Abstract
Purpose
Among men with either extracapsular disease or positive surgical margins after radical prostatectomy, immediate adjuvant therapy decreases the risk of biochemical recurrence at the cost of increased toxicity. We sought to further stratify these men into a low-risk group in whom watchful waiting after surgery may be preferred and a high-risk cohort in whom adjuvant therapy may be preferred.
Materials and Methods
We performed a retrospective analysis of 902 men treated by radical prostatectomy in the SEARCH database between 1988 and 2007 with positive surgical margins and/or extracapsular disease without seminal vesicle invasion or lymph node metastasis. The significant independent predictors of biochemical recurrence were determined using a multivariate Cox proportional hazards model. Based upon the recurrence risk generated from the multivariate Cox proportional hazards regression model, we generated tables to estimate the risk of recurrence at 1, 3 and 5 years after surgery.
Results
After a median of 3 years of follow-up, 346 (39%) patients developed a biochemical recurrence. On multivariate analysis, the significant predictors of biochemical recurrence included age >60 years, PSA >10 ng/ml, Gleason score 4+3 and 8–10, 2 or more sites of positive surgical margins and prostate specimen weight ≤30 grams. The overall predictive accuracy of the model as determined by the Concordance Index C was 0.67, which compared to 0.60 for the post-operative “Kattan nomogram” for this patient population.
Conclusions
We have developed a simple instrument, which once validated, may aid in the postoperative decision making process for men with intermediate risk of recurrence after prostatectomy.
Keywords: Prostate cancer, radical prostatectomy, biochemical recurrence
Introduction
The number of new cases of prostate cancer projected for 2007 is 218,890, which accounts for almost a third of all cancers in men excluding skin cancers. Radical prostatectomy is one of the most accepted and widely practiced management strategies for localized prostate cancer. Though survival rates for prostate cancer continue to be exceptionally good in comparison with other cancers, biochemical recurrence occurs in up to 30% of all men undergoing surgical treatment.1, 2 It is acknowledged those with organ confined cancer are least likely to recur while those with seminal vesicle invasion or lymph node metastases are at the highest risk for recurrence.3 It seems intuitive that men with organ-confined disease would benefit most from a watchful waiting approach while prior studies have shown that early aggressive treatment for the latter group improves overall survival, at least for those with lymph node involvement.4 However, for men with intermediate risk – those with either a positive surgical margin or extracapsular extension without seminal vesicle invasion – the optimal management remains unclear.
Recently, two large prospective clinical trials investigated the role of immediate adjuvant radiation therapy for men with either extracapsular disease or a positive surgical margin following radical prostatectomy.5, 6 Both studies found adjuvant therapy decreased the risk of prostate specific antigen (PSA) recurrence but at the cost of increased toxicity. While no metastasis-free or overall survival benefit was seen in either study, it is unknown whether this represents a lack of true long-term benefit from adjuvant treatment or was a function of modest patient numbers without sufficient follow-up time. Ultimately, balancing the benefit of reduced biochemical recurrence must be weighed against the potential for decrements in quality of life. Therefore, we sought to identify among those men with either extracapsular disease or a positive surgical margin, patients who were at low risk of recurrence and in whom close observation may be the preferred management approach while also identifying those who are at high-risk and in whom adjuvant therapy may be warranted. To accomplish this, we used the multi-center multi-ethnic Shared Equal-Access Regional Cancer Hospital (SEARCH) Database of men treated with radical prostatectomy at multiple Veterans Affairs Medical Centers across the United States.
