Abstract
Fetal Alcohol Syndrome (FAS) is a devastating disorder accompanied by numerous morphological and behavioral abnormalities. Human FAS has been modeled in laboratory animals including the zebrafish. Recently, embryonic exposure to low doses of ethanol has been shown to impair behavior without any gross morphological alterations in zebrafish. The exposed zebrafish showed reduced responses to animated conspecific images. The effect of embryonic ethanol exposure, however, has not been investigated in a real shoal and the potential mechanisms underlying the behavioral impairment are also unknown. Here we show that a 2 hour long immersion in 0.25% and 0.50% (vol/vol) alcohol at 24 hours post fertilization significantly increases the distance among members of freely swimming groups of zebrafish when measured at 70 days post fertilization. We also show that this impaired behavior is accompanied by reduced levels of dopamine, DOPAC, serotonin and 5HIAA as quantified by HPLC from whole brain extracts. Our results demonstrate that even very low concentrations of alcohol applied for a short period of time during the development of zebrafish can impair behavior and brain function. We argue that the observed behavioral impairment is not likely to be due to altered performance capabilities, e.g. motor function or perception, but possibly to social behavior itself. We also argue that our neurochemical data represent the first step towards understanding the mechanisms of this abnormality in zebrafish, which may lead to better modeling of, and ultimately perhaps better therapies for human FAS.
Keywords: zebrafish, fetal alcohol syndrome, dopamine, serotonin, social behavior, shoaling, ethanol
1. INTRODUCTION
Fetal Alcohol Syndrome (FAS) is a devastating disease that affects 1 in 200 children (Stratton et al., 1996). The syndrome is characterized by numerous anatomical abnormalities (Stratton et al., 1996) as well as behavioral aberrations including hyperactivity, deficits in executive functioning, abnormal social skills, increased aggression, delinquency, self-injury, inappropriate sexual behavior, and enhanced fear responses or anxiety in the affected children (e.g. Arenzana et al., 2006; Green, 2007; Steinhausen, 1995). The prevalence of milder forms of the disease in which prenatal alcohol (EtOH or ethyl alcohol) exposure was only modest, leading to less severe behavioral abnormalities, is likely to be much higher than 1 in 200, and these milder cases may remain undiagnosed or misdiagnosed.
FAS has been modeled in the laboratory successfully using animals, mostly rodents such as mice (e.g. Boehm et al., 1997; Becker et al., 1996), but even such model organisms as the fruit fly has been utilized in alcohol research (e.g. Guarnieri & Heberlein, 2003). The zebrafish may also hold great promise in this research area because it appears to strike an optimal compromise between system complexity (it is a vertebrate with basic anatomical, physiological and DNA nucleotide sequence similarities to mammals) and practical simplicity (it is small, easy to keep and breed in large numbers in the lab).
The zebrafish has been successfully employed in modeling FAS but most of these past studies administered high concentrations of alcohol and/or exposed the zebrafish embryo to the substance for extended period of time (Bilotta et al., 2004; Carvan III et al., 2004; Arenzana et al., 2006; Matsui et al., 2006). These fundamental pioneering studies confirmed the teratogenic effects of alcohol in zebrafish and found robust physical malformations that often resembled those seen in extreme severe cases of FAS. As such they demonstrated the promise of zebrafish in the analysis of the mechanisms underlying the gross anatomical changes seen in severe forms of FAS.
Most recently, however, zebrafish were also shown to respond to embryonic exposure to mild alcohol doses (Fernandes & Gerlai, 2009). This latter study revealed that if eggs are exposed to external alcohol concentrations up to 1% (vol/vol) for only 2 hours at 24 hour post fertilization (hpf), the exposed fish develop normally and show no apparent signs of anatomical malformations, and in fact no obvious behavioral defects either. However, when measured in a social preference task at their adult stage, the exposed zebrafish showed a behavioral abnormality that correlated with the concentration of alcohol to which they were exposed inside the egg. These fish showed increased distance from images of conspecifics, a result that was interpreted as impaired social behavior (Fernandes & Gerlai, 2009). This interpretation was based upon the fact that the authors did not find motor defects (the exposed fish swam the same distance and with the same speed as control), or visual perceptual impairment (the exposed fish did respond to the conspecific images by reducing the swim speed the same way as control, they just did not swim close enough to the images). Although suggestive, these results do not rule out alternative explanations. For example, it is possible that the computer monitor that was used presented the zebrafish images with a 60 Hz flicker rate and the artificial nature of this high frequency “vibration” affected the alcohol exposed fish more severely than the control. It is also not known whether other idiosyncratic features of the test situation inadvertently biased the outcome of the test against the alcohol treated fish. For example, the fish were measured singly in an open tank and although the fish images were provided for certain periods of time, the fear inducing aspect of the test tank may have contributed to the observed differences.
To address the above, in the current study we decided to test the effect of early embryonic alcohol exposure on zebrafish in a real freely moving shoal. In this task, zebrafish have the ability not only to see each other but also to perceive olfactory and auditory cues as well as lateral line signals (low frequency waves) from each other. This test situation is also less aversive as the fish are in a group and not alone in the test tank.
Notably, the mechanisms underlying the previously reported (Fernandes & Gerlai, 2009) behavioral impairment are not know. In the current study, therefore, we also started our investigation in this direction by measuring neurochemicals from whole brain extracts of adult zebrafish exposed to alcohol during their embryonic development. We chose to start this analysis with dopamine, and its metabolite DOPAC, and serotonin and its metabolite 5HIAA. The dopaminergic system has been known to be involved in several brain functions one of which is reward. Previously we have shown that the sight of conspecifics is rewarding for zebrafish (Al Imari & Gerlai, 2008). It is thus plausible that abnormal dopaminergic function may explain reduced social behavior. The serotoninergic system too has been shown to be involved in numerous brain functions, including the regulation of mood, e.g. fear responses and aggression (Olivier, 2006; Ruhe and Mason, 2007). Previously, it has also been shown that serotonin levels in the brain are affected by acute alcohol exposure (Chatterjee and Gerlai, 2009).Thus it is possible that altered serotoninergic responses contributed to the abnormal behavioral reactions towards conspecifics in the alcohol exposed zebrafish.
