Abstract
Cisplatin (cis-diamminedichloroplatinum II, CIS) is a potent and widely used chemotherapeutic agent to treat various malignancies, but its therapeutic use is limited because of the dose-dependent nephrotoxicity. Cell death and inflammation play key role in the development and progression of CIS-induced nephropathy. Sulforaphane (SFN), a natural constituent of cruciferous vegetables such as broccoli, Brussels sprouts, etc., has been shown to exert various protective effects in models of tissue injury and cancer. In this study, we have investigated the role of pro-survival, cell death and inflammatory signaling pathways using a rodent model of CIS-induced nephropathy, and explored the effects of SFN on these processes. Cisplatin triggered marked activation of stress signaling pathways (p53, Jun N-terminal kinase (JNK), and p38-α MAPK) and promoted cell death in the kidneys (increased DNA fragmentation, caspases-3/7 activity, TUNEL), associated with attenuation of various pro-survival signaling pathways (e.g. extracellular signal-regulated kinase (ERK) and p38-β MAPK). Cisplatin also markedly enhanced inflammation in the kidneys (promoted NF-κB activation, increased expression of adhesion molecules ICAM and VCAM, enhanced tumor necrosis factor-alpha (TNF-α) levels, and inflammatory cell infiltration). These effects were significantly attenuated by pre-treatment of rodents with SFN. Cisplatin-induced nephropathy is associated with activation of various cell death and pro-inflammatory pathways (p53, JNK, p38-α, TNF-α, and NF-κB) and impairments of key pro-survival signaling mechanisms (ERK and p38-β). SFN is able to prevent the CIS-induced renal injury by modulating these pathways, providing a novel approach for preventing this devastating complication of the chemotherapy.
Keywords: sulforaphane, natural compound, nephropathy, inflammation, cell death, cisplatin
1. Introduction
Cisplatin (cis-diamminedichloroplatinum II, CIS) is a widely used, potent antineoplastic agent to treat various forms of cancer; however, its therapeutic utility is limited by the development of dose-dependent nephrotoxicity in about 30% of the patients treated with this drug [1]. The mechanisms of the CIS-induced nephropathy are complex and may involve increased DNA damage, apoptotic and necrotic cell death, mitochondrial dysfunction and inflammation [1–17].
Multiple studies have demonstrated that the activation of various stress signaling pathways (e.g. mitogen-activated protein kinases (MAPKs), and p53), which are key regulators of proliferation, differentiation and cellular survival, contribute to the development and progression of CIS-induced nephropathy [18–22]. Emerging recent evidence also supports a key role of the inflammation in this process, which involves enhanced infiltration of leukocytes [8, 23, 24], increased expression of adhesion molecules (intercellular and vascular adhesion molecules (ICAM and VCAM)), and increased secretion of various inflammatory mediators by inflammatory or parenchyma cells (e.g. monocyte chemoattractant protein-1 (MCP-1), ED-1, and tumor necrosis factor-α (TNF-α) [9, 12, 25–27].
Sulforaphane (SFN) is a natural occurring isothiocyanate produced by the enzymatic action of the myrosinase on glucopharanine, a glucosinolate contained in cruciferous vegetables such as broccoli, brussel sprouts, cabbage, etc. [28, 29]. Experimental studies have demonstrated that SFN protects against renal fibrosis [30], kidney and intestinal ischemic-reperfusion injuries [31, 32], cytokine- and streptozotocin-induced beta-cell injury [33], bacterial lipopolysaccharide-induced inflammatory damage in human vascular endothelial cells [34], and inhibits angiogenesis [35]. Furthermore, increasing evidence based on various in vitro studies using cancer cell lines and rodent tumor models, demonstrating antiproliferative and pro-apoptotic effects of SFN in cancer cells or tumors, coupled with epidemiological studies suggesting that dietary intake of cruciferous vegetables is associated with lower cancer risk in bladder, lung, prostate, breast, kidney, ovarian cancers, and lymphoma support a potential benefit of SFN in various cancers and their prevention (reviewed in [36, 37]. In this study, we have explored the interplay of pro-survival, cell death and inflammatory signaling pathways using a rodent model of CIS-induced nephropathy, and explored the effects of SFN on these processes.
2. Material and methods
2.1. Animals and treatment
Male Wistar rats with an initial body weight of 230–260 g were used. The animals were maintained under 12-h light/dark cycles at controlled temperature, having free access to water and food. Experimental work was approved by the local ethical committee (Comité Institucional para el cuidado y uso y de animales de Laboratorio (CICUAL) approval ID 002/10) and followed the Guide for the Care and Use of Laboratory Animals, published by the United States National Institutes of Health (US-NIH), as well as the guidelines of Mexican Official Norm Guide (NOM) for the use and care of laboratory animals (NOM-062-ZOO-1999) and for the disposal of biological residues (NOM-087-ECOL-1995).
Four groups of rats were studied: [1] the control group (CT) was injected via jugular vein with DMSO/isotonic saline solution and then with isotonic saline solution by intraperitoneal injection; [2] sulforaphane group (SFN) (LKT laboratories. St. Paul, MN). SFN suspended in DMSO/isotonic saline solution was injected at a dose of 500 μg/kg as previously described [31], via jugular vein two times (24 h before and 24 h after isotonic saline solution injection); [3] the cisplatin group (CIS) (Sigma-Aldrich, St. Louis, MO) received a solution of CIS dissolved in isotonic saline solution by a single intraperitoneal injection (7.5 mg/kg) [38]. [4] The last group (CIS+SFN), SFN was suspended in DMSO/isotonic saline solution and injected at a dose of 500 μg/kg via jugular vein two times (24 h before and 24 h after 7.5 mg/kg CIS injection). Animals were sacrificed 72 h after CIS or vehicle injection and kidneys were collected.
