Abstract
The efficacy of magnetoencephalography (MEG) as an alternative to invasive methods for investigating the cortical representation of language has been explored in several studies. Recently, studies comparing MEG to the gold standard Wada procedure have found inconsistent and often less-than accurate estimates of laterality across various MEG studies. Here we attempted to address this issue among normal right-handed adults (N=12) by supplementing a well-established MEG protocol involving word recognition and the single dipole method with a sentence comprehension task and a beamformer approach localizing neural oscillations. Beamformer analysis of word recognition and sentence comprehension tasks revealed a desynchronization in the 10–18 Hz range, localized to the temporo-parietal cortices. Inspection of individual profiles of localized desynchronization (10–18 Hz) revealed left hemispheric dominance in 91.7% and 83.3% of individuals during the word recognition and sentence comprehension tasks, respectively. In contrast, single dipole analysis yielded lower estimates, such that activity in temporal language regions was left-lateralized in 66.7% and 58.3% of individuals during word recognition and sentence comprehension, respectively. The results obtained from the word recognition task and localization of oscillatory activity using a beamformer appear to be in line with general estimates of left hemispheric dominance for language in normal right-handed individuals. Furthermore, the current findings support the growing notion that changes in neural oscillations underlie critical components of linguistic processing.
Keywords: Magnetoencephalography, Language, Laterality, Neural Oscillation, Beamformer
1. Introduction
The utility of magnetoencephalography (MEG) as an alternative to traditional invasive methods for the study of the cortical representation of language has been explored in several studies which differ in their experimental design and analytical approach. Using a verbal continuous recognition memory (CRM) protocol, we have previously reported on the cardinal features of MEG-derived cortical activation maps for receptive language, marked by a greater degree of activity in the left temporo-parietal cortex (including the posterior portions of the middle and superior temporal gyri; and the supramarginal and angular gyri) [Breier et al., 1999; 2000; Papanicolaou et al., 1999]. Moreover, the efficacy of this protocol in assessing hemispheric dominance for language has been addressed in studies involving brain surgery candidates, the largest of which demonstrated a high degree of concordance (87%) between language laterality judgments made on the basis of MEG mapping and those made, independently, on the basis of the Wada procedure which involves anesthetization of each hemisphere while assessing language and which constitutes the “gold standard” (Papanicolaou et al., 2004).
In addition to the CRM protocol, studies employing alternative language tasks and source modeling techniques have also reported on the efficacy of MEG as a non-invasive means for establishing hemispheric dominance for language, with some degree of variability on laterality estimates among them. In an early study comparing MEG findings to the Wada, Szymanski and colleagues (2001) found 71% of right-handed tumor patients to be left-lateralized for language using the single dipole method of analysis. Some years later, Bowyer and colleagues (2004) found a higher level of concordance (89%) between MEG and Wada results among 27 epilepsy patients using a source density-imaging technique. In a later study by Kamada and colleagues (2007), MEG and fMRI-based laterality judgments from 87 patients with brain lesions were matched to Wada findings in 100% of the cases. However, the analysis used in this study and the subsequent level of agreement with the Wada would not have been possible using only one imaging modality. Even still, this study suggests that across a large sample, establishing language laterality non-invasively is possible. More recent studies have dissected the MEG signal into time-frequency components and utilized a beamformer to estimate the corresponding sources of activity. For example, Kim and Chung (2008) correctly established language laterality compared to the Wada procedure in 12 out of 17 (71%) epilepsy patients based on localization of activity in the left temporal-parietal cortex, employing an auditory verbal oddball paradigm and a spatial filtering method. Furthermore, using the posterior aspect of the inferior frontal gyrus (IFG) for the region of interest (ROI), the latter study demonstrated that correct laterality judgments were made in 94% (16 out of 17) of the cases studied. Similarly, Hirata et al. (2010) recently found an 85% degree of concordance between Wada and MEG-derived measures of laterality in the IFG, middle frontal gyrus (MFG), and the insular cortex, utilizing a silent word reading task and a beamformer among 60 patients. While MEG estimates of language dominance appear to be improving, there exists variability across the results of language studies published over the last decade due to differences in data analysis techniques and experimental paradigms.
