Abstract
Poor fetal growth is associated with decrements in muscle strength likely due to changes during myogenesis. We investigated the association of poor fetal growth with muscle strength, fatigue resistance, and the response to training in the isolated quadriceps femoris. Females (20.6 yrs) born to term but below the 10th percentile of ponderal index (PI)-for-gestational-age (LOWPI, n=14) were compared to controls (HIGHPI, n=14), before and after an 8-week training. Muscle strength was assessed as grip-strength and as the maximal isometric voluntary contraction (MVC) of the quadriceps femoris. Muscle fatigue was assessed during knee extension eercise. Body composition and the maximal oxygen consumption (VO2max) were also measured. Controlling for fat free mass (FFM), LOWPI versus HIGHPI women had ~11% lower grip-strength (P=0.023), 9–24% lower MVC values (P=0.042 pre-trained; P=0.020 post-trained), a higher rate of fatigue (pre- and post-training), and a diminished training response (P=0.016). Statistical control for FFM increased rather than decreased strength differences between PI groups. The PI was not associated with VO2max or measures of body composition. Strength and fatigue decrements strongly suggest that poor fetal growth affects the pathway of muscle force generation. This could be due to neuromotor and/or muscle morphologic changes during development e.g., fiber number, fiber type, etc. Muscle from LOWPI women may also be less responsive to training. Indirectly, results also implicate muscle as a potential mediator between poor fetal growth and adult chronic disease, given muscle’s direct role in determining insulin resistance, type II diabetes, physical activity, and so forth.
Keywords: Fetal programming, intrauterine growth retardation, myogenesis, grip-strength, birth-weight, fatigability, developmental origins of health and disease
INTRODUCTION
An association of early life conditions, specifically, low birth weight (BW), low ponderal index (PI, gm/cm3), and infant catch-up growth, with obesity and increased chronic disease later in life is well documented at the population level (1–6). The dominant explanation is the “fetal origins” hypothesis, which suggests that during critical periods of development, environmental stressors (especially under-nutrition) resulting in intrauterine growth retardation (IUGR), reprogram developmental pathways that permanently alter adult metabolism and subsequent chronic disease risk. These so-called fetal programming (FP) effects are well documented on body composition in a wide range of age groups. For example, studies of infants (7), children/adolescents (8, 9), and adults (10–17), all show that FP results in a reduction in the relative amount of lean tissue compared to body fat. Skeletal muscle may be particularly susceptible to FP effects as restricted nutrients tend to be partitioned preferentially to the heart, brain, and other more vital organ systems. Further, myogenesis is sensitive to environmental perturbation, occurs very early during embryo formation, and ultimately determines a number of relatively fixed traits in adulthood including the muscle fiber number, the fiber size, and the fiber type distribution (18–20). With respect to nutrient restriction, the animal data are consistent showing reductions in muscle fiber number as well as increases in type I versus type II fibers in adulthood (21–28). Such developmental changes on muscle morphology could underlie the well-known association of fetal growth restriction with insulin resistance and type II diabetes, as insulin resistance is fundamentally a characteristic of skeletal muscle (29, 30). Morphological/metabolic changes could also have implications for exercise performance and physical activity levels in both children and adults.
Several studies have investigated the association of FP with muscle strength and/or muscle performance (29, 31–35). Of these, three large-scale population studies showed positive associations of BW with grip strength, even after adjustment for various adult body size covariates (31–33). For example, the study by Kuh et al. (33) showed a positive association of BW with grip-strength even after adjustment for age, sex, and adult height. The effect was present in both men and women, and a 1-kg difference in BW in the cohort meant going from the 10th to 80th percentile of the grip-strength distribution. These effects are likely qualitative and independent of muscle size, as is suggested by two direct studies of isolated muscle function. In a 31P magnetic resonance spectroscopy (31PMRS) study of the isolated working flexor digitorum superficialis, low PI at birth was associated with reduced exercise duration and a faster rate of phosphocreatine (PCR) depletion (34). Another study by the same research group, using near-infrared spectroscopy, showed an increase in the rate of forearm muscle reoxygenation in low PI subjects after finger flexion exercise (29). In both studies, the authors concluded that low PI is associated with a delay in the activation of glycolysis/glycogenolysis at the commencement of strenuous muscle contraction, thus resulting in a rapid depletion of PCR stores.
Of the aforementioned studies, nearly all evaluated the performance of the finger flexors only as opposed to the performance of a larger isolated muscle group. Only one of these (35) evaluated a fitness outcome, including the performance on a static arm-pull test and a vertical jump test, but neither measure was associated with the BW and neither measure effectively isolated the performance of a single working muscle group. In the case of vertical jumping, other factors, including technique, are probably also important. To date, no human FP study has evaluated the fatigue characteristics of muscle, although hind-limb muscle fatigue has been evaluated in vivo in new-porn piglets (21). Also, no study has evaluated the responsiveness of skeletal muscle to resistance and/or endurance training. The latter issue is interesting with reference to the original thrifty-phenotype hypothesis (36), whereby FP is viewed as an adaptive response to fetal nutritional stress that results in a decreased investment in muscle given the high energy costs of muscle maintenance. It is not known whether the thrifty phenotype persists after muscle resistance training, or whether the phenotype resists the normal hypertrophy of skeletal muscle to an endurance or resistance training program.
The overall goal of this study was to determine whether birth-markers of FP were associated with the strength, fatigue resistance, and response to training of a large-muscle mass (the quadriceps femoris muscle complex). We focused on the PI as a marker of FP given the studies described above, which were successful at detecting FP effects in relatively modest samples of subjects. BW, as a marker of FP, may be more problematic in correctly identifying IUGR infants as not all IUGR babies are small-for-gestational-age, and not all small-for-gestational-age babies are small due to growth restriction. Also, the PI as a marker of thinness at birth appears to be better associated with catch-up growth in the first year of life and with obesity and insulin resistance later in adulthood (2, 37), also hallmarks of the FP complex. To achieve these study goals, twenty-eight college-aged women were recruited to participate in an 8-week strength and resistance training program. Fourteen of these women were identified as falling below the 10th percentile of the ponderal index (PI)-for-gestational age (38), a cutoff which has relatively high sensitivity and specificity to predict IUGR (39, 40). Fourteen of the women served as age, sex, and physical activity matched controls. The strength and fatigue characteristics of the right quadriceps femoris were evaluated pre- and post-training using a modified static-dynamic knee extension protocol that was first described by Lewis and Fulco (41). This protocol assesses both the maximal isometric strength of the quadriceps femoris, as well as progressive muscle fatigue following dynamic knee-extension exercise. In addition to this protocol, pre-training, post-training, and change (Δ) values with training for the aerobic capacity (VO2max) and the body composition were evaluated for each participant.
