Abstract
The airway epithelium plays a role in host defense through the binding of innate immune receptors, which leads to the activation of inflammatory mediators, including antimicrobial peptides. The active form of vitamin D, 1,25(OH)2D3, induces the expression of the antimicrobial peptide LL-37 in both myeloid cells and airway epithelial cells (AEC). Here, we demonstrate that mRNA encoding triggering receptor expressed on myeloid cells (TREM)-1 was induced up to 12-fold by 1,25(OH)2D3 in normal human bronchial epithelial (NHBE) cells and in well-differentiated cultures of six airway epithelial cell lines from patients with cystic fibrosis (CF) and healthy individuals. TREM-2 and DAP12 were also expressed in airway cultures, but not induced by vitamin D. Induction occurs through a vitamin D response element identified in its proximal promoter region, and was regulated by PU.1 expressed in the AEC. Activation of TREM-1 by a cross-linking antibody led to an induction of both human β-defensin-2 and tumor necrosis factor (TNF)-α mRNA, demonstrating its functionality in these cells. Our results expand on the role played by the airway epithelium in innate immunity and suggest that vitamin D can modulate the innate immune defense of the airway epithelium, and could potentially be developed as an adjunctive therapy for airway infections.
Keywords: Respiratory, vitamin D, cathelicidin, cystic fibrosis
Introduction
The innate immune system is responsible for recognizing and eliminating inspired pathogens from the airway.1 The initial line of defense against these micro-organisms is the airway surface fluid (ASF), a viscous, elastic material containing several antimicrobial substances.2 The respiratory secretions in ASF are active against a broad range of microorganisms, and include such antimicrobial peptides as β-defensins and the cathelicidin LL-37. The genes that encode these peptides are regulated; their expression is induced upon recognition of pathogens by innate immune receptors such as the toll-like receptors (TLRs).
In addition to the TLRs, whose induction and activation increases the host defense response to pathogens, other receptors, such as those in the triggering receptor expressed on myeloid cells (TREM) family differentially regulate the innate immune response. These receptors were initially identified on myeloid cells, including neutrophils and macrophages and their expression and activation appear to more finely adjust the innate immune response.3,4 For example, TREM-1 was initially identified as a receptor that was induced by lipopolysaccharide (LPS) on neutrophils and monocytes, and found highly expressed at sites of inflammation. While its activation can lead to proinflammatory cytokine secretion, it can act synergistically with TLRs to result in a large-scale inflammatory response.5–8 Evidence that it regulates the inflammatory response arises from studies that show a low dose of siRNA to TREM-1 increased survival from experimental sepsis, while a high dose increased mortality as a result of reduced neutrophil function.9 Furthermore, its activation can reduce the expression of some LPS-induced genes, while activating others.10 A soluble form of TREM-1 (sTREM-1) is induced in inflammation and appears to negatively regulate TREM-1 signaling. Another family member, TREM-2, regulates the activation of osteoclasts, microglial cells and dendritic cells,11 and appears to be a receptor for bacterial phagocytosis.12
TREM-1 expression is regulated by nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and PU.1 transcription factors.13 The ligand for TREM-1 has not yet been identified, but there is some evidence that it may be activated by binding of soluble proteins14 and some pathogens.15 Investigators have also used a cross-linking antibody that activates TREM-1.5 Once activated, it phosphorylates the intra-cellular mediators DAP12, phospholipase Cγ and extracellular signal-regulated kinase (ERK) 1/2, leads to activation of NF-κB and a rise in intracellular [Ca2+].5 Treatment of mice with the antibody enhanced the natural defense against Streptococcus pneumoniae infection in mouse lungs, leading to increased clearance of bacteria and increased survival.16 In cystic fibrosis (CF), expression of TREM-1 in monocytes was reduced and could not be induced by LPS, in contrast to controls. These CF monocytes appear to be maintaining an LPS-tolerance-like state, reducing their ability to adequately regulated innate immunity.17
Another problem found in CF is the chronically reduced levels of vitamin D in the serum.18 We recently demonstrated that physiological levels of the active form of vitamin D (1,25(OH)2 vitamin D3) can induce the expression of LL-37 in airway epithelial cells (AEC) and increase the antibacterial activity in the ASF of cells cultured in air-liquid interface (ALI).19 Further analysis demonstrated that airway epithelial cells can convert inactive 25(OH)D3 to the active form by expressing 1-α hydroxylase, thus producing active vitamin D, which can induce LL-37 expression.20 This suggested that reduced vitamin D levels could aid in the increased susceptibility to infection observed in the airways of CF patients and that vitamin D could be useful as a potential addition to antibacterial therapy. To further define the role of vitamin D in the innate immune defense of the airway, we examined the expression of LL-37 and other mediators in response to 1,25(OH)2D3 in cultured cells from CF and non-CF individuals.
