.8 s.e.m. m2 ha−1 for logged forest (tables 2 and 3). The relative contribution of dipterocarps to total basal area was approximately 60 per cent in unlogged forest when compared with about 30 per cent in logged forest. Trees greater than 90 cm d.b.h. were entirely absent from the transects in the logged forest but present in matched transect plots in the primary forest of Danum Valley (figure 4a). Differences in total basal area were owing to tree size and not tree density which was similar in the two forest types: the unlogged forest had a total of 410 stems in the surveyed hectare (sum of the four transect plots) versus 417 in the hectare of logged forest. The frequency of the smallest measured trees (10–20 cm) was lower in the logged forest (figure 4a).
Figure 4.
(a) Kernel density estimation (probability) of stand size distribution in logged (solid line) and unlogged (dotted line) forests. d.b.h. was measured ≥10 cm only. Note the increased density of mid-sized trees (30–80 cm) in selectively logged forest and the higher density of small (<20 cm) and large (>90 cm) trees in unlogged forests. (b) Estimates of total above-ground tree biomass (AGB; ±1 s.e.m.) for the background selectively logged forest of the Sabah Biodiversity Experiment (Malua Forest Reserve) and the primary unlogged forest of Danum Valley.
(c). Ecosystem functioning: carbon stocks
Because approximately 65 per cent (155 ± 10.3 s.e.m. Mg ha−1) of the total carbon of tropical lowland dipterocarp forest occurs in above-ground biomass, logging can have large impacts on the carbon stocks of these ecosystems by removing the larger species with denser wood. Estimates from our data of above-ground tree biomass of the selectively logged forest were only 272.1 Mg ha−1 (95% CI: 225.6–318.6) compared to 468.6 Mg ha−1 (261.6–675.7) in the primary forest of Danum Valley (figure 4b). As around 50 per cent of wood is assumed to be carbon [63], this leads to estimates of carbon stored of 136 Mg C ha−1 (±7.3 s.e.m.) in the selectively logged forest compared with 234.3 Mg C ha−1 (±32.5 s.e.m.) in the primary forest of Danum Valley (figure 5). Based on these estimates, the total area of 500 ha for the Sabah Biodiversity Experiment has an initial pre-enrichment planting total organic carbon content of 118.6 Gg C ± 4.2 (s.d.).
Figure 5.
Overview of the baseline carbon budget for the selectively logged forest of the Sabah Biodiversity Experiment. Values are means ±s.e.m. Mg C ha−1 (with the per cent contribution to total organic C stocks given in parentheses). Litterfall and soil respiration rates are reported as Mg C ha−1 yr−1.
(d). Species differences
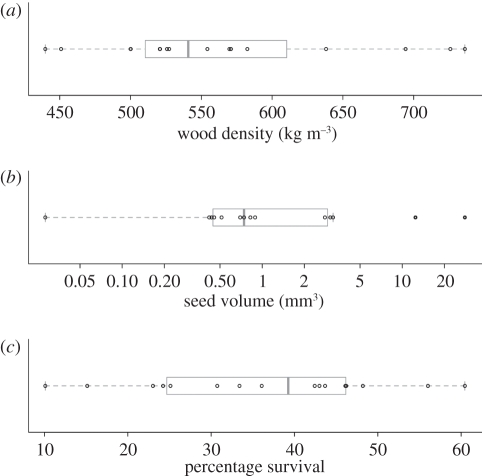
Preliminary inspection of our data on the traits of dipterocarps suggests substantial species differences. Wood density varies from 440 to 736 kg m−3 (figure 6a). For context, Borneo Ironwood or Belian (Eusideroxylon zwageri Teijsm. & Binnend.) has an air-dry density of 1000–1105 kg m−3, while early successional Macaranga species have a wood density ranging from 270 to 590 kg m−3 (at 15% moisture content). Seed size also varied widely across three orders of magnitude from 0.028 to 28 cm−3. Finally, survival of the enrichment-planted seedlings had a median value around 40 per cent but varied from approximately 10 to 60% (figure 6c).
Figure 6.
Variation in key functional traits ((a) wood density; (b) seed volume) and a demographic process ((c) percentage survival) of the dipterocarp species used for enrichment planting in the Sabah Biodiversity Experiment. Percentage survival is mean values from the first measure of first planting of the southern block.
4. Discussion
Overall, the results of our comparison of the selectively logged lowland dipterocarp forest of the Malua Forest Reserve with the nearby primary forest of Danum Valley support the expectation that logging has chronic impacts on forest composition, structure and functioning, at least for the first 22 years since harvesting.
