Abstract
Objective:
We evaluated the effect of the divalproex sodium formulation of valproic acid on brain volumes using MRI in people with mild to moderate Alzheimer disease (AD) and assessed for changes associated with behavioral and cognitive effects.
Methods:
Eighty-nine of 313 participants randomized to divalproex or placebo in a 24-month, parallel-group trial received MRI scans at baseline and 12 months. Interval MRI annual percent changes in whole brain, ventricular, and hippocampal volumes were the primary outcomes of interest. Change from baseline in clinical outcomes was assessed at 6-month intervals.
Results:
There were no baseline differences between active treatment and placebo groups in age, education, brain volumes, clinical rating scores, or APOE ϵ4 carrier status. The group treated with divalproex showed a greater rate of decline in left and right hippocampal and brain volumes (−10.9% and −12.4% vs −5.6% and −6.3%, and −3.5% vs −1.4%, respectively), and a greater rate of ventricular expansion (24.5% vs 9.9%) (p < 0.001). Mini-Mental State Examination scores showed a more rapid decline with divalproex through month 12 (placebo = −2.0 ± 4.3, divalproex = −3.9 ± 4.0) (p = 0.037), although there were no changes on other cognitive, behavioral, or functional ratings at 12 and 24 months.
Conclusions:
Divalproex treatment was associated with accelerated brain volume loss over 1 year and perhaps with greater cognitive impairment. The long-term clinical effects of these changes are not known.
Valproic acid (VPA) administered in the form of divalproex sodium is indicated for treatment of manic episodes in bipolar disorder, for seizure disorders, and as prophylaxis for migraine headaches (Depakote package insert, Abbott Laboratories, North Chicago, IL). It has also been studied for possible anti-agitation effects in Alzheimer disease (AD).1 There are no widely accepted or clearly effective drug therapies for behavioral management in AD.
In addition to symptomatic management, it has been hypothesized that VPA may have neuroprotective properties and reduce rates of disease progression in AD.2 Putative mechanisms for this include reduction of neurofibrillary tangle formation by inhibiting glycogen synthase kinase-3β (GSK-3β), a kinase that phosphorylates tau; protection against neuronal apoptosis by upregulation of B-cell CLL/lymphoma-2 protein (BCL2); and promotion of synaptogenesis by inhibition of histone deacetylases.3 In cultured rat hippocampal neurons, valproate prevents excessive cellular influx of Ca2+ due to glutamate and amyloid β-peptide (Aβ), protecting against excitotoxic injury.2 It inhibits Aβ production and neuronal plaque formation in both cellular and mouse models, while improving memory in mouse models of AD.4,5 In contrast to these putative neuroprotective effects, several studies have suggested that valproate therapy may cause reversible brain atrophy and cognitive impairment.6–10
This report presents results from an MRI substudy of a 24-month randomized, placebo-controlled clinical trial of divalproex sodium (divalproex) for mild to moderate AD, conducted by the Alzheimer's Disease Cooperative Study (ADCS).11 The goal of the parent study was to determine whether divalproex delayed or prevented emergence of agitation or psychosis and slowed cognitive or functional decline in AD. The goal of this MRI study was to examine the effects of divalproex treatment on brain volumes, and to assess the effects of divalproex on the relationship between brain morphometry, cognition, function, and neuropsychiatric symptoms.
METHODS
Participants.
Trial methods are described in detail elsewhere.11 Briefly, 313 participants with probable AD12 were recruited from 46 clinical sites in the United States, of which 19 sites and 172 volunteers participated in the MRI study from November 2005 through March 2009. The main inclusion criteria were age ≥55 years, weight at least 40 kg, Mini-Mental State Examination (MMSE) score 12–20 inclusive, and a requirement not to have experienced agitation or psychosis since illness onset.
Standard protocol approvals, registrations, and patient consents.
Results from the primary randomized treatment trial from which data for this observational MRI subanalysis were obtained are reported separately,11 and registered at clinicaltrials.gov (NCT00071721). Written informed consent was obtained from the caregiver and either the patient, if possible, or an authorized representative. The study was reviewed and approved by the institutional review board at each site.
Study design.
Participants were assigned, randomly, to double-blind treatment with either divalproex sodium or identically appearing placebo tablets for 24 months. The primary clinical endpoint was time to emergence of agitation or psychosis, combined with an assessment of the clinical significance of behavioral change rated by the study clinician.11 Structural MRI scans were acquired at baseline and at 12 months to assess treatment and disease-associated changes in whole brain, hippocampal, and ventricular volumes over time, as a planned secondary analysis in a subset of participants. Randomization was one-to-one for the 2 treatment groups in permuted blocks of 4.
Intervention.
A 250-mg enteric coated extended-release formulation of divalproex sodium or identical-appearing placebo tablet was provided by Abbott Laboratories. Participants received one tablet daily by mouth for 1 week, then 2 tablets daily for 1 week; further dose titration occurred in increments of 250 mg, increasing weekly, targeting 10–12 mg/kg/day with minimum dose targets adjusted to weight and to tolerability. After the initial titration phase, dose reduction was permitted if warranted clinically.
Clinical assessments.
Time to emergence of agitation or psychosis was assessed using the Neuropsychiatric Inventory (NPI).11,13 Secondary clinical aims included delaying the progression of cognitive, functional, other behavioral and global measures associated with AD. These secondary aims were assessed with the Alzheimer's Disease Assessment Scale–cognitive subscale (ADAS-cog), the Clinical Dementia Rating (CDR), MMSE, ADCS activities of daily living (ADCS-ADL), the ADCS Clinical Global Impression of Change scale (ADCS-CGIC), Cohen Mansfield Agitation Inventory (CMAI), and the Alzheimer's Disease Quality of Life scale (QOL-AD).
Imaging acquisition.