In the majority of clinical studies, estimates of laterality based on non-invasive neuroimaging methods result in a smaller percentage of left-hemisphere (LH) dominance than would be expected. This may be due to the fact that among patients, particularly candidates for epilepsy surgery, inter-hemispheric dominance shift resulting in more right hemisphere dominant patients is likely to occur (Pataraia et al., 2004; 2005). But this does not account for the lower percentage of LH dominance found using MEG compared to that found with the Wada procedure in the same patient group (Papanicolaou et al., 2004). Therefore, this marginal underestimation of the percentage of LH dominant cases found with MEG may be due to either the task or to the analysis procedure used. Accordingly, in this study we explored the possibility that more accurate laterality estimates could be derived by introducing a more naturalistic language activation task in conjunction with an alternative MEG analysis procedure to the one we are typically employing, with a sample of right-handed normal individuals. However, in order to assess the possible improvements associated with alternative task and analysis techniques, we must first adopt an independent and valid estimate of left-hemisphere dominance in the normal population as a guideline for our study.
In the general population, it has long been estimated that roughly 96% of right-handed individuals are left-dominant for language on the basis of a large sample of epilepsy patients (Rasmussen & Milner, 1977). While this estimate is often cited to explain language dominance in the normal population, a more realistic estimate may be derived by studying individuals with language impairment due to acute unilateral brain injury. Among these patients, the possibility of lesion-induced hemispheric dominance shift is eliminated. In a recent study by Moser and colleagues (2010), several large-scale studies were identified which reported on unilateral stroke resulting in aphasia. On the basis of these studies, they concluded that approximately 90% of normal individuals are left-lateralized for language regardless of handedness. Furthermore, when the proportion of left- and right-handed individuals in the general population is considered, the percentage of left-lateralized individuals rises to 94% among right-handed people. Accordingly, this percentage may serve as a more accurate estimate of hemispheric dominance for language among the general population.
In addition to clinical populations, hemispheric dominance for language has been studied with noninvasive functional imaging methods among neurologically intact individuals as well. For example, in a large-scale normative MEG study by Papanicolaou et al. (2006) using the single-word CRM paradigm and an automated dipole localization technique, it was found that hemispheric dominance for receptive language is mainly accounted for by sustained activity in the left middle temporal gyrus, a finding which did not vary as a function of age, gender or stimulus modality. Moreover, using a variant of the CRM paradigm, Mohamed et al. (2009) localized language-specific event-related desynchronization (ERD) to a region in the posterior superior temporal gyrus (STG) of the left hemisphere using a small normative sample. In addition, Cornelissen and colleagues (2009) used a passive word reading task and a similar analysis technique with a sample of healthy controls and found a preponderance of activation in the left posterior STG region. However, the aforementioned studies reported group averages and did not specify estimates of hemispheric dominance for each individual subject. Therefore, in this study we attempted to directly address this issue on a case-by-case basis by supplementing the well established CRM paradigm and the equivalent current dipole analysis with an alternative language task involving sentence comprehension and a source analysis involving time frequency (TF) and beamformer methods.
2. Results & Discussion
2.1 In-scanner task performance
Percent correct identification of targets was 95.1% ± 2.3 for the CRM task, demonstrating excellent accuracy across participants. For the sentence comprehension task, participants exhibited a 94.1% ± 4.8 rate of accuracy for detection of semantically correct sentences.
2.2 Dipole Analysis
The dipole fitting was applied to all twelve subjects during a specific period for each task and a paired samples t test was used to compare the total dipoles in the left and right hemispheres. The late component of the CRM task (150–700ms) showed significantly greater dipoles in the left middle temporal gyrus (MTG) compared to the right hemisphere (p=0.019). The mean number of dipoles across subjects was 10.6 ± 2.9 (± standard error of the mean) for the left hemisphere and 3.8 ± 1.3 for the right hemisphere. For the late component of the comprehension task (3000–3500ms) the same analysis yielded significantly greater dipoles in a region occupying inferior and middle temporal gyri of the left hemisphere compared to the right (p=0.024). The mean number of dipoles in the left hemisphere of this region was 25.2 ± 7.9 and 7.0 ± 2.6 in the right hemisphere. Wernicke’s area (posterior STG) was the primary region of interest across both tasks and methods since it is the area responsible for receptive language. Previous findings using the dipole method identified only the MTG as a region for estimating hemispheric language laterality (Papanicolaou et al., 2006). However, using the novel comprehension task, a preponderance of dipoles was also observed in inferior temporal regions which were included in the analysis of that task.
