Abstract
Introduction:
Previous studies have indicated that high sensation seekers are more sensitive to the reinforcing effects of nicotine, initiate smoking at an earlier age, and smoke greater amounts of cigarettes. This study examined the influence of sensation-seeking status on tobacco smoking following deprivation in regular tobacco users.
Methods:
Twenty healthy tobacco-smoking volunteers with low or high impulsive sensation-seeking subscale scores completed 2 consecutive test days per week for 3 consecutive weeks. Each week, a range of self-report, performance, and cardiovascular assessments were completed during ad libitum smoking on Day 1 and before and after the paced smoking of a tobacco cigarette containing 0.05, 0.6, or 0.9 mg of nicotine following 24 hr of tobacco deprivation on Day 2. In addition, self-administration behavior was analyzed during a 2-hr free access period after the initial tobacco administration.
Results:
In high sensation seekers, tobacco smoking independent of nicotine yield ameliorated deprivation effects, whereas amelioration of deprivation effects was dependent on nicotine yield among low sensation seekers. However, this effect was limited to a small subset of measures. Subsequent cigarette self-administration increased in a nicotine-dependent manner for high sensation seekers only.
Conclusions:
Compared with low sensation seekers, high sensation seekers were more sensitive to the withdrawal relieving effects of nonnicotine components of smoking following 24 hr of deprivation on selective measures and more sensitive to nicotine yield during subsequent tobacco self-administration. These results are consistent with studies suggesting that factors driving tobacco dependence may vary as a function of sensation-seeking status.
Introduction
Sensation seeking is defined as the seeking of varied, novel, complex, and intense sensations and experiences and the willingness to take physical, social, legal, and financial risks for the sake of such experience (Zuckerman, 1994). It is commonly reported that high sensation seekers initiate drug use at an earlier age, use greater amounts of drugs, are more likely to develop problems related to drug use, and are less likely to remain abstinent following drug treatment (reviewed in Zuckerman, 2007). Sensation-seeking scores among drug-naive adolescents predict sensitivity to the reinforcing effects of stimulant and sedative drugs as young adults (Kelly et al., 2009), suggesting that adolescents high in sensation seeking may be at greater vulnerability to repeated drug use. A growing body of literature also suggests that high sensation-seeking young adults are more sensitive to the reinforcing and other behavioral effects of a range of drugs including alcohol (Fillmore, Ostling, Martin, & Kelly, 2009; Magid, Maclean, & Colder, 2007), hallucinogens (Khavari, Mabry, & Humes, 1977), and stimulants (Bowling & Bardo, 1994; Kelly et al., 2006; Stoops et al., 2007). Tailoring prevention materials for high sensation seekers has been shown to increase intervention efficacy (e.g., Palmgreen, Donohew, Lorch, Hoyle, & Stephenson, 2001).
Several studies have examined the role of sensation seeking during various stages of nicotine/tobacco use. High sensation seekers are more likely to use tobacco during adolescence (Andrucci, Archer, Pancoast, & Gordon, 1989; Frankenberger, 2004). Perkins et al. (2000, 2008b) found that high scores on several personality dimensions related to sensation seeking (e.g., novelty seeking, experience seeking, disinhibition) were related to nicotine choice and increased scores on verbal reports of the reinforcing and aversive effects of an acute dose of nicotine among nonsmokers. However, individual differences in sensitivity to the behavioral effects of nicotine during acute nicotine exposure were limited to initial exposures, as this relationship was not replicated in regular smokers in this study. Among regular smokers, sensation seeking has been linked to a number of tobacco use variables, including craving response to smoking cues (Doran, Cook, McChargue, & Spring, 2009), magnitude of withdrawal effect following tobacco deprivation in ratings of negative affect and anhedonia (Carton, Le Houezec, Lagrue, & Jouvent, 2000; Leventhal et al., 2007), and higher tobacco relapse rates after a quit attempt (Kahler, Spillane, Metrik, Leventhal, & Monti, 2009), suggesting that sensation seeking plays a role in the initiation, escalation, and maintenance of tobacco use behavior.
Fewer studies have examined the role of sensation seeking as a predictor of nicotine dependence among regular smokers. Carton, Jouvent, and Widlocher (1994) found that after controlling for duration and frequency of smoking, subject-rated FTND scores correlated with experience seeking and disinhibition subscales of the Sensation-Seeking Scale in regular tobacco smokers. While consistent with a conclusion that high sensation-seeking chronic tobacco users may be more tobacco dependent than low sensation seekers, interpretation of these data must be tempered by the exclusive reliance on self-report measures, the absence of objective measures of tobacco deprivation, and a lack of experimental manipulation of the level of nicotine deprivation.
The purpose of this study was to examine the influence of sensation seeking on the effects of the nicotine yield of tobacco smoke (0.05, 0.6, and 0.9 mg) following tobacco deprivation across a range of self-administration, self-report, performance, and cardiovascular measures in a sample of regular tobacco users. We hypothesized that the deprivation period would engender nicotine deprivation effects on self-report, performance, and cardiovascular function and that tobacco cigarette smoking would ameliorate deprivation effects in a nicotine-yield dependent manner. Furthermore, based on previous reports of increased tobacco dependence among high sensation seekers, we hypothesized that high sensation seekers would show (a) greater effects of deprivation, (b) enhanced sensitivity to nicotine-yield and nonnicotine components of denicotinized cigarettes following tobacco deprivation, and (c) higher rates of cigarette smoking self-administration.
Methods
Participants
Twenty healthy adult tobacco-smoking volunteers (10 females, ages 18–38), recruited through advertisements placed on the University of Kentucky campus and in the local community, completed a six-session study that was approved by the University of Kentucky Medical Institutional Review Board. Volunteers responding to advertisements completed a brief telephone interview or an Internet-based questionnaire addressing general medical and legal status and the Impulsive Sensation-Seeking Scale of the Zuckerman–Kuhlman Personality Questionnaire (ZKPQ; Zuckerman, Kuhlman, Joireman, Teta, & Kraft, 1993). Those reporting good health and having Impulsive Sensation-Seeking Scale (IMP/SS) scores that fell in the upper (i.e., high sensation seekers: males ≥ 14, females ≥ 13) or lower (i.e., low sensation seekers: males ≤ 7, females ≤ 6) quartile of scores from a distribution of 2,969 college students (provided by M. Zuckerman, personal communication) were invited to participate in the study. The study sample size of 20 was chosen based on effect sizes from similar studies examining sensation-seeking group differences in the pharmacological effects of stimulant drugs (e.g., Kelly et al., 2006; Stoops et al., 2007).
During an orientation and medical screening day, volunteers completed a battery of medical and psychological questionnaires, including the Eysenck Personality Inventory (EPI; Eysenck & Eysenck, 1964), Beck Depression Inventory (Beck, Ward, Mendelson, Mock, & Erbaugh, 1961), locally developed Attention Deficit Hyperactivity Disorder (ADHD) and conduct disorder checklists, the Brief Symptom Inventory (Derogatis, 1993), and Form V of the Zuckerman Sensation-Seeking Scale (Zuckerman, Eysenck, & Eysenck, 1978), as well as blood chemistry, liver function, and urinalysis tests. Tobacco-smoking status was verified by assessing breath carbon monoxide (CO) levels. To be eligible to participate, subjects were required to have a CO level ≥ 12 ppm. Volunteers were excluded if they had a history of or current significant medical illness (e.g., cardiovascular disease, neurological or psychiatric disorder), excessive use of alcohol or caffeine, regular use of other drugs, pregnant or breastfeeding status, or any other condition that would increase risk for study participation. During a separate training session, participants practiced the study tasks until performance was consistent and accurate across consecutive trials. Twenty-two volunteers initiated the study; two participants dropped out for reasons unrelated to the study, and data from these two participants were not included in the final analysis.