One exception was that the alpha and beta diversities of the transect plots in the selectively logged forest were not reduced (figure 2) [73]. This was not a function of differences in tree stem and trunk density, which was very similar in the two forest types. Rather, this is probably partly because, by definition, selective logging removes a restricted range of species (here mainly dipterocarps) [74]. Selective logging may also have beneficial effects on the diversity of the pioneers and other non-harvested species through disturbance and release from competition with the large dipterocarp individuals that have been extracted [75]. Multi-variate analysis of the composition of the unlogged and selectively logged forest transect plots supports this explanation with apparent clustering of plots from the two forest types (figure 3), and while pioneer species were present in the selectively logged forest they were not detected in the samples from the unlogged forest.
These differences in composition had both structural and functional consequences 22 years after logging. As expected from its nature, selective logging reduced the frequency of large trees. Indeed, no trees greater than 90 cm d.b.h. were found in transect plots in the selectively logged forests, whereas trees up to twice this diameter were present in the samples from the primary forest of Danum Valley (figure 4a). It is important to point out that some larger trees do persist in the selectively logged forest because fewer species were selected for logging in the 1980s than in more recent times (e.g. the legume Koompassia excelsa (Becc.) Taub. is sometimes now logged for timber for parquet while previously the silica-rich wood was considered too brittle for any commercial use) and even individuals of desirable species sometimes occurred in inaccessible areas such as on steep slopes. However, it should be noted that the comparisons made here reflect the impact of one instance of selective logging only. Current logging methods include the use of helicopters that enable some of the remaining larger trees in inaccessible spots to be harvested. Comparison of Danum Valley with twice-logged areas of the Malua Forest Reserve would almost certainly reveal larger differences between the two areas and the estimates provided here are conservative in the sense that they consider the effects of only one round of selective logging when most areas have now undergone at least two rounds.
Through the use of bulldozers, dragging of logs on cables, establishment of log landings and so on, logging also damages and kills many seedlings [76] and exert a large impact on rooting and compaction in forest soils [76]. In addition to the reduced number of fruiting mother trees, and subsequent reduction in seedling recruitment, this probably explains the reduced levels of trees with current measurements in the smallest 10–20 cm d.b.h. size class. In contrast, measurements of d.b.h. in the range of approximately 20–80 cm were sometimes slightly above those in unlogged forest, perhaps reflecting reduced competition for seedlings that survived logging and benefited from the removal of larger individuals.
We estimate the total carbon stored in the selectively logged forest to be 237.2 Mg C ha−1 (±8.4 s.d.) with approximately two-thirds above and one-third below ground. The total can be divided into the following six major carbon pools by percentage (figure 5): above-ground tree (57%) and non-tree biomass (2%), below-ground roots (10%), forest floor litter (<1%), deadwood (6%) and soil (25%). Our results suggest that selective logging of dipterocarps has resulted in the above-ground biomass and carbon pools of the Malua lowland dipterocarp rainforest being depressed by approximately 40 per cent even 22 years after logging. However, indicators of nutrient and carbon turnover rates (dead standing wood, fine roots and litterfall) in the logged forest were not distinguishable from those observed in the neighbouring primary forest of the Danum Valley Conservation Area. This substantial reduction in carbon stocks 22 years after selective logging suggests that restoration and management practices that increase dipterocarp recruitment and basal area in logged dipterocarp forest do have the potential to increase carbon storage during this century by accelerating the return to pre-logging levels [58]. Based on our estimates from the Sabah Biodiversity Experiment (500 ha), enrichment planting in the Malua Forest Reserve (35 000 ha) and in the entire logged concession (1 million ha) has the potential to increase the amount of carbon stored by around 77 t C ha−1 in addition to having other potential associated benefits to biodiversity and other ecosystem processes and services. Although we cannot distinguish site from management effects, a survey of unlogged forest at Danum Valley and of the pre-logged forest of the Sabah Biodiversity Experiment completed in 1983 showed that estimated overall volume (unlogged: 178–230 m3 ha−1, logged: 193–221 m3 ha−1) and estimated dipterocarp volume (unlogged: 149–225 m3 ha−1, logged: 180–216 m3 ha−1) was comparable at both sites before logging in 1986 (Yayasan Sabah Forest Management Plan 1984–2032, unpublished data). We therefore assume that the effect of logging is real and approximately as indicated. For comparison, we also report three studies that independently confirm the effect of logging on approximately 20 years old forest: the study of Berry et al. [77] reported a reduction of −99.0 Mg C ha−1 (with 95% CI of −162.5 to −35.5), Pinard & Putz [78] −186.5 Mg C ha−1 (with 95% CI of −261.7 to −111.3) and Tangki & Chappell [79] −334.7 Mg C ha−1 (with 95% CI of −512.4 to −157.0).