2.3 Spectral Analysis
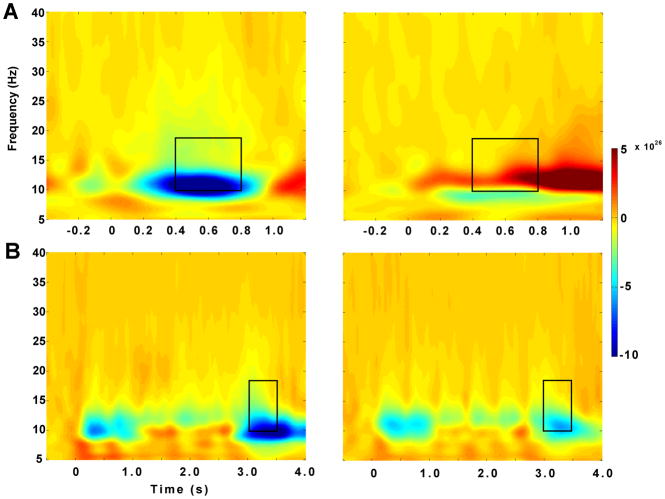
Group average TF plots for both tasks (Figure 1) show several event-related synchronizations (ERS) as well an event-related desynchronization (ERD) in posterior temporal sensors. Topographic plots of the average ERDs for both tasks were lateralized to a posterior region of the left hemisphere likely covering temporal regions. Figure 1 illustrates average TF plots across 22 MEG sensors which corresponded to the ERDs in the topographic plots. ERDs were observed at a frequency range of 10 to 18 Hz across both tasks for a duration of 400 ms (400–800ms) in the CRM task and 500 ms (3000–3500ms) in the reading comprehension task. The TF plot for the comprehension task (Figure 1B) shows six separate ERSs and ERDs bilaterally which correspond to the presentation of each of the six word stimuli. A dependent samples statistical test comparing pre-stimulus and post-stimulus spectral intensity across subjects identified a significant ERD at 14 Hz along the time windows mentioned above for each task. The black boxes in Figure 1 illustrate the resulting time-frequency ranges submitted to the beamformer analyses.
Figure 1.
A, A time-frequency plot for the CRM task showing an average across 22 MEG sensors covering the left and right lateral temporal region, respectively, with a frequency range of 5 to 40 Hz and a period of −0.5 to 1.2 s. The plot represents an average across all subjects. B, The equivalent time-frequency plot for the comprehension task with a frequency range of 5 to 40 Hz and a period of −0.5 to 4 s. Black boxes indicate time-frequency ranges used for beamformer analysis.
