Abstract
Objective:
This randomized, double-blind, placebo-controlled, dose-ranging phase 2 study explored safety, efficacy, and biomarker effects of ELND005 (an oral amyloid anti-aggregation agent) in mild to moderate Alzheimer disease (AD).
Methods:
A total of 353 patients were randomized to ELND005 (250, 1,000, or 2,000 mg) or placebo twice daily for 78 weeks. Coprimary endpoints were the Neuropsychological Test Battery (NTB) and Alzheimer's Disease Cooperative Study–Activities of Daily Living (ADCS-ADL) scale. The primary analysis compared 250 mg (n =84) to placebo (n =82) after an imbalance of infections and deaths led to early discontinuation of the 2 higher dose groups.
Results:
The 250 mg dose demonstrated acceptable safety. The primary efficacy analysis at 78 weeks revealed no significant differences between the treatment groups on the NTB or ADCS-ADL. Brain ventricular volume showed a small but significant increase in the overall 250 mg group (p =0.049). At the 250 mg dose, scyllo-inositol concentrations increased in CSF and brain and CSF Aβx-42 was decreased significantly compared to placebo (p =0.009).
Conclusions:
Primary clinical efficacy outcomes were not significant. The safety and CSF biomarker results will guide selection of the optimal dose for future studies, which will target earlier stages of AD.
Classification of evidence:
Due to the small sample sizes, this Class II trial provides insufficient evidence to support or refute a benefit of ELND005.
Cortical deposition of amyloid plaques is one of the pathologic hallmarks of Alzheimer disease (AD).1,2 Oligomers of Aβ peptides are hypothesized to exert toxic effects on neurons, initiating a cascade of events culminating in the classic “plaque and tangle” pathology characteristic of AD.
ELND005 (scyllo-inositol) is an endogenous inositol stereoisomer,3 which is not directly involved in phosphatidyl-inositol (PI) signaling.4,5 Although scyllo-inositol at pharmacologic doses may alter myo-inositol levels and indirectly affect PI signaling, its main effects are thought to be binding and inhibition of Aβ42 peptide aggregation and formation of Aβ fibrils.5,6 In transgenic animals, scyllo-inositol reduced brain Aβ concentrations and plaque burden, preserved synaptic density, and improved learning deficits.5,7 Scyllo-inositol also appears to neutralize toxic effects of Aβ oligomers,6 including amelioration of oligomer-induced synaptic loss, LTP inhibition, and memory deficits.8,9 A prior amyloid anti-aggregation agent failed to demonstrate efficacy in phase 3 trials,10 but several other amyloid-targeted therapies are currently being studied.11–13 ELND005 is an orally bioavailable small molecule, which achieves steady state in plasma within 5 days, and at 2,000 mg twice daily has shown CNS penetration in healthy volunteers.14 This profile makes ELND005 an attractive candidate as a potential disease-modifying oral treatment for AD.
This study evaluated safety, efficacy, and biomarker effects of ELND005 across a wide dose range. The doses of ELND005 (250, 1,000, and 2,000 mg) administered twice daily (BID) were based on cumulative phase 1 safety/pharmacokinetic data. Brain imaging and CSF biomarkers were incorporated to assess potential effects of ELND005 on disease pathology.
METHODS
This double-blind, parallel-arm, randomized, placebo-controlled, multicenter safety and efficacy study was conducted at 58 sites in North America between December 2007 and May 2010.
Patients.
The study enrolled patients 50–85 years of age with probable AD by National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) criteria,15 Mini-Mental State Examination (MMSE)16 score of 16–26, MRI scan consistent with AD and free of other pathologic findings, Rosen Modified Hachinski17 score ≤4, and no significant neurologic, psychiatric, or medical illnesses. Medications with potential cognitive effects were not permitted, with the exception of stable dosages of acetylcholinesterase inhibitors or memantine.
Standard protocol approvals, registrations, and patient consents.
The study protocol (ClinicalTrials.gov number NCT00568776) was approved by each site's institutional review board. Written informed consent was obtained from each patient (or legally authorized representative) and their study partners or caregivers.
Study design and treatment.
Patients were randomly assigned to 1 of 4 treatment arms: placebo or ELND005 (250, 1,000, or 2,000 mg) administered orally BID. Random assignment was performed with an interactive voice response system using a computer-generated randomization list, which ensured that study site personnel had no knowledge of which group a given patient would be allocated to when making the determination of that patient's study eligibility. The randomization was stratified by MMSE score (16–21 vs 22–26), APOE ε4 carrier status (1 or 2 alleles vs none), and use of approved AD symptomatic medications (yes vs no). Cognitive, functional, and MRI assessments were performed at baseline and weeks 12 (no MRI), 24, 48, and 78. An Independent Safety Monitoring Committee (ISMC) reviewed ongoing and final study results. Administrative analyses were conducted after all patients had completed 24 and 48 weeks on study (see appendix e-1 for details). Based on the 48-week safety review, patients in the 1,000 and 2,000 mg groups were withdrawn; no changes were made to study conduct for patients in the 250 mg or placebo groups.
Safety measures.
Safety assessments included adverse event (AE) monitoring, clinical laboratory tests, and electrocardiograms. MRI safety assessments included fluid-attenuated inversion recovery and gradient-echo MRI sequences18 performed every 6 months in conjunction with volumetric MRI studies; results were interpreted by site-affiliated local radiologists.
Efficacy outcome measures.
