Abstract
Objective
To test the existence of sex differences in cortical activation during verb generation when performance is controlled for.
Methods
20 male and 20 female healthy adults underwent fMRI using a covert verb generation task (BD-VGT) and its event-related version (ER-VGT) that allowed for intra-scanner recordings of overt responses. Task-specific activations were determined using the following contrasts: BD-VGT covert generation>finger-tapping; ER-VGT overt generation>repetition; ER-VGT overt>covert generation. Lateral cortical regions activated during each contrast were used for calculating language lateralization index scores. Voxelwise regressions were used to determine sex differences in activation, with and without controlling for performance. Each brain region showing male/female activation differences for ER-VGT overt generation>repetition (isolating noun-verb association) was defined as a region of interest (ROI). For each subject, the signal change in each ROI was extracted, and the association between ER-VGT activation related to noun-verb association and performance was assessed separately for each sex.
Results
Males and females performed similarly on language assessments, had similar patterns of language lateralization, and exhibited similar activation patterns for each fMRI task contrast. Regression analysis controlling for overt intra-scanner performance either abolished (BD-VGT) or reduced (ER-VGT) the observed differences in activation between sexes. The main difference between sexes occurred during ER-VGT processing of noun-verb associations, where males showed greater activation than females in the right middle/superior frontal gyrus (MFG/SFG) and the right caudate/anterior cingulate gyrus (aCG) after controlling for performance. Better verb generation performance was associated with increased right caudate/aCG activation in males and with increased right MFG/SFG activation in females.
Conclusions
Males and females exhibit similar activation patterns during verb generation fMRI, and controlling for intra-scanner performance reduces or even abolishes sex differences in language-related activation. These results suggest that previous findings of sex differences in neuroimaging studies that did not control for task performance may reflect false positives.
Keywords: sex differences, verb generation, semantic association, fMRI, language lateralization
1. Introduction
Language-related sex differences are a matter of debate. Evidence for a female advantage in language abilities exists early in childhood (Murray et al., 1990; Roulstone et al., 2002), persisting through adolescence (Lynn, 1992; Mann et al., 1990) and continuing into adulthood where females reportedly have slightly better language skills than males, particularly in the domain of verbal fluency (Hyde and Linn, 1988; Kimura, 1992; Weiss et al., 2003b). There is also evidence for differences between sexes in language processing strategies; for example, females employ categorical organization to recall words in memory tasks (Cox and Waters, 1986; Kimura, 1999; Kramer et al., 1997; Kramer et al., 1988), while males will recall words in order of presentation (Kimura, 1999; Kramer et al., 1988). These sex differences in language processing and skill level may be rooted in the differing neurodevelopmental changes that occur in males and females, particularly in the connections between various brain regions (Schmithorst and Holland, 2006; Schmithorst and Holland, 2007). Sex differences have been previously illustrated in the growth trajectory of white matter (De Bellis et al., 2001; Lenroot et al., 2007; Perrin et al., 2009) and axonal myelination (Perrin et al., 2009) during childhood and adolescence. These and other studies provide a structural and neurodevelopmental basis for the observed sex differences in language abilities.
Neuroimaging studies have produced mixed findings as to whether differences in language abilities between males and females are associated with differences in brain activation during language processing in children (Burman et al., 2008; Plante et al., 2006) and adults (Baxter et al., 2003; Frost et al., 1999; Gauthier et al., 2009; Shaywitz et al., 1995; Weiss et al., 2003a). The inconsistent findings have been attributed to differences in sample size, the language task utilized and differences in analysis methods (Harrington and Farias, 2008). An aim of the present study was to test another possible reason for the inconsistent findings, in particular, the failure to control for differences in task performance.
Our study focused on verbal fluency. Verbal fluency tasks require coordination of numerous brain systems including language, auditory, motor, attention, and memory. In a typical test of verbal fluency such as the Semantic Fluency Test, subjects are verbally instructed to say as many words as possible in one minute for a given category (Kozora and Cullum, 1995; Lezak, 1995). This requires auditory perception and attention to the auditorily presented category (Binder et al., 2008; Hickok and Poeppel, 2004) as well as linguistic processing to attach meaning to the category (Levelt et al., 1999; Pulvermuller, 2001). Word processing is followed by semantic retrieval in which the subject must access words from their lexicon and decide upon words that are semantically related to the category (Badre et al., 2005; Hillis et al., 2001; Thompson-Schill et al., 1997). In order to produce a response, the subject must then access the correct phonological representation of each word and convert it into a sequence of articulatory movements. This process includes appropriately sequencing phonemes for syllabification while holding the phonological representation in an output buffer (Hillis et al., 2001; Levelt et al., 1999; Nickels and Howard, 2004a; Nickels and Howard, 2004b). Finally, the speech motor network is engaged to verbalize the semantically appropriate words (Hillis et al., 2001; Levelt et al., 1999), and subjects monitor their responses before and during articulation as part of a self-correction strategy (Nickels and Howard, 2004a; Oomen et al., 2001). The overt responses, in turn, provide auditory feedback (e.g., from hearing one’s own verbal responses), an element which is thought to be involved in the control of articulation (Munhall, 2001). The memory system is also engaged as subjects must hold the category in memory throughout semantic processing and word retrieval (Shivde and Thompson-Schill, 2004). Using independent component analysis, a data-driven analysis method that does not require an a priori hypothesis, Karunanayaka et al. (2010) consolidated these processes and described in great detail a comprehensive model of verb generation comprised of word processing and word generation modules (Karunanayaka et al., 2010).
In order to accurately measure performance and determine the extent of cortical involvement during fMRI study of language including verbal fluency, subjects must be able to respond overtly during the scan itself, and their overt responses need to be recorded and later fully assessed. A number of language studies have utilized overt responses during fMRI (Barch et al., 2000; McCarthy et al., 1993; Phelps et al., 1997; Rosen et al., 2000; Yetkin et al., 1995), although many more have employed silent (i.e., covert) responses. Advances in neuroimaging methodology and technology, however, have allowed for the recording of verbal responses during sparse-sampling (Fridriksson et al., 2010; Fu et al., 2002; Fu et al., 2006; Schmithorst and Holland, 2004) or continuous fMRI acquisition (Birn et al., 2010; Crosson et al., 2009). Verb generation is an example of verbal fluency that is readily performed by both children and adults. Vannest et al. (2010) recently found that brain activation during both covert and overt verb generation correlate strongly with in-scanner performance on an overt verb generation task (Vannest et al., 2010). However, these studies have not yet examined whether differences in fMRI activation during language processing between males and females relate to differences in overt intra-scanner performance during a semantic fluency task.
Therefore, the objective of this study was to test whether there are any sex differences in verb generation during fMRI when overt intra-scanner performance is controlled for. Recently, Gauthier et al. (2009) tested individuals outside the scanner, selected them based on high- and low-fluency performance, then investigated sex differences between groups using a covert verbal fluency fMRI task (Gauthier et al., 2009). Though the authors found main effects of sex and performance (Gauthier et al., 2009), the stratification approach and covert nature of the task may have limited their ability to infer which brain regions are associated with increasing levels of task performance. Here we report results from an event-related verb generation task utilizing a sparse-sampling method for fMRI acquisition (Schmithorst and Holland, 2004) that allows for the recording and later analysis of overt responses. The event-related design of the task also allows for the isolation of brain activation related to different linguistic components involved in verb generation, e.g., processing of noun-verb semantic associations versus articulation and auditory processing (Vannest et al., 2010). Previous studies of verbal fluency have employed fMRI block-design tasks (e.g., (Birn et al., 2010; Gauthier et al., 2009; Harrington and Farias, 2008)), which may have masked subtle differences in language-related processing that exist between males and females. These subtleties may be better captured by an event-related study design that can isolate specific language components and processes (Rosen et al., 1998). We hypothesized that males and females would exhibit similar fMRI brain activation patterns overall related to verb generation, but that controlling for intra-scanner performance could reveal sex differences in language-related activation. Additionally, based on the proposed sex differences in language processing strategies, we predicted that the association between verb generation performance and the degree of activation in particular brain regions during the processing of noun-verb associations would also differ between males and females.
2. Methods
2.1 Subjects
Forty-six healthy adults (≥18 years old, 20 males) were recruited as part of a larger ongoing research study that was approved by the Institutional Review Board (NIH R01-NS04828). All subjects were native English speakers, right-handed with an Edinburgh Handedness Inventory (EHI) score above 50 (Oldfield, 1971), had no contraindications to receiving an MRI at 3T, had no prior history of psychiatric or neurological illness, and successfully completed both functional MRI verb generation tasks. Written informed consent was obtained from all participants. For this analysis we selected the 20 females that best matched the 20 males in age and handedness to facilitate statistical comparisons. SPSS 18.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses of demographic and performance data.
2.2 Language assessments
Language testing was performed prior to MRI scanning. The Peabody Picture Vocabulary Test, Fourth Edition (PPVT) was used to test receptive vocabulary; subjects were asked to select one out of a set of four pictures they thought best represented the meaning of the word orally presented by the examiner (Dunn and Dunn, 2007). The Boston Naming Test, Second Edition (BNT) was used to test word-finding and semantic retrieval processes; subjects were asked to name the picture presented by the examiner (Kaplan et al., 2001). The PPVT and BNT are each summarized as a standardized score based on correct responses. The Controlled Oral Word Association Test (COWAT) was used to test oral fluency; subjects were scored based on the number of words beginning with a given letter they could say in one minute (Lezak, 1995). The Semantic Fluency Test (SFT) was used to test verbal fluency and semantic retrieval; performance was scored based on the number of words the subject could say in one minute for a given category (Kozora and Cullum, 1995; Lezak, 1995). Subjects also completed the 12-item Complex Ideation subtest of the Boston Diagnostic Aphasia Examination (BDAE), in which they were asked to verbally agree or disagree about factual material that was orally presented by the examiner (Goodglass and Kaplan, 1972). The BDAE progressed from simple facts to more difficult material requiring reference to knowledge beyond word recall, and performance was scored based on the number of correct responses for the 12 items. Independent samples t-tests (two-tailed) were used to test for differences in performance between males and females.