METHODS
Subjects and recruitment
Twenty-eight female undergraduates born to term (>37 weeks to 42 weeks gestation) were recruited into the study on the basis of birth measures. For the formation of study groups, participants were compared to sex specific reference curves of the ponderal index (PI)-for-gestational age (38). Fourteen were at or below the 10th percentile of PI-for-gestational age (LOWPI, 2.2±0.1 gm/cm3) and fourteen were in the upper normal range (>10 percentile, HIGHPI, 2.7±0.1 gm/cm3). Participants were required to document birth measures via a hospital record and/or a birth certificate. Gestational-age was determined from the documented due date, if it was available in the medical record, or if not, from maternal recall. Maternal recall of birth measures have proven reliable for both clinical and epidemiological use (42), and our data showed the expected increase in both BW and PI with increasing gestational age from 37 to 42 weeks compared to standard reference populations (38, 43). All participants were interviewed to obtain a medical history. Exclusion criteria included current pregnancy or pregnancy within the previous year, asthma, cardiovascular disease, diabetes, musculoskeletal problems that would have contraindicated participation in the training program or study protocols, and anemia measured via a spot measure of hemoglobin concentration from finger-tip blood using a point-of-care hemoglobin analyzer (Hemocue, Angelholm, Sweden). This study was approved by the Institutional Review Board (IRB) of the University at Albany, SUNY. Participants gave written informed consent and were compensated for their time.
Study Design
On entry into the study and before performance evaluation, diet and physical activity patterns were assessed. To evaluate activity patterns, participants wore GT1M Actigraph accelerometers (Pensacola, Fla.) during waking hours over 3 continuous days (2 weekdays and 1 weekend day). Accelerometer count data were processed according to the 2-stage regression model of Crouter et al. (44) to produce MET values of daily energy expenditure. Diet was assessed via the diary method and detailed instructions were given on how to record all caloric intake over 2 weekdays and 1 weekend day. The dietary data were analyzed by the same investigator using the N2 Nutrition IV software package (N-Squared Computing, Salem, Oregon). After baseline evaluations, participants completed an 8-week training program with pre- and post-training evaluations of body composition, aerobic capacity, and muscle strength/fatigue characteristics.
Training
Training consisted of 1-hour sessions, three times a week, for eight weeks, with the goal of increasing VO2max and the strength of the quadriceps femoris muscle complex. The first 40 minutes of each session consisted of interval training on a stationary spin-bicycle. Participants wore heart-rate monitors and were required to keep heart rate values within prescribed limits during structured intervals that included warm-up/warm-down periods, as well as intervals of varying intensity that lasted from 1–4 minutes. In total, training was structured so that 25% of time was spent in light cycling (heart rate range at 50–70% of VO2max), 50% of time in moderate cycling (70–80% of VO2max), and 25% in hard cycling (80–90% of VO2max). Following cycling, participants performed knee extension resistance weight training on a standard knee-extension weight-bench (3 sets of 15 repetitions each, with approximately 5 minutes between sets). Participants were allowed to select the external weight settings for knee extension training and were instructed/encouraged that the final extensions of the final set should be a challenge to complete. Accordingly, participants were instructed/encouraged to increase the weight as their muscle strength increased over the training period. All participants kept a training log, and all participants completed the 24 sessions of the training program. For the measures described below the same equipment and protocols were used both pre- and post-training.
Body composition
Body density was determined by hydrodensitometry. The underwater weight was obtained using a suspended seat attached to an LC105 250/S Omega beam load cell force transducer (Omega Engineering, Stamford, Connecticut). The load cell was calibrated before and after each measurement and a continuous force signal was acquired by an REM/400M data acquisition system (CB Sciences, New Hampshire). Subjects were required to exhale to residual volume before submerging, and the underwater-weight was ascertained once stable and replicable weight values were obtained from multiple trials (typically 5–10 trials per subject). The residual volume (RV) was measured outside of the water tank in a seated position using an oxygen dilution technique (Wilmore et al. 1980). The Siri equation (45) was used to calculate body fat percentage from body density and fat and fat-free mass (FFM) were calculated from the total body-weight.
VO2max
Aerobic capacity was measured on a stationary cycle ergometer (Monarch 874E) using an incremental protocol. Subjects started with a workload of 1.0 kilogram resistance at 70 rpm for 3 minutes. For the second workload (also 3 minutes), resistance was incremented by 0.5 kilograms. For the third workload (2 minutes), resistance was incremented by 0.5 kg. Thereafter, the workload was incremented every minute by 0.25 kg until subject volitional fatigue. VO2max was defined as the highest level of oxygen consumption averaged over the final minute of the test concomitant with at least two of the following conditions: 1) a non-linear increase in exercise ventilation resulting in a respiratory exchange ratio greater than 1.10, 2) a plateau in the VO2-work rate relationship, or 3) a maximal heart rate within 10% of the age predicted maximum. During VO2 testing, subjects breathed through a low resistance breathing valve (Hans-Rudolph). The expired ventilation (VE, l·min−1 BTPS) as well as the fractional concentrations of O2 and CO2 in the expired air were processed by a Parvo-medics TrueMax metabolic measuring system (Sandy, Utah) to produce 1 minute interval calculations of VO2. Gas analyzers were calibrated with standard gases before each exercise test. The pneumotach used to measure ventilatory flow was also calibrated prior to each test with a 3-liter calibration syringe. Heart rate (HR) was continuously monitored via telemetry (Polar Electric Oy, Sweden) interfaced with the metabolic measuring system.
Muscle strength and muscle fatigue
A grip-strength test was used to determine the strength of the finger-flexors using a Lafayette hand-grip dynamometer (model 78010). Each participant was given 3 opportunities to produce a maximal grip-strength value using their dominant hand, and the best of these values was taken as the maximal grip-strength. In the leg, muscle strength and fatigue were determined in the isolated right quadriceps femoris following a modification of the protocol of Lewis and Fulco (24). Subjects were seated on a platform with their right leg affixed to a minimal friction cable-pulley system that suspended a variable weight load. A padded foot harness was used for the connection of foot-to-cable. An LC105 250/S Omega beam load cell force transducer (Omega Engineering, Stamford, Connecticut) was attached to the cable immediately behind the foot, and a continuous force signal was acquired by an REM/400M data acquisition system (CB Sciences, New Hampshire). In addition, a PT101 precision potentiometer position transducer (Nordisk Transducer Teknik, Hadsund, Denmark) was attached to the cable-pulley system to measure the distance of dynamic knee extension. A “stop” mechanism was introduced behind the force transducer with the purpose of preventing cable movement. This stop mechanism was activated to fix the leg at a 90° angle for the measurement of force output during maximal voluntary static (isometric) contraction of the quadriceps femoris (see below). Work rate (kg·m·s−1 converted to Joules) during dynamic knee extension was calculated by integration of the continuous force curve over time and the distance measurement.
