Abstract
One of a family of devastating lysosomal storage disorders, Krabbe disease is characterized by demyelination, psychosine accumulation, and inflammation. Affected infants rarely survive longer than two years. Using the twitcher mouse model of the disease, this study evaluated the potential of intrastriatal injection of adipose or bone marrow-derived mesenchymal stromal cells (MSCs) as a treatment option. Neonatal pups were injected with MSCs at 3–4 days of age and subjected to a battery of behavioral tests beginning at 15 days. While MSC injection failed to increase lifespan of twitchers, improvements in rotarod performance and twitching severity were observed at 27–38 days of age using MSCs derived from bone marrow. This study tested several different tasks developed in adult mice for evaluation of disease progression in immature twitchers. Rotarod was both reliable and extremely sensitive. Automated gait analysis using the Treadscan program was also useful for early evaluation of differences prior to overt gait dysfunction. Finally, this study represents the first use of the Stone T-maze in immature mice. Validation of rotarod and automated gait analysis for detection of subtle differences in disease progression is important for early stage efforts to develop treatments for juvenile disorders.
Keywords: Krabbe disease, twitcher mice, mesenchymal stem/stromal cells, adipose-derived stem/stromal cells, gait analysis, motor function
1. Introduction
Krabbe disease is an inherited, autosomal recessive condition resulting from lack of a functional galactosylceramidase (GALC) enzyme. Krabbe disease is a rare (1/100,000 births), but exceedingly devastating condition. Infants are born normal, but begin to show progressively worsening symptoms such as irritability, spasticity, loss of motor skills, hypersensitivity to external stimuli, stiffness of muscles, extension of arms and legs, clenched fingers, hypotonicity, blindness, deafness, growth arrest, and progressive microcephaly, usually culminating in death around 2 years of age [1–4]. GALC is a lysosomal enzyme which catabolizes psychosine and galactosylceramide, one of the primary structural components of myelin [5]. GALC and another lysosomal enzyme, GM1-ganglioside B-galactosidase, are both able to catabolize galactosylceramide; however, only GALC can metabolize psychosine [6, 7]. This observation has led to the psychosine hypothesis, relating much of the pathology of Krabbe disease to psychosine’s accumulation and toxic effects upon oligodendrocytes and Schwann cells [8, 9].
Currently, the only approved therapies for Krabbe disease are bone marrow transplantation (BMT) or umbilical cord blood transplantation (UCB). Engrafted donor hematopoietic stem cells appear to migrate to the brain and provide a source of GALC. Both treatments substantially improve outcome- if administered prior to symptom onset-but are not cures. BMT shortly after birth improves neurological function and extends lifespan past 3 years of age; however, BMT after symptom onset is characterized by continued progression and greater than 50% mortality by age 3 [10, 11]. UCB can be performed without a complete HLA match [12–14], but must still be accomplished prior to appearance of symptoms, and language and motor deterioration still seem to occur in a majority of patients [15]. In addition, the risk of Graft vs Host Disease (GVHD) is substantial in BMT and UCB. GVHD reportedly occurred in 8 of 11 asymptomatic and 5 of 14 symptomatic Krabbe patients transplanted with UCB in one study [10]. Since GVHD is the direct result of the donor lymphocytes mounting a cytotoxic immune reaction against the recipient, combination therapy with an immunosuppressive component could help to improve patient outcome following UCB or BMT treatment.
To date, other treatment options have resulted in delayed onset of symptoms, but not a cure, when tested in a murine model of Krabbe disease known as twitcher. These treatments include enzyme replacement, dietary intervention, stem cell therapy, gene therapy, substrate reduction, anti-inflammatory treatment, or assorted combinations of therapies [16–26]. Mesenchymal stromal cells (MSCs), derived from bone (BMSC) or adipose tissue (ASC), are capable of developing along neuronal lineages, and, in addition to being hypoimmunogenic and suitable for allogeneic transplantation, also appear to have pronounced immunosuppressive capabilities [27–35]. As such, MSCs may offer a unique treatment option, since they could potentially provide a source of GALC enzyme, differentiate to replace damaged oligodendrocytes, suppress chronic neuroinflammation by immune suppression and are unlikely to carry GVHD risks [36]. It may be that reduced neuroinflammation as a result of MSC injection could persist far longer than the MSC cells themselves, as other studies have shown that the neuroinflammatory condition of the brain is distinctly longer lasting than peripheral inflammatory status [37]
Nevertheless, many questions remain before the potential of MSC treatment of Krabbe can be definitively known. A prime area of investigation must be the optimal location of MSC injection for maximum clinical benefit, which may be a result of cell persistence, neuronal differentiation, more potent anti-inflammatory action, or some combination of the above. Twitcher mice are an authentic murine model of Krabbe disease that are an ideal system for initial investigation of possible treatments [38]. They were used for initial studies of BMT, resulting in a lifespan increase from 40 to 100 days [39, 40]. In human infants the CNS is primarily affected; however, in the twitcher mice the PNS is primarily affected [41, 42]. Thus, for early stage studies attempting to assess new treatments specifically targeted to the CNS, successful use of the Twitcher model requires the ability to detect very subtle differences in outcome as a result of improved CNS pathology that can be observed despite continued deterioration of the PNS.
Previous work has shown that injection of MSCs into the lateral ventricles of neonatal twitcher mice prior to disease onset can improve disease outcome. While the lifespan increase was marginal, significant improvements were noted using behavioral measurements such as wire hang and rear stride length [36]. Importantly, these improvements were observed despite disappearance of the MSCs 16–18 days after injection, and correlated with substantial reductions in inflammatory cytokines and inducible nitric oxide synthase (iNOS) in the brains at terminal stage [36]. These results suggest that MSCs have the potential to improve Krabbe outcome either alone or as a component of a combination therapy, but several factors must be optimized, including the site of injection, dosage (both volume and concentration of cells) and number and timing of injections. The Ripoll et al. experiments thus demonstrate a key point, namely that the ability to detect subtle alterations in disease progression may be essential in the early stages of determining optimal parameters for MSC injection.
The study reported here accomplishes two goals. First, MSCs injected into the ventricles became widely dispersed throughout the CNS and persisted for 8–10 days post-injection, but failed to engraft and differentiate [36]. To determine if injection directly into neuronal tissue could similarly improve outcome in the twitcher mouse model, MSCs (GFP labeled ASCs and BMSCs) were injected into the striatum of neonatal pups. The striatum is known to be important for motor function, suggesting its potential utility as a target. Second, an expanded spectrum of behavioral tests was performed to evaluate their ability to detect subtle improvements in motor and behavioral function. These results are reported here; studies are ongoing to determine MSC engraftment ability, differentiation potential, and effects on inflammation following striatal injection in these pups.
2. Material and Methods
2.1 Animals
Mice were housed in the AALAC-approved facility at Pennington Biomedical Research Center (PBRC). All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC). A breeding colony of heterozygous “Twitcher” mice carrying a natural mutation in the GALC gene was established using mice obtained from Tulane University, originally from Jackson Laboratories (B6.CE-Galctwi/J stock #000845). Genotyping was performed by PCR from anal swabs performed at weaning according to the molecular beacon protocol developed by Terrell et al. [43].
2.2 Murine eGFPTgASCs and eGFPTgBMSCs
The inbred transgenic strain C57Bl/6-Tg(UBC-GFP) was used to obtain ASCs from inguinal fat pads and BMSCs from bone marrow. These mice express enhanced green fluorescent protein ubiquitously and were a generous gift from Dr. David Welsh of Louisiana State University Health Sciences Center (Jackson Laboratories). All donors were individually euthanized by CO2 at 2–4 months old and cells were subsequently isolated and cultured as previously described [44].
2.3 Stereotaxic Surgery and Striatal Injection of MSCs
Surgeries were performed at PND 3–4 on all pups within a litter. eGFPTgASC and eGFPTgBMSC cells were harvested and resuspended at a concentration of 20,000 cells/ul in Hank’s Buffered Salt Solution (HBSS) (Fisher Scientific, Philadelphia PA). Control pups received an injection of HBSS. Each pup was cryoanesthetized by 10 minutes on ice, then moved to a stereotaxic frame. Preliminary experiments using India ink to confirm the site of injection were performed to determine the striatal injection coordinates, which were anterior-posterior −0.2, mediolateral +/− 1.2, and dorsoventral - 2.5. A pump was used to inject 1ul per hemisphere at a rate of 0.5ul/min, followed by 2 minutes of dwell time through a 30G stainless steel needle (Hamilton, Reno, NV). Following surgery, pups were removed to a heating pad and stimulated until they recovered sufficiently to be returned to their litter.
2.4 Longevity analysis
For purposes of survival measurements, a terminal point of 20% loss of maximum body weight was established. At this point, twitchers were euthanized and tissues collected. Normal littermates were euthanized at 39–42 days, corresponding to the time of euthanasia for the twitchers.
2.5 Behavioral Analysis
Based upon genotyping results, twitcher mice and their normal littermate controls were subjected to a comprehensive series of behavioral tests beginning at 15–18 days of age. They were weaned at 21–22 days old. Behavior tests were performed two times per week for the duration of the lifespan of the twitcher animals. Body weight was measured at each behavior testing time point, and every day for twitchers once they began to lose weight. The behavior tests are described in detail below.
2.5.1 Twitching Frequency and Severity
An observational scale was used to classify both the frequency and severity of twitching during disease progression [36]. For twitching frequency, the scale ranged between 0 and 3, with 0 representing complete absence, and 3 representing constant twitching. The scale for twitching severity was 0 to 4: 0- no twitching, 1- mild vibration, 2- moderate, 3- severe but retains control of head, 4- severe and has lost head control.