2.4 Beamformer
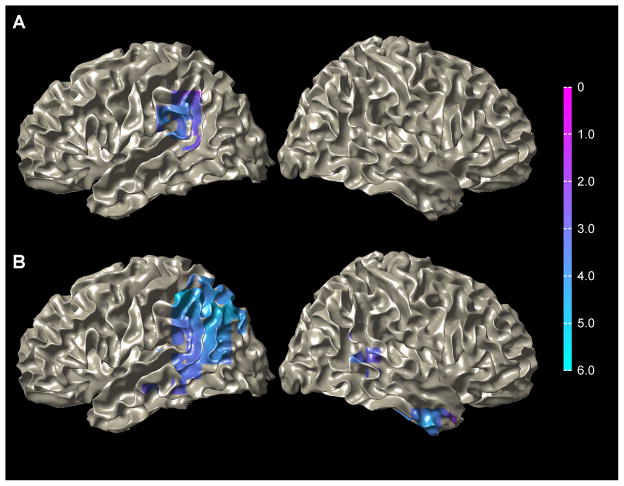
Group analysis of the dynamic imaging of coherent sources (DICS) beamformer localization of the time-frequency ranges identified in the TF plots yielded significant ERDs in several brain regions. The most prominent cluster across both tasks occupied a set of regions in the posterior perisylvian area of the left hemisphere (Figure 2) that included the supramarginal area, the posterior third of the STG, and the transverse temporal gyrus identified by the Talairach atlas. These regions may not necessarily play a simultaneous role in language processing, however, the disperse nature of the beamformer, especially at the group-level, does not discriminate across anatomical boundaries. In order to account for wide-spread activation in temporal regions, all three ROIs are used for the subsequent laterality index (LI) measure. A maximum t value of −4.31 in the group analysis of the CRM task was identified in the supramarginal gyrus (−52.0, −52.0, 38.0). A maximum t value of 5.90 was found in the comprehension task in a similar location (−60.0, −50.0, 40.0). Significant ERDs were also observed in parietal regions in the left hemisphere (angular gyrus, inferior parietal lobule, superior parietal lobule) and temporal regions in the right hemisphere (anterior inferior temporal gyrus, posterior superior temporal gyrus) in the reading comprehension task. Activation was not observed in any other regions other than the supramarginal gyrus, the posterior STG, and the transverse temporal gyrus in the CRM task (Figure 2).
Figure 2.
A, An ERD from the CRM task localized across subjects to temporal-parietal regions (left greater than right) using the DICS beamformer during the 400 to 800 ms period following stimulus onset and a frequency range of 10–18 Hz. B, Comprehension task ERD localized across subjects to similar regions as the CRM task during the 3000 to 3500 ms period following the first stimulus onset and a frequency range of 10–18 Hz. Data are superimposed onto a single subject’s MRI normalized to MNI coordinate space. Color intensity scale represents t-values across subjects.
2.5 Laterality Indices
The LI was calculated for each subject based on the dipole results and separately for the beamformer results. On the basis of the dipole analysis, 8 out of the 12 subjects (66.7%) lateralized to the left during the CRM task while only 7 out of the 12 subjects (58.3%) lateralized to the left in the comprehension task. Based on the beamformer analysis, 11 subjects (91.7%) lateralized to the left hemisphere for the CRM task. In the reading comprehension task, 10 subjects (83.3%) lateralized to the left hemisphere (Table 1). Overall, the CRM task gave a result which more closely matched the accepted percentage of left lateralized individuals (94%) compared to the comprehension task. Furthermore, the beamformer analysis method gave higher percentages of left lateralized subjects compared to the dipole method.
Table 1.
Laterality Indices1
| CRM | Comprehension | |||
|---|---|---|---|---|
| Subject | Beamformer | Dipole | Beamformer | Dipole |
| S1 | 0.815 | U | 0.948 | 1.000 |
| S2 | 1.000 | 1.000 | 0.275 | 1.000 |
| S3 | 1.000 | 1.000 | −0.021 | 0.806 |
| S4 | −0.696 | U | 1.000 | U |
| S5 | 0.977 | 0.200 | 0.745 | 0.388 |
| S6 | 0.650 | 0.379 | 0.852 | 0.586 |
| S7 | 0.998 | U | 0.169 | U |
| S8 | 0.453 | 1.000 | −0.262 | −1.000 |
| S9 | 0.872 | 0.310 | 0.994 | U |
| S10 | 1.000 | U | 0.971 | −0.385 |
| S11 | 0.377 | 0.936 | 0.977 | 1.000 |
| S12 | 0.984 | 0.314 | 1.000 | 0.667 |
Values represent a LI for each subject across both tasks and analysis methods. A U stands for undetermined, for cases in which the dipole method did not yield a sufficient amount of dipoles in the appropriate ROI of either hemisphere to compute a LI.