To begin the protocol participants were required to execute three repeat maximal voluntary contractions (MVCs), defined as the maximal isometric muscle force measured with the leg at a fixed position of 90°. An individual MVC lasted from 3–5 seconds and a 1-minute rest interval was given between trials. The maximal MVC was taken as the best of these three repeat efforts. Based on the maximal MVC, weight was applied to the pulley system such that dynamic knee extensions would produce a peak force of approximately 20% of maximal MVC. The fatigue protocol proceeded over three minutes by alternating 1-minute bouts of dynamic (concentric) knee extension exercise (from 90° up to 150° and back every 2 seconds i.e., 0.5 Hz) with static isometric MVC measurements. Muscle fatigue was ascertained as the decrease in MVC efforts over time.
Statistical Analysis
All outcome measures were evaluated for normality using the Kolmogorov-Smirnov test against a standard normal distribution using the Lilliefors 2-tail probability and were not significantly different from normal. ANOVA and ANCOVA were used to test for mean differences by PI-group. When appropriate, additional covariates were introduced e.g., gestational age, FFM, etc., as explained in the Results section below. A repeated measures ANCOVA was used to test for overall PI-group differences in grip-strength and MVC force during the leg-kick protocol, with PI as the between-subjects factor and strength over time as the within-subjects factor. For all ANCOVA analyses, variables representing the response to training (Δ training) were evaluated by controlling for the baseline (pre-training) values of these measures. This is the suggested approach for dealing with “change” variables, rather than performing analyses on percentage change or absolute change from baseline (46). For all tests, statistical significance was indicated at P≤0.05 for both main and interaction effects. Values are presented in both tables and figures as means ± SEM. All statistical analyses were performed using SPSS statistical software, version 17.0 for Macintosh (SPSS Inc., Chicago, Il).
RESULTS
The characteristics of study participants, including their birth/perinatal information, are given by study group in Table 1. The LOWPI group had lower PI due to both increased BL (p=0.012) and a trend towards decreased BW (p=0.255). In contrast, LOWPI and HIGHPI subgroups were well-matched on a number of other potential confounders including age, gestational age, birth order, maternal height, maternal age, subject height, body mass index (m/kg2), and pre-study physical activity i.e., energy expenditure (METs). Also, in the week preceding entry into the study, there were no significant differences detected between PI-groups in the caloric intake of carbohydrates, fats, or proteins expressed either as kcals/day or as a percentage of the overall caloric intake (data not shown).
Table 1.
General chacteristics of study samples
| LOWPI | HIGHPI | 1 p-values | |
|---|---|---|---|
| (n=14) | (n=14) | ||
| Age, yrs | 20.9 ± 0.6 | 20.4 ± 0.3 | 0.395 |
| BW, grams | 3073.3 ± 151.5 | 3287.6 ± 104.3 | 0.255 |
| BL, cm | 51.7 ± 0.6 | 49.5 ± 0.5 | 0.012 |
| PI, gm.cm-1 | 2.2 ± 0.1 | 2.7 ± 0.1 | <0.001 |
| Gestational Age, days | 275.3 ± 2.6 | 278.4 ± 2.4 | 0.383 |
| Birth order | 1.8 ± 0.2 | 2.0 ± 0.2 | 0.506 |
| maternal ht, cm | 164.0 ± 1.7 | 165.4 ± 1.8 | 0.576 |
| Maternal age, yrs | 28.9 ± 1.1 | 29.9 ± 1.1 | 0.539 |
| Subject Ht, cm | 161.7 ± 0.9 | 160.3 ± 1.5 | 0.449 |
| BMI, wt/ht2 | 24.4 ± 1.0 | 22.9 ± 0.7 | 0.246 |
| 2METs | 1.63 ± 0.04 | 1.59 ± 0.05 | 0.587 |
P-values from ANOVA
MET values are the average of three 24-hour days of activity monitoring
Body composition
As expected, body composition was responsive to training. While the total body weight changed minimally, the overall mean FFM of participants increased by 0.7 kg (P<0.01), and body fat percentage decreased by 1.1 percentage points (P<0.01). However, LOWPI vs. HIGHPI subgroups did not differ significantly for pre-training, post-training, or Δtraining values of body composition (Table 2).
Table 2.
Body composition and body composition changes with training
| LOWPI | HIGH PI | 1 P-values | |
|---|---|---|---|
| Wt, Kg-pre | 63.5 ± 2.8 | 58.8 ± 2.4 | 0.218 |
| Wt, Kg-post | 63.6 ± 2.8 | 58.9 ± 2.4 | 0.210 |
| 2Δ Wt | 0.2 ± 0.9 | 0.3 ± 1.0 | 0.969 |
| FFM-pre, kg | 42.2 ± 1.3 | 41.0 ± 1.4 | 0.538 |
| FFM-post, kg | 42.9 ± 1.3 | 41.7 ± 1.2 | 0.590 |
| Δ FFM | 0.7 ± 0.2 | 0.7 ± 0.4 | 0.994 |
| Body fat-pre, % | 32.8 ± 1.9 | 29.7 ± 1.3 | 0.198 |
| Body fat-post. % | 31.8 ± 1.9 | 28.6 ±1.4 | 0.189 |
| Δ Body fat | −1.0 ± 0.6 | −1.2 ± 0.3 | 0.826 |
| VO2max-pre, 1·min−1 | 2.00 ± 0.10 | 2.01 ± 0.07 | 0.898 |
| VO2max-post, 1·min−1 | 2.17 ± 0.10 | 2.26 ± 0.07 | 0.498 |
| Δ VO2max | 0.17 ± 0.04 | 0.25 ± 0.06 | 0.274 |
| VO2max-pre, 1·min−1·Kg-FFM−1 | 47.4 ± 2.1 | 49.2 ± 1.3 | 0.467 |
| VO2max-post, 1·min−1·Kg-FFM−1 | 50.5 ± 1.8 | 54.2 ± 1.0 | 0.082 |
| Δ VO2max | 3.1 ± 0.8 | 5.0 ± 1.3 | 0.226 |
P-values from ANOVA
ANCOVA models testing for change (Δ) control for the pre-training value.
Aerobic capacity
As expected, VO2max expressed as l·min−1 or ml·min·kg-FFM−1 increased by ~9% with training (P<0.01, Table 2). However, there were no significant PI-group differences in pre-training, post-training, or Δtraining values, with the exception of a non-significant trend towards higher post-training ml·min·kg-FFM−1 values in HIGHPI women (P=0.082).
