Abstract
Purpose
PSA doubling time (PSADT) following biochemical recurrence after radical prostatectomy is a powerful predictor of prostate cancer-specific and overall death. To calculate PSADT requires multiple PSA determinations unaltered by secondary therapy separated by sufficient time. Physicians and patients may be unwilling to wait before starting secondary therapy, especially for high-risk recurrences. Hence, those with calculable PSADT may represent a select lower-risk group relative to all men with biochemical recurrence.
Methods
We compared clinical and pathological features between patients with and without calculable PSADT and assessed time trends in the proportion with calculable PSADT among 535 patients with biochemical recurrence after radical prostatectomy at 5 VA medical centers comprising the SEARCH Database between 1988 and 2003.
Results
PSADT was not calculable in 187 (35%) patients, most commonly due to receipt of secondary therapy (155/187 (83%)). Over time, the proportion of patients with calculable PSADT declined significantly (p<0.001). The presence of adverse pathological features, faster time to recurrence, higher body mass index and differing surgical centers were associated with not having a calculable PSADT. Among all men who recurred in the most recent year of analysis the adjusted probability of having a calculable PSADT was only 43%: 61% in patients with favorable pathology, yet only 30% with seminal vesicle invasion.
Conclusions
Those with calculable PSADT represented a select, lower-risk cohort and the proportion of patients with calculable PSADT declined over time. This highlights the need for alternative markers for men with recurrent prostate cancer as one of our best current markers, PSADT, is only available in a limited number of patients.
Keywords: Prostate neoplasms, radical prostatectomy, prostate specific antigen, tumor markers
INTRODUCTION
PSA doubling time (PSADT) following biochemical recurrence after radical prostatectomy is a powerful predictor for prostate cancer-specific and overall death.1, 2 It has been suggested that PSADT may be a surrogate end-point for prostate cancer death.2 Consequently, PSADT is increasingly being used as a study end-point and by clinicians caring for recurrent prostate cancer patients. However, to calculate PSADT, patients must be followed long enough to have multiple PSA determinations separated by sufficient time. Treatment with any secondary therapy which alters PSA levels renders PSA values inaccurate for calculating PSADT. Thus, the dilemma arises when physicians and patients do not want to wait a sufficient period of time to calculate PSADT before providing secondary treatment. As a result, some patients will receive secondary therapy prior to having a calculable PSADT. In prior studies of men with biochemical recurrence after primary therapy, many patients did not have calculable PSADT.1, 3 Not only does this highlight the need for alternative prognostic markers for patients with recurrent prostate cancer, this raises the possibility that by limiting analyses to patients with calculable PSADT it may introduce selection bias. Moreover, as evidence grows to support additional therapies for high-risk cases, more patients will receive earlier treatment and even fewer patients will have calculable PSADT in the future.4, 5
The integrated computerized medical record within the Veterans Affairs (VA) system maximizes the ability to follow patients, minimizing patients lost to follow-up. Thus, we used the well-described, VA-based, Shared Equal Access Regional Cancer Hospital (SEARCH) database6 to assess time trends in the proportion of men with a calculable PSADT and compare clinical and pathological features between patients with and without calculable PSADT following biochemical recurrence after radical prostatectomy.
MATERIALS AND METHODS
Study population
After obtaining Institutional Review Board approval from each institution, data from patients undergoing radical prostatectomy between 1988 and 2006 at 5 VA Medical Centers across the country were combined into the SEARCH database.6 Patients treated with preoperative androgen deprivation therapy or radiation therapy were excluded. Of 1,950 patients within SEARCH, we excluded 71 (4%) patients with missing follow-up data and 1,255 (64%) who did not experience a biochemical recurrence, defined as a single PSA >0.2 ng/ml, 2 concentrations at 0.2 ng/ml, or secondary treatment for an elevated postoperative PSA. Those recurring after 2003 (n=74) were excluded to minimize the possibility that recent recurrences did not have calculable PSADT due to insufficient follow-up. Finally, we excluded patients with nodal metastases (n=15) as these patients were likely to receive immediate hormonal therapy based on evidence from Messing et al.7 This resulted in a study population of 535.
Follow-up
PSADT was calculated as the natural log of 2 divided by the slope of the linear regression line of logarithmically transformed PSA over time using all PSA values within 24 months after recurrence.1 Patients were required to have ≥2 PSA values separated by ≥3 months. Patients were followed until: a) PSADT was calculable; b) receipt of secondary treatment precluding PSADT calculation; or c) insufficient PSA follow-up data to calculate PSADT. Patients without a calculable PSADT were classified as to the reason why: received radiation treatment; received androgen deprivation therapy; received radiation and androgen deprivation therapy; or insufficient follow-up.
