Abstract
Objectives
Intra-articular fractures predispose patients to post-traumatic osteoarthritis (PTOA), with associated chronic joint pain and decreased function. The success of articular fracture management is dependent on how the fracture is treated and on fracture type and severity. The purpose of the present study was to correlate objective CT-based indices of intra-articular fracture severity with subsequent joint degeneration. It was hypothesized that an injury severity metric that included objective measures of articular disruption, of fracture energy, and of fragment displacement/dispersal would be a useful predictor of PTOA.
Methods
Novel CT-based image analysis techniques were utilized to quantify acute injury characteristics in a prospective series of twenty tibial plafond fractures managed by articulated external fixation, with later definitive surgical fracture reduction performed after soft-tissue swelling had sufficiently resolved. PTOA severity was assessed two years post-injury using the Kellgren-Lawrence (KL) radiographic grading scale. A predictive model was developed by linearly regressing these two-year KL outcomes on the CT-based severity metrics.
Results
A combined acute severity score involving articular disruption and fracture energy successfully predicted PTOA severity (R2 = 0.70), whereas fragment displacement / dispersal and surgeon opinion correlated much less well with degeneration (R2 = 0.42 and 0.47). The concordance between the combined metric and PTOA severity was 88%.
Conclusions
The findings of this study indicate that objective CT-based metrics of acute injury severity can reliably predict intermediate-term PTOA outcomes in this challenging class of articular fractures. Quantitative biomechanical assessment of injury characteristics provides new possibilities to improve fracture management and to guide PTOA research.
Keywords: fracture severity, intra-articular, post-traumatic arthritis
Introduction
Articular fractures vary in their degree of articular surface disruption, overall comminution, fragment displacement / dispersal, and associated soft tissue injury. The severity of a fracture is among the most important determinants of long-term joint function, but it has not been amenable to objective quantification. Clinicians use plain radiographs and computed tomography (CT) to classify fractures. However, fracture classification is subjective and known to be an unreliable assessment of injury severity [1-3]. A novel CT-based method to objectively measure fracture energy, and the degree of fragment displacement/dispersion, has been developed[4]. That study demonstrated excellent agreement between the CT-based metric and severity rank ordering assessments performed by experienced orthopaedic trauma surgeons [4].
While those findings helped to establish the credibility of CT-based metrics for quantifying fracture severity, the relationship between these measures of fracture severity and clinical or radiographic outcome was not studied. The purpose of the present study was to correlate objective, CT-based indices of intra-articular fracture severity with post-traumatic osteoarthritis (PTOA) and clinical outcome in a series of tibial plafond fracture patients. It was hypothesized that an injury severity metric that included objective measures of articular disruption, of fracture energy, and of fragment displacement / dispersal would be an accurate predictor of PTOA and functional outcomes after two years.
Materials and Methods
The study group consisted of twenty patients with isolated tibial plafond fractures (13 male, 7 female, ages from 20 to 64 years), selected from a larger group treated at our institution by three experienced orthopaedic surgeons. An Institutional Review Board approved the study protocol, and informed consent was obtained from all patients. To be included, patients had to have sustained an isolated closed or open unilateral fracture. Their initial CT scan, which was the index CT scan for the study, was obtained after provisional limb realignment with either a splint or a spanning external fixator. CT technicians were instructed to scan the entire length of the fractured tibia, and a comparable segment from the non-injured contralateral limb. Failure to do so precluded injury severity analysis and therefore necessitated exclusion from the study. One of the investigators, an orthopaedic trauma surgeon (JLM), chose cases to represent a spectrum of acute injury severity that included a range of fractures from OTA partial articular fractures (Type B2 and 3) to severely comminuted fractures involving the entire tibial plafond (Type C3 classification). Patients enrolled in the study received standard-of-care treatment and diagnostic plain radiographs and CT scans of their lower extremities. Fractures were treated using provisional spanning external fixation, with definitive reduction of the articular surface performed from several days to two weeks later, after soft-tissue swelling had sufficiently resolved. The reduced articular surface was internally fixed with small-fragment screws, with the aid of an intra-operative c-arm. The external fixator was used until healing, typically for three months. Although there were no fracture site infections, one patient developed a non-union and was ultimately excluded from the study.
