Abstract
DNA repair plays a critical role in human cancers. We hypothesized that DNA mismatch repair gene variants are associated with risk of pancreatic cancer. We retrospectively genotyped 102 single-nucleotide polymorphisms (SNPs) of 13 mismatch repair related genes in 706 patients with pancreatic cancer and 706 cancer-free controls using the mass spectroscopy–based MassArray method. Association of genotype with pancreatic cancer risk was tested by multivariate logistic regression models. A significance level of P≤0.0015 was set at the false discovery rate (FDR) <1% using the Beta-Uniform Mixture method. We found 28 SNPs related to altered pancreatic cancer risk (P<0.05). Adjusting for multiple comparisons, MGMT I143V AG/GG, PMS2 IVS1-1121C>T TC/TT, and PMS2L3 Ex1+118C>T CT/TT genotypes showed significant main effects on pancreatic cancer risk at FDR <1% with OR (95% CI) of 0.60 (0.46-0.80), 1.44 (1.14-1.81) and 5.54 (2.10-14.61), respectively (P≤0.0015). To demonstrate genotype-phenotype association, we measured O6-ethylguanosine (O6-EtGua) adduct levels in vitro by immunoslot blot assay in lymphocytes treated with N-ethyl-N-nitrosourea (ENU) in 297 case/control subjects. MGMT I143V GG, MGMT K178R GG, MSH6 G39E AG/AA, PMS2L3 IVS3+9A>G GA and TP73 IVS1-7449G>C CG/CC genotypes correlated with a higher level of ENU-induced DNA adducts. Haplotypes of MGMT, MSH6, PMS2, PMS2L3, and TP73 were significantly associated with pancreatic cancer risk (P≤0.0015). Our findings suggest that mismatch repair gene variants may affect susceptibility to pancreatic cancer.
Keywords: mismatch repair, single-nucleotide polymorphism, pancreatic cancer, susceptibility, haplotype
Introduction
Pancreatic cancer ranks as the fourth-leading cause of cancer mortality in the United States.[1] It is a notoriously aggressive and difficult-to-treat malignancy expressing global genomic instability (e.g., mutation, translocation, and deletion) and aneuploidy.[1,2] Identification of predisposing genetic factors and environmental and lifestyle risk factors is critical for the primary prevention of this fatal disease. Five to ten percent of pancreatic cancer patients have inherited germline disorders, including mutations of genes responsible for Peutz-Jeghers syndrome (STK11), hereditary pancreatitis (PRSS1, SPINK1), Lynch syndrome (MLH1, MSH2), familial atypical multiple-mole melanoma (p16), cystic fibrosis (CTFR), hereditary breast-ovarian cancer (BRCA1, BRCA2), familial adenomatous polyposis (APC), or family X site-specific pancreatic cancer (PALLD).[2-4] Some of these genes (e.g., MLH1, MSH2, BRCA1, and BRCA2) function in DNA repair and cellular response to DNA damage. Most pancreatic cancers harbor genetic alterations in KRAS2, p16, TP53, or SMAD4.[2-4] Moreover, DNA damage control and apoptosis pathways have been identified as part of the core signaling pathways in human pancreatic cancers.[5] Several case-control studies have examined the associations between risk of pancreatic cancer and genes involved in base excision repair, nucleotide excision repair, and homologous recombination DNA repair.[6-10] Although these genes were observed to have little significant main effect, several of them showed significant interactions with cigarette smoking, a well-established risk factor for pancreatic cancer.[11]
The genes of the mismatch repair (MMR) pathway have yet to be examined in these studies of DNA repair genes. As a multifaceted DNA-repair system, MMR improves the fidelity of DNA replication, maintains genome stability, and affects DNA damage signaling, apoptosis, and cell type–specific processes.[12,13] MMR deficiency confers a mutated phenotype susceptible to cancer.[12] Human MMR is complicated machinery encompassing several functional complexes.[12,13]
To test the hypothesis that genetic variants in MMR modify the risk of pancreatic cancer, we evaluated 13 genes encoding key nodes for DNA mismatch recognition and removal (MutS homolog 2 [MSH2], MSH3, MSH6, MutL homolog 1 [MLH1], MLH3, postmeiotic segregation increased 1 [PMS1], PMS2, PMS2-like 3 [PMS2L3], exonuclease I [EXO1], and three prime repair exonuclease 1 [TREX1]), or MMR interaction component O6-methylguanine-DNA methyltransferase [MGMT], or MMR-induced apoptosis transducer (tumor protein 73 [TP73]), or MMR regulator RecQ protein-like/DNA helicase Q1-like (RECQL).[14] MutSα homolog MSH2/MSH6 recognizes base-base mismatches and small insertion/deletion loops (IDLs).[12] MutSβ homolog MSH2/MSH3 recognizes small and large IDLs.[12] MutLα homolog MLH1/PMS2 forms a ternary complex with mismatched DNA and MutSα, increases discrimination between heteroduplexes and homoduplexes, and acts in meiotic recombination.[12] Loss of MLH1 or PMS2 brings about mutated and microsatellite instability phenotypes.[12] MMR (MLH1, MSH2, MSH6, and PMS2) germline mutations cause Lynch syndrome/hereditary nonpolyposis colorectal cancer.[12] PMS2L3 encodes a polypeptide homologous to PMS2.[15] As a member of the PMS2 family, PMS2L3 contains motifs conserved in MutL elements.[15] MutLβ homolog MLH1/PMS1 and MutLγ homolog MLH1/MLH3 act in meiotic recombination and as backup for MutLα in repairing base-base mismatches/small IDLs.[12] EXO1 encoding 5′-3′ exonuclease is critical in MMR and recombination by interacting with MSH2 and removing biosynthetic errors.[16] The TREX1 gene encodes 3′-5′ DNA exonuclease to proofread for DNA polymerase and degrade the 3′ ends of nicked DNA.[17,18] MGMT, involved in DNA direct reversal repair, defends against alkylating agents by interacting with MutSα, MutLα, and EXO1.[19,20] RECQL, a RecQ DNA helicase family member, has been found to interact with MMR proteins in regulating genetic recombination.[21] TP73 encodes p73, a p53 family member, which acts in cellular growth, development, and responses to DNA damage.[22,23] MMR-mediated apoptosis can be induced by p73.[24] We examined 102 single-nucleotide polymorphisms (SNPs) of the 13 genes in 706 pancreatic cancer patients and 706 controls.