2.3 Verb generation tasks
Subjects performed two fMRI verb generation tasks, both of which were programmed and presented using DirectRT (Version 2008; Empirisoft, www.empirisoft.com) during the same imaging session.
The first task was a block-design verb generation task (BD-VGT) based on a task introduced by Petersen et al. (1988) (Petersen et al., 1988). The BD-VGT task was used as a reference task to confirm that subjects displayed fMRI activation patterns during covert verb generation typical of those observed in previous studies by our group (Eaton et al., 2008; Holland et al., 2001; Karunanayaka et al., 2010; Szaflarski et al., 2006; Szaflarski et al., 2008; Tillema et al., 2008). The BD-VGT consisted of alternating 30-second test and control blocks. During each test block, the subject was aurally presented with a noun every 6 seconds (5 nouns per test block) and was instructed to silently generate verbs associated with each noun (covert generation). During each control block, the subject was aurally presented a target tone every 6 seconds (5 tones per control block) and was instructed to perform bilateral sequential finger tapping in response to each tone. The control blocks were designed to distract the subject and interrupt the process of covert verb generation in between active blocks and to control for the auditory presentation of the noun during the test condition. The task began with a control condition, followed by alternating test and control conditions (10 blocks each). Each subject performed two runs of the BD-VGT during fMRI, and was informed at the start that there would be a memory test on the nouns following the scan. The post-scan memory test consisted of 30 forced-choice questions in which the subject was instructed to identify those nouns that were presented during the test blocks of the BD-VGT.
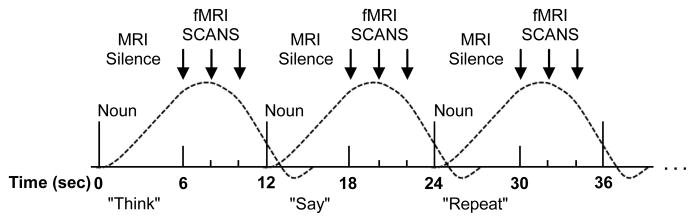
The second verb generation task was an event-related design (ER-VGT; Fig. 1) that employed a method of sparse sampling during fMRI acquisition (Schmithorst and Holland, 2004). The task began with a “Ready” screen presented for 4 seconds, followed by 45 task trials lasting 12-seconds each. The first 6 seconds of each trial occurred during MRI silence in which the subject was aurally presented with a concrete noun and given visual instructions to silently “think” of verbs associated with the noun (covert verb generation similar to the above described block design), “say” the related verbs out loud (overt verb generation), or “repeat” the noun out loud (overt noun repetition). MRI silence was followed by 6 seconds in which 3 fMRI volumes were acquired; during this time, the word “STOP” was visually presented for .5 seconds and then the word “SCAN” for the remaining 5.5 seconds. These prompts were issued to insure that the subjects would stop articulating prior to commencement of each scan acquisition in order to minimize the possibility of motion artifacts contaminating data. During the 6 seconds of MRI silence, the subject was able to clearly hear both the presented noun stimulus and their own verbal response, and the subject’s verbal responses were recorded. Then, during the 6 seconds of fMRI scanning that followed, the peak of the hemodynamic response was captured for each stimulus-response event that occurred during MRI silence. Each trial type was repeated 15 times, alternating in the following order: covert verb generation, overt verb generation, and overt noun repetition. The subject performed one run of the ER-VGT during fMRI. Audio recordings of overt responses were transcribed for analysis of the subject’s in-scanner performance.
Figure 1.
Schematic of the ER-VGT. Each trial consists of a 6-second period of MRI silence followed by a 6-second period where we acquire 3fMRI scans (arrows). During the silent period, the subject hears a noun and is visually instructed to covertly “think” of verbs associated with the noun, overtly “say” the associated verbs, or overtly “repeat” the noun and audio is recorded. During the fMRI scan period, the word “STOP” is visually presented for .5 seconds, followed by the word “SCAN” for 5.5 seconds, and we acquire images during the peak of the hemodynamic response (dotted curve) to each preceding stimulus-response event.
2.4 Magnetic Resonance Imaging
Neuromaging was performed on a 3 Tesla Clinical MRI system using an 8 channel phased array head coil equipped with an MRI compatible A/V system (Avotec, Inc.). Following pre-scanning procedures that include RF coil calibration, shimming and phase reference scans (Schmithorst et al., 2001), a T1-weighted 3D whole brain scan was acquired in the sagittal plane with the following parameters: TR/TE = 8.1/3.7 ms, FOV 25.0 × 21.1 × 18.0 cm, matrix 252 × 211, flip angle 8º, slice thickness = 1 mm isotropic. During the verb generation tasks, a gradient echo EPI (echo planar image) sequence was used to obtain whole brain T2*-weighted fMRI scans with the following parameters: TR/TE= 2000/38 ms, FOV = 24 × 24 cm, matrix = 64 × 64, slice thickness = 4 mm, 32 axial slices. We acquired 165 whole brain fMRI scans for each of the two BD-VGT runs (total scan time of 5 minutes and 30 seconds per run), and 137 whole brain fMRI scans were acquired during the single ER-VGT run (total scan time of 9 minutes and 4 seconds).
2.5 Behavioral data analysis for verb generation tasks
Performance on the BD-VGT was measured indirectly as the percentage of correctly remembered nouns on a memory test administered following the MRI scan session. For the ER-VGT, the audio recordings for the 15 trials of overt verb generation and the 15 trials of overt noun repetition were manually decoded for appropriate verbs or non-verbs based on the quality of the response. For example, the noun “ball” elicited the production of semantically related verbs such as “throw,” “bounce,” and “kick,” and were considered appropriate verbs; however, verbs such as “drink” and “write” that were semantically unrelated, nouns, adjectives, other grammatical categories, and utterances that were incomprehensible were counted as incorrect responses. If a subject repeated the same verb multiple times for a particular noun, this was counted as one appropriate response, but the repeat was not marked as an incorrect response. A correct trial for overt verb generation was one in which at least one appropriate verb was generated, and for overt noun repetition the noun had to be repeated at least once. For each condition, we counted the number of correct trials, the average response time for a correct trial (i.e., the time between the end of presentation of the noun and the beginning of the verbal response), and the total number of correct utterances. For the overt verb generation condition, we also calculated the total number of utterances (i.e., verbs, non-verbs, and repeated verbs) and verb generation accuracy (i.e., the proportion of the total number of appropriately generated verbs divided by the sum of the total number of verbs and non-verbs that were generated). Independent samples t-tests (two-tailed) were used to test for differences between males and females in BD-VGT noun recognition accuracy and in ER-VGT intra-scanner behavioral performance during overt verb generation and noun repetition.
2.6 Image processing and statistical analysis of fMRI data
Image reconstruction, pre-processing, statistical modeling and visualization of fMRI data for single subject and group analyses were performed using CCHIPS (Cincinnati Children’s Hospital Image Processing Software, https://irc.cchmc.org/software/cchips.php) developed in the Interactive Data Language software environment (www.ittvis.com). Following fMRI data reconstruction (Schmithorst et al., 2001), we removed the first two fMRI scans from the ER-VGT data, during which the “Ready” screen was presented, so as to not include it in event-related analysis. We also removed the first 15 fMRI scans from each run of the BD-VGT during the first control block to allow for T1 equilibrium. The two BD-VGT runs were then concatenated into a single dataset for pre-processing steps including motion-correction using a 12-parameter affine transformation (Thevenaz et al., 1998) and spatial normalization (Talairach and Tournoux, 1988) for each fMRI task dataset.
For each task, statistical analysis was performed using general linear modeling (GLM), while accounting for motion-correction parameters and low frequency (quadratic) signal drift of signal intensity, to calculate magnitude differences in the hemodynamic response between different conditions for each task (Worsley and Friston, 1995). GLM was used to estimate brain activation for each subject related to (1) covert verb generation on the BD-VGT by contrasting activation in test blocks relative to control blocks (covert generation>finger-tapping), (2) noun-verb semantic association, while controlling for articulation and auditory processing, on the ER-VGT by contrasting activation during overt verb generation relative to noun repetition (overt generation>repetition), and (3) articulation and auditory processing, while controlling for semantic association, on the ER-VGT by contrasting activation during overt relative to covert verb generation (overt>covert generation).
Three composite datasets (all subjects, male subjects only, and female subjects only) were constructed for each GLM contrast for use in group-level analysis. We performed a one-sample t-test to create statistical (i.e., z-score) maps showing group effects for each GLM contrast and applied a 4 mm full width half maximum Gaussian filter to each dataset. We performed random-effects regression analysis using the composite dataset of all subjects to directly compare task-related activation between males and females, with and without controlling for intra-scanner verb generation accuracy. ER-VGT verb generation accuracy was applied to each GLM contrast, including for the BD-VGT, with the assumption that intra-scanner performance during ER-VGT overt verb generation may be used to infer performance during covert verb generation in either task. Activation clusters on resultant z-score maps were comprised of at least 20 contiguous voxels (1280 μL) that were statistically significant at a corrected threshold of p<.05, determined using an alpha probability simulation algorithm in CCHIPS to account for multiple comparisons (Forman et al., 1995).