2.5.2 Wire Hang
Both forelimb strength and hindlimb control were assessed by wire hang ability, on a scale of 0–4, measuring the ability of the animal to hang onto a wire with their front feet, and to swing their rear legs up to grasp the wire [36]. Scores were assigned as follows: 0- no difficulty, 1- struggle grasping the wire with their rear paws, 2- inability to grasp with rear paws, but can hang by their forelimbs for greater than 3 sec, 3- falls within 3 sec, 4- falls immediately.
2.5.3 Rotarod
Rotarod (Med-Associates, St Albans, VT) was used to assess motor function and coordination. The machine consisted of a slowly accelerating rod, progressing from 4 to 40 rpm during a period of 300 sec, and the latency to fall was recorded for each animal. Mice were given 3 trials per day, with an intertrial interval of 30–40 min. Each trial lasted a maximum of 300 sec. An initial training session, where each mouse was acclimated first to the movement of the rod, then to its acceleration for 1 minute, was conducted the day before the first rotarod session.
2.5.4 Gait Analysis
A video treadmill system and the computer program, Treadscan (CleverSys Inc., Reston, VA), was used to finely assess the development of gait abnormalities with disease progression. For each animal, at each behavior session, 20 seconds of video of the animal walking on the treadmill was recorded. Due to the wide disparity between normal and twitcher mice, and to the rapid decline in walking ability of twitchers, speed was adjusted according to the individual capability of each animal. For normal animals, in order to ensure that the animals were walking, rather than galloping, a maximum speed of 20cm/s was established. Videos were recorded as avi files, then converted to mpg format for analysis. Since the disease typically presents with progressively increasing hindlimb paralysis, analysis focused on the rear limbs only. Each video was individually assessed, and only segments where the animal was walking consistently were selected for Treadscan analysis. After the automated software analysis, results were manually reviewed to ensure a minimum of 5 good strides per leg in order to be included in the dataset. Any stance time and swing time measures that appeared to vary dramatically from other step cycles in the analysis were manually reviewed and deleted if necessary. In addition, 5 footprints per rear paw were manually selected by marking each toe and the heel at the beginning of a step cycle, and these footprints were subsequently analyzed by the Treadscan program. The database feature of Treadscan enabled the export of grouped data by age for each individual parameter for statistical analysis of differences between groups.
2.5.5 Mouse Stone T-maze
In order to assess the ability of the twitchers and their normal littermates to acquire learning and retain the information, they were tested in the newly developed mouse Stone T-maze (STM). Details of this maze have been previously published [45]; however, some modifications were required due to the age and size of the mice. The amount of water in the maze was decreased to approximately 15mm to enable the young mice to wade. In addition, the straight run training protocol for the maze was modified to require only 8 successful trials, rather than the standard 15. The day after straight run training the mice were administered 15 maze trials in a single day. Mice were run in squads where every mouse received its 1st trial prior to the first mouse receiving its 2nd trial. This enabled each mouse to have an approximately 15–25 min intertrial interval during which they were placed into their holding cage with a dry towel. In addition, mice were given even longer breaks if they evinced evidence of fatigue, and food was provided in the bottom of the holding cage during the intertrial interval. Since the motor deficits of twitchers become quite severe after 30 days of age, straight run training was conducted at 21–24 days of age and the maze at 23–26 days of age. Successful completion of a trial in the maze was still determined as completion in less than 300 seconds. If a trial reached 300 sec, the trial was scored as a failure, and 3 failures during the 15 trials resulted in the mouse being removed from further analysis. Maze performance was recorded using Anymaze video software. Errors were counted as any instance in which the nose and ears of an animal entered an incorrect zone. Due to the rapid progression of the disease, the traditional retention period of 1 week was shortened to 2 days, occurring at PND 24–27. A subsequent maze experiment examined older animals at PND 27–29. These animals were not retention tested.
2.6 Statistical Analysis
Results were accepted as significant at or below p=0.05. A priori t-tests were performed for all tasks to test for differences between Twitcher-BMSC and Twitcher-HBSS, and between Twitcher-ASC and Twitcher-HBSS groups. Appropriate degrees of freedom were selected based upon the results of Levene’s test for equality of variances. For survival of different twitcher groups, the Log-rank (Mantel-Cox) test was used in Prism (GraphPad Prism 5). The n values for survival of each group were as follows: twitcher BMSC N=10 (plus 3 animals censored), twitcher ASC N= 8 (plus 3 animals censored), and twitcher HBSS N=13 (plus 7 animals censored). Censored animals occurred either as a result of early, random death, or tissue collection at PND32 for subsequent experiments. All other statistical analysis was performed with SPSS.
For the maze data, a Genotype X Injection X Trial Block ANOVA was used to test for differences between groups. Genotype and Injection were between-subject factors and Trial Block was a repeated measure. Each Trial Block includes 3 trials (1–3, 4–6, 7–9, 10–12 and 13–15), except for retention (RE, 5 trials). Group numbers were as follows: twitcher BMSC N=8, twitcher ASC N=9, twitcher HBSS N=14, normal BMSC N=12, normal ASC N=9, and normal HBSS N=8. The second set of maze data were obtained from uninjected genotypes, and was analyzed by Genotype X Trial Block ANOVA. All groups contained 8 animals. For wire hang, twitching frequency, and twitching severity, twitcher groups were compared using Injection X Age Block ANOVA, with Age Block being a repeated measure. For all other analysis, Genotype X Injection X Age Block ANOVAs were performed with Age Block as a repeated measure. Test results were grouped into 6 day age blocks (T1=15–20 days, T2=21–26 days, T3=27–32 days and T4=33–38 days). Animal numbers varied slightly between age blocks due to interference between maze and behavior testing and to mortality at T4. The range of group numbers was N=9–11 for twitcher BMSC, N=8–9 for twitcher ASC, N=10–18 for twitcher HBSS, N=13–16 for normal BMSC, N=9–13 for normal ASC, and N=8–10 for normal HBSS. For gait analysis, the age blocks were slightly different due to inability to obtain good data prior to day 18 and subsequent to day 35 for twitchers. The gait age blocks were T1=18–23, T2=24–29 and T3=30–35. Group numbers varied slightly between the two toe spread measures and the automated measures because of differences in quality of video required for analysis. For automated gait measures, the group numbers were: twitcher BMSC N=6–9, twitcher ASC N=9, twitcher HBSS N=10–15, normal BMSC N=15, normal ASC N=12–13, and normal HBSS N=7–9. For toe spread measures, group sizes were: twitcher BMSC N=8–9, twitcher ASC N=9, twitcher HBSS N=10–15, normal BMSC N=15, normal ASC N=12–13, and normal HBSS N=7–9.
If significant interactions between Genotype and/or Injection with Age Block were detected, then further analysis at each Age Block was conducted. Specifically, if the overall Genotype X Injection X Age Block interaction was significant, then lower order ANOVA for Genotype X Injection were conducted at each Age Block. If only Age Block X Injection was significant, then 1 way ANOVA for Injection was conducted at each Age Block, followed by Bonferonni post-hoc comparisons when appropriate. If only the Genotype X Injection interaction was significant, then t-tests comparing Genotype at each Age Block were conducted to determine how early differences between twitchers and normals could be detected. Levene’s test for equality of variance was used to determine the appropriate degrees of freedom for t-tests.
2.7 Cell Tracking
In order to assess the persistence and distribution of the injected BMSCs and ASCs, animals were sacrificed at different time points after surgery. One pup was sacrificed immediately after surgery in order to confirm that injected cells could be detected by GFP fluorescence. In addition, 3–4 pups were sacrificed at 8 days post-surgery in each group. These brains were drop-fixed in 4% paraformaldehyde overnight, then cryoprotected in 30% sucrose and frozen in OCT. At 20 days post-surgery, 3 twitchers and 3 normals per group were transcardially perfused under isoflurane anesthesia with PBS and 4% paraformaldehyde. Brains were then fixed and frozen as above. These brains were cryocut into sections 20µm thick, mounted on slides, and examined under a fluorescence microscope for the presence of GFP positive cells.
