Abstract
Although much attention has been devoted to resveratrol, a unique polyphenol produced by plants throughout the world and credited as potentially being responsible for the so-called “French paradox” given its broad spectrum activity, the hundreds of oligomeric materials derived from it have been largely ignored despite their similarly high biochemical potential. Challenges in achieving their isolation in quantity from natural sources, coupled with an inability to rationally prepare them in the laboratory, are the main culprits. Here we show that a programmable, controlled, and potentially scaleable synthesis of the resveratrol family is possible through a unique three-stage design. These efforts required novel tactics coupled with strategy- and reagent-guided functionalizations to differentiate two distinct cores possessing multiple sites with the same and/or similar reactivity, ultimately leading to five higher-order natural products. We anticipate that this work 1) demonstrates that challenging, positionally-selective functionalizations of complex materials are possible where biosynthetic studies have indicated otherwise, 2) provides materials and tools to finally unlock the full biochemical potential of the family, particularly from the standpoint of activity and drug-property optimization, and 3) affords an intellectual framework to potentially access other oligomeric families controllably.
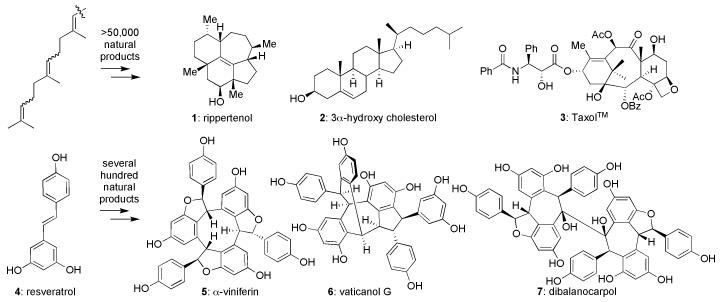
Resveratrol is to oligostilbenes what isoprene is to terpenes. Whether through highly evolved enzymatic pathways, diversification opportunities through uncontrolled reactions, or a combination thereof, these starting points lead to hundreds of unique frameworks (such as 1–3 and 5–7, Figure 1) with wide-ranging biological properties relevant to the treatment of cancer, AIDS, and bacterial infections, among other diseases.1-4 From the standpoint of laboratory synthesis, though strategies, tactics, and logic exist to controllably access most terpenes and thereby facilitate full biochemical and pharmaceutical explorations,3 the same cannot be said of resveratrol-derived oligostilbenes.5,6
Figure 1. The diversity of selected terpene and polyphenolic oligostilbene natural products: products of privileged starting materials.
Me = methyl, Ph = phenyl, Ac = acetate, Bz = benzoate.
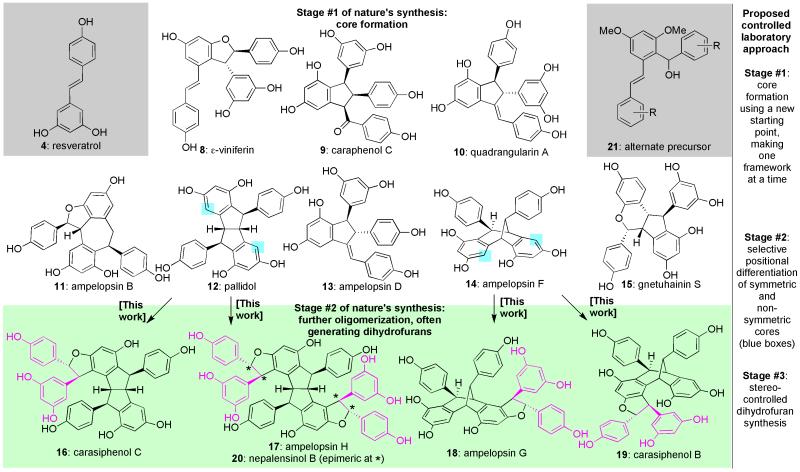
These unique materials are produced by plants throughout the world as phytoalexins, serving as a first line of chemical defense against stresses such as fungal infections. That role may derive from the fact that these molecules, including 8–20 (Figure 2), can be formed readily from resveratrol (4) through its self-merger anywhere between two, and upwards of ten, times through numerous distinct C-C and C-O bond constructions. Indeed, to put that bond-forming diversity into perspective, most other similarly-produced oligomer families have just one or two specific combination modes.6 Although the biogenesis of this family remains an open question, a number of studies4,7,8 point to a reasonable hypothesis based on two main phases of construction, stages that could be viewed as similar to the cyclase and oxidase paradigm of terpene biosynthesis.2,3 In the first, radical and/or cation-based reaction cascades convert resveratrol (4) into multiple dimeric frameworks, including 8–15. These cores may result either through direct dimerization of 4 or rearrangement reactions of a given dimer (especially ε-viniferin, 8) into additional frameworks under appropriate conditions to create an initial base of architectural and stereochemical diversity.7,8 Whether enzymes are involved in these processes is unknown, though the participation of some chiral entity at some stage seems reasonable given that many of these materials are isolated in an optically active form.