2. METHODS
2.1 Animals and Housing
Two hundred and eighty eight zebrafish (Danio rerio) of the AB strain were used in the two experiments outlined below. From these, 240 zebrafish were analyzed in the behavioral experiments, and 48 zebrafish were utilized in the neurochemical analysis with HPLC. The fish used in the behavioral experiments were housed in groups of 10. The 10 fish in each tank was considered a shoal. For each alcohol concentration (dose) 8 such shoals were quantified (as outlined below). Importantly, the unit of statistical analysis in our behavioral study is the shoal of 10 individuals, i.e. the sample size for each alcohol concentration group in the behavioral analysis was n = 8. For the neurochemical analysis, a total of 48 zebrafish were required to create n=8 samples across the three treatment groups as two brains were pooled per sample to minimize variability due to dissection error.
The fish originated from progenitors obtained from the Zebrafish International Research Centre (ZIRC) (Eugene, Oregon) and were bred in-house. All experiments described were approved by the University of Toronto Animal Care Committee. All fish used in this study were bred, raised and housed in the same environment. Gender could not visually be determined in a reliable fashion at the time of testing but we have found the gender ratio to be 50:50% on average in this strain.
Upon hatching, the animals were housed in groups of ten in 2.8l Plexiglas aquaria. At 15 days post fertilization the aquaria were connected to a recirculating filtration aquaculture rack system which had a mechanical, biological, and activated carbon filter as well as a UV sterilizing unit (Aquaneering Inc. (San Diego, Ca, USA). Prior to being connected to the aquaculture rack system, water changes were performed every other day to avoid debris buildup in the aquaria. Water was maintained at 27°C. The system water used on the rack as well as during the development and testing of the fish was reverse osmosis purified and was supplemented with 60mg/l Instant Ocean Sea Salt to achieve water chemistry appropriate for zebrafish.
Zebrafish were kept at a 12h light/12h dark cycle with lights on at 7:00 h and off at 19:00 h. All fish were fed twice daily with Larval Artificial Plankton 100 (particle size below 100 mm, ZeiglerBros, Inc., Gardners, PA, USA) until two weeks post fertilization, after which animals were fed twice daily with nauplii of brine shrimp (Artemia salina) until they were four weeks old. After this, the developing fry were fed a mixture of flake food (Tetramin Tropical fish flake food, Tetra Co, Melle, Germany) and powered spirulina (1 part, Jehmco Inc., Lambertville, NJ, USA).
2.2 Alcohol Treatment
The dosing regimen utilized for this study has been described previously (Fernandes and Gerlai, 2009), and is summarized briefly below. 288 zebrafish eggs were collected 2.5 hours after fertilization. The eggs were randomly divided into three equal groups. The eggs were placed in system water (deionized and sterilized water supplemented with 60 mg/ l Instant Ocean Sea Salt, Big Al’s Pet Store, Mississauga, ON, Canada) in 100 ml Petri dishes.
At 24 hour post-fertilization, each group of zebrafish eggs received one of the following concentrations of ethanol solution: 0.00%, 0.25%, or 0.50% (vol/vol %). These concentrations were chosen as they have shown not to cause any gross morphological deformities in a previous study (Fernandes and Gerlai, 2009). The timing of 24 hours post-fertilization also corresponds with a time used previously (Fernandes and Gerlai, 2009). The rationale for this timing of alcohol exposure was that at 24 hours post-fertilization, the development of major organs, including the brain, in zebrafish has begun but has not yet been completed. The duration of the treatment was the same as before (Fernandes & Gerlai, 2009), i.e. two hours, after which the eggs were washed with system water and placed in a 2.8l plexiglass aquaria. At this stage, the three treatment groups were subdivided into groups of ten fish, and each of these groups was placed in their own aquarium and labeled.
2.3.1. Experiment 1: Assessment of shoaling behavior at 70dpf
The purpose of this experiment was to investigate differences in shoal cohesion at 70 days post-fertilization (dpf) in zebrafish exposed to different concentrations of alcohol during embryonic development. This age was chosen because by this stage of development normal (control) zebrafish are expected to show robust shoaling responses (Fernandes & Gerlai, 2009; Buske & Gerlai, 2010; Buske & Gerlai, 2011) and thus any impairment in this behavior induced by embryonic alcohol exposure may be detected. The main goal of the experiment was to investigate whether impairments in social behavior are observable in freely moving shoals.
Upon reaching the 70dpf age, all 10 fish from a given shoal were released simultaneously in the center of a square plexi-glass tank, the open field, and were allowed to explore the field freely. The behavioral trial lasted seven minutes and was recorded with an overhead video camera (JVC Everio Hard Drive GZ-MG750BU). After the open field trial, the shoal was returned to its home tank. The arena size was kept proportional to the body length of the experimental fish similarly to what has been recommended for other fish species before (Vogel, 2008; Masuda et al, 2003; Gallego et al., 1995) and exactly as used in a previous experiment conducted with zebrafish (Buske and Gerlai, 2011). That is, the size of the open field (70 × 70 × 15 cm, length × width × depth) was chosen to be 28x the average body length of the 70 dpf old zebrafish. The behavioral observation trials were conducted between 9:00 h and 16:00 h, i.e. the middle of the light phase of the light cycle.
