Abstract
The causal relationship between atrazine exposure and the occurrence of breast cancer in women was evaluated using the framework developed by Adami et al. (2011) wherein biological plausibility and epidemiological evidence were combined to conclude that a causal relationship between atrazine exposure and breast cancer is “unlikely”. Carcinogenicity studies in female Sprague-Dawley (SD) but not Fischer-344 rats indicate that high doses of atrazine caused a decreased latency and an increased incidence of combined adenocarcinoma and fibroadenoma mammary tumors. There were no effects of atrazine on any other tumor type in male or female SD or Fischer-344 rats or in three strains of mice. Seven key events that precede tumor expression in female SD rats were identified. Atrazine induces mammary tumors in aging female SD rats by suppressing the luteinizing hormone surge, thereby supporting a state of persistent estrus and prolonged exposure to endogenous estrogen and prolactin. This endocrine mode of action has low biological plausibility for women because women who undergo reproductive senescence have low rather than elevated levels of estrogen and prolactin. Four alternative modes of action (genotoxicity, estrogenicity, upregulation of aromatase gene expression or delayed mammary gland development) were considered and none could account for the tumor response in SD rats. Epidemiological studies provide no support for a causal relationship between atrazine exposure and breast cancer. This conclusion is consistent with International Agency for Research on Cancer’s classification of atrazine as “unclassifiable as to carcinogenicity” and the United States Environmental Protection Agency's classification of atrazine as “not likely to be carcinogenic.”
Keywords: atrazine, mode of action, endocrine, breast cancer, weight-of-the-evidence, framework
Toxicology is rapidly being transformed from a descriptive science to one capable of prediction. Central to this progress is the understanding of key genomic, proteomic, biochemical, physiological, and pathological events on the pathway from chemical exposure to the expression of toxicity. The succession of key events following initial exposure, through intermediate states and ultimately to measurable adverse outcomes has been called a “mode of action” (Meek et al., 2003; Sonich-Mullin et al., 2001; USEPA, 2005). When the sequence of events is understood at a fundamental level of chemical-cell molecular interaction, the mode of action becomes a mechanism of toxicity. Mechanisms of toxicity are often postulated but in fact are rarely established. In the expression of toxicity, it is not uncommon for different facets of toxicity to be triggered at different dose levels (Slikker et al., 2004a,b) or through different mechanisms. Furthermore, differences between species with respect to absorption, distribution, metabolism, elimination, and target-specific susceptibility may render the extrapolation from in vitro models or even from in vivo animal data to humans difficult.
To accommodate the complexity of evaluating a mode of action, systematic approaches have been developed to evaluate the relevance of findings in animal studies to humans (Meek et al., 2003). Three key questions are: (1) Is there sufficient evidence in animal studies to propose a mode of action? (2) Is that mode of action operative in humans? and (3) Is the mode of action relevant to humans after considering differences between species with respect to pharmacokinetic and toxicodynamic factors operative at expected levels of human exposures?
Ultimately, the question of whether humans display toxicity following exposure to the chemical through ingestion, inhalation, or dermal contact can best be ascertained from observational epidemiology. Although there is a long history of interpreting epidemiology studies, only recently have methods been developed to systematically integrate animal data with data from observational epidemiology (Adami et al., 2011; European Center for Ecotoxicology and Toxicology of Chemicals, 2009; Swaen, 2006). This paper presents a case study utilizing the methodology described by Adami et al. (2011) wherein toxicological and epidemiological evidence were combined in a systematic framework to conclude whether a causal relationship exists between atrazine exposure and breast cancer in humans. Mode of action research using animal models (Brusick, 1994; Cooper et al., 2007; Eldridge and Wetzel, 2008; Stevens et al., 1994; Yi, Simpkins, and Breckenridge, in preparation) and epidemiology studies on breast cancer in women were evaluated. Breast cancer was selected for this case study because there were many mode of action research studies and a number of epidemiological studies on breast cancer that have been previously evaluated by regulatory authorities as part of a comprehensive cancer risk assessment (Australian Pesticides and Veterinary Medicines Authority [APVMA], 2004, 2008; Food and Agricultural Organization of the United Nations and the World Health Organization [FAO/WHO], 2009; USEPA, 2003b, 2006). A detailed review of the association between triazine exposure and any cancer has also just recently been published (Sathiakumar et al., 2011).
MATERIALS AND METHODS
The five-step method outlined by Adami et al. (2011) was followed including (1) collection of all relevant studies, (2) assessment of quality, (3) evaluation of the weight of evidence, (4) assignment of a scalable conclusion, and (5) placement on a causal relationship grid. All relevant toxicological and epidemiological studies were identified and study quality was assessed according to guidelines for evaluating toxicological (USEPA, 1993, 2001, 2003a) and epidemiology studies (London Principles, 1995: von Elm, 2007). Studies that characterize the effect of atrazine on the latency and the incidence of adenocarcinoma and fibroadenoma in the mammary glands of female Sprague-Dawley (SD) rats were summarized (Cooper et al., 2007; Eldridge and Wetzel, 2008; Stevens et al., 1994, 1999).
Studies relevant to the proposed mode of action underlying the effect of atrazine on mammary tumors were evaluated and a concordance analysis was conducted. The framework analysis also examined and rejected alternative modes of action including hypotheses relating to
Genotoxicity (Brusick, 1994);
Direct estrogenicity (Eldridge et al., 2008);
Induction of aromatase expression in vivo resulting in excess formation of estrogen (Sanderson et al., 2000, 2001; Yi, Simpkins, and Breckenridge, in preparation); and
Delayed mammary gland development leading to an extended period in which the mammary gland remains undifferentiated and vulnerable to genotoxic or viral carcinogens (Hovey et al., 2010; Russo et al., 2006).
Once the weight of the evidence assessment was completed, the relevance of the mode of action to humans was considered. This analysis included a consideration of the dose at which humans are likely to be exposed to atrazine. The most probable mode of action underlying the occurrence of mammary tumors in animal models was identified and whether it was plausible that the mode of action underlying the mammary tumor response in rats would be operative in humans.
Epidemiological studies that evaluated the association between human exposure to atrazine and the incidence of breast cancer were identified. The quality of the studies was characterized and whether collectively the results supported an inference of causality. The overall assessment was completed by using a causal inference grid and assigning atrazine to one of the four categories described by Adami et al. (2011).
RESULTS AND DISCUSSION
Effects of Atrazine on Mammary Tumors in Female SD Rats
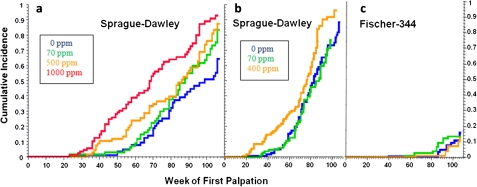
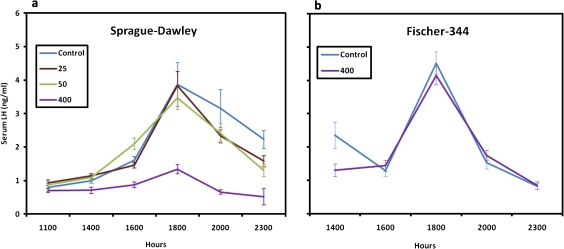
Stevens et al. (1999) summarized the results from nine carcinogenicity studies on atrazine in male and/or female SD rats (five studies), female Fischer 344 rats (two studies), and CD-1 mice (two studies). Additional non-guideline studies were conducted in SD and Fischer-344 rats and in two cross-bred mouse strains (C57Bl6 x C3H/Anf or AKR) (Table 1). In female SD rats, atrazine caused a decreased latency and/or increased combined incidence of mammary adenocarcinoma and fibroadenoma tumors (Figs. 1a and 1b) with no effect on the incidence of any other tumor type. There were no effects of atrazine at the maximum tolerated dose on the incidence of mammary tumors, on the incidence of any other tumor type in the female Fischer-344 rats (Fig. 1c) or on tumor incidence in three strains of mice (Table 1). Overall, the weight of the evidence indicates that the effect of atrazine on tumor incidence is specific to mammary tumors in female SD rats.
TABLE 1.
Effect of Oral Administration of Atrazine on the Incidence and/or Latency of Tumors in Rodents
| Study reference | Species | Strain | Sex | Levels tested (mg/kg/day) | Duration (months) | Results |
| Innes et al. (1969) (NTP Study) | Mouse | C57BL/6 X C3H/Anf; C57BL/6 X AKR | M and F | 21.5a | 18 | Negative |
| Stevens et al. (1999) (M1) | Mouse | CD-1 | M and F | 0, 10, 300, and 1000b | M 21, F 22 | Negative |
| Stevens et al. (1999) (M2) | Mouse | CD-1 | M and F | 0, 1.6, 47.9, 246.9, and 482.7 | 21 | Negative |
| Pintér et al. (1990) | Rat | Fischer-344 | M and F | 0, 375, and 750b | 29 | Inconclusive |
| Thakur et al. (1998) | Rat | Fischer-344 | F | 0, 0.7, 4.7, 13.6, and 27.4 | 24 | Negative |
| Thakur et al. (1998) | Rat | Fischer-344 | M and F | 0, 0.7, 4.7, 13.6, and 27.4 | 24 | Negative |
| Stevens et al. (1994) (Study 1) | Rat | SD | M and F | 0, 0.5, 5.5, and 60 | 24 | Male: negative |
| Female: positive (0.5, 60 mg/kg/day) | ||||||
| Increased incidence | ||||||
| Stevens et al. (1999) (SDR1) | Rat | SD | M and F | 0, 0.5, 3.5, 29.5, and 64.7 | 24 | Male: negative; Female: positive (3.5, 29.5, 64.7 mg/kg/day) |
| (Increased incidence) | ||||||
| Stevens et al. (1994) (Study 3) | Rat | SD | F | 0, 0.7, 3.5, and 37.6 | 24 | Negative |
| Stevens et al. (1999) (SDR2) | Rat | SD | F | 0, 4.3, and 25.6 | 24 | Positive: (early onset) at 25.6 mg/kg/day |
| No effect on incidence | ||||||
| Stevens et al. (1999) (SDR3) | Rat | SD | F | 0, 4.3, and 25.6 | 24 | Positive: (early onset) at 25.6 mg/kg/day |
| No effect on incidence | ||||||
| Stevens et al. (1999) (SDR5) | Rat | SD | F (intact) | 0, 1.5, 3.1, 4.2, and 24.4 | 12, 24 | Positive: (early onset) at 24.4 mg/kg/day |
| No effect on incidence | ||||||
| Stevens et al. (1999) (SDR4) | Rat | SD | F (ovex) | 0, 1.2, 2.5, 3.5, and 20.9 | 12, 24 | Negative |
| Pettersen and Turnier (1995)c | Rat | SD | F | 0, 0.8, 1.7, 2.8, 4.1, and 23.9 | 12 | Positive: (early onset) at 23.9 mg/kg/day |
| No effect on incidence |
Only female doses given.
Presented in ppm only, as conversion data were unavailable.
Study has not been published.
FIG. 1.
(a) Atrazine administered at dietary concentrations of 500 or 1000 ppm caused a decreased latency of the combined incidence of adenocarcinoma and fibroadenoma mammary tumors in female SD rats. The 500 ppm had no effect on the terminal incidence of mammary tumors; 70 ppm was the no observed effect level (NOEL) for both latency and incidence (Study SDR1, Stevens et al., 1999). (b) Atrazine administered at dietary concentration of 400 ppm caused a decreased latency of the combined incidence of adenocarinoma and fibroadenoma mammary tumors in female SD. The NOEL was 70 ppm (Studies SDR3; Stevens et al., 1999). (c) Atrazine administered at dietary concentration of 70 or 400 ppm had no effect on the latency or the combined incidence of adenocarinoma and fibroadenoma mammary tumors in female Fischer-344 rats (Study FR4; Stevens et al., 1999).
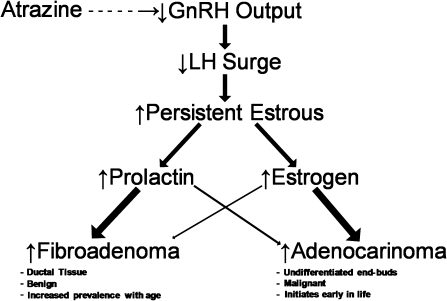
Key Events Underlying the Occurrence of Mammary Tumors in Female SD Rats
Key events (Fig. 2) leading to a decreased latency and an increased incidence of mammary tumors in atrazine-treated female SD rats have been proposed (Stevens et al., 1994) such that:
FIG. 2.
Key events associated with the earlier appearance and increased incidence of mammary tumors in atrazine-treated female SD rats.
Atrazine causes a dose-dependent effect on the synchronized activity of gonadotrophin-releasing hormone (GnRH) neurons during the estrogen-induced luteinizing hormone (LH) surge so that the amplitude of the LH surge is reduced (Foradori et al., 2009b).
The reduction in the LH surge amplitude and a reduced area under the LH curve causes a failure of the maturing ovarian follicles to ovulate their eggs.
The failure of ovulation results in continuous secretion of estrogen over successive days until the unovulated follicles undergo atresia.
Repeated failure of ovulation over successive days of the estrous cycle produces a persistent state of estrus resulting in prolonged exposure of the mammary gland and the pituitary to endogenous estrogen. Estrogen is known to be mitogenic and a metabolite of estradiol, 4-hydroxy estradiol, is possibly directly carcinogenic by redox recycling between a semiquinone and quinine form liberating supraoxide radicals (Jefcoate et al., 2000).
Under the influence of estrogen, proliferative changes are observed earlier in the mammary gland and in the pituitary. Prolonged stimulation of ductal tissue in the mammary gland by estrogen causes an increased incidence and a decreased latency of adenocarcinomas (Sielken et al., 2005).
Proliferative changes in the pituitary in response to increased exposure to endogenous estrogens are postulated to result in an earlier development of pituitary tumors resulting in hyperprolactinemia (O’Connor et al., 2000).
Prolonged exposure of ductal tissue in the mammary gland to endogenous prolactin is postulated to cause an earlier occurrence of mammary fibroadenoma (Eldridge et al., 1999a,b; Eldridge and Wetzel, 2008; Sielken et al., 2005; Stevens et al., 1994).