3. Results
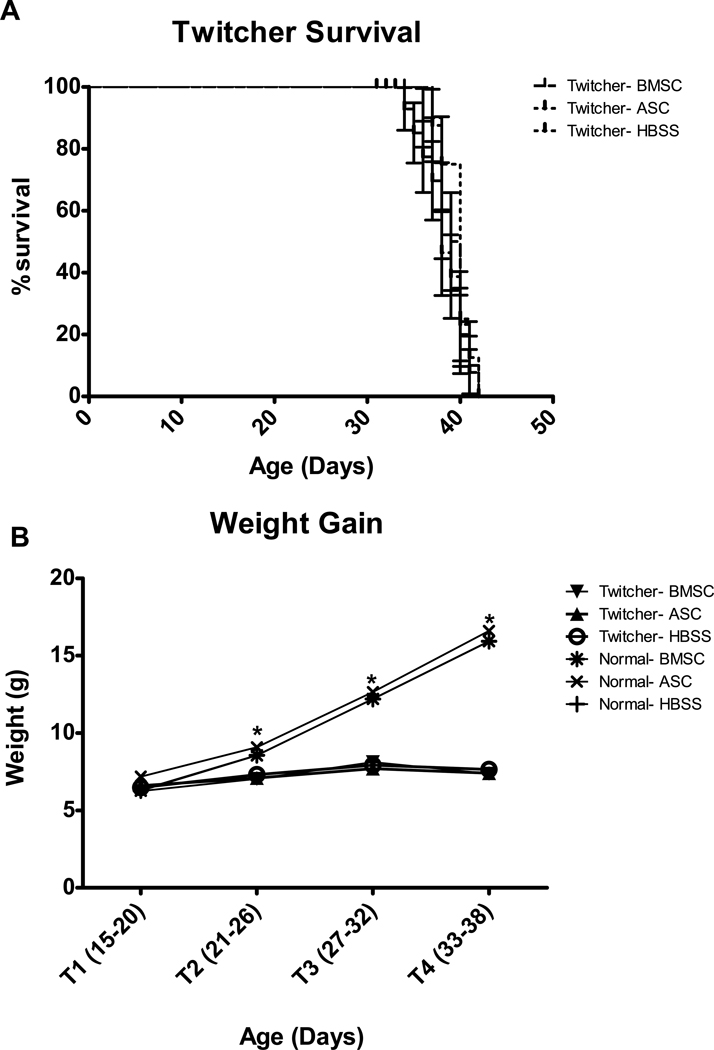
The largest impact of the twitcher genotype is loss of weight and death (a terminal point of 20% weight loss, usually occurring around day 40). Injection of ASC and BMSC had no significant impact on either survival or weight gain of twitchers (Figure 1). While no significant effects of injection were observed on weight gain, the difference between twitchers and normals was pronounced and dependent on age (Genotype X Age Block: (3,162) = 411.6, p < 0.001) and Genotype (F(1,54) = 187.3, p < 0.001). Subsequent t-tests for Genotype at each Age Block revealed that the difference in weight between twitchers and normals became significant at T2, T3 and T4 (t(61.1)= 5.5, p < 0.001, t(59.8) = 12.1, p < 0.001, and t(56.8)= 24.1, p < 0.001, respectively). At T1 there was no significant difference in weight between twitchers and normals (t(61) = 0.55, p = 0.584). The results of a priori t-tests comparing ASC and BMSC twitchers to HBSS twitchers were consistent with these results, showing no significant differences at any time point.
Fig. 1.
Injection of BMSC and ASC did not alter disease progression in twitchers as measured by A) survival analysis using a terminal point of 20% weight loss, and B) weight gain. Genotype differences in weight between twitcher and normal mice became significant at days 21–26 (T2). * indicates significant (p<0.001) difference between twitcher and normal mice. Details of the statistical tests employed are provided in the text.
3.1 Functional tests
3.1.1 Twitching and Wire Hang
Several parameters were examined to investigate the functional implications of striatal injections of BMSCs and ASCs. Twitching begins shortly after weaning in twitcher genotypes, progressing from mild vibrations to severe twitching before the animals reach terminal point. As evident in Figure 2A, there is no significant impact of ASC or BMSC injection on the frequency of twitching compared to HBSS controls (Injection: F(2,27)=0.02, p=0.98). There was a significant Age X Injection interaction (F(6,81)=2.8, p<0.05); however, subsequent ANOVA for Injection at each Age Block failed to detect significant injection effects at any age. A priori t-tests showed the same pattern, with no significant differences at any Age Block.
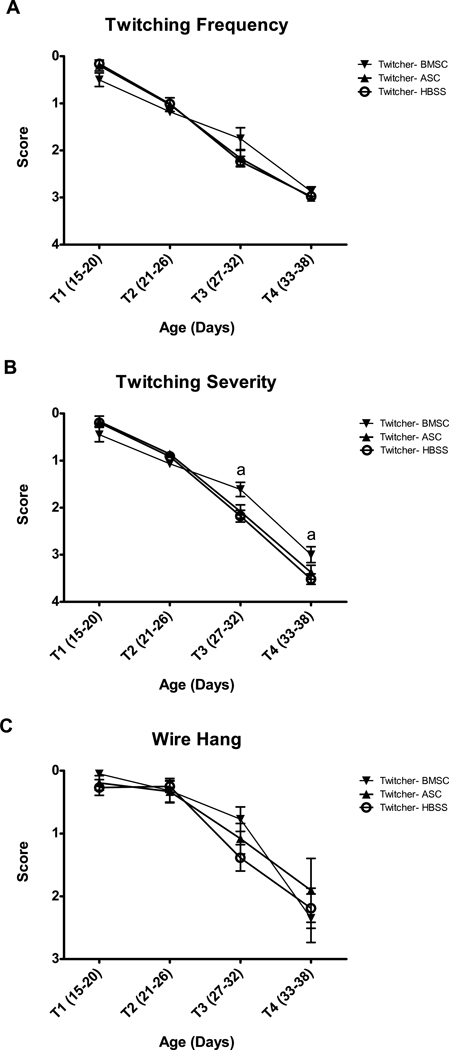
Fig. 2.
BMSC and ASC injection had no effect on A) twitching frequency in twitcher mice. BMSC, but not ASC, injection significantly lessened the severity of twitching (B) between days 27 and 38 (T3 and T4). Neither injection improved performance on the wire hang apparatus (C). ‘a’ denotes significant (p<0.05) difference between BMSC and HBSS twitchers by Bonferroni posthoc analysis and by a priori t-test.
Twitching severity (Figure 2B) was affected by the BMSC injections at T3 and T4. There was no overall Injection effect (F(2,27)=1.6, p=0.217); however, the Age X Injection effect was significant (F(6,81)=5.5, p<0.001). Injection ANOVA at each Age Block showed no significant impact of Injection at T1 or T2, but the Injection effect was significant at both T3 and T4 (F(2,35)=4.6, p=0.017 and F(2,28)=3.8, p=0.036, respectively). Bonferroni post-hoc analysis confirmed that the differences were between the BMSC and HBSS groups (p=0.017 and p=0.034 at T3 and T4), while the differences between ASC and HBSS and between BMSC and ASC were not significant. A priori t-tests confirmed these results, showing a significant difference between BMSC and HBSS twitchers at T3 (t(27)=2.9, p=0.008) and T4 (t(21=2.7, p=0.015), but no other significant differences.
A gross measure of both strength and coordination can be obtained by the wire hang procedure, which measures the animal’s ability to hang with its front paws and pull the rear paws up to grasp the wire. While all normal genotype groups were able to perform this task with ease (data not shown), twitcher genotypes underwent dramatic decline in ability as the disease progressed. Injection of ASC and BMSC was unable to significantly attenuate this decline (Figure 2C). The effect of Injection was not significant (F(2,27)=0.28, p=0.755), nor was there an Age Block X Injection interaction (F(6,81)=0.40, p=0.88). A priori t-tests revealed the same pattern, with only the T3 BMSC vs HBSS twitcher comparison being close to significance (t(27)=2.0, p=0.057).
3.1.2 Rotarod Performance
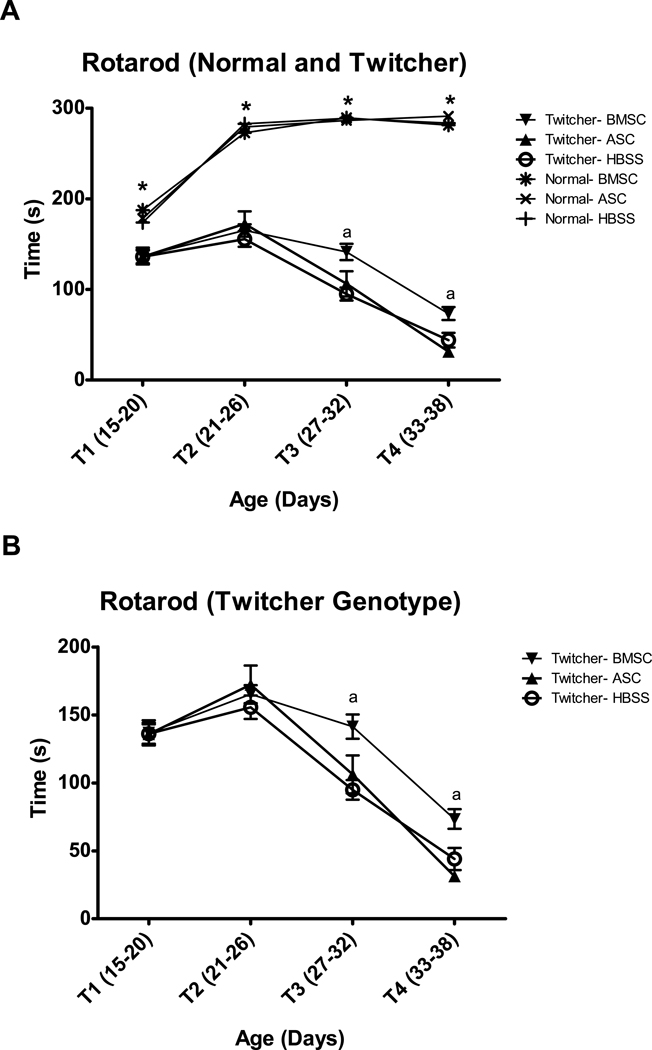
The rotarod is frequently used to measure motor coordination in mice. Despite their young age, both normal and twitcher genotypes were able to perform this task (Figure 3A). The effect of Genotype was significant (F(1,51)=785, p<0.001), although Injection was not (F(2,51)=1.0, p=0.370), and Genotype X Injection X Age Block fell narrowly short of achieving significance (F(6,153)=2.1, p=0.052). Subsequent t-tests for Genotype at each Age Block showed that rotarod performance was capable of discriminating between twitcher and normal genotypes at each time (T1- t(68)=5.7, p<0.001, T2-t(71)=16.4, p<0.001, T3-t(52.3)=25.6, p<0.001, and T4- t(64)=37.7, p<0.001). These results identify rotarod performance as an extremely sensitive measure for detecting early symptoms of functional decline in twitcher mice.