The coprimary efficacy endpoints were the changes from baseline to week 78 in Neuropsychological Test Battery (NTB)19z score and Alzheimer's Disease Cooperative Study–Activities of Daily Living (ADCS-ADL) score.20 Secondary clinical endpoints were the Alzheimer's Disease Assessment Scale Cognitive Subscale (ADAS-Cog),21 Clinical Dementia Rating–Sum of Boxes (CDR-SB),22,23 and Neuropsychiatric Inventory (NPI) scores.24 A 12-item ADAS-Cog version was used (75-point maximum score), including “concentration/distractibility” as the 12th item. Exploratory clinical outcomes were change from baseline to week 78 in MMSE16 score and 3 responder analyses: proportion of patients having either 1) >0.3-point worsening in NTB z score, 2) <6-point worsening in ADAS-Cog score, or 3) no change or improvement in NTB z score or ADCS-ADL score. All efficacy endpoints were analyzed by modified Intent-to-Treat (mITT) population and per protocol set (PPS).
Biomarker outcome measures.
The key imaging endpoint was change from baseline to week 78 in brain ventricular volume.25,26 Whole brain volume, hippocampal volume, and cortical ribbon thickness were also measured. All volumetric MRI assessments were performed by NeuroRx Research (Montreal) using previously described methods.27–29 All computer-generated results were reviewed and corrected as necessary. A subset of patients underwent magnetic resonance spectroscopy (MRS) for assessment of scyllo-inositol and myo-inositol brain levels. Lumbar punctures were performed on another subset of patients at baseline, week 24 (primary CSF biomarker endpoint), and week 78 for determination of CSF Aβx-40, Aβx-42, total tau, phospho-tau181 (p-tau),30 and ELND005 concentrations.
Statistical analysis.
Following the discontinuation of the 1,000 and 2,000 mg groups, the primary comparison was amended in the statistical analysis plan to include the 250 mg and placebo groups only. A repeated-measures model was used to compare the change from baseline between the 250 mg and placebo groups for all continuous efficacy and biomarker endpoints, with time included as a categorical variable; no assumptions or limitations were imposed on response trajectory (e.g., linearity). “Responder” proportions were analyzed using an exact unconditional version of Fisher exact test. The coprimary endpoints were tested at a significance level of 0.049 due to the administrative interim analyses performed by an independent statistical team and reviewed by sponsor personnel not involved in study conduct. All other statistical testing was performed at a significance level of 0.05. Analyses were carried out using SAS 9.1.3.
Analysis populations.
The safety population consisted of 351 patients who received at least one dose of study drug. The mITT population included 341 patients who received at least one dose of study drug and one postbaseline efficacy assessment. The PPS included 130 mITT patients who met all inclusion/exclusion criteria, completed the week 78 visit, and took at least 80% of assigned study drug.
Prespecified subgroup analyses.
Subgroup analyses specified in the protocol included mild AD (screening/baseline MMSE 23–26, in an attempt to define an even milder population), moderate AD (screening/baseline MMSE 16–22), and APOE ε4 carriers and noncarriers.
Safety analysis.
AEs were coded using the Medical Dictionary for Regulatory Activities (v 13.0). Treatment-emergent AEs (TEAEs), serious AEs (SAEs), clinical laboratory, ECG, and vital signs data were summarized by treatment group.
Pharmacokinetic and pharmacodynamic analysis.
ELND005 concentrations in plasma and CSF were summarized using descriptive statistics. Biomarkers were analyzed by repeated-measures analysis (see appendix e-1 on the Neurology® Web site at www.neurology.org for biomarker assay methodology).
Sample size.
As originally designed, with a sample size of 85 in each of 4 groups (total n =340) and a 0.050 level 2-sided t test of the average treatment effect of all 3 ELND005 groups vs placebo, the study had >90% power to detect differences of 0.2 on the NTB and 4.07 on the ADCS-ADL. Following discontinuation of the 2 highest dose groups, the study retained approximately 80% and 70% power to detect these same differences on the NTB and ADCS-ADL, respectively.
RESULTS
Patient disposition.
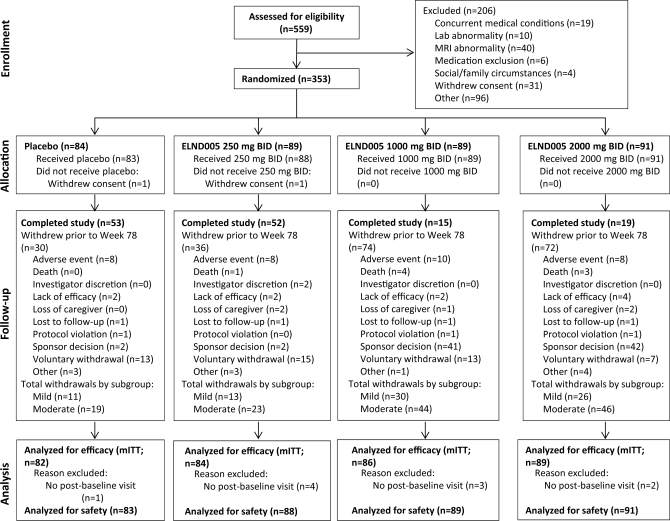
Figure 1 depicts the disposition of all screened and randomized patients.
Figure 1. Patient disposition.
mITT=modified intent-to-treat.
Demographics and baseline characteristics.
A total of 353 patients were enrolled and randomized and 351 received at least one dose of study drug (figure 1). Baseline measures were well-balanced across all groups (table 1). After discontinuation of the 2 high-dose groups, the primary analysis was based on 166 patients (n =84 250 mg; n =82 placebo).