Figure 2. The challenge of the resveratrol family in context: Nature’s putative biogenesis and a specific plan for achieving their controlled assembly.
Critical for the controlled synthesis of dimeric natural products in the class proved to be a unique starting material (21) well removed from resveratrol itself. From these cores, regioselective functionalizations and a dihydrofuran formation protocol with divergent stereocontrol were then needed. These final challenges are the subject of this work.
From these staging areas, further oligomerization can occur in a second synthesis stage, with one seemingly prevalent mode being the addition of new resveratrol units to generate dihydrofuran rings. As indicated by the representative structures shown for compounds 16–209-13 derived from the pallidol (12) and ampelopsin F (14) cores, these new ring systems are added with inconsistent regio- and stereospecificity. Though it is unknown the degree to which a given plant species can dictate the synthetic distribution of these variants (and again whether enzymes are even involved in such processes), from a global perspective the possibility of concurrently garnering such diversity is likely advantageous since it affords a broader range of architectures and potential biochemical properties. Indeed, initial screens have shown that activity is correlated with both size and stereochemistry. For example, while resveratrol (4) possesses broad spectrum activity in murine models (and is implicated in the “French paradox” in humans),14,15 the addition of increasing numbers of resveratrol units affords compounds with greater specificity and potency.16,17,18 Similarly, while ampelopsin H (17) and nepalensinol B (20) have the same cores, their stereochemical differences lead to different mechanisms of anti-cancer activity.19,20
Given this general mode and purpose of synthesis, these materials are ideal compound collections for biochemical screening if they can be obtained in quantity. Unfortunately, achieving their isolation from natural sources even in minute amounts is challenging and labor intensive as is modulating their multiple reactive groups in a laboratory flask. Indeed, biosynthetic explorations based on exposing resveratrol (4) or varied protected forms to a single chemical or oxidase usually lead to complex product mixtures since they cannot differentiate, at will, between the dozens of reaction pathways available as would be required to prepare a single structure.21-26 In fact, of those materials that can be isolated and characterized from such studies, most (and sometimes all) are non-natural, while those of natural origin encompass only a small percentage of the total family. The same outcome occurs if higher-order materials are exposed to resveratrol (4) under similar conditions.27 By contrast, wholly stepwise, non-biomimetic sequences empowered by retrosynthetic analysis can produce single dimeric members with control, but do not possess the strategic power to deliver a family-level solution for all frameworks.5,6,28,29,30,31 Herein, we establish an approach with the potential to lead to the entire resveratrol collection controllably, one member at a time.
Programmable Synthesis Design
As indicated within the box on the right-hand side of Figure 2, we anticipated that the clearly diverse, complicated, and potentially chaotic bond constructions of nature’s resveratrol oligomer synthesis could be regulated in a laboratory flask if three separate goals proved achievable. The first, the rational, systematic, and controlled synthesis of the various dimeric cores, was accomplished through the use of 21 as an alternate starting point. Indeed, we have already converted this material into structures 10–14, a reduced form of 8 and analogues of 9, as well as several additional unique dimeric natural products in both published32,33 and unpublished work; some of these syntheses have proceeded on gram-scale. The second and third objectives are the subject of this study, tasks for which we initially targeted compounds 16–19 to address the challenges anticipated in adding resveratrol units controllably to any core. This process required 1) the means to differentiate the highlighted positions within symmetric and non-symmetric frameworks 12 and 14 not only from all the other non-highlighted sites, but also from each other,34,35 and 2) a robust sequence that could potentially advance a given positionally-differentiated material into either trans-dihydrofuran diastereoisomer. While both goals are easily stated, they were expected to be quite challenging, the first in particular since it requires differentiation that nature may not deploy (or can perform only with enzymes) and that biomimetic approaches have not achieved.