Patients were followed for two years post-injury, with repeat imaging studies collected at regular intervals (4 months, 1 year, and 2 years following surgery). PTOA was assessed on weight bearing radiographs at two years after injury, using the Kellgren-Lawrence (KL) grading scale, a widely accepted radiographic arthrosis assessment instrument that has been shown to have good inter-observer reliability [5]. In a consensus grading session, an experienced orthopaedic trauma surgeon (JLM) and a senior resident (CVH) graded the cases according to the KL criteria. Ankle pain and function were assessed using a validated questionnaire, the Ankle Osteoarthritis Scale (AOS) [6]. For this assessment, patients completed a survey consisting of nine questions related to pain, and nine related to disability. The AOS questions are specific to symptoms and disability related to the ankle joint. Metrics for pain and disability are formulated from patient answers and are combined to generate the AOS score. The AOS score is scaled from 0 to 100, with higher scores indicating greater pain or disability.
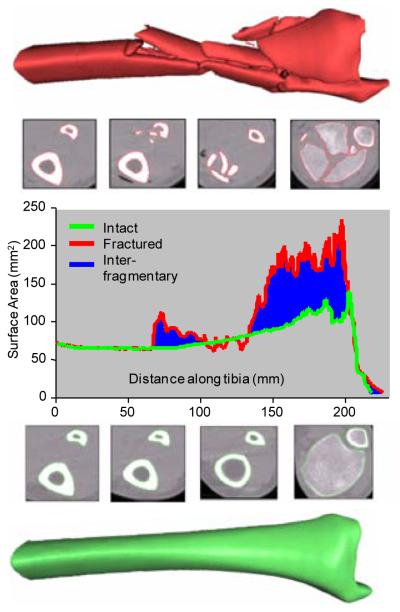
Three fundamental components of fracture severity (fracture energy, articular comminution and fragment displacement/dispersal) were objectively measured from CT scans, using advanced image analysis methods [4, 7-8]. Fracture energy was quantified by applying fracture mechanics principles, relating the energy absorbed to the fracture-liberated inter-fragmentary surface area [8]. Greater inter-fragmentary surface area accompanies greater degrees of fracture comminution. Tibia bone surface areas were determined for both limbs on the CT, and the liberated surface area was then calculated as the difference in fractured versus intact (datum) tibial surface areas (Figure 1) [4]. In order to assess the repeatability of the surface area measures, a validation study was performed by an operator who re-calculated the liberated surface area for one minor, one moderate, and one severe fracture case image six months after performing the first analysis. The second analysis differed from the first by less than 8%, showing the technique to be reasonably repeatable.
Figure 1.
The surface area for the fractured (red) and intact contralateral (green) are plotted along the length of the tibia. The total inter-fragmentary surface area is graphically represented by the blue area between the intact and fractured curves. The severe disruption and fragmentation visible in the fractured metaphysis illustrates comminution.
The fracture energy was derived from the liberated surface area measurement, by multiplying inter-fragmentary surface areas times the energy release rate, the latter being a material constant that was scaled from CT intensities to account for variation in bone density within and between patients [9]. The degree of articular comminution was quantified by determining the amount of inter-fragmentary surface area present within 1.5 mm of the articular surface. It was expressed as a percentage of the intact surface area over the same portion of the distal tibia. Fragment displacement /dispersal was indexed according to the volume of displaced soft tissues, using methods previously reported[4] (Figure 2). Quantifying gross fragment displacement required that translations be measured relative to an intact reference datum. This was accomplished by proximally aligning a mirrored image of the intact contralateral tibia to the fractured tibia. Displacement was then quantified by computing the difference in volumes occupied by the aggregate aligned intact (i.e. mirrored contralateral)/fractured bones and the intact bone alone.
Figure 2.