Table 1.
Patient demographics and baseline characteristics
Abbreviations: ADAS-Cog=Alzheimer's Disease Assessment Scale–Cognitive Subscale; ADCS-ADL=Alzheimer's Disease Cooperative Study–Activities of Daily Living; CDR-SB=Clinical Dementia Rating–Sum of Boxes; MMSE=Mini-Mental State Examination; NPI=Neuropsychiatric Inventory; NTB=Neuropsychological Test Battery.
All data are mean (SD) unless otherwise indicated.
Safety.
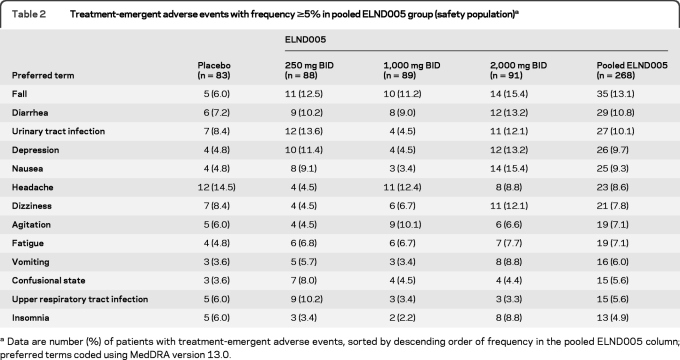
The overall incidence of TEAEs was similar across the 4 dose groups. TEAEs were reported for 91.6% and 87.5% of patients in the placebo and 250 mg groups, respectively. The most common AEs in the 250 mg group are shown in table 2. The safety and tolerability profiles in APOE ε4 carriers and noncarriers were similar.
Table 2.
Treatment-emergent adverse events with frequency ≥5% in pooled ELND005 group (safety population)a
Data are number (%) of patients with treatment-emergent adverse events, sorted by descending order of frequency in the pooled ELND005 column; preferred terms coded using MedDRA version 13.0.
The incidence of withdrawals due to AE was higher in the 1,000 mg (16.9%) and 2,000 mg (13.2%) groups than in the 250 mg (10.2%) and placebo (9.6%) groups. The incidence of SAEs was also higher in the ELND005 groups compared with placebo (23.1, 22.5, 21.6, and 13.3%, in the 2,000 mg, 1,000 mg, 250 mg, and placebo groups, respectively). The incidence of respiratory tract infections was higher in the 1,000 mg and 2,000 mg groups than in the placebo and 250 mg groups, even when adjusted for duration of exposure to account for early termination of the high dose groups. The overall incidence of SAEs was similar in the mild and moderate subgroups, except for serious infections and neurologic and psychiatric SAEs, which were lower in the mild subgroup.
At the week 48 ISMC review, more SAEs of infection were found in the 2,000 mg group compared to other groups, and a disproportionate number of deaths was seen in the 2 high-dose groups, with 0, 1, 5, and 4 deaths in the placebo, 250, 1,000, and 2,000 mg groups, respectively. Nine of the 10 deaths were assessed as not related to study drug by the reporting investigator. The patients who died tended to be older, and 9 of the 10 were in the moderate AD stratum (table e-3). The sponsor electively discontinued the 1,000 and 2,000 mg groups with the concurrence of the ISMC. The 250 mg group showed an acceptable safety profile and was continued. No additional deaths occurred in the 250 mg or placebo groups. There were no clinically relevant changes in vital signs or laboratory measures except for a dose-dependent decrease in uric acid. The mild and moderate subgroups had similar TEAE profiles, except for confusional episodes (predominantly in moderate patients).
Efficacy.
Primary endpoints.
In the overall mITT population that included patients with mild and moderate AD (MMSE 16–26), the NTB z score difference was 0.033 (95% CI −0.140, 0.205) and the ADCS-ADL difference was −1.4 (95% CI −5.4, 2.6) (figure 2; table e-2). Neither of these differences was significant. In the overall PPS population, treatment differences on the NTB and the ADCS-ADL scores were also not significant.
Figure 2. Primary outcome measures: Changes from baseline.
Least squares (LS) mean changes from baseline for the coprimary outcome measures (Neuropsychological Test Battery [NTB] and Alzheimer's Disease Cooperative Study–Activities of Daily Living [ADCS-ADL]) in overall (Mini-Mental State Examination [MMSE] 16–26) modified intent-to-treat (mITT) (A, B) and per protocol set (PPS) (C, D) populations, and for mild Alzheimer disease (AD) subgroup (MMSE 23–26) mITT (E, F) and PPS (G, H) populations. For all panels: upward (positive) direction represents better performance, downward direction (negative) indicates worse performance. Changes from baseline to week 78 in the ELND005 (purple) and placebo (green) treatment groups noted on graph.
Secondary and exploratory clinical endpoints.
In the overall mITT population, patterns of change similar to the primary outcomes were noted for CDR-SB, NPI, ADAS-Cog, and MMSE (table e-2), none of which achieved significance. The proportion of “responders” who did not decline on either primary endpoint was 38% in the 250 mg and 32% in placebo groups. When the 2 high-dose groups were discontinued, <25% of patients in those 2 groups had completed week 78, and clinical assessments of discontinuing patients were partially unblinded (investigators were aware that they were in the high-dose groups). Observed treatment effects at the 1,000 and 2,000 mg doses were not significant in comparison to the 250 mg dose or placebo on the primary endpoints (summary statistics of observed values are presented in appendix e-1).