Methods
Study population
After obtaining Institutional Review Board approval from each institution to abstract and combine data, data from patients undergoing radical prostatectomy at the Veterans Affairs Medical Centers in West Los Angeles, Palo Alto, San Francisco, and Augusta, Georgia, and Durham, North Carolina were combined into the SEARCH database.7 This database includes information on patient age at surgery, race, height, weight, clinical stage, grade of cancer on diagnostic biopsies, preoperative PSA, surgical specimen pathology (specimen weight, tumor grade, stage, and surgical margin status), and follow-up PSA data. Patients treated with preoperative androgen deprivation or radiation therapy were excluded. Of the 2,062 men within the SEARCH Database, we excluded 32 and 205 men who were at very high-risk of recurrence due to lymph node metastasis and seminal vesicle invasion, respectively. Due to the very low-risk of recurrence, we excluded men with organ confined disease and negative margins. This resulted in a study population of 902 men. Biochemical recurrence was defined as a single PSA >0.2 ng/ml, 2 concentrations at 0.2 ng/ml, or secondary treatment for an elevated postoperative PSA. The prostatectomy specimens were sectioned per each institution’s protocol.7 Margins were categorized as positive or negative at each given anatomic location (i.e. apex, bladder neck, left peripheral or right peripheral). Information regarding the number of positive foci at each location was not available, thus patients were defined as either positive or negative at each location. The number of positive margins was computed by adding the number of locations with a positive margin. Each location was considered mutually exclusive. For example, a man with an isolated left apical margin was considered to have a positive apical margin, but not a left lateral margin, for a total of one positive margin.
Statistical analysis
Significant risk factors for time to biochemical recurrence were examined using log-rank survivorship analysis and Cox proportional hazards regression. The variables considered for entry into the model included age, race, body mass index (BMI), year of surgery, SEARCH site, pre-operative serum PSA, pathological Gleason score, extracapsular extension, number of positive surgical margins, and prostate specimen weight. As our goal was to develop easy to use tables, this necessitated identifying cut-points to categorize the significant prognostic characteristics. Therefore, we performed univariate exploratory analyses among the identified significant variables using multiple clinically relevant cut points for each variable (e.g. age ≤ 50 vs. age >50, age ≤ 60 vs. age >60, etc.) and determined that grouping age ≤ 60 vs. >60, Gleason score as ≤3+4, 4+3, vs. 8–10, preoperative PSA ≤ 10 vs. >10 ng/ml, prostate specimen weight ≤ 30 vs. >30 grams and number of positive margins locations ≤ 2 vs. >2 provided the maximum likelihood χ2 ratio for estimating time to biochemical recurrence; therefore, these groupings were used in the multivariate model. For multivariable analysis, a step-wise Cox proportional hazards model was used with p<0.15 to determine which variables should be entered into the model at each step. The variable with the highest p value was then successively deleted until only variables with a p<0.1 remained. The predictive performance of the model was assessed and compared with the post-operative “Kattan nomogram”8 using the concordance index C.9 Tables were generated to predict the 1, 3 and 5-year actuarial risk of biochemical recurrence free survival using the coefficients of the multivariable Cox model. All statistical analyses were performed using STATA 9.2 (STATA Corp; College Station, TX) or SAS 9.1.3 (SAS Institute; Cary, NC) and P<0.05 was considered statistically significant.
Results
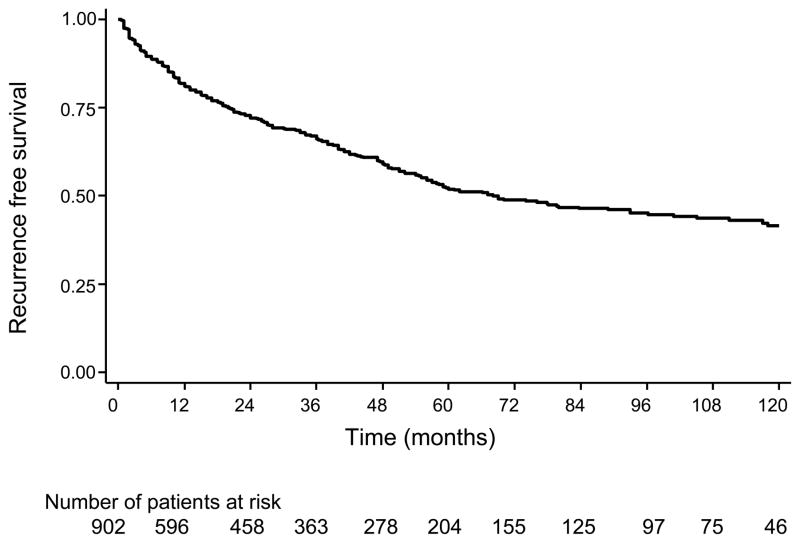
Table 1 lists the demographic and clinicopathological characteristics of the patients. Most patients were below 60 years of age (61%, n=554) had preoperative PSA values 10 ng/mL (65%, n= 586) and had a pathological Gleason score of 7 or less (87%, n=760). A relatively large proportion of the men were African American (40%, n=358). The mean (SD) and median follow-up among men who did not recur was 4.0 years (3.6) and 3.0 respectively. Overall, 346 (39%) patients experienced biochemical recurrence (figure 1). The 1-, 3-, and 5-year overall risk of biochemical recurrence was 19, 34 and 48%.