Experimental Evidence Supporting the Proposed Mode of Action
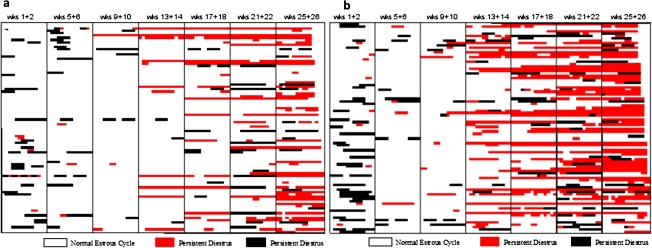
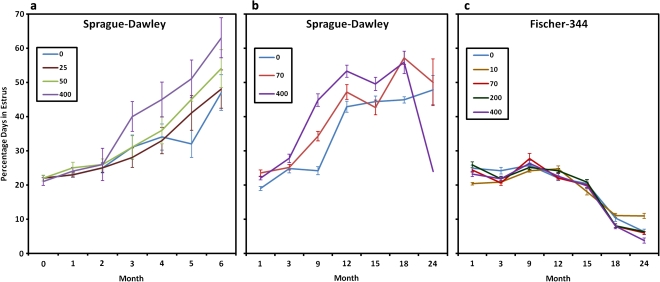
Figure 3 shows the number of estrous days in female control SD rats (Fig. 3a) and in those exposed to 400 ppm atrazine (Fig. 3b) after 26 weeks of treatment. High-dose atrazine-treated female SD rats displayed an increased proportion of days in constant estrus commencing after three months of treatment (week 13–14) in the 400 ppm dose group (Fig. 3b) compared with untreated controls (Fig. 3a). A dose-dependent relationship existed, with a modest increase in the percent days in estrus noted in the 70 ppm group after nine months of treatment (Fig. 4b); no effect was observed at feeding levels less than or equal to 50 ppm (Fig. 4a). The earliest appearance of disrupted estrous cycles in the 400 ppm treated groups occurred prior to the earliest appearance of the increased incidence of mammary tumors (week 26; Fig. 1b). There was no effect of 400 ppm atrazine on either mammary tumor incidence (Fig. 1c) or on the estrous cycle in female Fischer-344 rats (Fig. 4c).
FIG. 3.
(a) Characterization of the estrous cycle in individual control female SD rats (Study SDR3 Stevens et al., 1999) during treatment weeks 1 and 2, 9 and 10, 13 and 14, 17 and 18, 21 and 22, and weeks 25 and 26. A normal estrous cycle was defined as a cycle lasting four or five days. Persistent diestrus was defined as occurring when there were more than two successive days of diestrus; persistent estrus was defined as occurring when there were two or more successive days when the vaginal smear indicated that the rat was in estrus. Each row represents the result from 1 of 90 rats evaluated in each group. (b) Characterization of the estrous cycle in individual 400 ppm atrazine-treated female SD rats (Study SDR3, Stevens et al., 1999).
FIG. 4.
(a) Dose- and time-dependent effect of 0, 25, 50, or 400 ppm atrazine administered in the diet on the percent days in estrus in female SD rat; 50 ppm was the no observed effect level (Study SDR3, Stevens et al., 1999). (b) Comparison of the dose- and the time-dependent effects of atrazine administered at dietary concentrations of 0, 70, or 400 ppm on the percent days in estrus in female SD rats; 70 ppm was the no observed effect level (Study SDR3, Stevens et al., 1999). (c) Atrazine administered at dietary concentrations of 70 or 400 ppm had no effect on the percent days in estrus in female Fischer 344 rats (Study FR2, Stevens et al., 1999).
Studies investigating the effect of atrazine on the estrogen-induced LH surge in female SD and Fischer-344 rats indicate that doses of atrazine that disrupted the estrous cycle and caused a decreased latency and/or increased incidence of mammary tumors in female SD rats also suppressed the LH surge (Fig. 5a). In contrast, there was no effect of dietary concentrations of atrazine up to 400 ppm on the estrogen-induced LH surge in female Fischer-344 rats (Fig. 5b). This latter finding is consistent with the absence of an effect of atrazine on the estrous cycle (Fig. 4c) or on the latency or incidence of mammary tumors in female Fischer-344 rats (Fig. 1c).
FIG. 5.
(a) Atrazine administered for 6 months at dietary concentration of 400 ppm significantly suppressed the estrogen-induced LH surge in female SD rats; 50 ppm was the no observed effect level (Study SDR3, Stevens et al., 1999). (b) Atrazine administered for 6 months at a dietary concentration of 400 ppm had no effect on the estrogen-induced LH surge in female Fischer-344 rats (Study FR2, Stevens et al., 1999).
The weight of evidence indicates that atrazine induces mammary tumors in female SD rats by suppressing the LH surge, thereby supporting a state of persistent estrous and prolonged exposure to endogenous estrogen and prolactin. The results are consistent, within and across studies as discussed above, with respect to dose and duration of exposure needed to produce the neuroendocrine-mediated effects. The results are highly specific to the female SD rat because another strain of rat, the Fischer-344, is insensitive to this mode of action. Neither the LH surge, the estrous cycle, nor mammary tumor incidence are altered in atrazine-treated female Fischer-344 rats compared with untreated controls. The Fischer-344 rat is non-responsive because it maintains a normal estrous cycle through a greater portion of its life span, followed by a decline in ovarian estrogens similar to the human female (Table 2).
TABLE 2.
Species Differences in Reproductive Senescence (Adapted from Chapin et al., 1996)
| Parameter | SD rat | Fischer-344 rat | Women |
| Start of Senescence (% of normal lifespan) | 30–40% | 60–70% | 60–70% |
| Principal cause of senescence | Hypothalmic failure to stimulate LH/FSH | Hypothalmic failure to control prolactin surges | Depletion of ovarian follicle content |
| LH surge capability | Lost | Maintained | Maintained |
| Predominant cycle pattern | Persistent estrus | Pseudopregnancy episodes | Menopause |
| Estrogen/progesterone ratio | Elevated/prolonged | Reduced | Reduced |
| Prolactin secretion | Persistently elevated | Episodically elevated | Reduced |
| Spontaneous mammary tumor incidence (lifetime) | 30–40% | 2–5% | 8–10% |
| Principal known factors that increase MT Risk | Prolactin, estrogen, chemical mutagens | Prolactin, estrogen, chemical mutagens | Estrogen, nulliparity, family history |
| Prolactin dependence | High | Medium | None |
Structure-activity assessments indicate that the chlorotriazines share this common mechanism of action. When other functional groups are substituted for the chlorine atom on the triazine nucleus (S-methyl, O-methyl, and hydroxy), there was no effect of treatment on mammary tumor incidence in chronic bioassays in SD rats (Supplementary Figure S1). Terbumeton, which has an O-methyl group substitution, was the only exception (Stevens et al., 1994).
Relevance of the Proposed Mode of Action for Women
Folliculogenesis during the mammalian ovarian cycle is regulated by relatively low levels of circulating LH and follicule-stimulating hormone (FSH) produced by a “basal” mode of gonadotropin secretion, which is controlled by the negative feedback action of ovarian steroids, primarily estradiol. Ovulation, which occurs at the end of the follicular phase, is achieved by a massive discharge of LH that is generated by a “surge” mode of gonadotropin secretion. Both modes of gonadotropin secretion are dependent on hypophysiotropic stimulation from the hypothalamus in the form of GnRH. Basal gonadotropin secretion is dependent on the intermittent release of brief “pulses” of GnRH from the hypothalamus into the hypophysial portal circulation, and this pulsatile secretion occurs approximately every 20–60 min depending on species (Freeman, 2006; Zeleznik and Pohl, 2006). The neural mechanism that generates pulsatile GnRH release is resident in the medial-basal hypothalamus (MBH) (Blake and Sawyer, 1974; Krey et al., 1975) and is termed the GnRH pulse generator (Knobil, 1980). While incompletely understood, emerging models of GnRH pulse generation suggest similarity across species (Lehman et al., 2010; Wakabayashi et al., 2010). Intermittent GnRH stimulation of the gonadotropin-secreting cells of the pituitary, which is obligatory for sustained gonadotropin secretion (Belchetz et al., 1978), induces a corresponding pulsatile pattern of LH secretion into the systemic circulation. Thus, the pulsatile pattern of LH concentration in peripheral blood serves as a surrogate for the GnRH pulse generator (Plant, 1986). Surge gonadotropin secretion is achieved either by amplification of pituitary gonadotropin responsiveness to GnRH pulses or by activation of a hypothalamic GnRH surge generator that results in a large and relatively sustained discharge of GnRH into the hypophysial portal circulation. The relative importance of these mechanisms for generating gonadotropin surges in mammals is species dependent.
The estrous cycle in rodents is short, and the pre-ovulatory LH surge is brief, governed by the light-dark cycle, with the hypothalamus playing a key role in timing the pre-ovulatory LH surge (Freeman, 2006). Every afternoon during a critical period spanning ∼2 h, the rodent brain generates a circadian signal, which in combination with the positive feedback action exerted by the elevated levels of circulating estradiol on proestrus, activates the GnRH surge generator that triggers the LH surge. Thus, the role of the rodent brain in controlling the timing of ovulation may be viewed as deterministic. The hypothalamic neurons synthesizing GnRH in the rodent brain are located primarily in the pre-optic area (POA), with few in the more caudal MBH (Silverman et al., 1994). Release of GnRH into the portal circulation is dependent on increased activity of norepinephrine neurons located in the brainstem (Sawyer, 1995; Simpkins et al., 1979a,b; Herbison, 1997; Wise et al., 1997, 1999) and on kisspeptin neurons located in the anteroventral periventricular nucleus of the POA (Oakley et al., 2009), the site of positive feedback of ovarian estradiol (Goodman, 1978). As such, inhibition of neural activity early in the afternoon of proestrus by administration of barbiturate or other centrally-acting drugs blocks the pre-ovulatory LH surge in rats (Freeman, 2006, Goodman and Knobil, 1981). Another fundamental characteristic of the GnRH surge mechanism in the rodent is its susceptibility to perinatal programming by testicular androgens such that the hypothalamus of the adult male rat is unable to respond to the positive feedback of estradiol (Feder, 1981).
The human menstrual cycle is long and exhibits a protracted pre-ovulatory LH surge spanning 2–3 days and ends with menses, due to the involution of the corpus luteum and the resulting decline in estrogens and progesterone (Hall, 2009; Zeleznik and Pohl, 2006). In contrast to the rodent, the role of the primate brain, although obligatory in driving the menstrual cycle, is permissive rather than deterministic. In the human female, the preovulatory LH surge appears to occur in the absence of a GnRH surge (Hall et al., 1994; Martin et al., 1998; Ottowitz et al., 2008) and unfolds in the face of an unchanging frequency of the GnRH pulse generator, as reflected by pulsatile LH release (Adams et al., 1994; Martin et al., 1998). Thus, it must be concluded that the LH surge is timed and elicited by a positive feedback action of estradiol at the level of the pituitary to dramatically enhance responsiveness to pulsatile GnRH stimulation in women. Indeed, the spontaneous menstrual cycle can be recapitulated in women deficient in GnRH, simply by the exogenous administration of a series of pulses of GnRH (Hall, 2009; Filicori et al., 1986; Martin et al., 1998; Santoro et al., 1986). These findings indicate that the entire pattern of gonadotropin secretion throughout the human menstrual cycle is governed by the negative and positive feedback actions of ovarian estradiol at the level of the pituitary. Similar observations regarding pulsatile GnRH replacement have been made in female rhesus monkey following hypothalamic lesions that abolish endogenous GnRH release (Knobil et al., 1980; Zeleznik and Pohl, 2006). In the monkey, however, the preovulatory LH surge is associated with a GnRH surge (Pau et al., 1993), which, in contrast to the rat, the GnRH surge is timed solely by the positive feedback action of estradiol acting at the level of the MBH-pituitary unit (Karsch et al., 1973). Unlike the rodent, many GnRH neurons in primates are located in the MBH (Silverman et al., 1994).
In contrast to rodents (Simpkins et al., 1985), inhibition of neural activity by administration of barbiturates or other centrally acting drugs does not affect the estrogen induced pre-ovulatory LH surge in women or the female monkey (Knobil, 1974; Weiss et al., 1977). The hypothalamic control of the pre-ovulatory LH surge in primates is emancipated from perinatal programming by testicular androgen secretion, as reflected by the ability of estradiol to elicit LH surges in adult male rhesus monkeys castrated postpubertally, and either bearing a subcutaneous ovarian transplant (Norman and Spies, 1986) or receiving estradiol to mimic a late follicular phase pattern of circulating estradiol (Karsch et al., 1973).
Reproductive aging in rodents and women is also different. In female SD rats, reproductive senescence occurs as a result of a breakdown of the brain regulation of the LH surge, while the ovaries remain functional very late into life (Aschheim, 1976; Meites et al., 1978). The decline in reproductive function is primarily a result of (1) the inability of brain norepinephrine neurons to transmit the estrogen signal to GnRH neurons (Meites et al., 1978; Simpkins et al., 1979a,b; Weiss et al., 1977), (2) the inability to stimulate a pre-ovulatory LH surge resulting in the maintenance of ovarian follicles, and (3) the persistent secretion of estrogens. Increased secretion of estrogens causes a persistent state of hyperprolactinemia (Sarkar et al., 1982). Thus in the SD rat, reproductive senescence is characterized by persistent hyperestrogenemia and hyperprolactinemia with low levels of LH and FSH.
In contrast to female SD rats, which begin to display episodes of persistent estrus as early as six months of age, female Fischer-344 rats maintain normal 4–5 day estrous cycles through 18 months of age (Estes et al., 1982; Estes and Simpkins, 1984; Lu et al., 1979, 1980). At two years of age, Fischer-344 rats display normal estrous cycles, interspersed with periods where corpus lutea (CLs) are maintained for extended periods, accompanied by secretion of ovarian progesterone (Estes and Simpkins, 1984; Lu et al., 1980). Thus, in the aging Fischer-344 rat, the hypothalamic-pituitary-gonadal (HPG) axis maintains the ability to mediate an estrogen-induced LH surge although it cannot inhibit episodic prolactin surges after the LH surge has occurred (Estes and Simpkins, 1982). This creates an endocrine state called pseudopregnancy (Beach et al., 1975; Estes et al., 1982) because prolactin secretion is prolonged and CLs are maintained (Estes et al., 1982).