2.3.2. Recording and quantification of behavior
All digital video files were converted to AVI format using Cyberlink Powerdirector. Still images were obtained for every 5 seconds of the complete duration of the recorded trials (420 sec trial length, 5 sec sampling rate, 85 time samples starting with time point 0 sec and ending with time point 420 sec). From each still image, the exact coordinates of each member of the ten-member shoal were determined. These coordinates were then used to calculate the following measures using a custom software application developed in-house described previously (Miller and Gerlai, 2007). First we calculated the nearest neighbor distance. Nearest neighbor is the distance between a focal fish and another shoal member that is closest to it. Each shoal member has only one nearest neighbor thus this measure is not dependent on the size of the shoal (the number of shoal members) but it is also less sensitive to the distribution of fish within the shoal compared to two other measures we calculated (see below). The nearest neighbor values (N) were averaged (ΣN/9, a 10 member shoal has 9 nearest neighbor values because a fish cannot be its own neighbor) for each shoal. The mean and standard error of these values were then calculated for each dose group, which contained an n = 8 shoals, for each observation interval sampled. Another measure of shoal cohesion is the Interindividual distance among shoal members. This measure, unlike the nearest neighbor distance, is dependent upon the size of the shoal but more importantly it is also more sensitive to the distribution of the fish within the shoal. It was calculated as follows. First the distances between a focal fish and all other members of the shoal were determined (I). These distances were then averaged for the particular focal fish (ΣD/9). This calculation was performed for each shoal member. The values obtained for all shoal members were then averaged (Σ( ΣD/9)/10 also see figure 1). This measure takes into account all the distances between pairs of fish within a shoal and thus provides a full description of shoal cohesion characteristic of the particular shoal quantified. The mean and the standard error of the average interindividual distances (based upon the 8 shoals tested per alcohol dose group) were then calculated for every 5 second interval sampled. The variance of the distances between a focal fish and all of its shoal mates (V) was also calculated for each shoal member. The average of the ten variance values (ten member shoal) obtained was then calculated (ΣV/10). This value was then used to determine the mean and standard error of the 8 shoals tested per alcohol dose group. The “variance of distances” quantifies how homogeneously distributed are the members of the shoal. Last, we calculated the distance from center. This measure is expected to reveal potential thigmotactic behavior, considered a measure of fear. The distance of a focal fish from the center was first established ‘C’, and then these distance values were averaged (ΣC/10). The mean and standard error of these values were then obtained for the 8 shoal dose groups for each interval sampled. All of the above values are calculated in body length of the fish, a common practice in the analysis of shoaling that allows comparison across different age groups, i.e. differently sized fish. Fish of different alcohol dose groups were tested and their behavior quantified and analyzed in a fully randomized and blind manner as to their group designation.
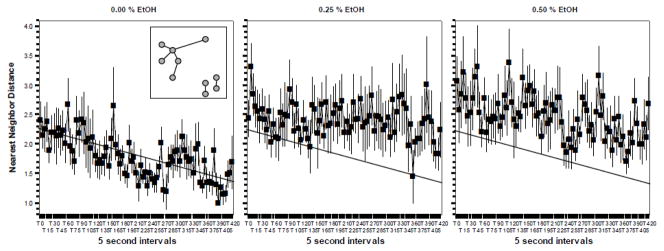
Figure 1.
The Nearest Neighbor Distance between fish did not significantly change in response to embryonic alcohol (EtOH) treatment when measured in 70 day post fertilization old zebrafish. Mean ± Standard Error are shown. Sample size n = 8 for each dose group (i.e. 8 shoals of ten fish each were quantified per alcohol dose). The concentration of embryonic alcohol treatment is shown above the graphs. The distance values are shown in body length. The inset in graph 1 shows the method of quantifying nearest neighbor distance. The grey circles represent individual fish in the shoal. The lines represent the nearest neighbor distance. Note that although there are seven lines, there are all together nine nearest neighbor distance values calculated because for the two “pairs” of fish on the lower right corner of the inset the nearest neighbor distance is the same for each member of the corresponding pair. Also note that the slanted straight line represents the best fit regression line for graph one. The same line is drawn on the other graphs to facilitate comparison of values. For further methodological details and results of statistical analyses, see Methods and Results.
2.4 Experiment 2: HPLC Analysis of whole brain samples
Zebrafish from all three alcohol dose groups (two alcohol exposed and one control group) were sacrificed at the same time to obtain a total of 48 brain samples (16 brains per dose group). Fish were sacrificed by decapitation between 1000 and 1500h, the brains were quickly dissected on ice and placed in a microcentrifuge tube which was then kept at −80°C. In order to obtain sufficient amount of whole brain tissue appropriate for neurotransmitter detection, we pooled 2 brain extracts and thus had n = 8 for each dose group for statistical analysis.
Our HPLC methods followed those developed recently for zebrafish (Chatterjee & Gerlai, 2009). Briefly samples were centrifuged and 5μl of the supernatant was analyzed using a BAS 460 MICROBORE-HPLC system with electrochemical detection (Bio-analytical Systems Inc., West Lafayette, IN, USA) together with a Uniget C-18 reverse phase microbore column as the stationary phase (BASi, Cat. No. 8949). Standard dopamine, DOPAC (Sigma Chemicals, St. Louis, MO, USA), serotonin and 5-HIAA (Sigma) were used to quantify and identify the peaks on the chromatographs. The total brain protein was found not significantly different between dose groups and the levels of neurochemicals are standardized accordingly, i.e. expressed as ng neurochemical per mg brain protein.
2.5 Statistical analysis
The behavioral data were analyzed using repeated measure variance analysis (ANOVA) with interval as the repeated measure (within subject) factor (85 levels) and Alcohol (3 levels) as the between subject factor. The unit of analysis for the behavioral study was the shoal, as explained above. The null hypothesis of “alcohol not increasing the distance among shoal members” was rejected when its probability was less then 0.05, a one tailed statistical test. The null hypothesis of “alcohol not changing the distance from center” was rejected when its probability was less then 0.05, a two tailed statistical test. The neurochemical data were analyzed with non-repeated ANOVA with Alcohol as the between subject factor. In case of significant alcohol effect (p < 0.05), post hoc multiple comparison test, the Tukey Honestly Significant Difference (HSD) test, was performed to investigate which alcohol dose group differed from which.