Table 1.
Preoperative clinical and pathological characteristics of men from the SEARCH database with either a positive surgical margin or extracapsular extension
| Characteristics | No. (%) |
|---|---|
| No. of patients | 902 |
| Age at time of surgery, mean (SD) | 62.0 (6.6) |
| Preoperative PSA, ng/mL | |
| Mean (SD) | 10.5 (11.0) |
| Median (Range) | 7.5 (0.56 – 140) |
| Race | |
| White | 493 (55) |
| Black | 358 (40) |
| Other | 45 (5) |
| Clinical stage | |
| T1 | 422 (52) |
| T2 | 379 (47) |
| T3 | 5 (1) |
| Biopsy Gleason score | |
| 2–6 | 526 (60) |
| 3+4 | 208 (24) |
| 4+3 | 69 (8) |
| 8–10 | 68 (8) |
| Pathological Stage | |
| pT2+ | 544 (60) |
| pT3 | 135 (15) |
| pT3+ | 223 (25) |
| Pathological Gleason score | |
| 2–6 | 283 (32) |
| 3+4 | 384 (44) |
| 4+3 | 93 (11) |
| 8–10 | 114 (13) |
| Extracapsular extension | 364 (43) |
| Positive surgical margins | 715 (84) |
| Prostate specimen weight, g | |
| Mean (SD) | 40.9 (20.0) |
| Median (Range) | 36.7 (8.2 – 232.5) |
| Lymph Node Status | |
| Negative | 643 |
| Unknown | 259 |
Figure 1.
Actuarial 10-year Kaplan-Meier estimates of biochemical recurrence rates in patients
On univariate exploratory analysis, the number of positive surgical margins, pathological Gleason score and pre-operative serum PSA levels were significantly associated with biochemical relapse (p<0.001 for all three) as was prostate specimen weight (p=0.002) and age at the time of surgery (p=0.03). No other variables were significantly associated with time to biochemical recurrence. On multivariate analysis, similar to the univariate analysis, only age at the time of surgery, pathological Gleason score, number of positive margins, PSA and prostate weight were significantly predictive of biochemical recurrence (table 2). The overall accuracy of this multivariate model to predict biochemical recurrence, as defined by the Concordance index C, was 0.67. This compared favorably with the post-operative “Kattan nomogram”8, which had a Concordance index C of 0.60 in our subset of patients.
Table 2.