3. Discussion
In this experiment we attempted to optimize the level of agreement between MEG results of hemispheric dominance for language and the accepted estimate of LH dominance in the normal population. Using two tasks and two analysis methods, estimates of laterality were computed. On the basis of these estimates, we identified an optimal combination of the well established CRM task and the DICS beamformer which produced a result (91.7%) closest to the estimate for LH dominance among healthy controls (94%), followed by the comprehension task and DICS beamformer (83.3%). The CRM task also provided a slightly better estimate of LH dominance than the comprehension task using the single dipole method.
Previous normative studies focusing on TF ranges using a beamformer (Mohamed et al., 2008; Cornelissen et al., 2009) do not report individual measures of laterality and thus do not provide a measurable level of laterality agreement among the general population. If we assume that all right-handed subjects in a small sample are left-lateralized for language based on the evidence (Moser et al., 2010) reporting an overwhelming percentage of left-lateralized individuals across large samples (94%), then individual measures of laterality may be reported. Moreover, the finding that 91.7% of the subjects in this sample lateralize to the LH suggests that a non-invasive measure of laterality is obtainable on a subject-by-subject basis among the general population. This combination of task and analysis may also be applied to clinical populations. In studies which established laterality non-invasively among patients using only MEG and alternative localization techniques to the dipole method, a varying range of LH dominance (71–94%) has been reported (Kim and Chung, 2008; Hirata et al., 2010). The approach described in this study may provide a more accurate measure to establish laterality within a larger patient populations among which Wada data exists to establish concordance. However, a direct comparison between the method of source localization (DICS beamformer) described here and other methods of source localizations such as the minimum-norm estimates (MNE) and alternative beamformers is necessary in order to fully assess the optimal combination of task and analysis methods. This study is the first step in completing that process but further studies involving larger subject samples and alternative tasks and data analysis methods may improve further estimates of hemispheric dominance for language.
4. Experimental Procedure
4.1 Subjects
Twelve healthy right-handed adults (5 males, mean age: 28.2 ± 5.5 years, range: 23–40 years) with normal or corrected-to-normal vision and normal hearing participated in this study. All participants were right-handed according to the Edinburgh Inventory with a mean handedness quotient of 87.5 ± 10.1%. All subjects were financially compensated for their participation and provided written consent. In accordance with the Declaration of Helsinki, the protocol was approved by the Institutional Review Board of the University of Texas Health Science Center at Houston.
4.2 CRM Task
The continuous recognition memory (CRM) task was performed during MEG recordings previously described by Papanicolaou et al. (2006). Subjects were asked to remember five target stimuli presented to them at the beginning of the recording session. The five target items were randomly placed among 40 other words in each of three blocks for a total of 135 trials. All word stimuli were produced by a native English speaker with a flat intonation and were digitized with a sampling rate of 22,000 Hz and 16-bit resolution. Mean duration of the stimuli was 450 ms (300–750 ms range) and the stimuli were delivered via two 5-meter-long plastic tubes terminating in ear inserts at an 80 dB sounds pressure level (SPL). Each trial lasted for 3.7 s with a random interval of 0 to 0.5 s between trials. Subjects were told to carefully listen to each word and were asked to push a button with their right index finger when a target word was presented.
4.3 Comprehension Task
A visual reading comprehension task was used either following or prior to the CRM task in random order across subjects. During this task, subjects were asked to silently read each word as it appeared on the screen, one at a time, and to decide if the sixth and final word fit the sentence semantically. To maintain attention throughout the task, in half of all trials a semantically incongruous sixth and final word was used while the other half of the trials contained a final word which was semantically congruous. Sentences with congruous and incongruous words were presented in random order. There were a total of 150 trials divided into 6 blocks of 25 trials each. Each of the first five words were displayed for 500 ms with the sixth word displayed for 1500 ms to give the subjects adequate time to decide if the final word fit the sentence. Following the presentation of the final word, a blank screen was presented informing subjects to make a button-pressing response if the sentence was semantically correct, and not to respond if it was not. This response period lasted for 3.5 seconds for a total trial time of 7.5 s and was followed by a random interval of 0 to 0.5 s before the next trial. A total of 322 unique words were used across all trials. All trials were included for data analysis. All word stimuli were presented in black Courier New font size 24 displayed on a gray background. Stimuli were projected onto a screen approximately 26” in front of the subject with a viewing angle of 30 degrees.