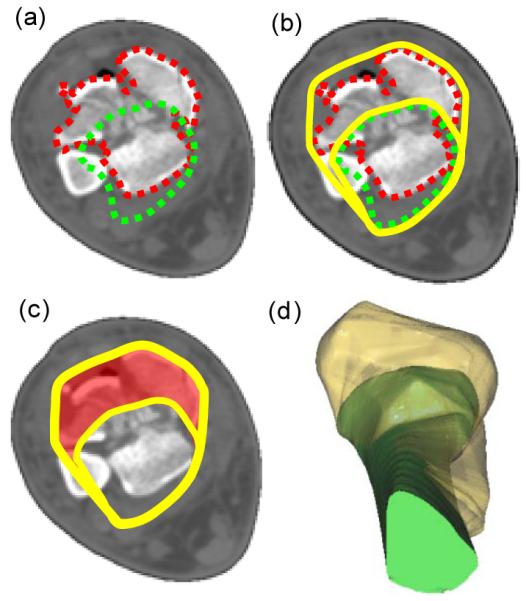
Method for quantifying fragment displacement[4]. Segmented intact and fractured bones (a) were circumscribed by convex hulls (b). The difference in area between convex hulls estimated in-plane tissue displacement (c). A volumetric quantity was formed by stacking the slices along the limb (d).
Linear regression was performed in order to establish the relationship between the three principal fracture characteristics (fracture energy, articular comminution, and fragment displacement/dispersal) and the severity of PTOA (as indexed by the patients’ two-year follow-up KL scores). To investigate which individual fracture characteristic best explained eventual PTOA severity, the KL scores were first separately regressed on each characteristic’s metric. Efforts to develop a comprehensive articular fracture severity metric predictive of joint degeneration began with normalizations of the three individual severity metrics. From the minimum and maximum observed values, severity metrics were scaled so that each ranged from the least severe (score 0), to the most severe observation (score 100). Multivariate linear regression was then performed using the three CT-based metrics as independent variables and the KL scores as the dependent variable. Recognizing that outcome is potentially affected by all three fracture severity characteristics, a PTOA predictive model was then developed based upon the regression.
Since the three measures combine ordinal and discrete values, statistical agreement between acute severity and arthrosis measures was also assessed using concordance -- the probability that the severity variable would correctly discriminate between a given pair of subjects’ arthrosis scores. The concordance measure ranges between 0 and 1, with 0.5 indicating chance agreement and 1 perfect agreement [10].
Results
The tibial plafond fractures included in this study ranged in severity from mild to severely comminuted, with contrasting characteristics reflected by the CT-based metrics (Table 1). The fracture energies ranged from 5.2 to 27.2 Joules, and the fragment displacement/dispersal metric ranged from 3.8 to 48.5 cm3. The increase in the free surface area of the fractured articular surface relative to its pre-fractured state (the metric of articular comminution) ranged from 51 to 156%. There was a loose association between fracture energy and fragment displacement (R2 = 0.61), but the severity of articular comminution did not correlate well with the other fracture severity measures (R2 = 0.18 and 0.20).
Table 1.
Case Data
Patient injury OTA fracture classifications, 2yr arthrosis grades, and severity measures.
| Case | OTA Classification |
KL | Fracture Energy (J) |
Displacement (cm3) |
Articular Comminution |
|---|---|---|---|---|---|
| A | C13 | 0 | 5.2 | 11.3 | 51% |
| B | B21 | 0 | 5.4 | 5.2 | 62% |
| C | B22 | 0 | 6.5 | 3.8 | 64% |
| D | N/A | 1 | 9.6 | 18.2 | 58% |
| E | B21 | 0 | 10.2 | 10.4 | 71% |
| F | C21 | 0 | 9.9 | 32.4 | 74% |
| G | B21 | 0 | 5.7 | 6.9 | 100% |
| H | B23 | 1 | 8.6 | 13.5 | 96% |
| I | C11 | 1 | 6.4 | 6.3 | 123% |
| J | C23 | 3 | 21.9 | 24.2 | 61% |
| K | C11 | 3 | 7.7 | 16.4 | 125% |
| L | C23 | 2 | 14.0 | 26.1 | 102% |
| M | C32 | 2 | 15.8 | 24.4 | 110% |
| N | C21 | 3 | 15.5 | 34.6 | 121% |
| O | C33 | 2 | 25.6 | 24.5 | 94% |
| P | B13 | 4 | 14.9 | 25.9 | 156% |
| Q | C33 | 4 | 24.4 | 47.4 | 119% |
| R | C11 | 2 | 25.8 | 27.5 | 114% |
| S | C32 | 2 | 27.2 | 48.5 | 124% |
| T | C31 | 3 | 25.9 | 27.7 | 137% |
Two years post-injury, 11 of the 20 patients had developed moderate to severe OA (KL grade ≥ 2). Regression analysis identified fracture energy and articular comminution individually as statistically significant PTOA predictors (p<0.01), whereas fragment displacement was not significant (p=0.35). Therefore, in a more refined analysis, only fracture energy and articular comminution were regressed to formulate the combined severity score. This regression model computed the optimal fracture energy weighting as 0.48 and the optimal weighting of articular comminution as 0.52, and it yielded an overall R2 of 0.70 (Figure 3).