Design
A double-blind, placebo-controlled, randomized design was used to examine the behavioral effects of nicotine yield (0.05 [low-yield nicotine cigarette was used as a “placebo” control], 0.6, and 0.9 mg) and time (pre- and postexperimental cigarette) in low and high sensation seekers following 24 hr of tobacco deprivation. A separate analysis of the effects of the 24-hr deprivation manipulation was also conducted.
Schedule
Each participant completed two consecutive test days per week for three consecutive weeks. At the start of test days, which occurred at the same time each day, participants answered open-ended questions regarding sleep, medication use, eating behavior, and health status during the preceding 24 hr and completed field sobriety, breath (Alcohol Sensor III, Intoximeters, Inc.; piCO Carbon Monoxide Monitor, Bedfont Scientific), and urine tests (cocaine, benzodiazepine, barbiturate, marijuana, amphetamine, and opiate drug use using OnTrak TesTstik, Varian, Inc.; pregnancy using Clearview HCG II, Unipath, Ltd). Thirty-minute sessions were completed on Day 1 following ad libitum smoking, on Day 2 following 24 hr of tobacco deprivation, as verified by breath (CO levels ≥ 10 ppm were required to complete Day 2 testing), and then repeated on Day 2 following paced smoking of eight puffs from one experimental cigarette. The paced smoking procedure was adapted from Kelly, Foltin, Rose, Fischman, and Brady (1990) and consisted of a 3-s preparation interval, a 3-s inhalation interval, and a 14-s exhale and rest period. This procedure was repeated eight times for each experimental cigarette. Following the postsmoking session, additional experimental cigarettes containing the nicotine yield administered that day could be self-administered for 2 hr. All cigarettes were smoked using a mouthpiece connected at the front and rear with PVC tubing to a volumetric transducer. The flow of air through the mouthpiece was measured to determine the duration and volume of each puff.
Upon completion of testing each week, subjects received $60. Upon successful completion of all three 2-day testing occasions, subjects received an additional $180 bonus plus task performance earnings. Total earnings were approximately $365; there were no group differences in earnings.
Drug
Commercially available cigarettes delivering 0.05 (Quest® Step 3), 0.6 (Quest® Step 1), and 0.9 mg (Kent®) of nicotine were prepared with black tape covering the brand name, so that all cigarettes looked the same, regardless of nicotine content. Doses (i.e., nicotine yield) were administered in a randomized order across the 3 weeks of the study.
Session Measures
During each session, self-report questionnaires (e.g., Foltin & Fischman, 1991), psychomotor and cognitive tasks (e.g., Roache, 1991), and cardiovascular measures were completed in the following order.
Wisconsin Smoking Withdrawal Scale
This 28-item questionnaire yields ratings on seven subscales: anger, anxiety, concentration, craving, hunger, sadness, and sleep on scales from 0 to 4. Each item was rated along a 5-point scale, from “Strongly disagree” to “Strongly agree.” This scale was used to assess the global effects of tobacco withdrawal (Welsch et al., 1999). Participants completed the Wisconsin Smoking Withdrawal Scale (WSWS) during the ad libitum session and during the pre-smoking session occurring following 24-hr deprivation.
Visual Analog Scale
Participants rated items (I feel stimulated, stressed, sedated, hungry, anxious, light-headed, thirsty, sleepy, sick to my stomach, down, high, drug effect, and I like the drug effect) presented individually on the computer by marking a 100-unit line anchored on the extremes by “Not at all” and “Extremely.”
Profile of Mood States
Participants completed an experimental version of the Profile of Mood States (POMS) (derived from McNair, Lorr, & Droppleman, 1971) consisting of 72 adjectives rated along a 5-point scale, from “not at all” to “extremely,” which yielded scores on 10 clusters: anxiety, depression, anger, vigor, fatigue, confusion, friendliness, elation, arousal, and total positive.
Addiction Research Center Inventory
The 49-item short form of the true–false inventory yielded information on five dimensions: lysergic acid diethylamide (LSD) Scale, Amphetamine (A) Scale, Benzedrine Group (BG) Scale, Morphine–Benzedrine Group (MBG) Scale, and the Pentobarbital, Chlorpromazine, Alcohol Group (PCAG) Scale (Martin, Sloan, Sapira, & Jasinski, 1971).
Minnesota Smoking Withdrawal Scale
Participants rated items associated with tobacco withdrawal (i.e., craving, difficulty concentrating, restlessness, headaches, drowsiness, and gastrointestinal symptoms) on a scale from 0 (not present), to 3 (severe intensity; Hughes & Hatsukami, 1986). This scale was used to assess the immediate effects of tobacco withdrawal and subsequent effects of smoking a cigarette.
Cardiovascular Assessment/Math Stress Task
Oscillometric systolic and diastolic blood pressure measures and heart rate were obtained (Sentry II, NBS Medical) before, during, and after completion of math addition problems. Subjects were paid 2 cents for each correct answer. The difficulty level of the addition problems (number of digits) and the duration of the time given to answer the problems were systematically manipulated based on participant performance accuracy in order to maintain a standard level of performance, regardless of math ability or experimental manipulations. As such, performance on this task was not analyzed.
Repeated Acquisition of Response Sequences
The acquisition phase required the subject to press four keys (1, 3, 7, and 9) on a numeric keypad to learn a new 10-response order (a “chain”). When the first correct key in the sequence was pressed, a “position” counter on the screen increased from 0 to 1. The position counter then increased by one each time the subject pressed the correct key in a given position in the sequence, but did not change if the subject pressed the incorrect key. If any key other than the correct key was pressed, a brief time out (blank screen) occurred. When the subject pressed the 10th and final key in the sequence, a “points” counter increased by one and the position counter was reset to 0, indicating that the first response in the order was again required. Subjects had 180 s to complete as many chains as possible. During the performance phase, the 10-response order remained the same across sessions and subjects had 60 s to complete as many chains as possible. The primary dependent measures for this task were the number of chains completed, the number of errors committed, and the percentage of correct responses. For the acquisition version of the task, the index of curvature for the number of errors committed was also calculated to determine how efficiently the 10-response order was acquired. Stimulant drug effects on repeated acquisition of response sequences (RA) task performance have been reported to vary as a function of sensation seeking status (Stoops et al., 2007).
Digit–Symbol Substitution Task
Participants completed a 1.5-min computerized version of the Digit–Symbol Substitution Task (DSST) adapted from McLeod, Griffiths, Bigelow, and Yingling (1982). Trial completion rate and accuracy was monitored on this psychomotor task.
Rapid Information Processing Task
Participants completed a 5-min computerized version of the rapid information processing task (RIP) (Fillmore et al., 2005). At the start of this task, single digits were presented in the center of the monitor at a rate of 90 digits/min, and subjects were instructed to press a key whenever three consecutive even or odd digits were presented. Each digit was displayed on the screen for 67 ms with an ISI of 600 ms. Following correct responses, the speed of digit presentation was increased, and following incorrect responses or missed signals, the speed of digit presentation was decreased. Information-processing capacity (working memory) was determined based on the rate of digit presentation and signal detection accuracy (proportion of hits).