All MRI scans were performed on 1.5-T scanners (not older than 8 years at the time of study launch) on various platforms across 19 sites. The imaging sequence used for morphometric analyses was an MPRAGE sequence developed for the Alzheimer's Disease Neuroimaging Initiative (ADNI),14 distributed to study sites from the Mayo Clinic, Rochester, MN. There were minor variations in the MRI protocol based on the specific hardware configuration on each scanner. The nominal parameters of the MPRAGE sequence were as follows: sagittal plane, repetition time/echo time/inversion time 2,400/3/1,000 msec, flip angle 8°, 24 cm field of view, 192 × 192 in-plane matrix, 1.2 mm slice thickness. A human and phantom scan was acquired during each scanner session. Scanner quality monitoring was performed at the Mayo Clinic using the ADNI phantom.14 All images were transferred for storage and processing over a secure FTP Internet transfer in DICOM-3 format to a central imaging laboratory at the San Francisco Veterans Administration Medical Center (SFVAMC).
Image processing.
All images were corrected for image distortion due to gradient nonlinearity using GradWarp15 and for intensity inhomogeneity using N316 using a software pipeline running at Mayo.14
Hippocampal volume segmentation was performed at the SFVAMC. Semiautomated hippocampal volumetry was carried out using a commercially available high-dimensional brain mapping tool (Medtronic Surgical Navigation Technologies, Louisville, CO), that has previously been validated and compared to manual tracing of the hippocampus.17 Measurement of hippocampal volume was achieved first by manually placing 22 control points as local landmarks for the hippocampus on the individual brain MRI data: 1 landmark at the hippocampal head, 1 at the tail, and 4 per image (i.e., at the superior, inferior, medial, and lateral boundaries) on 5 equally spaced images perpendicular to the long axis of the hippocampus. Second, fluid image transformation was used to match the individual brains to a template brain.18 The pixels corresponding to the hippocampus were then labeled and counted to obtain volumes. This method of hippocampal voluming has a documented reliability of an intraclass coefficient better than 0.94.17
Rates of whole brain atrophy and ventricular expansion were performed at the Mayo Clinic using a boundary shift integral (BSI) technique.19,20 Differences were calculated in pairwise fashion between the baseline scan and the follow-up scan. Following spatial registration of the follow-up scan to the baseline scan, intensity differences between the 2 scans at the brain-CSF boundary were used to compute change in brain volume. The ventricular atrophy rate was derived by creating a binary ventricular mask for each subject that selectively extracted ventricular change from the BSI. Quality control testing shows that the intraclass correlation coefficient for test-retest reproducibility of ventricle rate measurements from short interval serial MRI scans with this method is 0.91.21
Analysis.
Whole brain, total ventricular, and left and right hippocampal volumes were compared between placebo and treatment groups. Annualized percent changes were calculated based on specific individual durations between follow-up scans and baseline scans. This was done as an intent-to-treat analysis (ITT), with an α = 0.05. For cross-sectional analyses, raw volume measures of each structure at baseline and 12 months were standardized as a ratio to the subjects' total intracranial volume (TIV). Wilcoxon rank sum tests were used to compare TIV standardized baseline and month 12 MRI measures and annualized percent volume changes between groups.
Analysis of covariance (ANCOVA) models were used to evaluate the impact of potential covariates on group differences in MRI percent volume changes over 12 months. Covariates were included if a variable was unbalanced between groups (p < 0.05) and was associated with the volumetric outcome measure being assessed, determined by Spearman rank correlations or Wilcoxon rank sum where appropriate, using an α error criterion p = 0.1. Potential covariates assessed included age, gender, MMSE, total NPI, CDR sum of boxes score (CDR-SB), ADAS-cog, CMAI, ADCS-ADL, years of education, duration of dementia, APOE ϵ4 carrier status, and baseline standardized MRI volumes. Correlations between 12-month serum divalproex levels and annualized change in MRI volumes were assessed. Generalized estimating equations (GEE) modeling was used for longitudinal analysis, adjusting for covariates as needed, to assess group differences in all clinical measures over the entire 24-month study period between groups in this MRI subcohort. In addition, between-group differences in all clinical measures were assessed at baseline, 6-, 12-, 18-, and 24-month time points using Wilcoxon rank sum tests or Fisher exact tests. Survival analysis between groups evaluating the primary endpoint of emergence of neuropsychiatric symptoms over 24 months was independently evaluated in this MRI subgroup. Descriptive statistics were performed on demographic variables using 2-sided t tests and χ2, where appropriate.
RESULTS
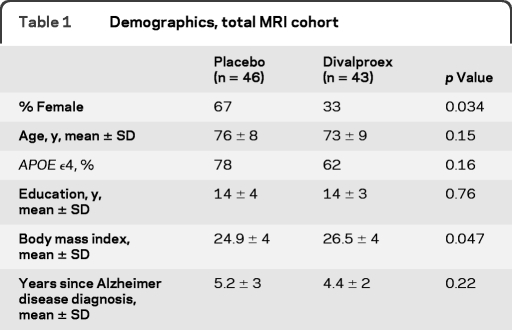
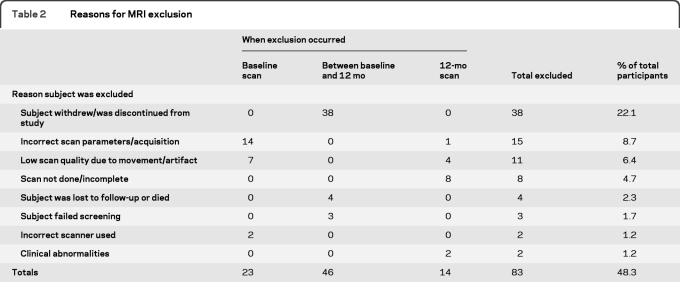
A total of 513 participants were screened into the 24-month treatment trial with 313 meeting all enrollment requirements and randomized into the trial (see the primary treatment trial results for details11). Of those enrolled, 172 consented for the MRI substudy and received baseline scans. A total of 149 baseline scans passed planned quality control measures, with 94 of those participants completing a 12-month follow-up MRI scan. Scans were excluded for either image quality/artifact issues or due to a change in scanner between baseline and follow-up. Five additional 12-month scans failed quality control; thus a total of 89 individuals were ultimately evaluated as part of this MRI substudy (46 placebo, 43 divalproex) (table 1). Reasons for MRI substudy dropouts are given in table 2. Eighty-eight individuals had MRI scans that passed quality control review for hippocampal measures at both timepoints (placebo = 45, divalproex = 43), 66 for whole brain measures (placebo = 35, divalproex = 31), and 71 for ventricular volumes (placebo = 38, divalproex = 33).