Materials and Methods
Study population and data collection
The study design and data collection have been described in detail previously.[25] This hospital-based case-control study included 706 patients with pathologically confirmed primary pancreatic ductal adenocarcinoma and 706 controls recruited at The University of Texas MD Anderson Cancer Center between 2000 and 2007.[7,26] The recruitment response rates of cases and controls were 80.6% and 76.9%, respectively.[7] All study subjects were U.S. residents and communicated in English. By personal interview, we collected information on demographics (age, sex, and self-reported race), cigarette smoking, alcohol consumption, medical history (e.g., diabetes), family history of cancer, and other risk factors. Control subjects were recruited from healthy spouses, friends, and non-blood relatives of patients with various types of cancers other than smoking-related or gastrointestinal cancers. Controls were frequency matched to cases by sex, race, and age at enrollment (±5 years). We collected a blood sample from each participant at enrollment. Cumulative smoking was calculated in “pack-years” [pack-years = (packs per day) × (years smoked)]. Alcohol consumption was calculated in terms of milliliters of ethanol consumed daily, with 12.0 oz of beer, 4.0 oz of wine, and 1.5 oz of hard liquor each considered to be equivalent to ~12.0 grams of ethanol. Body mass index (BMI, kg/m2) was calculated on the basis of self-reported weight and height at age 34 to 39 years in 363 cases and 425 controls (this information was collected only after January 2004). Family history of cancer in first-degree relatives was noted. Each study participant signed a written informed consent for the interview and blood sample donation. The study was approved by the MD Anderson Cancer Center institutional review board and conducted according to all current ethical guidelines.[7,27]
DNA extraction, SNP selection, and genotyping
DNA was extracted from peripheral-blood lymphocytes of 1385 study participants and from paraffin sections of normal tissues of 27 patients using Qiagen DNA isolation kits (Valencia, CA).[7,27] From the HapMap Project database (www.hapmap.org), we selected 45 tagging SNPs using the SNPbrowser (Applied Biosystems, Carlsbad, CA; www.allsnps.com/snpbrowser) with a cutoff of r2=0.8 and a minor allele frequency (MAF) ≥10% in Caucasians. For more comprehensive coverage of the polymorphisms, we chose 57 additional SNPs from the coding region (nonsynonymous or synonymous), untranslated region (UTR), promoter region, or splicing sites with a MAF ≥1% in Caucasians. The genes, nucleotide substitutions, functions (e.g., encoding amino acid changes), reference SNP identification numbers, and reported allele frequencies of the 102 SNPs were summarized in Supplemental Table 1. The mRNA transcripts, protein sequences, structures, homology models, and predicted functions for the SNPs were evaluated by F-SNP software (Queen’s University, Kingston, Ontario, Canada).[28] Genotyping was performed with the mass spectroscopy–based MassArray system (Sequenom, Inc, San Diego, CA). Twenty percent of the DNA samples were genotyped in duplicate, with 99.6% concordance. We excluded the inconsistent genotyping data from the final analysis.
In vitro ENU-Induced DNA adduct assay
As a phenotypic marker for the repair of DNA alkylation adducts, the N-ethyl-N-nitrosourea (ENU)-induced O6-ethylguanine (O6−EtGua) level was measured in peripheral blood lymphocytes that were treated with 0.64 mM ENU in vitro as previously described.[29] Semi-quantification of ENU-induced O6-EtGua was conducted by immunoslot blot.[29] 5μg DNA was slot-blotted onto Hybond-N+ Nylon membranes using Convertible Filtration Manifold System. After single-stranded DNA was immobilized onto the nitrocellular membranes, the membranes were blocked and hybridized with the (O6-EtGua)-specific mouse monoclonal antibody EM-2-3.[29] The membrane was hybridized with the secondary antibody goat anti-mouse horseradish peroxidase-labeled IgG. Enzymatic activity was visualized by chemiluminescence reaction with an ECL™ Western blotting detection kit. A standard for DNA measurement was generated by in vitro reaction of calf thymus DNA with 3H-labeled N-methyl-N-nitrosourea, and the DNA adduct levels were determined by scintillation counting. Standard curves were established from the series of DNA concentrations by using the immunoslot blot assay. The intensity of the band in each slot was compared with the standard curve to semi-quantify the adduct levels. Each sample was analyzed in duplicates and the arithmetic mean was computed from the parallel samples to represent the actual value of each sample.
Statistical analysis
We examined the genotype distribution for the Hardy-Weinberg equilibrium (HWE) by the goodness-of-fit χ2 test and calculated genotype/allele frequency of the SNPs by direct gene counting. We calculated haplotype diversity and linkage disequilibrium index (Lewontin’s |D’| and r2) by using SNPAlyze (DYNACOM Co., Ltd., Mobara, Japan) and Haplostats (Mayo Clinic, Rochester, MN). The heterozygous and homozygous genotypes were combined if the homozygous variant had a very low frequency (number of homozygote < 4) or if the homozygous and heterozygous genotypes exerted a similar effect on the risk for pancreatic cancer.
Odds ratio (OR) and 95% confidence interval (CI) were estimated by logistic regression analysis with adjustment for age, race, sex, smoking history (never, ≤20 pack-years, >20 pack-years), alcohol consumption (never, ≤60 grams of ethanol/day, >60 grams of ethanol/day), diabetes (yes or no), family history of cancer in first-degree relatives (yes or no), and BMI (≤25 kg/m2 or >25 kg/m2) in some analyses when appropriate. Non-drinkers and light drinkers were defined as consuming 0 gram and up to 60 grams of ethanol/day, respectively, and heavy drinkers as consuming >60 grams of ethanol/day. Statistical analysis was carried out by SPSS (SPSS Inc, Chicago, IL) and Stata (StataCorp LP, College Station, TX). We calculated the false discovery rate (FDR) using the Beta-Uniform Mixture method.[30] We found a P-value of 0.0015 corresponded to an FDR of 1%. We defined P ≤0.0015 in the genotype analysis as statistically significant.