4.4 MEG Data Acquisition
Brain activity was recorded using a whole-head magnetoencephalography system with 248 axial gradiometers (WH 3600, 4D Neuroimaging, San Diego, California, USA) housed in a magnetically shielded room. Signals were digitized at a sampling rate of 290 Hz and filtered online with a 0.1 Hz high-pass filter. Data were noise-reduced offline using an algorithm from the 4D-Neuroimaging software. The head position was computed prior to and at the end of acquisition (after both tasks) using coils placed at the nasion and ear canals bilaterally with an adapter to allow for the presentation of audio stimuli.
4.5 Data Analysis
4.5.1 Preprocessing and Dipole Analysis
Recorded signals were filtered with a low-pass filter of 20 Hz and individual MEG channels which presented excess drift or noise beyond an acceptable range (correlation with adjacent channels ≤ 0.6) were removed prior to dipole analysis. Epochs were inspected and those which contained artifacts were excluded from the dipole analysis. Dipole sources were reconstructed using an automated method of dipole analysis referred to as Automatic Channel Group Selection (ACGS ) previously described in detail by Papanicoloau et al. (2006). This routine performs an iterative single dipole analysis at each latency within an experimenter-specified temporal range and a goodness of fit ≥ 0.9. The resulting dipoles are co-registered with normalized T1-weighted structural MRI’s for each subject and transformed into MNI-standard (Montreal Neurological Institute) coordinate space. Normalized sources are then sorted into 41 Regions of Interest (ROIs) defined on the basis of the Anatomical Automatic Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002) and Brodmann areas within the MNI synthetic brain. While dipoles were computed in all 41 ROIs, for the purposes of this experiment, only the ROIs of the middle temporal gyrus (MTG) and the inferior temporal gyrus (ITG) were studied in accordance with previously reported findings using this method of dipole analysis which yielded optimal language lateralization results (Papanicolaou et al., 2006). Additionally, only dipoles computed between the 150 and 700 ms range post-stimulus were used in the analysis since this latency range has been shown to generally correspond to higher cognitive functions when studied in the context of a language task (Papanicolaou et al., 2006).
4.5.2 Preprocessing and Spectral Analysis
Data submitted to the spectral analyses and subsequent beamformer analyses underwent a similar series of preprocessing steps outlined in the dipole analysis section (4.5.1), however, a low-pass filter was not performed in order to preserve the frequency information required for the spectral analyses. All data were processed using the FieldTrip toolbox developed at the F.C. Donders Centre for Cognitive Neuroimaging (http://www.ru.nl/fcdonders/fieldtrip/). Trials which were contaminated by artifacts such as eye movement or other motor movements were removed from the data. Furthermore, an independent component analyses (ICA) algorithm was used to classify and remove cardiac artifacts and additional artifact components related to eye movements. The resulting artifact-free signals were used for all further analyses procedures. A time-frequency analysis was performed on the data to identify the time-frequency range which produced the most robust laterality based on observed ERDs. TF power maps were computed by applying a Morlet wavelet-based transform to the single-trial time-series of power with a window length of 1 and a step size of 0.5. Each wavelet was normalized to the pre-stimulus (baseline) and averaged across all trials such that the power in the TF plot represented an increase or decrease relative to the pre-stimulus level. Plots were constructed with a frequency range of 6 to 40 Hz and a period of 0 to 4 seconds (−500 to 0 ms baseline) for the comprehension task and 0 to 1.2 seconds (−500 to 0 ms baseline) for the CRM task.
4.5.3 Beamformer
A DICS beamformer (Gross et al., 2001) was applied to the data based on the time-frequency ranges selected from the spectral analysis. The DICS beamformer consists of an adaptive spatial filter created from the lead field matrices and the frequency component of the cross-spectral density matrix, obtained by applying a multi-taper fast Fourier Transform (FFT). The filter estimates the spatial distribution of power at each grid point of a brain volume, generated from each subject’s individual anatomical MRI. Unlike the dipole technique, the DICS beamformer comprises a distributed source estimation based on a predefined signal frequency range and time window. The sources are therefore estimated for a single latency range rather than individual time points which is the case with dipole source analysis. Furthermore, the distributed source computation relies on the distribution of frequency amplitude across MEG sensors rather than deriving a source based on opposite magnetic fields.