Tobacco Self-Administration Smoking Topography Measures
Smoking topography measures during the 2-hr smoking period included number of cigarettes smoked, puffs per cigarette, and total puff volume and duration. Participants also completed the Cigarette Rating Questionnaire (CQ; Westman, Levin, & Rose, 1992), which assesses tobacco smoke characteristics, including: good taste, calmed, awake, reduced hunger, irritability, nauseated, dizzy, reduced craving, and throat/chest sensations on 100-unit Visual Analog Scale (VAS). The CQ was administered twice, once after the experimental cigarette session and again after ad libitum smoking.
Data Analysis
Sensation-seeking group differences in demographic variables and CO levels were analyzed using independent t-tests. A preliminary analysis of the 24-hr deprivation period was conducted using a three-way mixed-model analysis of variance (ANOVA) with sensation-seeking status as a between-subjects factor, and test week (1–3) and session (pre- and postdeprivation) as within-subject factors. The results of the experimental cigarette administration were analyzed using a two-way mixed-model analysis of covariance (ANCOVA) with the 24-hr deprivation baseline used as the covariate, sensation-seeking status as the between-subjects factor, and nicotine yield (0.05, 0.6, 0.9 mg) as the within-subject factor. Type I error was minimized by examining significant interactions using simple-effects models and examining main effects using the Tukey–Kramer adjusted differences of least-squared means. Ad libitum smoking topography was analyzed using a 2 × 3 (sensation seeking × nicotine yield) mixed-model ANOVA. Due to equipment malfunction, topography measures were not available for two participants. Since collapsing across symptoms and analyzing only a total score can obscure specific effects during assessment of tobacco craving and withdrawal, analyses of individual assessment items has been recommended (Hughes & Hatsukami, 1998; Shiffman, West, & Gilbert, 2004). As such, items from the Minnesota Smoking Withdrawal Scale (MNWS), WSWS, and VAS were analyzed individually. All results were considered significant at p ≤ .05. Analyses were conducted using SAS version 9.1.
Results
Demographics
Table 1 presents participant demographics. Tobacco use frequency and nicotine yield of preferred cigarettes were similar in both groups. No participant reported being a regular smoker of either brands of cigarettes used in the study (i.e., Quest or Kent brands). No drug use or pregnancy was detected during daily urinalysis testing. As expected, two-sample t-tests confirmed that ZKPQ scores were significantly greater for high sensation seekers. Groups were also significantly different in total scores on form V of the Zuckerman Sensation-Seeking Scale (p < .01), the impulsivity subscale of the Addiction Research Center Maturation Scale (p < .05), and extraversion on the EPI (p < .01), but did not differ on any of the other screening questionnaires examining symptoms of behavioral undercontrol (e.g., ADHD or conduct disorder), mood disorders, or psychiatric symptoms.
Table 1.
Participant Demographic Information and CO Levels Before and Following 24 Hr of Tobacco Deprivation
| Low SS |
High SS |
|||||
| Mean | SD | Range | Mean | SD | Range | |
| ZKPQ IMP/SS score | 5.0 | 1.2 | 3–7 | 15.8*** | 2.1 | 13–19 |
| Age | 25.1 | 7.0 | 18–38 | 21.0 | 2.5 | 18–24 |
| Height (inches) | 67.8 | 4.6 | 62–74 | 71.1 | 4.7 | 64–77 |
| Weight (lbs) | 165.6 | 41.9 | 110–260 | 190.5 | 43.2 | 115–250 |
| Education | 14.1 | 2.1 | 12–17 | 13.5 | 1.4 | 12–17 |
| Tobacco (cigarettes/day) | 15.5 | 6.9 | 10–30 | 15.0 | 4.1 | 10–20 |
| Nicotine yield (mg/cigarette) | 0.9 | 0.3 | 0.5–1.6 | 1.0 | 0.3 | 0.6–1.6 |
| Caffeine (mg/day) | 139.1 | 167.5 | 0–580 | 95.0 | 106.5 | 0–350 |
| Alcohol (drinks/week) | 8.1 | 6.7 | 0–23 | 6.9 | 7.5 | 0–20 |
| Marijuana (occasions/month) | 0.3 | 0.9 | 0–3 | 0.1 | 0.3 | 0–1 |
| CO level | ||||||
| 0-hr deprivation | 24.8 | 9.6 | 13–47 | 21.4 | 7.4 | 14–35 |
| 24-hr deprivation | 3.9### | 1.1 | 3–6 | 5.1### | 1.9 | 3–9 |
Note. Total sample size was an N of 20 (10 per group). CO levels varied as a function of deprivation in both groups; however, there were no significant group differences in CO levels. CO = carbon monoxide; IMP/SS = Impuslive-Sensation Seeking subscale; SS = Sensation Seeking group; and ZKPQ = Zuckerman-Kuhlman Personality Questionnaire.
***p<.001 (difference between Low and HIgh SS); ###p<.001 (difference between 0-hr and 24-hr deprivation).
Deprivation Effects
The 24-hr deprivation period was used to engender a consistent level of tobacco deprivation upon which to examine the effects of smoking and nicotine yield. Compliance with the deprivation intervention was verified by breath CO (Table 1). There were no significant group differences in breath CO levels during ad libitum smoking or after 24 hr of smoking deprivation, as determined during intake assessments on test days.
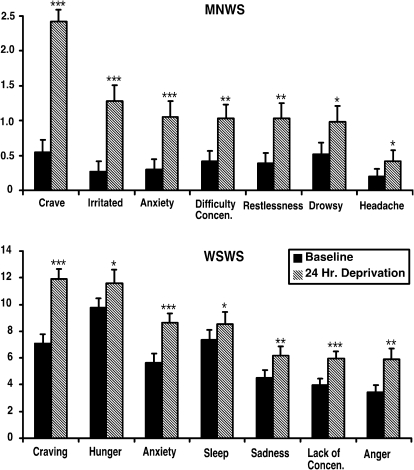
Figure 1 displays the results of the MNWS (Table 2) and the WSWS, which were used to examine the effects of 24 hr of smoking deprivation. As expected, significant increases on both measures were observed following 24 hr of deprivation. In addition, deprivation-induced changes were also observed on most of the other self-report measures (Table 2). No differences on self-report measures of 24-hr deprivation effects were observed as a function of sensation-seeking status.
Figure 1.
Presents significant effects of the 24-hr deprivation period on the Minnesota Smoking Withdrawal and Wisconsin Smoking Withdrawal Scales. Significant increases in mean subject-rated withdrawal effects were found on both measures. *p < .05, **p < .01, ***p < .001. Error bars represent 1 SE.
Table 2.