Figure 3.
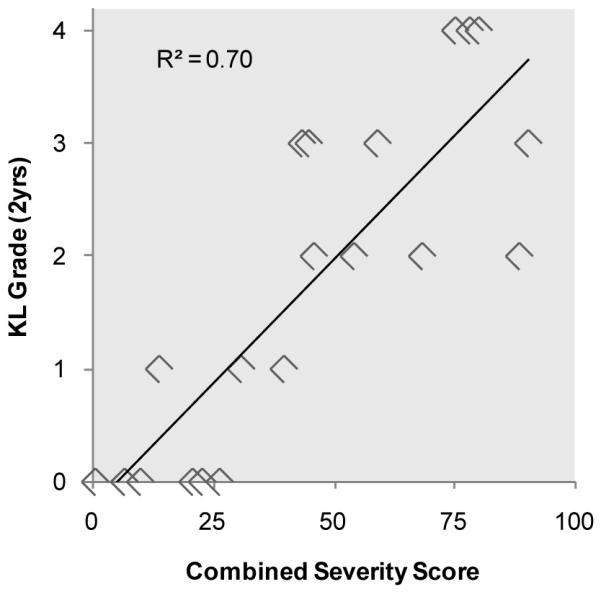
Linear regression modeled arthrosis scores as a function of measured severity characteristics. The combined severity score was formulated from the statistically significant characteristics, fracture energy and articular involvement.
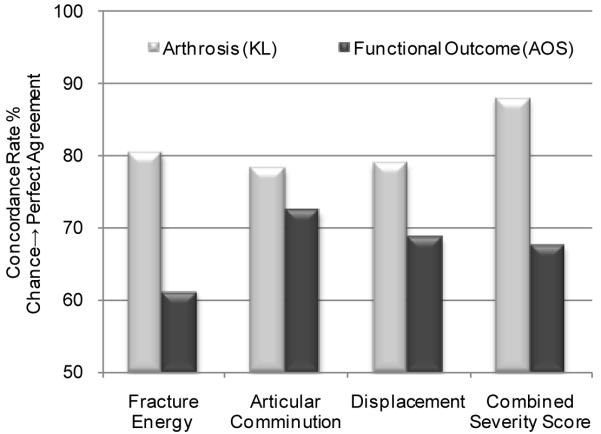
While the results of the regression models were encouraging, concordance provides a more easily interpretable measure for evaluating the CT-based metrics’ capacity for predicting the likelihood of joint degeneration. In this context, concordance is the probability that the computer accurately identifies which of two fractures will develop more severe PTOA symptoms after two years. The concordance analysis results suggest that CT-based acute severity metrics can effectively predict, and discriminate the likelihood of PTOA after a plafond fracture. The combined severity score had the best agreement with KL grade (concordance = 88%; 95% CI: 78 - 99%). Alone, the other severity measures performed less well, with concordances ranging from 78~81% (Figure 4). Since fracture energy and articular comminution were relatively weak predictors of PTOA when considered separately (R2=0.44 and 0.50, respectively), these data suggest that they act synergistically to influence the onset of PTOA.