3. RESULTS
The temporal changes throughout the 7 min behavioral recording session (85 time points with 5 sec sampling rate) in the nearest neighbor distance are shown in figure 1. ANOVA revealed a significant Interval effect (F(84, 1848) = 2.166, p <0.001) demonstrating that the nearest neighbor distances did not remain the same throughout the observation period. The Interval x Alcohol interaction was found non-significant (F(168, 1848) = 0.947, p > 0.65). Although figure 1 suggest an apparent increase of nearest neighbor distances in the two alcohol treated groups as compared to control, ANOVA found this difference not to reach statistical significance (Alcohol effect F(2, 22) = 1.987, p = 0.081).
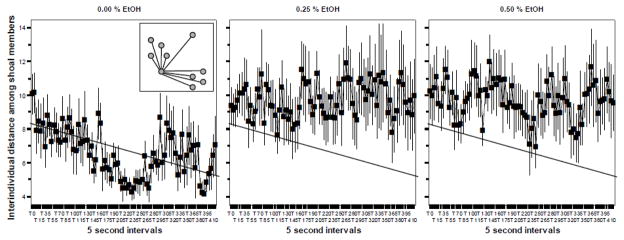
The Interindividual distance among all shoal members we consider a more sensitive measure of shoal cohesion as it takes each distance among shoal member pairs of all combinations into account. Variance analysis of temporal changes in this behavioral measure found a non-significant Interval effect (F(84, 1848) = 1.002, p > 0.45), a non-significant Interval x Alcohol interaction (F(168, 1848) = 0.918, p > 0.75), but a significant Alcohol effect (F(2, 22) = 2.943, p < 0.05). These results (also see figure 2) suggest that while the interindividual distances did not significantly vary with time during the recording session, alcohol increased these distances in an interval independent manner. The Tukey HSD multiple comparison test is inappropriate for the analysis of repeated measure type data and thus to analyze which alcohol group differed significantly from control (and whether they differed from each other), we first averaged the interval data and subsequently conducted a univariate non-repeated ANOVA with a Tukey HSD post hoc test on these averages. This analysis confirmed a significant Alcohol effect (ANOVA F(2, 22) = 2.943, p < 0.05) and showed that the two alcohol treated groups had significantly (one tailed p < 0.05) higher interindividual distance values as compared to control.
Figure 2.
The Interindividual Distance among all fish in the shoal significantly increased in response to embryonic alcohol (EtOH) treatment when measured in 70 day post fertilization old zebrafish. Mean ± Standard Error are shown. Sample size n = 8 for each dose group (i.e. 8 shoals of ten fish each were quantified per alcohol dose). The concentration of embryonic alcohol treatment is shown above the graphs. The distance values are shown in body length. The inset in graph 1 shows the method of quantifying nearest neighbor distance. The grey circles represent individual fish in the shoal. The lines represent the interindividual distances. The interindividual distances are shown only for one focal fish, but these distances were measured for each possible pair-wise combinations of fish. Note that the slanted straight line on the figures represents the best fit regression line for graph one. The same line is shown on the other graphs to facilitate comparison of values. For further methodological details and results of statistical analyses, see Methods and Results.
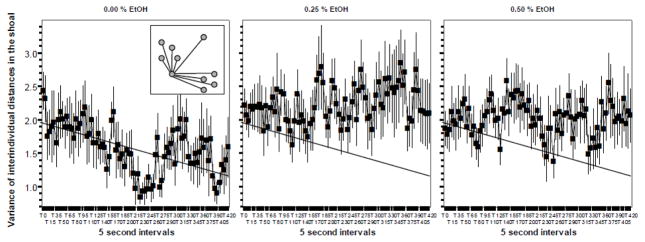
The pattern of results obtained for the measure ‘Variance of interindividual distances’ appears to be similar to that of the Interindividual distance (figure 3). ANOVA found no significant Interval effect (F(84, 1848) = 0.934, p > 0.60) and no significant Interval x Alcohol interaction (F(168, 1848) = 1.054, p > 0.30). Although the results appear to suggest that the variance of interindividual distances is increased by alcohol treatment, this alcohol effect did not reach statistical significance (F(2, 22) = 2.943, p = 0.088).
Figure 3.
The Variance of Interindividual Distance did not significantly change in response to embryonic alcohol (EtOH) treatment when measured in 70 day post fertilization old zebrafish. Mean ± Standard Error are shown. Sample size n = 8 for each dose group (i.e. 8 shoals of ten fish each were quantified per alcohol dose). The concentration of embryonic alcohol treatment is shown above the graphs. The distance values are shown in body length. The inset in graph 1 shows the method of quantifying nearest neighbor distance. The grey circles represent individual fish in the shoal. The lines represent the interindividual distances. The interindividual distances are shown only for one focal fish, but these distances were measured for each possible pair-wise combinations of fish. Note that there are nine distance values for one focal fish and these values differ. This variability is captured in the Variance of interindividual distances measure. Also note that the slanted straight line represents the best fit regression line for graph one. The same line is drawn on the other graphs to facilitate comparison of values. For further methodological details and results of statistical analyses, see Methods and Results.
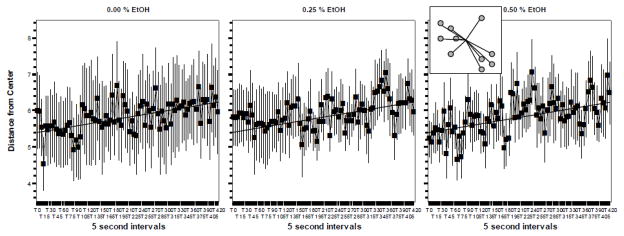
The last behavioral measure we consider is the distance from center (figure 4). ANOVA revealed no significant Interval (F(84, 1848) = 1.623, p <0.001), Interval × Alcohol Interaction (F(168, 1848) = 1.008, p > 0.45) or Alcohol (F(2, 22) = 0.003, p > 0.99) effects.
Figure 4.