In order to evaluate the reliability of the beamformer source localization for individual subjects, a statistical measure was incorporated such that a dependent samples non-parametric permutation test was computed for each subject comparing the post-stimulus to the pre-stimulus across all trials. This statistical test was followed by a spatial transformation of each subject’s MRI to MNI standard space. Only voxels which contained t values with an associated p < 0.05 were considered statistically significant. Group-level source maps were then computed for each task using the same statistical approach applied at the subject-level. A Bonferroni correction was implemented to correct for multiple comparisons.
4.5.4 Laterality Index
In order to assess language dominance, a laterality index (LI) was computed using the following formula: , where L represents the sum of all values within specified ROIs in the left hemisphere and R represents the sum of all values within those ROIs in the right hemisphere. The values for the dipole analysis consisted of dipole counts within the MTG for each hemisphere. For the beamformer, statistically significant t values of all voxels within the posterior perisylvian region (supramarginal, transverse temporal, and posterior third of the STG) were summed for each hemisphere. Across analysis methods, a LI greater than or equal to 0.1 was considered left-dominant while a value less than or equal to −0.1 was considered right-dominant, and a value between −0.1 and 0.1 was considered bilateral.
Highlights.
Word recognition task is optimal for assessing hemispheric dominance for language.
Language related activity is localized as a desynchronization at 10–18 Hz.
Changes in neural oscillations underlie critical components of linguistic processing.
Acknowledgments
This study was supported by Project 4 of grant P50 HD052117-03 awarded to ACP from the National Institute of Child Health and Human Development (NICHD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bogen JE, Bogen GM. Wernicke's region--Where is it? Ann N Y Acad Sci. 1976;280:834–43. doi: 10.1111/j.1749-6632.1976.tb25546.x. [DOI] [PubMed] [Google Scholar]
- Bowyer SM, Moran JE, Mason KM, Constantinou JE, Smith BJ, Barkley GL, Tepley N. MEG localization of language-specific cortex utilizing MR-FOCUSS. Neurology. 2004 Jun 22;62(12):2247–55. doi: 10.1212/01.wnl.0000130385.21160.7a. [DOI] [PubMed] [Google Scholar]
- Breier JI, Simos PG, Zouridakis G, Wheless JW, Willmore LJ, Constantinou JE, Maggio WW, Papanicolaou AC. Language dominance determined by magnetic source imaging: a comparison with the Wada procedure. Neurology. 1999 Sep 22;53(5):938–45. doi: 10.1212/wnl.53.5.938. [DOI] [PubMed] [Google Scholar]
- Breier JI, Simos PG, Zouridakis G, Papanicolaou AC. Lateralization of activity associated with language function using magnetoencephalography: a reliability study. Journal of Clinical Neurophysiology. 2000;17:503–510. doi: 10.1097/00004691-200009000-00010. [DOI] [PubMed] [Google Scholar]
- Cornelissen PL, Kringelbach ML, Ellis AW, Whitney C, Holliday IE, Hansen PC. Activation of the left inferior frontal gyrus in the first 200 ms of reading: evidence from magnetoencephalography (MEG) PLoS One. 2009;4(4):e5359. doi: 10.1371/journal.pone.0005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R. Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proc Natl Acad Sci U S A. 2001 Jan 16;98(2):694–9. doi: 10.1073/pnas.98.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata M, Goto T, Barnes G, Umekawa Y, Yanagisawa T, Kato A, Oshino S, Kishima H, Hashimoto N, Saitoh Y, Tani N, Yorifuji S, Yoshimine T. Language dominance and mapping based on neuromagnetic oscillatory changes: comparison with invasive procedures. J Neurosurg. 2010 Mar;112(3):528–38. doi: 10.3171/2009.7.JNS09239. [DOI] [PubMed] [Google Scholar]