2.7 Language-related lateralization index analysis
Based on the statistical maps resulting from the one-sample t-tests of composite datasets with all subjects, we constructed three functional regions of interest (ROI) for each GLM contrast. These ROIs were within the boundaries of significant activation found in (1) only the lateral frontal cortex, (2) only the lateral temporal and parietal cortices, and (3) the combined lateral frontal and temporal/parietal cortices. Each ROI was mirrored to include symmetrical left and right hemisphere clusters in the activated regions. The frontal and temporal/parietal ROIs encompassed regions of Broca’s and Wernicke’s areas respectively such that the combination was considered a global representation of language-related cortical activation, as described previously (Szaflarski et al., 2006). For each task, language-related lateralization index (LI) scores were determined for each subject using a voxel counting method. To determine the threshold for voxels used in the LI computation the mean z-score within each ROI is computed, and only voxels with a z-score greater than or equal to this mean are included in the LI calculation (Szaflarski et al., 2006). The LI score is defined by the formula LI = (ΣL - ΣR) / (ΣL + ΣR), where ΣL and ΣR are the number of super-threshold voxels in the left (L) and right (R) ROIs, respectively. Language-related lateralization was determined from the global LI. Adopting the convention used in previous verb generation studies; scores less than or equal to -.1 were considered right lateralized, scores ranging from -.1 and .1 were considered symmetric, and greater than or equal to .1 were considered left lateralized (Holland et al., 2001; Szaflarski et al., 2008). Independent samples t-tests (two-tailed) were used to compare male and female LI scores to test for sex differences in lateralization of language-related cortical activation.
2.8 Correlation analysis of intra-scanner performance and fMRI activation
To examine sex differences in the neural correlates of performance on overt verb generation, we focused on the ER-VGT contrast isolating noun-verb semantic association. Since performance may be a confounding variable when investigating sex differences in fMRI language activation, we included intra-scanner performance as a covariate in the regression analysis examining activation differences between sexes for ER-VGT noun-verb association. Significantly activated cluster resulting from this analysis were defined as a region of interest. For each subject, ROI values (i.e., the average signal change in the ROI relative to the group’s normalized mean signal intensity of the activated voxels in the ROI) were extracted. For each sex, the correlation between ROI values and verb generation accuracy were assessed using Pearson’s correlation coefficient.
3. Results
3.1 Subject demographics and performance
Demographic and performance variables for the 20 male and 20 female subjects are summarized in Table 1. Male and female subjects were closely matched in age (mean of 41.2 vs. 39.4 years) and handedness (mean EHI score of 92.0 vs. 94.5). Male and female subjects did not significantly differ in performance on any of the language assessments, although BDAE scores showed a trend towards significance at uncorrected p=.064. One male subject did not complete the post-scan noun recognition test for the BD-VGT. Females exhibited better recognition memory than males (uncorrected p=.006). Neither of these differences would be significant using a conservative Bonferroni-adjusted critical p-value of .0033. Furthermore, the difference in the post-scan noun recognition test is unlikely to be meaningful since the mean difference was only 1 out of 30 items (4.7%) for noun recognition accuracy. There were no other differences between males and females in ER-VGT behavioral performance measures.
Table 1.
Demographic and performance characteristics for the 40 study subjects.
| Males (n=20) | Females (n=20) | Mean Difference | 95% CI | p-valueb | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| Age, years | 41.2 | 13.8 | 39.4 | 11.2 | -1.8 | (-9.8 to 6.2) | .653 |
| EHI score | 92.0 | 11.4 | 94.5 | 7.6 | 2.6 | (-3.7 to 8.8) | .411 |
| PPVT IV score | 216.3 | 7.6 | 214.8 | 8.6 | -1.5 | (-6.6 to 3.7) | .575 |
| BNT score | 57.1 | 3.0 | 58.1 | 2.4 | 1.1 | (-.7 to 2.8) | .229 |
| COWAT score | 40.2 | 11.9 | 40.4 | 12.0 | .2 | (-7.5 to 7.9) | .958 |
| SFT score | 52.2 | 11.6 | 57.1 | 12.4 | 4.9 | (-2.8 to 12.6) | .205 |
| BDAE Complex Ideation score | 11.4 | 1.1 | 11.9 | .4 | .5 | (0 to 1.0) | .064 |
| BD-VGT noun recognition, % accuracya | 93.4 | 6.2 | 98.1 | 2.7 | 4.7 | (1.5 to 7.9) | .006 |
| ER-VGT overt noun repetition | |||||||
| Number of correct trials | 14.7 | 1.0 | 14.8 | .4 | .1 | (-.4 to .6) | .682 |
| Reaction time for correct trials, msec | 777.2 | 156.1 | 718.6 | 190.5 | -58.6 | (-170 to 52.9) | .294 |
| Number of repetitions | 59.8 | 21.4 | 64.7 | 19.3 | 4.9 | (-8.2 to 17.9) | .456 |
| ER-VGT overt verb generation | |||||||
| Number of correct trials | 13.1 | 2.3 | 14.0 | 1.7 | .9 | (-.4 to 2.2) | .171 |
| Reaction time for correct trials, msec | 1408.1 | 453.0 | 1401.6 | 236.9 | -6.5 | (-237.8 to 224.9) | .955 |
| Total number of utterances | 37.7 | 11.8 | 37.4 | 6.4 | -.3 | (-6.4 to 5.8) | .921 |
| % accuracy for verbs | 76.7 | 24.1 | 86.7 | 19.4 | 9.9 | (-4.1 to 24.0) | .159 |
Not assessed for one subject.
The critical p-value is .0033 after Bonferroni adjustment for multiple comparisons.
3.2 Functional MRI activation patterns
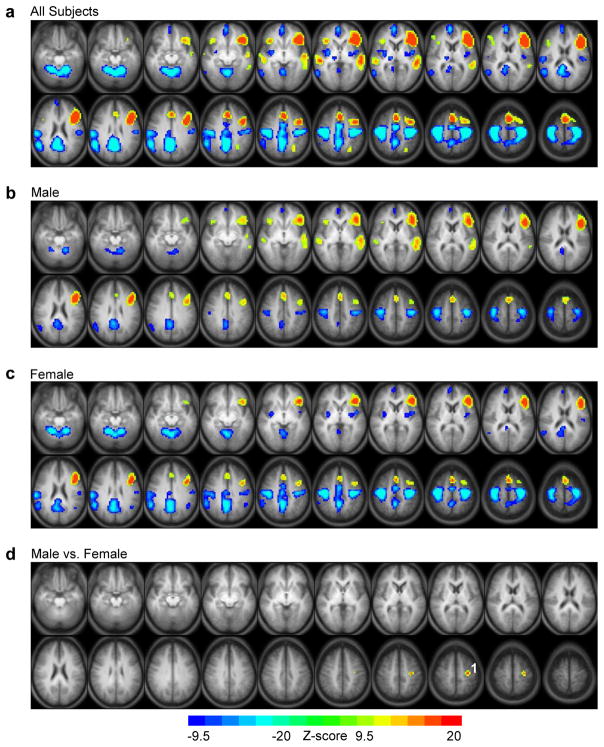
The BD-VGT statistical map for all subjects (Fig. 2a; Table 2) showed language-related activation (i.e., covert generation>finger-tapping) in bilateral (left > right) inferior/middle frontal gyrus (IFG/MFG), bilateral (left > right) middle/superior temporal gyrus (MTG/STG), left superior parietal lobule (SPL) extending into precuneus, and bilateral superior frontal/cingulate gyrus (SFG/CG). There was also bilateral motor-related activation (i.e., greater for finger-tapping than covert verb generation) in numerous regions including the cerebellum, precuneus extending into the posterior section of the cingulate gyrus (pCG) and paracentral lobule (PCL), putamen, insula, and pre/postcentral gyrus. Other regions showing activation greater for motor than for language included the right thalamus, right medial frontal gyrus (mFG) extending into the anterior portion of the cingulate gyrus (aCG) and right supramarginal gyrus (SMG) extending into the right inferior parietal lobule (IPL). Overall, male (Fig. 2b) and female (Fig. 2c) groups exhibited language- and motor-related activation that overlapped with the patterns shown for all subjects. Unlike in the statistical map of all subjects, neither group showed greater language activation in the left SPL/precuneus activation or greater motor activation in the right thalamus. Direct comparison of males and females (Fig. 2d) resulted in one cluster located in the left pre/postcentral gyrus that was more active in males than females. Under the assumption that intra-scanner performance during ER-VGT overt verb generation can be used to infer performance during BD-VGT covert verb generation (Vannest et al., 2010), we included ER-VGT verb generation accuracy in regression analysis. Given this assumption, no regions were found to significantly differ in task activation between males and females.
Figure 2.
Statistical maps for the block-design verb generation task (BD-VGT). Main effects of task in (a) all subjects, (b) males, and (c) females, with warm colors indicating activation greater for covert verb generation than finger tapping and cool colors indicating activation greater for finger tapping than covert genertaion. (d) Direct comparison of males and females for the BD-VGT; activation greater for males in (1) left precentral gyrus (centroid Tailarach coordinates x=-36, y=-15, z=47). Activated regions in statistical maps are significant at p<.05 corrected for multiple voxel comparisons, with nominal Z-scores ranging from -9.5 to -20 (cool colors) or 9.5 to 20 (warm colors). Each statistical map is presented in radiological convention and superimposed on an average T1-weighted image generated from all subjects. The 20 axial slices selected for each display panel range in Talairach coordinates from z=-17 mm (top left) to z=+59 mm (bottom right).
Table 2.