Table 1.
Demographics, total MRI cohort
Table 2.
Reasons for MRI exclusion
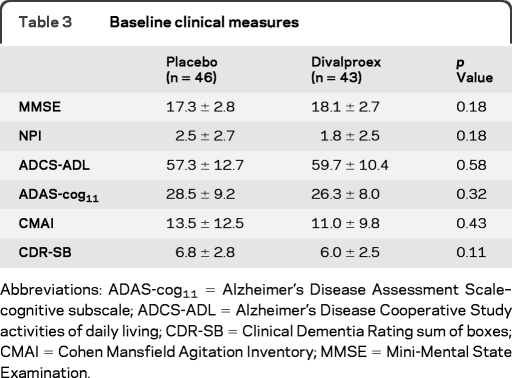
There were no significant between-group differences in baseline mean age, education, years since AD diagnosis, or APOE ϵ4 carrier status. There was a significantly higher body mass index (p = 0.047) and fewer female participants (p = 0.034) in the divalproex group compared to placebo (table 1). The treatment group had a mean divalproex serum concentration of 49.3 ± 22.5 μg/mL at 12 months. There were no significant differences in other baseline clinical measures (table 3). Comparison of demographic and clinical measures between the MRI subgroup and the 313 primary trial participants revealed no significant differences.
Table 3.
Baseline clinical measures
Abbreviations: ADAS-cog11 = Alzheimer's Disease Assessment Scale–cognitive subscale; ADCS-ADL = Alzheimer's Disease Cooperative Study activities of daily living; CDR-SB = Clinical Dementia Rating sum of boxes; CMAI = Cohen Mansfield Agitation Inventory; MMSE = Mini-Mental State Examination.
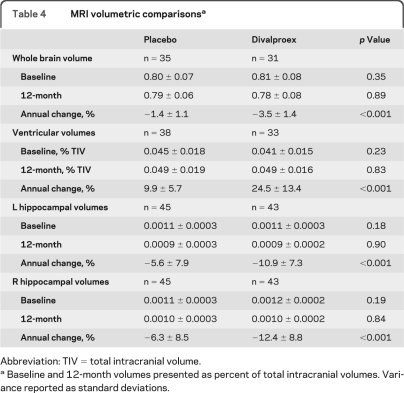
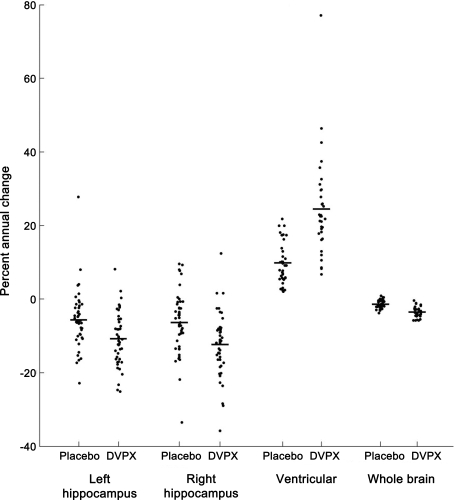
Analyses of imaging measures revealed no statistical differences between treatment groups at baseline or 12 months for hippocampal, whole brain, or ventricular standardized volumes. However, annualized percent volume changes showed significant group differences in all volumetric measures (table 4 and figure). The most pronounced difference was a change in ventricular volumes of +24.5 ± 13.4% in the divalproex-treated group compared to +9.9 ± 5.7% in the placebo group (p < 0.001). Brain volumes changed an average of −3.5 ± 1.4% in the divalproex group compared to −1.4 ± 1.1% in placebo-treated participants (p < 0.001). Annual hippocampal atrophy showed −10.9 ± 7.3% reduction on the left and −12.4 ± 8.8% on the right in the divalproex group compared to −5.6 ± 7.9% and −6.3 ± 8.5%, respectively, in the placebo group (p < 0.001).
Table 4.
MRI volumetric comparisonsa
Abbreviation: TIV = total intracranial volume.
Baseline and 12-month volumes presented as percent of total intracranial volumes. Variance reported as standard deviations.
Figure. Annualized percent volume changes.
Percent volume changes in placebo and divalproex treatment groups between baseline and month 12 MRI scans. Mean values represented by horizontal bar. All group differences are significant, p < 0.001. DVPX = divalproex sodium.
GEE models of clinical assessments over 24 months revealed no group differences in any measures. Similarly, no differences in the NPI primary outcome measure were found between groups in survival analyses. Evaluation of clinical and demographic measures revealed that sex and BMI, although unbalanced at baseline, were not associated with annual percent volume changes. At each 6-month time point between baseline and 24 months, there were no significant group differences in any clinical measures. However, when group differences in clinical measures were assessed for change from baseline at each trial time point, MMSE alone was found to have different rates of change between groups at month 6 and month 12. At month 6, the mean change in MMSE was −1.04 ± 2.6 for the placebo group and −2.4 ± 3.4 for the divalproex group (p = 0.034). At month 12 the change from baseline in MMSE was −2.0 ± 4.3 for the placebo group and −3.9 ± 4.0 in the divalproex group (p = 0.037). At 18 and 24 months, there were no longer group differences in MMSE change scores. When evaluating correlations of baseline ADAS-cog, CDR-SB, and MMSE scores with annualized volume changes, only MMSE showed a significant association. Baseline MMSE scores were correlated with right (Spearman rho = −0.38, p = 0.002) and left (Spearman rho = −0.34, p = 0.006) hippocampal annualized percent change, but not with whole brain or ventricular change. In addition, 12-month MMSE change scores correlated with annualized MRI percent change in ventricular volumes (Spearman rho = −0.34, p = 0.006) and whole brain volumes (Spearman rho = 0.3, p = 0.02), but not with hippocampal volumes. For this reason, 12-month MMSE change scores were added as covariates to ANCOVA models assessing group differences in MRI annual percent volume changes. All annual percent volume changes remained significantly different between groups in these models (p < 0.01). Finally, annual percent change in whole brain (rho = −0.46, p = 0.014) and ventricular volumes (rho = 0.61, p = 0.0005) but not hippocampal volumes correlated with month 12 divalproex serum concentrations.