- Kamada K, Sawamura Y, Takeuchi F, Kuriki S, Kawai K, Morita A, Todo T. Expressive and receptive language areas determined by a non-invasive reliable method using functional magnetic resonance imaging and magnetoencephalography. Neurosurgery. 2007 Feb;60(2):296–305. doi: 10.1227/01.NEU.0000249262.03451.0E. discussion 305–6. [DOI] [PubMed] [Google Scholar]
- Kim JS, Chung CK. Language lateralization using MEG beta frequencydesynchronization during auditory oddball stimulation with one-syllable words. Neuroimage. 2008 Oct 1;42(4):1499–507. doi: 10.1016/j.neuroimage.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Mohamed IS, Cheyne D, Gaetz WC, Otsubo H, Logan WJ, Carter Snead O, 3rd, Pang EW. Spatiotemporal patterns of oscillatory brain activity during auditory word recognition in children: a synthetic aperture magnetometry study. Int J Psychophysiol. 2008 May;68(2):141–8. doi: 10.1016/j.ijpsycho.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Moser DC, Papanicolaou AC, Swank P, Breier JI. Evidence for the solidarity of the expressive and receptive language systems: a retrospective study. J Int Neuropsychol Soc. 2011 Jan;17(1):62–8. doi: 10.1017/S1355617710001153. [DOI] [PubMed] [Google Scholar]
- Papanicolaou AC, Simos PG, Breier JI, Zouridakis G, Willmore LJ, Wheless JW, Constantinou JE, Maggio WW, Gormley WB. Magnetoencephalographic mapping of the language-specific cortex. J Neurosurg. 1999 Jan;90(1):85–93. doi: 10.3171/jns.1999.90.1.0085. [DOI] [PubMed] [Google Scholar]
- Papanicolaou AC, Simos PG, Castillo EM, Breier JI, Sarkari S, Pataraia E, Billingsley RL, Buchanan S, Wheless J, Maggio V, Maggio WW. Magnetocephalography: a noninvasive alternative to the Wada procedure. J Neurosurg. 2004 May;100(5):867–76. doi: 10.3171/jns.2004.100.5.0867. [DOI] [PubMed] [Google Scholar]
- Papanicolaou AC, Pazo-Alvarez P, Castillo EM, Billingsley-Marshall RL, Breier JI, Swank PR, Buchanan S, McManis M, Clear T, Passaro AD. Functional neuroimaging with MEG: normative language profiles. Neuroimage. 2006 Oct 15;33(1):326–4. doi: 10.1016/j.neuroimage.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Pataraia E, Simos PG, Castillo EM, Billingsley-Marshall RL, McGregor AL, Breier JI, Sarkari S, Papanicolaou AC. Reorganization of language-specific cortex in patients with lesions or mesial temporal epilepsy. Neurology. 2004 Nov 23;63(10):1825–32. doi: 10.1212/01.wnl.0000144180.85779.9a. [DOI] [PubMed] [Google Scholar]
- Pataraia E, Billingsley-Marshall RL, Castillo EM, Breier JI, Simos PG, Sarkari S, Fitzgerald M, Clear T, Papanicolaou AC. Organization of receptive language-specific cortex before and after left temporal lobectomy. Neurology. 2005 Feb 8;64(3):481–7. doi: 10.1212/01.WNL.0000150900.71773.E6. [DOI] [PubMed] [Google Scholar]
- Rasmussen T, Milner B. The role of early left-brain injury in determining lateralization of cerebral speech functions. Ann N Y Acad Sci. 1977 Sep 30;299:355–69. doi: 10.1111/j.1749-6632.1977.tb41921.x. [DOI] [PubMed] [Google Scholar]
- Szymanski MD, Perry DW, Gage NM, Rowley HA, Walker J, Berger MS, Roberts TP. Magnetic source imaging of late evoked field responses to vowels: toward an assessment of hemispheric dominance for language. J Neurosurg. 2001 Mar;94(3):445–53. doi: 10.3171/jns.2001.94.3.0445. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002 Jan;15(1):273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]