The Distance from the Center did not significantly change in response to embryonic alcohol (EtOH) treatment when measured in 70 day post fertilization old zebrafish. Mean ± Standard Error are shown. Sample size n = 8 for each dose group (i.e. 8 shoals of ten fish each were quantified per alcohol dose). The concentration of embryonic alcohol treatment is shown above the graphs. The distance values are shown in body length. The inset in graph 3 shows the method of quantifying the distance from the center. The grey circles represent individual fish in the shoal. The lines represent the distance values. Note that the slanted straight line represents the best fit regression line for graph one. The same line is drawn on the other graphs to facilitate comparison of values. For further methodological details and results of statistical analyses, see Methods and Results.
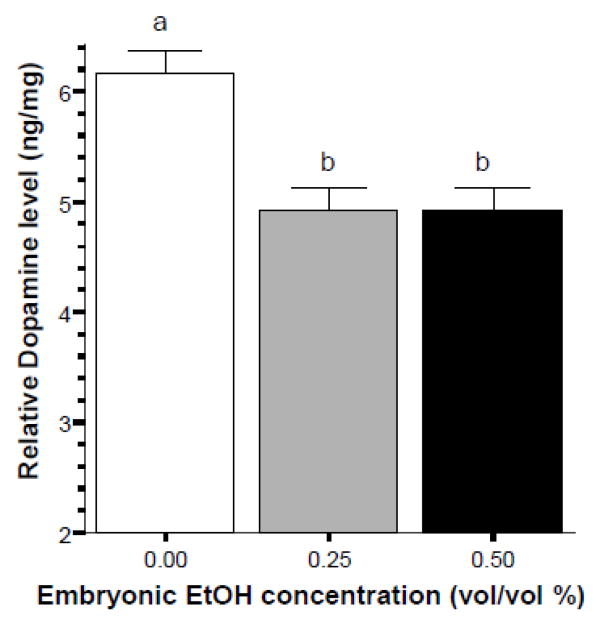
The next question we examined was whether embryonic alcohol treatment led to a lasting change in the amount of neurochemicals normalized to the weight of total brain protein. Figure 5 shows that dopamine is reduced in the alcohol treated groups of zebrafish, an observation confirmed by ANOVA, which found the Alcohol effect to be significant (F(2, 20) = 13.668, p < 0.001). Tukey HSD test showed that the control group (0% alcohol) is significantly different from both the 0.25% and 0.50% alcohol treated groups and that these latter two groups do not differ from each other.
Figure 5.
Embryonic alcohol exposure significantly reduces the level of dopamine in the brain of 70 day post fertilization old zebrafish. Mean ± Standard Error are shown. Sample sizes (n) = 8 for each alcohol (EtOH) dose group. Dopamine levels are expressed as ng per mg total brain protein. The small letters above the graph show the results of the Tukey HSD multiple comparison test. Bars that share the same letter designation are not significantly (p > 0.05) different from each other.
DOPAC levels appeared to diminish in a dose dependent manner in response to early embryonic alcohol exposure (figure 6), an effect that was found significant by ANOVA (F(2, 20) = 5.415, p < 0.05). Tukey HSD found the highest dose group (0.50 % alcohol) to significantly (p < 0.05) differ from control. The difference between control and the 0.25% alcohol group did not reach significance (p > 0.05), and the two alcohol groups also did not significantly differ from each other (p > 0.05)..
Figure 6.
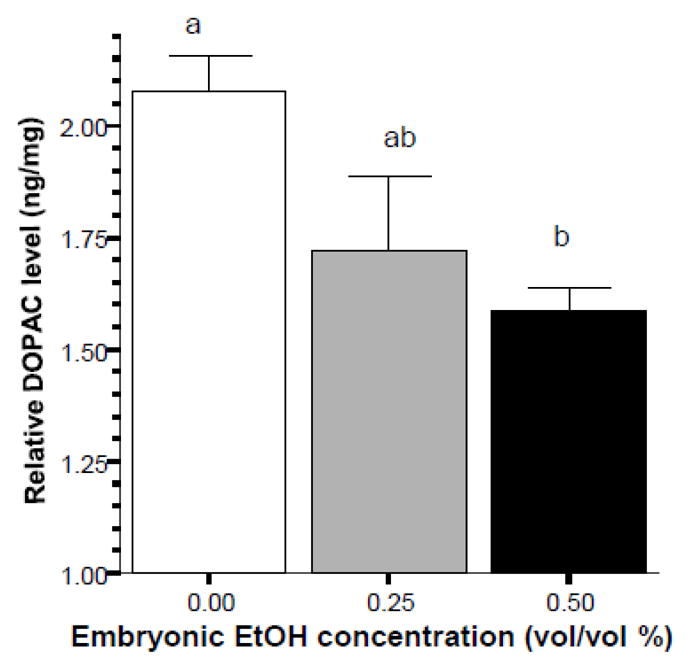
Embryonic alcohol exposure significantly reduces the level of DOPAC in the brain of 70 day post fertilization old zebrafish. Mean ± Standard Error are shown. Sample sizes (n) = 8 for each alcohol (EtOH) dose group. DOPAC levels are expressed as ng per mg total brain protein. The small letters above the graph show the results of the Tukey HSD multiple comparison test. Bars that share the same letter designation are not significantly (p > 0.05) different from each other.
Analysis of the effect of embryonic alcohol treatment on the levels of serotonin (figure 7) also showed significant alcohol effects (ANOVA F(2, 20) = 8.507, p < 0.01) and the Tukey HSD test revealed that the control group and the 0.25% alcohol treated group significantly (p < 0.05) differed from each other, while other differences were non-significant (p > 0.05).
Figure 7.