DISCUSSION
We observed unexpected increased rates of brain volume loss associated with divalproex sodium in elderly individuals with AD. Over 24 months we found no cognitive, neuropsychiatric, or functional differences noted between groups, and no overall differences in cognitive change over time, consistent with the negative findings in the larger primary 24-month treatment trial.11 However, the divalproex MRI subgroup experienced an accelerated decline in MMSE scores in the first 12 months of the trial, without similar findings in other global cognitive tests.
Reversible encephalopathy is a rare complication associated with VPA treatment.22,23 This is variably related to hepatotoxicity and hyperammonemia.24,25 Even in the absence of frank valproate-induced hyperammonemic encephalopathy, there are reports of reversible cognitive deficits associated with VPA use.7,10 One prospective study of 36 epilepsy patients (age ranging from 22 to 74) with VPA levels in the normal therapeutic range reported an 86% incidence of cognitive impairment, with degree of cognitive adverse effects highly associated with increased age. A total of 72% improved after discontinuing VPA.26 Since patients with AD are likely sensitive to other neurotoxic insults27,28 it is possible that the same potentially neurotoxic effects of divalproex associated with brain volume loss in the first 12 months of this longitudinal study also caused the initial rapid decline in MMSE scores observed here. Notably, analysis of adverse events and laboratory data from the primary ADCS divalproex treatment trial showed no evidence of significant neurotoxicity in any participants during the 24-month study.11
Transient reversible brain atrophy is a known, but rare, observed adverse reaction to valproate sodium and VPA therapy.6,7,10,29–31 The drug prescribing information states that “several reports have noted reversible cerebral atrophy and dementia associated with valproate therapy.” However, mechanisms underlying these brain changes are poorly understood, with no previous reports in elderly individuals with AD. Possibilities include divalproex-related osmotic changes, neurotoxicity, and acceleration of AD pathology. Valproate has been shown to decrease brain levels of myoinositol (MI), a known brain osmolyte, and upregulate glycine on magnetic resonance spectroscopy even in the absence of clear hyperammonemia.23,30 This may support a theory of global or localized osmotic shifts associated with the brain volume changes demonstrated in the present study. Despite evidence of potential neuroprotective properties of divalproex,3 there may be neurotoxic effects similar to those causing hepatotoxicity and hyperammonemia from divalproex-induced impairment of mitochondrial β-oxidation and urea cycle activity.30,32 Divalproex may further exacerbate mitochondrial toxicity in people with existing genetic sensitivity (such as Pro to Ser/MTATP8 mutations)7 or other mitotoxic disorders such as AD.33,34 One study recently demonstrated VPA to reversibly inhibit neurofilament production and neurite outgrowth.35 This suggests the possibility that VPA may exacerbate or accelerate the neurodegenerative process of AD. Finally, VPA may inhibit Aβ production and neuronal plaque formation,5 as well as accelerate microglial phagocytosis of Aβ1–42.36 No measure of CSF or cerebrocortical amyloid was performed in the current study. It is interesting to note, however, that AN1792, a synthetic amyloid-β peptide vaccine shown to reduce cortical amyloid in patients with AD, also resulted in brain, ventricular, and hippocampal changes similar to those seen in this study.36,37 Notably, the annual rates of cortical atrophy in our placebo group were consistent with previously reported rates in similar AD cohorts.38–41
Interpretations of these results are limited with respect to clinical and pathologic implications. In particular, we do not have imaging data after 24 months of divalproex treatment or after discontinuation of drug. Therefore it is not known if, like the MMSE scores, brain volumes later reverted to match the placebo group by the end of this 24-month trial, potentially representing a transient effect of divalproex treatment. And we do not know if these effects were reversible after divalproex discontinuation, or had an impact on future AD decline and prognosis. This pre-planned region-of-interest assessment of whole brain, ventricular, and hippocampal volumes does not allow us to infer that the morphologic effects associated with divalproex treatment were due to acceleration of AD pathology, vs nonspecific global neurotoxicity. We cannot advocate additional divalproex or VPA treatment trials in dementia given the risks and potentially detrimental findings presented here.
ACKNOWLEDGMENT
The authors thank Husseini Manji, MD, for scientific consultations in grant and study preparations, and Danielle Ripich, PhD, for creating the caregiver communications training program that was built into the study, and for grant and training manual preparation.
Glossary
GLOSSARY
- Aβ
amyloid β-peptide
- AD
Alzheimer disease
- ADAS-cog
Alzheimer's Disease Assessment Scale–cognitive subscale
- ADCS
Alzheimer's Disease Cooperative Study
- ADCS-ADL
Alzheimer's Disease Cooperative Study activities of daily living
- ADCS-CGIC
Alzheimer's Disease Cooperative Study Clinical Global Impression of Change scale
- ADNI
Alzheimer's Disease Neuroimaging Initiative
- ANCOVA
analysis of covariance
- BSI
boundary shift integral
- CDR
Clinical Dementia Rating
- CDR-SB
CDR sum of boxes score
- CMAI
Cohen Mansfield Agitation Inventory
- GEE
generalized estimating equation
- ITT
intent-to-treat analysis
- MMSE
Mini-Mental State Examination
- NPI
Neuropsychiatric Inventory
- QOL-AD
Alzheimer's Disease Quality of Life scale
- SFVAMC
San Francisco Veterans Administration Medical Center
- TIV
total intracranial volume
- VPA
valproic acid
AUTHOR CONTRIBUTIONS
Dr. Fleisher: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, statistical analysis, study supervision. Dr. Truran: drafting/revising the manuscript, analysis or interpretation of data, acquisition of data, study supervision. Dr. Mai: analysis or interpretation of data. Dr. Langbaum: drafting/revising the manuscript, analysis or interpretation of data, statistical analysis. Dr. Aisen: study concept or design, analysis or interpretation of data, study supervision. Dr. Cummings: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, statistical analysis, study supervision, obtaining funding. Dr. Jack: drafting/revising the manuscript, analysis or interpretation of data, contribution of vital reagents/tools/patients, acquisition of data. Dr. Weiner: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, study supervision. Dr. Thomas: drafting/revising the manuscript, statistical analysis, study supervision. Dr. Schneider: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data. Dr. Tariot: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, study supervision.