Differentiation of the Pallidol Core
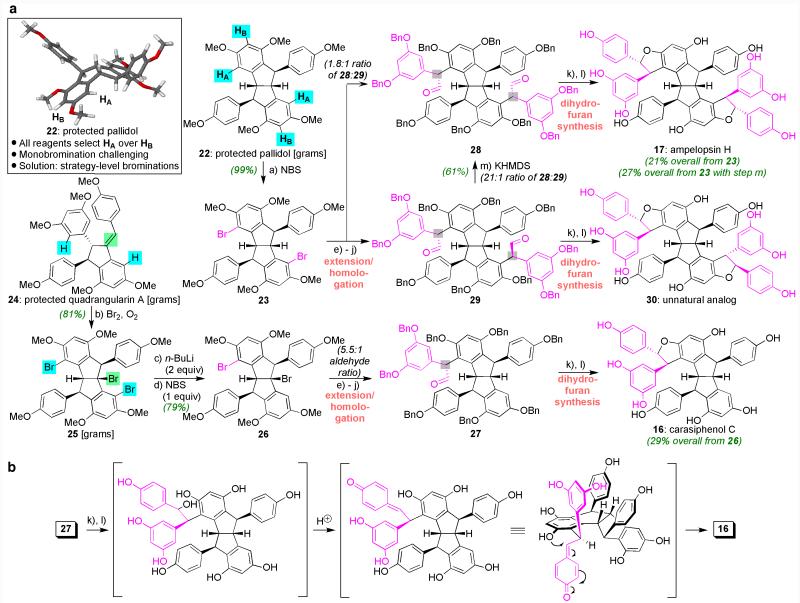
We began our studies by attempting to selectively functionalize the symmetric, protected pallidol core (22, Figure 3) using electrophilic aromatic substitution (EAS) with bromine as our preferred tool. To ultimately prepare the dihydrofurans relevant to the resveratrol class, this task required selective functionalization of HA over HB, with HA needing to be replaced selectively both one and two times. Although theoretical calculations [performed with the B3LYP functional in conjunction with the 6-31G(d) basis set] indicated that the atoms labeled as HA and HB were the most likely to be replaced among all those on the framework, electron density maps and electrostatic potentials were equivalent within error for HA versus HB. Based on inspection of various three-dimensional models, such as the one shown in the inset box, HB appeared to be the more accessible of the two due to the proximal nature of the hydrogens on the aliphatic core relative to HA. Based on experiment, however, HA proved to be the natural site of EAS with a synthesis of dibromide 23 achieved in 99% yield using 2 equivalents of NBS. Despite this selectivity, it proved difficult to obtain the corresponding monobromide in even 50% yield since the resultant material (not shown) readily underwent additional halogenation (generating 23) even when substoichiometric amounts of electrophilic bromine were employed. Thus, an alternate strategy was needed to desymmetrize compound 22 since the goal appeared outside the power of reagent control. A compound obtained previously (25, formed from 24 through three consecutive regiospecific brominations and a Friedel–Crafts cyclization)32 provided such a solution in that it was non-symmetric by virtue of its aliphatic bromine atom. Consequently, through initial removal of its aryl bromides followed by a site-selective rebromination as controlled by the aliphatic bromide, we formed compound 26 in 79% yield as an effective surrogate of the originally targeted monobrominated congener of 22.
Figure 3. A: Development of a strategy-level approach for selective brominations of the symmetric pallidol core followed by diastereocontrolled dihydrofuran formation to synthesize 16, 17, and 30. B: Critical details of the final dihydrofuran formation cascade.