Statistical analysis
Clinical and pathological characteristics were compared between patients with or without a calculable PSADT using t-tests and rank sum tests for normally and non-normally distributed continuous variables respectively, and χ-squared tests for categorical variables. The risk factors for not having a calculable PSADT were examined using multivariate backwards stepwise logistic regression analyses. Age at recurrence, race (white, black, non-white-non-black), body mass index (BMI in kg/m2: <25, 25.0–29.9, 30–34.9,≥35), clinical stage (T1, T2/T3), preoperative PSA (after log-transformation), biopsy and pathology Gleason score (2-6, 3+4,≥4+3), year of recurrence, surgical center (1–5) and time to biochemical recurrence (measured in months) were entered into the model. Information from the pathological sample was examined as a 3-tier categorical variable based upon risk of biochemical recurrence noted in a prior SEARCH database study as follows: i) organ confined margin negative; ii) organ confined margin positive or extracapsular extension with or without positive margins; or iii) seminal vesicle invasion.8 We successively eliminated the least significant variable, until only variables with p<0.05 remained. Based upon the final multivariate predictive model, we explored the adjusted probability of having a calculable PSADT over time stratified by pathological grouping. All statistical analyses were performed using STATA 9.2 (STATA Corp, College Station, TX).
RESULTS
Of the 535 patients, 187 (35%) did not have a calculable PSADT: 155 (29%) due to secondary treatment before PSADT was calculable and 32 (6%) due to missing follow-up. Of the 155 men who received secondary treatment, 29 (19%) received androgen deprivation therapy, 107 (69%) radiation therapy, and 19 (12%) combined radiation and androgen deprivation therapy.
Patients without calculable PSADT were younger (p=0.003), had higher BMI (p=0.02), underwent radical prostatectomy and recurred more recently (p<0.001), were more likely to have margin involvement, extracapsular extension or seminal vesicle invasion (p=0.002), had more sites of positive surgical margins (p=0.002), and recurred faster after surgery (p<0.001) (Table 1). Patients without calculable PSADT tended to have higher biopsy and pathological Gleason scores, yet lower clinical stage and preoperative PSAs, though these differences were not significant.
Table 1.
Demographic, clinical and pathological features among men with, and without calculable PSADT after biochemical recurrence (n=535)
| Characteristic | Incalculable PSADT 187 (35) | Calculable PSADT 348 (65) | p-value* |
|---|---|---|---|
| Mean age ± SD (years) | 61.9 ± 6.9 | 63.7 ± 6.1 | 0.003† |
|
| |||
| Race (%) | 0.54 | ||
| White | 89 (48) | 184 (53) | |
| Black | 83 (45) | 140 (40) | |
| Other | 14 (8) | 24 (7) | |
|
| |||
| Mean BMI ± SD (kg/m2) | 28.7 ± 4.7 | 27.6 ± 4.4 | 0.02† |
|
| |||
| Clinical stage (%) | 0.10 | ||
| T1 | 78 (45) | 113 (38) | |