2.2. Western blot analyses
Antibodies for cleaved caspase-3, p38-α, p38-β, phospho JNK, JNK, phospho ERK, ERK, p53, phospho NF-κB (p65) and NF-κB (p65) were obtained from Cell Signaling Technology Inc. (Danvers, MA). Antibodies for TNF-α, ICAM, VCAM were obtained from R&D Systems Inc. (Minneapolis, MN) and β-actin antibody was obtained from Chemicon (Temecula, CA). Protein was extracted from tissue homogenate using TREP lysis buffer, containing protease inhibitor cocktail set III and phosphatase inhibitor cocktail set I (Calbiochem, EMD Biosciences, San Diego, CA). The kidney protein samples were mixed in Laemmli buffer, boiled for 7 min, and then equal amounts (40 μg per lane) were fractionated on Criterion Bis-Tris gel and transferred onto Hybond-C Extra nitrocellulose membrane (GE Healthcare Bio-Sciences Corp., Piscataway, NJ) using a semidry transfer apparatus (Bio-Rad, Hercules, CA). The blocking was carried out for 2 hr in 5% nonfat dry milk in PBS. The primary antibodies were added as per the manufacturer’s recommended dilution in PBS buffer containing 0.1% Tween 20 and 5% bovine serum albumin (BSA) for overnight at 4°C. After three washes in PBS containing 0.1% Tween 20, secondary HRP conjugate (Pierce Biotechnology, Inc. Rockford, IL) was added, followed by three washes with PBS containing 0.1% Tween 20. The blots were detected with Supersignal West Pico chemiluminescent substrate (Pierce Biotechnology, Inc. Rockford, IL) and developed using Kodak Biomax film (PerkinElmer, Wellesley, MA).
2.3. Detection of apoptosis by terminal deoxynucleotidyl transferase-mediated uridine triphosphate (dUTP) nick-end labeling (TUNEL), renal DNA fragmentation and caspase 3/7 activity assays
Paraffin sections were dewaxed and in situ detection of apoptosis in the renal tissues was performed by TUNEL assay according to the instructions of the manufacturer (Roche Diagnostics, Indianapolis, IN, USA) and the number of apoptotic cells, as defined by chromatin condensation of nuclear fragmentation (apoptotic bodies), were counted. After TUNEL labeling, nuclei were labeled with Hoechst 33258 (Molecular Probes, Invitrogen) and the TUNEL-positive kidney cells were observed using an Olympus IX81 fluorescence microscope with a 20x objective at 2048 × 2048 resolution. The morphometric examination was performed by two independent, blinded investigators. The average number of apoptotic cells in each group was calculated by taking the average of TUNEL-positive apoptotic cells in 10 fields from each kidney sample with 200x magnification.
Caspase 3/7 activity in tissue lysate was measured using the Apo-One Homogenous Caspase-3/7 assay kit (Promega Corp., Madison, WI, USA). An aliquot of caspase reagent was added to each well and mixed on a plate shaker for 1 h at room temperature shielded from light, and the fluorescence was measured.
The DNA fragmentation assay was based on measuring the amount of mono- and oligonucleosomes in the cytoplasmic fraction of tissue extracts using a commercially available kit (Roche Diagnostics, Indianapolis, IN) according to the manufacturer’s instructions [9].
2.4. Myeloperoxidase (MPO) staining and Intercellular adhesion molecule-1 (ICAM-1) immunostaining