Although it is too early to assess the effects of dipterocarp diversity on ecosystem functioning in our experiment, the range of values for the probable functionally relevant traits (figure 6) is consistent with the type of species differences that lead to complementarity [80]. Increasing diversity may therefore be valuable in dipterocarp replanting and restoration schemes [81]. One mechanism by which diversity could enhance forest functioning is a simple spatial insurance effect [17]. Enrichment restoration has traditionally used a small number of species—typically those surviving in nurseries from the last major reproductive event (dipterocarps typically reproduce synchronously and irregularly [82])—and involved planting them in monoculture (or low-diversity combinations) in selectively logged forests. In the long term, this risks enriching forest with a small number of species, setting up a self-reinforcing cycle as these species are over-represented in the next round of reproduction and replanting. So long as no species can survive under all conditions, replanting with one species will result in recruitment failure in unfavourable areas. If density is low enough, this will result in recruitment failure by late-successional species (with higher wood density) and increase the area of forest dominated by pioneer species (with lower wood density). Planting areas with more speciose mixtures with a greater diversity of traits should reduce the risk of this type of recruitment failure through a spatial insurance effect. Thus, more diverse mixtures should spread risk and increase the chance of having the right species in the right place at the right time.
Selective logging directly and immediately reduces above-ground tree biomass carbon pools in our system by reducing total tree basal area [83,84]. It may have a long-term effect if its selective nature leads to a reduction in average wood density by increasing the abundance of early- relative to late-successional species as discussed above. Restoration and management practices for selectively logged forest that influence recruitment so as to increase total basal area and average wood density therefore have the potential to increase above-ground carbon storage during this century by accelerating the return to pre-logging levels. All else being equal, successful recruitment by species with higher than average wood density will result in greater carbon storage. However, all else may not be equal: enrichment-planting schemes focusing on species with high wood density (presuming a choice including such species is available) could reduce above-ground carbon if wood density trade-offs against other species traits such as growth or recruitment rate. In the long term, enrichment planting could have other feedbacks on forest structure and function via changes in recruitment conditions (light levels, etc.). Understanding the relationship between recruitment, growth rates and relevant functional traits (wood density, etc.) [85] is therefore key to predicting and managing the long-term effects of selective logging and enrichment planting on the structure of the forest and on the services that it provides. Predicting which species provide the most complementary community of species therefore remains a key challenge [43]—especially when multiple forest ecosystem functions are considered. We hope the Sabah Biodiversity Experiment will contribute towards achieving this goal for lowland dipterocarp forests.
Acknowledgements
We thank the Sabah Biodiversity Experiment and Danum Valley Field Centre research assistants for their work in the field, in particular Bernadus Bala Ola for tree identification; the Economic Planning Unit Sabah, Malaysia, and the Danum Valley Management Committee for research permission; the Forestry Department Sabah for tree identification; Romano Carlo for the forest drawing; Tim Paine and five reviewers for comments on the manuscript. This project was funded by the University of Zurich, The NERC Centre for Population Biology, Imperial College London, SNSF grant 31003A-125461/1 (www.projectdb.snf.ch/WebForms/Frameset.aspx) to A. Hector and DEFRA Darwin Initiative grant 16011 (http://darwin.defra.gov.uk/project/16011/?p=100&ob=dt&od=n) to G. Reynolds and A. Hector, and is part of the Royal Society South East Asia Rainforest Research Programme (Project No. RS243). This paper constitutes Publication Number A/581 of the Royal Society South East Asia Rainforest Research Programme and Publication Number 04 of the Sabah Biodiversity Experiment.