In women, reproductive aging and the occurrence of menopause is due to the exhaustion of ovarian follicles and a diminution of ovarian estrogen (Burger et al., 2007; Hale and Burger, 2005; Hale and Burger, 2009; Rance, 2009; Schiff and Wilson, 1978). During the menopause, however, the ability of exogenous estrogens to induce a pre-ovulatory LH surge is maintained, albeit slightly diminished (Santoro, 2005). In the absence of endogenous estrogen secretion, the feedback signal from the ovary governing the cyclic pattern of gonadotropin secretion is lost, and a constant hypergonadotropic state is produced as a result of GnRH pulse generator activity that is robustly maintained in the postmenopausal women. Post-menopausal estrogens and prolactin are very low, but LH and FSH secretions are greatly elevated (Burger et al., 2007; Hale and Burger, 2005; Hale and Burger, 2009; Rance, 2009; Schiff and Wilson, 1978). The major differences between aging of the human menstrual cycle and rodent estrous cycles are summarized in Table 2 and discussed specifically for atrazine in Chapin et al. (1996).
Possible Alternative Modes of Actions
Genotoxicity.
Atrazine is one of the most extensively tested chemicals for genotoxicity. Published studies include in vitro and in vivo experiments with mammalian cells, microbial systems, invertebrates, fish, and several plant species (Supplementary Tables S1 and S2; Brusick, 1994). Utilizing a weight of the evidence approach developed by the International Commission for Protection against Environmental Mutagens and Carcinogens (Brusick et al., 1992; Lohman et al., 1992), Brusick (1994) evaluated all published and registrant studies on the genotoxicity of atrazine. The evaluation was later expanded to include all studies published through December 2010. Seventeen of the 23 gene mutation studies following in vitro exposure of mammalian cell lines to atrazine were negative (Supplementary Table S1); gene mutation studies on atrazine metabolites (hydroxyatrazine, deethyl atrazine [DEA], deisopropylatrazine [DIA], and diaminochlorotriazine [DACT]) were also negative (Supplementary Table S3). With the exception of studies conducted in plant-based systems (Supplementary Table S4), the majority of the studies show that atrazine or its metabolites do not directly interact with DNA resulting in mutation. Hence, governmental agencies responsible for reviewing and interpreting toxicology data have concluded that atrazine is not genotoxic (APVMA, 2004, 2008; FAO/WHO, 2009; International Agency for Research on Cancer (IARC), 1999; USEPA, 2003a).
While the results shown in Supplementary Tables S1–S3 are predominately negative, there are a few isolated positive results. Isolated conflicting responses are not uncommon when a chemical is evaluated in a large number of studies. Such isolated occurrences are considered spurious responses due to normal variability or technical inadequacies (Brusick et al., 1998). Multiple positive responses tended to occur for those methods that detect chromosomal damage (structural or numerical changes) or DNA strand breakage (Gebel et al., 1997; Pino et al., 1988; Ribas et al., 1995; Singh et al., 2008). Unlike tests measuring gene mutation, these types of studies can give positive responses due to cytotoxic or other secondary effects that are not true indicators of a genotoxic mode of action. Atrazine has been reported to cause lipid peroxidation in vivo at high doses (Singh et al., 2011), and oxidative peroxidation is known to cause cell death and necrosis, which will yield false-positive effects in tests such as alkaline elution techniques and the comet assay (Choucroun et al., 2001; Pool-Zobel et al., 1999). A study by Singh et al. (2008) reported increased micronuclei and comet tail lengths in liver cells obtained from rats exposed to 300 mg/kg of atrazine for 7, 14, and 21 days. This dose level appeared to be high enough to induce oxidative damage in the target tissue, as the reported positive effects were minimized by the co-administration of an antioxidant. Consequently, the increased levels of DNA strand breakage in this and other elution assays may have been secondary to toxicity-induced apoptosis and not from direct effects of atrazine on liver cell DNA.
Some studies report chromosomal changes in atrazine-exposed cells evaluated using flow-cytometry (Biradar and Rayburn, 1995a,b; Rayburn et al., 2001; Taets et al., 1998). Flow cytometry quantifies changes in whole-cell DNA or individual chromosomes through analysis of coefficients of variation (CVs) in flow histograms. Biradar and Rayburn reported that atrazine caused a statistically significant increase in the CV of DNA content of G1 cells at concentrations as low as 0.005μM. In an assessment of the Biradar and Rayburn flow studies, Kligerman et al. (2000a) concluded that flow cytometry and the use of changes in CV is not a reliable method for the determination of clastogenicity. Application of flow analysis to chromosomal CV is generally subjected to computer-generated analysis of the results. Biradar and Rayburn (1995a) were unable to find statistically significant increases in the atrazine results using computer-based analysis and instead applied a unique manual approach to their studies to generate positive effects. When utilizing methods that permit the direct visualization of chromosomal damage, the vast majority of atrazine studies were negative (Adler, 1980; Basler and Rohrborn, 1978; Meisner et al., 1992; Kligerman et al., 2000a,b; Ribas et al., 1998).
Supplementary Table S4 gives the results of genotoxicity studies in plants and fungi. Atrazine is active in most plant-based tests as well as in a number of tests employing fungi. Compared with the data from tests in animals or animal cells, these results indicate that atrazine is handled differently in plant-based systems. Although there may be multiple reasons for the differences, clearly unique attributes of animal metabolism and detoxification processes not present in plant-based systems are likely most relevant. Thus, these studies are of minimal value in evaluating the genotoxicity potential of atrazine.
Except for studies on genotoxicity in plants and fungi, there is no reason to give more weight to in vivo studies versus in vitro studies because the chloro- and hydroxyl- metabolites of atrazine tested for mutagenic potential in in vitro are the predominant metabolites formed in vivo (Kim et al., 2010; McMullin et al., 2003). Oxidative metabolism of atrazine (Supplementary Figure S1) has been well characterized in vitro and in vivo in rodents (Hanoika et al., 1999), nonhuman primates (Hui et al., 2011; Maibach et al., 2001) and humans (Ademola et al., 1993; Buchholtz et al., 1999; Joo et al., 2010; Maibach et al., 2001). Although phase II metabolites such as glutathione conjugates and mercapturates (Supplementary Figure S2) are unique to in vivo systems and their formation in vivo might be a basis for assigning more weight to in vivo studies, these metabolites appear to be toxicologically less active (USEPA, 2002; Yi, Simpkins, and Breckenridge, in preparation) and are rapidly eliminated (Hui et al., 2011; Maibach et al., 2001). Overall, considering the results from both in vitro and in vivo models, the weight-of-evidence supports the conclusion that atrazine and its phase I and phase II metabolites are unlikely to be genotoxic.
Estrogen receptor agonist/antagonist.
Eldridge et al. (2008) recently summarized the results of more than 40 studies that evaluated the potential of atrazine to bind to or interact with estrogen receptors (ERs) in 17 estrogen-dependent systems in vivo or in more than a dozen different ER reporter or binding systems in vitro. None of the 24 studies that evaluated the in vivo expression of estrogen following atrazine exposure showed any estrogen-agonist effects, irrespective of the species or the strain of rat evaluated (Supplementary Table S5). In 10 of 15 studies, atrazine inhibited the action of estrogen in vivo, but only when the administered dose of atrazine was at least 100,000 times greater than the dose of estradiol. Four other studies showed no effect of atrazine at high concentrations and one study was inconclusive. None of the 18 studies reported any stimulatory effect of atrazine on estrogenic expression systems in vitro; weak inhibition of estrogen was reported in 3 of 10 studies (Supplementary Table S6). Weak competition between atrazine and estrogen for ERα or ERβ was noted in 10 of 17 studies that used donor tissues from a variety of species including two strains of rats (Supplementary Table S7). In some studies, weak competition was seen only when atrazine was pre-incubated with the receptor before estradiol was added to the media.
Overall, the weight of evidence indicates that atrazine does not act as an estrogen agonist. Atrazine may act as a weak ER antagonist if present in tissues at concentrations that are more than 100,000-fold greater than the concentration of estradiol. This is unlikely to occur under conditions of human exposure. For example, a 60 kg person consuming 2 l of water containing atrazine at the Maximum Contaminant Level (MCL) of 3 ppb would receive a dose of 0.1 μg atrazine/kg. Assuming the human plasma concentration scaled 1:1 to the plasma concentration determined in the rat (See Supplementary Figs. S4 and S5), then the expected steady state plasma concentration of total triazine in humans exposed at the MCL would be 0.1 ppb (4.8 × 10−10M). Total chlorotriazine concentration in plasma is expected to be approximately fivefold lower in humans because the concentration of total chlorotriazine eliminated in urine of men comprised only 14.4% of the administered dose (Maibach et al., 2001). Tissue concentration of atrazine is likely to be at parity with plasma at steady state because plasma:tissue partition coefficients are near 1.0 (P. S. Coder, unpublished data). The Kd for estradiol binding to the estrogen receptor is 7.7 × 10−10M (Laws et al., 2006; Rider et al., 2009). Thus, under conditions of human exposure, the concentration of atrazine equivalents in tissue is approximately the same as the Kd for estradiol. Therefore, it is unlikely that atrazine will antagonize the binding of estradiol to its receptor in vivo. Even if atrazine was a weak estrogen receptor antagonist in vivo, the consequence would likely be a diminution of estrogenic activity rather than an increase called for by the results of the carcinogenicity studies on atrazine.
Induction of messenger RNA for aromatase and/or aromatase enzyme activity.
In vitro studies (Sanderson et al., 2000, 2001) indicate that high concentrations of atrazine in 0.1% dimethyl sulfoxide increases aromatase activity and gene expression in the H295R adrenocorticocarcinoma cell line and in JEG-3 placental choriocarcinoma cells but not in the MCF-7 breast cancer cells or the R2C rat Leydig cell cancer cells (Heneweer et al., 2004; Supplementary Table S8). Carp hepatocytes treated with atrazine in vitro did not show altered vitellogenin synthesis (Sanderson et al., 2001). In the majority of these studies, statistically significant induction of aromatase activity or gene expression was observed only at the concentration near the solubility limit of atrazine (30μM; 6.5 ppm) although effects on aromatase have been reported in some studies at 10μM but not at 1μM of atrazine (Yi, Kim, Breckenridge, and Simpkins, in preparation). Ohno et al. (2004) reported that aromatase activity was unaffected by atrazine in KGN ovarian granulosa carcinoma cells and Keller and McClellan-Green (2004) found no effect of atrazine in an immortalized sea turtle cell line. The results from primary ovarian granulosa and endometrial stromal cells are mixed. Tinfo et al. (2011) reported a 2- to 2.5-fold increase in aromatase activity with atrazine but no change in aromatase message or protein in rat granulosa cells. Holloway et al. (2008) did not see a dose-response in human granulosa cells exposed ex vivo to atrazine and found no effect of atrazine on human endometrial stromal cells (Supplementary Table S8). Crain et al. (1997) and Spiteri et al. (1999) reported increased aromatase activity in alligator eggs painted with 14 ppm atrazine dissolved in ethanol. Even under these extreme conditions, not a single male was converted to a female. In contrast, when the male eggs were treated with 14 ppb of estradiol, 100% of the males were feminized. This indicates that the reported changes in aromatase in alligator eggs were of no functional consequence. A study in chicken eggs failed to show any effect on atrazine on aromatase activity (Matsushita et al., 2006).
The effect of other chlorotriazines such as simazine, as well as primary (Supplementary Figure S1) and secondary metabolites of atrazine (Supplementary Figure S2) on aromatase activity has been investigated (Supplementary Table S8). Exposure to DEA and DIA resulted in weaker responses than did exposure to atrazine, whereas DACT, the major animal chlorometabolite of atrazine, had no effect (Sanderson et al., 2000; Tinfo et al., 2011; Yi, Kim, Breckenridge, and Simpkins, in preparation). The glutathione and mercapturate conjugates of atrazine, which are secondary metabolites of atrazine, as well as hydroxyatrazine (HA) and ammeline, which are major plant metabolites, had no effect on aromatase messenger RNA (mRNA) in H295R or JEG3 cells (Yi, Kim, Breckenridge, and Simpkins, in preparation).
The weight of the evidence indicates that high micromolar concentrations of atrazine and to a lesser extent, its mono-dealkylated metabolites at concentrations above the solubility limit in aqueous media, cause an approximately twofold increase in expression or activity of aromatase in sensitive cell lines capable of steroidogenesis. Neither the major atrazine chlorometabolite, DACT, nor the glutathione or mecapturates of atrazine had any effect; HA or ammeline, the predominant plant metabolites of atrazine also were negative (Yi, Kim, Breckenridge, and Simpkins, in preparation). Because atrazine and the mono-dealkylated metabolites of atrazine are rapidly metabolized after ingestion, these chemicals are not expected to persist long enough to have any effect on aromatase activity in vivo (APVMA, 2010; FAO/WHO, 2009; Yi, Simpkins, and Breckenridge, in preparation). As discussed in the previous section, tissues concentration of atrazine equivalents will not be greater than 10−10M for humans exposed to atrazine at the MCL. In the rat, a repeated oral dose of 0.25 mg/kg for 5 days would be needed to produce a tissue concentration of 1μM atrazine (Supplementary Fig. S5), a concentration which clearly had no effect on aromatase activity or expression when evaluated in any in vitro system. However, atrazine is rapidly metabolized to less active metabolites, most notably DACT and the concentration of atrazine in plasma is short lived (tmax = 30 min; half-life of clearance from plasma is 30–45 min; Kim et al., 2010). In contrast, in the in vitro aromatase models, atrazine had to be present in media for at least 2 h at a concentration above 1μM to significantly affect aromatase mRNA expression (Yi, Kim, Breckenridge, and Simpkins, in preparation).
Studies on intact animals (Supplementary Table S9) indicate that neither the induction of mRNA aromatase gene expression nor the increased aromatase activity is observed in atrazine-treated animals. Hayes et al. (2002) argued that changes in circulating or whole body testosterone reflect an alteration in aromatase activity in atrazine-exposed Xenopus laevis. However, this cannot be taken as evidence that atrazine is altering aromatase expression because plasma hormone levels change dynamically from moment to moment and are subject to a host of variables (age, sex, season, time of day, and stress level) that are uncontrolled in environmental studies (Kang et al., 1995). Other studies have failed to observe a relationship between triazine exposure and testosterone levels in wild-caught Xenopus laevis (Hecker et al., 2004). Furthermore, the reported “feminization” effect of atrazine on amphibian gonadal development (Hayes et al., 2002), which has been attributed to an effect on aromatase, has not been replicated by other laboratories (Kloas et al., 2009a). Lastly, when changes in aromatase mRNA were evaluated in amphibians, no effects of atrazine exposure were found (Hecker et al., 2004, 2005a,b; Kloas et al., 2009b).