Kidney tissues were fixed in 4% buffered formalin. Paraffin-embedded sections were cut, deparaffinized, and hydrated in descending gradations of ethanol, followed by antigen retrieval with citrate buffer. Next, sections were incubated in 0.3% H2O2 in PBS to block endogenous peroxidase activity. The sections were then incubated with anti-MPO (Biocare Medical, Concord, CA), or anti-ICAM-1 (R&D Systems Inc., Minneapolis, MN) overnight at 4°C in a moist chamber. Biotinylated secondary antibodies and ABC reagent were added as per the kit’s instructions (Vector Laboratories, Burlingame, CA). Color development was induced by incubation with a DAB kit (Vector Laboratories, Burlingame, CA) for 3–5 min, and specific staining was visualized by light microscopy as described [9, 39–41]. After MPO staining and ICAM-1 immunostaining, nuclei were contrast with Nuclear Fast Red H-3403 (Vector Laboratories, Burlingame, CA).
2.5. Data analysis
Results were expressed as mean±SEM. Data were analyzed by ANOVA followed by Dunnett’s multiple comparisons test, using software Prism 5 (GraphPad, San Diego, CA). A p value <0.05 was considered significant. N represents number of separate experiments/rats.
3. Results
3.1. Sulforaphane prevents cisplatin-induced cellular death
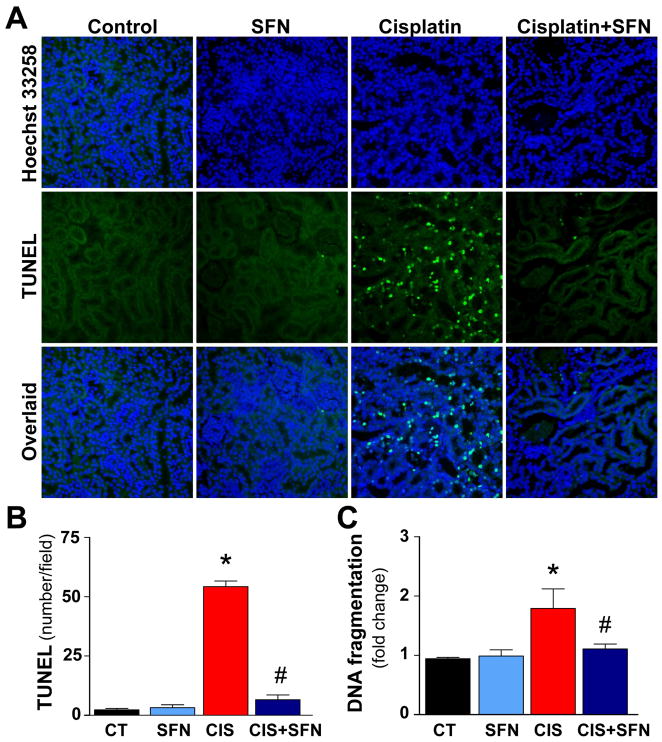
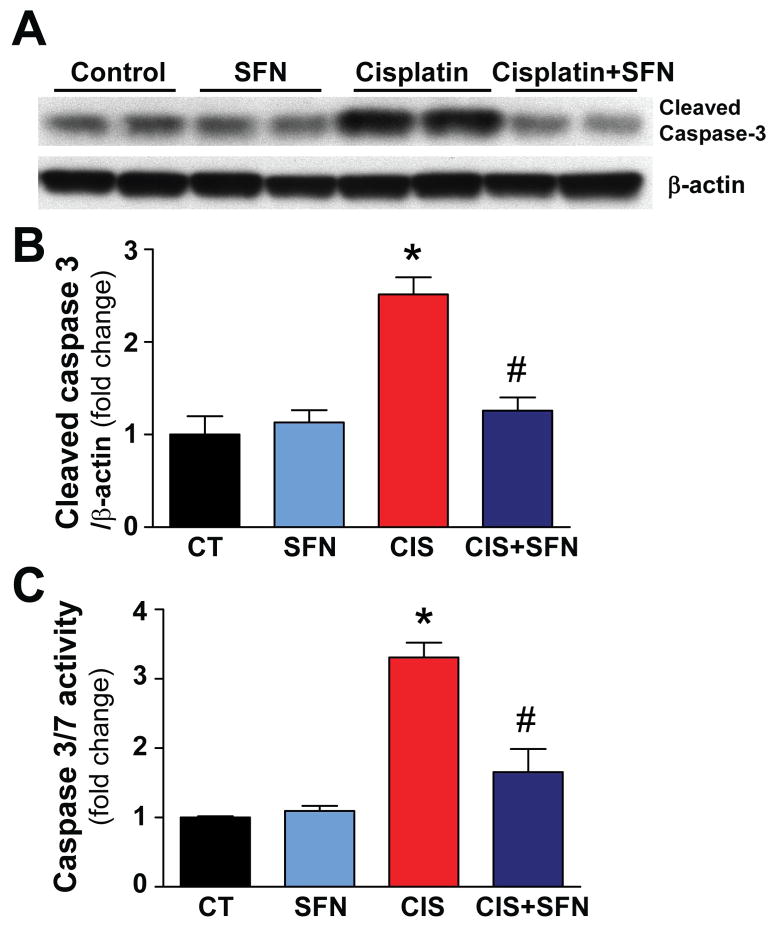
Cellular death was evaluated by TUNEL staining, DNA fragmentation, renal caspase-3 cleavage, caspase-3/7 activity assays (Figs. 1 and 2). As shown in Fig. 1, the TUNEL-positive (late apoptotic) cell number and DNA fragmentation were markedly increased in kidneys of CIS-treated rats (Fig. 1A–C). SFN-pre-treatment prevented the CIS-induced enhanced cell death (Fig. 1A–C). Caspase-3/7 activity and caspase-3 cleavage in kidney homogenates from CIS-treated rats were also markedly increased (Fig. 2A–C), which could be largely attenuated by SFN-pre-treatment. SFN had no effect in control (CT) animals on cell death (Figs. 1 and 2).
Fig. 1. SFN treatment prevents CIS-induced cell death.
(A–B) Late apoptotic cell death in kidney was evaluated by fluorescent TUNEL staining. As shown in representative images (200x original magnification), CIS markedly increased the number of TUNEL-positive cells (green) in the kidneys. (Middle) Nuclear staining with Hoechst (blue) and (right) colocalization with TUNEL. The number of TUNEL-positive cells was attenuated by SFN treatment. (C) CIS markedly increased DNA fragmentation; SFN treatment prevented this DNA damage. Results are means ±SEM, n=5. *p<0.001 vs. CT and SFN; #p<0.001 vs. CIS (B), *p<0.05 vs. CT and SFN; #p<0.05 vs. CIS (C).
Fig. 2. SFN treatment prevents CIS-induced cell death by decreasing caspase-3/7 activity and caspase-3 cleavage.
Caspase-3 cleavage and caspase-3/7 activity in kidney homogenates was markedly increased in CIS treated group. SFN treatment prevented significantly these increases. Results are means ±SEM, n=4 (B), n=5 (C). *p<0.001 vs. CT and SFN; #p<0.001 vs. CIS (B and C).
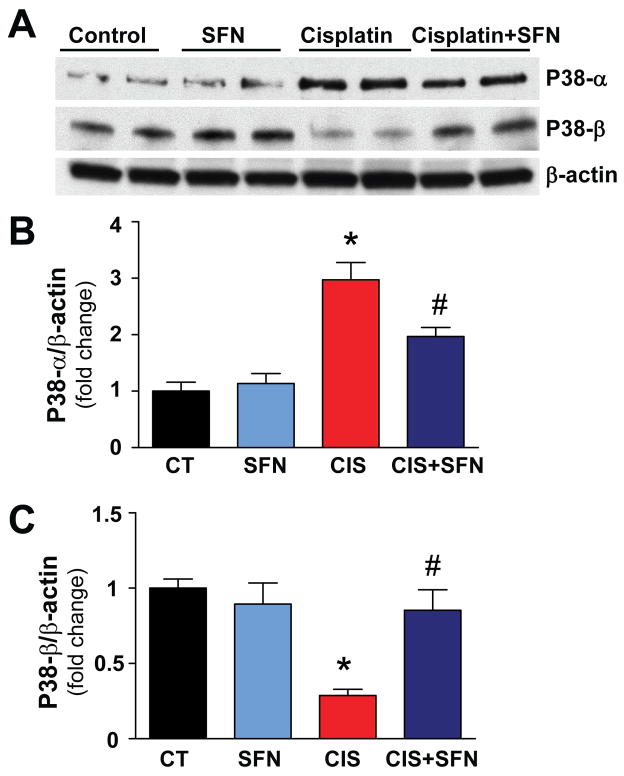
3.2. Sulforaphane prevents cisplatin-mediated increase in p38-α and decrease in p38-β MAPK
As shown in Figure 3, SFN significantly attenuated the CIS-induced increased expression of the pro-apoptotic p38-α in the kidneys, while prevented the abolished expression of the anti-apoptotic p38-β (Fig. 3A–C). SFN had no effect in control (CT) animals on p38-α or β (Figs. 3A–C).
Fig. 3. SFN treatment prevents CIS-mediated increase in p38-α and decrease in p38-β MAPK.
CIS-induced p38-α and p38-β expression in rat kidneys was prevented significantly by SFN treatment. Results are means ±SEM, n=4. *p<0.001 vs. CT and SFN; #p<0.05 vs. CIS (p38-α); *p<0.001 vs. CT and SFN; #p<0.05 vs. CIS (p38-β).