Presents the F Ratios of the 24-Hr Deprivation Analysis of Variance (First Column) and the Separate and Interactive Effects of Nicotine Yield and Sensation Seeking During the Experimental Cigarette ANCOVA (Columns 2–4)
| Measure | 24-hr deprivation, df (1,18) | Experimental cigarette analysis |
||
| Nicotine yield, df (2,36) | SS, df (1,18) | Nicotine yield × SS, df (2,36) | ||
| MNWS | ||||
| Crave | 82.65*** | 11.86*** | 1.54 | 0.54 |
| Irritated | 24.83*** | 1.80 | 1.38 | 0.14 |
| Anxious | 18.13*** | 0.89 | 0.05 | 0.07 |
| Difficulty concentrating | 20.78*** | 2.50 | 0.72 | 0.23 |
| Restlessness | 14.43** | 2.80 | 0.51 | 3.21* |
| Headache | 7.57* | 2.93 | 0.09 | 0.15 |
| Drowsy | 7.96* | 0.42 | 1.10 | 1.86 |
| VAS | ||||
| Stimulated | 9.50** | 13.16*** | 0.38 | 3.76* |
| Head rush | 5.04* | 15.79*** | 0.03 | 0.31 |
| Relaxed | 21.81*** | 2.71 | 1.88 | 0.17 |
| Pleasant feeling | 16.01** | 5.85** | 2.80 | 0.08 |
| Stressed | 7.36* | 2.78 | 1.12 | 0.88 |
| Anxious | 10.46** | 6.36** | 3.08 | 2.74 |
| Jittery | 6.28* | 0.08 | 0.12 | 1.28 |
| Sedated | 1.78 | 6.17** | 0.03 | 0.02 |
| Light headed | 0.01 | 14.26*** | 0.02 | 0.83 |
| High | 4.05 | 2.68 | 0.03 | 1.27 |
| Drug effect | 3.82 | 2.80 | 0.96 | 0.52 |
| Like drug | 4.11 | 3.42* | 1.34 | 0.76 |
| Thirsty | 0.93 | 0.89 | 1.79 | 0.67 |
| Sick to stomach | 0.00 | 2.20 | 1.24 | 0.27 |
| POMS | ||||
| Elation | 26.20*** | 8.26*** | 0.44 | 3.39* |
| Total positive | 18.55*** | 8.91*** | 0.88 | 1.93 |
| Vigor | 12.77** | 2.35 | 6.13* | 1.10 |
| Friendliness | 20.22*** | 3.60* | 1.52 | 0.01 |
| Anxiety | 13.53** | 5.90*** | 0.85 | 0.29 |
| Anger | 7.62* | 4.99* | 0.76 | 0.37 |
| Confusion | 8.86** | 0.69 | 0.17 | 0.11 |
| Depression | 2.62 | 4.35* | 0.56 | 0.00 |
| ARCI | ||||
| MBG | 25.27*** | 2.46 | 0.86 | 1.33 |
| A | 10.93** | 0.81 | 3.17 | 0.38 |
| BG | 10.96** | 1.35 | 5.76* | 1.11 |
| LSD | 13.98** | 4.62* | 0.53 | 1.72 |
| PCAG | 18.24*** | 0.91 | 5.10* | 3.32* |
| RA | ||||
| Acquisition | ||||
| Correct responses | 1.25 | 1.73 | 0.00 | 0.61 |
| Incorrect responses | 9.36** | 0.98 | 0.05 | 1.86 |
| Errors index of curvature | 4.21 | 0.43 | 0.27 | 0.24 |
| Performance | ||||
| Correct responses | 26.45*** | 3.91* | 0.96 | 0.70 |
| Incorrect responses | 1.23 | 3.30* | 0.19 | 3.64* |
| DSST | ||||
| Correct responses | 0.63 | 2.88 | 2.15 | 1.29 |
| Incorrect responses | 1.13 | 0.13 | 7.36* | 0.57 |
| RIP | ||||
| Characters per minute | 0.60 | 1.21 | 0.13 | 0.28 |
| Errors of commission | 0.15 | 1.29 | 1.96 | 0.49 |
| Errors of omission | 0.11 | 2.34 | 0.51 | 0.35 |
| Cardiovascular | ||||
| Baseline HR | 38.44*** | 43.30*** | 0.15 | 1.66 |
| Baseline systolic BP | 20.18*** | 1.35 | 0.27 | 1.34 |
| Baseline diastolic BP | 18.55*** | 0.74 | 2.61 | 0.56 |
Note. A = Amphetamine; ANCOVA = analysis of covariance; ARCI = Addiction Research Center Inventory; BG = Benzedrine Group; BP = blood pressure; DSST = Digit–Symbol Substitution Task; HR = heart rate; LSD=lysergic acid diethylamide; MBG = Morphine–Benzedrine Group; MNWS = Minnesota Smoking Withdrawal Scale; PCAG = Pentobarbital, Chlorpromazine, Alcohol Group; POMS = Profile of Mood States; RA = repeated acquisition of response sequences; RIP = rapid information processing; SS = Sensation Seeking group; and VAS = Visual Analog Scale.
*p < .05; **p < .01; ***p < .001.
The effects of 24-hr deprivation on RIP and DSST task performance varied as a function of sensation-seeking status. Significant sensation seeking × session interactions were observed on RIP proportion correct, F(1,18) = 6.904, p < .05, and correct commissions, F(1,18) = 9.669, p < .05, and DSST incorrect responses, F(1,18) = 6.891, p < .05. Simple effects indicated that deprivation-induced impairment occurred only among high sensation seekers. Deprivation-induced increases were also seen on errors during the acquisition phase of the RA task (p < .01) and correct responses on the performance phase of the RA task, but these effects did not differ as a function of sensation-seeking status.
The effects of 24-hr deprivation on systolic blood pressure also varied as a function of sensation-seeking status. Follow-up testing indicated that systolic pressure decreased following deprivation for high sensation seekers, only (p < .05). Deprivation also decreased cardiovascular measures of heart rate and diastolic blood pressure, but these effects were not different among low and high sensation seekers.
Smoking Effects
Subjective Measures
Minnesota Smoking Withdrawal Scale.
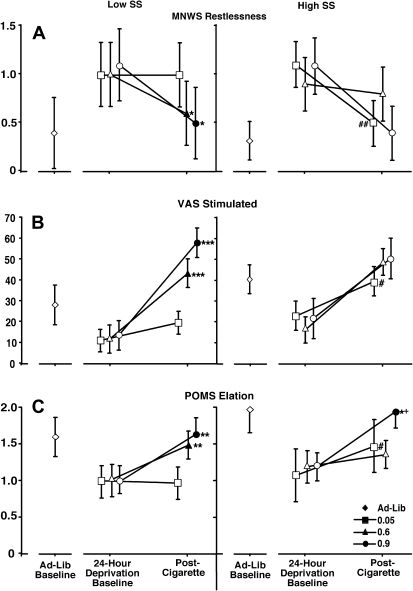
Effects of nicotine yield on ratings of restlessness from 24-hr deprivation baseline level varied as a function of sensation-seeking status (Table 2, Figure 2, Panel A). Simple effects analyses of the interaction indicated that decreases in ratings occurred as a function of nicotine yield in low sensation seekers alone, while high sensation seekers reported lower ratings of restlessness than low sensation seekers after smoking the 0.05-mg cigarette (i.e., changes in ratings among high sensation seekers occurred following smoking, regardless of nicotine yield; while changes among low sensation seekers were dependent on nicotine yield).
Figure 2.
Presents subject-rated effects of Minnesota Smoking Withdrawal Scale Restlessness (Panel A), Visual Analog Scale Stimulated (Panel B), and Profile of Mood States Elation (Panel C) during ad libitum smoking baseline and following 24 hr of deprivation prior to and after cigarette administration in low and high sensation seekers. A significant nicotine-yield × sensation-seeking interaction was present, with group differences present at the 0.05-mg postcigarette assessment and greater nicotine-induced changes in low sensation seekers for all three measures. Filled circles represent a significant change from the 0.05-mg nicotine yield postcigarette measurement, while asterisks (*) represent the magnitude of that change. *p < .05, **p < .01, ***p < .001. Crosses (+) represent a significant difference between 0.6- and 0.9-mg nicotine yield postcigarette measurement. +p < .05. Pound signs (#) represent a significant SS group difference at the 0.05-mg nicotine yield. #p < .10, ##p < .05. Error bars represent ±1 SE. SS = Sensation Seeking group.