Muscle strength
Maximal grip-strength and quadriceps femoris MVC values are given in Table 3, both as absolute values and after adjustment for FFM. It should be emphasized that we did not specifically train the finger-flexors during the 8-week training program, and while grip strength values were slightly higher after training, the increase was not significant (P=0.23). In contrast, the quadriceps femoris was trained in both a general manner (via cycling) and in a manner specific to the leg kick protocol (via knee extension resistance sets). Thus as expected, maximal MVC values post-training were significantly higher than pre-training values (P<0.01). For grip strength, the unadjusted mean values were not significantly different between LOWPI and HIGHPI groups despite a trend to lower grip-strength in the LOWPI women (Table 3A). However, when grip-strength values were adjusted for FFM (Table 3B), the difference between PI groups increased and became significant post-training (P=0.025) and nearly significant pre-training (P=0.059). A repeated measures analysis using both grip-strength measures and controlling for FFM, was significant at P=0.023. For the quadriceps femoris, maximal MVC values in a rested state tended to be lower in LOWPI women, and the difference increased after adjustment for the FFM, but these differences did not reach statistical significance. However, during the fatigue protocol (Fig 1.), MVC differences between groups became significant as described below.
Table 3.
Muscle strength values before and after training
| LOWPI | HIGHPI | 1 P-values | |
|---|---|---|---|
| A. Unadjusted mean values | |||
| Gripstrength-pre, kg | 27.11 ± 1.24 | 29.68 ± 1.37 | 0.177 |
| Gripstrength-post, kg | 27.57 ± 1.00 | 30.69 ± 1.65 | 0.112 |
| Δ Gripstrength, kg | 0.46 ±0.95 | 0.88 ± 0.52 | 0.707 |
| MVC-pre, kg | 28.4 ± 1.6 | 29.4 ± 1.0 | 0.435 |
| MVC-post, kg | 29.9 ± 1.4 | 31.6 ± 1.6 | 0.597 |
| 2 Δ MVC, kg | 1.5 ± 0.8 | 2.2 ± 0.9 | 0.557 |
| B. Marginal means adjusted for FFM | |||
| Gripstrength-pre, kg | 26.84 ± 1.12 | 30.10± 1.20 | 0.059 |
| Gripstrength-post, kg | 27.30 ± 1.13 | 30.99 ± 1.17 | 0.025 |
| Δ Gripstrength, kg | 0.46 ± 0.78 | 0.89 ± 0.81 | 0.708 |
| MVC-pre, kg | 28.2 ± 1.2 | 30.0 ± 1.3 | 0.396 |
| MVC-post, kg | 29.6 ± 1.4 | 32.0 ± 1.4 | 0.230 |
| Δ MVC, kg | 1.4 ± 0.9 | 2.0 ± 0.9 | 0.521 |
P-values from ANCOVA.
ANCOVA models testing for change (Δ) control for the pre-training value.
Fig 1.
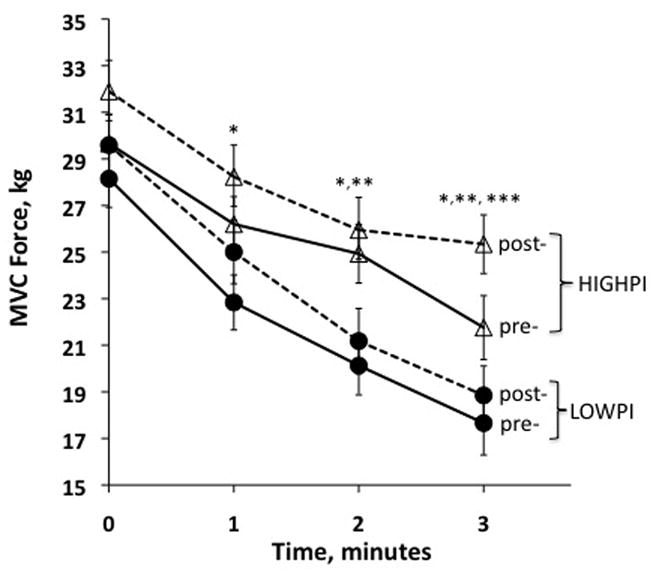
The maximal voluntary contraction (MVC) force decline of the quadriceps femoris during a fatigue protocol that alternated static measures of MVC with dynamic bouts of knee extension exercise (see Methods). The LOWPI and HIGHPI women are indicated by filled circles and open triangles, respectively. The training response is indicated by a solid-line (pre-training) versus a dashed line (post-training). The MVC measurements at time zero represent the maximal strength of the quadriceps femoris in a rested state. Overall, the MVC values plotted are the marginal means controlling for the FFM. *Significant differences between PI-groups for in the post-trained state. **Significant differences between PI-groups in the pre-trained stated. ***Significant differences between PI-groups in the change (Δ) of MVC after training.
Muscle Fatigue
Results from the leg-kick muscle fatigue protocol are shown in Fig. 1. The MVC values shown at time 0 are the same maximal MVC values from Table 3. Thereafter, subsequent (lower) MVC values at times 1, 2 and 3 minutes, are those produced after bouts of fatiguing dynamic knee extension exercise as described in the Methods. Overall, in absolute terms and adjusting for the FFM, MVC values were clearly lower in the LOWPI vs. HIGHPI women, both in the pre-trained (P=0.042) and post-trained (P=0.020) states. As the protocol progressed, the difference between PI groups widened, with significant time-point differences in MVC values emerging at minutes 2 and 3 (pre-training) and minutes 1, 2 and 3 (post-training). The training increase in MVC was similar between PI groups at times 0, 1, and 2, but HIGHPI women had a significantly greater increase at time 3 (1.08 vs. 3.69 kg in LOWPI vs. HIGHPI, respectively, P=0.016). Like the grip strength values, when MVC values were adjusted for the FFM, the magnitude of the difference between PI groups increased and the P-values decreased.
Associations with BW
Because both the BW and the PI have been used in previous studies as markers of FP, and because there is little consensus in the literature as to which birth measure is better, the associations with BW were also explored. For these analyses, the entire sample was used in a multivariate approach with BW as a continuous variable. Additional covariates were introduced into statistical models as appropriate e.g., gestational age, FFM, etc. These analyses failed to reveal any significant associations between BW and the study outcomes, pre-training, post-training, or in response to training. The observed power for these analyses was relatively low (~0.25), in part due to the distribution of BWs in the sample. That is, the mean BW for the total sample was 3,180±92 grams, similar to the female birth value in many reference populations, and only one participant was born below 2,500 grams, which is usually taken as the clinical cut-off for low BW.