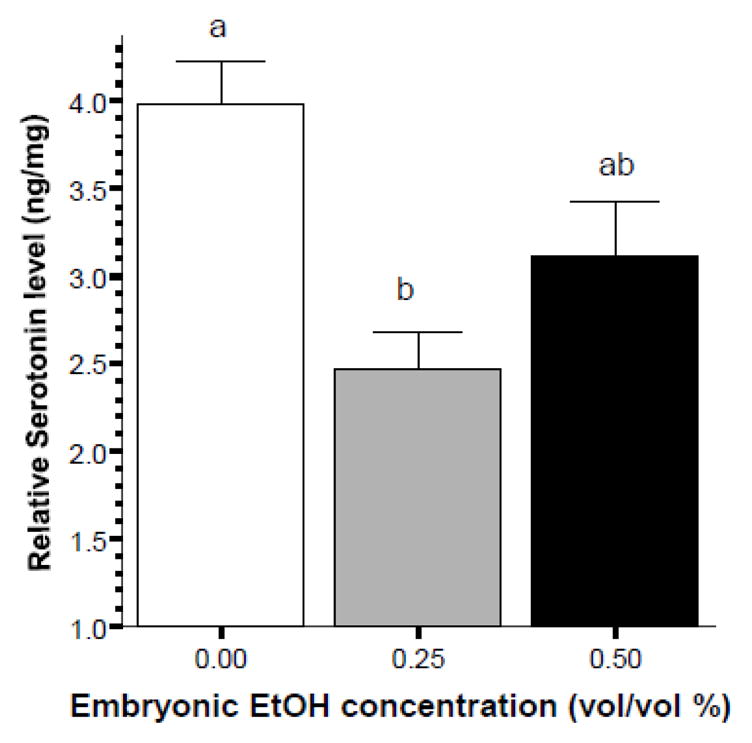
Embryonic alcohol exposure significantly reduces the level of serotonin in the brain of 70 day post fertilization old zebrafish. Mean ± Standard Error are shown. Sample sizes (n) = 8 for each alcohol (EtOH) dose group. Serotonin levels are expressed as ng per mg total brain protein. Bars that share the same letter designation are not significantly (p > 0.05) different from each other.
The effect of alcohol on the level of 5HIAA, a metabolite of serotonin, was also found significant (ANOVA F(2, 20) = 12.742, p < 0.001). Tukey HSD found the control group to significantly (p < 0.01) differ from both alcohol treated groups while these latter groups did not differ significantly (p > 0.05) from each other.
4. DISCUSSION
Embryonic alcohol exposure has been previously shown to have teratogenic effects at high doses in mammalian model organisms (e.g. Becker et al., 1996) as well as in zebrafish (e.g. Arenzana et al., 2006). Most recently, low doses of alcohol administered during embryonic development has been shown to induce significant behavioral impairments in zebrafish without gross anatomical malformations (Fernandes & Gerlai, 2009). The impairments manifested as abnormal responses to social stimuli. The nature of the impairment was somewhat questionable, however, due to the artificial aspect of the stimulus (computer animated zebrafish images) used and to the potential fear inducing aspect of the test situation. In the current paper abnormal social behavior (increased distance among shoal members, i.e. reduced shoal cohesion) induced by embryonic alcohol exposure has now been confirmed by the analysis of freely moving groups of zebrafish. We analyzed several different measures of shoaling responses. While nearest neighbor distance only showed a statistically marginal increase in the alcohol treated groups, analysis of the interindividual distance did reveal a significant alcohol effect.
Nearest neighbor distance has been widely used as a measure of shoal cohesion (e.g. Partridge, 1981) because it is independent of shoal size and thus can be employed even when the exact number of shoal members is not controlled or not known. However, this measure is only an approximation of shoal cohesion as it does not take into account all distance values between pairs of fish in a shoal. Interindividual distance, however, does and as such, this measure does not loose information but provides a more precise description of shoal cohesion. We argue that the more precise nature of this measure is what allowed us to detect a significant alcohol effect in this relatively small sample sized study (8 shoals per alcohol treatment). The variance of interindividual distance within a shoal also showed a marginal alcohol effect. It is important to note that this measure reflects the variance within the particular shoal studied, i.e. it is dependent upon how uniformly distributed the fish are within the shoal (the more uniform the distribution the smaller the variance). Although the alcohol effect only approached significance, this finding is also notable and will be further investigated in the future. In summary, the current results replicated our previous behavioral findings, and with the use of a different experimental set up and a different method of quantification of behavior showed that shoaling behavioral performance is impaired by embryonic alcohol exposure.
In the current study, the stimuli available to the shoal members were all natural and multimodal, which is important to note for the following reasons. Exposure to high doses of alcohol during embryonic development has been shown to impair the visual system in zebrafish (e.g. Arenzana et al., 2006) and thus it is conceivable that such impairments could occur, albeit to a lesser degree, in response to low doses of alcohol such as employed here. However, shoaling has been demonstrated in blind fish, i.e. the visual system is not a necessary requirement for appropriate shoaling to be maintained. Blind cave fish or blinded shoaling fish of other species can keep appropriate distances among themselves when in a group by using their lateral line, i.e. by sensing low frequency waves reflected back from their neighbors in a manner similar to echo-location (e.g. Gregson & Burt de Perera T., 2007; Partridge & Pitcher, 1980). It is unlikely that both the visual and lateral line perceptual abilities of the alcohol treated fish were affected by the alcohol treatment. This suggestion is supported by a previous study in which normal vision based responses were detected in zebrafish exposed to low concentrations of alcohol during their embryonic development (Fernandes & Gerlai, 2009).
Another issue with our previous study was that it measured the behavior of single experimental subjects. A single fish may exhibit agonistic and/or fear responses to the appearance of visual stimuli (see e.g. Gerlai et al., 2000) such as employed previously (Fernandes & Gerlai, 2009). But fish swimming in a shoal, as in the current study, are less likely to perform aggressive behaviors or influenced by fear inducing stimuli. Unaltered fear responses are also suggested by the lack of alcohol effects on the distance fish swam from the center. Fish, including zebrafish (Champagne et al., 2010), and other vertebrates (e.g. Treit & Fundytus, 1988), exhibit thigmotaxis under stressful or fear inducing conditions, i.e. stay close to the edge of their tank or cage, which we did not observe in the current study. Based on the above arguments and since we detected impaired shoaling responses in the freely swimming fish shoals, we conclude that it is likely that social cohesion, i.e. the preference to stay close to conspecifics, was impaired by the embryonic alcohol exposure employed, and other factors (impaired perception, altered aggression and or fear) are less likely to have contributed to this alteration.