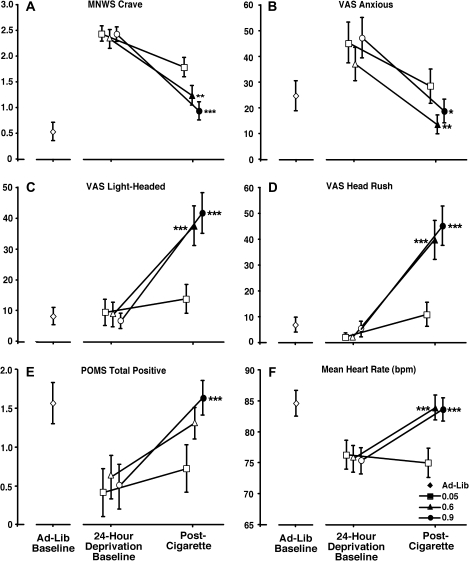
Nicotine reduced ratings on other MNWS similarly among low and high sensation seekers. Ratings on the MNWS Craving scale, pooled for sensation-seeking status, are presented in Panel A of Figure 3. Ratings were significantly lower after smoking the 0.6- and 0.9-mg cigarettes when compared with ratings following the 0.05-mg cigarette. Similar effects were observed on the other MNWS.
Figure 3.
Presents subject-rated (Panels A–E) and cardiovascular (Panel F) measures that varied as a function of nicotine yield. Filled circles represent a significant change from the 0.05-mg nicotine yield postcigarette measurement, while asterisks (*) represent the magnitude of that change. *p < .05, **p < .01, ***p < .001. Error bars represent ±1 SE.
Visual Analog Scale.
Nicotine effects on VAS stimulated varied as a function of sensation-seeking status (Figure 2, Panel B). Simple effects indicated that increases in ratings occurred as a function of nicotine yield in low sensation seekers alone, while high sensation seekers reported higher ratings of stimulated than low sensation seekers after smoking the 0.05-mg cigarette (p = .07). These results were similar to those on MNWS restlessness, with changes in ratings among high sensation seekers dependent on smoking but independent of nicotine yield, and changes among low sensation seekers dependent on nicotine yield.
Nicotine effects on other VAS items did not differ as a function of sensation-seeking status. Panel B of Figure 3 presents the pooled effects of nicotine yield on ratings of anxious. Significant effects of nicotine yield were found, with follow-up testing indicating reductions in deprivation-induced ratings of anxious at 0.6- and 0.9-mg yields relative to 0.05 mg. Panel C of Figure 3 presents a main effect of nicotine yield on ratings of light headed. Follow-up testing indicated increases in ratings of light-headed at the 0.6- and 0.9-mg yields relative to 0.05 mg. Similar effects were found on ratings of head rush (Figure 3, Panel D), stimulated, like drug, pleasant feeling, and sedated.
Profile of Mood States.
Nicotine effects on POMS elation varied as a function of sensation-seeking status (Figure 2, Panel C). Simple effects indicated nicotine-induced increases in both low and high sensation seekers, with a larger magnitude increase observed in low sensation seekers. In addition, high sensation seekers reported higher ratings of elation than low sensation seekers after smoking the 0.05-mg cigarette (p = .07). While group differences were found on ratings of vigor, with high sensation seekers reporting higher ratings than low sensation seekers, no main effects of nicotine yield or nicotine yield by group interactions were observed.
Nicotine effects on other POMS scales did not differ as a function of sensation-seeking status. Figure 3 (Panel E) presents the pooled ratings of total positive. A main effect of nicotine yield was observed, with ratings at the 0.9-mg nicotine yield significantly increased compared with the 0.05-mg yield. Similar effects were observed on ratings of friendliness. Nicotine yield-dependent decreases were observed on ratings of anxiety, anger, and depression.
Addiction Research Center Inventory.
The effects of nicotine yield on the PCAG Scale varied as a function of sensation-seeking status, with simple effects indicating a nicotine-yield dependent decrease among high sensation seekers only. In addition, high sensation seekers reported lower PCAG scores for both the 0.05- and 0.9-mg nicotine yields compared with low sensation seekers. Group differences were also found on BG scale ratings, with high sensation seekers reporting higher ratings than low sensation seekers, but no main effects of nicotine yield or nicotine yield by group interactions were observed.
A main effect of nicotine yield was found on the LSD scale, with follow-up testing indicating that LSD scores were lower at the 0.6-mg nicotine yield compared with the 0.05-mg yield.
Cigarette rating questionnaire.
Cigarettes ratings varied as a function of nicotine yield for all subscales, as evidenced by a main effect of nicotine yield. However, CQ ratings were unrelated to sensation-seeking status.
Performance Measures
RA task—acquisition.
No significant effects of group or nicotine yield were observed.
RA task—performance.
A significant nicotine yield × sensation seeking group interaction was observed for incorrect responses. Simple effects indicated a nicotine-yield dependent decrease in incorrect responding at both active nicotine yields in low sensation seekers, whereas incorrect responses were decreased only at the 0.6-mg nicotine yield among high sensation seekers.
A main effect of nicotine yield was present for correct responses, with increases observed at both active nicotine yields relative to the 0.05-mg yield.
Digit–Symbol Substitution Task.
High sensation seekers committed more errors on the DSST compared with low sensation seekers. Nicotine yield had no effects on DSST performance.
Rapid information processing.
No significant effects of group or nicotine yield were observed.
Cardiovascular Measures
Figure 3 (Panel F) presents the effects of nicotine yield on heart rate. Heart rate was increased after smoking the 0.6- and 0.9-mg cigarettes compared with the 0.05-mg yield. No differences in other cardiovascular measures were observed.
Tobacco Self-administration
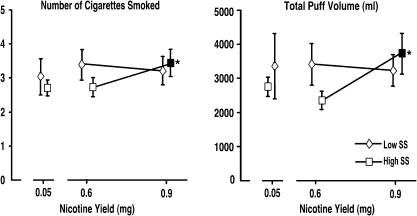
No changes in total cigarettes consumed, total number of puffs, or puff duration were observed, but puff volume increased in a nicotine-dependent manner, F(2,36.8) = 4.34, p < .05]. Figure 4 presents the number of cigarettes consumed and total puff volume over the 2-hr self-administration session as a function of sensation-seeking status. To examine change in tobacco self-administration as a function of active nicotine yield, a supplemental 2 × 2 ANCOVA was performed using cigarette smoking topography at the 0.05-mg yield condition as a covariate. The supplemental analysis revealed significant sensation seeking × nicotine yield interactions on number of cigarettes smoked, F(1,20) = 5.16, p < .05, total puff volume, F(1,18) = 4.78, p < .05, and puff duration, F(1,18) = 5.47, p < .05. Simple effects indicated that the number of cigarettes smoked, total puff volume, and puff duration increased during the 0.9-mg cigarette self-administration period in high sensation seekers only.
Figure 4.
Presents cigarette consumption and puff topography as a function of sensation-seeking status. Filled symbols indicate significant difference between 0.6- and 0.9-mg nicotine yields, while asterisks (*) represent the magnitude of that change. *p < .05. Error bars represent ±1 SE. SS = Sensation Seeking group.