Discussion
In young adult women who likely experienced IUGR, this study showed lower grip-strength and quadriceps femoris MVC strength values, both in absolute terms (for the MVC) and adjusting for the FFM (grip strength and MVC). In addition to the strength differences, LOWPI women showed evidence of diminished strength responsiveness to training and lower fatigue resistance during dynamic knee extension exercise. In contrast, a low PI at birth was not associated with the pre-, post- or Δtraining values of VO2max or body composition, including the increase in the mean FFM and the decrease in the body-fat percentage that our participants experienced with training. None of the study outcome measures were associated with BW, although the BW analysis was exploratory and the sample size and range of BW values were not sufficient to provide high statistical power. In general, our findings are similar to the findings of several other larger epidemiological studies that have suggested decreases in adult muscle strength as a consequence IUGR and FP. Our study extends these findings by demonstrating strength deficits by the PI as opposed to BW, and by showing these deficits in a large muscle mass apart from the finger-flexors. In addition to the strength effects, our results suggest that poor fetal growth may also be related to a diminished training responsiveness of muscle and a decreased resistance to fatigue.
The epidemiological studies that preceded this study showed that low BW was associated with decrements in adult grip strength independent of body size i.e., the strength differences persisted even after adjustment for later-life height and weight (31–33, 47). This led Kuh et al. to conclude that strength differences were due at least in part to qualitative effects of FP on muscle morphology during myogenesis (33). However, adjusting for adult height or weight as a proxy for adult muscle mass is not a perfect solution to support this conclusion. A muscle’s force generating capacity is strongly related to its cross-sectional area, and covariate measures of adult body-size can only capture some of this causal relationship. In other words, it is possible that the reduced strength values with FP reflect a reduced muscle mass per se rather than morphological changes during muscle development. This is particularly true given the well-documented effect of FP to reduce adult lean body mass relative to the fat mass (10–17). However muscle size differences are likely not the explanation for the reduced muscle strength values of the LOWPI women in our study. First, the LOWPI women were well-matched to control participants on adult body size and body composition. Second, the grip-strength and MVC differences actually increased after adjustment for the FFM, and the FFM is certainly a much better proxy for the adult muscle mass than is height or weight. Although we did not have a direct measure of the contracting muscle mass, the increased strength of association after control for the FFM strongly suggests morphological and/or neuromotor differences between the LOWPI and HIGHPI women.
With respect to the fatigue protocol, inspection of Fig. 1 clearly reveals lower MVC values in LOWPI women, both before and after training. These differences can be viewed as an extension of the overall strength differences discussed above. The fatigue resistance of the quadriceps femoris was assessed as the rate of decline of MVC over time. As the protocol progressed, LOWPI women showed an accelerated rate of muscle fatigue (both pre- and post-training), and there is also some indication that they were less responsive to 8-weeks of training. That is, only the HIGHPI women showed any evidence of improvement in fatigue resistance after training, although this conclusion is driven mostly by a significant group difference in Δtraining at minute 3 of the protocol. These results contrast with the only other study of fatigue resistance in the literature using an animal model of FP (21). In that study, new-born piglets with IUGR had better fatigue resistance during isometric contractions of the hindlimb plantar flexors using an in vivo muscle stimulation preparation. The authors attributed this difference to a higher proportion of type I (fatigue resistant fibers) in the IUGR group. However, the study made no adjustments for the large absolute force and muscle size differences between the IUGR and control piglets, and so the results are difficult to interpret in a size independent manner. Also, the research model differed as muscle characteristics were assessed at birth rather than in adulthood.
There are several lines of research that are relevant with respect to understanding how poor fetal growth could lead to a size-independent deficit in muscle strength and fatigue resistance. In humans, one line of research shows that IUGR has qualitative effects on neuromotor development (48, 49). These neurophysiologic studies are at a very early stage, but several motor deficits have been described with IUGR, including altered corticospinal excitability in adulthood (49). Unfortunately, to date, these deficits have not been directly linked to the force generating capacity of the skeletal muscle. In animals, another line of research shows that poor fetal growth induced by nutrient restriction reduces muscle fiber number, fiber density, muscle capillary density, and alters fiber-type proportions (22, 24, 25, 28). Changes in fiber-type proportions, in particular, could affect many aspects of human physical performance given the fundamental differences between fiber types in force generating capacity, metabolic characteristics, and fatigue resistance (50). For example, type I fibers are slow twitch, have a higher oxidative capacity, generate less force, and are more resistant to fatigue compared to type IIx (IIb) and IIa fibers. The animal literature is reasonably consistent in this area showing that fetal nutrient restriction reduces the total muscle fiber number, primarily as a consequence of a reduction in the formation of secondary versus primary muscle fibers during myogenesis (23, 24, 51–53). Because secondary muscle fibers are the precursors of adult type II fibers (54), fetal nutrient restriction may also increase the proportion of type I to type II fibers in neonatal animals (21, 27, 28). The overall reduction in muscle fibers formed during development may in turn also lead to compensatory increases in muscle fiber size which enhances the conversion of type I to type IIa and IIx (IIb) fiber types (22, 28, 55). Only one study in the human literature has evaluated muscle fiber-types on the basis of BW (30). In that study, young men born below the 10th percentile for BW-for-gestational age showed an increased proportion of type IIx fibers at the expense of IIa fibers, with no significant differences in the proportion of type I fibers. In humans, IIx fibers express a higher glycolytic and lower oxidative capacity compared to type IIa fibers, and also fatigue more readily. Thus a shift towards type IIx fibers in our LOWPI women could explain the decrease in fatigue resistance, but not necessarily the deficits in muscle strength.
Only two studies in the literature, by the same research group, have directly interrogated human muscle function at the metabolic level (29, 34). These researchers used the same basic research design that we used here with a focus on the PI rather than BW as a marker of IUGR and FP. However, low PI subjects were defined as those in the lower half of an opportunistic sample, rather than identified a priori as those falling below a recognized cutoff for IUGR using an external reference population. Additionally, gestational age was not taken into account. Nevertheless, the study results were significant and informative. One study, of flexor digitorum superficialis, using 31PMRS, showed that low PI was associated with reduced exercise duration and a faster rate of phosphocreatine (PCR) depletion (34). The other study, using near-infrared spectroscopy, showed an increase in the forearm muscle reoxygenation rate in low PI subjects after finger flexion exercise. In both studies it was concluded that low PI subjects have a delayed activation of glycolysis/glycogenolysis at the onset of hard exercise stressing the anaerobic system, and thus deplete PCR stores more readily. As a general result, a rapid depletion of muscle PCR stores would certainly help explain the decrease in the fatigue resistance of LOWPI women, but some distinction must be made between the onset of exercise in a small muscle mass (i.e., PCR depletion over the course of seconds) compared to the more dynamic state-state conditions of knee-extension exercise using a large muscle mass. Although our protocol lasted only 3 minutes, the knee extension work rate was held constant (at about 1000 J/s) and required about 40% percent of a subject’s VO2max once a steady-state was achieved i.e., after the 2nd minute. This is very different from the onset conditions that are evaluated by 31PMRS, and so direct comparisons should be made with caution.