The value of O6-EtGua measurement was square-root transformed to ensure the normal distribution for parametric testing and for stabilizing the group variance.[29] An analysis of covariance (ANCOVA) was used to compare the difference in mean adduct levels between genotypes adjusting for age, sex, race, smoking and alcohol consumption. The heterozygous and homozygous genotype was combined when both showed the same effect on the level of adducts. In the multiple linear regression, the percentage of the variance of dependent variable (adduct level) explained by the polymorphism and other variable was evaluated by subtracting the r2 value for the full model from the r2 for a model that excludes the test variable. The full models contained covariates age, race, sex, alcohol, smoking and the polymorphisms terms. Separate models were run for the nonsmokers and the smokers. Multiplicity adjusted P value was calculated.[29]
Results
Characteristics of study subjects
The demographics and risk factors in the study population were summarized in Table 1. Cases and controls were well matched by sex, age, and race. As reported previously, smoking, diabetes, obesity, and family history of cancer had been associated with increased risk of pancreatic cancer.[25]
Table 1. Characteristics of the study population.
| Variables | Case, n (%) (N=706) |
Control, n (%) (N=706) |
OR1 (95% CI) | P |
|---|---|---|---|---|
| Sex | Matching Factor | |||
| Female | 281 (39.8) | 269 (38.1) | ||
| Male | 425 (60.2) | 437 (61.9) | ||
| Race | Matching Factor | |||
| White | 624 (88.4) | 630 (89.2) | ||
| Nonwhite | 82 (11.6) | 76 (10.8) | ||
| Age, years | Matching Factor | |||
| < 50 | 77 (10.9) | 99 (14.0) | ||
| 50-60 | 173 (24.5) | 197 (27.9) | ||
| 60-70 | 265 (37.5) | 251 (35.6) | ||
| ≥ 70 | 191 (27.1) | 159 (22.5) | ||
| Diabetes | ||||
| No | 518 (73.4) | 627 (88.8) | 1.0 | |
| Yes | 188 (26.6) | 79 (11.2) | 2.77 (2.04-3.76) | <0.001 |
| Smoking | ||||
| Never | 285 (40.4) | 360 (51.0) | 1.0 | |
| ≤ 20 pack years | 175 (24.8) | 175 (24.8) | 1.32 (0.99-1.76) | 0.057 |
| > 20 pack years | 246 (34.8) | 171 (24.2) | 1.68 (1.27-2.22) | <0.001 |
| Alcohol consumption2 | ||||
| Never | 319 (46.9) | 325 (46.2) | 1.0 | |
| ≤ 420 g/week | 284 (41.8) | 324 (46.1) | 1.05 (0.81-1.35) | 0.72 |
| > 420 g/week | 77 (11.3) | 54 (7.7) | 1.44 (0.94-2.20) | 0.09 |
| 0-420 g/week vs. >420 g/week | 1.48 (1.03-2.13) | 0.03 | ||
| Family history of cancer3 | ||||
| No | 262 (37.3) | 318 (45.4) | 1.0 | |
| Yes | 441 (62.7) | 382 (54.6) | 1.56 (1.24-1.96) | <0.001 |
| Body mass index (kg/m2)4 | ||||
| < 25 | 188 (51.8) | 254 (59.8) | 1.0 | |
| 25-30 | 130 (35.8) | 144 (33.9) | 1.22 (0.90-1.65) | 0.20 |
| ≥ 30 | 45 (12.4) | 27 (6.4) | 2.25 (1.35-3.76) | 0.002 |
Odds ratios and P values were from logistic regression analysis adjusted for sex, race, age (continuous), diabetes, smoking (non-smoker, ≤20 pack years and >20 pack years), alcohol consumption (0-420 g/week vs. >420 g/week), body mass index, and family history of cancer in a first-degree relative.
Missing values from 26 cases and 3 controls.
Missing values from 3 cases and 6 controls.
Information was available for only 363 cases and 425 controls.
Genotype distribution and allele frequencies
The observed MAFs of the 102 SNPs in the study population were comparable to those reported in the general population (Supplemental Table 1). Linkage disequilibrium data were presented in Supplemental Table 2. Most of the 102 SNPs followed HWE; exceptions were MGMT IVS4-44836G>A, MLH1 I219V, MSH2 IVS11-62G>A, PMS2 P470S, N775S, −153C>G, PMS2L3 IVS3+9A>G, and RECQL IVS8+190A>G in both cases and controls (P<0.05).
Main effect of genotype
Twenty-eight SNPs of MGMT, MLH1, MLH3, MSH2, MSH3, PMS2, PMS2L3, RECQL, TP73, and TREX1 correlated with altered risk of pancreatic cancer after adjusting for other risk factors (P≤0.05, Table 2). MGMT I143V AG/GG, PMS2 IVS1-1121C>T TC/TT, and PMS2L3 Ex1+118C>T CT/TT remained statistically significant predictors for altered pancreatic cancer risk after adjusting for multiple comparisons (P≤0.0015). The corresponding ORs (95% CI) were 0.60 (0.46-0.80), 1.44 (1.14-1.81), and 5.54 (2.10-14.61), respectively (Table 2).
Table 2. Association of genotype with pancreatic cancer risk.