Reagents and conditions: a) NBS, THF, −78 °C; b) Br2, CH2Cl2, −78→25 ° C; c) n-BuLi, THF, −78 °C; d) NBS, THF, −78→2 5 ° C; e) n-BuLi, 3,5-dimethoxybenzaldehyde, THF, −78→25 °C; f) Dess-Martin periodinane, NaHCO3, CH2Cl2, 25 °C, g) BBr3, CH2Cl2, 70 °C; h) BnBr, n-Bu4NI, K2CO3, acetone, 70 °C; i) n-BuLi, Me3SI, THF, 0 °C; j) ZnI2, benzene, 25 °C; k) 4-benzyloxyphenylmagnesium bromide, THF, 25 °C; l) H2, 30% Pd/C, EtOAc/MeOH (1:1), 25 °C, then Amberlite IR-12-OH, 25 °C; m) KHMDS, −78 °C. NBS = N-bromosuccinimide, THF = tetrahydrofuran, n-BuLi = n-butyllithium, Bn = benzyl, KHDMS = potassium (bis)hexamethyldisilazide.
With this critical stage of selective functionalizations complete, we turned next to dihydrofuran formation. Although many methods exist to forge such rings from aryl bromides,32 our efforts revealed that these approaches do not proceed on the highly electron-rich and sterically-encumbered frameworks of the resveratrol class. Nevertheless, we were able to identify a reliable and novel solution to these challenges. These operations began (Figure 3A) through the initial incorporation of additional 3,5-dimethoxyphenyl ring systems and a protecting group exchange to afford diaryl ketones (see Supporting Information section for structures); the exchange in phenol protecting groups was made at this stage to ensure that mild deprotection conditions could be deployed with sensitive and highly polar late-stage intermediates. These steps were then followed by a Corey–Chaykovski epoxidation and Lewis acid-induced rearrangement (ZnI2 or BF3•OEt2) to generate aldehydes 27–29 from 26 and 23, respectively. While these operations collectively are seemingly conventional, they were designed to both respond to, and take advantage of, the uniqueness of the resveratrol system while also ensuring effective diastereocontrol. Indeed, because of the electron-wealth of the neighboring aromatic rings, the ketone precursors to 27–29 could not be engaged by aryl-containing organometallic, Wittig, or diazo-forming reagents; they could, however, react with a sulfonium-ylide, and once converted into aldehydes, that adjoining electron-wealth proved invaluable in that it likely prevented 27–29 from epimerizing readily as often occurs with such materials. For example, in the case of 29 only exposure to strong base (KHMDS) in THF altered that stereochemistry, with this moderately yielding reaction indicating the inherent reprotonation bias of this system.
Thus, with the ability to set these initial stereocenters, the stereodetermining portion of the sequence was complete given that the remaining operations (Grignard addition and a one-pot deprotection/cyclization sequence through the specific intermediates shown in Figure 3B for 16) afforded the desired trans-disposed dihydrofuran rings of carasiphenol C (16), ampelopsin H (17), and non-natural analog 30. Crude 1H NMR spectra revealed that these materials were generated with >15:1 preference over any materials, with the very minor, unassigned products potentially being cis-dihydrofurans based on comparison to published NMR coupling constant values. We attribute the overall high level of trans-selectivity to a desire to minimize strain as shown in the precyclization intermediate in Figure 3B, though we cannot rule out the possibility of equilibration of any cis-disposed products into the likely more thermodynamically-stable trans-materials under the acidic conditions used.37 Finally, we note that while we have not explicitly attempted a large scale synthesis of any of these final natural products (most reactions were performed with fewer than 100 mg of material for the sake of convenience, though the dimeric cores are available in gram quantities), we did advance enough material to obtain a 55 mg isolate from the final deprotection/cyclization sequence containing carasiphenol C (16) with at least 90% purity (based on 1H and 13C NMR analysis). To put that amount of synthetic material in context, only 10 mg of 16 was obtained from 70 kg of dried plant material in the original isolation effort.9