An alternate possibility to explain the strength and fatigability differences between our PI study groups is that they differed at baseline in their habitual or leisure time physical activity patterns and thus the training status of their muscle groups. This possibility cannot be ruled out but it is not likely as subjects were not engaged in any specific resistance training for at least 1-year prior to the study. In addition, the LOWPI and HIGHPI subgroups were well-matched on prior activity levels and had nearly identical baseline 24-hour daily energy expenditure values (METs, Table 1) and baseline VO2max values (Table 3). Additionally, we would have expected a greater training response in LOWPI if they had been poorly trained, and that was clearly not the case. However, because several studies now suggest that poor fetal growth results in reduced levels of physical activity in rats and humans (56–58), we took the additional step of introducing MET values into statistical models as a potential mediating variable. This step did not substantively change our study results, nor was there any evidence that physical activity mediated the association of PI with muscle strength or fatigue resistance.
In summary, this study shows clear deficits in muscle strength and fatigue resistance in college-aged women who were born with a PI lower than the 10th percentile for-gestational-age. At this level, the PI is a sensitive marker of IUGR, and thus our case-control approach offers a valid model for testing FP effects in human subjects. Because of the size independence of these performance deficits, it is likely that FP alters aspects of muscle morphology and/or metabolism during fetal myogenesis. However, the direct studies to test this hypothesis have yet to be conducted in humans, including studies that quantify muscle cross-sectional area, fiber-type, fiber proportion, capillarity, and so forth. Our study also suggests that poor fetal growth leads to a decreased responsiveness of muscle to a training stimulus. Although this is a tentative conclusion, if true, there are additional public health issues to consider. Poor fetal growth by itself constitutes a significant risk factor for adult chronic disease. A powerful way to attenuate that risk is to prescribe exercise as part of an active lifestyle. However, it could be that the baseline risk for chronic disease with IUGR is compounded by the additional risk of having a non-responsive muscle phenotype in adulthood, with implications for exercise performance, physical activity, and compliance with physical activity interventions.
Acknowledgments
The authors would like to thank the research subjects for their time and effort, Diane Sisto from the Albany Medical Center, and Walter Ensel from the Center for Social and Demographic analysis, University at Albany, SUNY. This research was supported by NIH 1 R03 HD055314 to T.D.B and T.B.G. The study sponsors had no role in the study design, in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Abbreviations
- FFM
fat free mass
- MVC
maximum voluntary isometric contraction
- FP
fetal programming
- IUGR
intrauterine growth retardation
- 31PMRS
31P magnetic resonance spectroscopy
- PCR
phosphocreatine
- BW
birth weight
- PI
ponderal index
- Δ
change with training
- VO2max
maximal O2 consumption
- MET
metabolic equivalent
Footnotes
Conflict of interest statement: None to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fall CH, Osmond C, Barker DJ, Clark PM, Hales CN, Stirling Y, et al. Fetal and infant growth and cardiovascular risk factors in women. Bmj. 1995 Feb 18;310(6977):428–32. doi: 10.1136/bmj.310.6977.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips DI, Barker DJ, Hales CN, Hirst S, Osmond C. Thinness at birth and insulin resistance in adult life. Diabetologia. 1994 Feb;37(2):150–4. doi: 10.1007/s001250050086. [DOI] [PubMed] [Google Scholar]
- 3.Barker DJ, Martyn CN, Osmond C, Hales CN, Fall CH. Growth in utero and serum cholesterol concentrations in adult life. Bmj. 1993 Dec 11;307(6918):1524–7. doi: 10.1136/bmj.307.6918.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phipps K, Barker DJ, Hales CN, Fall CH, Osmond C, Clark PM. Fetal growth and impaired glucose tolerance in men and women. Diabetologia. 1993 Mar;36(3):225–8. doi: 10.1007/BF00399954. [DOI] [PubMed] [Google Scholar]
- 5.Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993 Jan;36(1):62–7. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- 6.Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, et al. Fetal and infant growth and impaired glucose tolerance at age 64. Bmj. 1991 Oct 26;303(6809):1019–22. doi: 10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lapillonne A, Braillon P, Claris O, Chatelain PG, Delmas PD, Salle BL. Body composition in appropriate and in small for gestational age infants. Acta Paediatr. 1997 Feb;86(2):196–200. doi: 10.1111/j.1651-2227.1997.tb08868.x. [DOI] [PubMed] [Google Scholar]
- 8.Labayen I, Moreno LA, Blay MG, Blay VA, Mesana MI, Gonzalez-Gross M, et al. Early programming of body composition and fat distribution in adolescents. J Nutr. 2006 Jan;136(1):147–52. doi: 10.1093/jn/136.1.147. [DOI] [PubMed] [Google Scholar]
- 9.Singhal A, Wells J, Cole TJ, Fewtrell M, Lucas A. Programming of lean body mass: a link between birth weight, obesity, and cardiovascular disease? Am J Clin Nutr. 2003 Mar;77(3):726–30. doi: 10.1093/ajcn/77.3.726. [DOI] [PubMed] [Google Scholar]
- 10.Eriksson J, Forsen T, Tuomilehto J, Osmond C, Barker D. Size at birth, fat-free mass and resting metabolic rate in adult life. Horm Metab Res. 2002 Feb;34(2):72–6. doi: 10.1055/s-2002-20518. [DOI] [PubMed] [Google Scholar]