High doses of atrazine (50 or 200 mg/kg/day) administered daily to 60-day-old male Wistar rats for 1, 2, 3, 4, or 21 days resulted in slight and highly variable increases in serum androstenedione, testosterone, estradiol, estrone, corticosterone, and progesterone, as quantified by radio-immuno-assay (Modic et al., 2004). Although the acute effect of high doses of atrazine on corticosterone and progesterone have been confirmed in subsequent studies (Fraites et al., 2009; Laws et al., 2009), the effect on androstenedione testosterone, estradiol, and estrone has not been confirmed by liquid chromatography with tandem-mass spectrometry (LC-MS-MS) using internal 13C-stable label for identification and unlabeled standards for quantification (Handa, 2010).
Overall, the weight of the evidence indicates that high micromolar concentrations of atrazine and its mono-dealkylated metabolites, but not DACT, caused an approximate twofold increase in aromatase mRNA and activity in immortalized cell cultures in vitro. The absence of effects in vivo is likely attributed to the rapid metabolism to inactive metabolites (Kim et al., 2010). Hence, the in vitro mode of action is not likely relevant to intact mammals.
Delayed development of the mammary gland
It has been proposed that a delay in the development and differentiation of mammary gland increases susceptibility to physical, chemical, or viral agents that initiate cancer (Russo et al., 1992; Trichopoulos et al., 2008), whereas factors that delay the onset of menarche are protective (Russo et al., 2001, 2006). In utero exposure of female Long-Evans rats to 100 mg/kg/day atrazine (Rayner et al., 2005) or a mixture of atrazine and its metabolites (Enoch et al., 2007) has been reported to delay postnatal mammary gland development using, for the most part, subjective rating scales. When an unbiased, blinded quantitative assessment of mammary glands (ductal length, ductal area, epithelial area, epithelial density, and cell proliferation) was used, there was no evidence of a delay in mammary gland development at doses of atrazine up to 100 mg/kg/day (Hovey et al., 2010). Hovey and colleagues controlled for the effect of treatment-related reductions in body weight gain in the pregnant dams on subsequent pup development, as well as for atrazine-induced delay in sexual maturation, which has been reported in atrazine-treated females (Ashby et al., 2002; Laws et al., 2000). Delay in sexual maturation is known to affect mammary gland development because growth during puberty is allometric and sensitive to hormonal changes during the estrous cycle (Hovey et al., 2002). The results from a second laboratory confirmed the absence of an atrazine effect on postnatal mammary gland development at doses as high as 100 mg/kg/day (Davis et al., 2011). In addition, female SD rats exposed to atrazine in utero and throughout life did not have an increased incidence of mammary tumors compared with controls, suggesting that in utero exposure to atrazine did not contribute to an increased risk of developing mammary tumors later in life (Stevens et al., 1999). However, this study was underpowered (N = 30 per group); thus small effects may not have been detectable.
Overall, there is no compelling evidence to indicate that in utero exposure to atrazine increases the incidence of mammary tumors in rodents. There is a clear trend among females in developed countries to experience an earlier onset of puberty, leading to an earlier rather than a delayed development of breasts in young girls. The prolongation of exposure to endogenous estrogen increases breast cancer risk later in life (Russo et al., 2006; Trichopoulos et al., 2008).
Conclusions of Animal Mode of Action Research
The hypothesis that decreased latency and increased incidence of mammary tumors in female SD rats is mediated through the key events shown in Figure 2, is strongly supported by the data. This conclusion is consistent with a detailed analysis conducted by FAO/WHO (2009; Appendix 1, pp 118–124).
The mechanism of action underlying the effect of atrazine on the HPG axis is unknown because the molecular target(s) have yet to be identified. Some hypotheses have been ruled out but none has been established (Cooper et al., 2007). Recent work has allowed for a comparison of the effect of atrazine on the HPG and HPA axes in female Wistar, Long-Evans, and SD rats. Although the Wistar and inbred strains of Long-Evans rats have not been evaluated in traditional carcinogenicity studies, both SD and Long-Evans rats were derived from the original Wistar albino rat (Lindsey, 1979) and both Wistar and Long-Evans rats respond to high doses of atrazine with reduced LH surges (Cooper et al., 2000; Foradori et al., 2009a,b). New mechanistic studies suggest that the effect of atrazine on the LH surge may be secondary to an activation of corticosterone release in the rat (Fraites et al., 2009; Laws et al., 2009). However, the effect of atrazine on the estrogen plus progesterone induced LH surge in female Wistar rats was unaffected by adrenalectomy, whereas the suppressive effect of atrazine on pulsatile LH release was abolished (Foradori et al., 2011). This suggests that the effect of atrazine on the final common hypothalamic pathway (i.e., suppression of pulsatile versus surge release of GnRH/LH) may be mediated via distinct mechanisms in rodents. This dichotomy in the response to atrazine is intriguing because the control mechanism of pulsatile GnRH release in nonhuman primate and women is similar to the rodent control mechanisms. In contrast, major differences exist in the regulation of the LH surge in rodents compared with nonhuman primates and women. In the human female, the preovulatory LH surge appears to occur in the absence of a GnRH surge (Hall et al., 1994; Martin et al., 1998; Ottowitz et al., 2008).
The proposed mode of action underlying the decreased latency and increased incidence of mammary tumor response in the female SD rat is unlikely to be relevant to women due to the differences in reproductive aging of rodents and women. Although it is plausible that high doses of atrazine could suppress the LH surge in women by interfering with GnRH pulse generation, the consequence of that suppression would not result in an increased incidence of breast cancer in women. Therefore, atrazine was placed in the lower half of the biological plausibility scale described by Adami et al. (2011). There is little uncertainty in this conclusion because of the quality and the breadth of the studies that underlie the assessment. This conclusion is consistent with independent reviews by regulatory authorities around the world (AVMPA, 2004, 2008; FAO/WHO, 2009; IARC, 1999; USEPA, 2003b, 2006).
The evidence supporting alternative modes of action evaluated in this paper (genotoxicity, estrogenicity, aromatase, and delayed mammary gland development) is not compelling and do not indicate that these alternate modes of action play a role in the mammary tumor response observed in atrazine-treated female SD rats. These alternate modes of action are also unlikely to contribute to the occurrence of breast cancer in women, especially at doses to which women could conceivably be exposed.
Atrazine exposure and breast cancer: epidemiological evidence.
The potential association between atrazine exposure and the risk of breast cancer has been evaluated in epidemiological studies of incidence or mortality among women involved in triazine manufacture (MacLennan et al., 2002, 2003); women who lived on farms (Engel et al., 2005) or in proximity to farms and/or were associated with agricultural work (Reynolds et al., 2004); and women potentially exposed to atrazine via drinking water (McElroy et al., 2007). Ecological studies have also been conducted in which breast cancer incidence was examined across geographic areas defined by triazine exposure (Hopenhayn-Rich et al., 2002; Kettles et al., 1997; Mills and Yang, 2006; Muir et al., 2004; Reynolds et al., 2005).
Cohort studies on women involved with triazine manufacture.
Breast cancer incidence was examined in women employed in the manufacture and formulation of triazines (primarily atrazine). Among 184 women employed in a Louisiana plant who were followed from 1985 to 1997, one was diagnosed with breast cancer (expected = 1.5) (MacLennan et al., 2002). In a parallel study of mortality at this same plant, there was one death from breast cancer during 1970–97 among 211 female employees (expected = 0.6) (MacLennan et al., 2003).
The quality of these studies was classified as acceptable. One strength was that exposure to atrazine at this production facility could be quantified using job histories in a fashion similar to that employed by Hessel et al. (2004) in a study of prostate cancer at this facility. The major weakness was that there were only a few breast cancer cases and thus the study did not have adequate statistical power.
Cohort studies on women with potential exposure to atrazine or triazines on farms.
Spouses of professional pesticide applicators, recruited to the Agricultural Health Study in Iowa and North Carolina from 1993 through 1997, provided information about their own herbicide exposures and potential risk factors for breast cancer (Engel et al., 2005). Of the 309 women who went on to be diagnosed with breast cancer through the year 2000, only 11 (3.6%) had directly used atrazine; this was similar to the percentage for controls diagnosed with breast cancer (4.4%, relative risk [RR] = 0.7, 95% confidence interval [CI] 0.4–1.2). Among women who did not apply any pesticide, the percentage of their husbands who had done so was similar for cases and controls (74.8 vs. 73.8%, respectively, RR 1.1, 95% CI 0.7–1.6). Similar results were obtained when the assessment was based on 13 women with breast cancer who reported using triazines (Supplementary Table S10).
The study was classified as acceptable. The strength of the study was its prospective design with exposure assessment prior to disease diagnosis. The weakness was that there were too few cases through the end of 2000 to provide adequate statistical power.
The California Teachers study evaluated residential proximity to areas of simazine application and incidence of breast cancer in these regions (Reynolds et al., 2004). In this study, 1552 participants were diagnosed with breast cancer during 1996 through 1999. Using data obtained in the California Pesticide Use Reporting System for 1993 through 1995, the incidence of breast cancer was examined in relation to the amount of various pesticides and herbicides applied in the area within one-half mile of the residence of each participant. Breast cancer incidence did not vary appreciably across levels of simazine application compared with women living in an area in which less than one pound of simazine per square mile had been applied; the relative incidence was 0.9, 0.9, and 1.1 for women living in areas of simazine application of 1–13 pounds per square mile, 14–40 pounds per square mile and 41 or more pounds per square mile, respectively. A subset analysis of women who lived within 0.5 mile radius of the section of land sprayed or who lived within the section of land sprayed, yielded hazard ratios of 1.17 (CI = 0.82–1.67) and 1.44 (CI = 1.01–2.05), respectively, based upon an unspecified numbers of cases.
This study was categorized as acceptable. Strengths of the study included a large number of breast cancer cases and the control for suspected risk factors for breast cancer. However, the relationship between residential proximity to application of simazine and actual exposure was not quantified. Furthermore, confounding for co-exposure to other pesticides, which were used in proximity to the residence of women with breast cancer, was not controlled.
Case-control study on women with potential exposure to atrazine via groundwater.
This study evaluated the amount of atrazine in the groundwater near residences of women with and without breast cancer (McElroy et al., 2007). Female residents of rural Wisconsin (ages 20 through 79 years) who had been diagnosed with breast cancer during 1987 through 2000 were identified (n = 3275), as were 3669 demographically-similar control women. All women were interviewed with respect to exposure and characteristics relevant to the risk of breast cancer. Using data from surveys of atrazine levels in well water in 1994, 1996, and 2001, the investigators assigned an atrazine level to each woman’s own well water, based on the proximity of the well to those wells for which atrazine concentration had been determined. The distribution of estimated levels of atrazine in well water was nearly identical between the wells of cases and those of the controls. For women whose water was estimated to contain less than 0.15 ppb of atrazine, the relative risk of breast cancer in those whose water had 1–3 ppb atrazine was 1.1 (95% CI 0.9–1.4), adjusted for other breast cancer risk factors. Only nine women (five cases and four controls) had wells with an atrazine concentration estimated at greater than 3 ppb, too small a number to draw any conclusion.
Study quality was classified as acceptable. Strengths included the fact that 83 percent (11,001) of eligible case women and 83.1% (11,494) of eligible age-matched control women participated in the study. A non-biased approach was used to assign residence and well water concentration of atrazine or atrazine plus its chlorometabolites, but there was no control for duration of time in residence. The authors controlled for confounding by potential breast cancer risk factors (Supplementary Table S11). Weaknesses in the study included the fact that atrazine exposure was not assessed during a period of time that was etiologically relevant to development of breast cancer. In general, atrazine concentrations in groundwater were low (0.15–3 ppb) and varied over too narrow a concentration range to plausibly permit the documentation of a dose-response.
Ecological studies on breast cancer and triazines use in geographical regions.
Because ecological studies are generally hypothesis generating rather than hypothesis testing, the quality of individual studies in this category are not discussed except for the study of Muir et al. (2004), which appeared to have major limitations.
Reynolds et al. (2005) characterized residences in each square mile of the state of California in terms of estimated pesticide application during 1990 through 1997. The incidence of breast cancer during 1988 through 1997 bore no relation to the quantity of simazine application: rates were identical among women living in areas in the upper-fourth of the distribution of simazine application and those living in areas where virtually no simazine had been applied (RR 1.0, 95% CI 0.95–1.07).
A county-level study in California among Latin women during 1988 through 2000 similarly found no association between the incidence of breast cancer and the quantity of application of either simazine or atrazine (Mills and Yang, 2006). After adjusting for age, socioeconomic status, and fertility rates, the RRs for low, mid, and high “exposed” groups ranged from 0.83 to 0.87, 0.94 to 0.91, and 0.86 to 0.87, respectively, for assessment periods from 1988 to 1993 and from 1994 to 1999.
Two studies in Kentucky correlated breast cancer incidence with county-level exposure to triazines. The latter was estimated by jointly considering triazine levels in water samples, the amount of corn crops grown and county-specific pesticide use data. The first of these studies (Kettles et al., 1997) observed that in 1991 through 1992, the adjusted incidence of breast cancer in the 24 counties with the greatest estimated triazine exposure was nine percent higher than in the 54 counties with the lowest exposure. In 1993–1994, the corresponding figure was a 22% increase. In contrast, the second study (Hopenhayn-Rich et al., 2002) observed a 2% lower breast cancer incidence during 1993 through 1997 in counties with the highest, compared with the lowest, estimated atrazine exposures.
One additional ecologic study of breast cancer and atrazine (and cyanazine), in groupings of electoral wards in two districts of England (Muir et al., 2004), was not considered because the incidence rates varied across geographic groupings to such an implausibly large degree, more than 24-fold, that chance must have been exerting a strong influence. This variability was probably due to the evaluation of small geographic areas and the narrow window of time (1989 through 1991).
In summary, the association between exposure to atrazine or triazines exposure and breast cancer was largely null. These results are consistent with conclusions reached by various regulatory agencies that atrazine or the triazines are “unclassifiable as to carcinogenicity” (IARC, 1999) or that atrazine is not likely to be carcinogenic (USEPA, 2003b, 2006) with regard to any cancer.