Prospectively defined subgroup analyses.
Demographic characteristics for the subgroups analyzed were generally balanced (table e-1). APOE ε4 carrier status had no consistent effect on treatment outcomes. No significant treatment differences were seen in the moderate subgroup. As shown in table e-2, differences between 250 mg and placebo in the mITT analysis of the mild subgroup were 0.200 on the NTB (95% CI −0.046, 0.446; p =0.110) and 2.3 on the ADCS-ADL (95% CI −3.4, 7.9; p =0.426). In the PPS analysis of the mild subgroup, treatment–placebo difference of 0.403 (95% CI 0.111, 0.695) on the NTB was significant (p =0.007), but the ADCS-ADL difference of 2.3 (95% CI −3.8, 8.3) was not (p =0.459). The CDR-SB treatment differences of 0.87 (95% CI −0.44, 2.19) in the mild mITT (p =0.189) and 0.95 (95% CI −0.50, 2.40) in the mild PPS (p =0.195) were not significant, but were directionally consistent with the NTB (figure 2; table e-2). The ADAS-Cog treatment difference was not significant. Exploratory responder analyses in the mild subgroup favored treatment over placebo and were statistically significant in the PPS, but not mITT analysis (see table e-2).
Imaging biomarker endpoints.
In the overall mITT population, the change from baseline in ventricular volume was greater (3.2 mL; 95% CI 0.01, 6.4; p =0.049) in the 250 mg group (14.1 mL; 95% CI 11.7, 16.4) compared to the placebo group (10.9 mL; 95% CI 8.6, 13.2). Whole brain volume, hippocampal volume, and cortical ribbon thickness treatment differences were not significant (not shown).
CSF biomarkers.
At the week 24 (primary) time point, changes from baseline in Aβ40, Aβ42, tau, and p-tau concentrations were not significant. The week 78 samples from 20 patients showed a significant reduction in Aβ42 in the 250 mg group compared to placebo (−191.3 pg/mL [95% CI −329.6, −53.0; p =0.009]). The decrease in tau (−39.9, 95% CI −160.7, 80.9; p =0.497) was not significant (figure 3).
Figure 3. CSF biomarker changes from baseline.
Baseline values and change from baseline (CBL) to weeks 24 and 78 for CSF Aβx-42 (left) and tau (right). Least squares (LS) means were compared using a repeated-measures analysis; median values were compared using Wilcoxon rank-sum test.
Plasma and CSF pharmacokinetics of ELND005.
Plasma concentrations increased proportionately with dose and reached steady state between weeks 2 and 12 (not shown). CSF concentrations at week 24 were 13.8 μg/mL (95% CI 12.3, 315.4; n =19) in the 250 mg group, 31.4 μg/mL (95% CI 28.5, 34.4; n =16) in the 1,000 mg group, and 35.1 μg/mL (95% CI 28.7, 41.5; n =15) in the 2,000 mg group. MRS showed a dose-dependent increase in brain scyllo-inositol levels (data not shown).
DISCUSSION
The imbalance in the number of deaths and serious infections at the week 48 administrative analysis resulted in discontinuation of the 2 highest dose groups. The overall number and causes of death and the nature of infections were similar to rates reported in epidemiologic studies31,32 and in AD trials of similar duration.33 The mechanistic relationship, if any, between high doses and increase in infections remains unclear but is under investigation. The 250 mg group displayed an acceptable safety profile, which was not affected by patients' APOE ε4 carrier status. There were no reports of cerebral vasogenic edema18 at any dose, as assessed by local site radiologists.
The differences between the 250 mg and placebo groups (overall mITT, n =166) were not significant for the coprimary (NTB and ADCS-ADL) or secondary endpoints. In the prespecified subgroup analyses, there were no consistent efficacy trends in the moderate AD or APOE ε4 carrier or noncarrier subgroups.
In the prespecified subgroup of mild patients who completed the study and were compliant (PPS analysis), the 250 mg dose showed a significant and clinically relevant treatment effect on the NTB. The CDR-SB treatment difference of 0.95, although not significant, was directionally consistent with the NTB. The rate of CDR-SB decline on placebo (2.17 points over 78 weeks) was similar to that observed in the ADNI mild AD cohort (1.6 points over 52 weeks).34
The ADAS-Cog treatment–placebo difference was not significant but was directionally opposite to the NTB. The ADAS-Cog treatment–placebo difference was largely driven by minimal decline on placebo (2 points over 78 weeks), which is one-third the rate from the ADNI mild AD cohort (4.3 points over 52 weeks).34 The low rate of placebo worsening on ADAS-Cog was also inconsistent with the rates of placebo worsening on the NTB, CDR-SB, and ADCS-ADL in this study.
The NTB was chosen as the study's primary cognitive outcome measure because of its greater sensitivity in patients with mild AD.19,35 The ADAS-Cog is most sensitive to change in patients with moderate disease.36,37 Since the NTB captures changes in delayed memory and executive function which are not well-covered by the ADAS-Cog, our findings support the choice of the NTB for studies in mild AD.
There is a growing consensus that amyloid-targeted agents may provide more meaningful benefit when introduced at early stages of the disease.38 The positive cognitive trends in compliant mild patients are consistent with the preclinical effects of ELND005. In TgCRND8 mice, scyllo-inositol showed a more robust reduction of plaque accumulation when treatment was started at an earlier age.7
In patients with mild to moderate disease, the ventricular volume increase was significantly larger in the 250 mg group but was of small magnitude. In the mild group, the increase in ventricular volume was smaller and not significantly different from placebo. Although counterintuitive, similar findings were observed with other amyloid-targeted therapies.39,40 The observed ventricular enlargement could be due to inositol-related osmotic effects, to blockage of the arachnoid villi during the process of amyloid clearance, or could reflect “ex vacuo“ changes due to amyloid clearance leading to a decrease in brain volume.