Location of activated brain regions for all subjects during BD-VGT in Figure 2. Also included are the extent of activation clusters for all subjects, presence of the activation in males or females, and brain regions showing activation differences between males and females as a result of direct comparison.
| Location | BA | X, Y, Z | Voxels/Cluster | Males | Females | Difference |
|---|---|---|---|---|---|---|
| Covert verb generation > Finger tapping
| ||||||
| R. IFG/MFG/insula | 45–47, 13 | 32, 23, 3 | 99 | yes | yes | - |
| L. IFG/MFG/insula | 45–47, 13 | -43, 19, 23 | 839 | yes | yes | - |
| R. STG | 22 | 51, -23, 3 | 71 | yes | - | - |
| L. STG/MTG | 21, 22 | -58, -11, 3 | 188 | yes | yes | - |
| L./R. SFG/CG | 6, 32 | -2, 11, 59 | 259 | yes | yes | - |
| L. SPL/precuneus | 7 | -24, -68, 43 | 33 | - | - | - |
|
| ||||||
| Finger tapping > Covert verb generation
| ||||||
| cerebellum | -21, -49, -17 | 624 | yes | yes | - | |
| R. mFG/aCG | 9, 10, 32 | 2, 56, 11 | 180 | yes | yes | - |
| L./R. precuneus/pCG/PCL | 23, 30, 31, 7, 5 | 6, -49, 35 | 898 | yes | yes | - |
| R. putamen/insula | 13 | 28, -4, -1 | 54 | - | yes | - |
| L. putamen/insula | 13 | -43, 0, 7 | 46 | - | yes | - |
| R. thalamus | 13, -19, 7 | 22 | - | - | - | |
| R. SMG/IPL | 40 | 54, -49, 31 | 282 | yes | yes | - |
| R. precentral/postcentral gyrus | 4, 6, 3 | 36, -11, 55 | 683 | yes | yes | - |
| L. precentral/postcentral gyrus | 4, 6, 3 | -36, -15, 55 | 560 | yes | yes | M > F |
Abbreviations: BA= Brodmann’s Area; L=left; R=right; M=male; F=female; IFG=inferior frontal gyrus; MFG=middle frontal gyrus; SFG=superior frontal gyrus; mFG=medial frontal gyrus; STG=superior temporal gyrus; MTG=middle temporal gyrus; CG=cingulate gyrus; aCG=cingulate gyrus (anterior); pCG=cingulate gyrus (posterior); SMG=supramarginal gyrus; IPL=inferior parietal lobule; SPL=superior parietal lobule; PCL=paracentral lobule.
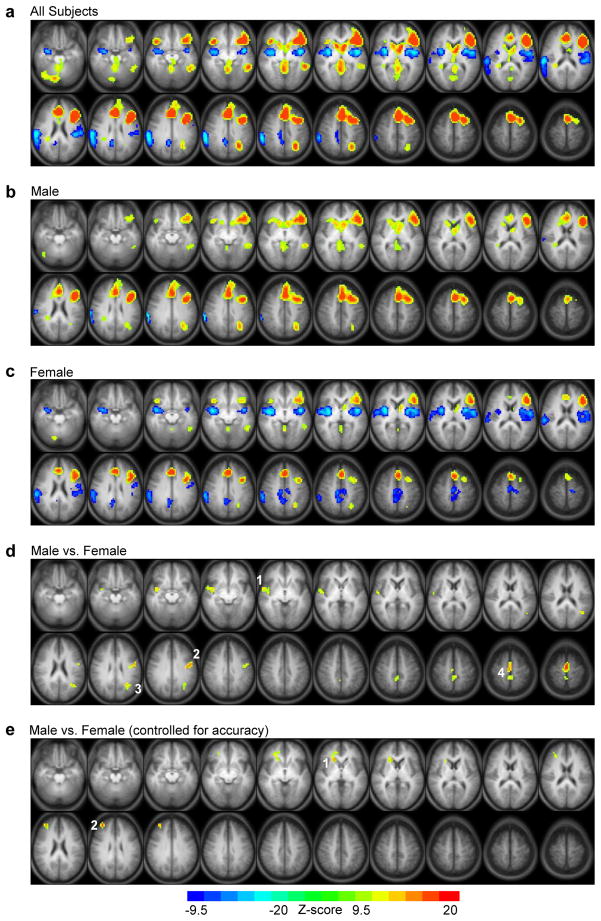
Similar to our previous study utilizing this task (Vannest et al., 2010), the ER-VGT statistical map of activation related to noun-verb association (i.e., overt generation>repetition) for all subjects (Fig. 3a; Table 3) showed left>right activation in the IFG/insula. Additional activation was observed in the left MTG, left precuneus extending into IPL/SPL, bilateral SFG extending into mFG and CG, right cerebellum, bilateral caudate, and bilateral lingual gyrus (LG) extending into the cuneus/pCG. Regions more active for overt repetition than generation included the bilateral STG extending into the insular cortex and the putamen, bilateral STG/IPL, as well as the right CG extending into the precuneus. Patterns of activation in males (Fig. 3b) and females (Fig. 3c) overlapped with the patterns shown for all subjects, although males only exhibited a single cluster of activation greater for repetition than generation in the right STG/postcentral gyrus/IPL. Direct comparison of males and females (Fig. 3d) resulted in males having greater activation related to noun-verb association than females in the right STG/insula, left precentral gyrus, left MTG/STG, and the bilateral mFG/SFG. Regression analysis accounting for verb generation accuracy (Fig. 3e) revealed two regions that were significantly more active in males than females: one cluster was located in the right caudate head extending into the aCG, while the second cluster was in the right MFG/SFG (BA 9).
Figure 3.
Statistical maps for the ER-VGT isolating noun-verb semantic association. Main effects of task in (a) all subjects, (b) males, and (c) females, with warm colors indicating activation greater for overt generation than repetition and cool colors indicating activation greater for repetition than overt generation. (d) Direct comparison of males and females, with activation greater for males than females in the (1) right superior temporal gyrus/insula (centroid Tailarach coordinates x=39, y=-11, z=-1), (2) left precentral gyrus (centroid Tailarach coordinates x=-51, y=-4, z=35), (3) left middle/superior temporal gyrus (centroid Tailarach coordinates x=-36, y=-56, z=19), and (4) bilateral medial/superior frontal gyrus (centroid Tailarach coordinates x=-2, y=-4, z=59). (e) Controlling for intra-scanner task performance (i.e., verb generation accuracy) when comparing males and females results in greater activation for males in the (1) right head of the caudate extending into the anterior cingulate cortex (centroid Tailarach coordinates x=15, y=22, z=3) and (2) right middle/superior frontal gyrus (BA 9; centroid Tailarach coordinates x=32, y=45, z=27). Activated regions in statistical maps are significant at p<.05 corrected for multiple voxel comparisons, with nominal Z-scores ranging from -9.5 to -20 (cool colors) or 9.5 to 20 (warm colors). Each statistical map is presented in radiological convention and superimposed on an average T1-weighted image generated from all subjects. The 20 axial slices selected for each display panel range in Talairach coordinates from z=-17 mm (top left) to z=+59 mm (bottom right).
Table 3.
Location of activated brain regions for all subjects during the ER-VGT contrast isolating processing related to noun-verb association in Figure 3. Also included are the extent of activation clusters for all subjects, presence of the activation in males or females, and brain regions showing activation differences between males and females as a result of direct comparison.
| Location | BA | X, Y, Z | Voxels/Cluster | Males | Females | Difference |
|---|---|---|---|---|---|---|
| Overt verb generation > Overt repetition
| ||||||
| cerebellum | 32, -60, -29 | 627 | yes | yes | - | |
| R. IFG/insula | 47, 13 | 32, 23, -5 | 81 | yes | yes | - |
| L. IFG/insula/MFG | 45–47, 13, 9 | -47, 19, 23 | 966 | yes | yes | - |
| L. MTG | 21 | -51, -38, -1 | 107 | yes | yes | - |
| R. caudate | 13, 15, 7 | 102 | yes | - | - | |
| L. caudate | -9, 4, 7 | 101 | yes | yes | - | |
| L./R. LG/cuneus/pCG | 18, 30 | 6, -57, 6 | 148 | yes | yes | - |
| L./R. SFG/mFG/CG/aCG | 8, 32, 24 | -2, 19, 43 | 720 | yes | yes | - |
| L. precuneus/IPL/SPL | 39, 7 | -28, -58, 39 | 121 | yes | yes | M > F |
|
| ||||||
| Overt repetition > Overt verb generation
| ||||||
| R. STG/insula/putamen | 21, 13 | 43, 0, -11 | 243 | - | yes | M > F |
| L. STG/insula/putamen | 21, 13 | -39, 0, -1 | 272 | - | yes | - |
| R. STG/postcentral gyrus/IPL | 42, 2, 40 | 58, -30, 35 | 372 | yes | yes | - |
| L. STG/IPL | 41, 40 | -43, -30, 23 | 114 | - | yes | M > F |
| R. CG/precuneus | 31 | 9, -49, 27 | 84 | - | yesa | M > Fa |
Abbreviations: BA= Brodmann’s Area; L=left; R=right; M=male; F=female; IFG=inferior frontal gyrus; MFG=middle frontal gyrus; SFG=superior frontal gyrus; mFG=medial frontal gyrus; STG=superior temporal gyrus; MTG=middle temporal gyrus; CG=cingulate gyrus; aCG=cingulate gyrus (anterior); pCG=cingulate gyrus (posterior); LG=lingual gyrus; IPL=inferior parietal lobule; SPL=superior parietal lobule.
bilateral.