Differentiation of the Ampelopsin F Core
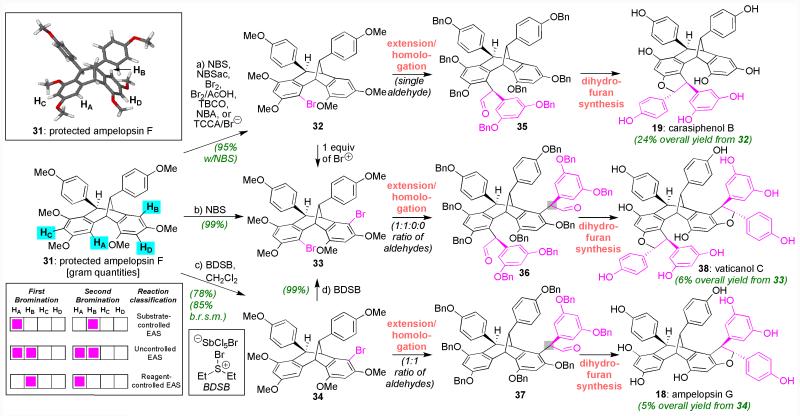
To assess whether these two final stages of synthesis had family-level applicability, we decided next to target natural products 18, 19, and 38 (Figure 4) starting from the non-symmetric ampelopsin F core (in form 31). Synthesizing these compounds was viewed as an even higher challenge in positional selectivity as it necessitated the site-selective replacement of positions HA and HB in either order among four highly electron-rich positions. Indeed, physical models revealed no obvious differences between these sites with calculations at the same level of theory denoted above indicating that all four hydrogens denoted within 31 were equally likely to be replaced. Experimentation soon revealed, however, that position HA could be readily functionalized with a variety of bromine sources (including acidic conditions), affording 32 in varied, but usually high, yield (95% with slightly less than 1 equivalent of NBS); addition of an extra equivalent of reagent then smoothly provided dibromide 33 (in 99% yield using NBS). Given the consistency of this outcome with reagents of varying size and reactivity, this functionalization pattern (HA then HB) likely reflects substrate control. Thus, accessing 34 (i.e. HB first) required reagent control, a result we felt was potentially achievable given that the use of a collidine/Br2 complex afforded 34 along with roughly equal amounts of 32 in an uncontrolled EAS reaction. After much experimentation, we found that a bromonium source we recently developed, Et2SBrβSbCl5Br (BDSB)38, was uniquely able to afford 34 cleanly in CH2Cl2; the yield for this process was 78% (85% based on recovered starting material) when we performed the reaction on >0.5 g scale. Only a small amount of 33 was formed from this process, with no 32 discernible by 1H NMR analysis of the crude reaction mixture. To the best of our knowledge, this reaction constitutes the most complex, positionally-selective reagent-based bromination described to date, complimenting other catalyst-controlled processes.39 We are currently investigating the basis for this selectivity, an issue of import since it may point to BDSB’s potential to provide other uniquely functionalized aromatic building blocks.
Figure 4. Use of substrate- and reagent-guided halogenations to synthesize three resveratrol trimers and tetramers (18, 19, and 38) from protected ampelopsin F (31).
Reagents and conditions: a) NBS, CH2Cl2, −78→25 °C; b) NBS, CH2Cl2, −78→25 °C; c) BDSB, CH2Cl2, −78 °C; d) BDSB, CH2Cl2, −78 °C. AcOH = acetic acid, NBSac = N-bromosaccharin, TBCO = tetrabromocyclohexadienone, NBA = N-bromoacetamide, TCCA = trichloroisocyanuric acid, BDSB = bromodiethylsulfide bromopentachloroantimonate.
Pleasingly, application of the same dihydrofuran formation sequence with these bromides led to diastereocontrolled syntheses of ampelopsin G (18), carasiphenol B (19), and vaticanol C (38), with the final of these compounds being an exciting lead for cancer treatment and prophylaxis.17 We found that high control in aldehyde stereochemistry was always obtained at position HA (such as for 35), but was consistently a 1:1 mixture at position HB as highlighted in 36 and 37, suggesting different inherent biases of the system. Strategically, however, it is important to note that we were also able to successfully advance the undesired aldehyde epimer from the ampelopsin G sequence into the diastereomeric dihydrofuran, synthesizing what is, at present, a non-natural analog (see Supporting Information). Thus, though we have not delineated a specific solution to forging these epimeric aldehydes controllably (since natural products were the specific goal of this study), we believe the robustness of the terminating sequence provides a family-level solution for the clean preparation of any trans-dihydrofuran dependent only on initially controlling aldehyde stereochemistry. As such, the potential exists to access resveratrol-based diversity and biochemical reactivity that has not yet been isolated from nature.