The 250 mg dose achieved CSF concentrations similar to those associated with improved learning in animal models.7 This dose also demonstrated a significant reduction of CSF Aβ42 at 78 weeks, which may reflect a gradual reduction of brain amyloid pathology consistent with findings in transgenic animals.5,7 In contrast to the CSF Aβ42 decline that is associated with greater plaque burden during the early stages of AD, the anti-aggregation effects of ELND005 are thought to result in clearance of soluble Aβ peptides and in decreased brain amyloid burden. The decrease in amyloid burden may be reflected in lower CSF Aβ42 levels, and possibly in larger ventricular volume at week 78.
The study's limitations include the decreased power to test the coprimary endpoints due to discontinuation of 2 dose groups and the small sample size of the prespecified subgroups and the CSF substudy. There were no statistical corrections for the multiple analyses.
Despite the limitations, and the fact that the study did not achieve its primary objective, these results will inform the design of future studies. The safety findings at the highest doses helped define the AEs to be carefully monitored in future studies. The 250 mg dose demonstrated acceptable safety and tolerability, CNS penetration, and target engagement (Aβ42 reduction), and showed potential cognitive benefit in patients with mild disease. These results will help optimize the dose range and choice of biomarkers, and will aid the selection of the appropriate patient population. Our findings support the concept that amyloid-targeted therapies may have their greatest benefit in patients at earlier stages of AD.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the investigators and their coworkers at the 58 participating study centers (listed in appendix e-2), as well as the patients and families who participated in the study. Stephanie Moore, MS, Susan Strobel, PhD, and Kimberly Jochman, PhD, coordinated the development of the manuscript. The authors also thank Drs. Tony Cruz, Dale Schenk, and Eliseo Salinas for their review of and contributions to the manuscript.
GLOSSARY
- Aβ
β-amyloid
- AD
Alzheimer disease
- ADAS-Cog
Alzheimer's Disease Assessment Scale–Cognitive subscale
- ADCS-ADL
Alzheimer's Disease Cooperative Study–Activities of Daily Living
- ADNI
Alzheimer's Disease Neuroimaging Initiative
- AE
adverse event
- CDR-SB
Clinical Dementia Rating–Sum of Boxes
- ISMC
Independent Safety Monitoring Committee
- LTP
long-term potentiation
- MedDRA
Medical Dictionary for Regulatory Activities
- mITT
modified intent-to-treat
- MMSE
Mini-Mental State Examination
- MRS
magnetic resonance spectroscopy
- NINCDS-ADRDA
National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association
- NPI
Neuropsychiatric Inventory
- NTB
Neuropsychological Test Battery
- p-tau
phospho-tau181
- PI
phosphatidyl-inositol
- PPS
per protocol set
- SAE
serious adverse event
- TEAE
treatment-emergent adverse event
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Salloway: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, study supervision. Dr. Sperling: drafting/revising the manuscript, analysis or interpretation of data, acquisition of data. Dr. Keren: drafting/revising the manuscript, analysis or interpretation of data, acquisition of data, study supervision. Dr. Porsteinsson: drafting/revising the manuscript, analysis or interpretation of data, acquisition of data, study supervision. Dr. van Dyck: drafting/revising the manuscript, analysis or interpretation of data, acquisition of data, study supervision. Dr. Tariot: drafting/revising the manuscript, analysis or interpretation of data, acquisition of data. Dr. Gilman: drafting/revising the manuscript, acquisition of data, study supervision. Dr. Arnold: drafting/revising the manuscript, analysis or interpretation of data, acquisition of data. Dr. Abushakra: drafting/revising the manuscript, analysis or interpretation of data, statistical analysis, study supervision. Dr. Hernandez: study concept or design, analysis or interpretation of data acquisition of data, statistical analysis. Dr. Crans: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, statistical analysis. Dr. Liang: drafting/revising the manuscript, study concept or design, analysis or interpretation of data. Dr. Quinn: drafting/revising the manuscript, analysis or interpretation of data. Dr. Bairu: drafting/revising the manuscript, analysis or interpretation of data, study supervision, obtaining funding. Dr. Pastrak: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, statistical analysis, study supervision. Dr. Cedarbaum: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, study supervision.