References
- 1.Mooney H. 2005. Foreword. In Forest diversity and function. Temperate and boreal systems (eds Scherer-Lorenzen M., Körner C., Schulze E.), p. VI Berlin, Heidelberg: Springer [Google Scholar]
- 2.Chazdon R. L. 2008. Beyond deforestation: restoring forests and ecosystem services on degraded lands. Science 320, 1458–1460 10.1126/science.1155365 (doi:10.1126/science.1155365) [DOI] [PubMed] [Google Scholar]
- 3.Guariguata M. R., Balvanera P. 2009. Tropical forest service flows: improving our understanding of the biophysical dimension of ecosystem services. Forest Ecol. Manag. 258, 1825–1829 10.1016/j.foreco.2009.06.025 (doi:10.1016/j.foreco.2009.06.025) [DOI] [Google Scholar]
- 4.Cardinale B. J., Matulich K., Hooper D. U., Byrnes J. E., Duffy E., Gamfeldt L., Balvanera P., O'Connor M. I., Gonzalez A. 2011. The functional role of producer diversity in ecosystems. Am. J. Bot. 98, 572–592 10.3732/ajb.1000364 (doi:10.3732/ajb.1000364) [DOI] [PubMed] [Google Scholar]
- 5.Duffy J. E. 2009. Why biodiversity is important to the functioning of real-world ecosystems. Front. Ecol. Environ. 7, 437–444 10.1890/070195 (doi:10.1890/070195) [DOI] [Google Scholar]
- 6.Naeem S., Bunker D. E., Hector A., Loreau M., Perrings C. 2009. Biodiversity, ecosystem functioning, and human wellbeing: an ecological and economic perspective. Oxford, UK: Oxford University Press [Google Scholar]
- 7.Hector A. 2009. Darwin's ‘principle of divergence’ and the link between biodiversity and ecosystem functioning. In Darwin und die botanik (eds Stocklin J., Hoxterman E.), pp. 182–191 Ransdorf: Basilisken Presse [Google Scholar]
- 8.Hector A., Hooper R. E. 2002. Darwin and the first ecological experiment. Science 295, 639–640 10.1126/science.1064815 (doi:10.1126/science.1064815) [DOI] [PubMed] [Google Scholar]
- 9.Gravel D., Bell T., Barbera C., Bouvier T., Pommier T., Venail P., Mouquet N. 2011. Experimental niche evolution alters the strength of the diversity–productivity relationship. Nature 469, 89–92 10.1038/nature09592 (doi:10.1038/nature09592) [DOI] [PubMed] [Google Scholar]
- 10.Hector A. 2011. Ecology: diversity favours productivity. Nature 472, 45–46 10.1038/472045a (doi:10.1038/472045a) [DOI] [PubMed] [Google Scholar]
- 11.Cardinale B. J., Srivastava D. S., Emmett Duffy J., Wright J. P., Downing A. L., Sankaran M., Jouseau C. 2006. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 443, 982–989 10.1038/nature05202 (doi:10.1038/nature05202) [DOI] [PubMed] [Google Scholar]
- 12.Cardinale B. J., Wrigh J. P., Cadotte M. W., Carroll I. T., Hector A., Srivastava D. S., Loreau M., Weis J. J. 2007. Impacts of plant diversity on biomass production increase through time because of species complementarity. Proc. Natl Acad. Sci. USA 104, 18 123–18 128 10.1073/pnas.0709069104 (doi:10.1073/pnas.0709069104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmid B., Balvanera P., Cardinale B. J., Godbold J., Pfisterer A. B., Raffaelli D., Srivastava Solan D. S. 2009. Consequences of species loss for ecosystem functioning: meta-analysis of data from biodiversity experiments. In Biodiversity, ecosystem functioning and human wellbeing (eds Naeem S., Bunker D., Hector A., Loreau M., Perrings C.), pp. 14–29 Oxford, UK: Oxford University Press [Google Scholar]
- 14.Gamfeldt L., Hillebrand H., Jonsson P. R. 2008. Multiple functions increase the importance of biodiversity for overall ecosystem functioning. Ecology 89, 1223–1231 10.1890/06-2091.1 (doi:10.1890/06-2091.1) [DOI] [PubMed] [Google Scholar]
- 15.Hector A., Bagchi R. 2007. Biodiversity and ecosystem multifunctionality. Nature 448, 188–190 10.1038/nature05947 (doi:10.1038/nature05947) [DOI] [PubMed] [Google Scholar]
- 16.Zavaleta E. S., Pasari J. R., Hulvey K. B., Tilman G. D. 2010. Sustaining multiple ecosystem functions in grassland communities requires higher biodiversity. Proc. Natl Acad. Sci. USA 107, 1443–1446 10.1073/pnas.0906829107 (doi:10.1073/pnas.0906829107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hector A., et al. 2010. General stabilizing effects of plant diversity on grassland productivity through population asynchrony and overyielding. Ecology 91, 2213–2220 10.1890/09-1162.1 (doi:10.1890/09-1162.1) [DOI] [PubMed] [Google Scholar]