Conclusions and discussions
This work demonstrates, for the first time, that the controlled synthesis of higher-order resveratrol oligomers (and potentially the entire class) can be achieved. Salient features of the design are its reliance on a unique starting point coupled with the need for challenging functionalizations on diverse and complex cores. Indeed, only through the introduction of a novel reagent (BDSB) was appropriate positional control achieved on one framework, while a strategic solution was required on the other. As such, these complex and positionally-selective approaches indicate that the same and/or similar functionalities can be differentiated even on highly complex frameworks, countering the notion that such processes could not be achieved based on previous biosynthetic explorations. Moreover, the developed sequences provide opportunities to optimize biological activity and alter physicochemical properties by making analogs otherwise inaccessible from resveratrol alone.
To put the developed approach for the resveratrol class in strategic context, it reflects a hybrid of retrosynthetic analysis40 and divergent/diversity-oriented synthesis,41,42 a combination that could be argued as obvious in terms of the bond connections desired, but non-obvious in terms of the tools needed to execute those reactions (particularly positionally-selective transforms). Whether or not this overall strategy can be extended to the synthesis of other oligomer families remains to be established. What is certain is the evaluation of that question will offer many opportunities for reagent and reaction discovery, particularly as more complex brominative functionalizations are evaluated with natural products, compounds pertinent to materials research, and building blocks needed for pharmaceuticals.
Methods Summary
All reactions were carried out under an argon atmosphere with dry solvents under anhydrous conditions; dry tetrahydrofuran, toluene, benzene, diethyl ether, and dichloromethane were obtained by passing commercially available pre-dried, oxygen-free formulations through activated alumina columns. Yields refer to chromatographically and spectroscopically (1H and 13C NMR) homogeneous materials, unless otherwise stated. Reagents were purchased at the highest commercial quality and used without further purification, unless otherwise stated. Reactions were magnetically stirred and monitored by thin-layer chromatography. For full experimental details, including procedures for all reactions and characterization of all new compounds (1H NMR, 13C NMR, mass spectrometry, infrared, Rf value), see Supplementary Information.
Supplementary Material
Acknowledgments
We thank Dr. George Sukenick of The Memorial Sloan Kettering Cancer Research Institute and Dr. John Decatur of Columbia University for NMR assistance, Dr. Yasuhiro Itagaki for mass spectrometric assistance, Mr. Adel ElSohly for theoretical calculations and helpful discussions, and Ms. Katharina Shaw and Mr. Jonathan Boyce for preparing some starting materials Financial support was provided by Columbia University, the National Institutes of Health (R01-GM84994), Bristol-Myers Squibb, Eli Lilly, the Research Corporation for Science Advancement (Cottrell Scholar Award to S.A.S.), and the Austrian Science Fund (FWF, Schrödinger postdoctoral fellowship J2986-N19 to A.G.).
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
References
- 1.Fischbach MA, Clardy J. One pathway, many products. Nature: Chem. Biol. 2007;3:353–355. doi: 10.1038/nchembio0707-353. [DOI] [PubMed] [Google Scholar]
- 2.Christianson DW. Structural biology and chemistry of the terpenoid cyclases. Chem. Rev. 2006;106:3412–3442. doi: 10.1021/cr050286w. [DOI] [PubMed] [Google Scholar]