Materials and methods
Bronchial epithelial cell culture
Normal bronchial epithelial cells (NHBE) were obtained from Lonza (Basel, Switzerland); bronchial epithelial cell lines (UNC-N1, 2, 3 and UNC-CF1, 2, 3, gifts from Dr Scott Randell, University of North Carolina Medical School) were cultured with Clonetics Bronchial Epithelial Growth medium (BEGM, Lonza) as described.21 ALI cultures were grown for approximately 10–15 d before any experiments were performed.
Western blot analysis
Trypsinized cultures were lysed in cold RIPA buffer in the presence of protease inhibitors (Roche, Indianapolis, IN, USA), using standard techniques. Nuclear extracts were isolated using the NE-PER nuclear and cytoplasmic extraction reagents (Thermo Scientific, Rockford, IL, USA) as per the manufacturer’s protocol. Protein samples were electrophoresed in equal amounts through sodium dodecyl sulfate poly-acrylamide gel electrophoresis (SDS-PAGE) Tris-Tricine 10–20% gradient gels (Bio-Rad, Hercules, CA, USA) and transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad). Western blot was performed using monoclonal mouse antibodies against LL-37 (1:250 dilution, Santa Cruz Biochemicals, Santa Cruz, CA, USA) or TREM-1 (1:750 dilution; R&D systems, Minneapolis, MN, USA) overnight. Anti-tubulin was used as a loading control. Bound antibodies were detected with an anti-monoclonal horse-radish peroxidase (HRP)-conjugated goat antibody (Thermo Scientific, Waltham, MA, USA), and visualized by chemiluminescence using Thermo Scientific Super Signal West Dura Extended Duration Substrate (Thermo Scientific).
Immunofluoresence
The NHBE cells were seeded on chamber slides (Nunc, Rochester, NY, USA) and grown for 1–2 d to ~80% confluence. The cells were treated with either 0.01% ethanol or 10−8 M 1,25(OH)2D3 for 24 h and fixed in 1% paraformaldehyde in phosphate buffered saline (PBS). After PBS washes, they were blocked for 10 min at 25°C in 10% goat serum (Invitrogen, Carlsbad, CA, USA), washed in PBS and incubated overnight at 4°C with anti-TREM-1 (FL234) (Santa Cruz Biochemicals) (1:200) or with rabbit serum at the same concentration as an isotype control (Santa Cruz Biochemicals). After PBS washes, incubation in secondary antibody (goat anti-rabbit IgG-AlexaFluor 488; Invitrogen) for 1.5 h at room temperature and further PBS washes, the slides were mounted with Vectashield mounting medium. The cells were visualized using an Olympus D70 fluorescent microscope and associated software (Olympus, Center Valley, PA, USA).
Reverse-transcription and real-time quantitative PCR
Total RNA was isolated using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA), and was reversed-transcribed with Superscript II reverse transcriptase with an oligo(dT) primer (Invitrogen). Controls without reverse transcriptase were carried out to demonstrate no non-specific amplification.
Quantification of mRNA levels was carried out relative to the housekeeping gene, β-2-microglobulin (B2M), using the MyiQ iCycler (Bio-Rad Laboratories) as previously described.19 The real-time quantitative PCR (RTQ-PCR) primers used were: TREM-1 forward: 5′-TGGTCTTCTCTGTCCTGT TTG-3′ and reverse 5′-ACTCCCTGCCTTTTACCT C-3′; LL-37 forward, 5′–GTCACCAGAGGATTGTG ACTTCAA-3′ and reverse 5′-TTGAGGGTCACTGT CCCCATA3′; TREM-2 forward, 5′-GCTTCTGCCC TTGGCTGGGG-3′ and reverse, 5′-CGCCACGCCCTGGAACACTG-3′; PU.1 forward, 5′-CCCACCGAGGCAGGGGATCT-3′ and reverse, 5′-GGGCACCAGGTCTTCTGATGGC-3′; DAP12 forward 5′-GAGACCGAGTCGCCTTATC-3′ and reverse 5′-ATACGGCCTCTGTGTGTTG-′3′; β2–Microglobulin (B2M) forward 5′-CTCCGTGGCCTTAGCTGTG-3′ and reverse 5′-TTTGGAGTACGCTGGATAGCCT-3′. The PCR fragments were amplified for 50 cycles (15 s at 95°C and 1 min at 60°C) and quantified based on the 2−ΔΔCT value compared to that of the housekeeping genes.