| Function | Genotype | Case, n/n | Control, n/n | OR1 (95% CI) | P1 | |
|---|---|---|---|---|---|---|
| Recognition | O6G | MGMT I143V (AA vs. AG/GG) | 501/112 | 472/170 | 0.60 (0.46-0.80) | <0.001 |
| MGMT K178R (AA vs. AG/GG) | 533/170 | 490/215 | 0.73 (0.57-0.93) | 0.01 | ||
| MGMT IVS5+23129G>A (AA vs. AG/GG) | 226/473 | 185/517 | 0.76 (0.60-0.97) | 0.027 | ||
| MutSα | MSH2 IVS9-9A>T (AA/TA vs. TT) | 525/18 | 547/5 | 3.81 (1.36-10.68) | 0.01 | |
| MSH6 G39E (GG vs. AG/AA) | 430/247 | 382/284 | 0.73 (0.58-0.92) | 0.008 | ||
| MSH6 IVS4-101G>C (CC vs. CG/GG) | 371/215 | 335/274 | 0.70 (0.55-0.89) | 0.0037 | ||
| MutSβ | MSH3 I79V (GG vs. GA/AA) | 367/245 | 344/166 | 1.33 (1.03-1.72) | 0.027 | |
| MSH3 Ex24-318G>A (GG/GA vs. AA) | 689/17 | 694/4 | 3.39 (1.11-10.3) | 0.03 | ||
| MutLα (γ) | MLH1 IVS3-659A>C (AA vs. CA/CC) | 231/472 | 187/516 | 0.73 (0.57-0.92) | 0.009 | |
| MLH1 IVS12-169C>T (CC vs. CT/TT) | 224/482 | 187/519 | 0.74 (0.59-0.91) | 0.006 | ||
| MLH1 IVS14-19A>G (AA vs. AG/GG) | 200/489 | 171/534 | 0.78 (0.61-1.00) | 0.05 | ||
| MLH3 P844L (CC vs. TC/TT) | 216/480 | 183/515 | 0.74 (0.60-0.92) | 0.007 | ||
| PMS2 T485K (CC vs. CA/AA) | 620/81 | 584/118 | 0.67 (0.49-0.91) | 0.01 | ||
| PMS2 IVS1-1121C>T (CC vs. TC/TT) | 260/354 | 317/322 | 1.44 (1.14-1.81) | 0.0015 | ||
| PMS2 IVS7+442G>T (GG vs. GT/TT) | 345/236 | 326/279 | 0.77 (0.60-0.98) | 0.036 | ||
| PMS2L3 Ex1+118C>T (CC vs. CT/TT) | 653/30 | 674/5 | 5.54 (2.10-14.61) | <0.001 | ||
| PMS2L3 IVS2-1578A>G (GG/AA vs. AG) | 365/238 | 322/301 | 0.70 (0.55-0.88) | 0.0027 | ||
| PMS2L3 IVS3+9A>G (AA/GG vs. GA) | 271/434 | 219/486 | 0.70 (0.56-0.89) | 0.0029 | ||
| Excision | Exonuclease | TREX1 Ex14+782T>C (TT/CT vs. CC) | 677/24 | 662/44 | 0.55 (0.32-0.93) | 0.026 |
| Apoptosis | TP73 | TP73 A610A (GA/AA vs. GG) | 629/69 | 643/50 | 1.54 (1.03-2.29) | 0.03 |
| TP73 IVS1-7449G>C (GG vs. CG/CC) | 251/438 | 210/476 | 0.73 (0.58-0.93) | 0.0097 | ||
| Regulation | RECQL | RECQL -5349A>G (GG/GA vs. AA) | 627/63 | 640/41 | 1.54 (1.01-2.34) | 0.046 |
| RECQL IVS1-92C>T (CC/TC vs. TT) | 599/43 | 592/25 | 1.74 (1.02-2.95) | 0.04 | ||
| RECQL IVS4-795C>T (CC/TT vs. CT) | 375/255 | 290/276 | 0.72 (0.57-0.91) | 0.006 | ||
| RECQL IVS5-717C>A (CC/CA vs. AA) | 651/48 | 666/30 | 1.63 (1.00-2.65) | 0.05 | ||
| RECQL IVS8+190A>G (AA vs. AG/GG) | 292/401 | 338/359 | 1.27 (1.02-1.58) | 0.03 | ||
| RECQL IVS11+582T>A (TT/AA vs. TA) | 293/230 | 219/250 | 0.68 (0.53-0.89) | 0.004 | ||
| RECQL *1236A>G (AA vs. AG/GG) | 212/283 | 146/261 | 0.73 (0.55-0.97) | 0.029 | ||
O6G, O(6)-alkyl-guanine; OR, Odds ratio.
OR and P values were from logistic regression analysis adjusted for sex, race, age, diabetes, smoking, alcohol consumption, and family history of cancer.
SNPs with P>0.05 are not listed.
Genotype association with O6-EtGua levels by smoking status
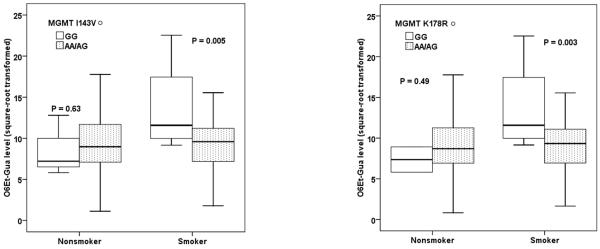
To demonstrate whether the genotypes are associated with the DNA repair capacity, we compared the O6-EtGua levels between genotypes for those SNPs that showed altered risk of pancreatic cancer as listed in Table 2. The square-root transformed value (mean ± SE) of O6-EtGua (fmol/μg DNA) was 9.3 ± 0.4 in 123 patients with pancreatic cancer and 8.8 ± 0.2 in 122 non-cancer controls (p=0.19). For power consideration, we analyzed the pooled data from both cases and controls, and the results were comparable when the analysis was conducted in controls only (Table 3). We found that MGMT I143V, MGMT K178R, MSH6 G32E, PMS2L3 IVS3+9A>G and TP73 IVS1-7449G>C genotypes correlated with O6-EtGua level (p<0.05). Multiple linear regression analyses showed a differential genotype effect on DNA adduct level by smoking status. MGMT and MSH6 genotypes correlated with DNA adduct levels mainly in smokers; while PMS2L3 and TP73 genotypes affected the DNA adduct levels mainly in nonsmokers. For example, MGMT 143V GG and MGMT K178R GG genotypes had higher adduct levels in smokers but lower adduct levels in non-smokers than their counterparts (Fig. 1).
Table 3. Association of genotype with DNA damage level.