Fig. 3.
Rotarod is a sensitive measure for detecting differences in motor performance between twitcher and normal mice (A), even at the earliest time points. The twitcher groups are presented alone in panel B to improve resolution. BMSC, but not ASC, injection significantly improves rotarod performance of twitchers from days 27–38 (T3 and T4). * indicates significant (p<0.001) difference between twitcher and normal mice. ‘a’ denotes significant (p<0.05) difference between BMSC and HBSS twitchers by a priori t-test.
A priori tests were conducted to examine the expected differences between MSC injected twitchers and HBSS twitcher controls. As evident in Figure 3B, the BMSC, but not the ASC, injection slowed the decline in rotarod performance observed as twitchers age. BMSC twitchers performed significantly better than control HBSS twitchers on the rotarod at PND 27–32 (t(25)=3.9, p=0.001) and at PND 33-38 (t(18)=2.7, p=0.015).
3.2 Genotype Differences by Gait Analysis
Gait analysis is another technique for detection of subtle motor pathology in mouse models, and the development of new video imaging capabilities has enhanced its usefulness. We used an automated software system (Treadscan) to analyze differences in gait between groups. An initial challenge was determining if sufficiently consistent stride patterns could be obtained from these young animals. In our hands, prior to day 18 both twitcher and normal pups were generally non-compliant. After day 35, the motor pathology in twitchers was too severe to allow capture of an adequate period of video data. Several gait parameters were therefore assessed for age blocks of 18–23 (T1), 24–29 (T2) and 30–35 (T3) days. Since the speed at which twitchers were able to walk varied dramatically as the disease progressed, video was captured at different speeds and analysis focused on speed independent measures. Speed-sensitive measures such as swing time were expressed as percentages of stride time in an effort to partially compensate for speed changes, while recognizing that differences in speed do reflect differences in disease progression. In this experimental design, animals were videotaped on the treadmill twice weekly. Such repeated testing in a treadmill task has been shown to alter gait in normal mice [46, 47]; however, it is beyond the scope of the current study to investigate the interaction between the effects of repeat testing and disease progression in twitcher mice. Analysis is rather focused on whether there are detectable differences between normal and twitcher genotypes, and between different twitcher injection groups. In Figures 4 and 5, selected gait measures are presented.
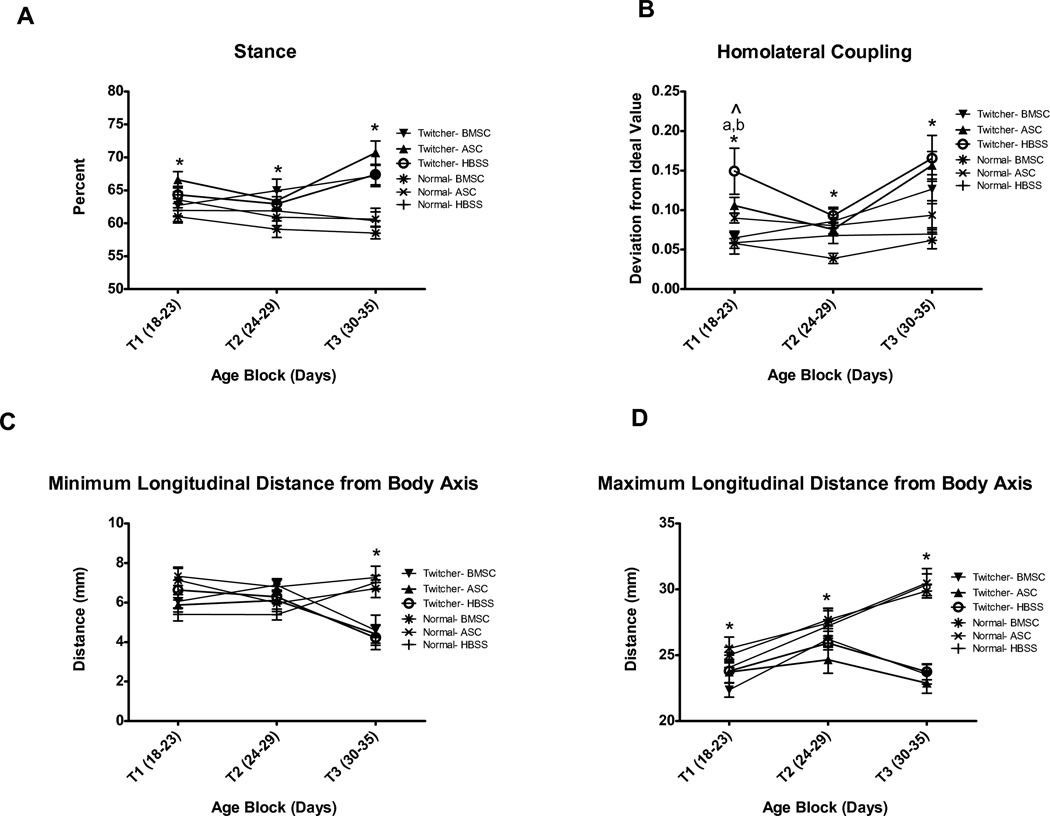
Fig. 4.
Automated gait analysis by Treadscan software is capable of discriminating between twitcher and normal genotypes by several measures. A) Percent Stance is significantly different between twitchers and normals at all ages tested. B) Homolateral coupling detects differences between twitchers and normals at all ages tested, but may be a somewhat variable measure to use prior to T2. C) Minimum longitudinal distance from body axis is less sensitive, discriminating between twitchers and normals only at T3 (days 30–35). D) Maximum longitudinal distance from body axis clearly detects genotype differences at all ages. * indicates significant (p<0.05) difference between twitcher and normal mice. ^ indicates significant (p<0.05) injection effect. ‘a’ denotes significant (p<0.05) difference between BMSC and HBSS twitchers by Bonferroni posthoc analysis and by a priori t-test. ‘b’ denotes significant (p<0.05) difference between BMSC and ASC twitchers by Bonferroni posthoc analysis.
Fig. 5.
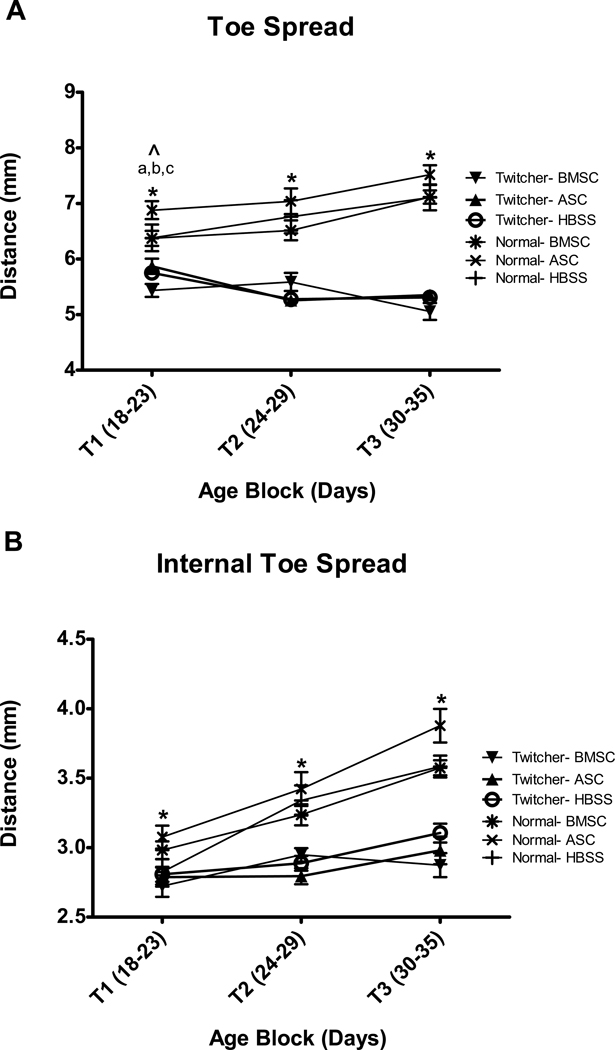
Treadscan analysis of footprint parameters following manual footprint selection is a sensitive means to detect genotype differences. In A) toe spread is significantly different between genotypes at all ages tested, but may be less reliable at T1 (days 18–23) than internal toe spread (B), which appears more consistent at T1. * indicates significant (p<0.001) difference between twitcher and normal mice. ^ indicates significant (p<0.05) injection effect. ‘a’ denotes significant (p<0.05) difference between BMSC and HBSS twitchers by a priori t-test. ‘b’ denotes significant (p<0.05) difference between BMSC and ASC twitchers by Bonferroni posthoc analysis. ‘c’ denotes significant (p<0.05) difference between ASC and HBSS twitchers by Bonferroni posthoc analysis.
3.2.1 Percent Stance and Swing
Percent Stance and Percent Swing reflect the two halves of the gait cycle. Percent Stance (Figure 4A) represents the portion of the stride where the foot is in contact with the floor. Percent Swing (data not shown) is the portion of the stride where the paw is in the air. The effect of Genotype is significant (F(1,50)=29.0, p<0.001), and is influenced by age (Age Block X Genotype: F(2,100)=13.9, p<0.001). T-tests at each Age Block were performed and confirmed that twitchers were significantly different than normals at each age (T1-t(60)=2.3, p=0.027, T2-t(65)=2.9, p=0.005, and T3-t(65)=7.3, p<0.001). The effect of Injection was not significant (F(2,50)=1.0, p=0.374) and it did not significantly interact with Genotype or Age Block. No significant differences were apparent between either BMSC or ASC twitchers and HBSS twitcher controls by a priori t tests.