Notably, the above behavioral changes were induced by low concentrations of alcohol, i.e. immersion in 0.25 and 0.50% (vol/vol) alcohol solution. Previously, we have found that inside the egg the alcohol concentration achieved was about 1/30th of the external concentration for the above doses (Fernandes & Gerlai, 2009). Although we have not quantified exactly how much alcohol may have reached the brain of the embryo inside the egg, this previous finding suggests that the alcohol concentration inside the egg must have been around 0.009% (for the 0.25% group) and 0.015% (for the 0.50% group). It is likely that the alcohol concentration inside the brain of the embryo was even less than these concentrations (for example, the concentration of blood alcohol has been found to be about 60% of the external concentration after 60 min immersion into the alcohol solution in adults, see e.g. Chaterjee & Gerlai, 2009; Fernandes & Gerlai, 2009 and references therein). Notably, the above alcohol concentrations are well below the legal limit (0.08% blood alcohol level) established for alcohol consumption in humans. Thus our results suggest that even low amounts of alcohol consumption may be deleterious for the developing vertebrate embryo, including perhaps the human fetus too.
The mechanisms underlying the impaired shoaling behavior performance are not known at this point. In the current study we have started the analysis of such potential mechanisms. Notably, our results are only correlative and can be regarded as pilot at best given that we only investigated two neurotransmitter systems and only from the perspective of overall neurochemical levels. Nevertheless, our results demonstrate that exposure to even low levels alcohol during embryonic development led to a lasting effect observable in the adults not only at the level of behavior but also at the level of neurochemistry. The embryonic alcohol treatment reduced all neurochemicals tested including dopamine levels, DOPAC, serotonin and 5HIAA. This reduction was not associated with changes in total brain weight (data not shown). Thus the relative decrease in the amount of these neurochemicals represents a hypofunctional or underdeveloped dopaminergic and serotoninergic system in the alcohol treated fish.
Previously, we have shown that the sight of conspecifics is rewarding for zebarfish (Al Imari & Gerlai, 2008) and several studies have shown that dopamine plays a pivotal role in reward (e.g. Kuhar et al., 1991). Serotonin has been shown to be involved in fear as well as aggression related responses (Olivier, 2006; Ruhe and Mason, 2007), behaviors that may alter shoaling. Also notably the levels of serotonin and of 5HIAA have been found to diminish in response to acute alcohol treatment in zebrafish (Chatterjee and Gerlai, 2009). Thus it is conceivable that the hypofunction of the dopaminergic system or of the serotoninergic system or the combination of the two may underlie the observed behavioral impairment. Whether other neurotransmitter systems may also be affected and how they may have contributed to the behavioral abnormalities will be investigated in the future. Other levels of investigation will also be necessary. For example, numerous gene expression changes that correspond to mechanisms downstream or upstream of neurotransmitter release and synthesis or neurotransmitter receptor function will also be investigated. The neuroanatomical locale of these potential changes will also need to be established.
Clearly, our current results only represent the beginning of such studies, but we argue they do provide enough rationale for the establishment of the above delineated research lines, which may ultimately lead to the unraveling of the mechanisms of mild fetal alcohol syndrome.
Figure 8.
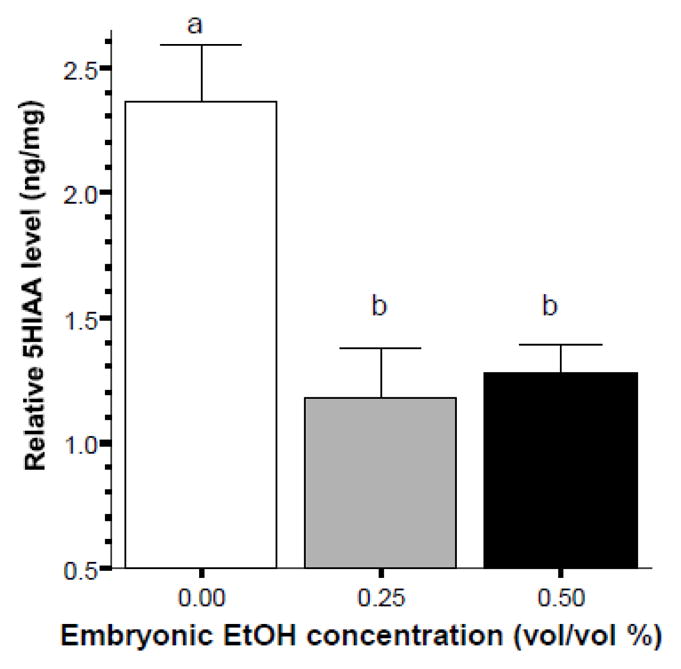
Embryonic alcohol exposure significantly reduces the level of 5HIAA in the brain of 70 day post fertilization old zebrafish. Mean ± Standard Error are shown. Sample sizes (n) = 8 for each alcohol (EtOH) dose group. 5HIAA levels are expressed as ng per mg total brain protein. Bars that share the same letter designation are not significantly (p > 0.05) different from each other.
Highlights.
Short exposure to low levels of alcohol at embryonic stage impairs behavior in adult fish.