|
O6-EtGua§ (Mean ± SE) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Case/control | Control | |||||||
| Genotype | N | All | Nonsmoker | Smoker | N | All | Nonsmoker | Smoker |
| MGMTI143V | ||||||||
| GG | 7 | 11.5 ± 2.1 | 8.6 ± 2.1 | 13.7 ± 3.0 | 6 | 12.2 ± 2.3 | 9.3 ± 3.5 | 13.7 ± 3.0 |
| AG | 55 | 9.4 ± 0.4 | 9.5 ± 0.6 | 9.2 ± 0.5 | 48 | 9.4 ± 0.4 | 9.5 ± 0.7 | 9.4 ± 0.6 |
| AA | 179 | 9.3 ± 0.2 | 9.5 ± 0.3 | 9.0 ± 0.3 | 147 | 9.2 ± 0.3 | 9.5 ± 0.4 | 8.8 ± 0.4 |
| AA/AG | 9.3 ± 0.2 | 9.5 ± 0.3 | 9.1 ± 0.3 | 9.2 ± 0.2 | 9.5 ± 0.3 | 8.9 ± 0.3 | ||
| p* | 0.02 | 0.63 | 0.005 | 0.03 | 0.94 | 0.007 | ||
| MGMTK178R | ||||||||
| GG | 6 | 11.6 ± 2.4 | 7.4 ± 1.5 | 13.7 ± 3.0 | 6 | 11.6 ± 2.3 | 7.4 ± 1.5 | 13.7 ± 3.0 |
| AG | 78 | 9.0 ± 0.4 | 9.2 ± 0.5 | 8.8 ± 0.5 | 65 | 9.0 ± 0.4 | 9.2 ± 0.6 | 8.7 ± 0.6 |
| AA | 211 | 8.8 ± 0.2 | 8.9 ± 0.3 | 8.8 ± 0.3 | 163 | 8.8 ± 0.3 | 9.1 ± 0.4 | 8.5 ± 0.4 |
| AA/AG | 8.9 ± 0.2 | 9.0 ± 0.3 | 8.8 ± 0.2 | 8.9 ± 0.2 | 9.2 ± 0.3 | 8.5 ± 0.3 | ||
| p | 0.05 | 0.49 | 0.003 | 0.058 | 0.46 | 0.004 | ||
| MSH6 G39E | ||||||||
| AG/AA | 116 | 9.6 ± 0.3 | 9.5 ± 0.5 | 9.7 ± 0.5 | 98 | 9.8 ± 0.4 | 9.9 ± 0.5 | 9.4 ± 0.5 |
| GG | 158 | 8.8 ± 0.2 | 9.1 ± 0.4 | 8.6 ± 0.3 | 117 | 8.6 ± 0.3 | 9.0 ± 0.4 | 8.2 ± 0.4 |
| p | 0.009 | 0.51 | 0.047 | 0.01 | 0.15 | 0.03 | ||
| PMS2L3 IVS3+9A>G | ||||||||
| GA | 243 | 9.1 ± 0.2 | 9.3 ± 0.3 | 8.9 ± 0.3 | 201 | 9.1 ± 0.2 | 9.4 ± 0.3 | 8.8 ± 0.4 |
| AA/GG | 54 | 8.1 ± 0.4 | 7.5 ± 0.5 | 8.8 ± 0.6 | 34 | 8.0 ± 0.5 | 7.7 ± 0.6 | 8.3 ± 0.8 |
| p | 0.05 | 0.01 | 0.86 | 0.077 | 0.046 | 0.60 | ||
| TP73 IVS1-7449G>C | ||||||||
| CG/CC | 193 | 9.3 ± 0.2 | 9.5 ± 0.3 | 9.2 ± 0.3 | 151 | 9.3 ± 0.3 | 9.6 ± 0.4 | 9.1 ± 0.4 |
| GG | 89 | 8.4 ± 0.3 | 8.1 ± 0.5 | 8.7 ± 0.5 | 72 | 8.4 ± 0.4 | 8.4 ± 0.5 | 8.4 ± 0.5 |
| p | 0.03 | 0.03 | 0.38 | 0.058 | 0.098 | 0.33 | ||
O6-EtGua, O6-ethylguanosine; SE, standard error.
Square-root transformed value of O6-EtGua original level (fmol/μg DNA).
P was from ANCOVA F test adjusted for age, race, sex, smoking, and alcohol consumption.
Fig.1. Box-plot of adduct level by genotype and smoking status.
The square-root transformed value of original DNA O6Et-Gua-adduct levels were associated with MGMT I143V (left panel) and MGMT K178R (right panel) in 297 subjects. P value was from ANCOVA F test. The solid line indicated median; the box extents marked the 25th and 75th percentile of the observed value, and the capped bars indicated the 10th and 90th percentile. Symbol indicated the outliers of adduct level.
Association of haplotype diversity with pancreatic cancer risk
MGMT, MSH6, PMS2, PMS2L3, and TP73 haplotypes correlated with pancreatic cancer risk after adjusting for multiple comparisons (P≤0.0015, Table 4). The associations manifested the effects of MGMT AATA of IVS4-44836G>A, IVS4-75473G>A; IVS4-7901C>T, IVS5+23129G>A; MSH6 G39E AG/AA (protective), IVS4-101G>C CC; PMS2 IVS1-1121C>T TC/TT, IVS7+442G>T GT/TT; PMS2L3 IVS3+9A>G GA/GG, IVS2-1578A>G AG, Ex1+118C>T CT/TT; and TP73 IVS1-7449G>C CG/CC (protective) and A610A GA/AA genotypes on pancreatic cancer risk. Other haplotypes with P>0.0015 but <0.05 in logistic regression models were shown in Supplemental Table 3.
Table 4. Association of haplotype with pancreatic cancer risk.
| Haplotype | Frequency (Case/Control) | OR1 (95% CI) | P1 |
|---|---|---|---|
| MGMT | |||
| GGCG | 0.1268/0.1113 | 1.0 | |
| AATG | 0.0912/0.0756 | 2.95 (2.09-4.18) | <0.001 |
| GGTA | 0.0766/0.0955 | 0.54 (0.37-0.78) | 0.001 |
| AATA | 0.094/0.05 | 3.07 (1.74-5.41) | <0.001 |
| MSH6 | |||
| GTCGA | 0.1908/0.2241 | 1.0 | |
| GTCCA | 0.1709/0.1216 | 1.93 (1.35-2.75) | <0.001 |
| ATCGA | 0.1116/0.126 | 0.61 (0.45-0.82) | 0.001 |
| PMS2 | |||
| TACCGGTA | 0.1382/0.2323 | 1.0 | |
| CACTTTTA | 0.0948/0.0776 | 2.03 (1.41-2.92) | <0.001 |
| TACCGGCG | 0.07/0.0464 | 4.11 (2.74-6.16) | <0.001 |
| CGGCTGTA | 0.061/0.048 | 3.13 (2.04-4.80) | <0.001 |
| CACTTGCG | 0.0502/0.0322 | 6.83 (3.83-12.1) | <0.001 |
| CACCTGTA | 0.0495/0.0368 | 2.98 (1.84-4.83) | <0.001 |
| CAGCTGTA | 0.0482/0.0198 | 3.00 (1.92-4.69) | <0.001 |
| CACCTTCG | 0.0466/0.0406 | 2.81 (1.78-4.43) | <0.001 |
| PMS2L3 | |||
| GAACC | 0.3663/0.3768 | 1.0 | |
| GAGCC | 0.1997/0.178 | 2.85 (2.24-3.63) | <0.001 |
| GGACC | 0.1923/0.1749 | 1.82 (1.35-2.50) | <0.001 |
| GGGCC | 0.1278/0.1048 | 3.67 (2.82-4.78) | <0.001 |
| TP73 | |||
| GCCCTGCG | 0.2632/0.2626 | 1.0 | |
| GCCCACCG | 0.0846/0.1148 | 0.51 (0.38-0.68) | <0.001 |
| GCCCTCCG | 0.0473/0.0535 | 0.32 (0.22-0.47) | <0.001 |
Odds ratios and P values were from logistic regression analysis adjusted for sex, race, age, diabetes, smoking, alcohol consumption, and family history of cancer. Haplotypes with P>0.0015 but <0.05 in logistic regression models are listed in Supplemental Table 3.