| T2/T3 | 94 (55) | 188 (62) | |
|
| |||
| Biopsy Gleason Score (%) | 0.35 | ||
| <7 | 85 (47) | 175 (54) | |
| 3+4 | 43 (24) | 68 (21) | |
| ≥4+3 | 52 (29) | 83 (25) | |
|
| |||
| Median PSA (ng/mL) at surgery (range) | 8.9 (1.2–69) | 10.1 (0.8–114) | 0.32Φ |
|
| |||
| Median year of surgery | 1999 | 1995 | <0.001Φ |
|
| |||
| Pathological Gleason Sum (%) | 0.08 | ||
| <7 | 45 (25) | 113 (33) | |
| 3+4 | 65 (36) | 122 (36) | |
| ≥4+3 | 71 (39) | 106 (31) | |
|
| |||
| Number of positive surgical margins (%) | 0.002 | ||
| 0 | 56 (36) | 142 (52) | |
| 1 | 39 (25) | 71 (26) | |
| 2 | 40 (25) | 41 (15) | |
| 3 | 18 (11) | 15 (6) | |
| 4 | 4 (3) | 5 (2) | |
|
| |||
| Median months to biochemical recurrence (range) | 11 (0.5–111) | 21 (0.5–143) | <0.001Φ |
|
| |||
| Median year of recurrence | 2000 | 1998 | <0.001Φ |
|
| |||
| Pathological Grouping | 0.002 | ||
| Organ confined (T2), margin negative (M−) | 30 (17) | 104 (31) | |
| T2M+ or T3 +/−M+, No SV invasion (SV−) | 102 (57) | 162 (48) | |
| Seminal Vesicle Invasion (SV+) | 47 (26) | 70 (21) | |
|
| |||
| No. Patients at each surgical center (%) | <0.001 | ||
| Center #1 | 57 (30) | 88 (26) | |
| Center #2 | 27 (14) | 59 (17) | |
| Center #3 | 16 (9) | 62 (18) | |
| Center #4 | 17 (9) | 55 (16) | |
| Center #5 | 70 (37) | 84 (24) | |
p-value from chi-squared test unless otherwise indicated
p-value from t-test
p-value from rank-sum test
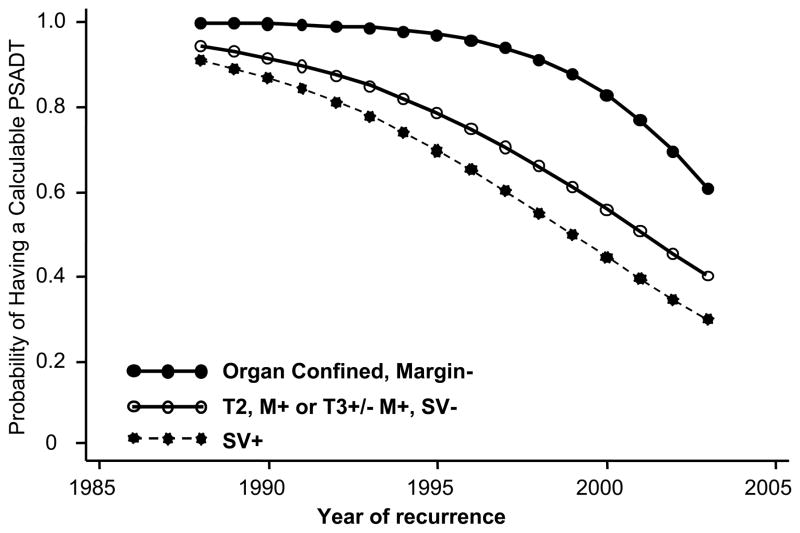
On multivariate analysis, the factors independently associated with not having a calculable PSADT were more recent year of recurrence, faster time to recurrence, higher BMI, adverse pathological features at radical prostatectomy, and differing surgical centers (Table 2). The association between year of recurrence, stratified by pathological findings, and likelihood of having a calculable PSADT are shown in figure 1. Among all men who recurred in 2003, the most recent year of analysis, the adjusted probability of having a calculable PSADT was only 43%: 61% in patients with favorable pathology, yet only 30% in patients with seminal vesicle invasion.
Table 2.
Factors predicting not having a calculable PSADT
| Characteristic | Odds Ratio | 95% CI | p-value |
|---|---|---|---|
| More recent year of recurrence | 1.29 | 1.18–1.41 | <0.001 |
|
| |||
| BMI (kg/m2) | |||
| <25 | 1.00 | --- | --- |
| 25.0 – 29.9 | 2.00 | 1.05–3.79 | 0.03 |
| 30.0 – 34.9 | 1.44 | 0.70–2.95 | 0.32 |
| ≥35.0 | 2.67 | 0.94–7.54 | 0.07 |
|
| |||
| Surgical Center | |||
| Center #1 | 1.00 | --- | --- |
| Center #2 | 0.56 | 0.26–1.22 | 0.14 |
| Center #3 | 0.75 | 0.33–1.66 | 0.47 |
| Center #4 | 0.21 | 0.09–0.50 | <0.001 |
| Center #5 | 1.58 | 0.81–3.09 | 0.18 |
|
| |||
| Pathological Grouping | |||
| Organ confined (T2), Margin negative (M−) | 1.00 | --- | --- |
| T2M+ or T3 +/−M+, No SV invasion (SV−) | 2.64 | 1.39–4.98 | 0.003 |
| Seminal Vesicle Invasion (SV+) | 3.72 | 1.73–7.98 | 0.001 |
|
| |||
| Time to PSA recurrence (months) | 0.99 | 0.98–1.00 | 0.007 |
Figure 1.