Figure 4.
Agreement between measures of acute injury severity and long term outcomes are evaluated using their rates of concordance. Severity of arthrosis had excellent agreement with the combined severity score (88% concordance). Acute severity measures agreed less well with functional outcomes (68% concordances).
Cases that were graded by KL to have either an absence or doubtful presence of OA (KL<2) were separated from those whose radiographs unambiguously indicated the presence of OA (KL≥ 2). Within these groupings, the magnitudes of each injury severity measure were averaged, and between-group differences were assessed using a two-sample, independent t-test. Each severity metric was identified to be different between the two groups, to varying degrees: fragment displacement/dispersal (p=0.007), fracture energy (p=0.003), and articular comminution (p=0.031) (Figure 5).
Figure 5.
Cases were grouped according to their PTOA status at 2 years post injury (OA absent: KL < 2, else OA Present). Injury severity and functional outcome measures were averaged within each PTOA group for comparison. Significant differences were observed in injury severity measures between these groups, but not in functional outcomes (AOS) (* p<0.05).
Functional outcome data collected from the AOS questionnaire showed a linkage between injury severity and long-term pain and disability. There was a concordance of 68% between the combined severity score and the AOS score (Figure 4). Between all individual severity metrics, articular comminution had the best agreement with the functional outcomes (73% concordance) (Figure 4). Furthermore, the group of patients identified with radiographic changes of PTOA reported worse functional outcomes than those patients who did not (Figure 5). Patients with KL grades ≥ 2 had a 37.6±18.8 AOS score, whereas those with KL<2 scored 21.4±20. However, these differences were not statistically significant (p = 0.12).
Discussion
The purpose of this study was to utilize objective CT-based fracture severity assessments to investigate the relationship between acute fracture severity and the subsequent onset of PTOA, in a series of tibial plafond fracture patients treated with the same surgical techniques. While all articular fractures are susceptible to PTOA, the plafond fracture of the distal tibia is an especially useful investigative clinical model. The ankle rarely develops primary OA, whereas PTOA very often develops within just a few years following tibial plafond fracture. With its high propensity for PTOA, the fractures of the tibial plafond provide a useful model to identify factors that predispose the joint to degeneration. However, since the pathomechanical etiology of PTOA shares common mechanisms across different load-bearing joints, the CT-based methodology utilized is in principle also suitable for other severe articular fractures, such as those of the tibial plateau or the acetabulum.
Anderson et al. 2008[4] introduced new objective CT-based techniques for quantifying acute fracture severity. As an intermediate indicator of their validity, CT-based metrics were shown to agree well with the subjective opinion of experienced orthopaedic trauma surgeons. Although such findings supported using CT-based metrics for fracture severity assessment, the present study advances the methodologies, based on new combined severity indices, that correlate with measures of patient outcome. These data provide evidence that articular comminution and fracture energy are strong predictors of subsequent PTOA. A linear regression model derived from these two metrics explained 70% of the variability in PTOA severity (KL grade) and had 88% discrimination accuracy (concordance). Fracture severity not only predicted the KL grade, but it was also able to modestly predict patient function after 2 years. With a 68% probability, fracture severity correctly discriminated between subjects with different AOS scores.
These CT-based biomechanical assessments have important potential applications to orthopaedic trauma research. In the future, this technique could make it possible to compare the results of different treatments of articular fractures after objectively measuring fracture severity, to assess differences or similarities between treatment groups at baseline. Similarly, multi-center pooling of datasets stratified for injury severity could enable the study of large numbers of articular fractures, grouped in ways not previously possible.
The existence of a measurable threshold of injury severity above which PTOA is highly likely could have implications for treating severe articular fractures. For instance, in fractured joints identified as being most highly at risk to develop PTOA, surgical interventions might concentrate on early reconstruction, including joint fusion. By contrast, in cases that are near the severity threshold, meticulously anatomic reduction to avoid additional deleterious mechanical forces would be more appropriate. A severity threshold might also be used as an indicator for the use of new biologic or pharmaceutical agents aimed at forestalling PTOA. For these potential applications to be realized, further translational research will be required to identify the relative mechanical effects of acute fracture severity (as measured in this study) and chronic increased contact stress arising from imperfect articular reduction (which was not accounted for in the data presented).