Discussion
With this study, we demonstrated a significant association between MMR network gene variants and risk of pancreatic cancer. MGMT, PMS2, PMS2L3 and TP73 genotypes and haplotypes showed effects on susceptibility to pancreatic cancer. As a multifunctional system, MMR maintains genome stability by removing mismatched or distorted DNA structures and stimulates the apoptosis cascade when cells are overwhelmed with genotoxic or cytotoxic damage.[12,31] MMR dysfunction causes genomic instability and abnormal DNA damage response.[31] Through failure to recognize or repair the mismatched base-pairs/IDLs or failure to activate apoptosis signaling, MMR dysfunction may promote tumor development by accumulating replication errors. [12,31] Individuals with Lynch syndrome have increased risk of pancreatic cancer.[32] The observed association between MMR genetic variation and risk of pancreatic cancer supports the notion that MMR may contribute to pancreatic carcinogenesis.
A recent field synopsis on low-penetrance variants in DNA repair genes and cancer susceptibility revealed sparse association signals with strong epidemiological credibility.[33] Among the DNA repair genes studied in various types of human cancers, MGMT I143V showed credibility in prostate cancer.[33] We observed that MGMT I143V and K178R (in linkage) variant alleles were significantly related to decreased risk of pancreatic cancer. Consistently, the I143V variant has been reported as acting alone[34] or interacting with dietary factors[35] to reduce risk of colorectal cancer or breast cancer.[36] We found that MGMT I143V GG and K178R GG genotypes correlated with increased DNA damage level. MGMT is the major enzyme to remove O6Et-Gua. I143V polymorphism is located close to the alkyl group acceptor pocket at codon 145, which may affect the acceptance of an alkyl group. We infer that the 143V might repair the DNA damage less effectively. We observed the interaction of I143V with smoking. The protective effect of MGMT 143V/178R genotype on risk of pancreatic cancer may not simply explained by its function in DNA adduct removal, however, it may reflex the activation of apoptosis signaling triggered by the high level of DNA damage.
The variant allele of PMS2 IVS1-1121C>T, which was associated with increased pancreatic cancer risk, had also been associated with increased risk of ovarian cancer.[37] IVS1-1121C>T is located close to the R20Q variant, which is defective in activating the p73-dependent apoptotic response to cisplatin.[38] PMS2L3 Ex1+118C>T (5′UTR) and IVS3+9A>G were related to pancreatic cancer risk. These SNPs were in linkage with the splice site variant IVS1-8C>T, which was predicted to cause frame-shift mutation.[28] PMS2L3 IVS3+9A>G GA genotype correlated with increased DNA damage level. GA genotype carriers may be less active in recognizing O6Et-Gua-mimic mismatches but more effective to activate apoptosis to clear the damaged cells.
We found that haplotypes of MGMT, MSH6, PMS2, PMS2L3, and TP73 correlated with pancreatic cancer risk. mutSα and mutSβ initiate repair of base-base mismatches and IDLs. MSH6 haplotype demonstrated the protective effect of the G39E variant against pancreatic cancer. G39E had been related consistently to decreased breast cancer risk.[39] G39E AG/AA genotype showed increased DNA damage level, which may be less effective to recognize O6Et-Gua-mimic mismatches to initiate the MMR machinery, but act more efficiently to activate apoptosis pathway to maintain the genome stability. p73 is a determinant of apoptosis and a therapeutic target in cancer treatment.[40] Interaction of p73 isoform TA with truncated ΔN regulates differentiation, proliferation, and apoptosis.[41] We found that TP73 genetic variation may modify cancer susceptibility, which supports a role for p73 in pancreatic carcinogenesis. TP73 IVS1-7449G>C CG/CC genotype showed increased DNA damage level, which may be more effective in inducing apoptosis with the DNA damage accumulation and genome instability.
Previously we reported that RECQL Ex15+159A>C predicted clinical outcome in pancreatic cancer.[42] Currently we found that several other RECQL SNPs might be associated with altered pancreatic cancer risk. Genomic instability is detectable in chronic pancreatitis PanIN lesions.[43] RecQ helicases are caretakers of the genome to maintain genomic stability by acting at interface between DNA replication and DNA repair.[44] The role of RECQL as a regulator for MMR and cell cycle checkpoints in pancreatic carcinogenesis[45] needs further exploration.
The strengths of our study include hypothesis-driven gene selection and comprehensive gene/SNP coverage. The limitations include a small sample size, the potential for false-positive findings due to multiple comparisons, lack of a replication set, and lack of SNP function investigation. To mitigate this, we applied a stringent FDR (1%)-controlled P-value. Because the MAFs of the functional SNPs were relatively low, a larger sample size is needed to demonstrate their associations with risk of cancer. Although we used FDR-p-cut-off to correct for multiple comparisons, our findings need to be confirmed in different populations. The associated SNPs in our study may not be functional but in linkage with other functional variants, since in candidate-gene studies most of the variants assayed are not causal but tagging causal ones. Another limitation is that we did not analyze the interaction of genotypes with risky factors (e.g. smoking, alcohol, diabetes). To clarify the gene-environment interaction, we need a larger sample size to get statistical power. Moreover, the study was conducted in a single tertiary referral hospital; our findings may not be applicable to the general population. Furthermore, several genes correlated with patient survival,[27] and thus the association with pancreatic cancer risk might be biased if we missed those patients who died rapidly of the aggressive tumor. However, the three SNPs with P≤0.0015 were not associated with overall survival, which indicated these genotypes’ potential predictive value for pancreatic cancer risk. Finally, eight SNPs deviated from the HWE might indicate the genotyping artifact, selection bias in recruitment, or causal effect on pancreatic cancer pathogenesis. Among those, PMS2L3 IVS3+9A>G showed protective effect on pancreatic cancer risk. However, none of the 8 SNPs remained significant after adjusting for multiple comparisons. So the major effects of those SNPs that followed HWE on pancreatic cancer risk were unlikely biased.