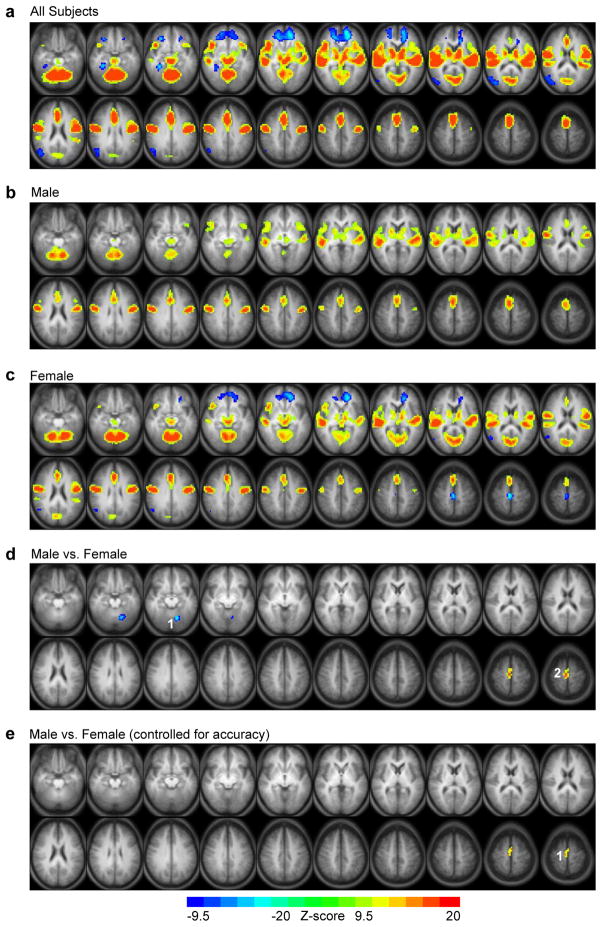
The ER-VGT statistical map of activation related to articulation/auditory processing (i.e., overt >covert generation) for all subjects (Fig. 4a; Table 4) showed bilateral activation in the midbrain, cerebellum, subcortical regions, as well as widespread bilateral activation in frontal, temporal, visual and motor cortices. Activation greater for covert than overt generation occurred bilaterally in the orbital frontal cortex, right parahippocampal gyrus, and right MTG/angular gyrus. Again, activation in males (Fig. 4b) and females (Fig. 4c) largely overlapped except males did not exhibit any posterior visual cortex activation, and females did not elicit left IFG activation related to articulation/auditory processing. Furthermore, only females showed greater activation for covert than overt generation, including an additional region in the bilateral PCL, although not in the right parahippocampal gyrus or right SMG/IPL. Direct comparison of males and females (Fig. 4d) showed males had greater activation related to articulation/auditory processing than females in the PCL and females had greater activation than males in the left cerebellum. However, regression analysis accounting for verb generation accuracy (Fig. 4e) revealed that only the PCL cluster was significantly more active in males than females.
Figure 4.
Statistical maps for the ER-VGT isolating articulation and auditory processing. Main effects of task in (a) all subjects, (b) males, and (c) females, with warm colors indicating activation greater for overt than covert generation and cool colors indicating activation greater for covert than overt generation. (d) Direct comparison of males and females, with activation greater for females than males in the (1) left cerebellum (centroid Tailarach coordinates x=-13, y=-56, z=-5), and activation greater for males than females in the (2) medial frontal gyrus (centroid Tailarach coordinates x=-2, y=-4, z=55). (e) Controlling for intra-scanner task performance (i.e., verb generation accuracy) when comparing males and females results in greater activation for males in only the (1) medial frontal gyrus. Activated regions in statistical maps are significant at p<.05 corrected for multiple voxel comparisons, with nominal Z-scores ranging from -9.5 to -20 (cool colors) or 9.5 to 20 (warm colors). Each statistical map is presented in radiological convention and superimposed on an average T1-weighted image generated from all subjects. The 20 axial slices selected for each display panel range in Talairach coordinates from z=-17 mm (top left) to z=+59 mm (bottom right).
Table 4.
Location of activated brain regions for all subjects during the ER-VGT contrast isolating articulation and auditory processing in Figure 4. Also included are the extent of activation clusters for all subjects, presence of the activation in males or females, and brain regions showing activation differences between males and females as a result of direct comparison.
| Location | BA | X, Y, Z | Voxels/Cluster | Males | Females | Difference |
|---|---|---|---|---|---|---|
| Overt > Covert verb generation
| ||||||
| cerebellum | -17, -56, -17 | 997 | yes | yes | M < F | |
| midbrain | -6, -23, -5 | 238 | yes | yes | - | |
| R. thalamus/caudate/pallidum | 13, -4, 15 | 173 | yes | yes | - | |
| L. thalamus/caudate/pallidum | -9, 4, 11 | 191 | yes | yes | - | |
| R. IFG/insula/precentral gyrus | 45, 47, 13, 6 | 47, -8, 27 | 648 | yes | yes | - |
| L. IFG/insula/precentral gyrus | 45, 47, 13, 6 | -39, -11, 31 | 653 | yes | yesa | - |
| R. MTG/STG/TTG | 21, 22, 41 | 47, -23, 7 | 378 | yes | yes | - |
| L. MTG/STG/TTG | 21, 22, 41 | -41, -23, 11 | 303 | yes | yes | - |
| L./R. aCG/CG/SFG | 24, 32, 8 | 6, 15, 51 | 628 | yes | yes | - |
| L./R. LG/cuneus/pCG | 18, 30 | -17, -60, 7 | 387 | - | yes | - |
|
| ||||||
| Covert > Overt verb generation
| ||||||
| R. aCG/mFG/SFG | 32, 10 | 17, 38, 3 | 167 | - | yes | - |
| L. aCG/mFG/SFG | 32, 10 | -13, 53, 7 | 199 | - | yes | - |
| R. parahippocampal gyrus | 36 | 28, -41, -5 | 50 | - | - | - |
| R. MTG/angular gyrus | 39 | 47, -60, 35 | 133 | - | yes | - |
| L./R. PCLb | 6 | 2, -30, 55 | 45 | - | yes | M > F |
Abbreviations: BA= Brodmann’s Area; L=left; R=right; M=male; F=female; IFG=inferior frontal gyrus; MFG=middle frontal gyrus; SFG=superior frontal gyrus; mFG=medial frontal gyrus; STG=superior temporal gyrus; MTG=middle temporal gyrus; TTG=transverse temporal gyrus; CG=cingulate gyrus; aCG=cingulate gyrus (anterior); pCG=cingulate gyrus (posterior); LG=lingual gyrus; PCL=paracentral lobule.
no IFG activation;
deactivation present in composite map of females only.
3.3 Language lateralization
LI scores did not significantly differ between male and female subjects (Table 5). Similar numbers of males and females were identified as exhibiting leftward lateralization of language-related activation during BD-VGT verb generation and ER-VGT noun-verb association, and also showing predominantly symmetrical activation during ER-VGT articulation/auditory processing.
Table 5.
LI scores and lateralization for the 20 male and 20 female subjects performing the verb generation tasks.
| BD-VGT | ER-VGT | ER-VGT | ||||
|---|---|---|---|---|---|---|
| Covert Verb Generation | Noun-Verb Association | Articulation/Auditory Processing | ||||
|
|
||||||
| Males | Females | Males | Females | Males | Females | |
| LI scoresa | ||||||
| Frontal | .48 (.30) | .45 (.28) | .41 (.25) | .35 (.26) | -.03 (.17) | -.07 (.17) |
| Temporal/Parietal | .29 (.38) | .20 (.30) | .41 (.29) | .37 (.29) | 0 (.16) | 0 (.11) |
| Global | .45 (.26) | .39 (.26) | .41 (.23) | .35 (.25) | 0 (.15) | -.01 (.10) |
|
| ||||||
| Lateralizationb | ||||||
| Left | 17 (85) | 19 (95) | 17 (85) | 16 (80) | 5 (25) | 4 (20) |
| Right | 0 (0) | 1 (5) | 0 (0) | 1 (5) | 3 (15) | 2 (10) |
| Symmetric | 3 (15) | 0 (0) | 3 (15) | 3 (15) | 12 (60) | 14 (70) |
Note. Data are reported as mean (SD) for LI scores and as frequency (percentages) for lateralization.
No significant difference between male and female LI scores; p>.3 for all comparisons.
Lateralization was determined from the global laterality index with LI > .1 as left-lateralized, LI < -.1 as right-lateralized and -.1 ≤LI ≤.1 as symmetric.
3.4 Relationships between ROI activation and intra-scanner performance
The two clusters showing significantly more activation in males than females after accounting for verb generation accuracy (Fig. 3e) were explored to determine if task performance was differentially associated with the degree of activation in these brain regions between males and females. Correlation analysis revealed a significant positive relationship between activation in the right caudate/aCG and verb generation accuracy in males (Pearson’s r=.495, p=.026), which did not exist in females (Pearson’s r=-.012, p=.961). There was also a positive trend approaching significance between activation in the right MFG/SFG (BA 9) and verb generation accuracy in females (Pearson’s r=.434, p=.056), but not in males (Pearson’s r=.082, p=.732).
4. Discussion
4.1 Summary
The goal of this study was to determine whether there are sex differences in cortical involvement during verb generation after controlling for intra-scanner performance. To achieve these objectives, we used a novel ER-VGT fMRI task that allowed for intra-scanner overt response monitoring. We found that males and females matched for age and handedness performed similarly on language assessments (Table 1), had similar fMRI language lateralization (Table 5), and exhibited overall similar activation patterns (Fig. 2–4). Direct comparison of male and female activation during the verb generation tasks yielded very few sex-specific differences in activation (Fig. 2d, 3d, 4d). We had proposed that controlling for intra-scanner performance and use of an event-related task design may unmask subtle sex differences that were not evident in previous studies. To the contrary, controlling for performance abolished the single cluster showing a difference in activation between sexes for BD-VGT (Fig. 2d) and reduced the number of clusters showing significant differences for ER-VGT (Fig. 3d-e, 4d-e). These results suggest that previous studies showing sex differences in language-related activation, in which task performance was not controlled for, may reflect false positive findings. Therefore, not controlling for performance may, in part, account for the inconsistent findings of previous neuroimaging studies investigating sex differences in language processing.