CONCLUSIONS
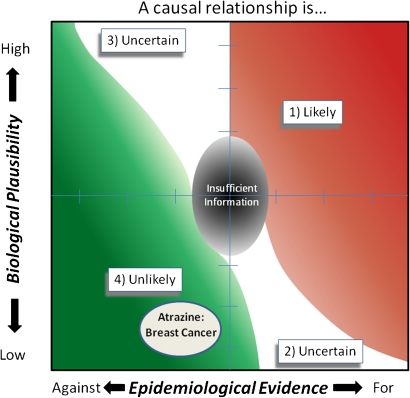
Using the framework outlined by Adami et al. (2011), atrazine is placed within the lower left hand quadrant of the framework categorical diagram (Fig. 6), which classifies atrazine in Category 4 unlikely. This conclusion is based upon (1) epidemiological evidence suggesting that exposure to atrazine is not associated with the occurrence of breast cancer in women and (2) the lack of a plausible mode of action for the occurrence of atrazine-related breast cancer in women.
FIG. 6.
Summary of the weight of evidence of the mode of action (biological plausibility) and the epidemiological evidence for a causal relationship between atrazine exposure and the occurrence of breast cancer in women.
This classification took into account a substantial body of literature on the mode of action plus the absence of positive epidemiological evidence, recognizing that the majority of the relevant studies were underpowered and might not detect small changes in breast cancer incidence. This conclusion is consistent with previous schemes for the classification of the carcinogenic potential of atrazine in humans (Table 3). These assessments collectively have taken into account the animal and all the human epidemiology evidence (IARC, 1999; USEPA, 2003b, 2006). However, the integrated approach developed by Adami et al. (2011) and used here provides explicit guidance on how to weigh study quality and how to integrate toxicological and epidemiology evidence. This approach has the additional advantage of qualitatively characterizing the uncertainty associated with any inference of causality and identifying whether additional mechanistic studies or epidemiological research would be more effective in reducing uncertainty.
TABLE 3.
Framework Assessment of the Animal Mode of Action, the Relevance of the MOA to Breast Cancer in Women and the Epidemiology Evidence
| EPA human cancer classification | Carcinogenic | Likely | Suggestive | Not likely |
| Human evidence | ||||
| Causation established | No | |||
| Association established | No | |||
| Animal evidence | ||||
| Multi versus single (sex, species, strain, and site) | Yes | |||
| Mode of action | ||||
| Key events proposed | Yes | |||
| Concordance of dose response relationships | Yes | |||
| Temporal association of key events | Yes | |||
| Strength, consistency, and specificity of association | Yes | |||
| Biological plausibility and coherence | Yes | |||
| Alternative MOAs evaluated | Yes | |||
| Human relevance | ||||
| MOA operative at any dose | No | |||
| MOA operative at plausible human dose | No | |||
| Framework: unlikely (category 4) | ||||
FUNDING
The regulatory studies on atrazine summarized in this manuscript were funded in their entirety by Syngenta Crop Protection, LLC., a registrant and basic manufacturer of atrazine. The research studies on atrazine carried out in the laboratories of James W. Simpkins, J. Charles Eldridge, Bob Handa, and Russ Hovey were also funded by Syngenta Crop Protection, LLC., as were some or all of the environmental studies on atrazine carried out in the laboratories of John Geisy, Ron Kendall, Lois Du Preez, and Werner Kloas.
Supplementary Material
Acknowledgments
All other studies were conducted by laboratories independent of Syngenta. All authors of this review have either served as consultants to Syngenta Crop Protection, LLC. (J.W.S., J.A.S., N.W., D.B., J.C.E., R.J.H., and T.M.P.) or were past (J.T.S.) or are current employees of Syngenta (T.P.P. and C.B.B.).
References
- Adami H-O, Berry C, Breckenridge C, Smith L, Swenberg J, Trichopoulos D, Weiss N, Pastoor T. Toxicology and epidemiology: improving the science with a framework for combining toxicological and epidemiological evidence to establish causal inference. Toxicol. Sci. 2011;22:223–234. doi: 10.1093/toxsci/kfr113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JM, Taylor AE, Schoenfeld DA, Crowley WF, Jr, Hall JE. The midcycle gonadotropin surge in normal women occurs in the face of an unchanging gonadotropin-releasing hormone pulse frequency. J. Clin. Endocrinol. Metab. 1994;79:858–864. doi: 10.1210/jcem.79.3.7521353. [DOI] [PubMed] [Google Scholar]
- Ademola JI, Sedik LE, Wester RC, Maibach HI. In vitro percutaneous adsorption and metabolism in man of 2-chloro-4-ethylamino-6-isopropylamine-s-triazine (atrazine) Arch. Toxicol. 1993;67:85–91. doi: 10.1007/BF01973676. [DOI] [PubMed] [Google Scholar]
- Adler ID. A review of the coordinated research effort on the comparison of test systems for the detection of mutagenic effects. Mutat. Res. 1980;74:77–93. doi: 10.1016/0165-1161(80)90234-4. [DOI] [PubMed] [Google Scholar]
- APVMA (Australian Pesticides and Veterinary Medicines Authority) The Reconsideration of Approvals of the Active Constituent Atrazine, Registrations of Products Containing Atrazine, and Their Associated Labels. 2004. APVMA, Kingston, ACT, Australia. [Google Scholar]
- APVMA (Australian Pesticides and Veterinary Medicines Authority) Atrazine Technical Report. The Reconsideration of the Active Constituent Registration of Products Containing Atrazine and Approval of Their Associated Labels. 2008. Available at: http://www.apvma.gov.au. Accessed August 12, 2011. [Google Scholar]
- APVMA (Australian Pesticides and Veterinary Medicines Authority) Atrazine Toxicity: Analysis of Potential Modes of Action. 2010. Available at: http://www.apvma.gov.au/products/review/docs/atrazine_moa_june_2010.pdf. Accessed August 12, 2011. [Google Scholar]
- Aschheim P. Aging in the hypothalamic-hypophyseal ovarian axis in the rat. In: Everitt AV, Burgess JA, editors. Hypothalamus, Pituitary and Aging. Springfield, IL: Charles C. Thomas Company; 1976. pp. 376–418. [Google Scholar]
- Ashby J, Tinwell H, Stevens J, Pastoor T, Breckenridge CB. The effects of atrazine on the sexual maturation of female rats. Regul. Toxicol. Pharmacol. 2002;35:468–73. doi: 10.1006/rtph.2002.1571. [DOI] [PubMed] [Google Scholar]
- Basler A, Rohrborn G. SCE Test In Vivo: Chromosome Aberrations in Bone Marrow Cells. FRG, Denmark: University of Düsseldorf; 1978. Contract Report (No. 175-77-1 ENV D). University of Düsseldorf, FRG, Denmark. [Google Scholar]
- Beach JE, Tyrey L, Everett JW. Serum prolactin and LH in early phases of delayed and direct pseudopregnancy in the rat. Endocrinology. 1975;96:243–346. doi: 10.1210/endo-96-5-1241. [DOI] [PubMed] [Google Scholar]
- Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E. Hypophysial responses to continuous and intermittent delivery of hypothalamic gonadotropin-releasing hormone. Science. 1978;202:631–633. doi: 10.1126/science.100883. [DOI] [PubMed] [Google Scholar]
- Biradar DP, Rayburn AL. Flow cytogenetic analysis of whole cell clastogenicity of herbicides found in groundwater. Arch. Environ. Contam. Toxicol. 1995a;28:13–17. doi: 10.1007/BF00213963. [DOI] [PubMed] [Google Scholar]
- Biradar DP, Rayburn AL. Chromosomal damage induced by herbicide contamination at concentrations observed in public water supplies. J. Environ. Qual. 1995b;24:1222–1225. [Google Scholar]
- Blake CA, Sawyer CH. Effects of hypothalamic deafferentation on the pulsatile rhythm in plasma concentrations of luteinizing hormone in ovariectomized rats. Endocrinology. 1974;94:730–736. doi: 10.1210/endo-94-3-730. [DOI] [PubMed] [Google Scholar]
- Brusick DJ. An assessment of the genetic toxicity of atrazine: relevance to human health and environmental effects. Mutat. Res. 1994;317:133–144. doi: 10.1016/0165-1110(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Brusick DJ, Albertini R, McRee D, Peterson D, Williams G, Hanawalt P, Preston J. Genotoxicity of radiofrequency radiation, DNA/Genetox Expert Panel. Environ. Mol. Mutagen. 1998;32:1–16. doi: 10.1002/(sici)1098-2280(1998)32:1<1::aid-em1>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Brusick DJ, Lohman PHM, Mendelsohn ML, Moore D, Waters MD. A method for comparing, combining and interpreting short-term genotoxicity test data. Mutat. Res. 1992;266:1–6. doi: 10.1016/0027-5107(92)90217-p. [DOI] [PubMed] [Google Scholar]
- Buchholz BA, Fultz E, Haack KW, Vogel JS, Gilman SD, Gee SJ, Hammock BD, Hui X, Wester RC, Maibach HI. HPLC-acelerator MS measurement of atrazine metabolites in human urine after dermal exposure. Anal. Chem. 1999;71:3519–3525. doi: 10.1021/ac990152g. [DOI] [PubMed] [Google Scholar]
- Burger HG, Hale GE, Robertson DM, Dennerstein L. A review of hormonal changes during the menopausal transition: focus on findings from the Melbourne Women's Midlife Health Project. Hum. Reprod. Update. 2007;13:559–565. doi: 10.1093/humupd/dmm020. [DOI] [PubMed] [Google Scholar]
- Chapin RE, Stevens JT, Hughes CL, Kelse WR, Hess RA, Daston GP. Symposium overview: Endocrine Modulation of Reproduction. Fundam. Appl. Toxicol. 1996;29:1–17. doi: 10.1006/faat.1996.0001. [DOI] [PubMed] [Google Scholar]
- Chollet MC, Degraeve N, Gilot-Delhalle J, Colizzi A, Moutschen J, Moutschen-Dahmen M. Mutagenic efficiency of atrazine with and without metabolic activation. Mutat. Res. 1982;97:237–238. [Google Scholar]
- Chou TS, Weber DS. The effect of atrazine on sister-chromatid exchange in maize. Genetics. 1981;97:521. [Google Scholar]
- Choucroun P, Gillet D, Dorange G, Sawickie B, Dewitte JD. Comet assay and early apoptosis. Mutat. Res. 2001;478:89–96. doi: 10.1016/s0027-5107(01)00123-3. [DOI] [PubMed] [Google Scholar]
- Chouroulinkov I. Progress Report (Jan.–Oct. 1982). Contract Report (No. CEE-ENV. 545 F (RS) Villejuif, France: French National Center for Research and Technology; 1982. [Google Scholar]
- Clements C, Ralph S, Petras M. Genotoxicity of select herbicides in Rana catesbeiana tadpoles using the alkaline single-cell gel DNA electrophoresis (Comet) assay. Environ. Mol. Mutagen. 1997;29:277–288. doi: 10.1002/(sici)1098-2280(1997)29:3<277::aid-em8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Connor K, Howell J, Chen I, Liu H, Berhane K, Sciarretta C, Safe S, Zacharewski T. Failure of chloro-s-triazine-derived compounds to induce estrogen receptor-mediated responses in vivo and in vitro. Fundam. Appl. Toxicol. 1996;30:93–101. [PubMed] [Google Scholar]
- Cooper RL, Laws SC, Das PC, Narotsky MG, Goldman JM, Lee Tyrey E, Stoker TE. Atrazine and reproductive function: mode and mechanism of action studies. Birth Defects Res. B Dev. Reprod. Toxicol. 2007;80:98–112. doi: 10.1002/bdrb.20110. [DOI] [PubMed] [Google Scholar]
- Cooper RL, Stoker TE, Tyrey L, Goldman JM, McElroy WK. Atrazine disrupts the hypothalamic control of pituitary-ovarian function. Toxicol. Sci. 2000;53:297–307. doi: 10.1093/toxsci/53.2.297. [DOI] [PubMed] [Google Scholar]
- Crain DA, Guillette LJ, Jr, Rooney AA, Pickford DB. Alterations in steroidogenesis in alligators (Alligator mississippiensis) exposed naturally and experimentally to environmental contaminants. Environ. Health Perspect. 1997;105:528–538. doi: 10.1289/ehp.97105528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzo B. Environmental xenobiotics may disrupt normal endocrine function by interfering with the binding of physiological ligands to steroid receptors and binding proteins. Environ. Health Perspect. 1997;105:294–301. doi: 10.1289/ehp.97105294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LK, Murr AS, Best DS, Fraites MJP, Zorrilla LM, Narotsky MG, Stoker TE, Goldman JE, Cooper RL. The effects of prenatal exposure to atrazine on pubertal and postnatal reproductive indices in the female rat. Reprod. Toxicol. 2011;32:43–51. doi: 10.1016/j.reprotox.2011.04.004. [DOI] [PubMed] [Google Scholar]
- de Veer I, Moriske HJ, Rüden H. Photochemical decomposition of organic compounds in water after UV-irradiation: investigation of positive mutagenic effects. Toxicol. Lett. 1994;72:113–119. doi: 10.1016/0378-4274(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Della Croce CD, Morichetti E, Intorre L, Soldani G, Bertini S, Bronzetti G. Biochemical and genetic interactions of two commercial pesticides with the monooxygenase system and chlorophyllin. J. Environ. Pathol. Toxicol. Oncol. 1996;15:21–28. [PubMed] [Google Scholar]
- Dunkelberg H, Fuchs J, Hengstler JG, Klein E, Oesch F, Struder K. Genotoxic effects of the herbicides alachlor, atrazine, pendimethaline, and simazine in mammalian cells. Bull. Environ. Contam. Toxicol. 1994;52:498–504. doi: 10.1007/BF00194135. [DOI] [PubMed] [Google Scholar]