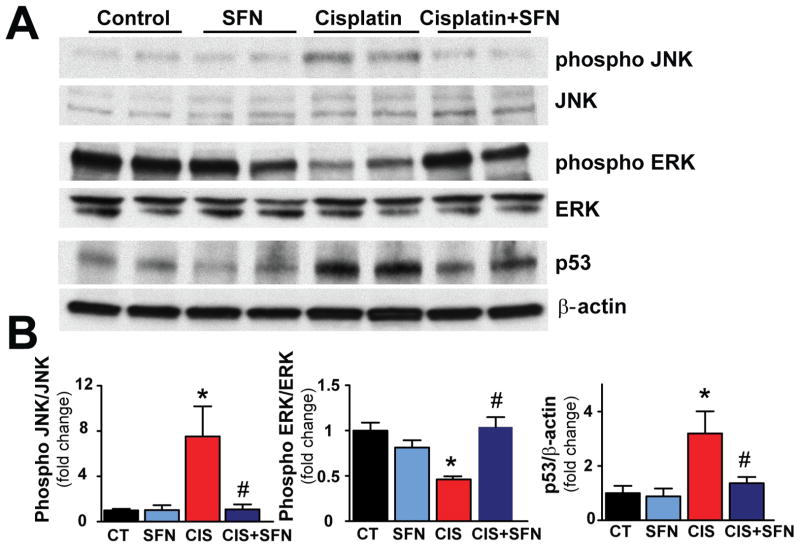
3.3. The cisplatin-induced MAPKs activation is attenuated by sulforaphane treatment
CIS treatment induced marked phosphorylation of renal phospho JNK and induction of p53 (7.52- and 3.19 fold, respectively, as compared with CT group) and dephosphorylation of phospho ERK (decreased to 0.46 fold vs. CT) (Fig. 4). SFN treatment significantly attenuated these alterations (Fig. 4). SFN had no effect in control (CT) animals on any of these variables (Fig. 4).
Fig. 4. CIS-induced MAPKs activation is attenuated by SFN treatment.
CIS-induced marked phosphorylation of JNK and p53, and dephosphorylation of ERK was attenuated by SFN treatment in CIS+SFN group. Results are means ±SEM, n=4. *p<0.05 vs. CT and SFN; #p<0.05 vs. CIS (phospho JNK); *p<0.001, *p<0.05 vs. CT and SFN, respectively; #p<0.05 vs. CIS (phospho ERK); *p<0.05 vs. CT and SFN; #p<0.05 vs. CIS (p53).
3.4. Sulforaphane treatment prevents inflammatory response in rat kidneys
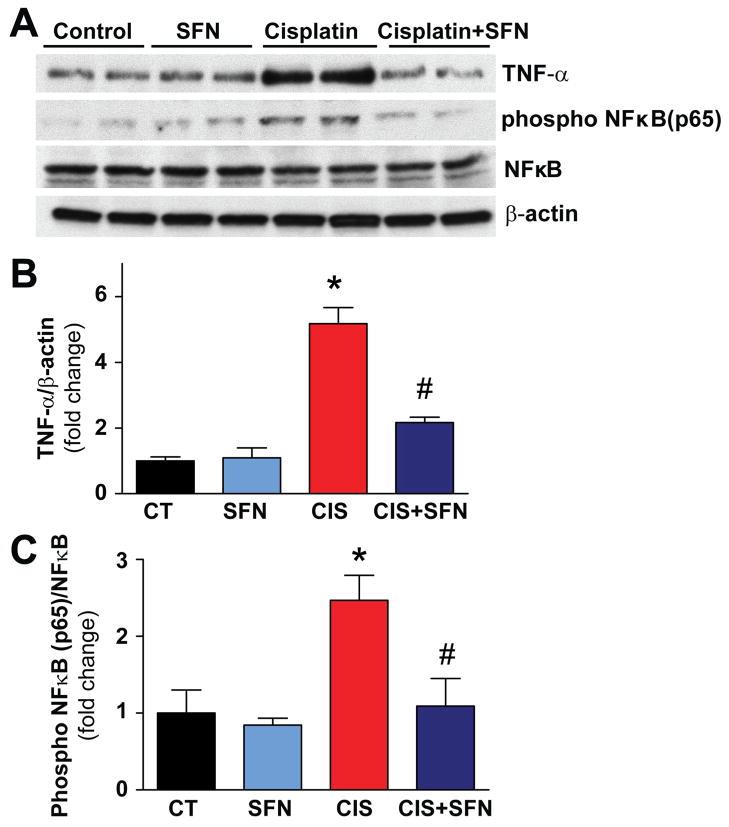
CIS triggered markedly increased inflammatory response in the kidneys (elevated TNF-α levels and NF-κB activation (Fig. 5). SFN treatment significant reduced these pro-inflammatory changes (Fig. 5). SFN had no effect in control (CT) animals on any of these variables (Fig. 5).
Fig. 5. SFN treatment prevents inflammatory response in rat kidneys.
CIS-induced marked phosphorylation of TNF-α and phospho NF-κB (p65) was prevented by SFN treatment in CIS+SFN group. Results are means ±SEM, n=4. *p<0.001 vs. CT and SFN; #p<0.001 vs. CIS (TNF-α); *p<0.001 vs. CT and SFN; #p<0.05 vs. CIS (phospho NF-κB (p65).
3.5. Sulforaphane treatment prevents cisplatin-induced enhanced renal adhesion molecule expression and renal inflammatory cell infiltration
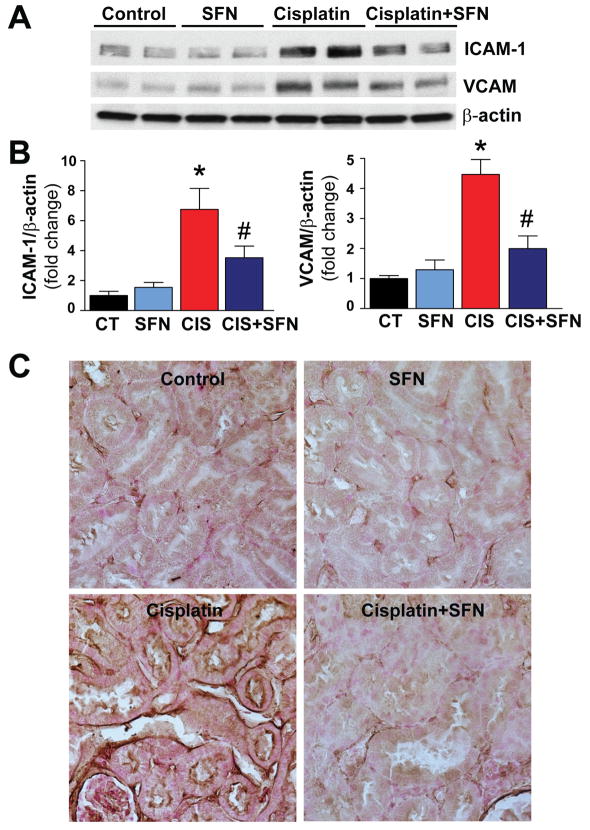
As shown in Fig. 6A–B, CIS treatment triggered increased expression of adhesion molecules ICAM-1 and VCAM-1 (6.8 and 4.5 fold, respectively) as compared to CT group. ICAM-1 expression is evident by an increased intensity of staining in kidney tissue in CIS-treated group (Fig. 6C). These increases of expression of ICAM-1, VCAM-1 and ICAM-1 staining were significantly attenuated by SFN pre-treatment (Fig. 6A–C). SFN had no effect in control (CT) animals on ICAM-1 or VCAM-1 expressions (Fig. 6).