Discussion
This study examined the self-report, physiological, psychomotor, and reinforcing effects of nicotine in smoked tobacco following 24 hr of tobacco deprivation in high and low sensation seekers with similar baseline rates of cigarette smoking. Tobacco deprivation engendered expected withdrawal effects on self-report, performance, and cardiovascular measures while tobacco smoking ameliorated many of these effects in a nicotine dose-dependent manner in both low and high sensation seekers. In high sensation seekers, deprivation effects on selective measures (e.g., increases in MNWS restlessness and decreases in VAS stimulated, Addiction Research Center Inventory [ARCI], PCAG, and POMS elation) were ameliorated by tobacco smoking independent of nicotine yield, whereas amelioration of these deprivation effects were dependent on nicotine yield among low sensation seekers. This study also examined tobacco self-administration as a function of nicotine yield following 24 hr of tobacco deprivation in high and low sensation seekers. Smoking behavior increased in a nicotine-dependent manner only among high sensation seekers. Although interactions of sensation seeking and nicotine yield were limited in quantity, the significant results were consistent across measures and suggest that, among smokers with similar baseline smoking rates, high sensation seekers are less sensitive than low sensation seekers to effects of cigarette nicotine yield following 24 hr of deprivation. In addition, high sensation seekers are more sensitive to nicotine yield during subsequent tobacco self-administration. Therefore, smokers high in sensation seeking may obtain more withdrawal relief from the nonnicotine effects of smoking, but may obtain more reinforcement from the nicotine effects of smoking.
Heishman, Taylor, and Henningfield (1994) have articulated the importance of establishing quantitative baseline measures of tobacco deprivation effects prior to evaluating deprivation interventions. In this study, the quantitative effects of the 24-hr deprivation manipulation were determined by comparing measures obtained during an ad libitum smoking with those following deprivation. Consistent with previous studies, 24 hr of tobacco deprivation increased self-report measures associated with tobacco withdrawal, impaired psychomotor task performance, and decreased heart rate and blood pressure (Evans & Drobes, 2008; Heishman et al., 1994; Hughes, 2007). These results confirm that the 24-hr deprivation manipulation in this study generated expected tobacco withdrawal effects.
Nicotine ameliorated the effects of 24 hr of deprivation in a dose-dependent manner on several verbal-report measures, heart rate, and psychomotor performance on the repeated acquisition task (see representative measures in Figure 3). In addition, nicotine increased verbal-report measures associated with the positive reinforcing effects of nicotine that were only minimally affected by tobacco deprivation (Figure 3, Panels D and E). These effects are comparable to those obtained in other tobacco deprivation studies that have examined the effects of nicotine administered via smoking and intravenous and intranasal routes (e.g, Atzori, Lemmonds, Kotler, Durcan, & Boyle, 2008; Jones, Garrett, & Griffiths, 1999; Kalman & Smith, 2005; Myers, Taylor, Moolchan, & Heishman, 2008; Parrott & Garnham, 1998).
Among smokers in the current study, the effects of tobacco deprivation varied as a function of sensation-seeking status on several measures. High sensation seekers exhibited greater decrements in RIP and DSST task performance and had lower systolic blood pressure after 24 hr of nicotine deprivation. Previous studies have demonstrated that high sensation seekers have greater negative affect and anhedonia during tobacco abstinence (Carton et al., 2000), and higher relapse rates after initiating smoking cessation (Kahler et al., 2009). Similarly, Carton et al. (1994) found that after controlling for duration and frequency of smoking, subject-rated FTND scores correlated with experience seeking and disinhibition scores on the Sensation-Seeking Scale (Form V) in regular tobacco smokers. The deprivation-related findings did not match those of Carton et al. with regards to high sensation seekers displaying greater negative affect and anhedonia. This discordance might be due to a variety of factors (e.g., level of nicotine dependence), but it is important to note that the present study was not specifically designed to evaluate the effects of tobacco deprivation per se, as no control conditions were included. However, these results do suggest that sensitivity to some tobacco deprivation effects, after controlling for tobacco smoking rates, is related to sensation-seeking status, though further research is warranted.
High sensation seekers were also less sensitive than low sensation seekers to the effects of nicotine yield in cigarettes on verbal report measures during tobacco deprivation. Another way to explain this difference is that high sensation seekers were more responsive to effects of smoking per se, independent of nicotine intake. Smoking low nicotine-yield (0.05 mg) cigarettes reduced 24-hr deprivation effects on MNWS restlessness, VAS stimulated, POMS elation (Figure 2), and ARCI PCAG in high but not low sensation seekers. Previous studies have demonstrated that cigarette smoking, independent of nicotine content, can ameliorate craving, negative mood, withdrawal, and psychomotor task performance impairment associated with tobacco deprivation (Barrett, 2010; Perkins et al., 2008a, 2010; Robinson, Houtsmuller, Moolchan, & Pickworth, 2000). The present study suggests that the conditioned negative reinforcing effects of nonnicotine components of smoking can vary as a function of sensation-seeking status.
A growing body of literature suggests that high sensation-seeking young adults are more sensitive to the reinforcing and other behavioral effects of a range of drugs including alcohol (Fillmore et al., 2009; Magid et al., 2007), hallucinogens (Khavari et al., 1977), and other stimulants (Bowling and Bardo, 1994; Kelly et al., 2006; Stoops et al., 2007). Specific to nicotine, Perkins et al. (2000, 2008b) have demonstrated that verbal reports of mood and nicotine choice behavior are correlated with several subscales of sensation seeking among healthy nonsmokers. In the current study, tobacco cigarette self-administration following 24 hr of tobacco deprivation was robust in both low and high sensation seekers. However, an increase in tobacco self-administration was observed as a function of nicotine yield among high sensation seekers only. Cigarette brands varied with nicotine yield (Quest 3 and Quest 1 cigarettes for the 0.05- and 0.6-mg nicotine yields and Kent for the 0.9-mg nicotine yield), but Cigarette Questionnaire ratings did not vary as a function of sensation-seeking status, suggesting that group differences in self-administration were related to the nicotine yields of the cigarettes. Moreover, because the nicotine yield of preferred cigarettes was not different between low and high sensation seekers, group differences in cigarette self-administration likely were not due to these study cigarettes being more similar to the preferred brands of either group. These results suggest that, rather than being more sensitive to all effects of nicotine in cigarettes, high sensation seeking smokers may be not only more sensitive to the reinforcing effect of nicotine in tobacco smoke but also more sensitive to the withdrawal relieving effects of the nonnicotine components of cigarette smoking.
There were limitations to the present study that should be noted. First, while an extreme groups design was used to maximize the opportunity to detect interactions between sensation-seeking status and the nicotine yield of cigarettes tested, group differences in nicotine effects were observed on only a limited number of measures, and the statistical power to detect such effects was limited due to the sample size. The sample size for the present study was chosen based upon effect sizes observed in studies that examined the reinforcing and behavioral effects of amphetamine in low and high sensation seekers (e.g., Kelly et al., 2006; Stoops et al., 2007). However, it is possible that the effects of sensation-seeking status might have been more modest in this study due to the tobacco dependent status of the study population. Using effect sizes from the current study, a power analysis revealed that an increase in sample size to an N of 30 would increase the power to detect several key effects of interest that were not found in this study (e.g., MNWS craving, difficulty concentrating, VAS like drug, head rush, and DSST performance). Thus, future studies might need to recruit a larger sample size in order to replicate and extend the current findings. Second, group differences in extraversion and impulsivity were obtained in this study. Given established correlations between sensation-seeking status and impulsivity (Zuckerman et al., 1993), these differences are not surprising. Furthermore, supplementary analyses controlling for extraversion by including scores on that dimension as a covariate did not change any significant effects reported in this manuscript. Nonetheless, it is possible that other variables not measured in this study could have differed among groups. Third, a separate brand of cigarette (Kent) was used for the 0.9-mg nicotine yield compared with Quest 3 and Quest 1 cigarettes for the 0.05- and 0.6-mg nicotine-yields. Although a double-blind procedure was used, it is possible that cues or tobacco ingredients specific to each brand (aside from nicotine yield) may have influenced behavior. Cigarette Questionnaire ratings did not vary as a function of brand, per se, but no direct assessment was utilized to verify that participants remained blind to brand. Finally, although several nicotine yields were assessed in this study, the range tested does not encompass the full range of yields in cigarettes currently available.