DISCLOSURE
Dr. Salloway serves on the scientific advisory boards of Elan Corporation, sanofi-aventis, Pfizer Inc, and Bristol-Myers Squibb; served on the scientific advisory for Eisai Inc.; serves as Associate Editor for Journal of Neuropsychiatry and Clinical Neurosciences; receives publishing royalties for The Frontal Lobes and Neuropsychiatric Illness (American Psychiatric Press Inc., 2001), The Neuropsychiatry of Limbic and Subcortical Disorders (American Psychiatric Press Inc., 1997), and Vascular Dementia (Humana Press, 2004); receives honoraria from Eisai Inc., Pfizer Inc, Novartis, Forest Laboratories, Inc., Elan Corporation, and Athena Diagnostics, Inc.; holds corporate appointments with Merck Serono and Medivation, Inc.; receives research support from Elan Corporation, Janssen Alzheimer's Immunotherapy, Bayer Schering Pharma, Wyeth, Bristol-Myers Squibb, Pfizer Inc, and Eisai Inc.; received research support from Myriad Genetics, Inc., GlaxoSmithKline, Neurochem-Alzhemed, Cephalon, Inc., Forest Laboratories Inc., and Voyager; and receives research support from the NIH/NIA, the Norman and Rosalie Fain Family Foundation, the Champlin Foundation, and the John and Happy White Foundation. Dr. Sperling received a speaker honorarium from Pfizer Inc.; serves on the editorial board of Alzheimer's disease and Associated Disorders; has served as a consultant for Elan Corporation, Wyeth, Janssen, Pfizer Inc, Avid Radiopharmaceuticals, Inc., Bayer Schering Pharma, and Bristol-Myers Squibb; has received research support from Elan Corporation, Janssen, and Bristol-Myers Squibb, NIH/NIA, Alzheimer's Association, American Health Assistance Foundation, and an Anonymous Foundation; and her husband has served as a consultant for Bristol-Myers Squibb and Janssen, and receives research support from Pfizer Inc, Janssen, and Avid Radiopharmaceuticals, Inc. Dr. Keren serves on scientific advisory boards for Pfizer Inc, Janssen, Wyeth, and Novartis; has received funding for travel or speaker honoraria from Pfizer Inc, Janssen, and Novartis; and serves as a consultant for Elan Corporation. Dr. Porsteinsson serves on scientific advisory boards for Elan Corporation, Janssen AI, Medivation, Inc., Pfizer Inc, Toyama Chemical Co., Ltd., and Transition Therapeutics Inc.; serves on the speakers' bureau for Forest Laboratories, Inc.; receives research support from Baxter International Inc., Bristol-Myers Squibb, Elan Corporation, Janssen AI, Medivation, Inc., Pfizer Inc, and Toyama Chemical Co., Ltd.; received research support from Eisai Inc., Eli Lilly and Company, Forest Laboratories, Inc., Janssen, GlaxoSmithKline, Merck Serono, Mitsubishi Tanabe Pharma Corporation, Myriad Genetics, Inc., Neurochem Inc, Ono Pharmaceutical Co. Ltd., and Wyeth; and receives research support from the NIH (NIA, NIMH). Dr. van Dyck has served on scientific advisory boards for Elan Corporation, Pfizer Inc, GlaxoSmithKline, Bristol-Myers Squibb, and Forest Laboratories, Inc.; has received funding for travel and speaker honoraria from Forest Laboratories, Inc.; his spouse owns or has applied for patents re: Use of guanfacine in the treatment of behavioral disorders, Use of lofexidine in the treatment of behavioral disorders, Chelerythrine, analogs thereof and their use in the treatment of bipolar disorder and other cognitive disorders (formerly licensed to Marinus Pharmaceuticals, Inc.); his spouse receives publishing royalties for The Neuropharmacology of Stimulant Drugs: Implications for AD/HD (Oxford University Press, 2000); serves as a consultant for Elan Corporation, Pfizer Inc, GlaxoSmithKline, Bristol-Myers Squibb, Forest Laboratories, Inc., and Merck Serono, and his spouse serves as a consultant for Shire plc; served on the speakers' bureau for Forest Laboratories, Inc.; receives/has received research support from Wyeth, Eli Lilly and Company, Pfizer Inc, Bristol-Myers Squibb, Medivation, Inc., Bayer Schering Pharma, Abbott, Elan Corporation, GlaxoSmithKline, Myriad Genetics, Inc., Neurochem Inc, Sanofi-Synthelabo Research, Janssen, Eisai Inc., Merck Serono, Mitsubishi Tanabe Pharma Corporation, the NIH (NIA, NIMH), Alzheimer's Association, American Health Assistance Foundation, and the National Alliance for Research on Schizophrenia and Affective Disorders (NARSAD); his spouse receives research support from Shire plc, the NIH (NIA, NINDS), the Kavli Neuroscience Institute at Yale, and NARSAD; and his spouse has received license fee payments and receives royalties from Shire plc for a patent re: Use of guanfacine in the treatment of behavioral disorders. Dr. Tariot serves/served on scientific advisory boards for ACADIA Pharmaceuticals, AC Immune SA, Allergan, Inc., Eisai Inc., Genentech, Inc., Novartis, sanofi-aventis, Schering-Plough Corp., Abbott, AstraZeneca, Bristol-Myers Squibb, Elan Corporation, GlaxoSmithKline, Eli Lilly and Company, Medivation, Inc., Merck Serono, Pfizer Inc, and Wyeth; has received funding for travel from Elan Corporation; serves on the editorial boards of CNS Spectrums, Expert Opinion on Investigational Drugs, and International Journal of Geriatric Psychiatry F1000 (Faculty of 1000); is author on a patent re: Biomarkers of Neurodegenerative disease; has received speaker honoraria from Banner Health; serves as a consultant