- 11.Gale CR, Martyn CN, Kellingray S, Eastell R, Cooper C. Intrauterine programming of adult body composition. J Clin Endocrinol Metab. 2001 Jan;86(1):267–72. doi: 10.1210/jcem.86.1.7155. [DOI] [PubMed] [Google Scholar]
- 12.Kahn HS, Narayan KM, Williamson DF, Valdez R. Relation of birth weight to lean and fat thigh tissue in young men. Int J Obes Relat Metab Disord. 2000 Jun;24(6):667–72. doi: 10.1038/sj.ijo.0801211. [DOI] [PubMed] [Google Scholar]
- 13.Kensara OA, Wootton SA, Phillips DI, Patel M, Jackson AA, Elia M. Fetal programming of body composition: relation between birth weight and body composition measured with dual-energy X-ray absorptiometry and anthropometric methods in older Englishmen. Am J Clin Nutr. 2005 Nov;82(5):980–7. doi: 10.1093/ajcn/82.5.980. [DOI] [PubMed] [Google Scholar]
- 14.Loos RJ, Beunen G, Fagard R, Derom C, Vlietinck R. Birth weight and body composition in young women: a prospective twin study. Am J Clin Nutr. 2002 Apr;75(4):676–82. doi: 10.1093/ajcn/75.4.676. [DOI] [PubMed] [Google Scholar]
- 15.Loos RJ, Beunen G, Fagard R, Derom C, Vlietinck R. Birth weight and body composition in young adult men--a prospective twin study. Int J Obes Relat Metab Disord. 2001 Oct;25(10):1537–45. doi: 10.1038/sj.ijo.0801743. [DOI] [PubMed] [Google Scholar]
- 16.Phillips DI. Relation of fetal growth to adult muscle mass and glucose tolerance. Diabet Med. 1995 Aug;12(8):686–90. doi: 10.1111/j.1464-5491.1995.tb00570.x. [DOI] [PubMed] [Google Scholar]
- 17.Yliharsila H, Kajantie E, Osmond C, Forsen T, Barker DJ, Eriksson JG. Body mass index during childhood and adult body composition in men and women aged 56–70 y. Am J Clin Nutr. 2008 Jun;87(6):1769–75. doi: 10.1093/ajcn/87.6.1769. [DOI] [PubMed] [Google Scholar]
- 18.Maltin CA, Delday MI, Sinclair KD, Steven J, Sneddon AA. Impact of manipulations of myogenesis in utero on the performance of adult skeletal muscle. Reproduction. 2001 Sep;122(3):359–74. doi: 10.1530/rep.0.1220359. [DOI] [PubMed] [Google Scholar]
- 19.Gollnick PD, Timson BF, Moore RL, Riedy M. Muscular enlargement and number of fibers in skeletal muscles of rats. J Appl Physiol. 1981 May;50(5):936–43. doi: 10.1152/jappl.1981.50.5.936. [DOI] [PubMed] [Google Scholar]
- 20.Hoh JF. Myogenic regulation of mammalian skeletal muscle fibres. News Physiol Sci. 1991 Feb;6:1–6. doi: 10.1152/physiologyonline.1991.6.1.1. [DOI] [PubMed] [Google Scholar]
- 21.Wank V, Bauer R, Walter B, Kluge H, Fischer MS, Blickhan R, et al. Accelerated contractile function and improved fatigue resistance of calf muscles in newborn piglets with IUGR. Am J Physiol Regul Integr Comp Physiol. 2000 Feb;278(2):R304–10. doi: 10.1152/ajpregu.2000.278.2.R304. [DOI] [PubMed] [Google Scholar]
- 22.Zhu MJ, Ford SP, Means WJ, Hess BW, Nathanielsz PW, Du M. Maternal nutrient restriction affects properties of skeletal muscle in offspring. J Physiol. 2006 Aug 15;575(Pt 1):241–50. doi: 10.1113/jphysiol.2006.112110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ward SS, Stickland NC. Why are slow and fast muscles differentially affected during prenatal undernutrition? Muscle Nerve. 1991 Mar;14(3):259–67. doi: 10.1002/mus.880140310. [DOI] [PubMed] [Google Scholar]
- 24.Quigley SP, Kleemann DO, Kakar MA, Owens JA, Nattrass GS, Maddocks S, et al. Myogenesis in sheep is altered by maternal feed intake during the peri-conception period. Anim Reprod Sci. 2005 Jul;87(3–4):241–51. doi: 10.1016/j.anireprosci.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Costello PM, Rowlerson A, Astaman NA, Anthony FE, Sayer AA, Cooper C, et al. Peri-implantation and late gestation maternal undernutrition differentially affect fetal sheep skeletal muscle development. J Physiol. 2008 May 1;586(9):2371–9. doi: 10.1113/jphysiol.2008.150987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dwyer CM, Madgwick AJ, Ward SS, Stickland NC. Effect of maternal undernutrition in early gestation on the development of fetal myofibres in the guinea-pig. Reprod Fertil Dev. 1995;7(5):1285–92. doi: 10.1071/rd9951285. [DOI] [PubMed] [Google Scholar]
- 27.Bauer R, Gedrange T, Bauer K, Walter B. Intrauterine growth restriction induces increased capillary density and accelerated type I fiber maturation in newborn pig skeletal muscles. J Perinat Med. 2006;34(3):235–42. doi: 10.1515/JPM.2006.042. [DOI] [PubMed] [Google Scholar]
- 28.Fahey AJ, Brameld JM, Parr T, Buttery PJ. The effect of maternal undernutrition before muscle differentiation on the muscle fiber development of the newborn lamb. J Anim Sci. 2005 Nov;83(11):2564–71. doi: 10.2527/2005.83112564x. [DOI] [PubMed] [Google Scholar]
- 29.Thompson CH, Sanderson AL, Sandeman D, Stein C, Borthwick A, Radda GK, et al. Fetal growth and insulin resistance in adult life: role of skeletal muscle morphology. Clin Sci (Lond) 1997 Mar;92(3):291–6. doi: 10.1042/cs0920291. [DOI] [PubMed] [Google Scholar]
- 30.Jensen CB, Storgaard H, Madsbad S, Richter EA, Vaag AA. Altered skeletal muscle fiber composition and size precede whole-body insulin resistance in young men with low birth weight. J Clin Endocrinol Metab. 2007 Apr;92(4):1530–4. doi: 10.1210/jc.2006-2360. [DOI] [PubMed] [Google Scholar]
- 31.Sayer AA, Cooper C. Fetal programming of body composition and musculoskeletal development. Early Hum Dev. 2005 Sep;81(9):735–44. doi: 10.1016/j.earlhumdev.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Sayer AA, Cooper C, Evans JR, Rauf A, Wormald RP, Osmond C, et al. Are rates of ageing determined in utero? Age Ageing. 1998 Sep;27(5):579–83. doi: 10.1093/ageing/27.5.579. [DOI] [PubMed] [Google Scholar]
- 33.Kuh D, Bassey J, Hardy R, Aihie Sayer A, Wadsworth M, Cooper C. Birth weight, childhood size, and muscle strength in adult life: evidence from a birth cohort study. Am J Epidemiol. 2002 Oct 1;156(7):627–33. doi: 10.1093/aje/kwf099. [DOI] [PubMed] [Google Scholar]