- European Center for Ecotoxicology and Toxicology of Chemicals. Framework for the integration of human and animal data in chemical risk assessment. 2009. Technical Report No. 104. Brussels, Belgium. [Google Scholar]
- Eldridge JC, Stevens JT, Breckenridge CB. Atrazine interaction with estrogen expression systems. Rev. Environ. Contam. Toxicol. 2008;196:147–160. doi: 10.1007/978-0-387-78444-1_6. [DOI] [PubMed] [Google Scholar]
- Eldridge JC, Wetzel LT. Mode of action of atrazine for mammary tumor formation in the female Sprague-Dawley rat. In: LeBaron HM, McFarland JE, Burnside OC, editors. The Triazine Herbicides: 50 Years Revolutionizing Agriculture. San Diego, CA: Elsevier; 2008. pp. 399–411. [Google Scholar]
- Eldridge JC, Wetzel LT, Stevens JT, Simpkins JW. The mammary tumor response in triazine-treated female rats: a threshold-mediated interaction with strain and species-specific reproductive senescence. Steroids. 1999a;64:672–678. doi: 10.1016/s0039-128x(99)00051-3. [DOI] [PubMed] [Google Scholar]
- Eldridge JC, Wetzel LT, Tyrey L. Estrous cycle patterns of Sprague-Dawley rats during acute and chronic atrazine administration. Reprod. Toxicol. 1999b;13:491–499. doi: 10.1016/s0890-6238(99)00056-8. [DOI] [PubMed] [Google Scholar]
- Engel LS, Hill DA, Hoppin JA, Lubin JH, Lynch CF, Pierce J, Samanic C, Sandler DP, Blair A, Alavanja MC. Pesticide use and breast cancer risk among farmers' wives in the Agricultural Health Study. Am. J. Epidemiol. 2005;161:121–135. doi: 10.1093/aje/kwi022. [DOI] [PubMed] [Google Scholar]
- Enoch RR, Stanko JP, Greiner SN, Youngblood GL, Rayner JL, Fenton SE. Mammary gland development as a sensitive end point after acute prenatal exposure to an atrazine metabolite mixture in female Long-Evans rats. Environ. Health Perspect. 2007;115:541–547. doi: 10.1289/ehp.9612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes KS, Simpkins JW. Age-related alternatives in catecholamine activity with microdissected brain regions of ovariectomized Fischer 344 rats. J. Neurosci. Res. 1984;11:4045–417. doi: 10.1002/jnr.490110408. [DOI] [PubMed] [Google Scholar]
- Estes KS, Simpkins JW, Kalra SP. Normal LHRH neuronal function and hyperprolactinemia in old pseudopregnant Fischer 344 rats. Neurobiol. Aging. 1982;3:247–252. doi: 10.1016/0197-4580(82)90047-1. [DOI] [PubMed] [Google Scholar]
- FAO/WHO (Food and Agricultural Organization of the United Nations (FAO) and the World Health Organization (WHO) Joint FAO/WHO Meeting held in 2007. Pesticide Residues in Food, Toxicology Evaluation, Atrazine. Geneva, Switzerland: WHO; 2009. pp. 37–138. [Google Scholar]
- Feder HH. Hormonal actions on the sexual differentiation of the genitalia and the gonadotropin-regulating systems. In: Adler NT, editor. Neuroendocrinology of Reproduction. Physiology and Behavior. 1981. Plenum Press, New York. [Google Scholar]
- Filicori M, Santoro N, Merriam GR, Crowley WF., Jr Characterization of the physiological pattern of episodic gonadotropin secretion throughout the human menstrual cycle. J. Clin. Endocrin. Metab. 1986;62:1136–1144. doi: 10.1210/jcem-62-6-1136. [DOI] [PubMed] [Google Scholar]
- Foradori CD, Hinds LR, Hanneman WH, Handa RJ. Effects of atrazine and its withdrawal on gonadotropin-releasing hormone neuroendocrine function in the adult female Wistar rat. Biol. Reprod. 2009a;81:1099–1105. doi: 10.1095/biolreprod.109.077453. [DOI] [PubMed] [Google Scholar]
- Foradori CD, Hinds LR, Hanneman WH, Legare ME, Clay CM, Handa RJ. Atrazine inhibits pulsatile luteinizing hormone release without altering pituitary sensitivity to a gonadotropin-releasing hormone receptor agonist in female Wistar rats. Biol. Reprod. 2009b;81:40–45. doi: 10.1095/biolreprod.108.075713. [DOI] [PubMed] [Google Scholar]
- Foradori CD, Hinds LR, Quihuis AM, Lacagnina AF, Breckenridge CB, Handa RJ. The differential effect of atrazine on luteinizing hormone release in adrenalectomized adult female Wistar rats. Biol. Reprod. 2011 doi: 10.1095/biolreprod.111.092452. . Advance Access published on June 15, 2011; doi:10.1095/biolreprod.111.092452. [DOI] [PubMed] [Google Scholar]
- Fraites MJ, Cooper RL, Buckalew A, Jayaraman S, Mills L, Laws SC. Characterization of the hypothalamic-pituitary-adrenal axis response to atrazine and metabolites in the female rat. Toxicol. Sci. 2009;112:88–99. doi: 10.1093/toxsci/kfp194. [DOI] [PubMed] [Google Scholar]
- Franekic JG, Hulina J, Kniewald J, Alaoevic M. Environ. Mol. Mutagen. 1989. Atrazine and the genotoxicity of its metabolites. 14, 62. [Google Scholar]
- Freeman ME. Neuroendocrine control of the ovarian cycle of the rat. In: Neill JD, editor. Knobil and Neill’s Physiology of Reproduction. 3rd ed. Vol. 2. San Diego, CA.: Elsevier; 2006. pp. 2327–2388. Chapter 43. [Google Scholar]
- Fujimoto N, Honda H. Effects of environmental estrogenic compounds on growth of a transplanted estrogen responsive pituitary tumor cell line in rats. Food Chem. Toxicol. 2003;41:1711–1717. doi: 10.1016/s0278-6915(03)00198-4. [DOI] [PubMed] [Google Scholar]
- Gebel T, Kevekordes S, Pav K, Edenharder R, Dunkelberg H. In vivo genotoxicity of selected herbicides in the mouse bone-marrow micronucleus test. Arch. Toxicol. 1997;71:193–197. doi: 10.1007/s002040050375. [DOI] [PubMed] [Google Scholar]
- Gentile JM, Plewa J. A bioassay for screening host-mediated proximal mutagens in agriculture (Abstract) Mutat. Res. 1975;31:317. [Google Scholar]
- George SE, Chadwick RW, Kohan MJ, Allison JC, Warren SH, Williams RW. Atrazine treatment potentiates excretion of mutagenic urine in 2,6-dinitrotoluene treated Fischer 344 rats. Environ. Mol. Mutagen. 1995;26:178–184. doi: 10.1002/em.2850260212. [DOI] [PubMed] [Google Scholar]
- Ghiazza G, Zavarise G, Lanero M, Ferraro G. Sister chromatid exchanges induced in human lymphocyte chromosomes by trifluralin, atrazine and simazine. Boll. Soc. Ital. Biol. Sper. 1984;60:2149–2153. [PubMed] [Google Scholar]
- Goodman RL. The site of the positive feedback action of estradiol in the rat. Endocrinology. 1978;102:151–159. doi: 10.1210/endo-102-1-151. [DOI] [PubMed] [Google Scholar]
- Goodman RL, Knobil E. The sites of action of ovarian steroids in the regulation of LH secretion. Prog. Neuroendocrinol. 1981;32:57–63. doi: 10.1159/000123130. [DOI] [PubMed] [Google Scholar]
- Graumann K, Breithofer A, Jungbauer A. Monitoring of estrogen mimics by a recombinant yeast assay: synergy between natural and synthetic compounds. Sci. Total Environ. 1999;225:69–79. doi: 10.1016/s0048-9697(99)80018-7. [DOI] [PubMed] [Google Scholar]
- Hale GE, Burger HG. Perimenopausal reproductive endocrinology. Endocrinol. Metab. Clin. North Am. 2005;34:907–922. doi: 10.1016/j.ecl.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Hale GE, Burger HG. Hormonal changes and biomarkers in late reproductive age, menopausal transition and menopause. Best Pract. Res. Clin. Obstet. Gynaecol. 2009;23:7–23. doi: 10.1016/j.bpobgyn.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Hall JE. Neuroendocrine control of the menstrual cycle. In: Strauss JF III, Barbieri RL, editors. Yen and Jaffe’s Reproductive Endocrinology. Physiology, Pathophysiology, and Clinical Management. 6th ed. 2009. pp. 139–154. [Google Scholar]
- Hall JE, Taylor AE, Martin KA, Rivier J, Schoenfeld DA, Crowley WF., Jr. Decreased release of gonadotropin-releasing hormone during the preovulatory midcycle luteinizing hormone surge in normal women. Proc. Natl. Acad. Sci. U.S.A. 1994;91:6894–6898. doi: 10.1073/pnas.91.15.6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa R. Effects of Atrazine on Neuroendocrine Function in Male and Female Rats. 2010. USEPA Science Advisory Panel Meeting, September 14–17, Washington, DC. Available at: http://www.epa.gov/scipoly/sap/ Accessed Augsut 12, 2011. [Google Scholar]
- Hayes TB, Collins A, Lee M, Mendoza M, Noriega N, Stuart AA, Vonk A. Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. Proc. Natl. Acad. Sci. U.S.A. 2002;99:5476–80. doi: 10.1073/pnas.082121499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker M, Geisy JP, Park JW, Jones PD, Jooste AM, Carr JA, Solomon KR, Smith EE, Van Der Kraak G, Kendall RJ, et al. Plasma sex steroid concentrations and gonadal aromatase activities in African clawed frogs (Xenopus laevis) from South Africa. Environ. Toxicol. Chem. 2004;23:1996–2007. doi: 10.1897/03-450. [DOI] [PubMed] [Google Scholar]
- Hecker M, Kim WJ, Park JW, Murphy MB, Villeneuve D, Coady KK, Jones PD, Solomon KR, Van Der Kraak G, Carr JA, et al. Plasma concentrations of estradiol and testosterone, gonadal aromatase activity and ultrastructure of the testis in Xenopus laevis exposed to estradiol or atrazine. Aquat. Toxicol. 2005a;72:383–396. doi: 10.1016/j.aquatox.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Hecker M, Park JW, Murphy MB, Jones PD, Solomon KR, Van Der Kraak G, Carr JA, Smith EE, du Preez L, Kendall R J, et al. Effects of atrazine on CYP19 gene expression and aromatase activity in testes and on plasma sex steroid concentrations of male African clawed frogs (Xenopus laevis) Toxicol. Sci. 2005b;86:273–280. doi: 10.1093/toxsci/kfi203. [DOI] [PubMed] [Google Scholar]
- Heneweer M, van den Berg M, Sanderson JT. A comparison of human H295R and rat R2C cell lines as in vitro screening tools for effects on aromatase. Toxicol. Lett. 2004;146:183–194. doi: 10.1016/j.toxlet.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Herbison AE. Noradrenergic regulation of cyclic GnRH secretion. Rev. Reprod. 1997;2:1–6. doi: 10.1530/ror.0.0020001. [DOI] [PubMed] [Google Scholar]
- Hessel PA, Kalmes R, Smith TJ, Lau E, Mink PJ, Mandel J. A nested case-control study of prostate cancer and atrazine exposure. J. Occup. Environ. Med. 2004;46:379–85. doi: 10.1097/01.jom.0000121128.73921.a1. [DOI] [PubMed] [Google Scholar]
- Holloway AC, Anger DA, Crankshaw DJ, Wu M, Foster WG. Atrazine-induced changes in aromatase activity in estrogen sensitive target tissues. J. Appl. Toxicol. 2008;28:260–270. doi: 10.1002/jat.1275. [DOI] [PubMed] [Google Scholar]
- Hopenhayn-Rich C, Stump ML, Browning SR. Regional assessment of atrazine exposure and incidence of breast and ovarian cancers in Kentucky. Arch. Environ. Contam. Toxicol. 2002;42:127–136. doi: 10.1007/s002440010300. [DOI] [PubMed] [Google Scholar]
- Hovey RC, Coder PS, Wolf JC, Sielke RL, Jr, Tisdel MO, Breckenridge CB. Quantitative assessment of mammary gland development in female Long Evans rats following in utero exposure to atrazine. Toxicol. Sci. 2010;119:380–390. doi: 10.1093/toxsci/kfq337. [DOI] [PubMed] [Google Scholar]
- Hovey RC, Trott JF, Vonderhaar BK. Establishing a framework for the functional mammary gland: from endocrinology to morphology. J. Mammary Gland Biol. Neoplasia. 2002;7(1):17–38. doi: 10.1023/a:1015766322258. [DOI] [PubMed] [Google Scholar]
- Hui X, Wester RC, Maibach HI. Pharmacokinetics of [14C]-atrazine in rhesus monkeys, single-dose intravenous and oral administration. Toxicol. Environ. Chem. 2011;93:370–382. [Google Scholar]
- IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans. Evaluation and re-evaluation of some agents which target specific organs in rodent bioassays. Vol. 73. Lyon, France: IARC; 1999. pp. 59–113. [Google Scholar]
- Innes JRM, Ulland BM, Valerio MG, Pertrucelli L, Fishbein L, Hart ER, Pallota AJ, Bates RR, Falk HL, Gart JJ, et al. Bioassay of pesticides and industrial chemicals for tumorigenicity in mice: a preliminary note. J. Natl. Cancer Inst. 1969;42:1101–1114. [PubMed] [Google Scholar]
- Jefcoate CR, Liehr JG, Santen RJ, Sutter TR, Yager JD, Wei Y, Santner SJ, Tekmal R, Demers L, Pauley R, et al. Tissue-specific synthesis and oxidative metabolism of estrogens. J. Natl. Cancer Inst. Monogr. 2000:95–112. doi: 10.1093/oxfordjournals.jncimonographs.a024248. No. 27, Chapter 5. [DOI] [PubMed] [Google Scholar]
- Joo H, Choi K, Hodgson E. Human metabolism of atrazine. Pestic. Biochem. Physiol. 2010;98:73–79. [Google Scholar]
- Kang L, Marin M, Kelley D. Androgen biosynthesis and secretion in developing Xenopus laevis. Gen. Comp. Endocrinol. 1995;100:293–307. doi: 10.1006/gcen.1995.1160. [DOI] [PubMed] [Google Scholar]
- Karsch FJ, Dierschke DJ, Knobil E. Sexual differentiation of pituitary function: apparent difference between primates and rodents. Science. 1973;179:484–486. doi: 10.1126/science.179.4072.484. [DOI] [PubMed] [Google Scholar]
- Keller JM, McClellan-Green P. Effects of organochlorine compounds on cytochrome P450 aromatase activity in an immortal sea turtle cell line. Mar. Environ. Res. 2004;58:347–351. doi: 10.1016/j.marenvres.2004.03.080. [DOI] [PubMed] [Google Scholar]