Fig. 6. SFN treatment prevents CIS-induced enhanced renal adhesion molecule (ICAM-1, VCAM-1) expression.
(A–B) CIS-induced ICAM-1 and VCAM-1 expression in rat kidneys was prevented significantly by SFN treatment in CIS+SFN group. Results are means ±SEM, n=4. *p<0.001 vs. CT and SFN; #p<0.05 vs. CIS (ICAM-1); *p<0.001 vs. CT and SFN; #p<0.001 vs. CIS (VCAM-1). (C) ICAM-1 expression is evident by an increased intensity of staining in kidney tissue in CIS-treated groups. This expression was prevented by SFN treatment in CIS+SFN group (n=5).
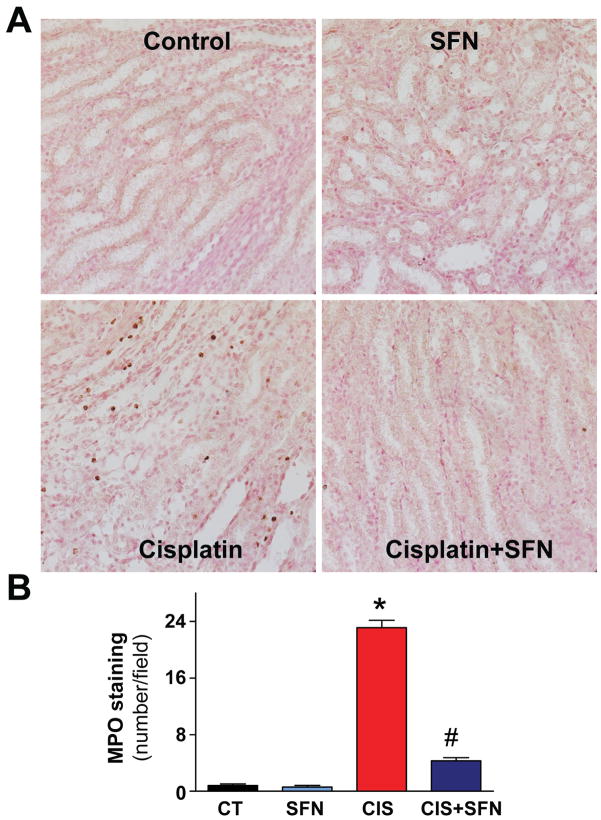
CIS treatment significantly increased the number of MPO-positive inflammatory cells around the damaged areas in the kidneys (indicative of neutrophil infiltration) to ~33 cells/field, as compared to CT group (Fig. 7). Neutrophil infiltration was markedly attenuated by SFN pre-treatment (Fig. 7). SFN had no effect in control (CT) animals on MPO positive infiltrating cells (Fig. 7).
Fig. 7. SFN treatment prevents CIS-induced renal inflammatory cell infiltration.
CIS significantly increased the number of renal MPO-positive cells around the damage tubular structures (400× original magnification). SFN treatment prevented significantly leukocyte infiltration in the CIS+SFN group. Results are means ±SEM, n=5. *p<0.001 vs. CT and SFN; #p<0.001 vs. CIS.
4. Discussion
In this study, we demonstrate that the CIS-induced nephropathy is associated with marked activation of stress signaling and inflammatory pathways coupled with attenuation of pro-survival signaling mechanisms promoting cell death in tubular cells. We also show that SFN, a natural constituent of cruciferous vegetables such as broccoli, cabbage, etc., is able to provide significant protection against CIS-induced cell death by attenuating pro-apoptotic pathways and inflammation and by promoting activation of pro-survival mechanisms.
Several studies have demonstrated that the cisplatin-induced nephropathy is associated with markedly increased caspase-dependent apoptosis, however the signaling mechanisms were not explored in details [3, 8, 42].
p38 MAPK exists in four isoforms: p38-α, -β, -γ, -δ. Two of the four isomers of p38 MAPK (-α, -β), seems to play opposing regulatory roles in myocardiac ischemic-reperfusion injury, diabetic cardiomyopathy and preconditioning [43]. While p38-β is anti-apoptotic p38-α promotes cell death [18, 21]. Consistently with these results CIS induced marked activation of pro-apoptotic p38-α in the kidneys and inactivation of pro-survival p38-β pathway. SFN treatment significantly reduced the CIS-induced enhanced p38α signaling and prevented the abolished p38-β activation. We also found that CIS induced increased activation of JNK and diminished phosphorylation of ERK, which can promote cell death [25, 44]. p53 is a powerful tumor suppressors gene in human cancer and is also importantly implicated in cell death associated with CIS-induced nephrotoxicity [19, 45]. Indeed, CIS-induced nephropathy was associated with increased activation of p53. The above mentioned CIS-induced alterations in signaling processes could largely be attenuated by SFN pre-treatment.
JNK and p38 signaling pathways can be activated by pro-inflammatoy cytokines or in response to cellular stress such as oxidative, osmotic or genotoxic stress [45]. CIS induces increased renal expression of the inflammatory cytokine TNF-α [9, 12, 27], which can also trigger activation of NF-κB by increased reactive oxygen species generation [23]. Indeed, as also suppoted by our data, NF-κB activation plays an important role in CIS-induced renal injury [45]. In accordance with previous studies mentioned above CIS-induced TNF-α induction and subsequent phosphorylation of NF-κB (p65), which were largely prevented by SFN pre-treatment (Fig. 5).
Inflammation plays an important physiological role in CIS-induced nephrotoxicity and it has been demonstrated that CIS administration increases the infiltration of leukocytes and macrophages within 72 hours in damaged renal tissues [8, 23], as well as it increases the expression of adhesion molecules (e.g. ICAM-1 and VCAM-1), that play an important role in the pathophysiology of acute kidney injury [26, 45]. We found increasd expression of ICAM-1 and VCAM-1 in kidneys of CIS-treated rats (Fig. 6A–B), as well as increased number of myeloperoxydase positive inflammatory cell, which could be attenuated by pretreatment with SFN (Figs. 6 and 7).