Behavioral impairment found confirms previous results and suggests abnormal shoaling behavior is induced
Behavioral abnormality is accompanied by reduced dopamine, DOPAC, serotonin and 5HIAA levels from whole brain extracts
Behavioral and neurochemical abnormalities are correlative only but open new lines of research for fetal alcohol syndrome model development
Acknowledgments
This study was supported by an NIH/NIAAA R01 grant to R.G. We thank Diptendu Chatterjee for technical help.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Imari L, Gerlai R. Sight of conspecifics as reward in associative learning in zebrafish (Danio rerio) Behav Brain Res. 2008;189:216–219. doi: 10.1016/j.bbr.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Arenzana FJ, Carvan MJ, III, Aijon J, Sanchez-Gonzalez R, Arevalo R, Porteros A. Teratogenic effects of ethanol exposure on zebrafish visual system development. Neurotox Terat. 2006;28:342–348. doi: 10.1016/j.ntt.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Becker HC, Díaz-Granados JL, Randall CL. Teratogenic actions of ethanol in the mouse: a minireview. Pharmacol Biochem Behav. 1996;55:501–513. doi: 10.1016/s0091-3057(96)00255-9. [DOI] [PubMed] [Google Scholar]
- Bilotta J, Barnett JA, Hancock L, Saszik S. Ethanol exposure alters zebrafish development: a novel model of fetal alcohol syndrome. Neurotox and Terat. 2004;26:737–743. doi: 10.1016/j.ntt.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Boehm SL, Lundahl KR, Caldwell J, Gilliam DM. Ethanol teratogenesis in the C57BL/6J, DBA/2J, and A/J inbred mouse strains. Alcohol. 1997;14:389–395. doi: 10.1016/s0741-8329(97)87950-5. [DOI] [PubMed] [Google Scholar]
- Buske C, Gerlai R. Progress in Neuropsychopharmacology & Biological Psychiatry. Elsevier Inc; 2011. Shoaling develops with age in Zebrafish (Danio rerio) in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buske C, Gerlai R. Maturation of shoaling behavior is accompanied by changes in the dopaminergic and serotoninergic systems in zebrafish. Developmental Psychobiology. 2011 doi: 10.1002/dev.20571. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvan M, III, Loucks E, Weber D, Williams F. Ethanol effects on the developing zebrafish: neurobehavior and skeletal morphogenesis. Neurotoxicology and Teratology. 2004;26:757–768. doi: 10.1016/j.ntt.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Champagne DL, Hoefnagels CC, de Kloet RE, Richardson MK. Translating rodent behavioral repertoire to zebrafish (Danio rerio): relevance for stress research. Behav Brain Res. 2010;214:332–342. doi: 10.1016/j.bbr.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Chatterjee D, Gerlai R. High precision liquid chromatography analysis of dopaminergic and serotoninergic responses to acute alcohol exposure in zebrafish. Behav Brain Res. 2009;200:208–213. doi: 10.1016/j.bbr.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes Y, Gerlai R. Long-term behavioral changes in response to early developmental exposure to ethanol in zebrafish. Alcoholism, clin and exp res. 2009;33:601–609. doi: 10.1111/j.1530-0277.2008.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego A, Heath MR. The development of schooling behaviour in Atlantic herring Clupea harengus. Journal of Fish Biology. 1994;45:569–588. [Google Scholar]
- Gerlai R, Lahav M, Guo S, Rosenthal A. Drinks like a fish: Zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol Biochem Behav. 2000;67:773–782. doi: 10.1016/s0091-3057(00)00422-6. [DOI] [PubMed] [Google Scholar]
- Green J. Fetal alcohol spectrum disorder: Understanding the effects of prenatal alcohol exposure and supporting students. J Sch Health. 2007;77:103–108. doi: 10.1111/j.1746-1561.2007.00178.x. [DOI] [PubMed] [Google Scholar]
- Gregson JNS, Burt de Perera T. Shoaling in eyed and blind morphs of the characin Astyanax fasciatus under light and dark conditions. J of Fish Biology. 2007;70:1615–1619. [Google Scholar]
- Guarnieri DJ, Heberlein U. Drosophila melanogaster, a genetic model system for alcohol research. Int Rev Neurobiol. 2003;54:199–228. doi: 10.1016/s0074-7742(03)54006-5. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- Masuda R, Shoji J, Nakayama S, Tanaka M. Development of schooling behavior in Spanish mackerel Scomberomorus niphonius during early ontogeny. Fisheries Science. 2003;69:772–776. [Google Scholar]
- Matsui JI, Egana AL, Sponholtz TR, Adolph AR, Dowling JE. Effects of ethanol on photoreceptors and visual function in developing zebrafish. Inv Ophthalm Visual Sci. 2006;46:4589–4597. doi: 10.1167/iovs.05-0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NY, Gerlai R. Quantification of shoaling behaviour in zebrafish (Danio rerio) Behavioural Brain Research. 2007;184:157–166. doi: 10.1016/j.bbr.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Olivier B. Serotonin and Aggression. Annals of the New York Academy of Sciences. 2006;1036:382–392. doi: 10.1196/annals.1330.022. [DOI] [PubMed] [Google Scholar]
- Partridge BL, Pitcher TJ. The sensory basis of fish schools: relative roles of lateral line and vision. Journal of Comparative Physiology. 1980;135:315–325. [Google Scholar]
- Partridge BL. Internal dynamics and the interrelations of fish in schools. Journal of Comparative Physiology. 1981;144:313–325. [Google Scholar]
- Ruhe H, Mason N. Molecular Psychiatry - Abstract of article: Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Molecular psychiatry. 2007;12:331–359. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- Steinhausen HC. Children of alcoholic parents. A review Eur Child Adolesc Psychiatry. 1995;4:143– 152. doi: 10.1007/BF01980453. [DOI] [PubMed] [Google Scholar]
- Stratton K, Howe C, Battaglia F. Fetal Alcohol Syndrome: Diagnosis, Epidemiology, Prevention and Treatment. Washington, DC: National Academy Press; 1996. p. 213. [Google Scholar]
- Streissguth AP. Fetal Alcohol Syndrome: A Guide for Families and Communities Paul H. Brookes Publishing Co; Baltimore, MD: 1997. p. 306. [Google Scholar]
- Treit D, Fundytus M. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol Biochem Behav. 1988;31:959–962. doi: 10.1016/0091-3057(88)90413-3. [DOI] [PubMed] [Google Scholar]
- Vogel S. Modes and scaling in aquatic locomotion. Integrative and Comparative Biology. 2008;48:702–712. doi: 10.1093/icb/icn014. [DOI] [PubMed] [Google Scholar]