- 3.Chen K, Baran PS. Total synthesis of eudesmane terpenes by site-selective C–H oxidations. Nature. 2009;459:824–828. doi: 10.1038/nature08043. [DOI] [PubMed] [Google Scholar]
- 4.Sotheeswaran S, Pasupathy V. Distribution of resveratrol oligomers in plants. Phytochemistry. 1993;32:1083–1092. [Google Scholar]
- 5.Quideau S, Deffieux D, Douat-Casassus C, Pouységu L. Plant polyphenols: chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011;50:586–621. doi: 10.1002/anie.201000044. [DOI] [PubMed] [Google Scholar]
- 6.Snyder SA, ElSohly AM, Kontes F. Synthetic approaches to oligomeric natural products. Nat. Prod. Rep. 2011;28:897–924. doi: 10.1039/c1np00001b. [DOI] [PubMed] [Google Scholar]
- 7.Takaya Y, Yan K-X, Terashima K, Ito J, Niwa M. Chemical determination of the absolute structures of resveratrol dimers, ampelopsins A, B, D and F. Tetrahedron. 2002;58:7259–7265. [Google Scholar]
- 8.Takaya Y, Yan K-X, Terashima K, He Y-H, Niwa M. Biogenetic reactions on stilbenetetramers from Vitaceaeous plants. Tetrahedron. 2002;58:9265–9271. [Google Scholar]
- 9.Wang S, Ma D, Hu C. Three new compounds from the aerial parts of Caragana sinica. Helvetica Chimica Acta. 2005;88:2315–2321. [Google Scholar]
- 10.Tanaka T, et al. Six new heterocyclic stilbene oligomers from stem bark of Shore hemsleyana. Heterocycles. 2001;55:729–740. [Google Scholar]
- 11.Wang S, Ma D, Hu C. Two new oligostilbenes from Caragana sinica. J. Asian Nat, Prod. Res. 2004;6:241–248. doi: 10.1080/10286020310001653309. [DOI] [PubMed] [Google Scholar]
- 12.Oshima Y, Ueno Y, Hisamichi K, Takeshita M. Ampelopsins F and G, novel bridged plant oligostilbenes from Ampelopsis brevipedunculata var. hancei roots (vitaceae) Tetrahedron. 1993;49:5801–5804. [Google Scholar]
- 13.Tanaka T, Ito T, Nakaya K, Iinuma M, Riswan S. Oligostilbenoids in stem bark in Vatica rassak. Phytochemistry. 2000;54:63–69. doi: 10.1016/s0031-9422(00)00026-1. [DOI] [PubMed] [Google Scholar]
- 14.Jang M, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 15.Milne JC, et al. Small molecules activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–717. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsukamoto T, et al. Vaticanol C, a resveratrol tetramer, activates PPARα and PPARβ/γ in vitro and in vivo. Nutrition & Metabolism. 7 doi: 10.1186/1743-7075-7-46. online only (2010), DOI: 10.1186/1743-7075-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito T, et al. Antitumor effect of resveratrol oligomers against human cancer cell lines and the molecular mechanism of apoptosis induced by vaticanol C. Carcinogenesis. 2003;24:1489–1497. doi: 10.1093/carcin/bgg105. [DOI] [PubMed] [Google Scholar]
- 18.Abe N, et al. Resveratrol oligomers from Vatica albiramis. J. Nat. Prod. 2010;73:1499–1506. doi: 10.1021/np1002675. [DOI] [PubMed] [Google Scholar]
- 19.Atun S, Aznam N, Arianingrum R, Takaya Y, Niwa M. Resveratrol derivatives from stem bark of Hopea and their biological activity test. Journal of Physical Science. 2008;19:7–21. [Google Scholar]
- 20.Yamada M, et al. Stilbenoids of Kobresia nepalensis (Cyperaceae) exhibiting DNA topoisomerase II inhibition. Phytochemistry. 2006;67:307–313. doi: 10.1016/j.phytochem.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Langcake P, Pryce RJ. Oxidative dimerisation of 4-hydroxystilbenes in vitro: production of a grapevine phytoalexin mimic. J. Chem. Soc., Chem. Commun. 1977:208–210. [Google Scholar]
- 22.Sako M, Hosokawa H, Ito T, Iinuma M. Regioselective oxidative coupling of 4-hydroxystilbenes: synthesis of resveratrol and ε-viniferin (E)-dehydrodimers. J. Org. Chem. 2004;69:2598–2600. doi: 10.1021/jo035791c. [DOI] [PubMed] [Google Scholar]
- 23.Li W, Li H, Hou Z. Total synthesis of (±)-quadrangularin A. Angew. Chem. Int. Ed. 2006;45:7609–7611. doi: 10.1002/anie.200603097. [DOI] [PubMed] [Google Scholar]