Adjusted probability of having a calculable PSADT - stratified by pathologic group
Finally, we repeated the analyses excluding men who recurred within 3 months after radical prostatectomy as these patients may have had PSA persistence as opposed to recurrence. In doing so, of the 535 men in our study population, 46 patients without and 42 with a calculable PSADT were excluded. The trends in clinical and pathological differences between the two groups and the decline in the proportion with calculable PSADT over time were the same as those observed within the full cohort (data not shown).
DISCUSSION
Given that over 200,000 men are diagnosed with prostate cancer annually,9 of which approximately half are treated with radical prostatectomy,10 and a third of which will experience a PSA recurrence after surgery,11 the need for accurate prognostic markers in this setting is great. PSADT following biochemical recurrence after radical prostatectomy is an excellent prognostic marker. It is strongly associated with prostate cancer-specific and overall survival,1, 2 and as such is being increasingly used as a prognostic tool by physicians and as an intermediate endpoint in clinical studies.2, 12–14
However, increasingly the dilemma emerges in the setting of rising PSA after radical prostatectomy when physicians and patients do not want to wait a sufficient period of time to calculate PSADT before providing secondary treatment. Indeed, in prior studies, many patients did not have calculable PSADT.1, 3 Not only does this suggest a need for alternative prognostic markers in recurrent prostate cancer, but raises the possibility of selection bias between patients with and without calculable PSADT.
To explore this, we examined clinical and pathological features among men with biochemical recurrence after radical prostatectomy who did or did not have a calculable PSADT. We found two concerning trends. First, the percentage of patients with calculable PSADT declined significantly over time. Second, patients with calculable PSADT represented a select, lower-risk cohort. Thus, restricting analyses to patients with calculable PSADT not only reduces patient numbers but may introduce selection bias and we anticipate this will become more pronounced in the future.
By capitalizing on the computerized medical record system within the VA, we were able to accurately follow patients regardless of their follow-up location. We had overall follow-up information on 96% of patients. Among recurrent patients, the focus of this study, we had accurate post-recurrence follow-up information in 94%. Therefore, the number of patients lost to follow-up was extremely low, thus reducing bias from inadequate follow-up.
Evidence from prospective randomized controlled trials is growing that adjuvant treatments improve PSA control,4, 7 and retrospective studies suggest, early receipt of secondary treatment may improve outcomes.5, 15 While mortality data remain immature, the earlier use of secondary treatment is becoming more common. Indeed, in the current multicenter study, 29% of recurrent patients received secondary treatment prior to having a calculable PSADT. Furthermore, the proportion with an incalculable PSADT increased significantly over time, with secondary treatment being the primary reason for incalculable PSADT. In 2003, the most recent year of analysis, only 43% of patients had calculable PSADT. As enthusiasm for early secondary therapies after radical prostatectomy grows, it is likely that even fewer men will have calculable PSADT in the future.
Given that evidence supporting use of secondary therapy is strongest for patients with high risk pathological features, our finding that patients with calculable PSADT had significantly lower risk features is not surprising. In the lower risk setting, patients and physicians may be more willing to observe PSA changes rather than treat aggressively with secondary therapy. Thus, patients with calculable PSADT represented a select, lower-risk group. If confirmed in other studies, this has potential profound implications for studies using PSADT as an intermediate end-point. For example, recurrent patients represent a select cohort with an overall more aggressive biology relative to non-recurrent patients. However, within this select group of men with recurrent disease, if those with the most aggressive biology are selectively culled by early secondary treatment, then those who are left for post-recurrence PSADT analysis represent an intermediate-risk cohort. As such, examination of the association between any given factor and disease aggressiveness only among selected patients with intermediate-risk disease (i.e. aggressive enough to recur, but not so aggressive to receive very early secondary treatment) may not be fully reflective of the overall association between the factor and disease biology. As we move into an era of translational research in prostate cancer, it becomes increasingly important to correlate molecular data with clinical outcomes. By restricting analyses only to men with calculable PSADT, we limit our ability to fully understand the biology of new disease markers.
We also noted significant differences in the proportion of patients with computable doubling times between surgical centers. As this analysis controlled for multiple clinical and pathological features, it is likely these inter-center differences in proportion with calculable PSADT are due to varying usage of secondary therapies. Given this variation among centers, it is important that authors report the proportion of patients with calculable PSADT and the clinical and pathological composition of patients with calculable PSADT relative to those without calculable PSADT.