Cox proportional hazards analysis of factors estimating time to biochemical recurrence following radical prostatectomy
| Variables | Hazard Ratio (95% CI) | Coefficient β | P value |
|---|---|---|---|
| Patient age >60 vs. ≤ 60 | 1.57 (1.17 – 2.11) | 0.45 | 0.003 |
|
| |||
| Preoperative PSA>10 vs. ≤ 10 ng/mL | 1.59 (1.21 – 2.09) | 0. 46 | 0.001 |
|
| |||
| Pathological Gleason score (vs. ≤3+4) | |||
| 4+3 | 1.57 (1.06 – 2.32) | 0.45 | 0.025 |
| 8 – 10 | 2.17 (1.51 – 3.10) | 0.77 | <0.001 |
|
| |||
| Number of positive margins >2 vs. ≤ 2 | 1.96 (1.49 – 2.57) | 0.67 | <0.001 |
|
| |||
| Prostate weight ≤ 30 vs. >30g | 1.72 (1.29 – 2.30) | 0.55 | <0.001 |
Abbreviations: CI – Confidence Interval; PSA – Prostate Specific Antigen
We used the significant risk factors identified in table 2 to generate tables to estimate the risk of biochemical recurrence free survival at 1-, 3-, and 5-years after surgery (table 3). For example, the 1-, 3-, and 5-year risk of biochemical recurrence for a man with age ≤ 60, preoperative serum PSA level ≤ 10 ng/mL, pathological Gleason score of 3+4 or lower, with ≤ 2 positive margins and a prostate specimen weight of >30g are 93, 87 and 79%, respectively. This compares to a 1-, 3-, and 5-year risk of PSA recurrence of 26, 7, and 1%, respectively, for a man with age >60, preoperative serum PSA level >10 ng/mL, pathological Gleason score ranging from 8 through 10, with >2 positive margins and a prostate specimen weight of ≤ 30g.
Table 3.
Estimate of risk of biochemical recurrence free survival following prostatectomy*
| YEAR 1 | Risk Estimate, % (95% Confidence Interval) | |||||
|---|---|---|---|---|---|---|
| Margins ≤ 2 | Margins > 2 | |||||
| Prostate weight >30g | Prostate weight ≤ 30g | Prostate weight >30g | Prostate weight ≤ 30g | |||
| Age ≤ 60 | PSA ≤ 10 ng/mL | Gleason score ≤ 3+4 | 93 (91–95) | 88 (84–92) | 87 (82–91) | 78 (72–86) |
| Gleason score 4+3 | 89 (84–94) | 82 (75–91) | 80 (72–89) | 68 (56–82) | ||
| Gleason score 8–10 | 85 (79–92) | 76 (67–87) | 74 (64–85) | 59 (46–75) | ||
| PSA>10 ng/mL | Gleason score ≤ 3+4 | 89 (85–93) | 82 (76–89) | 80 (73–87) | 68 (58–79) | |
| Gleason score 4+3 | 83 (76–91) | 73 (62–86) | 70 (59–83) | 54 (40–74) | ||
| Gleason score 8–10 | 78 (69–88) | 65 (53–80) | 61 (49–77) | 43 (29–63) | ||
| Age > 60 | PSA ≤ 10 ng/mL | Gleason score ≤ 3+4 | 89 (86–92) | 82 (77–88) | 80 (74–86) | 68 (59–78) |
| Gleason score 4+3 | 84 (78–90) | 74 (64–85) | 71 (60–83) | 55 (41–73) | ||
| Gleason score 8–10 | 78 (71–86) | 65 (54–78) | 62 (50–76) | 43 (30–63) | ||
| PSA>10 ng/mL | Gleason score ≤3+4 | 83 (79–88) | 73 (65–82) | 70 (62–79) | 54 (43–69) | |
| Gleason score 4+3 | 75 (67–85) | 61 (48–78) | 57 (45–73) | 38 (24–61) | ||
| Gleason score 8–10 | 68 (58–79) | 51 (38–69) | 46 (34–63) | 27 (15–48) | ||
Discussion
Despite being one of the most effective methods for managing organ confined disease radical prostatectomy is still plagued with a recurrence rate ranging from 15 – 40 percent.1, 2 While pathological stage and grade are useful determinants of recurrence risk, many men will find themselves in an intermediate risk group based upon standard pathological features. For these men, adjuvant radiation has been suggested as a potential modality to reduce biochemical recurrence risk though at the cost of increased toxicity.5, 6 In order to better risk stratify these men, we examined men with extracapsular disease or a positive margin after radical prostatectomy and determined significant risk factors for biochemical recurrence. We found that along with the standard prognostic variables of PSA and Gleason grade, that the number of positive margins as well as prostate size and patient age were all significant predictors of biochemical recurrence. Using these features, we developed tables that estimate the risk of biochemical recurrence at 1-, 3-, and 5-years after surgery. It is hoped that once these tables are validated, that their use can help guide men and their physicians toward identifying those at the greatest and lowest need for adjuvant therapy.