Once performance was taken into account, we did identify two brain regions (i.e., the right MFG/SFG and right caudate/aCG) in which males showed greater activation than females for ER-VGT processing of noun-verb associations. Subsequent correlation analysis revealed that improved verb generation performance was associated with increased right caudate/aCG activation in males and with increased right MFG/SFG activation in females, which may reflect differences in language processing strategies between sexes (Cox and Waters, 1986; Kimura, 1999; Kramer et al., 1997; Kramer et al., 1988). However, these findings should be interpreted with caution, as replication is warranted before any firm conclusions can be made. Overall, we demonstrated that there are very few differences between males and females with regard to activation during verb generation, especially after accounting for performance.
4.2 Functional MRI task differences
The ER-VGT was designed to address the possible shortcomings of previous studies that have explored language-related sex differences. However, since this task is not well characterized in healthy individuals, discussion of the findings in the context of other fMRI tasks is relevant. We collected data using a well-established BD-VGT as a reference and to document normal activation patterns during covert verb generation in the sample of subjects included in the study, although a covert task may not be the most precise method of studying verbal fluency. The activation pattern for the BD-VGT in all subjects (Fig. 2a) was consistent with previous studies (Eaton et al., 2008; Holland et al., 2001; Karunanayaka et al., 2010; Szaflarski et al., 2006; Szaflarski et al., 2008; Tillema et al., 2008). The ER-VGT contrast for overt generation>repetition to isolate processing of noun-verb associations (Fig. 3a) were similar to that observed for the BD-VGT contrast for covert generation>finger-tapping since both were aimed at isolating word-level semantic processing.
The symmetrical activation observed for the overt>covert generation contrast during the ER-VGT, corresponds to articulation and auditory processing and is consistent with previous work describing cortical involvement in speech and hearing. In addition to motor regions that are involved in speech articulation, we noted very robust insular activation. Such activation may be due to the role this region has been posited to play in articulation and articulatory planning (Dronkers, 1996; Hiraga et al., 2010; Karunanayaka et al., 2010; Price, 2010). As described in the theoretical model of verbal fluency, overt responses further provide auditory feedback that is also involved in articulatory control (Munhall, 2001). ER-VGT activation related to articulation/auditory processing also occurred in frontal and temporal regions previously shown to be involved in the auditory processing of language (Binder et al., 2008; Karunanayaka et al., 2010) and music (Schmithorst, 2005). Medial frontal activation was also observed in this contrast, likely due to attentional and executive functions involved in producing an overt verbal response. The activation in visual regions we observed is consistent with activation related to visual imagery thought to be a part of the verb generation process (Ganis et al., 2004; Karunanayaka et al., 2010), more specifically, part of the word generation module described by Karunanayaka et al. (2010). An increase in this region during overt versus covert verb generation may be related to the degree of effort.
Differences between BD-VGT and ER-VGT are apparent in Figures 2 and 3. ER-VGT activation related to processing noun-verb associations in all subjects (Fig. 2a) was increased in the left MTG rather than in the left STG as observed in BD-VGT covert generation contrast (Fig. 3a). While the STG activation during BD-VGT may be attributed to how the subjects switched between types of aurally presented stimuli, e.g., switching between hearing nouns and hearing tones (Schmithorst, 2005), the verb generation circuit proposed by Karunanayaka et al. (2010) suggested that the STG is involved in accessing semantic and phonological information in memory while the MTG is involved more with generating semantic representations of the presented nouns (Karunanayaka et al., 2010). Since the two conditions (i.e., overt generation and overt repetition) used to isolate processing of noun-verb associations during ER-VGT are both linguistic in nature, access of semantic and phonological information and related STG activation is common for both conditions and cancel each other out. However, MTG recruitment allowing for generation of semantic representations for the nouns is necessary to facilitate generation of associated verbs and is therefore isolated in the contrast. We noted increased visual cortex activation during ER-VGT noun-verb association that was not present during BD-VGT, which is consistent with the model of verb generation processes that involves visual imagery (Karunanayaka et al., 2010). Activation of these visual brain regions in our analyses is consistent with other studies that have evaluated cognitive processes, e.g., memory search (Cabeza et al., 2002), indicating that subjects rely on visualization or visual imagery in order to generate and express verbs associated with nouns (Ganis et al., 2004). These results support the notion that an event-related design can better isolate specific language components and processes than the block design. Using the event-related design, we were able to identify brain regions that were differentially recruited by males and females during verb generation after controlling for individual subject performance.
4.3 Similarities and differences in language-related activation
Perhaps the most remarkable finding in this study is how few sex differences in language-related activation exist when directly comparing males and females that are matched for age and performance (Fig. 2d, 3d, 4d). This observation is consistent with previous studies showing similar language-related activation in both sexes (Buckner et al., 1995; Frost et al., 1999; Garn et al., 2009; Haut and Barch, 2006; Weiss et al., 2003a). Consistent with previous studies (Baxter et al., 2003; Buckner et al., 1995; Frost et al., 1999; Gauthier et al., 2009), we also found that activation differences between sexes were primarily related to greater overall activation in males than females. Unique to our study was our ability to control for intra-scanner performance (i.e., verb generation accuracy) when investigating differences between sexes in language-related activation. Controlling for performance reduced the number of brain regions showing differences in activation (Fig. 3e, 4e) or resulted in no significant difference between sexes, as observed in the BD-VGT. Our results suggest caution when interpreting findings from previous neuroimaging studies that did not take intra-scanner performance into account when exploring sex differences in cortical involvement during language production. Furthermore, it appeared that males had more symmetrical inferior frontal activation than females during BD-VGT covert verb generation (Fig. 2b, 2c), but direct comparison of task-related activation between males and females revealed only a single region where activation is significantly different between the sexes (Fig. 2d). In order to better capture the lateralization patterns for each sex, functional ROIs were defined based on the statistical maps of all subjects, and these were used in calculating LI. Although average LI scores (frontal, temporal/parietal and global) were consistently greater in males than females, the differences were not statistically significant (Table 5). Therefore, while some studies showed that language-related cortical activation may be more left-lateralized in males than females (Baxter et al., 2003; Clements et al., 2006; Shaywitz et al., 1995), our results provide additional evidence for similar language lateralization between sexes (Frost et al., 1999; Harrington and Farias, 2008; Weiss et al., 2003a) and are consistent with meta-analyses of neuroimaging studies that showed no effect of sex on language lateralization (Sommer et al., 2004; Sommer et al., 2008).
As stated previously, we found few differences between males and females in language-related activation for both verb generation tasks, and controlling for performance either abolished (as in the BD-VGT) or reduced (as in the ER-VGT) the number of activation clusters that were different between the sexes. Even after controlling for performance, however, there remained activation differences between males and females during ER-VGT processing of noun-verb associations. Specifically, males showed greater activation than females in the right caudate/aCG and right MFG/SFG (BA 9). Correlation analysis revealed that males, but not females, exhibited increased recruitment of the right caudate/aCG with increasing verb generation accuracy. The caudate is part of the fronto-cortical-striatal circuit involved in decision-making (Simard et al., 2010), while the anterior cingulate cortex is thought to be involved in attentional processes (Fichtenholtz et al., 2004) and in regulating behavior by monitoring performance and detecting errors (Carter et al., 1998; Kerns et al., 2004). In contrast, increased verb generation accuracy in females, but not in males, was associated with increased right MFG/SFG (BA 9) recruitment; activation in this region of the prefrontal cortex is associated with sustained attention (Cabeza and Nyberg, 2000; Kim et al., 2006; Lawrence et al., 2003) and with increasing working memory load and organizational demand (Wendelken et al., 2008). Furthermore, Lawrence et al. (2003) found that right MFG activation increased with better performance on a rapid visual information processing task (Lawrence et al., 2003). Taken together, these results suggest that males and females activate a similar network of brain regions during language processing, but may differ somewhat in the attentional and cognitive control strategies they employ. These differences in strategies may be reflected in the recruitment of the same brain regions to different degrees in order to achieve better verb generation performance, as implied by the results of the correlation analyses. The model of verbal fluency described in the introduction involves coordination of multiple brain systems for successful performance. Although we did not examine functional connectivity in this analysis, evidence from studies of sexual dimorphisms that occur during development of the human brain lead us to posit that the subtle difference in cortical recruitment by males and females may be mediated by underlying structural connectivity differences between brain regions involved in the various neural networks (De Bellis et al., 2001; Lenroot et al., 2007; Perrin et al., 2009; Perrin et al., 2009; Schmithorst et al., 2008).
4.4 Study limitations and future directions
The small to moderate sample sizes typically used in neuroimaging studies are believed to contribute to false positive findings and lack of generalizability of sex differences (Harrington and Farias, 2008; Ihnen et al., 2009), and likely limit the ability to replicate results between studies. Language studies with large numbers of adult subjects (Frost et al., 1999; Haut and Barch, 2006) and meta-analyses that pooled together subjects from neuroimaging studies to address the issue of language-related sex differences (Sommer et al., 2004; Sommer et al., 2008) have found little in the way of sex differences. The implications of these studies are twofold: 1) language-related sex differences may be small and, therefore, subtle effects are difficult to detect, and 2) the differences reported by studies with small sample sizes may be false positives. The results of our current study primarily support the findings of these larger studies, with the exception of activation in the right caudate/aCG and right MFG/SFG (BA 9) that showed greater activation in males than females after controlling for performance. Given our moderate sample size of 40 subjects, we cannot discount the possibility that these observed sex differences might be false positive results that may not be present in a similar study with a larger number of subjects. Therefore, these results should be interpreted with caution until they are replicated in a larger study. Additionally, we observed a non-significant positive correlation between right MFG/SFG activation and verb generation accuracy in females; this may or may not be a false negative result that could also be evaluated in a larger study. Future studies that replicate our results in a larger sample of males and females are necessary to test the validity of these sex-specific activation differences and how they relate to verb generation performance.