COINVESTIGATORS
Members of the Alzheimer's Disease Cooperative Study Group: Participating Investigators: Sandra Vicari, PhD (Southern Illinois University, Springfield, Illinois, Site Investigator); Lon Schneider, MD (University of Southern California, Los Angeles, California, Site Investigator); Jody Corey-Bloom, MD, PhD (University of California, La Jolla, California, Site Investigator); Nancy Barbas, MD, MSW (University of Michigan, Ann Arbor, Michigan, Site Investigator); Karen Bell, MD (Columbia University, New York, New York, Site Investigator); Ranjan Duara, MD (Wien Center for Clinical Research, Miami Beach, Florida, Site Investigator); Paul Rosenberg, MD (Johns Hopkins University, Baltimore, Maryland, Site Investigator); M. Saleem Ismail, MD (University of Rochester Medical Center, Rochester, New York, Site Investigator); Ruth Mulnard, RN, DNSc, FAAN (University of California, Irvine, California, Site Investigator); Myron Weiner, MD (University of Texas Southwestern Medical Center, Dallas, Texas, Site Investigator); Larry Tune, MD (Emory University, Atlanta, Georgia, Site Investigator); Jeffrey Burns, MD (University of Kansas, Kansas City, Kansas, Site Investigator); John Ringman, MD (University of California, Los Angeles, California, Site Investigator); Martin Farlow, MD (Indiana University, Indianapolis, Indiana, Site Investigator); Geoffrey Ahern, MD, PhD (Arizona Health sciences Center, Tucson, Arizona, Site Investigator); Paul Solomon, PhD (The Memory Clinic, Bennington, Vermont, Site Investigator); John (Chuang-Kuo) Wu, MD, PhD (Northwestern University, Chicago, Illinois, Site Investigator); Jacobo Mintzer, MD (Medical University of South Carolina, North Charleston, South Carolina, Site Investigator); Walter Martinez, MD (Premiere Research Institute, West Palm Beach, Florida, Site Investigator); David Geldmacher, MD (University of Virginia, Charlottesville, Virginia, Site Investigator); George Grossberg, MD (Saint Louis University, St. Louis, Site Investigator); Zinaida Lebedeva, MD (North East Ohio Health Services, Beachwood, Ohio, Site Investigator); Marwan Sabbagh, MD, FAAN, CCR (Banner Sun Health Research Institute, Sun City, Arizona, Site Investigator); Thomas Obisesan, MD, MPH, FAAFP (Howard University, Washington, District of Columbia, Site Investigator); Stephen Thein, PhD (Pacific Research Network, San Diego, California, Site Investigator); Andrius Baskys, MD, PhD (VA Healthcare System, Long Beach, California, Site Investigator); Alan Lerner, MD (Case Western Reserve University, Cleveland, Ohio, Site Investigator); William Petrie, MD (Psychiatric Consultants, Franklin, Tennessee, Site Investigator); Smita Kittur, MD (Neurological Care of CNY, Syracuse, New York, Site Investigator); Sanjay Gupta, MD (Global Research and Consulting, Olean, New York, Site Investigator); Vernice Bates, MD (Dent Neurologic Institute, Amherst, New York, Site Investigator); Yuval Zabar, MD (Lahey Clinic, Inc, Burlington, Massachusetts, Site Investigator); Earl Zimmerman, MD (Albany Medical College, Albany, New York, Site Investigator); Kevin Foley, MD (Saint Mary's Health Care, Grand Rapids, Michigan, Site Investigator); Susan Schultz, MD (University of Iowa, Iowa City, Iowa, Site Investigator); Brian Ott, MD (Rhode Island Hospital, Providence, Rhode Island, Site Investigator); Jerome Yesavage, MD (Stanford/PAIRE, Palo Alto, California, Site Investigator); Charles Bernick, MD (Cleveland Clinic Lou Ruvo Center for Brain Health, Las Vegas, Nevada, Site Investigator); Jason Karlawish, MD (University of Pennsylvania, Philadelphia, Pennsylvania Site Investigator). Members of the Data and Safety Monitoring Board: Karl Kieburtz, MD (University of Rochester Medical Center, Rochester, NY, DSMB chair); Bruce Miller, MD (University Of California San Francisco, CA, DSMB member); Richard Kryscio, PhD (University of Kentucky, Lexington, DSMB member); George Alexopoulos, MD (Weill Cornell Medical College, New York, NY, DSMB member). Clinical monitors: Karen Croot, BA (University of California, San Diego, clinical monitor); Viviana Messick, BS (University of California, San Diego, clinical monitor); Alan Pamoleras, BA (University of California, San Diego, clinical monitor); Rebecca Ryan-Jones, PhD (University of California, San Diego, clinical monitor); Gina Garcia-Camilo, MD (Mount Sinai Hospital, New York, clinical monitor); Mario Schittini, MD, MPH (Mount Sinai Hospital, New York, clinical monitor); Kris Gravanda Brugger, BA (Georgetown University, Washington DC, clinical monitor); Pamela A. Saunders, PhD (Georgetown University, Washington DC, clinical monitor); Janet Kastelan, MA (New York University, New York, clinical monitor).