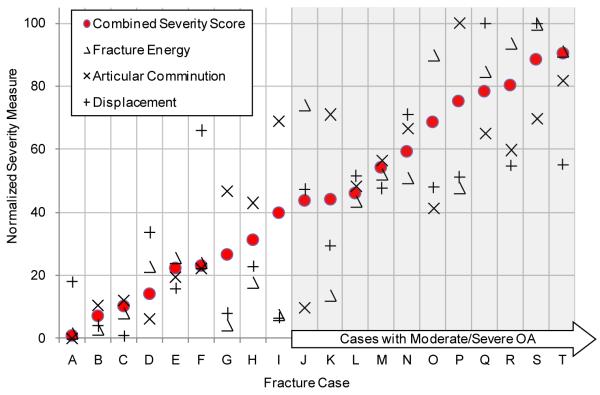
The CT-based methodology suggests that a fracture severity threshold does indeed exist. Figure 6 shows the cases studied, ordered according to the combined acute fracture severity measure. These data support the existence of a severity threshold (apparently, somewhere in the vicinity of 40) above which joint degeneration is likely. While the presently limited number of cases (n=20) provides only modest statistical power for acceptance vs. rejection of the hypothesis of threshold existence, this relationship merits further investigation.
Figure 6.
CT-based measures of injury severity are shown for each patient. Cases are ordered according to their combined severity score. Moderate to severe OA developed in patients with a combined severity score greater than 40, suggesting the existence of a severity threshold.
This study did not attempt to control for other important variables that might affect patient outcome. For instance, residual articular displacement is another important mechanical determinant of subsequent PTOA. It has previously been shown that the severity of articular injury and the quality of articular reduction are closely linked [11]. It is therefore possible that abnormal contact loading was another pathomechanical pathway leading to PTOA in the more severely injured cases in our study. Members of our research group have reported an association between increased contact loading assessed on finite element models and cartilage thinning in some of these same cases, further establishing this link (Van Hofwegen et al.; AAOS 2009). It is of course unknown whether different rates of PTOA would have resulted with different treatment techniques and/or different fracture reductions. However, objective measurements of injury severity have the potential to allow these questions to be addressed in future studies.
Despite the statistical relationships established in this study, limitations of the measurement techniques need to be acknowledged. The dynamic and complex nature of high-energy fractures complicates objective CT-based measurements of injury severity. Many patient and surgical variables are either difficult or simply intractable to measure. For example, biologic and anatomic variability certainly affects bone’s fracture behavior. However, much (albeit not all) of this variability was accounted for with patient-specific bone density estimations. In addition, the present metrics assume that no bone volume was lost during fracture. While certain fracture conditions may produce bone impaction, our experience has been that healthy bone largely conserves volume during high-energy, rapid loading events like those producing tibial plafond fractures. This assumption is computationally supported, as there was less than a 5% volumetric difference between the fractured and intact bones in this patient series. The fragment displacement/dispersal metric is also subject to variability, based on the accuracy to which the limb is realigned at the time of the index CT scan. Ideally, one would want to have that measurement be made at the instant of maximal displacement, but obviously this is not feasible. The articular comminution metric is an approximate measure for disruption, since it assumes that the articular fragments are all within close proximity to the joint, even though this may not be valid in some instances. Although not observed in this particular patient series, articular fragments can sometimes (especially in very high-energy trauma) be displaced away from the joint region and therefore not captured by this metric. This latter limitation is being addressed with the development of an algorithm that can distinguish articular from non-articular bone, enabling derivation of a metric to focus on articular disruption, regardless of the fragments’ displacement or geometry. In addition, post-surgical mal-alignments would certainly influence a patient’s outcome. However, as there were no cases in this series with gross mal-alignment, so this was therefore not specifically measured and accounted for in this study. Finally, the present average of 8-10 hours of analyst time required to quantify injury severity poses a significant barrier to implementing these techniques clinically. Even though much of the algorithm is automated, certain operations (e.g., bone boundary segmentation) need greater automation, to further reduce user interaction and total processing time.