- 18.Yachi S., Loreau M. 1999. Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proc. Natl Acad. Sci. USA 96, 1463–1468 10.1073/pnas.96.4.1463 (doi:10.1073/pnas.96.4.1463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hubbell S. P. 2001. The unified neutral theory of biodiversity and biogeography. Princeton, NJ: Princeton University Press [Google Scholar]
- 20.Chesson P. 2000. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 31, 343–366 10.1146/annurev.ecolsys.31.1.343 (doi:10.1146/annurev.ecolsys.31.1.343) [DOI] [Google Scholar]
- 21.Purves D., Turnbull L. A. 2010. Different but equal: the implausible assumption at the heart of neutral theory. J. Anim. Ecol. 79, 1215–1225 10.1111/j.1365-2656.2010.01738.x (doi:10.1111/j.1365-2656.2010.01738.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turnbull L. A., Rees M., Purves D. W. 2008. Why equalising trade-offs are not always neutral. Ecol. Lett. 11, 1037–1046 10.1111/j.1461-0248.2008.01214.x (doi:10.1111/j.1461-0248.2008.01214.x) [DOI] [PubMed] [Google Scholar]
- 23.Bunker D. E., Naeem S. 2006. Species diversity and ecosystem functioning. Science 312, 846–847 10.1126/science.312.5775.846b (doi:10.1126/science.312.5775.846b) [DOI] [PubMed] [Google Scholar]
- 24.Nadrowski K., Wirth C., Scherer-Lorenzen M. 2010. Is forest diversity driving ecosystem function and service? Curr. Opin. Environ. Sustain. 2, 75–79 10.1016/j.cosust.2010.02.003 (doi:10.1016/j.cosust.2010.02.003) [DOI] [Google Scholar]
- 25.Scherer-Lorenzen M., Potvin C., Koricheva J., Schmid B., Hector A., Bornik Z., Reynolds G., Schulze E.-D. 2005. The design of experimental tree plantations for functional biodiversity research. In Forest diversity and function. Temperate and boreal systems (eds Scherer-Lorenzen M., Körner C., Schulze E.), pp. 347–376 Berlin, Heidelberg: Springer [Google Scholar]
- 26.Riihimäki J., Kaitaniemi P., Koricheva J., Vehviläinen H. 2005. Testing the enemies hypothesis in forest stands: the important role of tree species composition. Oecologia 142, 90–97 10.1007/s00442-004-1696-y (doi:10.1007/s00442-004-1696-y) [DOI] [PubMed] [Google Scholar]
- 27.Liang J. J., Buongiorno J., Monserud R. A., Kruger E. L., Zhou M. 2007. Effects of diversity of tree species and size on forest basal area growth, recruitment and mortality. Forest Ecol. Manag. 243, 116–127 10.1016/j.foreco.2007.02.028 (doi:10.1016/j.foreco.2007.02.028) [DOI] [Google Scholar]
- 28.Vila M., Pino J., Font X. 2007. Regional assessment of plant invasions across different habitat types. J. Veg. Sci. 18, 35–42 10.1111/j.1654-1103.2007.tb02513.x (doi:10.1111/j.1654-1103.2007.tb02513.x) [DOI] [Google Scholar]
- 29.Amichev B. Y., Burger J. A., Rodrigue J. A. 2008. Carbon sequestration by forests and soils on mined land in the Midwestern and Appalachian coalfields of the U.S. Forest Ecol. Manag. 256, 1949–1959 10.1016/j.foreco.2008.07.020 (doi:10.1016/j.foreco.2008.07.020) [DOI] [Google Scholar]
- 30.Guckland A., Jacob M., Flessa H., Thomas F. M., Leuschner C. 2009. Acidity, nutrient stocks and organic-matter content in soils of a temperate deciduous forest with different abundance of European beech (Fagus sylvatica L.). J. Plant Nutr. Soil Sci. 172, 500–511 10.1002/jpln.200800072 (doi:10.1002/jpln.200800072) [DOI] [Google Scholar]
- 31.Talkner U., Jansen M., Beese F. O. 2009. Soil phosphorus status and turnover in central-European beech forest ecosystems with differing tree species diversity. Eur. J. Soil Sci. 60, 338–346 10.1111/j.1365-2389.2008.01117.x (doi:10.1111/j.1365-2389.2008.01117.x) [DOI] [Google Scholar]
- 32.Cesarz S., Fahrenholz N., Migge-Kleian S., Platner C., Schaefer M. 2007. Earthworm communities in relation to tree diversity in a deciduous forest. Eur. J. Soil Biol. 43, S61–S67 10.1016/j.ejsobi.2007.08.003 (doi:10.1016/j.ejsobi.2007.08.003) [DOI] [Google Scholar]