DISCLOSURE
Dr. Fleisher serves on scientific advisory boards for the NIH, Eli Lilly & Company, and Elan Corporation; has served as Guest Editor for Behavioral Neurology; has served as a consultant for Elan Corporation, Pfizer Inc, Janssen, and Wyeth; and receives research support from Avid Radiopharmaceuticals, Inc., Eli Lilly and Company, and the NIH/NIA. Dr. Truran, Dr. Mai, and Dr. Langbaum report no disclosures. Dr. Aisen serves on a scientific advisory board for NeuroPhage and Novartis; serves on the editorial boards of BMC Medicine and Alzheimer's Research & Therapy; is listed as inventor on a patent re: DHA therapy for apolipoprotein E4 negative Alzheimer's disease (potential royalties assigned in full to UCSD); serves as a consultant to Elan Corporation, Wyeth, Eisai Inc., Schering-Plough Corp., Bristol-Myers Squibb, Eli Lilly and Company, NeuroPhage, Merck & Co., Roche, Amgen, Genentech, Inc., Abbott, Pfizer Inc, Novartis, Bayer Schering Pharma, Astellas Pharma Inc., Dainippon Sumitomo Pharma, BioMarin Pharmaceutical Inc., Solvay Pharmaceuticals, Inc., Otsuka Pharmaceutical Co., Ltd., Daiichi Sankyo, AstraZeneca, Janssen, and Medivation, Inc.; receives research support from Pfizer Inc, Bayer Schering Pharma, Baxter International Inc., and the NIH/NIA; and has received stock options from Medivation, Inc. and NeuroPhage. Dr. Cummings has served on scientific advisory boards for Abbott, ACADIA Pharmaceuticals, Accera, Inc., Adamas Pharmaceuticals, Anavex Life Sciences Corp., Astellas Pharma Inc., Avanir Pharmaceuticals, Baxter International Inc., Bristol-Myers Squibb, CoMentis, Inc., Eisai Inc., Elan Corporation, EnVivo Pharmaceuticals, Forest Laboratories, Inc., Genentech, Inc., GlaxoSmithKline, Janssen, Eli Lilly and Company, Lundbeck Inc., Medivation, Inc., Medtronic, Inc., Merck Serono, Merz Pharmaceuticals, LLC, Myriad Genetics, Inc., Neuren Pharmaceuticals Limited, Neurokos, Novartis, Noven Pharmaceuticals, Inc., Orion Corporation Pfizer Inc, Prana Biotechnology Limited, QR Pharma, Inc., reMYND, Schering-Plough Corp., Signum Biosciences, Sonexa Therapeutics, Inc., Takeda Pharmaceutical Company Limited, Wyeth, and Toyama Chemical, Co., Ltd.; has received funding for travel and speaker honoraria from Abbott, Astellas Pharma Inc., Avanir Pharmaceuticals, Baxter International Inc., Bristol-Myers Squibb, Genentech, Inc., Lundbeck Inc., Novartis, and Pfizer Inc.; serves on the editorial boards of Psychogeriatrics, Practical Neurology, Dementia and Geriatric Cognitive Disorders, Internal Medicine Thailand, Cognitive and Behavioral Neurology, Middle-Eastern Journal of Age and Aging, Middle-Eastern Journal of Family Medicine, Clinical Neurology and Neurosurgery, Translational Neuroscience, Clinical Trial Magnifier, Translational Neuroscience, and Neuroscience Pathways; serves as a consultant for Ambassador Nursing Homes Silverado Assisted Living; serves on speakers' bureaus for Eisai Inc., Forest Laboratories, Inc., Janssen, Novartis, Pfizer Inc, and Lundbeck Inc.; receives research support from the NIH/NIA and the Sidell-Kagan Foundation; owns stock in ADAMAS, Prana Biotechnology Limited, Sonexa Therapeutics, Inc., MedAvante, Inc., NeuroTrax, Neurokos, and QR Pharma, Inc.; receives license fee payments for use of the Neuropsychiatric Inventory; and has provided expert witness consultation in medico-legal cases. Dr. Jack serves on scientific advisory boards for Elan/Janssen AI, Eli Lilly & Company, GE Healthcare, and Eisai Inc.; receives research support from Baxter International Inc., Allon Therapeutics, Inc., Pfizer Inc, the NIH/NIA, and the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Foundation; and holds stock/stock options in Johnson & Johnson. Dr. Weiner serves on scientific advisory boards for Bayer Schering Pharma, Eli Lilly and Company, CoMentis, Inc., Neurochem Inc, Eisai Inc., Avid Radiopharmaceuticals Inc., Aegis Therapies, Genentech, Inc., Allergan, Inc., Lippincott Williams & Wilkins, Bristol-Myers Squibb, Forest Laboratories, Inc., Pfizer Inc, McKinsey & Company, Mitsubishi Tanabe Pharma Corporation, and Novartis; has received funding for travel from Nestlé and Kenes International and to attend conferences not funded by industry; serves on the editorial board of Alzheimer's & Dementia; has received honoraria from the Rotman Research Institute and BOLT International; serves as a consultant for Elan Corporation; receives research support from Merck & Co., Radiopharmaceuticals Inc., the NIH, the Veterans Administration, and the State of California; and holds stock in Synarc and Elan Corporation. Dr. Thomas has served on a scientific advisory board for Myriad Genetics, Inc.; has served as a consultant for Medivation, Inc., Myriad Genetics, Inc., Bristol-Meyers Squibb, and Neurochem Inc.; and receives research support from the US Department of Defense and the NIH/NIA. Dr. Schneider served as an editor on the Cochrane Collaborations Dementia and Cognitive Improvement Group; serves on scientific advisory boards for AstraZeneca, AC Immune SA, Allon Therapeutics, Inc., Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Merck Serono, and Roche; serves on editorial boards for Alzheimer's & Dementia, Current Alzheimer's Research, International Journal of