Finally, a major aim of the present study was to investigate if the failure to control for task performance may be another reason for the inconsistent findings regarding sex differences in brain activation during language processing. Our findings strongly suggest that task performance is an important factor in acquiring variable results. We showed that controlling for in-scanner performance consistently reduced the number of observed sex differences in activation during verb generation when directly comparing males and females, suggesting that accounting for task performance may help to minimize false positive results. Taking task performance into account may also minimize false negative findings; by controlling for the variability of individual subject performance, small subtle differences in language processing that possibly exist between males and females may be unmasked. Therefore, future neuroimaging studies investigating language-related sex differences should utilize neuroimaging tasks that monitor intra-scanner performance, particularly when studying verbal fluency. Overall, we believe that conducting studies with larger sample sizes and controlling for task performance will allow for more consistent results between studies, making it possible to draw some firm conclusions regarding sex differences in language-related activation.
Acknowledgments
Support for this study was provided by NIH R01 NS04828. This study was presented in part at the 16th Annual Meeting of the Organization for Human Brain Mapping, Barcelona, Spain.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47 (6):907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Sabb FW, Noll DC. Anterior cingulate and the monitoring of response conflict: evidence from an fMRI study of overt verb generation. Journal of Cognitive Neuroscience. 2000;12 (2):298–309. doi: 10.1162/089892900562110. [DOI] [PubMed] [Google Scholar]
- Baxter LC, Saykin AJ, Flashman LA, Johnson SC, Guerin SJ, Babcock DR, Wishart HA. Sex differences in semantic language processing: a functional MRI study. Brain and Language. 2003;84 (2):264–272. doi: 10.1016/s0093-934x(02)00549-7. [DOI] [PubMed] [Google Scholar]
- Binder JR, Swanson SJ, Hammeke TA, Sabsevitz DS. A comparison of five fMRI protocols for mapping speech comprehension systems. Epilepsia. 2008;49 (12):1980–1997. doi: 10.1111/j.1528-1167.2008.01683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Kenworthy L, Case L, Caravella R, Jones TB, Bandettini PA, Martin A. Neural systems supporting lexical search guided by letter and semantic category cues: a self-paced overt response fMRI study of verbal fluency. Neuroimage. 2010;49 (1):1099–1107. doi: 10.1016/j.neuroimage.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Raichle ME, Petersen SE. Dissociation of human prefrontal cortical areas across different speech production tasks and gender groups. Journal of Neurophysiology. 1995;74 (5):2163–2173. doi: 10.1152/jn.1995.74.5.2163. [DOI] [PubMed] [Google Scholar]
- Burman DD, Bitan T, Booth JR. Sex differences in neural processing of language among children. Neuropsychologia. 2008;46 (5):1349–1362. doi: 10.1016/j.neuropsychologia.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Dolcos F, Graham R, Nyberg L. Similarities and differences in the neural correlates of episodic memory retrieval and working memory. Neuroimage. 2002;16 (2):317–330. doi: 10.1006/nimg.2002.1063. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12 (1):1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280 (5364):747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Clements AM, Rimrodt SL, Abel JR, Blankner JG, Mostofsky SH, Pekar JJ, Denckla MB, Cutting LE. Sex differences in cerebral laterality of language and visuospatial processing. Brain and Language. 2006;98 (2):150–158. doi: 10.1016/j.bandl.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Cox D, Waters HS. Sex differences in the use of organization strategies: A developmental analysis. Journal of Experimental Child Psychology. 1986;41 (1):18–37. doi: 10.1016/0022-0965(91)90066-2. [DOI] [PubMed] [Google Scholar]
- Crosson B, Moore AB, McGregor KM, Chang YL, Benjamin M, Gopinath K, Sherod ME, Wierenga CE, Peck KK, Briggs RW, Rothi LJ, White KD. Regional changes in word-production laterality after a naming treatment designed to produce a rightward shift in frontal activity. Brain and Language. 2009;111 (2):73–85. doi: 10.1016/j.bandl.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM. Sex differences in brain maturation during childhood and adolescence. Cerebral Cortex. 2001;11 (6):552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- Dronkers NF. A new brain region for coordinating speech articulation. Nature. 1996;384 (6605):159–161. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn DM. Peabody Picture Vocabulary Test. 4. NCS Pearson, Inc; 2007. [Google Scholar]
- Eaton KP, Szaflarski JP, Altaye M, Ball AL, Kissela BM, Banks C, Holland SK. Reliability of fMRI for studies of language in post-stroke aphasia subjects. Neuroimage. 2008;41 (2):311–322. doi: 10.1016/j.neuroimage.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtenholtz HM, Dean HL, Dillon DG, Yamasaki H, McCarthy G, LaBar KS. Emotion-attention network interactions during a visual oddball task. Brain Research Cognitive Brain Research. 2004;20 (1):67–80. doi: 10.1016/j.cogbrainres.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33 (5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fridriksson J, Bonilha L, Baker JM, Moser D, Rorden C. Activity in preserved left hemisphere regions predicts anomia severity in aphasia. Cerebral Cortex. 2010;20 (5):1013–1019. doi: 10.1093/cercor/bhp160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost JA, Binder JR, Springer JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Language processing is strongly left lateralized in both sexes. Evidence from functional MRI. Brain. 1999;122(Pt 2):199–208. doi: 10.1093/brain/122.2.199. [DOI] [PubMed] [Google Scholar]
- Fu CH, McIntosh AR, Kim J, Chau W, Bullmore ET, Williams SC, Honey GD, McGuire PK. Modulation of effective connectivity by cognitive demand in phonological verbal fluency. Neuroimage. 2006;30 (1):266–271. doi: 10.1016/j.neuroimage.2005.09.035. [DOI] [PubMed] [Google Scholar]
- Fu CH, Morgan K, Suckling J, Williams SC, Andrew C, Vythelingum GN, McGuire PK. A functional magnetic resonance imaging study of overt letter verbal fluency using a clustered acquisition sequence: greater anterior cingulate activation with increased task demand. Neuroimage. 2002;17 (2):871–879. [PubMed] [Google Scholar]
- Ganis G, Thompson WL, Kosslyn SM. Brain areas underlying visual mental imagery and visual perception: an fMRI study. Brain Research Cognitive Brain Research. 2004;20 (2):226–241. doi: 10.1016/j.cogbrainres.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Garn CL, Allen MD, Larsen JD. An fMRI study of sex differences in brain activation during object naming. Cortex. 2009;45 (5):610–618. doi: 10.1016/j.cortex.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Gauthier CT, Duyme M, Zanca M, Capron C. Sex and performance level effects on brain activation during a verbal fluency task: a functional magnetic resonance imaging study. Cortex. 2009;45 (2):164–176. doi: 10.1016/j.cortex.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. The Assessment of Aphasia and Related Disorders. Lea & Febiger; 1972. [Google Scholar]
- Harrington GS, Farias ST. Sex differences in language processing: functional MRI methodological considerations. Journal of Magnetic Resonance Imaging. 2008;27 (6):1221–1228. doi: 10.1002/jmri.21374. [DOI] [PubMed] [Google Scholar]
- Haut KM, Barch DM. Sex influences on material-sensitive functional lateralization in working and episodic memory: men and women are not all that different. Neuroimage. 2006;32 (1):411–422. doi: 10.1016/j.neuroimage.2006.01.044. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92 (1–2):67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Kane A, Tuffiash E, Ulatowski JA, Barker PB, Beauchamp NJ, Wityk RJ. Reperfusion of specific brain regions by raising blood pressure restores selective language functions in subacute stroke. Brain and Language. 2001;79 (3):495–510. doi: 10.1006/brln.2001.2563. [DOI] [PubMed] [Google Scholar]
- Hiraga A, Tanaka S, Kamitsukasa I. Pure dysarthria due to an insular infarction. Journal of Clinical Neuroscience. 2010;17 (6):812–813. doi: 10.1016/j.jocn.2009.10.026. [DOI] [PubMed] [Google Scholar]
- Holland SK, Plante E, Weber Byars A, Strawsburg RH, Schmithorst VJ, Ball WS., Jr Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage. 2001;14 (4):837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Hyde JS, Linn MC. Gender Differences in Verbal Ability: A Meta-Analysis. Psychological Bulletin. 1988;104 (1):53–69. [Google Scholar]
- Ihnen SK, Church JA, Petersen SE, Schlaggar BL. Lack of generalizability of sex differences in the fMRI BOLD activity associated with language processing in adults. Neuroimage. 2009;45 (3):1020–1032. doi: 10.1016/j.neuroimage.2008.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. 2. Lippincott Williams & Wilkins; 2001. [Google Scholar]
- Karunanayaka P, Schmithorst VJ, Vannest J, Szaflarski JP, Plante E, Holland SK. A group independent component analysis of covert verb generation in children: a functional magnetic resonance imaging study. Neuroimage. 2010;51 (1):472–487. doi: 10.1016/j.neuroimage.2009.12.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303 (5660):1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kim J, Whyte J, Wang J, Rao H, Tang KZ, Detre JA. Continuous ASL perfusion fMRI investigation of higher cognition: quantification of tonic CBF changes during sustained attention and working memory tasks. Neuroimage. 2006;31 (1):376–385. doi: 10.1016/j.neuroimage.2005.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura D. Sex differences in the brain. Scientific American. 1992;267 (3):118–125. doi: 10.1038/scientificamerican0992-118. [DOI] [PubMed] [Google Scholar]
- Kimura D. Sex and Cognition. The MIT Press; 1999. [Google Scholar]