Finally, we noted that obese patients were less likely to have calculable PSADT. Though recent literature has shown overweight patients to have more aggressive disease,16 this only became widely-appreciated after the publication in 2003 of a landmark study on obesity and cancer mortality.17 We have previously noted that obese patients are more likely to have positive surgical margins in the absence of other signs of advanced disease,18 and this may have influenced the likelihood of an overweight patient receiving salvage radiation therapy. Further study is needed to better assess the association between BMI, number and extent of positive surgical margins, and receipt of secondary therapy precluding calculating PSADT.
It should be noted, the proportion of patients with incalculable PSADT also depends on the number of PSA values required to calculate PSADT and over what minimum time interval. Requirement of a greater number of PSA values or a greater time interval between PSA values would decrease the number of patients with calculable PSADT and would likely lead to even greater differences between those with and without calculable PSADT.
This study has some limitations. We contrasted clinical and pathological features of those with and without calculable PSADT and concluded those with PSADT tended to have less aggressive features. Ideally, we would compare time to metastases and time to cancer specific mortality controlling for receipt of any secondary therapies, as this would provide a more accurate assessment of the aggressiveness of tumors in these two groups. At this time, this information is not available in the SEARCH database. However, the clinical and pathological variables we used have been previously shown to correlate with time to metastases and prostate cancer specific mortality.3, 19 In addition, our data are integrated from multiple surgical centers. While this has the potential to introduce heterogeneity, we feel it is more illustrative of the real world differences that exist between centers in use of secondary therapies and thus the proportion of men in whom a PSADT is calculable. Also, as our study is retrospective in nature, we infer the reason for most men to not have a calculable PSADT was because physicians and patients opted for early secondary therapy due to concerns about the aggressiveness of the recurrent disease. Without surveying patients and physicians at the time of treatment decision we cannot be certain about the motivation behind early receipt of secondary therapy. The attitudes and beliefs at the time of biochemical recurrence warrant further study. Regardless of the exact reasons why men did or did not have a calculable PSADT, the observation remains that men with a calculable PSADT appeared to represent a select lower-risk cohort.
Finally, it is important to re-iterate the most meaningful end-point in recurrent prostate cancer remains overall mortality. To date, our best predictor or surrogate for this end-point remains PSADT. In the current study, we did not specifically examine the ability of PSADT to predict this important outcome, though in prior studies it has been shown to predict overall survival.19 However, the current report does suggests there may be limitations in relying on PSADT given that only a limited number of men have usable data. This was especially true among men with high-risk disease – i.e. those at the greatest risk of a poor outcome. Therefore, it is essential new markers be developed, ideally based upon pathological analysis of the tissue and thus available immediately following surgery. To this end, recent studies have demonstrated this approach accurately predicts PSA recurrence,20 but whether it accurately predicts more delayed end-points remains to be determined. However, given the limitations that not all men have PSADT data, it is clear that this approach is desperately needed.
CONCLUSIONS
In conclusion, the current data, if confirmed in other studies, suggest restricting analyses to patients with calculable PSADT not only reduces patient numbers but introduces selection bias. With support increasing for early institution of secondary therapies, these trends will likely become more pronounced in the future. This underscores the need to develop alternative study end-points and prognostic markers as one of our best current markers, PSADT, is only available in a limited number of patients.