Geriatric Psychiatry, Psychogeriatrics, and BMC Psychiatry; receives publishing royalties for Evidence-based Dementia Practice (Blackwell, 2003); serves as a consultant for Abbott, AC Immune SA, Allergan, Inc., Allon Therapeutics, Inc., Alzheimer Drug Discovery Foundation, AstraZeneca, Baxter International Inc., Bristol-Myers Squibb, Elan Corporation, Eli Lilly and Company, Exonhit, GlaxoSmithKline, Ipsen, Johnson & Johnson, Myriad Genetics, Inc., MedAvante, Inc., Merck Serono, Novartis, Pfizer Inc, Roche, Servier, Targacept, Inc., Toyama Chemical, Co., Ltd., Accera, Inc., Forest Laboratories, Inc., Lundbeck Inc., Medivation, Inc., sanofi-aventis, Schering-Plough Corp., Schwabe Pharma, Teva Pharmaceutical Industries Ltd., Voyager Pharmaceutical Corporation, Wyeth, and Transition Therapeutics Inc.; receives research support from AstraZeneca, Elan Pharmaceuticals, Forest Laboratories, Inc., Johnson & Johnson, Myriad Genetics, Inc., Takeda Pharmaceutical Company Limited, Wyeth, Baxter, Eli Lilly and Company, Novartis, Pfizer, the NIH (NIA, NIMH), and the Alzheimer's Association; and has prepared expert reports for medico-legal cases. Dr. Tariot serves/served on scientific advisory boards for ACADIA Pharmaceuticals, AC Immune SA, Allergan, Inc., Eisai Inc., Genentech, Inc., Novartis, sanofi-aventis, Schering-Plough Corp., Abbott, AstraZeneca, Bristol-Myers Squibb, Elan Corporation, GlaxoSmithKline, Eli Lilly and Company, Medivation, Inc., Merck Serono, Pfizer Inc, and Wyeth; has received funding for travel from Elan Corporation; serves on the editorial boards of CNS Spectrums, Expert Opinion on Investigational Drugs, and International Journal of Geriatric Psychiatry F1000 (Faculty of 1000); is author on a patent re: Biomarkers of neurodegenerative disease; has received speaker honoraria from Banner Health; serves as a consultant for Adamas Pharmaceuticals, Avid Radiopharmaceuticals, Inc., Baxter International Inc., EPIX Pharmaceuticals Inc, Forest Laboratories, Inc., MedAvante, Inc., Myriad Genetics, Inc., Roche, Transition Therapeutics Inc., Worldwide Clinical Trials, ACADIA Pharmaceuticals, AC Immune SA, Allergan, Inc., Eisai Inc., Genentech, Inc., Novartis, sanofi-aventis, Schering-Plough Corp., Abbott, AstraZeneca, Bristol Myers Squibb, Elan Corporation, GlaxoSmithKline, Eli Lilly and Company, Medivation, Inc., Merck Serono, Pfizer Inc, and Wyeth; receives research support from Baxter International Inc., Johnson & Johnson, Takeda Pharmaceutical Company Limited, Abbott, AstraZeneca, Avid Radiopharmaceuticals, Inc., Bristol-Myers Squibb, Elan Corporation, GlaxoSmithKline, Janssen, Eli Lilly and Company, Medivation, Inc., Merck Serono, Pfizer Inc, Toyama Chemical, Co., Ltd., Wyeth, the Alzheimer's Association, and the Arizona Department of Health; and holds stock options in MedAvante, Inc. and Adamas Pharmaceuticals.
REFERENCES
- 1. Tariot PN, Raman R, Jakimovich L, et al. Divalproex sodium in nursing home residents with possible or probable Alzheimer disease complicated by agitation: a randomized, controlled trial. Am J Geriatr Psychiatry 2005;13:942–949 [DOI] [PubMed] [Google Scholar]
- 2. Mark RJ, Ashford JW, Goodman Y, Mattson MP. Anticonvulsants attenuate amyloid beta-peptide neurotoxicity, Ca2+ deregulation, and cytoskeletal pathology. Neurobiol Aging 1995;16:187–198 [DOI] [PubMed] [Google Scholar]
- 3. Tariot PN, Loy R, Ryan JM, Porsteinsson A, Ismail S. Mood stabilizers in Alzheimer's disease: symptomatic and neuroprotective rationales. Adv Drug Deliv Rev 2002;54:1567–1577 [DOI] [PubMed] [Google Scholar]
- 4. Su Y, Ryder J, Li B, et al. Lithium, a common drug for bipolar disorder treatment, regulates amyloid-beta precursor protein processing. Biochemistry 2004;43:6899–6908 [DOI] [PubMed] [Google Scholar]
- 5. Qing H, He G, Ly PT, et al. Valproic acid inhibits Abeta production, neuritic plaque formation, and behavioral deficits in Alzheimer's disease mouse models. J Exp Med 2008;205:2781–2789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guerrini R, Belmonte A, Canapicchi R, Casalini C, Perucca E. Reversible pseudoatrophy of the brain and mental deterioration associated with valproate treatment. Epilepsia 1998;39:27–32 [DOI] [PubMed] [Google Scholar]
- 7. Galimberti CA, Diegoli M, Sartori I, et al. Brain pseudoatrophy and mental regression on valproate and a mitochondrial DNA mutation. Neurology 2006;67:1715–1717 [DOI] [PubMed] [Google Scholar]
- 8. Papazian O, Canizales E, Alfonso I, Archila R, Duchowny M, Aicardi J. Reversible dementia and apparent brain atrophy during valproate therapy. Ann Neurol 1995;38:687–691 [DOI] [PubMed] [Google Scholar]
- 9. Straussberg R, Kivity S, Weitz R, Harel L, Gadoth N. Reversible cortical atrophy and cognitive decline induced by valproic acid. Eur J Paediatr Neurol 1998;2:213–218 [DOI] [PubMed] [Google Scholar]
- 10. Yamanouchi H, Ota T, Imataka G, Nakagawa E, Eguchi M. Reversible altered consciousness with brain atrophy caused by valproic acid. Pediatr Neurol 2003;28:382–384 [DOI] [PubMed] [Google Scholar]
- 11. Tariot PN, Schneider LS, Cummings JL, et al. Chronic divalproex sodium to attenuate agitation and clinical progression of Alzheimer disease. Arch Gen Psychiatry 2011;68:853–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944 [DOI] [PubMed] [Google Scholar]