for Adamas Pharmaceuticals, Avid Radiopharmaceuticals, Inc., Baxter International Inc., EPIX Pharmaceuticals Inc, Forest Laboratories, Inc., MedAvante, Inc., Myriad Genetics, Inc., Roche, Transition Therapeutics Inc., Worldwide Clinical Trials, ACADIA Pharmaceuticals, AC Immune SA, Allergan, Inc., Eisai Inc., Genentech, Inc., Novartis, sanofi-aventis, Schering-Plough Corp., Abbott, AstraZeneca, Bristol Myers Squibb, Elan Corporation, GlaxoSmithKline, Eli Lilly and Company, Medivation, Inc., Merck Serono, Pfizer Inc, and Wyeth; receives research support from Baxter International Inc., Johnson & Johnson, Takeda Pharmaceutical Company Limited, Abbott, AstraZeneca, Avid Radiopharmaceuticals, Inc., Bristol-Myers Squibb, Elan Corporation, GlaxoSmithKline, Janssen, Eli Lilly and Company, Medivation, Inc., Merck Serono, Pfizer Inc, Toyama Chemical, Co., Ltd., Wyeth, the Alzheimer's Association, and the Arizona Department of Health; and holds stock options in MedAvante, Inc. and Adamas Pharmaceuticals. Dr. Gilman serves on scientific advisory boards for Elan Corporation, Janssen AI, Pfizer Inc, Allergan, Inc., and Teva Pharmaceutical Industries Ltd.; has received funding for travel from GlaxoSmithKline; serves as Editor-in-Chief of and receives publishing royalties for Contemporary Neurology Series (Oxford University Press, 1982–present), MedLink Neurology (MedLink Corporation, 1992–present), and Experimental Neurology (Elsevier, 2003–present); and receives research support from GE Healthcare and the NIH/NINDS. Dr. Arnold serves on scientific advisory boards for Biogen Idec, Genentech, Inc., Roche, and Teva Pharmaceutical Industries Ltd.; has received speaker honoraria from Biogen Idec, Bayer Schering Pharma, CD-Pharma Interactive Medical Production, Genentech, Inc., EMD Serono, Inc., MS Forum, Sanofi-Aventis, GlaxoSmithKline, Merck Serono, and Teva Pharmaceutical Industries Ltd.; holds a patent re: Method of evaluating the efficacy of drug on brain nerve cells; has served as a consultant for Bayer Schering Pharma, Eisai Inc., Biogen Idec, NeuroRx Research Inc., Elan Corporation, Genentech, Inc., Genzyme Corporation, Eli Lilly and Company, GlaxoSmithKline, and Roche; has received research support from Biogen Idec, Bayer Schering Pharma, the Canadian Institutes of Health Research, and the Multiple Sclerosis Society of Canada; and holds stock in NeuroRx Research Inc. Dr. Abushakra is an employee of and holds stock and stock options in Elan Corporation; and was an employee of and held stock in Allergan, Inc. (2006–2099). Dr. Hernandez is an employee of and holds stock and stock options in Elan Corporation. Dr. Crans is an employee of and holds stock and stock options in Elan Corporation. Dr. Liang is an employee of and holds stock and stock options in Elan Corporation. Dr. Quinn is an employee of and holds stock and stock options in Elan Corporation. Dr. Bairu is an employee of and holds stock and stock options in Elan Corporation. Dr. Pastrak is an employee of Transition Therapeutics Inc. Dr. Cedarbaum was an employee of and held stock and stock options in Elan Corporation at the time of manuscript preparation.
REFERENCES
- 1. Hardy J, Duff K, Hardy KG, Perez-Tur J, Hutton M. Genetic dissection of Alzheimer's disease and related dementias: amyloid and its relationship to tau. Nat Neurosci 1998;1:355–358 [DOI] [PubMed] [Google Scholar]
- 2. Gandy S. The role of cerebral amyloid β accumulation in common forms of Alzheimer disease. J Clin Invest 2005;115:1121–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaiser LG, Schuff N, Cashdollar N, Weiner MW. Scyllo-inositol in normal aging human brain: 1H magnetic resonance spectroscopy study at 4 Tesla. NMR Biomend 2005;18:51–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Takenawa T, Egawa K. CDP-diglyceride:inositol transferase from rat liver. Purification and properties J Biol Chem 1977;252:5419–5423 [PubMed] [Google Scholar]
- 5. Fenili D, Brown M, Rappaport R, McLaurin J. Properties of scyllo-inositol as a therapeutic treatment of AD-like pathology. J Mol Med 2007;85:603–611 [DOI] [PubMed] [Google Scholar]
- 6. McLaurin J, Golomb R, Jurewicz A, Antel PJ, Fraser PE. Inositol stereoisomers stabilize an oligomeric aggregate of Alzheimer amyloid β peptide and inhibit Aβ-induced toxicity. J Biol Chem 2000;275:18495–18502 [DOI] [PubMed] [Google Scholar]
- 7. McLaurin J, Kierstead ME, Brown ME, et al. Cyclohexanehexol inhibitors of Aβ aggregation prevent and reverse Alzheimer phenotype in a mouse model. Nat Med 2006;12:801–808 [DOI] [PubMed] [Google Scholar]
- 8. Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-β protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci 2007;27:2866–2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Townsend M, Cleary JP, Mehta T, et al. Orally available compound prevents deficits in memory caused by the Alzheimer amyloid-β oligomers. Ann Neurol 2006;60:668–676 [DOI] [PubMed] [Google Scholar]
- 10. Gauthier S, Aisen PS, Ferris SH, et al. Effect of tramiprosate in patients with mild-to-moderate Alzheimer's disease: exploratory analyses of the MRI sub-group of the Alphase study. J Nutr Health Aging 2010;13:550–557 [DOI] [PubMed] [Google Scholar]