- 34.Taylor DJ, Thompson CH, Kemp GJ, Barnes PR, Sanderson AL, Radda GK, et al. A relationship between impaired fetal growth and reduced muscle glycolysis revealed by 31P magnetic resonance spectroscopy. Diabetologia. 1995 Oct;38(10):1205–12. doi: 10.1007/BF00422370. [DOI] [PubMed] [Google Scholar]
- 35.Te Velde SJ, Twisk JW, Van Mechelen W, Kemper HC. Birth weight, adult body composition, and subcutaneous fat distribution. Obes Res. 2003 Feb;11(2):202–8. doi: 10.1038/oby.2003.32. [DOI] [PubMed] [Google Scholar]
- 36.Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992 Jul;35(7):595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 37.Holmes GE, Miller HC, Hassanein K, Lansky SB, Goggin JE. Postnatal somatic growth in infants with atypical fetal growth patterns. Am J Dis Child. 1977 Oct;131(10):1078–83. doi: 10.1001/archpedi.1977.02120230024003. [DOI] [PubMed] [Google Scholar]
- 38.Lehingue Y, Remontet L, Munoz F, Mamelle N. Birth ponderal index and body mass index reference curves in a large population. American Journal of Human Biology. 1998;10:327–40. doi: 10.1002/(SICI)1520-6300(1998)10:3<327::AID-AJHB8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 39.Dombrowski MP, Berry SM, Johnson MP, Saleh AA, Sokol RJ. Birth weight-length ratios, ponderal indexes, placental weights, and birth weight-placenta ratios in a large population. Arch Pediatr Adolesc Med. 1994 May;148(5):508–12. doi: 10.1001/archpedi.1994.02170050066012. [DOI] [PubMed] [Google Scholar]
- 40.Vintzileos AM, Lodeiro JG, Feinstein SJ, Campbell WA, Weinbaum PJ, Nochimson DJ. Value of fetal ponderal index in predicting growth retardation. Obstet Gynecol. 1986 Apr;67(4):584–8. [PubMed] [Google Scholar]
- 41.Lewis SF, Fulco CS. A new approach to studying muscle fatigue and factors affecting performance during dynamic exercise in humans. Exerc Sport Sci Rev. 1998;26:91–116. [PubMed] [Google Scholar]
- 42.Adegboye AR, Heitmann B. Accuracy and correlates of maternal recall of birthweight and gestational age. Bjog. 2008 Jun;115(7):886–93. doi: 10.1111/j.1471-0528.2008.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lubchenco LO, Hansman C, Dressler M, Boyd E. Intrauterine Growth as Estimated from Liveborn Birth-Weight Data at 24 to 42 Weeks of Gestation. Pediatrics. 1963 Nov;32:793–800. [PubMed] [Google Scholar]
- 44.Crouter SE, Clowers KG, Bassett DR., Jr A novel method for using accelerometer data to predict energy expenditure. J Appl Physiol. 2006 Apr;100(4):1324–31. doi: 10.1152/japplphysiol.00818.2005. [DOI] [PubMed] [Google Scholar]
- 45.Siri WE. The gross composition of the body. Adv Biol Med Phys. 1956;4:239–80. doi: 10.1016/b978-1-4832-3110-5.50011-x. [DOI] [PubMed] [Google Scholar]
- 46.Kaiser L. Adjusting for baseline: change or percentage change? [see comments] Stat Med. 1989;8(10):1183–90. doi: 10.1002/sim.4780081002. [DOI] [PubMed] [Google Scholar]
- 47.Sayer AA, Syddall HE, Gilbody HJ, Dennison EM, Cooper C. Does sarcopenia originate in early life? Findings from the Hertfordshire cohort study. J Gerontol A Biol Sci Med Sci. 2004 Sep;59(9):M930–4. doi: 10.1093/gerona/59.9.m930. [DOI] [PubMed] [Google Scholar]
- 48.Pitcher J, Henderson-Smart D, Robinson J. Prenatal Programming of Human Motor Function. In: Wintour E, Owens J, editors. Early LIfe Origins of Health and Disease. Springer; 2006. [Google Scholar]
- 49.Pitcher JB, Robertson AL, Cockington RA, Moore VM. Prenatal growth and early postnatal influences on adult motor cortical excitability. Pediatrics. 2009 Jul;124(1):e128–36. doi: 10.1542/peds.2008-1638. [DOI] [PubMed] [Google Scholar]
- 50.Zierath JR, Hawley JA. Skeletal muscle fiber type: influence on contractile and metabolic properties. PLoS Biol. 2004 Oct;2(10):e348. doi: 10.1371/journal.pbio.0020348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu MJ, Ford SP, Nathanielsz PW, Du M. Effect of maternal nutrient restriction in sheep on the development of fetal skeletal muscle. Biol Reprod. 2004 Dec;71(6):1968–73. doi: 10.1095/biolreprod.104.034561. [DOI] [PubMed] [Google Scholar]
- 52.Dwyer CM, Stickland NC, Fletcher JM. The influence of maternal nutrition on muscle fiber number development in the porcine fetus and on subsequent postnatal growth. J Anim Sci. 1994 Apr;72(4):911–7. doi: 10.2527/1994.724911x. [DOI] [PubMed] [Google Scholar]
- 53.Wigmore PM, Stickland NC. Muscle development in large and small pig fetuses. J Anat. 1983 Sep;137( Pt 2):235–45. [PMC free article] [PubMed] [Google Scholar]
- 54.Beermann DH, Cassens RG, Hausman GJ. A second look at fiber type differentiation in porcine skeletal muscle. J Anim Sci. 1978 Jan;46(1):125–32. doi: 10.2527/jas1978.461125x. [DOI] [PubMed] [Google Scholar]
- 55.Daniel ZC, Brameld JM, Craigon J, Scollan ND, Buttery PJ. Effect of maternal dietary restriction during pregnancy on lamb carcass characteristics and muscle fiber composition. J Anim Sci. 2007 Jun;85(6):1565–76. doi: 10.2527/jas.2006-743. [DOI] [PubMed] [Google Scholar]
- 56.Andersen LG, Angquist L, Gamborg M, Byberg L, Bengtsson C, Canoy D, et al. Birth weight in relation to leisure time physical activity in adolescence and adulthood: meta-analysis of results from 13 nordic cohorts. PLoS ONE. 2009;4(12):e8192. doi: 10.1371/journal.pone.0008192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab. 2000 Jul;279(1):E83–7. doi: 10.1152/ajpendo.2000.279.1.E83. [DOI] [PubMed] [Google Scholar]
- 58.Vickers MH, Breier BH, McCarthy D, Gluckman PD. Sedentary behavior during postnatal life is determined by the prenatal environment and exacerbated by postnatal hypercaloric nutrition. Am J Physiol Regul Integr Comp Physiol. 2003 Jul;285(1):R271–3. doi: 10.1152/ajpregu.00051.2003. [DOI] [PubMed] [Google Scholar]