- 24.Li W, Li H, Luo Y, Yang Y, Wang N. Biosynthesis of resveratrol dimers by regioselective oxidative coupling reaction. Synlett. 2010:1247–1250. [Google Scholar]
- 25.Velu SS, et al. Regio- and stereoselective biomimetic synthesis of oligostilbenoid dimers from resveratrol analogues: influence of the solvent, oxidant, and substitution. Chem. Eur. J. 2008;14:11376–11384. doi: 10.1002/chem.200801575. [DOI] [PubMed] [Google Scholar]
- 26.Takaya Y, et al. Biomimetic transformation of resveratrol. Tetrahedron. 2005;61:10285–10290. [Google Scholar]
- 27.He Y-H, Takaya Y, Terashima K, Niwa M. Determination of absolute structure of (+)-davidiol A. Heterocycles. 2006;68:93–100. [Google Scholar]
- 28.Kim I, Choi J. A versatile approach to oligostilbenoid natural products - synthesis of permethylated analogues of viniferifuran, malibatol A, and shoreaphenol. Org. Biomol. Chem. 2009;7:2788–2795. doi: 10.1039/b901911a. [DOI] [PubMed] [Google Scholar]
- 29.Kraus GA, Gupta V. A new synthetic strategy for the synthesis of bioactive stilbene dimers. A direct synthesis of amurensin H. Tetrahedron Lett. 2009;50:7180–7183. [Google Scholar]
- 30.Jeffrey JL, Sarpong R. Concise synthesis of paucifloral F unsing a Larock annulation. Org. Lett. 2009;11:5450–5453. doi: 10.1021/ol902141z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicolaou KC, Kang Q, Wu TR, Lim CS, Chen DY-K. Total synthesis and biological evaluation of the resveratrol-derived polyphenol natural products hopeanol and hopeahainol A. J. Am. Chem. Soc. 2010;132:7540–7548. doi: 10.1021/ja102623j. [DOI] [PubMed] [Google Scholar]
- 32.Snyder SA, Zografos AL, Lin Y. Total synthesis of resveratrol-based natural products: a chemoselective approach. Angew. Chem. Int. Ed. 2007;46:8186–8191. doi: 10.1002/anie.200703333. [DOI] [PubMed] [Google Scholar]
- 33.Snyder SA, Breazzano SP, Ross AG, Lin Y, Zografos A. Total synthesis of diverse carbogenic complexity within the resveratrol class from a common building block. J. Am. Chem. Soc. 2009;131:1753–1765. doi: 10.1021/ja806183r. [DOI] [PubMed] [Google Scholar]
- 34.Sculimbrene BR, Morgan AJ, Miller SJ. Enantiodivergence in small-molecule catalysis of asymmetric phosphorylation: concise total syntheses of the enantiomeric d-myo-inositol-1-phosphate and d-myo-inositol-3-phosphate. J. Am. Chem. Soc. 2002;124:11653–11656. doi: 10.1021/ja027402m. [DOI] [PubMed] [Google Scholar]
- 35.Lewis CA, Miller S. Site-selective derivatization and remodeling of erythromycin A by using peptide-based chiral catalysts. Angew. Chem. Int. Ed. 2006;45:5616–5619. doi: 10.1002/anie.200601490. [DOI] [PubMed] [Google Scholar]
- 36.Betolini F, Pineschi M. Recent progress in the synthesis of 2,3-dihydrofurans. Org. Prep. Proceed. Int. 2009;41:385–418. [Google Scholar]
- 37.Kurosawa W, Kobayashi H, Kan T, Fukuyama T. Total synthesis of (−)- ephedradine A: an efficient construction of optically active dihydrobenzofuran-ring via C-H insertion reaction. Tetrahedron. 2004;60:9615–9628. [Google Scholar]
- 38.Snyder SA, Treitler DS, Brucks AP. Simple reagents for direct halonium-induced polyene cyclization. J. Am. Chem. Soc. 2010;132:14303–14314. doi: 10.1021/ja106813s. [DOI] [PubMed] [Google Scholar]
- 39.Gustafson J, Lim D, Miller SJ. Dynamic kinetic resolution of biaryl atropisomers via peptide-catalyzed asymmetric bromination. Science. 2010;328:1251–1255. doi: 10.1126/science.1188403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corey EJ, Cheng XM. The Logic of Chemical Synthesis. Wiley; 1995. [Google Scholar]
- 41.Boger DL, Brotherton CE. Total synthesis of azafluoranthene alkaloids: rufescine and imeluteine. J. Org. Chem. 1984;49:4050–4055. [Google Scholar]
- 42.Burke MD, Schreiber SL. A planning strategy for diversity-oriented synthesis. Angew. Chem. Int. Ed. 2004;43:46–58. doi: 10.1002/anie.200300626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.