3.2.2 Stride Coupling
Treadscan computes three different coupling measures (homolateral, homologous and diagonal). We found homolateral -to be the most consistent trait for comparison of twitcher and normal mice (Figure 4B); it reflects the coordinated pattern of movement of the paws on the same side of the body. There was an effect of Genotype (F(1,50)=32.1, p<0.001), and a Genotype X Injection X Age Block interaction (F(4,100)=3.1, p=0.019); however, there was no effect of Injection (F(2,50)=2.5, p=0.096). Genotype X Injection ANOVA at each Age Block was consequently performed. At T1, the effect of both Genotype (F(1,50)=9.3, p=0.004) and Injection (F(2,50)=3.5, p=0.037) was significant, and the two factors interacted (F(2,50)=3.9, p=0.026). Bonferroni posthoc analysis showed a significant difference between ASC and BMSC (p=0.043) and between BMSC and HBSS (p=0.007), but not between ASC and HBSS. At T2, Genotype was still significant (F(1,50)=7.1, p=0.01), but there was no effect of Injection or Age Block X Injection. This pattern was repeated at T3, where the effect of Genotype was significant (F(1,50)=26.2, p<0.001), and Injection and Age Block by Injection were not significant. Consistent with the ANOVA analysis, a priori t-tests show a significant difference between BMSC Twitchers and HBSS Twitchers (t(10)=2.8, p=0.018) at time 18–23, but no subsequent differences. No significant differences between ASC and HBSS twitchers were detected. The pattern for homolateral coupling shows initially a higher deviation from the ideal value for twitcher mice at T1, then they improve at T2, before becoming considerably worse at T3. Further study is warranted to determine if this represents a sensitive detection of developmental delay in gait development, or if it indicates excessive variability of this particular Treadscan measure at this age.
3.2.3 Longitudinal Distance from Body Axis
Minimum (Figure 4C) and maximum (Figure 4D) longitudinal distances (LD) reflect the closest and farthest respectively that the rear limbs got from the body axis. For minimum LD, Genotype is a significant factor (F(1,50)=7.4, p=0.009) and is influenced by Age (Genotype X Age interaction F(2,100)=20.3, p<0.001). There is no effect of Injection (F(2,50)=1.1, p=0.341), nor does Injection interact with Age Block (F(4,100)=0.531, p=0.714). Genotype X Injection is significant (F(2,50)=3.5, p=0.036), but the a priori t-tests reveal no differences between MSC injected and HBSS injected twitchers. Genotype t-tests at each Age Block show a significant difference between twitcher and normal genotypes at T3 (t(65)=5.9, p<0.001); however, this particular gait parameter is not sensitive enough to detect differences at T1 (t(60)=1.5, p=0.140) or T2 (t(65)=0.69, p=0.492). The difference at T3 reflects a tendency of advanced stage twitchers to place their feet farther forward under their body.
Maximum LD appears to be a more sensitive trait to use for evaluation of disease progression in twitchers. Genotype is significant here (F(1,50)=35.9, p<0.001), and influenced by age (Genotype X Age Block, F(2,100)=36.0, p<0.001). There is not a significant effect of Injection, or any interaction between Age Block and Injection. T-tests for Genotype at each Age Block were performed, and show significant differences at all three blocks (T1- t(60)=2.6, p=0.012, T2- t(63.4)=2.7, p=0.01, T3- t(65)=10.9, p<0.001). While maximum longitudinal distance would be expected to increase as animals grow, this trait actually decreases in twitchers after days 24–29 (T2). It is always less in twitchers than normals; however, the difference is moderate, prior to the precipitous drop at days 30–35 (T3). This decrease probably results both from the cessation of growth and from impaired ability to extend and to control their rear limbs. No differences were found between ASC or BMSC twitchers and HBSS twitchers by a priori t-tests.
3.2.4 Toe Spread
Footprint analysis in Treadscan was performed by manually marking the toes and heels for 5 footprints for each rear paw of each video. The software then analyzed the distance between the outermost toes (Toe Spread, Figure 5A) and between the 2nd and 4th toe (Internal Toe Spread, Figure 5B). Both measures increase as normal animals grow.
For Toe Spread, Genotype (F(1,53)=152.3, p<0.001) and Injection (F(2,53)=3.3, p=0.044) are both significant, and interact (Age Block X Genotype X Injection: F(4,106)=4.6, p=0.002). However, Bonferroni posthoc analysis only detects a significant difference between ASC and HBSS (p=0.022). Genotype X Injection ANOVA at each Age Block was performed to further explore the data. At T1 (days 18–23), Genotype (F(1,53)=49.1, p<0.001) and Injection (F(2,53)=6.0, p=0.005) are significant, but do not interact (Genotype by Injection: F(2,53)=1.4, p=0.263). Bonferroni posthoc analysis detects a significant difference between ASC and BMSC (p=0.015) and ASC and HBSS (p=0.021), but not between HBSS and BMSC. Toe Spread actually decreases for twitcher animals after days 18–23, reflecting not only a cessation in growth, but also an inability to spread their toes. At T2, Genotype is significant (F(1,53)=85.8, p<0.001) and interacts with Injection (F(2,53)=4.8, p=0.012), although Injection is not a significant factor (F(2,53)=0.65, p=0.526). At T3, Genotype is significant (F(1,53)=254.8, p<0.001), and there is no significant effect or interaction of Injection. The lack of a consistent injection response suggests that the supposed T1 injection response is unlikely to be linked to a change in progression of disease in twitchers. Indeed, a priori t-tests fail to uncover any significant differences between ASC and HBSS twitchers, while the difference between BMSC and HBSS twitchers is significant only at days 18–23 (T1: t(17)=2.3, p=0.032), when BMSC twitchers actually have a lower toe spread distance.
Internal toe spread essentially remains constant for twitchers, while increasing steadily for normal mice. There is a significant effect of Genotype (F(1,53)=152.3, p<0.001), but not of either Injection (F(2,53)=1.5, p=0.236) or Genotype X Injection interaction (F(2,53)=2.3, p=0.110). The effect of Genotype is influenced by age (Genotype X Age Block: F(2,106)=22.8, p<0.001). T-tests for Genotype at each Age Block show that Internal Toe Spread is capable of differentiating between twitchers and normals at each age tested (T1: t(60)=3.4, p=0.001, T2: t(55.3)=6.7, p<0.001, and T3: t(65)=9.0, p<0.001). A priori t tests show no differences except for between BMSC twitchers and HBSS twitchers at days 30–35 (t(20)=2.1, p=0.050), when the value for BMSC twitchers is slightly less than for HBSS twitchers.
3.3 Mouse Stone T-maze (STM)
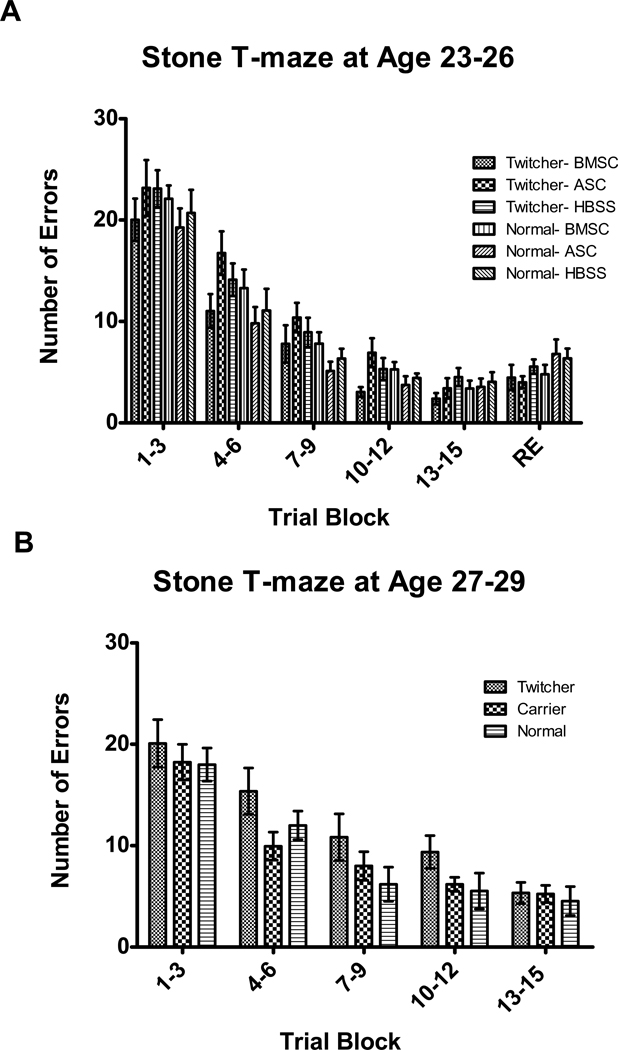
To test whether the demyelination and brain inflammation characteristic of Krabbe disease resulted in impairment of learning in twitcher mice, we used a novel maze task to assess learning [45]. In this task, it is the number of errors at the final Trial Block that indicates learning impairment. To assess whether intrastriatal injection of ASC or BMSC altered learning in twitcher mice, the performance of ASC and BMSC injected twitcher and normal mice was compared to the HBSS injected groups (Figure 6A). These experiments were performed at days 23–26 to allow testing of retention at days 25–29. No Genotype (F(1,53)=0.51, p=0.477) or Injection (F(2,53)=0.87, p=0.424) differences in performance were observed by ANOVA; however, there was a Genotype X Trial Block interaction (F(5,265)=2.9, p=0.014). Consequently, t-tests for Genotype were carried out at Trial Block 13–15 (the last acquisition trials), and at Trial Block RE (the retention trials). There were no significant genotype differences at either Trial Block. A priori t-tests also indicate that there is no difference between BMSC or ASC twitchers and HBSS twitchers at Trial Blocks 13–15 or RE.