- 13. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308–2314 [DOI] [PubMed] [Google Scholar]
- 14. Jack CR, Jr, Bernstein MA, Fox NC, et al. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging 2008;27:685–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jovicich J, Czanner S, Greve D, et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage 2006;30:436–443 [DOI] [PubMed] [Google Scholar]
- 16. Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 1998;17:87–97 [DOI] [PubMed] [Google Scholar]
- 17. Hsu YY, Schuff N, Du AT, et al. Comparison of automated and manual MRI volumetry of hippocampus in normal aging and dementia. J Magn Reson Imaging 2002;16:305–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Christensen GE, Joshi SC, Miller MI. Volumetric transformation of brain anatomy. IEEE Trans Med Imaging 1997;16:864–877 [DOI] [PubMed] [Google Scholar]
- 19. Freeborough PA, Fox NC. The boundary shift integral: an accurate and robust measure of cerebral volume changes from registered repeat MRI. IEEE Trans Med Imaging 1997;16:623–629 [DOI] [PubMed] [Google Scholar]
- 20. Gunter JL, Shiung MM, Manduca A, Jack CR., Jr Methodological considerations for measuring rates of brain atrophy. J Magn Reson Imaging 2003;18:16–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jack CR, Jr, Shiung MM, Weigand SD, et al. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology 2005;65:1227–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Segura-Bruna N, Rodriguez-Campello A, Puente V, Roquer J. Valproate-induced hyperammonemic encephalopathy. Acta Neurol Scand 2006;114:1–7 [DOI] [PubMed] [Google Scholar]
- 23. Ziyeh S, Thiel T, Spreer J, Klisch J, Schumacher M. Valproate-induced encephalopathy: assessment with MR imaging and 1H MR spectroscopy. Epilepsia 2002;43:1101–1105 [DOI] [PubMed] [Google Scholar]
- 24. Chicharro AV, de Marinis AJ, Kanner AM. The measurement of ammonia blood levels in patients taking valproic acid: looking for problems where they do not exist? Epilepsy Behav 2007;11:361–366 [DOI] [PubMed] [Google Scholar]
- 25. Panda S, Radhakrishnan K. Two cases of valproate-induced hyperammonemic encephalopathy without hepatic failure. J Assoc Physicians India 2004;52:746–748 [PubMed] [Google Scholar]
- 26. Armon C, Shin C, Miller P, et al. Reversible parkinsonism and cognitive impairment with chronic valproate use. Neurology 1996;47:626–635 [DOI] [PubMed] [Google Scholar]
- 27. Run X, Liang Z, Zhang L, Iqbal K, Grundke-Iqbal I, Gong CX. Anesthesia induces phosphorylation of tau. J Alzheimers Dis 2009;16:619–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xie Z, Tanzi RE. Alzheimer's disease and post-operative cognitive dysfunction. Exp Gerontol 2006;41:346–359 [DOI] [PubMed] [Google Scholar]
- 29. Abreu LN, Issler C, Lafer B. Valproate-induced reversible pseudoatrophy of the brain and hyperammonemic encephalopathy in a bipolar patient. Aust NZ J Psychiatry 2009;43:484–485 [PubMed] [Google Scholar]
- 30. Garcia M, Huppertz HJ, Ziyeh S, Buechert M, Schumacher M, Mader I. Valproate-induced metabolic changes in patients with epilepsy: assessment with H-MRS. Epilepsia 2009;50:486–492 [DOI] [PubMed] [Google Scholar]
- 31. Evans MD, Shinar R, Yaari R. Reversible dementia and gait disturbance after prolonged use of valproic acid. Seizure Epub 2011 Mar 22 [DOI] [PubMed] [Google Scholar]
- 32. Petroff OA, Rothman DL, Behar KL, Hyder F, Mattson RH. Effects of valproate and other antiepileptic drugs on brain glutamate, glutamine, and GABA in patients with refractory complex partial seizures. Seizure 1999;8:120–127 [DOI] [PubMed] [Google Scholar]
- 33. Albers DS, Beal MF. Mitochondrial dysfunction and oxidative stress in aging and neurodegenerative disease. J Neural Transm Suppl 2000;59:133–154 [DOI] [PubMed] [Google Scholar]
- 34. Chen JX, Yan SD. Amyloid-beta-induced mitochondrial dysfunction. J Alzheimer Dis 2007;12:177–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Qian Y, Zheng Y, Tiffany-Castiglioni E. Valproate reversibly reduces neurite outgrowth by human SY5Y neuroblastoma cells. Brain Res 2009;1302:21–33 [DOI] [PubMed] [Google Scholar]
- 36. Smith AM, Gibbons HM, Dragunow M. Valproic acid enhances microglial phagocytosis of amyloid-beta(1–42). Neuroscience 2010;169:505–515 [DOI] [PubMed] [Google Scholar]
- 37. Fox NC, Black RS, Gilman S, et al. Effects of Abeta immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology 2005;64:1563–1572 [DOI] [PubMed] [Google Scholar]
- 38. Jack CR, Jr, Lowe VJ, Weigand SD, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain 2009;132:1355–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fox NC, Freeborough PA. Brain atrophy progression measured from registered serial MRI: validation and application to Alzheimer's disease. J Magn Reson Imaging 1997;7:1069–1075 [DOI] [PubMed] [Google Scholar]
- 40. Jack CR, Jr, Petersen RC, Xu Y, et al. Rate of medial temporal lobe atrophy in typical aging and Alzheimer's disease. Neurology 1998;51:993–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jobst KA, Smith AD, Szatmari M, et al. Rapidly progressing atrophy of medial temporal lobe in Alzheimer's disease. Lancet 1994;343:829–830 [DOI] [PubMed] [Google Scholar]