- Kozora E, Cullum CM. Generative naming in normal aging: Total output and qualitative changes using phonemic and semantic constraints. The Clinical Neuropsychologist. 1995;9 (4):313–320. [Google Scholar]
- Kramer JH, Delis DC, Kaplan E, O’Donnell L, Prifitera A. Developmental sex differences in verbal learning. Neuropsychology. 1997;11 (4):577–584. doi: 10.1037//0894-4105.11.4.577. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Delis DC, Daniel M. Sex differences in verbal learning. Journal of Clinical Psychology. 1988;44 (6):907–915. [Google Scholar]
- Lawrence NS, Ross TJ, Hoffmann R, Garavan H, Stein EA. Multiple neuronal networks mediate sustained attention. The Journal of Cognitive Neuroscience. 2003;15 (7):1028–1038. doi: 10.1162/089892903770007416. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36 (4):1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levelt WJ, Roelofs A, Meyer AS. A theory of lexical access in speech production. Behavioral and Brain Sciences. 1999;22(1):1–38. doi: 10.1017/s0140525x99001776. discussion 38–75. [DOI] [PubMed] [Google Scholar]
- Lezak M. Neuropsychological Assessment. Oxford University Press; 1995. [Google Scholar]
- Lynn R. Sex Differences on the Differential Aptitude Test in British and American Adolescents. Educational Psychology. 1992;12 (2):101–102. [Google Scholar]
- Mann VA, Sasanuma S, Sakuma N, Masaki S. Sex differences in cognitive abilities: a cross-cultural perspective. Neuropsychologia. 1990;28 (10):1063–1077. doi: 10.1016/0028-3932(90)90141-a. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Blamire AM, Rothman DL, Gruetter R, Shulman RG. Echo-planar magnetic resonance imaging studies of frontal cortex activation during word generation in humans. Proceedings of the National Academy of Sciences of the United States of America. 1993;90 (11):4952–4956. doi: 10.1073/pnas.90.11.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munhall KG. Functional imaging during speech production. Acta Psychologica (Amsterdam) 2001;107 (1–3):95–117. doi: 10.1016/s0001-6918(01)00026-9. [DOI] [PubMed] [Google Scholar]
- Murray AD, Johnson J, Peters J. Fine-tuning of utterance length to preverbal infants: effects on later language development. The Journal of Child Language. 1990;17 (3):511–525. doi: 10.1017/s0305000900010862. [DOI] [PubMed] [Google Scholar]
- Nickels L, Howard D. Correct responses, error analyses, and theories of word production: A response to Martin. Cognitive Neuropsychology. 2004a;21 (5):531–536. doi: 10.1080/02643290442000059. [DOI] [PubMed] [Google Scholar]
- Nickels L, Howard D. Dissociating Effects of Number of Phonemes, Number of Syllables, and Syllabic Complexity on Word Production in Aphasia: It’s the Number of Phonemes that Counts. Cognitive Neuropsychology. 2004b;21 (1):57–78. doi: 10.1080/02643290342000122. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9 (1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oomen CC, Postma A, Kolk HH. Prearticulatory and postarticulatory self-monitoring in Broca’s aphasia. Cortex. 2001;37 (5):627–641. doi: 10.1016/s0010-9452(08)70610-5. [DOI] [PubMed] [Google Scholar]
- Perrin JS, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, Veillette S, Pausova Z, Paus T. Sex differences in the growth of white matter during adolescence. Neuroimage. 2009;45 (4):1055–1066. doi: 10.1016/j.neuroimage.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988;331 (6157):585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Hyder F, Blamire AM, Shulman RG. FMRI of the prefrontal cortex during overt verbal fluency. Neuroreport. 1997;8 (2):561–565. doi: 10.1097/00001756-199701200-00036. [DOI] [PubMed] [Google Scholar]
- Plante E, Schmithorst VJ, Holland SK, Byars AW. Sex differences in the activation of language cortex during childhood. Neuropsychologia. 2006;44 (7):1210–1221. doi: 10.1016/j.neuropsychologia.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Annals of the New York Academy of Sciences. 2010;1191 (1):62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Pulvermuller F. Brain reflections of words and their meaning. Trends in Cognitive Sciences. 2001;5 (12):517–524. doi: 10.1016/s1364-6613(00)01803-9. [DOI] [PubMed] [Google Scholar]
- Rosen BR, Buckner RL, Dale AM. Event-related functional MRI: past, present, and future. Proceedings of the National Academy of Sciences of the United States of America. 1998;95 (3):773–780. doi: 10.1073/pnas.95.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Ojemann JG, Ollinger JM, Petersen SE. Comparison of brain activation during word retrieval done silently and aloud using fMRI. Brain and Cognition. 2000;42 (2):201–217. doi: 10.1006/brcg.1999.1100. [DOI] [PubMed] [Google Scholar]
- Roulstone S, Loader S, Northstone K, Beveridge M. The Speech and Language of Children Aged 25 Months: Descriptive Data from the Avon Longitudinal Study of Parents and Children. Early Child Development and Care. 2002;172 (3):259–268. [Google Scholar]
- Schmithorst VJ. Separate cortical networks involved in music perception: preliminary functional MRI evidence for modularity of music processing. Neuroimage. 2005;25 (2):444–451. doi: 10.1016/j.neuroimage.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Dardzinski BJ, Holland SK. Simultaneous correction of ghost and geometric distortion artifacts in EPI using a multiecho reference scan. IEEE Transactions on Medical Imaging. 2001;20 (6):535–539. doi: 10.1109/42.929619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK. Event-related fMRI technique for auditory processing with hemodynamics unrelated to acoustic gradient noise. Magnetic Resonance in Medicine. 2004;51 (2):399–402. doi: 10.1002/mrm.10706. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK. Functional MRI evidence for disparate developmental processes underlying intelligence in boys and girls. Neuroimage. 2006;31 (3):1366–1379. doi: 10.1016/j.neuroimage.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK. Sex differences in the development of neuroanatomical functional connectivity underlying intelligence found using Bayesian connectivity analysis. Neuroimage. 2007;35 (1):406–419. doi: 10.1016/j.neuroimage.2006.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK, Dardzinski BJ. Developmental differences in white matter architecture between boys and girls. Human Brain Mapping. 2008;29 (6):696–710. doi: 10.1002/hbm.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Constable RT, Skudlarski P, Fulbright RK, Bronen RA, Fletcher JM, Shankweiler DP, Katz L, Gore JC. Sex differences in the functional organization of the brain for language. Nature. 1995;373 (6515):607–609. doi: 10.1038/373607a0. [DOI] [PubMed] [Google Scholar]
- Shivde G, Thompson-Schill SL. Dissociating semantic and phonological maintenance using fMRI. Cognitive, Affective, & Behavioral Neuroscience. 2004;4 (1):10–19. doi: 10.3758/cabn.4.1.10. [DOI] [PubMed] [Google Scholar]
- Simard F, Joanette Y, Petrides M, Jubault T, Madjar C, Monchi O. Fronto-striatal Contribution to Lexical Set-Shifting. Cerebral Cortex. 2010 doi: 10.1093/cercor/bhq182. [DOI] [PubMed] [Google Scholar]
- Sommer IE, Aleman A, Bouma A, Kahn RS. Do women really have more bilateral language representation than men? A meta-analysis of functional imaging studies. Brain. 2004;127 (Pt 8):1845–1852. doi: 10.1093/brain/awh207. [DOI] [PubMed] [Google Scholar]
- Sommer IE, Aleman A, Somers M, Boks MP, Kahn RS. Sex differences in handedness, asymmetry of the planum temporale and functional language lateralization. Brain Research. 2008;1206:76–88. doi: 10.1016/j.brainres.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Holland SK, Jacola LM, Lindsell C, Privitera MD, Szaflarski M. Comprehensive presurgical functional MRI language evaluation in adult patients with epilepsy. Epilepsy and Behavior. 2008;12 (1):74–83. doi: 10.1016/j.yebeh.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Holland SK, Schmithorst VJ, Byars AW. fMRI study of language lateralization in children and adults. Human Brain Mapping. 2006;27 (3):202–212. doi: 10.1002/hbm.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. Thieme Medical Publishers; 1988. [Google Scholar]
- Thevenaz P, Ruttimann UE, Unser M. A pyramid approach to subpixel registration based on intensity. IEEE Transactions on Image Processing. 1998;7 (1):27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proceedings of the National Academy of Sciences of the United States of America. 1997;94 (26):14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillema JM, Byars AW, Jacola LM, Schapiro MB, Schmithorst VJ, Szaflarski JP, Holland SK. Cortical reorganization of language functioning following perinatal left MCA stroke. Brain and Language. 2008;105 (2):99–111. doi: 10.1016/j.bandl.2007.07.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannest J, Rasmussen J, Eaton KP, Patel K, Schmithorst V, Karunanayaka P, Plante E, Byars A, Holland S. FMRI activation in language areas correlates with verb generation performance in children. Neuropediatrics. 2010;41 (5):235–239. doi: 10.1055/s-0030-1267982. [DOI] [PubMed] [Google Scholar]
- Weiss EM, Siedentopf C, Hofer A, Deisenhammer EA, Hoptman MJ, Kremser C, Golaszewski S, Felber S, Fleischhacker WW, Delazer M. Brain activation pattern during a verbal fluency test in healthy male and female volunteers: a functional magnetic resonance imaging study. Neuroscience Letters. 2003a;352 (3):191–194. doi: 10.1016/j.neulet.2003.08.071. [DOI] [PubMed] [Google Scholar]
- Weiss EM, Kemmler G, Deisenhammer EA, Fleischhacker WW, Delazer M. Sex differences in cognitive functions. Personality and Individual Differences. 2003b;35 (4):863–875. [Google Scholar]
- Wendelken C, Bunge SA, Carter CS. Maintaining structured information: An investigation into functions of parietal and lateral prefrontal cortices. Neuropsychologia. 2008;46 (2):665–678. doi: 10.1016/j.neuropsychologia.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited--again. Neuroimage. 1995;2 (3):173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Yetkin FZ, Hammeke TA, Swanson SJ, Morris GL, Mueller WM, McAuliffe TL, Haughton VM. A comparison of functional MR activation patterns during silent and audible language tasks. American Journal of Neuroradiology. 1995;16 (5):1087–1092. [PMC free article] [PubMed] [Google Scholar]