Acknowledgments
Supported by the Department of Defense, Prostate Cancer Research Program (RJH & SJF); Department of Veterans Affairs, National Institute of Health R01CA100938 (WJA), NIH Specialized Programs of Research Excellence Grant P50 CA92131-01A1 (WJA), the Georgia Cancer Coalition (MKT), and the American Urological Association Foundation/Astellas Rising Star in Urology Award (SJF). Views and opinions of, and endorsements by the author(s) do not reflect those of the US Army or the Department of Defense. All results herein are original and have not been presented elsewhere in any format. Financial Disclosures: Dr. Freedland – Astra Zeneca – Speaker/Advisory Board
ABBREVIATIONS
- BMI
Body Mass Index
- PSA
Prostate Specific Antigen
- PSADT
Prostate Specific Antigen Doubling Time
- VA
Veterans Affairs
- SEARCH
Shared Equal Access Regional Cancer Hospital Database
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 2.D’Amico AV, Moul JW, Carroll PR, Sun L, Lubeck D, Chen MH. Surrogate end point for prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Natl Cancer Inst. 2003;95:1376. doi: 10.1093/jnci/djg043. [DOI] [PubMed] [Google Scholar]
- 3.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 4.Bolla M, van Poppel H, Collette L, van Cangh P, Vekemans K, Da Pozzo L, et al. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911) Lancet. 2005;366:572. doi: 10.1016/S0140-6736(05)67101-2. [DOI] [PubMed] [Google Scholar]
- 5.Stephenson AJ, Shariat SF, Zelefsky MJ, Kattan MW, Butler EB, Teh BS, et al. Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA. 2004;291:1325. doi: 10.1001/jama.291.11.1325. [DOI] [PubMed] [Google Scholar]
- 6.Freedland SJ, Amling CL, Dorey F, Kane CJ, Presti JC, Jr, Terris MK, et al. Race as an outcome predictor after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. Urology. 2002;60:670. doi: 10.1016/s0090-4295(02)01847-2. [DOI] [PubMed] [Google Scholar]
- 7.Messing EM, Manola J, Sarosdy M, Wilding G, Crawford ED, Trump D. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med. 1999;341:1781. doi: 10.1056/NEJM199912093412401. [DOI] [PubMed] [Google Scholar]
- 8.Freedland SJ, Aronson W, Presti JC, Jr, Kane CJ, Terris MK, Elashoff D, et al. Should a positive surgical margin following radical prostatectomy be pathological stage T2 or T3? Results from the SEARCH database. J Urol. 2003;169:2142. doi: 10.1097/01.ju.0000061760.23169.be. [DOI] [PubMed] [Google Scholar]
- 9.American Cancer Society. Cancer Facts & Figures 2007. Atlanta: American Cancer Society; 2007. [Google Scholar]
- 10.Cooperberg MR, Grossfeld GD, Lubeck DP, Carroll PR. National practice patterns and time trends in androgen ablation for localized prostate cancer. J Natl Cancer Inst. 2003;95:981. doi: 10.1093/jnci/95.13.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004;172:910. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 12.Zhou P, Chen MH, McLeod D, Carroll PR, Moul JW, D’Amico AV. Predictors of prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Clin Oncol. 2005;23:6992. doi: 10.1200/JCO.2005.01.2906. [DOI] [PubMed] [Google Scholar]
- 13.Lee AK, D’Amico AV. Utility of prostate-specific antigen kinetics in addition to clinical factors in the selection of patients for salvage local therapy. J Clin Oncol. 2005;23:8192. doi: 10.1200/JCO.2005.03.0007. [DOI] [PubMed] [Google Scholar]
- 14.D’Amico AV, Moul J, Carroll PR, Sun L, Lubeck D, Chen MH. Prostate specific antigen doubling time as a surrogate end point for prostate cancer specific mortality following radical prostatectomy or radiation therapy. J Urol. 2004;172:S42. doi: 10.1097/01.ju.0000141845.99899.12. [DOI] [PubMed] [Google Scholar]
- 15.Moul JW, Wu H, Sun L, McLeod DG, Amling C, Donahue T, et al. Early versus delayed hormonal therapy for prostate specific antigen only recurrence of prostate cancer after radical prostatectomy. J Urol. 2004;171:1141. doi: 10.1097/01.ju.0000113794.34810.d0. [DOI] [PubMed] [Google Scholar]
- 16.Freedland SJ, Platz EA. Obesity and Prostate Cancer: Making Sense out of Apparently Conflicting Data. Epidemiol Rev. 2007 doi: 10.1093/epirev/mxm006. [DOI] [PubMed] [Google Scholar]
- 17.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 18.Freedland SJ, Aronson WJ, Kane CJ, Presti JC, Jr, Amling CL, Elashoff D, et al. Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: a report by the Shared Equal Access Regional Cancer Hospital database study group. J Clin Oncol. 2004;22:446. doi: 10.1200/JCO.2004.04.181. [DOI] [PubMed] [Google Scholar]
- 19.Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, et al. Death in patients with recurrent prostate cancer after radical prostatectomy: prostate-specific antigen doubling time subgroups and their associated contributions to all-cause mortality. J Clin Oncol. 2007;25:1765. doi: 10.1200/JCO.2006.08.0572. [DOI] [PubMed] [Google Scholar]
- 20.Cordon-Cardo C, Kotsianti A, Verbel DA, Teverovskiy M, Capodieci P, Hamann S, et al. Improved prediction of prostate cancer recurrence through systems pathology. J Clin Invest. 2007;117:1876. doi: 10.1172/JCI31399. [DOI] [PMC free article] [PubMed] [Google Scholar]