Sensation seeking plays a role at multiple stages of tobacco use, including initiation, escalation, maintenance, and cessation. Previous studies investigating the relationship between sensation seeking and tobacco/nicotine have demonstrated that high sensation seekers are more likely to use tobacco during adolescence (Andrucci et al., 1989; Frankenberger, 2004), are more sensitive to the initial reinforcing and subjective effects of nicotine (Perkins et al., 2000; 2008b), display a stronger craving response to smoking cues (Doran et al., 2009), have greater ratings of negative affect and anhedonia during tobacco abstinence (Carton et al., 2000), have higher scores on ratings of tobacco dependence (Carton et al., 1994), and are more likely to resume smoking after a quit attempt (Kahler et al., 2009). Furthermore, those high in novelty seeking are more sensitive to the effects of tobacco withdrawal (Leventhal et al., 2007). The finding that high sensation seeking smokers were more sensitive to some of the withdrawal relieving effects of denicotinized cigarettes suggests that denicotinized cigarettes may be a more effective treatment in high versus low sensation seekers (e.g., Rose, Behm, Westman, & Kukovich, 2006). Tailoring prevention materials for high sensation seekers has been shown to enhance the efficacy of marijuana interventions (e.g., Palmgreen et al., 2001). Similar approaches could be used to enhance tobacco prevention and treatment interventions.
Funding
The National Institute on Drug Abuse (P50 DA05312).
Declaration of Interests
Dr Perkins reports that he has consulted with Cypress Bioscience on treatments for smoking cessation.
Acknowledgments
We would like to thank Richard Charnigo for consultation on statistical issues and Cleeve Emurian, Beth Eaves, and Ashley Yingling for their assistance with study support and data management. This work was written with full investigator access to all relevant data and was not under editorial review by a sponsor prior to submission.
References
- Andrucci GL, Archer RP, Pancoast DL, Gordon RA. The relationship of MMPI and sensation seeking scales to adolescent drug use. Journal of Personality Assessment. 1989;53:253–266. doi: 10.1207/s15327752jpa5302_4. doi:10.1207/s15327752jpa5302. [DOI] [PubMed] [Google Scholar]
- Atzori G, Lemmonds CA, Kotler ML, Durcan MJ, Boyle J. Efficacy of a nicotine (4 mg)-containing lozenge on the cognitive impairment of nicotine withdrawal. Journal of Clinical Psychopharmacology. 2008;28:667–674. doi: 10.1097/JCP.0b013e31818c9bb8. doi:10.1097/JCP.0b013e31818c9bb8. [DOI] [PubMed] [Google Scholar]
- Barrett SP. The effects of nicotine, denicotinized tobacco, and nicotine-containing tobacco on cigarette craving, withdrawal, and self-administration in male and female smokers. Behavioural Pharmacology. 2010;21:144–152. doi: 10.1097/FBP.0b013e328337be68. doi:10.1097/FBP.0b013e328337be68. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bowling SL, Bardo MT. Locomotor and rewarding effects of amphetamine in enriched, social, and isolate reared rats. Pharmacology Biochemistry and Behavior. 1994;48:459–464. doi: 10.1016/0091-3057(94)90553-3. doi:10.1016/0091-3057(94)90553-3. [DOI] [PubMed] [Google Scholar]
- Carton S, Jouvent R, Widlocher D. Sensation seeking, nicotine dependence, and smoking motivation in female and male smokers. Addictive Behaviors. 1994;19:219–227. doi: 10.1016/0306-4603(94)90026-4. doi:10.1016/0306-4603(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Carton S, Le Houezec J, Lagrue G, Jouvent R. Relationships between sensation seeking and emotional symptomatology during smoking cessation with nicotine patch therapy. Addictive Behaviors. 2000;25:653–662. doi: 10.1016/s0306-4603(00)00067-8. doi:10.1016/S0306-4603(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. Brief symptom inventory: Administration, scoring and procedures manual. Minneapolis, MN: National Computer Systems; 1993. [Google Scholar]
- Doran N, Cook J, McChargue D, Spring B. Impulsivity and cigarette craving: Differences across subtypes. Psychopharmacology. 2009;207:365–373. doi: 10.1007/s00213-009-1661-x. doi:10.1007/s00213-009-1661-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DE, Drobes DJ. Nicotine self-medication of cognitive-attentional processing. Addiction Biology. 2008;14:32–42. doi: 10.1111/j.1369-1600.2008.00130.x. doi:10.1111/j.1369-1600.2008.00130.x. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ, Eysenck SBG. Eysenck personality inventory. San Diego, CA: Educational and Industrial Testing Service; 1964. [Google Scholar]
- Fillmore MT, Kelly TH, Martin CA. Effects of d-amphetamine in human models of information processing and inhibitory control. Drug and Alcohol Dependence. 2005;77:151–159. doi: 10.1016/j.drugalcdep.2004.07.013. doi:10.1016/j.drugalcdep.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Ostling EW, Martin CA, Kelly TH. Acute effects of alcohol on inhibitory control and information processing in high and low sensation-seekers. Drug and Alcohol Dependence. 2009;100:91–99. doi: 10.1016/j.drugalcdep.2008.09.007. doi:10.1016/j.drugalcdep.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Assessment of abuse liability of stimulant drugs in humans: A methodological survey. Drug and Alcohol Dependence. 1991;28:3–48. doi: 10.1016/0376-8716(91)90052-z. doi:10.1016/0376-8716(91)90052-Z. [DOI] [PubMed] [Google Scholar]
- Frankenberger KD. Adolescent egocentrism, risk perceptions, and sensation seeking among smoking and nonsmoking youth. Journal of Adolescent Research. 2004;19:576–590. doi:10.1177/074355840326004. [Google Scholar]
- Heishman SJ, Taylor RC, Henningfield JE. Nicotine and smoking: A review of the effects on human performance. Environmental and Clinical Psychopharmacology. 1994;2:345–395. doi:10.1037/1064-1297.2.4.345. [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Errors in using tobacco withdrawal scale. Tobacco Control. 1998;7:92–93. doi: 10.1136/tc.7.1.92a. doi:10.1136/tc.7.1.92a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: Valid symptoms and time course. Nicotine and Tobacco Research. 2007;9:315–327. doi: 10.1080/14622200701188919. doi:10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Jones HE, Garrett BE, Griffiths RR. Subjective and physiological effects of intravenous nicotine and cocaine in cigarette smoking cocaine abusers. The Journal of Pharmacology and Experimental Therapeutics. 1999;288:188–197. Retrieved from http://www.jpet.org. [PubMed] [Google Scholar]
- Kahler CW, Spillane NS, Metrik J, Leventhal AM, Monti PM. Sensation seeking as a predictor of treatment compliance and smoking cessation treatment outcomes in heavy social drinkers. Pharmacology, Biochemistry and Behavior. 2009;93:285–290. doi: 10.1016/j.pbb.2009.01.003. doi:10.1016/j.pbb.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman D, Smith SS. Does nicotine do what we think it does? A meta-analytic review of the subjective effects of nicotine in nasal spray and intravenous studies with smokers and nonsmokers. Nicotine and Tobacco Research. 2005;7:317–333. doi: 10.1080/14622200500125385. doi:10.1080/14622200500125385. [DOI] [PubMed] [Google Scholar]