Fig. 6.
The mouse Stone T-maze can be used to measure learning ability in young mice; however, neither BMSC nor ASC injection significantly affects learning (A). Twitcher mice have no learning impairments at either days 23–26 (A), or at days 27–29 (B).
It is possible that learning impairments in twitcher mice do not become evident until later ages than the 23–26 days tested in the above experiment. Therefore, the performance of twitcher mice at days 27–29, well into disease progression, was compared to the performance of their unaffected siblings. At 27–29 days of age, twitchers are still able to successfully complete testing in the STM (Figure 6B). There were no significant differences in performance between twitcher mice and their normal and heterozygous siblings (Genotype: F(2,21)=2.8, p=0.086, Genotype X Trial Block: F(8,84)=0.67, p=0.716). These results were confirmed by the results of a priori t-tests for difference between normal and twitcher mice at the last Trial Block (trials 13–15), which showed no significant difference. This indicates that there are no significant impairments in learning in twitcher mice, at least prior to the dramatic physical declines that occur after day 30.
3.4 Cell Tracking
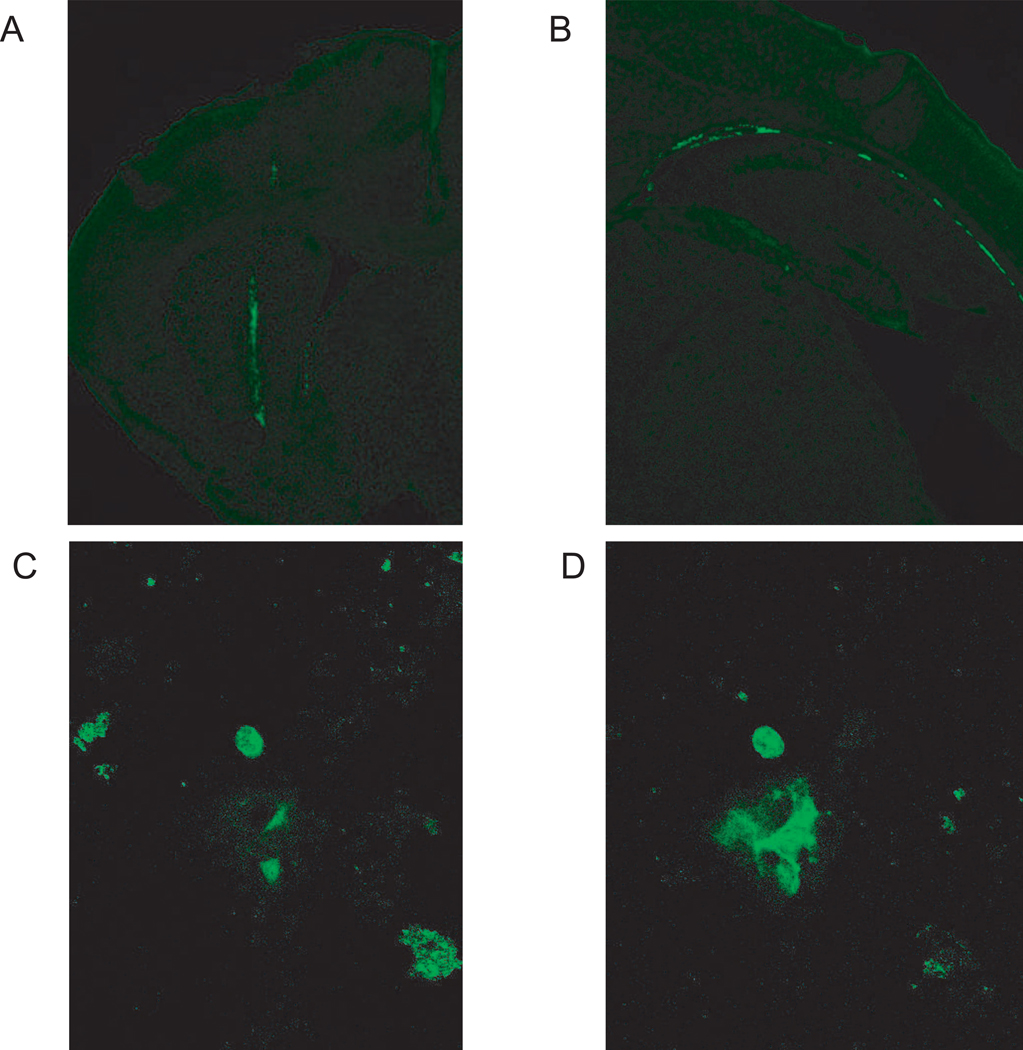
GFP fluorescence was used to detect the presence and distribution of injected ASCs and BMSCs. This approach has been used by Ripoll et al. (36). Immediately after injection, numerous GFP positive cells can be seen at the injection site in the striatum, as well as some GFP cells already moving to other sites in the brain (Figure 7A, 7B). At 8 days post-injection, it was still possible to detect GFP positive cells scattered throughout BMSC-injected brains (Figure 7C); however, there was considerable variation in the number of cells that we were able to detect in different animals. Despite screening numerous slices, we did not find GFP positive cells in ASC-injected animals. At 20 days post-injection, slices from 3 ASC normals, 3 BMSC normals, 3 ASC twitchers, and 3 BMSC twitchers were examined; however, no GFP positive cells were found. It is possible that some ASC and BMSC cells remain, but that GFP fluorescence microscopy is not a sensitive enough technique to detect low numbers of cells. Nevertheless, these results do suggest that there are very few, if any, injected cells remaining by 20 days post-surgery.
Fig. 7.
Detection of injected ASCs and BMSCs by GFP fluorescence. GFP positive cells are apparent immediately after injection into the striatum at 4X magnification (A). Cells can also be detected at more posterior brain locations at this time at 4X (B). At 8 days post-surgery, some BMSC cells could be detected by GFP signal at 20X (representative images in C & D). GFP positive cells were not detected at 20 days post-surgery.
4. Discussion
Intrastriatal injections of MSCs had no impact on either weight gain or lifespan in twitcher mice. However, the PNS is more severely affected in the murine twitcher model than the human disease [48, 49]. Any beneficial effects of the injections would be confined to the CNS. Hence, injections targeted to the striatum could be expected to result in more subtle impacts on disease progression in mice, since the PNS will be unaffected by this intervention. In addition, dependent upon the persistence and engraftment of the injected cells, the impacts could be temporally limited, dissipating as the injected cells disappear. It is for these reasons that it is important to establish sensitive functional measures capable of detecting minor improvements to build upon in future steps towards developing a treatment.
During this study several potential measures of disease progression in twitcher mice were evaluated. Results are summarized in Table 1. Many of these functional tasks have been developed and used primarily in adult mice, so it was important to validate their use for the young twitchers and their normal littermates. Twitching frequency and severity scores are qualitative measures, potentially subject to variation depending upon the observer. Even wire hang scores involve a certain amount of judgment to assess mild impairments at early stages. Rotarod performance was the most sensitive motor task, and was capable of detecting differences not only between twitcher and normal genotypes, but also between BMSC- and HBSS- injected twitchers. Overall, intrastriatal injection of BMSC and ASC cells failed to substantially affect disease progression, so the ability of the rotarod task to detect such subtle differences underscores its potential utility as an early and extremely sensitive detector of motor dysfunction in young mice. The high sensitivity of this assay is particularly encouraging as it is relatively quick to perform, suited to repeat measures, and provides a quantitative, unambiguous measure of performance.
Table 1.
Summary of effects of different behavioral and physiological tests. Differences between twitcher and normal genotypes, and between untreated twitchers and the different twitcher injection groups. NS- not significant, NA- not applicable.
| Behavioral Test | Twitcher vs Normal Effects |
Twitcher vs ASC Treated Twitcher |
Twitcher vs BMSC Treated Twitcher |
|---|---|---|---|
| General Parameters | T1= 15–20 days, T2= 21–26 days, T3= 27–32 days, T4= 33–38 days | ||
| Weight Loss | p<0.001 from T2, T3, T4, NS at T1 | NS | NS |
| Survival | -- | NS | NS |
| Twitching Frequency | -- | NS | NS |
| Twitching Severity | -- | NS | p<0.05 at T3, T4, NS at T1, T2 |
| Wire Hang | -- | NS | NS |
| Rotarod | p<0.001 at T1, T2, T3, T4 | NS | p<0.05 at T3, T4 |
| Gait Parameters | T1= 18–23 days, T2= 24–29 days, T3= 30–35 days | ||
| Percent Stance | p<0.05 at T1, T2,T3 | NS | NS |
| Homolateral Coupling | p<0.01 at T1, T2, T3 | NS | p<0.05 at T1, NS at T2, T3 |
| Minimum Longitudinal Distance | p<0.001 at T3, NS at T1, T2 | NS | NS |
| Maximum Longitudinal Distance | p<0.05 at T1, T2, T3 | NS | NS |
| Toe Spread | p<0.001 at T1, T2, T3 | NS | p<0.05 at T1, NS at T2, T3 |
| Internal Toe Spread | p<0.001 at T1, T2, T3 | NS | p=0.05 at T3, NS at T1, T2 |
| Stone T-maze, days 23–26 | NS | NS | NS |
| Stone T-maze, 27–29 days, uninjected mice | NS | NA | NA |
This study also validated use of the relatively new Treadscan system for detection of motor deficits in developing mice. Prior studies have used adult mice [46, 47, 50]; using the system to compare twitcher and normal mice presented several challenges, including noncompliance prior to day 18, differences in size and the necessity of repeat testing. Twitcher mice were smaller than normal mice, and were not capable of maintaining the same speed, particularly at later stages of the disease. Nevertheless, Treadscan was able to successfully differentiate between normal and twitcher genotypes at early stages of the disease, prior to noticeable gait aberrations. Maximum LD, percent stance and percent swing were all significantly different even at the earliest, day 18–23, timepoint (T1). Coupling measures were more subject to variability due to less consistent performance of the young mice; however, homolateral coupling was able to detect differences between genotypes. Toe spread and internal toe spread were both able to detect genotype differences at early stages, when it was often difficult to obtain long periods of consistent walking behavior. Toe spread results were somewhat inconsistent at the first timepoint (days 18–23). Internal toe spread seemed to give more consistent results than toe spread, while still providing high sensitivity. Since toe spread and internal toe spread measures can be obtained from Treadscan following manual marking of toes, data can be obtained from poorer quality video than is possible with other traits. Of course, this must be balanced against the greater investment in time and labor needed for footprint analysis.