Although intuitively plausible, biomechanical measurement of injury characteristics such as fracture energy, peri-articular comminution, and fragment displacement / dispersal has become practical only in the present era of high computer power and advanced image processing capabilities. With further programming work – grounded in continued surveillance of clinical outcomes - the quantitative biomechanical assessment of injury characteristics offers new possibilities for contributing to improved clinical research, to more accurate assessment of patient prognosis, and to better injury management decisions. A better (i.e., quantitative) basis for assessing the likelihood that a patient is destined to progress to PTOA could better inform orthopaedic decision-making. For emergency management personnel, for surgeons-in-training, and for established surgeons who have limited experience in managing these difficult injuries, meaningful quantitative measurements of key characteristics of individual injuries could facilitate more effective consultation with experts, both in person and electronically. Presently, these pragmatic clinical applications still lie ahead, because these assessment methods are still too unwieldy for use in the routine clinical setting. Additional streamlining is therefore underway. Finally, for purposes of research design in laboratory or translational settings, it should become possible to focus attention more directly upon the specific characteristics of injury which are truly most important for patient outcome, as opposed to diverting effort to less influential co-variates. These and other attractions provide strong impetus for ongoing work to further develop this novel biomechanical paradigm
Acknowledgments
Funding in support of this project was received from the NIH (AR46601, AR48939 and AR55533), the Arthritis Foundation, the Orthopaedic Trauma Association, and the AO Foundation, Switzerland.
Footnotes
Presentation of Material: This study was presented in part at the Annual Meeting of the Orthopaedic Trauma Association, Denver, CO, 2008.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dirschl DR, Adams GL. A critical assessment of factors influencing reliability in the classification of fractures, using fractures of the tibial plafond as a model. J Orthop Trauma. 1997;11(7):471–476. doi: 10.1097/00005131-199710000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Humphrey CA, Dirschl DR, Ellis TJ. Interobserver reliability of a CT-based fracture classification system. J Orthop Trauma. 2005;19(9):616–22. doi: 10.1097/01.bot.0000177107.30837.61. [DOI] [PubMed] [Google Scholar]
- 3.Swiontkowski MF, Sands AK, Agel J, et al. Interobserver variation in the AO/OTA fracture classification system for pilon fractures: Is there a problem. J Orthop Trauma. 1997;11(7):467–70. doi: 10.1097/00005131-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Anderson DD, Mosqueda TV, Thomas TP, et al. Quantifying tibial plafond fracture severity: Absorbed energy and fragment displacement agree with clinical rank ordering. Journal of Orthopaedic Research. 2008;26(8):1046–52. doi: 10.1002/jor.20550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Domsic RT, Saltzman CL. Ankle osteoarthritis scale. Foot & ankle international. 1998;19(7):466–471. doi: 10.1177/107110079801900708. [DOI] [PubMed] [Google Scholar]
- 7.Beardsley CL, Bertsch CR, Marsh JL, et al. Interfragmentary surface area as an index of comminution energy: proof of concept in a bone fracture surrogate. Journal of Biomechanics. 2002;35(3):331–338. doi: 10.1016/s0021-9290(01)00214-7. [DOI] [PubMed] [Google Scholar]
- 8.Beardsley CL, Anderson DD, Marsh JL, et al. Interfragmentary surface area as an index of comminution severity in cortical bone impact. J Orthop Res. 2005;23(3):686–90. doi: 10.1016/j.orthres.2004.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson LJ. Biomechanics of cellular solids. J Biomech. 2005;38(3):377–99. doi: 10.1016/j.jbiomech.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 10.Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. Springer; Verlag NY: 2001. [Google Scholar]
- 11.Williams TM, Nepola JV, DeCoster TA, et al. Factors affecting outcome in tibial plafond fractures. Clin Orthop Relat Res. 2004;423:93–8. doi: 10.1097/01.blo.0000127922.90382.f4. [DOI] [PubMed] [Google Scholar]