- Kettles MA, Browning SR, Prince TS, Horstman SW. Triazine herbicide exposure and breast cancer incidence: An ecologic study of Kentucky counties. Env. Health Perspect. 1997;105:1222–1227. doi: 10.1289/ehp.971051222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Pastoor T, Clewell H, Andersen M. Pharmacokinetics of Atrazine and Chlorinated Metabolites. 2010. USEPA Science Advisory Panel Meeting, 14–17 September, Washington, DC. Available at: http://www.epa.gov/scipoly/sap/meetings/2010/091410meeting.html. Accessed August 10, 2011. [Google Scholar]
- Kligerman AD, Doerr CL, Tennant AH, Zucker RM. Cytogenetic studies of three triazine herbicides I. In vitro studies. Mutat. Res. 2000a;465:53–59. doi: 10.1016/s1383-5718(99)00211-9. [DOI] [PubMed] [Google Scholar]
- Kligerman AD, Doerr CL, Tennant AH, Zucker RM. Cytogenetic studies of three triazine herbicides. II. In vivo micronucleus studies in mouse bone marrow. Mutat. Res. 2000b;471:107–112. doi: 10.1016/s1383-5718(00)00124-8. [DOI] [PubMed] [Google Scholar]
- Kloas W, Lutz I, Springer T, Krueger H, Wolf J, Holden L, Hosmer A. Does atrazine influence larval development and sexual differentiation in Xenopus laevis? Toxicol. Sci. 2009a;107:376–84. doi: 10.1093/toxsci/kfn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloas W, Lutz I, Urbatzka R, Springer T, Krueger H, Wolf J, Holden L, Hosmer A. Does atrazine affect larval development and sexual differentiation of South African clawed frogs? Ann. N. Y. Acad. Sci. 2009b;1163:437–40. doi: 10.1111/j.1749-6632.2009.04456.x. [DOI] [PubMed] [Google Scholar]
- Knobil E. On the control of gonadotropin secretion in the rhesus monkey. Recent Prog. Horm. Res. 1974;30:1–36. doi: 10.1016/b978-0-12-571130-2.50005-5. [DOI] [PubMed] [Google Scholar]
- Knobil E. The neuroendocrine control of the menstrual cycle. Recent Prog. Horm. Res. 1980;36:53–58. doi: 10.1016/b978-0-12-571136-4.50008-5. [DOI] [PubMed] [Google Scholar]
- Knobil E, Plant TM, Wildt L, Belchetz PE, Marshall G. Control of the rhesus monkey menstrual cycle: permissive role of hypothalamic gonadotropin-releasing hormone. Science. 1980;207:1371–1373. doi: 10.1126/science.6766566. [DOI] [PubMed] [Google Scholar]
- Krey LC, Butler WR, Knobil E. Surgical disconnection of the medial basal hypothalamus and pituitary function in the rhesus monkey. I. Gonadotropin secretion. Endocrinology. 1975;96:1073–1087. doi: 10.1210/endo-96-5-1073. [DOI] [PubMed] [Google Scholar]
- Laws SC, Ferrell JM, Stoker TE, Schmid J, Cooper RL. The effects of atrazine on female Wistar rats: An evaluation of the protocol for assessing pubertal development and thyroid function. Toxicol. Sci. 2000;58:366–376. doi: 10.1093/toxsci/58.2.366. [DOI] [PubMed] [Google Scholar]
- Laws SC, Hotchkiss M, Ferrell J, Jayaraman S, Mills L, Modic W, Tinfo N, Fraites M, Stoker T, Cooper R. Chlorotriazine herbicides and metabolites activate an ACTH-dependent release of corticosterone in male Wistar rats. Toxicol. Sci. 2009;112:78–87. doi: 10.1093/toxsci/kfp190. [DOI] [PubMed] [Google Scholar]
- Laws SC, Yavanhxay S, Cooper RL, Eldridge C. Nature of the binding interaction for 50 structurally diverse chemicals with rat estrogen receptors. Toxicol. Sci. 2006;94:46–56. doi: 10.1093/toxsci/kfl092. [DOI] [PubMed] [Google Scholar]
- Legler J, Dennekamp M, Vethaak AD, Brouwer A, Koeman JH, van der Burg B, Murk AJ. Detection of estrogenic activity in sediment-associated compounds using in vitro reporter gene assays. Sci. Total Environ. 2002;293:69–83. doi: 10.1016/s0048-9697(01)01146-9. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Merkley CM, Coolen LM, Goodman RL. Anatomy of the kisspeptin neural network in mammals. Brain Res. 2010;1364:90–102. doi: 10.1016/j.brainres.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey JR. Historical Foundations. In: Baker HJR, Russell Lindsey J, Weisbroth SH, editors. The Laboratory Rat. Volume I. Biology and Disease. New York: Academic Press; 1979. pp. 1–36. [Google Scholar]
- Lohman PHM, Mendelsohn ML, Moore DH, Waters MD, Brusick DJ, Ashby J, Lohman WJA. A method for comparing, combining short-term genotoxicity test data: the basic system. Mutat. Res. 1992;266:7–25. doi: 10.1016/0027-5107(92)90218-q. [DOI] [PubMed] [Google Scholar]
- London Principles. Principles for Evaluating Epidemiologic Data in Regulatory Risk Assessment, ISBN 0-9654148-0-9, and “Recommendations for Implementing the 'London Principles' for Risk Assessment Guidance, Federal Focus 1996. 1995. Available at: http://www.fedfocus.org/science/london.html. Accessed August 12, 2011. [Google Scholar]
- Lu JKH, Gilman DP, Judd HL, Sawyer CH. Differential patterns of gonadotropin responses to ovarian steroids and to LH-releasing hormone between constant-estrous and pseudopregnant states in aging rats. Biol. Repro. 1980;23:345–351. doi: 10.1095/biolreprod23.2.345. [DOI] [PubMed] [Google Scholar]
- Lu KH, Hopper BR, Vargo TM, Yen SSC. Chronological changes in sex steroid, gonadotropin and prolactin secretion in aging female rats displaying different reproductive states. Biol. Repro. 1979;21:193–203. doi: 10.1095/biolreprod21.1.193. [DOI] [PubMed] [Google Scholar]
- MacLennan PA, Delzell E, Sathiakumar N, Myers SL. Mortality among triazine herbicide manufacturing workers. J. Toxicol. Environ. Health A. 2003;66:501–17. doi: 10.1080/15287390306356. [DOI] [PubMed] [Google Scholar]
- MacLennan PA, Delzell E, Sathiakumar N, Myers SL, Cheng H, Grizzle W, Chen VW, Wu XC. Cancer incidence among triazine herbicide manufacturing workers. J. Occup. Environ. Med. 2002;44:1048–1058. doi: 10.1097/00043764-200211000-00011. [DOI] [PubMed] [Google Scholar]
- Maibach HB, Stevens JT, Hui X, Breckenridge CB, Wester R. Comparison of the absorption, metabolism and elimination of atrazine administered orally to man or orally or intravenously to rhesus monkey. Toxicologist. 2001;60:294. [Google Scholar]
- Martin KA, Welt CK, Taylor AE, Smith JA, Crowley WF, Jr, Hall JE. Is GnRH reduced at the midcycle surge in the human. Evidence from a GnRH-deficient model. Neuroendocrinol. 1998;67:363–369. doi: 10.1159/000054334. [DOI] [PubMed] [Google Scholar]
- Matsushita S, Yamashita J, Iwasawa T, Tomita T, Ikeda M. Effects of in ovo exposure to imazalil and atrazine on sexual differentiation in chick gonads. Poult. Sci. 2006;85:1641–1647. doi: 10.1093/ps/85.9.1641. [DOI] [PubMed] [Google Scholar]
- McElroy JA, Gangnon RE, Newcomb PA, Kanarek MS, Anderson HA, Brook JV, Trentham-Dietz A, Remington PL. Risk of breast cancer for women living in rural areas from adult exposure to atrazine from well water in Wisconsin. J. Expo. Sci. Environ. Epidemiol. 2007;17:207–214. doi: 10.1038/sj.jes.7500511. [DOI] [PubMed] [Google Scholar]
- McMullin TS, Brzezicki JM, Cranmer BK, Tessari JD, Andersen ME. Pharmacokinetic modeling of disposition and time-course studies with [14C] atrazine. J. Toxicol. Environ. Health A. 2003;66:941–964. doi: 10.1080/15287390306454. [DOI] [PubMed] [Google Scholar]
- Meek ME, Bucher JR, Cohen SM, Dellarco V, Hill RN, Lehman-McKeeman LD, Longfellow DG, Pastoor T, Seed J, Patton DE. A framework for human relevance analysis of information on carcinogenic modes of action. Crit. Rev. Toxicol. 2003;33:591–653. doi: 10.1080/713608373. [DOI] [PubMed] [Google Scholar]
- Meisner LF, Belluck DA, Roloff BD. Cytogenetic effects of alachlor and/or atrazine in vivo and in vitro. Environ. Mol. Mutagen. 1992;19:77–82. doi: 10.1002/em.2850190110. [DOI] [PubMed] [Google Scholar]
- Meites J, Huang HH, Simpkins JW. Recent studies on neuroendocrine control of reproductive senescence in rats. In: Scheider EL, editor. The Aging Reproductive System. New York: Raven Press; 1978. pp. 213–235. [Google Scholar]
- Miller A, Ebert E, Gast A. Cytogenetic studies with atrazine on plants. Experientia. 1972;28:704–705. doi: 10.1007/BF01944990. [DOI] [PubMed] [Google Scholar]
- Mills PK, Yang R. Regression analysis of pesticide use and breast cancer incidence in California Latinas. J. Environ. Health. 2006;68:15–22. [PubMed] [Google Scholar]
- Modic W, Ferrell J, Wood C, Laskey J, Cooper R, Laws S. Atrazine alters steroidogenesis in male Wistar rats. Toxicologist. 2004;78:117. [Google Scholar]
- Muir K, Rattanamongkolgul S, Smallman-Raynor M, Thomas M, Downer S, Jenkinson C. Breast cancer incidence and its possible spatial association with pesticide application in two counties of England. Public Health. 2004;118:513–520. doi: 10.1016/j.puhe.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Norman RL, Spies HG. Cyclic ovarian function in a male macaque: additional evidence for a lack of sexual differentiation in the physiological mechanisms that regulate the cyclic release of gonadotropins in primates. Endocrinology. 1986;118:2608–2610. doi: 10.1210/endo-118-6-2608. [DOI] [PubMed] [Google Scholar]
- Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocrine Rev. 2009;30:713–743. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor JC, Plowchalk DR, Van Pelt CS, Davis LG, Cook JC. Role of prolactin in chloro-s-triazine rat mammary tumorigenesis. Drug Chem. Toxicol. 2000;23:575–601. doi: 10.1081/dct-100101972. [DOI] [PubMed] [Google Scholar]
- Ohno K, Araki N, Yanase T, Nawata H, Iida H. A novel nonradioactive method for measuring aromatase activity using a human ovarian granulosa-like tumor cell line and an estrone ELISA. Toxicol. Sci. 2004;82:443–450. doi: 10.1093/toxsci/kfh292. [DOI] [PubMed] [Google Scholar]
- Ottowitz WE, Dougherty DD, Fischman AJ, Hall JE. 2-fluoro-2-deoxy-D glucose positron emission tomography demonstration of estrogen negative and positive feedback on luteinizing hormone secretion in women. J. Clin. Endocrinol. Metab. 2008;93:3208–3214. doi: 10.1210/jc.2008-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pau KY, Berria M, Hess DL, Spies HG. Preovulatory gonadotropin-releasing hormone surge in ovarian-intact rhesus macaques. Endocrinology. 1993;133:1650–1656. doi: 10.1210/endo.133.4.8404606. [DOI] [PubMed] [Google Scholar]
- Pettersen JC, Turnier JC. 1-Year Chronic Toxicity Study with Atrazine Technical in Rats: Final Report. 1995. Rep. N°: F-00171, 8.12.1995. Syngenta Crop Protection LLC. Greensboro, NC. [Google Scholar]
- Pino A, Maura A, Grillo P. DNA damage in stomach, kidney, liver and lung of rats treated with atrazine. Mutat. Res. 1988;209:145–147. doi: 10.1016/0165-7992(88)90032-2. [DOI] [PubMed] [Google Scholar]
- Pintér A, Török G, Börzsönyl M, Surján A, Csík M, Kelecsényi Z, Kocsis Z. Long-term carcinogenicity bioassay of the herbicide atrazine in F 344 rats. Neoplasma. 1990;37:533–544. [PubMed] [Google Scholar]
- Plant TM. Gonadal regulation of hypothalamic gonadotropin-releasing hormone release in primates. Endocrine Rev. 1986;7:75–88. doi: 10.1210/edrv-7-1-75. [DOI] [PubMed] [Google Scholar]
- Pool-Zobel BL, Abrahamse SL, Collins AR, Kark W, Gugler R, Oberreuther D, Siegel EG, Treptow-van Lishaut S, Rechkemmer G. Analysis of DNA strand breaks, oxidized bases, and glutathione S-transferase P1 in human cells. Cancer Epidemiol. Biomarkers Prev. 1999;8:609–614. [PubMed] [Google Scholar]
- Rance NE. Menopause and the human hypothalamus: evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides. 2009;30:111–122. doi: 10.1016/j.peptides.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayburn AL, Bouma J, Northcott CA. Comparing the clastogenic potential of atrazine with caffeine using Chinese hamster ovary (CHO) cells. Toxicol. Lett. 2001;121:69–78. doi: 10.1016/s0378-4274(01)00318-6. [DOI] [PubMed] [Google Scholar]
- Rayner JL, Enoch RR, Fenton SE. Adverse effects of prenatal exposure to atrazine during a critical period of mammary gland growth. Toxicol. Sci. 2005;87:255–266. doi: 10.1093/toxsci/kfi213. [DOI] [PubMed] [Google Scholar]
- Reynolds P, Hurley SE, Goldberg DE, Yerabati S, Gunier RB, Hertz A, Anton-Culver H, Bernstein L, Deapen D, Horn-Ross PL, et al. Residential proximity to agricultural pesticide use and incidence of breast cancer in the California Teachers Study cohort. Environ. Res. 2004;96:206–18. doi: 10.1016/j.envres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Reynolds P, Hurley SE, Gunier RB, Yerabati S, Quach T, Hertz A. Residential proximity to agricultural pesticide use and incidence of breast cancer in California, 1988–1997. Environ. Health Perspect. 2005;113:993–1000. doi: 10.1289/ehp.7765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas G, Frenzilli G, Barale R, Marcos R. Herbicide-induced DNA damage in human lymphocytes evaluated by the single-cell gel electrophoresis (SCGE) assay. Mutat. Res. 1995;344:41–54. doi: 10.1016/0165-1218(95)90037-3. [DOI] [PubMed] [Google Scholar]