Learning tasks have generally been developed in adult mice. This is the first application of the mouse STM in immature mice; however, they perform well with a few minor modifications, detailed in the methods section. In this task, mice wade through water while learning the correct sequence of turns to navigate the maze. Since performance is measured by number of errors, rather than by latency to escape, learning can be assessed without confounds from impaired motor performance. Twitchers were able to perform well up to day 30, despite considerable motor impairments. The ability of twitchers to perform as well as normal mice may indicate that the striatum is not a primary focal point of disease-related damage. Nevertheless, the validation of this maze task in immature mice is an important demonstration of the task’s potentially widespread utility for assessment of learning ability.
5. Conclusions
Overall, this study demonstrates improvements to rotarod performance and twitching severity at certain stages in twitcher mice following striatal BMSC injection. We have identified rotarod performance and automated gait analysis using the Treadscan program as sensitive tools for monitoring subtle improvements in disease progression for early stage development of potential treatments. The lack of more robust improvements may indicate that the striatum is not an optimal target for MSC injection. Pathology in the twitcher brain begins in the hindbrain and progresses forward, suggesting that the hindbrain may be more important in disease progression than the striatum.
Previous experiments by Ripoll et al. (36) demonstrated physiological improvements, including improvements in weight gain, following intraventricular injections of an equivalent cell dose. They could not detect cells more than 20 days post-surgery. Our analysis of GFP positive cells by microscopy suggests that the cells migrate from the striatum throughout the brain; however, even at 8 days post-surgery, GFP positive cells were present in very low abundance in BMSC animals, and undetectable in ASC animals. Taken together, these two studies suggest that intraventricular targets are a better site for injection than the striatum, even though cells did migrate out from the striatum. Further studies should test both different injection sites, such as the hindbrain, and also higher cell doses to improve persistence and engraftment.
Highlights.
Intrastriatal mesenchymal stromal cell injection in twitcher mouse model of Krabbe.
Minor improvements in rotarod and twitching severity at certain stages.
Automated gait analysis by Treadscan software detects early gait abnormalities.
Successful use of Stone T-maze to assess learning in immature mice.
Acknowledgments
The authors gratefully acknowledge the technical assistance of Gang Yu, Xiying Wang, and Forum Shah. Cynthia Ripoll and Mette Flaat are thanked for helpful advice and discussions. Laura Dallam provided excellent editorial assistance.
Role of the Funding Source
J.M.G., P.J.P., S.E.W., and B.A.B. were supported by grant 1 R21 NS059665 to B.A.B. This work used Animal Metabolism and Behavior Core facilities supported by a NORC Center Grant # 1P30 DK072476 entitled “Nutritional Programming: Environmental and Molecular Interactions” sponsored by NIDDK. Funding entities did not contribute to study design, data analysis, manuscript writing or publication decisions.
Abbreviations
- MSC
mesenchymal stromal cells
- GALC
galactosylceramidase
- BMT
bone marrow transplant
- BMSC
bone marrow stromal cell
- ASC
adipose-derived stromal cell
- GVHD
Graft vs Host disease
- UCB
umbilical cord blood transplantation
- HLA
human leukocyte antigen
- CNS
central nervous system
- PNS
peripheral nervous system
- iNOS
inducible nitric oxide synthase
- GFP
green fluorescent protein
- AAALAC
Association for Assessment and Accreditation of Laboratory Animal Care
- IACUC
Institutional Animal Care and Usage Committee
- PND
postnatal day
- HBSS
Hank’s Buffered Salt Solution
- STM
Stone T-maze
- ANOVA
analysis of variance
- SPSS
Statistical Package for the Social Sciences
- T1
age block 1
- T2
age block 2
- T3
age block 3
- T4
age block 4
- LD
longitudinal distance
- T
twitcher
- N
normal
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Shawna E. Wicks, Email: shawna.wicks@pbrc.edu.
Heaven E. Londot, Email: hlondo1@tigers.lsu.edu.
Bo Zhang, Email: bzhang9@jhu.edu.
Jennifer Dowden, Email: jennifer.dowden@pbrc.edu.
Jessica Klopf-Eiermann, Email: jeierman@tulane.edu.
Jeanne M. Fisher-Perkins, Email: jfisher2@tulane.edu.
Cynthia B. Trygg, Email: ctrygg@tulane.edu.
Brittni A. Scruggs, Email: bscruggs@tulane.edu.
Xiujuan Zhang, Email: xzhang3@tulane.edu.
Jeffrey M. Gimble, Email: jeffrey.gimble@pbrc.edu.
Bruce A. Bunnell, Email: bbunnell@tulane.edu.
Paul J. Pistell, Email: ppistell@towson.edu.
References
- 1.Zlotogora J, Regev R, Hadar S, Iancu TC. Growth pattern in Krabbe's disease. Acta Paediatr Scand. 1986;75:251–254. doi: 10.1111/j.1651-2227.1986.tb10194.x. [DOI] [PubMed] [Google Scholar]
- 2.D'Agostino AN, Sayre GP, Hayles AB. Krabbe's disease. Globoid cell type of leukodystrophy. Arch Neurol. 1963;8:82–96. doi: 10.1001/archneur.1963.00460010098012. [DOI] [PubMed] [Google Scholar]
- 3.Hagberg B, Sourander P, Svennerholm L. Diagnosis of Krabbe's infantile leucodystrophy. J Neurol Neurosurg Psychiatry. 1963;26:195–198. doi: 10.1136/jnnp.26.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gullotta F, Pavone L, Mollica F, Grasso S, Valenti C. Krabbe's disease with unusual clinical and morphological features. Neuropadiatrie. 1979;10:395–400. doi: 10.1055/s-0028-1085341. [DOI] [PubMed] [Google Scholar]
- 5.Sandhoff K. The biochemistry of sphingolipid storage diseases. Angew Chem Int Ed Engl. 1977;16:273–285. doi: 10.1002/anie.197702733. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi T, Shinnoh N, Goto I, Kuroiwa Y. Hydrolysis of galactosylceramide is catalyzed by two genetically distinct acid beta-galactosidases. J Biol Chem. 1985;260:14982–14987. [PubMed] [Google Scholar]
- 7.Wenger DA. Murine, canine and non-human primate models of Krabbe disease. Mol Med Today. 2000;6:449–451. doi: 10.1016/s1357-4310(00)01800-1. [DOI] [PubMed] [Google Scholar]
- 8.Svennerholm L, Vanier MT, Mansson JE. Krabbe disease: a galactosylsphingosine (psychosine) lipidosis. J Lipid Res. 1980;21:53–64. [PubMed] [Google Scholar]
- 9.Igisu H, Suzuki K. Progressive accumulation of toxic metabolite in a genetic leukodystrophy. Science. 1984;224:753–755. doi: 10.1126/science.6719111. [DOI] [PubMed] [Google Scholar]
- 10.Escolar ML, Poe MD, Provenzale JM, Richards KC, Allison J, Wood S, et al. Transplantation of umbilical-cord blood in babies with infantile Krabbe's disease. N Engl J Med. 2005;352:2069–2081. doi: 10.1056/NEJMoa042604. [DOI] [PubMed] [Google Scholar]
- 11.Caniglia M, Rana I, Pinto RM, Fariello G, Caruso R, Angioni A, et al. Allogeneic bone marrow transplantation for infantile globoid-cell leukodystrophy (Krabbe's disease) Pediatr Transplant. 2002;6:427–431. doi: 10.1034/j.1399-3046.2002.02026.x. [DOI] [PubMed] [Google Scholar]
- 12.Kurtzberg J, Laughlin M, Graham ML, Smith C, Olson JF, Halperin EC, et al. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. N Engl J Med. 1996;335:157–166. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- 13.Laughlin MJ, Eapen M, Rubinstein P, Wagner JE, Zhang MJ, Champlin RE, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265–2275. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- 14.Laughlin MJ, Barker J, Bambach B, Koc ON, Rizzieri DA, Wagner JE, et al. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. N Engl J Med. 2001;344:1815–1822. doi: 10.1056/NEJM200106143442402. [DOI] [PubMed] [Google Scholar]
- 15.Duffner PK, Caviness VS, Jr, Erbe RW, Patterson MC, Schultz KR, Wenger DA, et al. The long-term outcomes of presymptomatic infants transplanted for Krabbe disease: report of the workshop held on July 11 and 12, 2008, Holiday Valley, New York. Genet Med. 2009;11:450–454. doi: 10.1097/GIM.0b013e3181a16e04. [DOI] [PubMed] [Google Scholar]