In the current study of men with intermediate risk, the overall 5-year biochemical recurrence free survival was 55%. Therefore, nearly half the men in this group will recur while half will not by 5-years. This is in line with prior studies that found the risk of recurrence among men with positive surgical margins was similarly near 50%.10 This highlights the uncertain future for these men who are at high-risk of recurrence.
Analogous to multiple prior studies, we found men with higher pre-operative PSA and higher grade disease were at increased risk of biochemical recurrence.11, 12 Apart from these well discussed variables, we found the number of margins to be strongly associated with the risk of biochemical failure. Specifically, the risk of recurrence rose in those with 2 or more positive margins while men with 1 or 0 positive margins had similar outcomes. Across many studies positive margins have been strongly associated with recurrence,12, 13 but studies reporting the number of margins as a risk factor for recurrence are infrequent.14, 15 Lowe et al found that men with more than one positive margins were at increased risk of recurrence in univariate analysis.14 However, no multivariate analysis was performed. In the current study, the number of positive margins remained a significant predictor of recurrence on multivariate analysis. Our findings are similar to those by Obek et al who found that 2 or more positive margins were associated with 2.5 times increased risk for biochemical recurrence.15 Therefore, future studies should include not just whether the overall surgical margins are positive, but the number of positive margins.
Several prior studies have found that younger men tend to have more favorable clinical outcomes after radical prostatectomy.16, 17 In agreement with these prior studies including a prior study from the SEARCH database,17 we also found younger men had better outcomes. Though invariably age is on a continuum, we found that a cut-off of age 60 provided the best separation between “young” and “old”. However, in different patient populations the exact cutoff may vary. Whether age 60 is truly the optimal cut-off remains to be determined.
We18 and others19 have previously found that men with smaller prostates are at increased risk of recurrence. The current study lends further support to the growing literature suggesting a clinically important and statistically significant association between small prostate size and poor outcome. It has previously been suggested much of this association may be driven by larger prostates producing more PSA from the benign elements leading to early detection creating a lead time bias.19 However, it has also been suggested there may be a true biological link between small prostate size and aggressive prostate cancer. If true, it is unknown whether this results from a local paracrine effect of the benign elements suppressing the aggressive cancer or a small prostate creating a hostile environment where only the most aggressive cancers can survive18 or an alternative explanation. However, regardless of the reason why, the clinical significance is that small prostate size appears to be associated with poorer outcomes among men with prostate cancer.
Ultimately, putting all of the significant risk factors together we derived tables to estimate the risk of recurrence at 1-, 3-, and 5-years after surgery. The tables have the advantage of being able to estimate the risk of biochemical recurrence based on easily available clinicopathological characteristics and does not involve complex calculations. The accuracy of these tables was 67% for predicting time to biochemical recurrence. Overall, this compared well with the Kattan postoperative nomogram which had a predictive accuracy of only 60% among our patients.8 However, the modest predictive accuracy of 67% highlights the difficulty in predicting outcome among men who are intermediate risk. It is hoped that in the future, molecular markers will be available to further aide risk stratification within this group.
Our study has several limitations. First and foremost, though our model to predict recurrence appeared to perform better than the commonly used Kattan nomogram, this was based upon the same population from which our model was derived. Therefore, external validation will be required to assess whether this holds true in other populations as well. Also, in many of the subsets in table 3, the confidence intervals are wide. This likely reflects small patient numbers, but also the fact that currently available clinical and pathological information is inexact in predicting outcome. Future studies are needed to better identify molecular markers that can improve risk stratification. The follow-up of our patients is relatively short. Longer follow-up with more individuals in the higher risk categories will enable us to derive more robust conclusions. We must bear in mind that although an acceptable clinical endpoint, biochemical recurrence is not a perfect surrogate for more concrete endpoints like metastases or cancer specific death since biochemical recurrence has a highly variable natural history. However, early biochemical recurrence is linked with increased risk for prostate cancer death.20 The current study used non-centralized pathologic evaluation of surgical specimens from multiple institutions by multiple pathologists, which may make pooling of pathologic findings problematic. However, this may better represent what most practicing urologists can expect in their practice and as such, may have broader applicability than single institutional series. Finally, while our model may be able to better risk stratify patients the amount of risk which should be the threshold to treat is difficult to determine. This decision may be influenced by a multitude of factors including life expectancy, comorbidities and individual patient preference. Ultimately, it is the discretion of the patient and treating physician to decide on treatment options.