Although our study has shown that administration of SFN was able to attenuate the CIS-induced kidney injury, it has not addressed the question of potential interference of SFN with the chemotherapeutic efficacy of CIS, which is a potential limitation. However, on the basis of multiple reports demonstrating anticancer effects of SFN by itself (comprising of inhibition of histone deacetylation, cell cycle arrest, inhibition of cell growth and induction of apoptosis, among many other mechanisms) in cell culture studies of human liver, colon, prostate, breast, colon, ovarian, bladder and immune cell malignancies, and studies indicating efficacy of broccoli, broccoli sprouts and SF in animal models of cancer (reviewed in [36]), it is more likely that SFN would rather enhance the chemotherapeutic efficacy of CIS. The latter is also supported by SFN’s reported antiangiogenic properties [35] and its inhibitory effect on NF-κB signal transduction pathway observed in our report. Furthermore, it has recently been demonstrated that SFN is not decreasing the antitumor potential of CIS [46] and in cancer stem cells it in fact can even enhance its cytotoxicity [47].
Another important consideration to address is if the dose of SFN administered in our study bears any human clinical relevance. This is a very difficult question to answer, because of the possibly differences in the kinetics, distribution and metabolism of the compound in humans and in rats (for example human T1/2 of SFN has been reported to be more than 10 times longer than in rats [48, 49]). However, the SFN plasma levels in rats reach 46±19 ng/ml after administration of 0.5 mg/kg of this isothiocyanate [50], the same dose used in the present study. Similar plasma levels of SFN were reported in humans after broccoli consumption: 12±1.6 ng/ml [51] and 18.2±6.5 ng/ml [52], suggesting that the dose used in the present study may have clinical relevance. In addition, in support of the idea that nutritional amounts of broccoli might exerts therapeutic effects, a pilot study has found that consumption of only 68 g of broccoli sprouts was able to inhibit histone deacetylase (a putative target of SFN’s anticancer properties) in peripheral blood mononuclear cells [53]. Importantly, the dose of SFN used in our study is also in the range of the amounts reported to exert antitumor effects in animal models of various cancers [36].
Collectively, we demonstrated that the cisplatin-induced nephropathy is associated with activation of various cell death and pro-inflammatory pathways (p53, JNK, p38α, TNF-α, and NF-κB) and impairments of key pro-survival signaling mechanisms (ERK and p38-β); and these pathological alterations can be prevented by a natural, low cost, antioxidant/anti-inflammatory constituent from cruciferous vegetables (e.g. broccoli, Brussels sprouts, etc), providing a novel approach for preventing this devastating complication of the chemotherapy. These results, coupled with the potent antitumor effect of SFN reported in multiple preclinical studies, and epidemiological evidence suggesting that dietary intake of cruciferous vegetables may lower cancer risk in humans, are particularly exciting. Well-powered future clinical trials to explore the potential benefit of SFN supplementation and/or whole broccoli consumption in cancer prevention, treatment, or against cisplatin-induced nephropathy are warranted.
Acknowledgments
Grants, sponsors, and funding sources: This work was supported by the Intramural Research Program of NIH/NIAAA (to Pacher P), Consejo Nacional de Ciencia y Tecnología (CONACYT) 129838 and Dirección General de Asuntos del Personal Académico (DGAPA) 201910 (to Pedraza-Chaverri J). Dr. Horváth was supported by a Hungarian Research Scientific Fund (OTKA) Fellowship (MB08-A 80238). The study sponsors had no role in the study design, in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Guerrero-Beltrán CE dedicates this study to his beloved family: Eddy, Carlos, Arturo and Antonio.
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 2.Baek SM, Kwon CH, Kim JH, Woo JS, Jung JS, Kim YK. Differential roles of hydrogen peroxide and hydroxyl radical in cisplatin-induced cell death in renal proximal tubular epithelial cells. J Lab Clin Med. 2003;142:178–86. doi: 10.1016/S0022-2143(03)00111-2. [DOI] [PubMed] [Google Scholar]
- 3.Cummings BS, Schnellmann RG. Cisplatin-induced renal cell apoptosis: caspase 3-dependent and -independent pathways. J Pharmacol Exp Ther. 2002;302:8–17. doi: 10.1124/jpet.302.1.8. [DOI] [PubMed] [Google Scholar]
- 4.Jiang DJ, Jia SJ, Dai Z, Li YJ. Asymmetric dimethylarginine induces apoptosis via p38 MAPK/caspase-3-dependent signaling pathway in endothelial cells. J Mol Cell Cardiol. 2006;40:529–39. doi: 10.1016/j.yjmcc.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 5.Liu H, Baliga R. Endoplasmic reticulum stress-associated caspase 12 mediates cisplatin-induced LLC-PK1 cell apoptosis. J Am Soc Nephrol. 2005;16:1985–92. doi: 10.1681/ASN.2004090768. [DOI] [PubMed] [Google Scholar]