- 33.Sobek S., Gossner M. M., Scherber C., Steffan-Dewenter I., Tscharntke T. 2009. Tree diversity drives abundance and spatiotemporal beta-diversity of true bugs (Heteroptera). Ecol. Entomol. 34, 772–782 10.1111/j.1365-2311.2009.01132.x (doi:10.1111/j.1365-2311.2009.01132.x) [DOI] [Google Scholar]
- 34.Sobek S., Steffan-Dewenter I., Scherber C., Tscharntke T. 2009. Spatiotemporal changes of beetle communities across a tree diversity gradient. Divers. Distrib. 15, 660–670 10.1111/j.1472-4642.2009.00570.x (doi:10.1111/j.1472-4642.2009.00570.x) [DOI] [Google Scholar]
- 35.Vehviläinen H., Koricheva J., Ruohomaki K. 2007. Tree species diversity influences herbivore abundance and damage: meta-analysis of long-term forest experiments. Oecologia 152, 287–298 10.1007/s00442-007-0673-7 (doi:10.1007/s00442-007-0673-7) [DOI] [PubMed] [Google Scholar]
- 36.Vehviläinen H., Koricheva J., Ruohomaki K. 2008. Effects of stand tree species composition and diversity on abundance of predatory arthropods. Oikos 117, 935–943 10.1111/j.2008.0030-1299.15972.x (doi:10.1111/j.2008.0030-1299.15972.x) [DOI] [Google Scholar]
- 37.Bauhus J., Van der Meer P., Kanninen M. 2010. Ecosystem goods and services from plantation forests. London: Earthscan [Google Scholar]
- 38.Richards A. E., Forrester D. I., Bauhus J., Scherer-Lorenzen M. 2010. The influence of mixed tree plantations on the nutrition of individual species: a review. Tree Physiol. 30, 1192–1208 10.1093/treephys/tpq035 (doi:10.1093/treephys/tpq035) [DOI] [PubMed] [Google Scholar]
- 39.Piotto D., Viquez E., Montagnini F., Kanninen M. 2004. Pure and mixed forest plantations with native species of the dry tropics of Costa Rica: a comparison of growth and productivity. Forest Ecol. Manag. 190, 359–372 10.1016/j.foreco.2003.11.005 (doi:10.1016/j.foreco.2003.11.005) [DOI] [Google Scholar]
- 40.Menalled F. D., Kelty M. J., Ewel J. J. 1998. Canopy development in tropical tree plantations: a comparison of species mixtures and monocultures. Forest Ecol. Manag. 104, 249–263 10.1016/S0378-1127(97)00255-7 (doi:10.1016/S0378-1127(97)00255-7) [DOI] [Google Scholar]
- 41.Erskine P. D., Lamb D., Bristow M. 2006. Tree species diversity and ecosystem function: can tropical multi-species plantations generate greater productivity? Forest Ecol. Manag. 233, 205–210 10.1016/j.foreco.2006.05.013 (doi:10.1016/j.foreco.2006.05.013) [DOI] [Google Scholar]
- 42.Ewel J. J. 2006. Species and rotation frequency influence soil nitrogen in simplified tropical plant communities. Ecol. Appl. 16, 490–502 10.1890/1051-0761(2006)016[0490:SARFIS]2.0.CO;2 (doi:10.1890/1051-0761(2006)016[0490:SARFIS]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 43.Ewel J. J., Mazzarino M. J. 2008. Competition from below for light and nutrients shifts productivity among tropical species. Proc. Natl Acad. Sci. USA 105, 18 836–18 841 10.1073/pnas.0807216105 (doi:10.1073/pnas.0807216105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Healy C., Gotelli N. J., Potvin C. 2008. Partitioning the effects of biodiversity and environmental heterogeneity for productivity and mortality in a tropical tree plantation. J. Ecol. 96, 903–913 10.1111/j.1365-2745.2008.01419.x (doi:10.1111/j.1365-2745.2008.01419.x) [DOI] [Google Scholar]
- 45.Potvin C., Gotelli N. J. 2008. Biodiversity enhances individual performance but does not affect survivorship in tropical trees. Ecol. Lett. 11, 217–223 10.1111/j.1461-0248.2007.01148.x (doi:10.1111/j.1461-0248.2007.01148.x) [DOI] [PubMed] [Google Scholar]
- 46.Ruiz-Jaen M. C., Potvin C. 2010. Tree diversity explains variation in ecosystem functioning in a neotropical forest in Panama. Biotropica 42, 638–647 10.1111/j.1744-7429.2010.00631.x (doi:10.1111/j.1744-7429.2010.00631.x) [DOI] [Google Scholar]
- 47.Murphy M., Balser T., Buchmann N., Hahn V., Potvin C. 2008. Linking tree biodiversity to belowground process in a young tropical plantation: impacts on soil CO2 flux. Forest Ecol. Manag. 255, 2577–2588 10.1016/j.foreco.2008.01.034 (doi:10.1016/j.foreco.2008.01.034) [DOI] [Google Scholar]