To conclude, we observed an association between MMR polymorphism and pancreatic cancer risk, providing supporting evidence of a role for MMR dysfunction in pancreatic carcinogenesis. Our findings need to be validated in diverse populations. If confirmed, such information may help to identify individuals at high risk who may benefit from the primary prevention of pancreatic cancer.
Supplementary Material
Acknowledgments
Grant Sponsor: National Institutes of Health (NIH) RO1 grant CA098380 (to D.L.), SPORE P20 grant CA101936 (to J.L.A.), NIH Cancer Center core grant CA16672, and a research grant from the Lockton Research Funds (to D.L.).
Abbreviations
- ANCOVA
analysis of covariance
- BMI
Body mass index
- CI
confidence interval
- ENU
N-ethyl-N-nitrosourea
- FDR
false discovery rate
- HWE
Hardy-Weinberg equilibrium
- IDL
insertion/deletion loop
- MAF
minor allele frequency
- MMR
mismatch repair
- O6-EtGua
O6-ethylguanine
- OR
odds ratio
- SNP
single-nucleotide polymorphism
- UTR
untranslated region
References
- 1.Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2(12):897–909. doi: 10.1038/nrc949. [DOI] [PubMed] [Google Scholar]
- 2.Jaffee EM, Hruban RH, Canto M, Kern SE. Focus on pancreas cancer. Cancer Cell. 2002;2(1):25–28. doi: 10.1016/s1535-6108(02)00093-4. [DOI] [PubMed] [Google Scholar]
- 3.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20(10):1218–1249. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 4.Pogue-Geile KL, Chen R, Bronner MP, et al. Palladin mutation causes familial pancreatic cancer and suggests a new cancer mechanism. PLoS Med. 2006;3(12):e516. doi: 10.1371/journal.pmed.0030516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321(5897):1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McWilliams RR, Bamlet WR, de Andrade M, Rider DN, Cunningham JM, Petersen GM. Nucleotide excision repair pathway polymorphisms and pancreatic cancer risk: evidence for role of MMS19L. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1295–1302. doi: 10.1158/1055-9965.EPI-08-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li D, Suzuki H, Liu B, et al. DNA repair gene polymorphisms and risk of pancreatic cancer. clinical Cancer Res. 2009;15(2):740–746. doi: 10.1158/1078-0432.CCR-08-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McWilliams RR, Bamlet WR, Cunningham JM, et al. Polymorphisms in DNA repair genes, smoking, and pancreatic adenocarcinoma risk. Cancer Res. 2008;68(12):4928–4935. doi: 10.1158/0008-5472.CAN-07-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiao L, Hassan MM, Bondy ML, et al. XRCC2 and XRCC3 gene polymorphism and risk of pancreatic cancer. Am J Gastroenterol. 2008;103(2):360–367. doi: 10.1111/j.1572-0241.2007.01615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duell EJ, Holly EA, Bracci PM, Wiencke JK, Kelsey KT. A population-based study of the Arg399Gln polymorphism in X-ray repair cross- complementing group 1 (XRCC1) and risk of pancreatic adenocarcinoma. Cancer Res. 2002;62(16):4630–4636. [PubMed] [Google Scholar]
- 11.Duell EJ, Bracci PM, Moore JH, Burk RD, Kelsey KT, Holly EA. Detecting pathway-based gene-gene and gene-environment interactions in pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(6):1470–1479. doi: 10.1158/1055-9965.EPI-07-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiricny J. The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol. 2006;7(5):335–346. doi: 10.1038/nrm1907. [DOI] [PubMed] [Google Scholar]
- 13.Constantin N, Dzantiev L, Kadyrov FA, Modrich P. Human mismatch repair: reconstitution of a nick-directed bidirectional reaction. J Biol Chem. 2005;280(48):39752–39761. doi: 10.1074/jbc.M509701200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong X, Li Y, Hess KR, Abbruzzese JL, Li D. DNA mismatch repair gene polymorphisms affect survival in pancreatic cancer. Oncologist. 16(1):61–70. doi: 10.1634/theoncologist.2010-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicolaides NC, Carter KC, Shell BK, Papadopoulos N, Vogelstein B, Kinzler KW. Genomic organization of the human PMS2 gene family. Genomics. 1995;30(2):195–206. doi: 10.1006/geno.1995.9885. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Yuan F, Presnell SR, et al. Reconstitution of 5′-directed human mismatch repair in a purified system. Cell. 2005;122(5):693–705. doi: 10.1016/j.cell.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 17.Yang YG, Lindahl T, Barnes DE. Trex1 exonuclease degrades ssDNA to prevent chronic checkpoint activation and autoimmune disease. Cell. 2007;131(5):873–886. doi: 10.1016/j.cell.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Wang CJ, Lam W, Bussom S, Chang HM, Cheng YC. TREX1 acts in degrading damaged DNA from drug-treated tumor cells. DNA Repair (Amst) 2009;8(10):1179–1189. doi: 10.1016/j.dnarep.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klapacz J, Meira LB, Luchetti DG, et al. O6-methylguanine-induced cell death involves exonuclease 1 as well as DNA mismatch recognition in vivo. Proc Natl Acad Sci U S A. 2009;106(2):576–581. doi: 10.1073/pnas.0811991106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaina B, Christmann M, Naumann S, Roos WP. MGMT: key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA Repair (Amst) 2007;6(8):1079–1099. doi: 10.1016/j.dnarep.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Doherty KM, Sharma S, Uzdilla LA, et al. RECQ1 helicase interacts with human mismatch repair factors that regulate genetic recombination. J Biol Chem. 2005;280(30):28085–28094. doi: 10.1074/jbc.M500265200. [DOI] [PubMed] [Google Scholar]
- 22.Stiewe T. The p53 family in differentiation and tumorigenesis. Nat Rev Cancer. 2007;7(3):165–168. doi: 10.1038/nrc2072. [DOI] [PubMed] [Google Scholar]