- 6.Liu L, Yang C, Herzog C, Seth R, Kaushal GP. Proteasome inhibitors prevent cisplatin-induced mitochondrial release of apoptosis-inducing factor and markedly ameliorate cisplatin nephrotoxicity. Biochem Pharmacol. 2010;79:137–46. doi: 10.1016/j.bcp.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Mukhopadhyay P, Rajesh M, Batkai S, Patel V, Kashiwaya Y, Liaudet L, et al. CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin-induced cardiomyopathy and in human cardiomyocytes. Cardiovasc Res. 2010;85:773–84. doi: 10.1093/cvr/cvp369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukhopadhyay P, Rajesh M, Pan H, Patel V, Mukhopadhyay B, Batkai S, et al. Cannabinoid-2 receptor limits inflammation, oxidative/nitrosative stress, and cell death in nephropathy. Free Radic Biol Med. 2010;48:457–67. doi: 10.1016/j.freeradbiomed.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan H, Mukhopadhyay P, Rajesh M, Patel V, Mukhopadhyay B, Gao B, et al. Cannabidiol attenuates cisplatin-induced nephrotoxicity by decreasing oxidative/nitrosative stress, inflammation, and cell death. J Pharmacol Exp Ther. 2009;328:708–14. doi: 10.1124/jpet.108.147181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park HM, Cho JM, Lee HR, Shim GS, Kwak MK. Renal protection by 3H-1,2-dithiole-3-thione against cisplatin through the Nrf2-antioxidant pathway. Biochem Pharmacol. 2008;76:597–607. doi: 10.1016/j.bcp.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 11.Park MS, De Leon M, Devarajan P. Cisplatin induces apoptosis in LLC-PK1 cells via activation of mitochondrial pathways. J Am Soc Nephrol. 2002;13:858–65. doi: 10.1681/ASN.V134858. [DOI] [PubMed] [Google Scholar]
- 12.Ramesh G, Reeves WB. TNFR2-mediated apoptosis and necrosis in cisplatin-induced acute renal failure. Am J Physiol Renal Physiol. 2003;285:F610–8. doi: 10.1152/ajprenal.00101.2003. [DOI] [PubMed] [Google Scholar]
- 13.Ramesh G, Reeves WB. p38 MAP kinase inhibition ameliorates cisplatin nephrotoxicity in mice. Am J Physiol Renal Physiol. 2005;289:F166–74. doi: 10.1152/ajprenal.00401.2004. [DOI] [PubMed] [Google Scholar]
- 14.Ries F, Klastersky J. Nephrotoxicity induced by cancer chemotherapy with special emphasis on cisplatin toxicity. Am J Kidney Dis. 1986;8:368–79. doi: 10.1016/s0272-6386(86)80112-3. [DOI] [PubMed] [Google Scholar]
- 15.Santos NA, Catao CS, Martins NM, Curti C, Bianchi ML, Santos AC. Cisplatin-induced nephrotoxicity is associated with oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Arch Toxicol. 2007;81:495–504. doi: 10.1007/s00204-006-0173-2. [DOI] [PubMed] [Google Scholar]
- 16.Sugiyama S, Hayakawa M, Kato T, Hanaki Y, Shimizu K, Ozawa T. Adverse effects of anti-tumor drug, cisplatin, on rat kidney mitochondria: disturbances in glutathione peroxidase activity. Biochem Biophys Res Commun. 1989;159:1121–7. doi: 10.1016/0006-291x(89)92225-0. [DOI] [PubMed] [Google Scholar]
- 17.Tsuruya K, Ninomiya T, Tokumoto M, Hirakawa M, Masutani K, Taniguchi M, et al. Direct involvement of the receptor-mediated apoptotic pathways in cisplatin-induced renal tubular cell death. Kidney Int. 2003;63:72–82. doi: 10.1046/j.1523-1755.2003.00709.x. [DOI] [PubMed] [Google Scholar]
- 18.Das S, Fraga CG, Das DK. Cardioprotective effect of resveratrol via HO-1 expression involves p38 map kinase and PI-3-kinase signaling, but does not involve NFkappaB. Free Radic Res. 2006;40:1066–75. doi: 10.1080/10715760600833085. [DOI] [PubMed] [Google Scholar]
- 19.Jiang M, Wei Q, Pabla N, Dong G, Wang CY, Yang T, et al. Effects of hydroxyl radical scavenging on cisplatin-induced p53 activation, tubular cell apoptosis and nephrotoxicity. Biochem Pharmacol. 2007;73:1499–510. doi: 10.1016/j.bcp.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo J, Tsuji T, Yasuda H, Sun Y, Fujigaki Y, Hishida A. The molecular mechanisms of the attenuation of cisplatin-induced acute renal failure by N-acetylcysteine in rats. Nephrol Dial Transplant. 2008;23:2198–205. doi: 10.1093/ndt/gfn090. [DOI] [PubMed] [Google Scholar]
- 21.Mukherjee S, Lekli I, Das M, Azzi A, Das DK. Cardioprotection with alpha-tocopheryl phosphate: amelioration of myocardial ischemia reperfusion injury is linked with its ability to generate a survival signal through Akt activation. Biochim Biophys Acta. 2008;1782:498–503. doi: 10.1016/j.bbadis.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi R, Huang Q, Zhu X, Ong YB, Zhao B, Lu J, et al. Luteolin sensitizes the anticancer effect of cisplatin via c-Jun NH2-terminal kinase-mediated p53 phosphorylation and stabilization. Mol Cancer Ther. 2007;6:1338–47. doi: 10.1158/1535-7163.MCT-06-0638. [DOI] [PubMed] [Google Scholar]
- 23.Sung MJ, Kim DH, Jung YJ, Kang KP, Lee AS, Lee S, et al. Genistein protects the kidney from cisplatin-induced injury. Kidney Int. 2008;74:1538–47. doi: 10.1038/ki.2008.409. [DOI] [PubMed] [Google Scholar]
- 24.Zhuang S, Lu B, Daubert RA, Chavin KD, Wang L, Schnellmann RG. Suramin promotes recovery from renal ischemia/reperfusion injury in mice. Kidney Int. 2009;75:304–11. doi: 10.1038/ki.2008.506. [DOI] [PubMed] [Google Scholar]
- 25.Francescato HD, Costa RS, da Silva CG, Coimbra TM. Treatment with a p38 MAPK inhibitor attenuates cisplatin nephrotoxicity starting after the beginning of renal damage. Life Sci. 2009;84:590–7. [PubMed] [Google Scholar]
- 26.Kang KP, Kim DH, Jung YJ, Lee AS, Lee S, Lee SY, et al. Alpha-lipoic acid attenuates cisplatin-induced acute kidney injury in mice by suppressing renal inflammation. Nephrol Dial Transplant. 2009;24:3012–20. doi: 10.1093/ndt/gfp242. [DOI] [PubMed] [Google Scholar]
- 27.Ramesh G, Reeves WB. TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest. 2002;110:835–42. doi: 10.1172/JCI15606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dinkova-Kostova AT, Talalay P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol Nutr Food Res. 2008;52 (Suppl 1):S128–38. doi: 10.1002/mnfr.200700195. [DOI] [PubMed] [Google Scholar]
- 29.Fahey JW, Talalay P. Antioxidant functions of sulforaphane: a potent inducer of Phase II detoxication enzymes. Food Chem Toxicol. 1999;37:973–9. doi: 10.1016/s0278-6915(99)00082-4. [DOI] [PubMed] [Google Scholar]