- 48.Oelmann Y., Potvin C., Mark T., Werther L., Tapernon S., Wilcke W. 2010. Tree mixture effects on aboveground nutrient pools of trees in an experimental plantation in Panama. Plant Soil 326, 199–212 10.1007/s11104-009-9997-x (doi:10.1007/s11104-009-9997-x) [DOI] [Google Scholar]
- 49.Zeugin F., Potvin C., Jansa J., Scherer-Lorenzen M. 2010. Is tree diversity an important driver for phosphorus and nitrogen acquisition of a young tropical plantation? Forest Ecol. Manag. 260, 1424–1433 10.1016/j.foreco.2010.07.020 (doi:10.1016/j.foreco.2010.07.020) [DOI] [Google Scholar]
- 50.Scherer-Lorenzen M., Bonilla J. L., Potvin C. 2007. Tree species richness affects litter production and decomposition rates in a tropical biodiversity experiment. Oikos 116, 2108–2124 10.1111/j.2007.0030-1299.16065.x (doi:10.1111/j.2007.0030-1299.16065.x) [DOI] [Google Scholar]
- 51.Hubbell S. P. 2006. Neutral theory and the evolution of ecological equivalence. Ecology 87, 1387–1398 10.1890/0012-9658(2006)87[1387:NTATEO]2.0.CO;2] (doi:10.1890/0012-9658(2006)87[1387:NTATEO]2.0.CO;2]) [DOI] [PubMed] [Google Scholar]
- 52.Whitmore T. C. 1998. An introduction to tropical rain forests. Oxford, UK: Oxford University Press [Google Scholar]
- 53.Gouvenain R. C. D., Silander J. A. 2003. Do tropical storm regimes influence the structure of tropical lowland rain forests? Biotropica 35, 166–180 10.1111/j.1744-7429.2003.tb00276.x (doi:10.1111/j.1744-7429.2003.tb00276.x) [DOI] [Google Scholar]
- 54.Marsh C. W., Greer A. G. 1992. Forest land-use in Sabah, Malaysia: an introduction to Danum Valley. Phil. Trans. R. Soc. Lond. B 335, 331–339 10.1098/rstb.1992.0025 (doi:10.1098/rstb.1992.0025) [DOI] [Google Scholar]
- 55.Majalap N., Chu N. H. 1992. Laboratory manual for chemical analysis. Sandakan, Malaysia: Forest Research Centre, Forestry Department [Google Scholar]
- 56.Buringh P. 1979. Introduction to the study of soils in tropical and subtropical regions. Wageningen, The Netherlands: PUDOC, Center for Agricultural Publishing and Documentation [Google Scholar]
- 57.Saner P. 2009. Ecosystem carbon dynamics in logged forest of Malaysian Borneo. PhD thesis, University of Zurich, Zurich: See https://www.zora.uzh.ch/25404 [Google Scholar]
- 58.Chan H. T., Shamsudin I., Ismail P. 2008. An in-depth look at enrichment planting (Malaysian forest records no. 47) (eds Chan H. T., Shamsudin I., Ismail P.) Kuala Lumpur, Malaysia: Forest Research Institute Malaysia [Google Scholar]
- 59.Oksanen J., Guillaume Blanchet F., Kindt R., Legendre P., O'Hara R. B., Simpson G. L., Solymos P. M., Stevens H. H., Wagner H. 2011. vegan: Community Ecology Package. R package version 1–17-8
- 60.Magurran A. E. 2004. Measuring biological diversity. Oxford, UK: Blackwell Publishing [Google Scholar]
- 61.R Development Core Team 2011. A language and environment for statistical computing. Vienna, Austria: R foundation for Statistical Computing [Google Scholar]
- 62.Pinard M. A. 1995. Carbon retention by reduced-impact logging. PhD thesis, University of Florida: Gainesville, FL [Google Scholar]
- 63.Brown S., Gillespie A. J. R., Lugo A. E. 1989. Biomass estimation methods for tropical forests with applications to forest inventory data. Forest Sci. 35, 881–902 [Google Scholar]
- 64.Nepstad D. C., et al. 1994. The role of deep roots in the hydrological and carbon cycles of Amazonian forests and pastures. Nature 372, 666–669 10.1038/372666a0 (doi:10.1038/372666a0) [DOI] [Google Scholar]
- 65.Pinard M., Putz F. 1997. Monitoring carbon sequestration benefits associated with reduced-impact logging project in Malaysia. Mitigation Adapt. Strateg. Global Change 2, 203–215 10.1007/BF02437204 (doi:10.1007/BF02437204) [DOI] [Google Scholar]
- 66.Delaney M., Brown S., Lugo A. E., Torres-Lezama A., Quintero N. B. 1998. The quantity and turnover of dead wood in permanent forest plots in six life zones of Venezuela. Biotropica 30, 2–11 10.1111/j.1744-7429.1998.tb00364.x (doi:10.1111/j.1744-7429.1998.tb00364.x) [DOI] [Google Scholar]
- 67.Saner P., Lim R., Burla B., Ong R. C., Scherer-Lorenzen M., Hector A. 2009. Reduced soil respiration in gaps in logged lowland dipterocarp forests. Forest Ecol. Manag. 258, 2007–2012 10.1016/j.foreco.2009.07.048 (doi:10.1016/j.foreco.2009.07.048) [DOI] [Google Scholar]