- 11. Lannfelt L, Blennow K, Zetterberg H, et al. Safety, efficacy, and biomarker findings of PBT2 in targeting Aβ as a modifying therapy for Alzheimer's disease: a phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol 2008;7:779–786 [DOI] [PubMed] [Google Scholar]
- 12. Querfuth HW, LaFerla FM. Alzheimer's disease. N Engl J Med 2010;362:329–344 [DOI] [PubMed] [Google Scholar]
- 13. Frisardi V, Solfrizzi V, Imbimbo BP, et al. Towards disease-modifying treatment of Alzheimer's disease: drugs targeting β-Amyloid. Curr Alzheimer Res 2010;7:40–55 [DOI] [PubMed] [Google Scholar]
- 14. Garzone P, Koller M, Pastrak A, et al. Oral amyloid anti-aggregating agent ELND005 is measurable in CSF and brain of healthy adult men. Alzheimers Dement 2009;5(4 suppl):P323.Abstract [Google Scholar]
- 15. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944 [DOI] [PubMed] [Google Scholar]
- 16. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 17. Rosen WG, Terry RD, Fuld PA, Katzman R, Peck A. Pathological verification of ischemic score in differentiation of dementias. Ann Neurol 1980;7:486–488 [DOI] [PubMed] [Google Scholar]
- 18. Salloway S, Sperling R, Gilman S, et al. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology 2009;73:2061–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harrison J, Minassian SM, Jenkins L, Black RS, Koller M, Grundman M. The NTB: A neuropsychological test battery for use in Alzheimer's Disease Clinical Trials. Arch Neurol 2007;64:1323–1329 [DOI] [PubMed] [Google Scholar]
- 20. Galasko D, Bennett D, Sano M, et al. for the Alzheimer's Disease Cooperative Study. An inventory to assess activities of daily living clinical trials in Alzheimer's disease. Alz Dis Assoc Disord 1997;11(suppl 2):S33–S39 [PubMed] [Google Scholar]
- 21. Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry 1984;141:1356–1364 [DOI] [PubMed] [Google Scholar]
- 22. Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Brit J Psychiatry 1982;140:566–572 [DOI] [PubMed] [Google Scholar]
- 23. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414 [DOI] [PubMed] [Google Scholar]
- 24. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308–2314 [DOI] [PubMed] [Google Scholar]
- 25. Fox NC, Cousens S, Scahill R, Harvey RJ, Rossor MN. Using serial registered brain magnetic resonance imaging to measure disease progression in Alzheimer disease. Arch Neurol 2000;57:339–344 [DOI] [PubMed] [Google Scholar]
- 26. Smith SM, Rao A, DeStefano N, et al. Longitudinal and cross-sectional analysis of atrophy in Alzheimer's disease: cross-validation of BSI, SIENS and SIENAX. Neuroimage 2007;36:1200–1206 [DOI] [PubMed] [Google Scholar]
- 27. Smith SM, Zhang Y, Jenkinson M, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage 2002;17:479–489 [DOI] [PubMed] [Google Scholar]
- 28. Collins DL, Pruessner JC. Towards accurate, automatic segmentation of the hippocampus and amygdala from MRI by augmenting ANIMAL with a template library and label fusion. Neuroimage 2010;52:1355–1366 [DOI] [PubMed] [Google Scholar]
- 29. Chen JT, Narayanan S, Collins DL, Smith SM, Matthews PM, Arnold DL. Relating neocortical pathology to disability progression in multiple sclerosis using MRI. Neuroimage 2004;23:1168–1175 [DOI] [PubMed] [Google Scholar]
- 30. Galasko D, Chang L, Motter R, et al. High cerebrospinal fluid tau and low amyloid beta42 levels in the clinical diagnosis of Alzheimer disease and relation to apolipoprotein E genotype. Arch Neurol 1998;55:937–945 [DOI] [PubMed] [Google Scholar]
- 31. Ganguli M, Dodge HH, Shen C, Pandav RS, DeKosky ST. Alzheimer disease and mortality: a 15-year epidemiological study. Arch Neurol 2005;62:779–784 [DOI] [PubMed] [Google Scholar]
- 32. Brunnstrom HR, Englund EM. Causes of death in patients with dementia disorders. Eur J Neurol 2009;16:488–492 [DOI] [PubMed] [Google Scholar]
- 33. Feldman HH, Doody RS, Kivipelto M, et al. Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology 2010;74:956–964 [DOI] [PubMed] [Google Scholar]
- 34. Petersen RC, Aisen PS, Beckett LA, et al. Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology 2010;74:201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gilman S, Koller M, Black RS, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology 2005;64:1553–1562 [DOI] [PubMed] [Google Scholar]
- 36. Doraiswamy PM, Kaiser L, Bieber F, Garman RL. The Alzheimer's Disease Assessment Scale: evaluation of psychometric properties and patterns of cognitive decline in multicenter clinical trials of mild to moderate Alzheimer's disease. Alzheimer Dis Assoc Disord 2001;15:174–183 [DOI] [PubMed] [Google Scholar]
- 37. Benge JF, Balsis S, Geraci L, Massman PJ, Doody RS. How well do the ADAS-cog and its subscales measure cognitive dysfunction in Alzheimer's disease? Dement Geriatr Cogn Disord 2009;28:63–69 [DOI] [PubMed] [Google Scholar]
- 38. Aisen PS. Alzheimer's disease therapeutic research: the path forward. Alzheimers Res Ther 2009;1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fox NC, Black RS, Gilman S, et al. Effects of Abeta immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology 2005;10:1563–1572 [DOI] [PubMed] [Google Scholar]
- 40. Rinne JO, Brooks DJ, Rossor MN, et al. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer's disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol 2010;9:363–372 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.