- 23.Morgan MA, Parsels LA, Parsels JD, Mesiwala AK, Maybaum J, Lawrence TS. Role of checkpoint kinase 1 in preventing premature mitosis in response to gemcitabine. Cancer Res. 2005;65(15):6835–6842. doi: 10.1158/0008-5472.CAN-04-2246. [DOI] [PubMed] [Google Scholar]
- 24.Urist M, Tanaka T, Poyurovsky MV, Prives C. p73 induction after DNA damage is regulated by checkpoint kinases Chk1 and Chk2. Genes Dev. 2004;18(24):3041–3054. doi: 10.1101/gad.1221004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hassan MM, Bondy ML, Wolff RA, et al. Risk factors for pancreatic cancer: case-control study. Am J Gastroenterol. 2007;102(12):2696–2707. doi: 10.1111/j.1572-0241.2007.01510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong X, Javle M, Hess KR, Shroff R, Abbruzzese JL, Li D. Insulin-like growth factor axis gene polymorphisms and clinical outcomes in pancreatic cancer. Gastroenterology. 139(2):464–473. doi: 10.1053/j.gastro.2010.04.042. 473 e461-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong X, Jiao L, Li Y, et al. Significant associations of mismatch repair gene polymorphisms with clinical outcome of pancreatic cancer. J Clin Oncol. 2009;27(10):1592–1599. doi: 10.1200/JCO.2008.20.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee PH, Shatkay H. F-SNP: computationally predicted functional SNPs for disease association studies. Nucleic Acids Res. 2008;36(Database issue):D820–824. doi: 10.1093/nar/gkm904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiao L, Chang P, Firozi PF, Lai D, Abbruzzese JL, Li D. Polymorphisms of phase II xenobiotic-metabolizing and DNA repair genes and in vitro N-ethyl-N-nitrosourea-induced O6-ethylguanine levels in human lymphocytes. Mutat Res. 2007;627(2):146–157. doi: 10.1016/j.mrgentox.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pounds S, Cheng C. Improving false discovery rate estimation. Bioinformatics. 2004;20(11):1737–1745. doi: 10.1093/bioinformatics/bth160. [DOI] [PubMed] [Google Scholar]
- 31.Shewach DS, Lawrence TS. Antimetabolite radiosensitizers. J Clin Oncol. 2007;25(26):4043–4050. doi: 10.1200/JCO.2007.11.5287. [DOI] [PubMed] [Google Scholar]
- 32.Kastrinos F, Mukherjee B, Tayob N, et al. Risk of pancreatic cancer in families with Lynch syndrome. Jama. 2009;302(16):1790–1795. doi: 10.1001/jama.2009.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vineis P, Manuguerra M, Kavvoura FK, et al. A field synopsis on low-penetrance variants in DNA repair genes and cancer susceptibility. J Natl Cancer Inst. 2009;101(1):24–36. doi: 10.1093/jnci/djn437. [DOI] [PubMed] [Google Scholar]
- 34.Tranah GJ, Bugni J, Giovannucci E, et al. O6-methylguanine-DNA methyltransferase Leu84Phe and Ile143Val polymorphisms and risk of colorectal cancer in the Nurses’ Health Study and Physicians’ Health Study (United States) Cancer Causes Control. 2006;17(5):721–731. doi: 10.1007/s10552-006-0005-y. [DOI] [PubMed] [Google Scholar]
- 35.Loh YH, Mitrou PN, Bowman R, et al. MGMT Ile143Val polymorphism, dietary factors and the risk of breast, colorectal and prostate cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk study. DNA Repair (Amst) 9(4):421–428. doi: 10.1016/j.dnarep.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Shen J, Terry MB, Gammon MD, et al. MGMT genotype modulates the associations between cigarette smoking, dietary antioxidants and breast cancer risk. Carcinogenesis. 2005;26(12):2131–2137. doi: 10.1093/carcin/bgi179. [DOI] [PubMed] [Google Scholar]
- 37.Song H, Ramus SJ, Quaye L, et al. Common variants in mismatch repair genes and risk of invasive ovarian cancer. Carcinogenesis. 2006;27(11):2235–2242. doi: 10.1093/carcin/bgl089. [DOI] [PubMed] [Google Scholar]
- 38.Marinovic-Terzic I, Yoshioka-Yamashita A, Shimodaira H, et al. Apoptotic function of human PMS2 compromised by the nonsynonymous single-nucleotide polymorphic variant R20Q. Proc Natl Acad Sci U S A. 2008;105(37):13993–13998. doi: 10.1073/pnas.0806435105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conde J, Silva SN, Azevedo AP, et al. Association of common variants in mismatch repair genes and breast cancer susceptibility: a multigene study. BMC Cancer. 2009;9:344. doi: 10.1186/1471-2407-9-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lunghi P, Costanzo A, Mazzera L, Rizzoli V, Levrero M, Bonati A. The p53 family protein p73 provides new insights into cancer chemosensitivity and targeting. Clin Cancer Res. 2009;15(21):6495–6502. doi: 10.1158/1078-0432.CCR-09-1229. [DOI] [PubMed] [Google Scholar]
- 41.Rosenbluth JM, Pietenpol JA. The jury is in: p73 is a tumor suppressor after all. Genes Dev. 2008;22(19):2591–2595. doi: 10.1101/gad.1727408. [DOI] [PubMed] [Google Scholar]
- 42.Li D, Frazier M, Evans DB, et al. Single nucleotide polymorphisms of RecQ1, RAD54L, and ATM genes are associated with reduced survival of pancreatic cancer. J Clin Oncol. 2006;24(11):1720–1728. doi: 10.1200/JCO.2005.04.4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baumgart M, Werther M, Bockholt A, et al. Genomic instability at both the base pair level and the chromosomal level is detectable in earliest PanIN lesions in tissues of chronic pancreatitis. Pancreas. 39(7):1093–1103. doi: 10.1097/MPA.0b013e3181dc62f6. [DOI] [PubMed] [Google Scholar]
- 44.Chu WK, Hickson ID. RecQ helicases: multifunctional genome caretakers. Nat Rev Cancer. 2009;9(9):644–654. doi: 10.1038/nrc2682. [DOI] [PubMed] [Google Scholar]
- 45.Wu Y, Brosh RM., Jr Distinct roles of RECQ1 in the maintenance of genomic stability. DNA Repair (Amst) 9(3):315–324. doi: 10.1016/j.dnarep.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.