Conclusions
In conclusion, we have developed an easy to use device which after validation in other patient populations should enable both physicians and patients to assess the risk of recurrence in a subgroup of patients with intermediate risk after radical prostatectomy. This will serve to guide the decision making process specifically regarding the need for adjuvant therapy.
Table 4.
| YEAR 3 | Risk Estimate, % (95% Confidence Interval) | |||||
|---|---|---|---|---|---|---|
| Margins ≤2 | Margins>2 | |||||
| Prostate weight >30g | Prostate weight ≤ 30g | Prostate weight >30g | Prostate weight ≤ 30g | |||
| Age ≤ 60 | PSA ≤10 ng/mL | Gleason score ≤3+4 | 87 (83–91) | 78 (72–85) | 76 (68–83) | 62 (52–73) |
| Gleason score 4+3 | 80 (72–89) | 68 (56–82) | 64 (52–80) | 46 (32–68) | ||
| Gleason score 8–10 | 73 (63–85) | 59 (46–75) | 54 (41–72) | 35 (22–56) | ||
| PSA>10 ng/mL | Gleason score ≤3+4 | 80 (73–87) | 67 (58–79) | 64 (54–75) | 47 (34–62) | |
| Gleason score 4+3 | 70 (59–83) | 54 (40–74) | 50 (36–69) | 30 (17–54) | ||
| Gleason score 8–10 | 61 (49–76) | 43 (28–64) | 38 (25–58) | 19 (9–40) | ||
| Age > 60 | PSA 10 ≤ng/mL | Gleason score ≤3+4 | 80 (75–85) | 68 (60–77) | 64 (56–74) | 47 (36–61) |
| Gleason score 4+3 | 70 (61–81) | 54 (41–72) | 50 (37–68) | 30 (17–54) | ||
| Gleason score 8–10 | 61 (51–74) | 43 (30–61) | 38 (26–57) | 19 (9–39) | ||
| PSA>10 ng/mL | Gleason score ≤3+4 | 70 (63–78) | 54 (43–68) | 50 (39–62) | 30 (19–48) | |
| Gleason score 4+3 | 57 (45–72) | 38 (24–60) | 33 (21–53) | 15 (6–37) | ||
| Gleason score 8–10 | 46 (34–62) | 26 (15–47) | 22 (12–39) | 7 (2–23) | ||
Table 5.