- 68.Burgess P. F. 1966. Timbers of Sabah. Sandakan, Malaysia: Sabah Forestry Department [Google Scholar]
- 69.Chave J., et al. 2005. Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 145, 87–99 10.1007/s00442-005-0100-x (doi:10.1007/s00442-005-0100-x) [DOI] [PubMed] [Google Scholar]
- 70.Newman M. F., Burgess P. F., Whitmore T. C. 1996. Manuals of Dipterocarps for foresters. Borneo island light hardwoods. Edinburgh, UK: Royal Botanical Gardens Edinburgh and CIFOR [Google Scholar]
- 71.Newman M. F., Burgess P. F., Whitmore T. C. 1998. Manuals of dipterocarps for foresters. Borneo island medium and heavy hardwoods. Edinburgh, UK: Royal Botanic Garden, Edinburgh and CIFOR [Google Scholar]
- 72.Dzulkifli D. 2011. Growth and survival trade-offs in seedlings of 16 Dipterocarpaceae species. Masters Thesis, University of Zurich, Zurich [Google Scholar]
- 73.Cannon C. H., Peart D. R., Leighton M. 1998. Tree species diversity in commercially logged Bornean rainforest. Science 281, 1366–1368 10.1126/science.281.5381.1366 (doi:10.1126/science.281.5381.1366) [DOI] [PubMed] [Google Scholar]
- 74.Berry N. J., Phillips O. L., Ong R. C., Hamer K. C. 2008. Impacts of selective logging on tree diversity across a rainforest landscape: the importance of spatial scale. Landsc. Ecol. 23, 915–929 10.1007/s10980-008-9248-1 (doi:10.1007/s10980-008-9248-1) [DOI] [Google Scholar]
- 75.Bischoff W., Newbery D. A., Lingenfelder M., Schnaeckel R., Petol G. H., Madani L., Ridsdale C. E. 2005. Secondary succession and dipterocarp recruitment in Bornean rain forest after logging. Forest Ecol. Manag. 218, 174–192 10.1016/j.foreco.2005.07.009 (doi:10.1016/j.foreco.2005.07.009) [DOI] [Google Scholar]
- 76.Nussbaum R., Anderson J., Spencer T. 1996. Planting Dipterocarps for rehabilitation of log landings and skid trails in Sabah, Malaysia. In Proc. 5th round-table Conf. on dipterocarps (eds Appanah S., Khoo K. C.), p. 542 Kuala Lumpur, Malaysia: Forest Research Institute Malaysia [Google Scholar]
- 77.Berry N. J., et al. 2010. The high value of logged tropical forests: lessons from northern Borneo. Biodivers. Conserv. 19, 985–997 10.1007/s10531-010-9779-z (doi:10.1007/s10531-010-9779-z) [DOI] [Google Scholar]
- 78.Pinard M. A., Putz F. E. 1996. Retaining forest biomass by reducing logging damage. Biotropica 28, 278–295 10.2307/2389193 (doi:10.2307/2389193) [DOI] [Google Scholar]
- 79.Tangki H., Chappell N. A. 2008. Biomass variation across selectively logged forest within a 225-km2 region of Borneo and its prediction by Landsat TM. Forest Ecol. Manag. 256, 1960–1970 10.1016/j.foreco.2008.07.018 (doi:10.1016/j.foreco.2008.07.018) [DOI] [Google Scholar]
- 80.Loreau M., Hector A. 2001. Partitioning selection and complementarity in biodiversity experiments. Nature 412, 72–76 10.1038/35083573 (doi:10.1038/35083573) [DOI] [PubMed] [Google Scholar]
- 81.Kettle C. J. 2010. Sowing seeds for REDD+. Science 330, 584. 10.1126/science.330.6004.584-a (doi:10.1126/science.330.6004.584-a) [DOI] [PubMed] [Google Scholar]
- 82.Curran L. M., Trigg S. N., McDonald A. K., Astiani D., Hardiono Y. M., Siregar P., Caniago I., Kasischke E. 2004. Lowland forest loss in protected areas of Indonesian Borneo. Science 303, 1000–1003 10.1126/science.1091714 (doi:10.1126/science.1091714) [DOI] [PubMed] [Google Scholar]
- 83.Kirby K. R., Potvin C. 2007. Variation in carbon storage among tree species: implications for the management of a small-scale carbon sink project. Forest Ecol. Manag. 246, 208–221 10.1016/j.foreco.2007.03.072 (doi:10.1016/j.foreco.2007.03.072) [DOI] [Google Scholar]
- 84.Balvanera P., Kremen C., Martinez-Ramos M. 2005. Applying community structure analysis to ecosystem function: examples from pollination and carbon storage. Ecol. Appl. 15, 360–375 10.1890/03-5192 (doi:10.1890/03-5192) [DOI] [Google Scholar]
- 85.Hooper D., et al. 2005. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr. 75, 3–35 10.1890/04-0922 (doi:10.1890/04-0922) [DOI] [Google Scholar]