- Kelly TH, Foltin RW, Rose AJ, Fischman MW, Brady JV. Smoked marijuana effects on tobacco cigarette smoking behavior. The Journal of Pharmacology and Experimental Therapeutics. 1990;252:934–944. Retrieved from http://jpet.aspetjournals.org/content/252/3/934.short. [PubMed] [Google Scholar]
- Kelly TH, Robbins G, Martin CA, Fillmore MT, Lane SD, Harrington NG, et al. Individual differences in drug abuse vulnerability; d-Amphetamine and sensation-seeking status. Psychopharmacology. 2006;189:17–25. doi: 10.1007/s00213-006-0487-z. doi:10.1007/s00213-006-0487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly TH, Delzer TA, Martin CA, Harrington NG, Hays LR, Bardo M. Performance and subjective effects of diazepam and d-amphetamine in high and low sensation seekers. Behavioural Pharmacology. 2009;20:505–517. doi: 10.1097/FBP.0b013e3283305e8d. doi:10.1097/FBP.0b013e3283305e8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khavari KA, Mabry E, Humes M. Personality correlates of hallucinogen use. Journal of Abnormal Psychology. 1977;86:172–178. doi: 10.1037//0021-843x.86.2.172. doi:10.1037/0021-843X.86.2.172. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Boyd S, Moolchan ET, Heishman SJ, Lerman C, et al. Associations between Cloninger's temperament dimensions and acute tobacco withdrawal. Addictive Behaviors. 2007;32:2976–2989. doi: 10.1016/j.addbeh.2007.06.014. doi:10.1016/j.addbeh.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magid V, Maclean MG, Colder CR. Differentiating between sensation seeking and impulsivity through their mediated relations with alcohol use and problems. Addictive Behaviors. 2007;32:2046–2061. doi: 10.1016/j.addbeh.2007.01.015. doi:10.1016/j.addbeh.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clinical Pharmacology and Therapeutics. 1971;12:245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling J. An automated version of the digit symbol substitution test (DSST) Behavioral Research Methods, Instruments and Computers. 1982;14:463–466. doi:10.3758/BF03203313. [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Profile of mood states (manual) San Diego, CA: Educational and Industrial Testing Services; 1971. [Google Scholar]
- Myers CS, Taylor RC, Moolchan ET, Heishman SJ. Dose-related enhancement of mood and cognition in smokers administered nicotine nasal spray. Neuropsychopharmacology. 2008;33:588–598. doi: 10.1038/sj.npp.1301425. doi:10.1038/sj.npp.1301425. [DOI] [PubMed] [Google Scholar]
- Palmgreen P, Donohew L, Lorch EP, Hoyle RH, Stephenson MT. Television campaigns and adolescent marijuana use: Tests of sensation seeking targeting. American Journal of Public Health. 2001;91:292–296. doi: 10.2105/ajph.91.2.292. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1446528/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott AC, Garnham NJ. Comparative mood states and cognitive skills of cigarette smokers, deprived smokers and nonsmokers. Human Psychopharmacology: Clinical and Experimental. 1998;13:367–376. doi:10.1002/(SICI)1099-1077(199807)13:5<367::AID-HUP10>3.0.CO;2-2. [Google Scholar]
- Perkins KA, Gerlach D, Broge M, Grobe JE, Wilson A. Greater sensitivity to subjective effects of nicotine in nonsmokers high in sensation seeking. Experimental and Clinical Psychopharmacology. 2000;8:462–471. doi: 10.1037//1064-1297.8.4.462. doi:10.1037//1064-1297.8.4.462. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Lerman C, Coddington SB, Jetton C, Karelitz JL, Scott JA, et al. Initial nicotine sensitivity in humans as a function of impulsivity. Psychopharmacology. 2008b;200:529–544. doi: 10.1007/s00213-008-1231-7. doi:10.1007/s00213-008-1231-7. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Ciccocioppo M, Conklin CA, Milanak ME, Grottenthaler A, Sayette MA. Mood influences on acute smoking responses are independent of nicotine intake and dose expectancy. Journal of Abnormal Psychology. 2008a;117:79–93. doi: 10.1037/0021-843X.117.1.79. doi:10/1037/0021-843X.117.1.79. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Conklin CA, Sayette MA, Giedgowd GE. Acute negative affect relief from smoking depends on the affect situation and measure but not on nicotine. Biological Psychiatry. 2010;67:707–714. doi: 10.1016/j.biopsych.2009.12.017. doi:10.1016/j.biopsych.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roache JD. Performance and physiological measures in abuse liability evaluation. British Journal of Addiction. 1991;86:1595–1600. doi: 10.1111/j.1360-0443.1991.tb01753.x. doi:10.1111/j.1360-0443.1991.tb01753.x. [DOI] [PubMed] [Google Scholar]
- Robinson ML, Houtsmuller EJ, Moolchan ET, Pickworth WB. Placebo cigarettes in smoking research. Experimental and Clinical Psychopharmacology. 2000;8:326–332. doi: 10.1037//1064-1297.8.3.326. doi:10.1037//1064-1297.8.3.326. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Kukovich P. Precessation treatment with nicotine skin patch facilitates smoking cessation. Nicotine and Tobacco Research. 2006;8:89–101. doi: 10.1080/14622200500431866. doi:10.1080/14622200500431866. [DOI] [PubMed] [Google Scholar]
- Shiffman S, West RJ, Gilbert DG. Recommendation for the assessment of tobacco craving and withdrawal in smoking cessation trials. Nicotine and Tobacco Research. 2004;6:599–614. doi: 10.1080/14622200410001734067. doi:10.1080/14622200410001734067. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Robbins CG, Martin CA, Rush CR, Kelly TH. The reinforcing, subject-rated, performance, and cardiovascular effects of d-amphetamine: Influence of sensation-seeking status. Addictive Behaviors. 2007;32:1177–1188. doi: 10.1016/j.addbeh.2006.08.006. doi:10.1016/j.addbeh.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Experimental and Clinical Psychopharmacology. 1999;7:354–361. doi: 10.1037//1064-1297.7.4.354. doi:10.1037/1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- Westman EC, Levin ED, Rose JE. Smoking while wearing the nicotine patch: Is smoking satisfying or harmful? Clinical Research. 1992;40:871A. [Google Scholar]
- Zuckerman M, Eysenck SB, Eysenck HJ. Sensation seeking in England and America: Cross-cultural, age, and sex comparisons. Journal of Consulting and Clinical Psychology. 1978;46:139–149. doi: 10.1037//0022-006x.46.1.139. doi:10.1037/0022-006X.46.1.139. [DOI] [PubMed] [Google Scholar]
- Zuckerman M, Kuhlman DM, Joireman J, Teta P, Kraft M. A comparison of three structural models of personality: The big three, the big five, and the alternative five. Journal of Personality and Social Psychology. 1993;65:757–768. doi:10.1037/0022-3514.65.4.757. [Google Scholar]
- Zuckerman M. Behavioral expressions and biosocial bases of sensation seeking. New York: Cambridge University Press; 1994. [Google Scholar]
- Zuckerman M. Sensation seeking and risky behavior. Washington, DC: American Psychological Association; 2007. [Google Scholar]