| YEAR 5 | Risk Estimate, % (95% Confidence Interval) | |||||
|---|---|---|---|---|---|---|
| Margins<2 | Margins>2 | |||||
| Prostate weight >30g | Prostate weight ≤ 30g | Prostate weight >30g | Prostate weight ≤ 30g | |||
| Age ≤ 60 | PSA ≤ 10 ng/mL | Gleason score ≤3+4 | 79 (74–86) | 67 (59–77) | 64 (54–74) | 46 (35–60) |
| Gleason score 4+3 | 70 (58–83) | 54 (40–73) | 49 (35–69) | 30 (16–54) | ||
| Gleason score 8–10 | 61 (48–76) | 42 (28–63) | 38 (24–59) | 18 (9–40) | ||
| PSA>10 ng/mL | Gleason score ≤3+4 | 69 (61–79) | 53 (42–68) | 49 (38–63) | 29 (18–47) | |
| Gleason score 4+3 | 56 (43–74) | 37 (22–61) | 32 (19–55) | 14 (5–38) | ||
| Gleason score 8–10 | 45 (31–65) | 25 (13–49) | 21 (10–42) | 7 (2–23) | ||
| Age > 60 | PSA ≤10 ng/mL | Gleason score ≤3+4 | 70 (63–76) | 53 (44–65) | 49 (40–61) | 29 (19–45) |
| Gleason score 4+3 | 57 (45–71) | 38 (24–59) | 33 (20–54) | 15 (6–37) | ||
| Gleason score 8–10 | 45 (33–62) | 26 (15–46) | 21 (11–40) | 7 (2–22) | ||
| PSA>10 ng/mL | Gleason score ≤3+4 | 56 (48–66) | 37 (26–53) | 32 (22–46) | 14 (7–30) | |
| Gleason score 4+3 | 40 (28–58) | 21 (10–44) | 17 (8–36) | 5 (1–21) | ||
| Gleason score 8–10 | 29 (18–46) | 12 (4–30) | 9 (3–23) | 1 (<1–9) | ||
How this should be explained to a patient is that, Mr. X, given your PSA, age, prostate weight, number of positive surgical margins, and your Gleason sum, our best guess is that you have an X (actual value from the table) percentage chance of not developing a PSA recurrence at 1, 3, and 5 years. We are 95% confident that your chances of not recurring are somewhere between x and y (the lower and upper limits of the 95% confidence interval).
Acknowledgments
Supported by the Department of Veterans Affairs, National Institute of Health R01CA100938 (WJA), NIH Specialized Programs of Research Excellence Grant P50 CA92131-01A1 (WJA), the Georgia Cancer Coalition (MKT), the Department of Defense, Prostate Cancer Research Program, (SJF), and the American Urological Association Foundation/Astellas Rising Star in Urology Award (SJF). Views and opinions of, and endorsements by the author(s) do not reflect those of the US Army or the Department of Defense.
Key for Abbreviations
- CI
confidence interval
- OR
odds ratio
- PSA
prostate-specific antigen
- SD
standard deviation
- SEARCH
Shared Equal-Access Regional Cancer Hospital
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Han M, Partin AW, Pound CR, Epstein JI, Walsh PC. Long-term Biochemical Disease-Free and Cancer-specific Survival Following Anatomic Radical Retropubic Prostatectomy: The 15-Year Johns Hopkins Experience. Urol Clin North Am. 2001;28:555. doi: 10.1016/s0094-0143(05)70163-4. [DOI] [PubMed] [Google Scholar]
- 2.Roehl KA, Han M, Ramos CG, Antenor JAV, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004;172:910. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 3.D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Schnall M, Tomaszewski JE, et al. A Multivariate Analysis of Clinical and Pathological Factors that Predict for Prostate Specific Antigen Failure after Radical Prostatectomy for Prostate Cancer. J Urol. 1995;154:131. [PubMed] [Google Scholar]
- 4.Messing EM, Manola J, Sarosdy M, Wilding G, Crawford ED, Trump D. Immediate Hormonal Therapy Compared with Observation after Radical Prostatectomy and Pelvic Lymphadenectomy in Men with Node-Positive Prostate Cancer. N Engl J Med. 1999;341:1781. doi: 10.1056/NEJM199912093412401. [DOI] [PubMed] [Google Scholar]
- 5.Thompson IM, Jr, Tangen CM, Paradelo J, Lucia MS, Miller G, Troyer D, et al. Adjuvant Radiotherapy for Pathologically Advanced Prostate Cancer: A Randomized Clinical Trial. JAMA. 2006;296:2329. doi: 10.1001/jama.296.19.2329. [DOI] [PubMed] [Google Scholar]