- 30.Shin DH, Park HM, Jung KA, Choi HG, Kim JA, Kim DD, et al. The NRF2-heme oxygenase-1 system modulates cyclosporin A-induced epithelial-mesenchymal transition and renal fibrosis. Free Radic Biol Med. 2010;48:1051–63. doi: 10.1016/j.freeradbiomed.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoon HY, Kang NI, Lee HK, Jang KY, Park JW, Park BH. Sulforaphane protects kidneys against ischemia-reperfusion injury through induction of the Nrf2-dependent phase 2 enzyme. Biochem Pharmacol. 2008;75:2214–23. doi: 10.1016/j.bcp.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 32.Zhao HD, Zhang F, Shen G, Li YB, Li YH, Jing HR, et al. Sulforaphane protects liver injury induced by intestinal ischemia reperfusion through Nrf2-ARE pathway. World J Gastroenterol. 2010;16:3002–10. doi: 10.3748/wjg.v16.i24.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song MY, Kim EK, Moon WS, Park JW, Kim HJ, So HS, et al. Sulforaphane protects against cytokine- and streptozotocin-induced beta-cell damage by suppressing the NF-kappaB pathway. Toxicol Appl Pharmacol. 2009;235:57–67. doi: 10.1016/j.taap.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Shan Y, Zhao R, Geng W, Lin N, Wang X, Du X, et al. Protective effect of sulforaphane on human vascular endothelial cells against lipopolysaccharide-induced inflammatory damage. Cardiovasc Toxicol. 2010;10:139–45. doi: 10.1007/s12012-010-9072-0. [DOI] [PubMed] [Google Scholar]
- 35.Davis R, Singh KP, Kurzrock R, Shankar S. Sulforaphane inhibits angiogenesis through activation of FOXO transcription factors. Oncol Rep. 2009;22:1473–8. doi: 10.3892/or_00000589. [DOI] [PubMed] [Google Scholar]
- 36.Jeffery EH, Keck AS. Translating knowledge generated by epidemiological and in vitro studies into dietary cancer prevention. Mol Nutr Food Res. 2008;52 (Suppl 1):S7–17. doi: 10.1002/mnfr.200700226. [DOI] [PubMed] [Google Scholar]
- 37.Cheung KL, Kong AN. Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J. 2010;12:87–97. doi: 10.1208/s12248-009-9162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chirino YI, Sanchez-Gonzalez DJ, Martinez-Martinez CM, Cruz C, Pedraza-Chaverri J. Protective effects of apocynin against cisplatin-induced oxidative stress and nephrotoxicity. Toxicology. 2008;245:18–23. doi: 10.1016/j.tox.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 39.Batkai S, Osei-Hyiaman D, Pan H, El-Assal O, Rajesh M, Mukhopadhyay P, et al. Cannabinoid-2 receptor mediates protection against hepatic ischemia/reperfusion injury. FASEB J. 2007;21:1788–800. doi: 10.1096/fj.06-7451com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moon KH, Hood BL, Mukhopadhyay P, Rajesh M, Abdelmegeed MA, Kwon YI, et al. Oxidative inactivation of key mitochondrial proteins leads to dysfunction and injury in hepatic ischemia reperfusion. Gastroenterology. 2008;135:1344–57. doi: 10.1053/j.gastro.2008.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mukhopadhyay P, Rajesh M, Batkai S, Kashiwaya Y, Hasko G, Liaudet L, et al. Role of superoxide, nitric oxide, and peroxynitrite in doxorubicin-induced cell death in vivo and in vitro. Am J Physiol Heart Circ Physiol. 2009;296:H1466–83. doi: 10.1152/ajpheart.00795.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar S, Allen DA, Kieswich JE, Patel NS, Harwood S, Mazzon E, et al. Dexamethasone ameliorates renal ischemia-reperfusion injury. J Am Soc Nephrol. 2009;20:2412–25. doi: 10.1681/ASN.2008080868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajesh M, Mukhopadhyay P, Batkai S, Patel V, Saito K, Matsumoto S, et al. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J Am Coll Cardiol. 2010;56:2115–25. doi: 10.1016/j.jacc.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang D, Westerheide SD, Hanson JL, Baldwin AS., Jr Tumor necrosis factor alpha-induced phosphorylation of RelA/p65 on Ser529 is controlled by casein kinase II. J Biol Chem. 2000;275:32592–7. doi: 10.1074/jbc.M001358200. [DOI] [PubMed] [Google Scholar]
- 45.Akcay A, Nguyen Q, Edelstein CL. Mediators of inflammation in acute kidney injury. Mediators Inflamm. 2009;2009:137072. doi: 10.1155/2009/137072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Di Pasqua AJ, Hong C, Wu MY, McCracken E, Wang X, Mi L, et al. Sensitization of non-small cell lung cancer cells to cisplatin by naturally occurring isothiocyanates. Chem Res Toxicol. 2010;23:1307–9. doi: 10.1021/tx100187f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kallifatidis G, Labsch S, Rausch V, Mattern J, Gladkich J, Moldenhauer G, et al. Sulforaphane Increases Drug-mediated Cytotoxicity Toward Cancer Stem-like Cells of Pancreas and Prostate. Mol Ther. 2011;19:188–95. doi: 10.1038/mt.2010.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu R, Hebbar V, Kim BR, Chen C, Winnik B, Buckley B, et al. In vivo pharmacokinetics and regulation of gene expression profiles by isothiocyanate sulforaphane in the rat. J Pharmacol Exp Ther. 2004;310:263–71. doi: 10.1124/jpet.103.064261. [DOI] [PubMed] [Google Scholar]
- 49.Shapiro TA, Fahey JW, Dinkova-Kostova AT, Holtzclaw WD, Stephenson KK, Wade KL, et al. Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: a clinical phase I study. Nutr Cancer. 2006;55:53–62. doi: 10.1207/s15327914nc5501_7. [DOI] [PubMed] [Google Scholar]
- 50.Hanlon N, Coldham N, Gielbert A, Kuhnert N, Sauer MJ, King LJ, et al. Absolute bioavailability and dose-dependent pharmacokinetic behaviour of dietary doses of the chemopreventive isothiocyanate sulforaphane in rat. Br J Nutr. 2008;99:559–64. doi: 10.1017/S0007114507824093. [DOI] [PubMed] [Google Scholar]
- 51.Hanlon N, Coldham N, Gielbert A, Sauer MJ, Ioannides C. Repeated intake of broccoli does not lead to higher plasma levels of sulforaphane in human volunteers. Cancer Lett. 2009;284:15–20. doi: 10.1016/j.canlet.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 52.Vermeulen M, Klopping-Ketelaars IW, van den Berg R, Vaes WH. Bioavailability and kinetics of sulforaphane in humans after consumption of cooked versus raw broccoli. J Agric Food Chem. 2008;56:10505–9. doi: 10.1021/jf801989e. [DOI] [PubMed] [Google Scholar]
- 53.Myzak MC, Tong P, Dashwood WM, Dashwood RH, Ho E. Sulforaphane retards the growth of human PC-3 xenografts and inhibits HDAC activity in human subjects. Exp Biol Med (Maywood) 2007;232:227–34. [PMC free article] [PubMed] [Google Scholar]