- Ribas G, Surralles J, Carbonell E, Creus A, Xamena N, Marcos R. Lack of genotoxicity of the herbicide atrazine in cultured human lymphocytes. Mutat. Res. 1998;416:93–99. doi: 10.1016/s1383-5718(98)00081-3. [DOI] [PubMed] [Google Scholar]
- Rider CV, Hartig PC, Cardon MC, Wilson VS. Development of a competitive binding assay system with recombinant estrogen receptors from multiple species. Toxicol. Lett. 2009;184:85–89. doi: 10.1016/j.toxlet.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Russo J, Balogh GA, Chen J, Fernandez SV, Fernbaugh R, Heulings R, Mailo DA, Moral R, Russo PA, Sheriff F, et al. The concept of stem cell in the mammary gland and its implication in morphogenesis, cancer and prevention. Frontiers Biosci. 2006;11:151–172. doi: 10.2741/1788. [DOI] [PubMed] [Google Scholar]
- Russo J, Hu YF, Silva ID, Russo IH. Cancer risk related to mammary gland structure and development. Microsc. Res. Tech. 2001;52:204–23. doi: 10.1002/1097-0029(20010115)52:2<204::AID-JEMT1006>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Russo J, Rivera R, Russo IH. Influence of age and parity on the development of the human breast. Breast Cancer Res. Treat. 1992;22:211–218. doi: 10.1007/BF01833517. [DOI] [PubMed] [Google Scholar]
- Sanderson JT, Letcher RJ, Heneweer M, Giesy JP, van den Berg M. Effects of chloro-s-triazine herbicides and metabolites on aromatase activity in various human cell lines and on vitellogenin production in male carp hepatocytes. Environ. Health Perspect. 2001;109:1027–1031. doi: 10.1289/ehp.011091027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson JT, Seinen W, Giesy JP, van den Berg M. 2-chloro-s-triazine herbicides induce aromatase (CYP19) activity in H295R human adrenocortical carcinoma cells: A novel mechanism for estrogenicity. Toxicol. Sci. 2000;54:121–127. doi: 10.1093/toxsci/54.1.121. [DOI] [PubMed] [Google Scholar]
- Santoro N, Wierman ME, Filicori M, Waldstreicher J, Crowley WF., Jr Intravenous administration of pulsatile gonadotropin-releasing hormone in hypothalamic amenorrhea: effects of dosage. J. Clin. Endocrinol. Metab. 1986;62:109–116. doi: 10.1210/jcem-62-1-109. [DOI] [PubMed] [Google Scholar]
- Santoro N. The menopausal transition. Am. J. Med. 2005;118(Suppl. 12B):8–13. doi: 10.1016/j.amjmed.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Sarkar DK, Gottschall PE, Meites J. Damage to hypothalamic dopaminergic neurons is associated with development of prolactin-secreting pituitary tumors. Science. 1982;218:684–686. doi: 10.1126/science.7134966. [DOI] [PubMed] [Google Scholar]
- Sathiakumar N, MacLennan PA, Mandel J, Delzell E. A review of the epidemiologic studies of triazine herbicides and cancer. Crit. Reviews Toxicol. 2011;41(Suppl. 1):1–34. doi: 10.3109/10408444.2011.554793. [DOI] [PubMed] [Google Scholar]
- Sawyer CH. First Geoffrey Harris Memorial Lecture. Some recent developments in brain-pituitary-ovarian physiology. Neuroendocrin. 1995;17:97–124. doi: 10.1159/000122347. [DOI] [PubMed] [Google Scholar]
- Schiff I, Wilson E. Clinical Aspects of aging of the female reproductive system. In: Scheider EL, editor. The Aging Reproductive System. New York, NY: Raven Press; 1978. pp. 9–28. [Google Scholar]
- Sielken RL, Valdez-Flores C, Holden L, Breckenridge C, Stevens J. Statistical inferences about the mechamism of action in carcinogenicity. Scand. J. Work Environ. Health. 2005;31(Suppl. 1):151–155. [PubMed] [Google Scholar]
- Silverman A-J, Livne I, Witkin JW. The gonadotropin-releasing hormone (GnRH), neuronal systems: Immunocytochemistry and in situ hybridization. In: Knobil E, Neill JD, Greenwald GS, Markert CL, Pfaff DF, editors. The Physiology of Reproduction. 2nd ed. Volume 1. New York, NY: Raven Press; 1994. pp. 1683–1709. [Google Scholar]
- Simmon VF, Poole D. In Vitro and In Vivo Microbiological Assays of Six Ciba-Geigy Chemicals: Final Report. 1977. Rep. N°: SRI Project LSC-5686. (Unpublished study received Aug 13, 1981 under 7F1983; prepared by Stanford Research Institute, Menlo Park, CA. Submitted by Ciba-Geigy Corp., Greensboro, N.C.; CDL: 070213-I) [Google Scholar]
- Simpkins JW, Advis JP, Hodson CA, Meites J. Blockade of steroid induced LH release by selective depletion of anterior hypothalamic norepinephrine activity. Endocrinology. 1979a;104:506–509. doi: 10.1210/endo-104-2-506. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Eldridge JC, Wetzel LT. Ballantine LG, McFarland JE, Hackett DS, editors. Role of strain-specific reproductive patterns in the appearance of mammary tumors in atrazine-treated rats. Triazine Herbicides: Risk Assessment. 1998;Vol. 683:399–413. ACS Symposium Series. American Chemical Society, Washington, DC. [Google Scholar]
- Simpkins JW, Huang HH, Advis JP, Meites J. Evaluation of changes in NE and DA turnover during progesterone induced LH and prolactin surges in ovariectomized, estrogen primed rats. Biol. Reprod. 1979b;20:625–632. doi: 10.1095/biolreprod20.3.625. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Millard WJ, Gabriel SM, Soltis EE. Noradrenergic methods in neuroendocrinology. In: Steger RW, Johns A, editors. Handbook of Pharmacologic Methodologies for the Study of the Neuroendocrine System. Boca Raton, FL: CRC Press; 1985. pp. 1–63. [Google Scholar]
- Singh M, Pushpindar K, Sandhir R, Kiran R. Protective effects of vitamin E against atrazine-induced genotoxicity in rats. Mutat. Res. 2008;654:145–149. doi: 10.1016/j.mrgentox.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Singh M, Sandhir R, Kiran R. Effects on antioxidant status of liver following atrazine exposure and its attenuation by vitamin E. Exp. Toxicol. Pathol. 2011;63:269–276. doi: 10.1016/j.etp.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Slikker W, Jr, Andersen ME, Bogdanffy MS, Bus JS, Cohen SD, Conolly RB, David RM, Doerrer NG, Dorman DC, Gaylor DW, et al. Dose-dependent transitions in mechanisms of toxicity. Toxicol. Appl. Pharmacol. 2004a;201:203–225. doi: 10.1016/j.taap.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Slikker W, Jr., Andersen ME, Bogdanffy MS, Bus JS, Cohen SD, Conolly RB, David RM, Doerrer NG, Dorman DC, Gaylor DW, et al. Dose-dependent transitions in mechanisms of toxicity: case studies. Toxicol. Appl. Pharmacol. 2004b;201:226–294. doi: 10.1016/j.taap.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Sonich-Mullin C, Fielder R, Wiltse J, Baetcke K, Dempsey J, Fenner-Crisp P, Grant D, Hartley M, Knaap A, Kroese D, et al. IPCS conceptual framework for evaluating a mode of action for chemical carcinogenesis. Reg. Toxicol. Phamacol. 2001;34:146–152. doi: 10.1006/rtph.2001.1493. [DOI] [PubMed] [Google Scholar]
- Spiteri ID, Guillette LJ, Jr, Crain DA. The functional and structural observations of the neonatal reproductive system of alligators exposed in ovo to atrazine, 2,4-d, or estradiol. Toxicol. Ind. Health. 1999;15:181–186. doi: 10.1191/074823399678846565. [DOI] [PubMed] [Google Scholar]
- Stevens JT, Breckenridge CB, Wetzel LT, Gillis JH, Luempert LG, 3rd, Eldridge JC. Hypothesis for mammary tumorigenesis in Sprague-Dawley rats exposed to certain triazine herbicides. J. Toxicol. Environ. Health. 1994;43:139–153. doi: 10.1080/15287399409531911. [DOI] [PubMed] [Google Scholar]
- Stevens JT, Breckenridge CB, Wetzel LT, Thakur AK, Liu C, Werner C, Luempert LC, III, Eldridge JC. A risk characterization for atrazine: oncogenicity profile. J. Toxicol. Environ. Health Part A. 1999;56:69–109. doi: 10.1080/009841099158169. [DOI] [PubMed] [Google Scholar]
- Swaen GMH. A framework for using epidemiology data for risk assessment. Hum. Exp. Toxicol. 2006;25:147–155. doi: 10.1191/0960327106ht600oa. [DOI] [PubMed] [Google Scholar]
- Taets C, Aref S, Rayburn AL. The clastogenic potential of triazine herbicide combinations found in potable water supplies. Environ. Health Perspect. 1998;106:197–205. doi: 10.1289/ehp.98106197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur AK, Wetzel LT, Voelker RW, Wakefield AE. Results of a two-year oncogenicity study in fischer 344 rats with atrazine. In: Ballantine LG, MacFarland JE, Hackett DS, editors. Triazine Herbicides: Risk Assessment. Washington, DC: American Chemical Society; 1998. pp. 384–398. 30 ACS Symposium Series 683. [Google Scholar]
- Tinfo NS, Hotchkiss MG, Buckalew AR, Zorrilla LM, Cooper RL, Laws SC. Understanding the effects of atrazine on steroidogenesis in rat granulosa and H295R adrenal cortical carcinoma cells. Reprod. Toxicol. 2011;31:84–93. doi: 10.1016/j.reprotox.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Trichopoulos D, Adami HO, Ekbom A, Hsieh CC, Lagiou P. Early life events and conditions and breast cancer risk: From epidemiology to etiology. Int. J. Cancer. 2008;122:481–485. doi: 10.1002/ijc.23303. [DOI] [PubMed] [Google Scholar]
- U.S. EPA. Pesticide Reregistration Rejection Rate Analysis—Toxicology. National Service Center for Environmental Publications; 1993. 738R93004. United States Environmental Protection Agency, Washington, DC. [Google Scholar]
- U.S. EPA. HED Standard Operating Procedure: Executive Summaries for Toxicology Data Evaluation Records (DERs) 2001. SOP 2001.02. United States Environmental Protection Agency, Washington, DC. [Google Scholar]
- U.S. EPA. Office of Pesticide Programs, Health Effects Division. The Grouping of a Series of Triazine Pesticides Based on a Common Mechanism of Toxicity. 2002. United States Environmental Protection Agency, Washington, DC. [Google Scholar]
- U.S. EPA. Interim Reregistration Eligibility Decision for Atrazine. Special Review and Reregistration Division. 2003a. Report No.: Case No. 0062, 31.01.2003. United States Environmental Protection Agency, Washington, DC. [Google Scholar]
- U.S. EPA. A Summary of General Assessment Factors for Evaluating the Quality of Scientific and Technical Information. 2003b. Science Policy Council, Washington, DC EPA 100/B-03/001 Available at: . http://www.epa.gov/spc/pdfs/assess2.pdf. [Google Scholar]
- U.S. EPA. Guidelines for Carcinogenic Risk Assessment; Risk Assessment Forum. 2005. EPA/630/P-03/001B, United States Environmental Protection Agency, Washington, DC. Available at: http://www.epa.gov/raf/publications/pdfs/CANCER_GUIDELINES_FINAL_3-25-05.PDF. Accessed August 10, 2011. [Google Scholar]
- U.S. EPA. Triazine Cumulative Risk Assessment. HED Human Health Risk Assessment in Support of the Reregistration Eligibility Decisions for Atrazine, Simazine and Propazine. PC Codes: 080808, 080803, 080807. 2006. DP 317976. Health Effects Division (7509C), Benefits and Economic Analysis Division (7503C), and Environmental Fate and Effects Division (7507C). March 28, 2006. Office of Prevention, Pesticides and Toxic Substances. United States Environmental Protection Agency, Washington, DC. [Google Scholar]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The strengthening of the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med. 2007;4:1623–1627. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K-I, et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J. Neurosci. 2010;30:3124–3132. doi: 10.1523/JNEUROSCI.5848-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G, Schmidt C, Kleinberg DL, Ganguly M. Positive feedback of oestrogen on LH secretion in women in neuroleptic drugs. Clin. Endocrinol. (Oxf.) 1977;7:423–427. doi: 10.1111/j.1365-2265.1977.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Wise PM. Neuroendocrine modulation of the “menopause”: insights into the aging brain. Am. J. Physiol. 1999;277:E965–E970. doi: 10.1152/ajpendo.1999.277.6.E965. [DOI] [PubMed] [Google Scholar]
- Wise PM, Kashon ML, Krajnak KM, Rosewell KL, Cai A, Scarbrough K, Harney JP, McShane T, Lloyd JM, Weiland NG. Aging of the female reproductive system: a window into brain aging. Recent Prog. Horm. Res. 1997;52:279–303. [PubMed] [Google Scholar]
- Zeleznik AJ, Pohl CR. Control of follicular development, corpus luteum function, the maternal recognition of pregnancy, and the neuroendocrine regulation of the menstrual cycle in higher primates. In: Neill JD, editor. Knobil and Neill’s Physiology of Reproduction. 3rd ed. Vol. 2. San Diego, CA: Elsevier; 2006. pp. 2449–2510. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.