- 6.Bolla M, van Poppel H, Collette L, van Cangh P, Vekemans K, Da Pozzo L, et al. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911) The Lancet. 366:572. doi: 10.1016/S0140-6736(05)67101-2. [DOI] [PubMed] [Google Scholar]
- 7.Freedland SJ, Amling CL, Dorey F, Kane CJ, Presti JC, Terris MK, et al. Race as an outcome predictor after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. Urology. 2002;60:670. doi: 10.1016/s0090-4295(02)01847-2. [DOI] [PubMed] [Google Scholar]
- 8.Stephenson AJ, Scardino PT, Eastham JA, Bianco FJ, Jr, Dotan ZA, DiBlasio CJ, et al. Postoperative Nomogram Predicting the 10-Year Probability of Prostate Cancer Recurrence After Radical Prostatectomy. J Clin Oncol. 2005;23:7005. doi: 10.1200/JCO.2005.01.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harrell FE, Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247:2543. [PubMed] [Google Scholar]
- 10.Karakiewicz PI, Eastham JA, Graefen M, Cagiannos I, Stricker PD, Klein E, et al. Prognostic impact of positive surgical margins in surgically treated prostate cancer: multi-institutional assessment of 5831 patients. Urology. 2005;66:1245. doi: 10.1016/j.urology.2005.06.108. [DOI] [PubMed] [Google Scholar]
- 11.Partin AW, Piantadosi S, Sanda MG, Epstein JI, Marshall FF, Mohler JL, et al. Selection of men at high risk for disease recurrence for experimental adjuvant therapy following radical prostatectomy. Urology. 1995;45:831. doi: 10.1016/S0090-4295(99)80091-0. [DOI] [PubMed] [Google Scholar]
- 12.Bauer JJ, Connelly RR, Seterhenn IA, Deausen J, Srivastava S, McLeod DG, et al. Biostatistical modeling using traditional preoperative and pathological prognostic variables in the selection of men at high risk for disease recurrence after radical prostatectomy for prostate cancer. The Journal of Urology. 1998;159:929. [PubMed] [Google Scholar]
- 13.Epstein JI. Incidence and significance of positive margins in radical prostatectomy specimens. Urol Clin North Am. 1996;23:651. doi: 10.1016/s0094-0143(05)70343-8. [DOI] [PubMed] [Google Scholar]
- 14.Lowe BA, Lieberman SF. Disease recurrence and progression in untreated pathologic stage t3 prostate cancer: selecting the patient for adjuvant therapy. J Urol. 1997;158:1452. [PubMed] [Google Scholar]
- 15.Obek C, Sadek S, Lai S, Civantos F, Rubinowicz D, Soloway MS. Positive surgical margins with radical retropubic prostatectomy: anatomic site-specific pathologic analysis and impact on prognosis. Urology. 1999;54:682. doi: 10.1016/s0090-4295(99)00204-6. [DOI] [PubMed] [Google Scholar]
- 16.Khan MA, Han M, Partin AW, Epstein JI, Walsh PC. Long-term cancer control of radical prostatectomy in men younger than 50 years of age: update 2003. Urology. 2003;62:86. doi: 10.1016/s0090-4295(03)00404-7. [DOI] [PubMed] [Google Scholar]
- 17.Freedland SJ, Presti JC, Kane CJ, Aronson WJ, Terris MK, Dorey F, et al. Do younger men have better biochemical outcomes after radical prostatectomy? Urology. 2004;63:518. doi: 10.1016/j.urology.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 18.Freedland SJ, Isaacs WB, Platz EA, Terris MK, Aronson WJ, Amling CL, et al. Prostate size and risk of high-grade, advanced prostate cancer and biochemical progression after radical prostatectomy: a search database study. J Clin Oncol. 2005;23:7546. doi: 10.1200/JCO.2005.05.525. [DOI] [PubMed] [Google Scholar]
- 19.D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Tomaszewski JE, Wein A. A prostate gland volume of more than 75 cm3 predicts for a favorable outcome after radical prostatectomy for localized prostate cancer. Urology. 1998;52:631. doi: 10.1016/s0090-4295(98)00228-3. [DOI] [PubMed] [Google Scholar]
- 20.Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Partin AW. Time to prostate specific antigen recurrence after radical prostatectomy and risk of prostate cancer specific mortality. J Urol. 2006;176:1404. doi: 10.1016/j.juro.2006.06.017. [DOI] [PubMed] [Google Scholar]