- 16.Lin D, Donsante A, Macauley S, Levy B, Vogler C, Sands MS. Central nervous system-directed AAV2/5-mediated gene therapy synergizes with bone marrow transplantation in the murine model of globoid-cell leukodystrophy. Mol Ther. 2007;15:44–52. doi: 10.1038/sj.mt.6300026. [DOI] [PubMed] [Google Scholar]
- 17.Dolcetta D, Perani L, Givogri MI, Galbiati F, Amadio S, Del Carro U, et al. Design and optimization of lentiviral vectors for transfer of GALC expression in Twitcher brain. J Gene Med. 2006;8:962–971. doi: 10.1002/jgm.924. [DOI] [PubMed] [Google Scholar]
- 18.Pellegatta S, Tunici P, Poliani PL, Dolcetta D, Cajola L, Colombelli C, et al. The therapeutic potential of neural stem/progenitor cells in murine globoid cell leukodystrophy is conditioned by macrophage/microglia activation. Neurobiol Dis. 2006;21:314–323. doi: 10.1016/j.nbd.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Shen JS, Watabe K, Ohashi T, Eto Y. Intraventricular administration of recombinant adenovirus to neonatal twitcher mouse leads to clinicopathological improvements. Gene Ther. 2001;8:1081–1087. doi: 10.1038/sj.gt.3301495. [DOI] [PubMed] [Google Scholar]
- 20.Meng XL, Shen JS, Watabe K, Ohashi T, Eto Y. GALC transduction leads to morphological improvement of the twitcher oligodendrocytes in vivo. Mol Genet Metab. 2005;84:332–343. doi: 10.1016/j.ymgme.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Croitoru-Lamoury J, Williams KR, Lamoury FM, Veas LA, Ajami B, Taylor RM, et al. Neural transplantation of human MSC and NT2 cells in the twitcher mouse model. Cytotherapy. 2006;8:445–458. doi: 10.1080/14653240600879152. [DOI] [PubMed] [Google Scholar]
- 22.Biswas S, Biesiada H, Williams TD, LeVine SM. Substrate reduction intervention by L-cycloserine in twitcher mice (globoid cell leukodystrophy) on a B6;CAST/Ei background. Neurosci Lett. 2003;347:33–36. doi: 10.1016/s0304-3940(03)00633-5. [DOI] [PubMed] [Google Scholar]
- 23.Biswas S, LeVine SM. Substrate-reduction therapy enhances the benefits of bone marrow transplantation in young mice with globoid cell leukodystrophy. Pediatr Res. 2002;51:40–47. doi: 10.1203/00006450-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Galbiati F, Givogri MI, Cantuti L, Rosas AL, Cao H, van Breemen R, et al. Combined hematopoietic and lentiviral gene-transfer therapies in newborn Twitcher mice reveal contemporaneous neurodegeneration and demyelination in Krabbe disease. J Neurosci Res. 2009;87:1748–1759. doi: 10.1002/jnr.22006. [DOI] [PubMed] [Google Scholar]
- 25.Kagitani-Shimono K, Mohri I, Fujitani Y, Suzuki K, Ozono K, Urade Y, et al. Anti-inflammatory therapy by ibudilast, a phosphodiesterase inhibitor, in demyelination of twitcher, a genetic demyelination model. J Neuroinflammation. 2005;2:10. doi: 10.1186/1742-2094-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pannuzzo G, Cardile V, Costantino-Ceccarini E, Alvares E, Mazzone D, Perciavalle V. A galactose-free diet enriched in soy isoflavones and antioxidants results in delayed onset of symptoms of Krabbe disease in twitcher mice. Mol Genet Metab. 100:234–240. doi: 10.1016/j.ymgme.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 27.Le Blanc K, Ringden O. Mesenchymal stem cells: properties and role in clinical bone marrow transplantation. Curr Opin Immunol. 2006;18:586–591. doi: 10.1016/j.coi.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Le Blanc K, Ringden O. Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:321–334. doi: 10.1016/j.bbmt.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Puissant B, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129:118–129. doi: 10.1111/j.1365-2141.2005.05409.x. [DOI] [PubMed] [Google Scholar]
- 30.Ringden O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lonnies H, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 31.Porada CD, Zanjani ED, Almeida-Porad G. Adult mesenchymal stem cells: a pluripotent population with multiple applications. Curr Stem Cell Res Ther. 2006;1:365–369. doi: 10.2174/157488806778226821. [DOI] [PubMed] [Google Scholar]
- 32.McIntosh K, Zvonic S, Garrett S, Mitchell JB, Floyd ZE, Hammill L, et al. The immunogenicity of human adipose-derived cells: temporal changes in vitro. Stem Cells. 2006;24:1246–1253. doi: 10.1634/stemcells.2005-0235. [DOI] [PubMed] [Google Scholar]
- 33.Yanez R, Lamana ML, Garcia-Castro J, Colmenero I, Ramirez M, Bueren JA. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells. 2006;24:2582–2591. doi: 10.1634/stemcells.2006-0228. [DOI] [PubMed] [Google Scholar]
- 34.Safford KM, Hicok KC, Safford SD, Halvorsen YD, Wilkison WO, Gimble JM, et al. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem Biophys Res Commun. 2002;294:371–379. doi: 10.1016/S0006-291X(02)00469-2. [DOI] [PubMed] [Google Scholar]
- 35.Safford KM, Safford SD, Gimble JM, Shetty AK, Rice HE. Characterization of neuronal/glial differentiation of murine adipose-derived adult stromal cells. Exp Neurol. 2004;187:319–328. doi: 10.1016/j.expneurol.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 36.Ripoll CB, Flaat M, Klopf-Eiermann J, Fisher-Perkins JM, Trygg CB, Scruggs BA, et al. Mesenchymal-Lineage Stem Cells Have Pronounced Anti-Inflammatory Effects in the Twitcher Mouse Model of Krabbe's Disease. Stem Cells. 2011;29:67–77. doi: 10.1002/stem.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzuki K. The twitcher mouse. A model of human globoid cell leukodystrophy (krabbe's disease) Am J Pathol. 1983;111:394–397. [PMC free article] [PubMed] [Google Scholar]
- 39.Hoogerbrugge PM, Poorthuis BJ, Romme AE, van de Kamp JJ, Wagemaker G, van Bekkum DW. Effect of bone marrow transplantation on enzyme levels and clinical course in the neurologically affected twitcher mouse. J Clin Invest. 1988;81:1790–1794. doi: 10.1172/JCI113521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeager AM, Brennan S, Tiffany C, Moser HW, Santos GW. Prolonged survival and remyelination after hematopoietic cell transplantation in the twitcher mouse. Science. 1984;225:1052–1054. doi: 10.1126/science.6382609. [DOI] [PubMed] [Google Scholar]
- 41.Jacobs JM, Scaravilli F, De Aranda FT. The pathogenesis of globoid cell leucodystrophy in peripheral nerve of the mouse mutant twitcher. J Neurol Sci. 1982;55:285–304. doi: 10.1016/0022-510x(82)90127-7. [DOI] [PubMed] [Google Scholar]
- 42.Taniike M, Suzuki K. Spacio-temporal progression of demyelination in twitcher mouse: with clinico-pathological correlation. Acta Neuropathol. 1994;88:228–236. doi: 10.1007/BF00293398. [DOI] [PubMed] [Google Scholar]
- 43.Terrell KA, Rasmussen TA, Trygg C, Bunnell BA, Buck WR. Molecular beacon genotyping for globoid cell leukodystrophy from hair roots in the twitcher mouse and rhesus macaque. J Neurosci Methods. 2007;163:60–66. doi: 10.1016/j.jneumeth.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ripoll CB, Bunnell BA. Comparative characterization of mesenchymal stem cells from eGFP transgenic and non-transgenic mice. BMC Cell Biol. 2009;10:3. doi: 10.1186/1471-2121-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pistell PJ, Ingram DK. Development of a water-escape motivated version of the Stone T-maze for mice. Neuroscience. 2010;166:61–72. doi: 10.1016/j.neuroscience.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wooley CM, Xing S, Burgess RW, Cox GA, Seburn KL. Age, experience and genetic background influence treadmill walking in mice. Physiol Behav. 2009;96:350–361. doi: 10.1016/j.physbeh.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wooley CM, Sher RB, Kale A, Frankel WN, Cox GA, Seburn KL. Gait analysis detects early changes in transgenic SOD1(G93A) mice. Muscle Nerve. 2005;32:43–50. doi: 10.1002/mus.20228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Powell HC, Knobler RL, Myers RR. Peripheral neuropathy in the Twitcher mutant. A new experimental model of endoneurial edema. Lab Invest. 1983;49:19–25. [PubMed] [Google Scholar]
- 49.Suzuki K. The twitcher mouse: a model for Krabbe disease and for experimental therapies. Brain Pathol. 1995;5:249–258. doi: 10.1111/j.1750-3639.1995.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 50.Beare JE, Morehouse JR, DeVries WH, Enzmann GU, Burke DA, Magnuson DS, et al. Gait analysis in normal and spinal contused mice using the TreadScan system. J Neurotrauma. 2009;26:2045–2056. doi: 10.1089/neu.2009.0914. [DOI] [PMC free article] [PubMed] [Google Scholar]