PCR array
Ribonucleic acid from stimulated or control ALI cultures of UNC-N1 cells was reverse-transcribed with the RT2 First Strand Kit (SABioscience, Frederick, MD, USA), and the cDNA was added to the RT2 qPCR Master Mix (SABioscience) as described by the manufacturer. The cDNA was amplified for 40 cycles (15 s at 95°C and 1 min at 60°C) in the array plates. The data were analyzed using the SABioscience PCR array analysis web portal (http://www.SABioscience.compcrarraydataanalysis.php).
Plasmid constructs and transfections
A549 cells in 24-well plates were co-transfected with 100 ng/well of the pTREM-1.luc plasmid, containing 1.3kb flanking the TREM-1 gene in the luciferase expression vector, pGL4.17, and a PU.1 expression vector (both gifts of R. Sadikot) followed by treatment with 0.01% ethanol or 10−8 M 1,25(OH)2D3 for 24 h. Empty vectors were used to keep the total DNA concentrations the same, using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol.
The luciferase assay was carried out as described,13 by normalizing to co-transfected Renilla luciferase. Firefly luciferase activity was quantified using the Dual-Glo luciferase assay kit (Promega, Madison, WI, USA) and measured in a luminometer.
Results
Induction of innate immunity genes by 1,25(OH)2D3
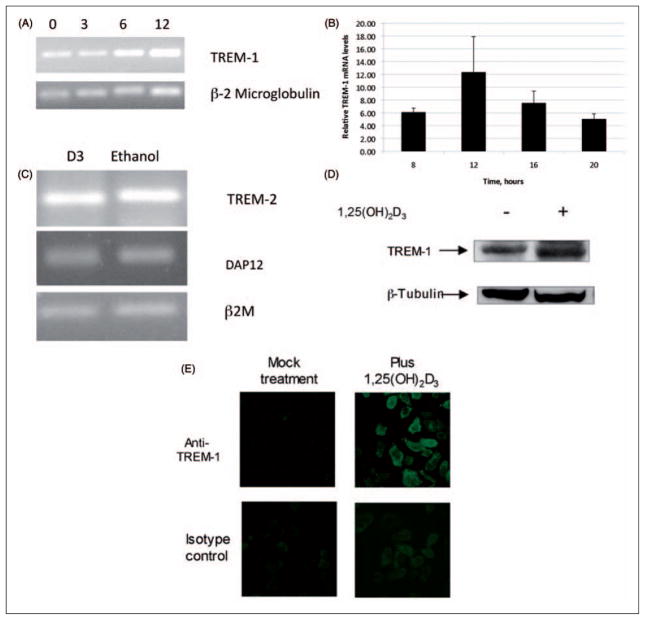
To determine whether other genes involved in innate immunity were regulated by vitamin D, we stimulated NHBE cells with 10−8 M 1,25(OH)2D3 or ethanol for 24 h. Total mRNA was isolated, transcribed into cDNA and subjected to PCR microarray analysis using RT2 Profiler Human Innate and Adaptive Immune Response Array (SABioscience). The results shown in Table 1 identify three genes stimulated by 1,25(OH)2D3. In addition to LL-37, induction of CD14 in the airway has already been demonstrated.20 The third gene induced to a great extent was TREM-1. To confirm and quantify this effect, we stimulated NHBE cells with 1,25(OH)2D3, and quantified the expression of TREM-1 by RT-QPCR. The results shown in Figure 1(A) demonstrate that TREM-1 is induced by 1,25(OH)2D3 in a time-dependent manner, with a maximum induction at 12 h (Figure 1B). We also observed expression of TREM-2 and DAP12 in these cells, but levels did not change in response to 1,25(OH)2D3 treatment (Figure 1C). This was verified by quantitative RT-PCR (data not shown). Similarly increased levels of both LL-37 and TREM-1 mRNA were also observed in NHBE cells cultured in ALI and stimulated by the addition of 10−8 M 1,25(OH)2D3 on the apical surface in 100 μl (data not shown). Western blot analysis of whole cell lysates of ALI cultures stimulated with 1,25(OH)2D3 on the basolateral surface confirms that protein levels rise in response to 1,25(OH)2D3 as well (Figure 1D). We did not observe a band in western blots of airway surface fluid, suggesting that the TREM-1 protein is maintained in its membrane-bound form. Vitamin D-treatment increases the surface expression of TREM-1 as observed by immunofluoresence analysis of NHBE cells stimulated with 1,25(OH)2D3 for 24 h (Figure 1E).
Table 1.
Induction of innate immunity genes in airway cells by 1,25(OH)2D3
| Up-regulated genes | Fold increase |
|---|---|
| CAMP (LL-37) | 38.2132 |
| CD14 | 46.3983 |
| TREM-1 | 20.7638 |
Figure 1.
Induction of TREM-1 in airway epithelial cells in response to 1,25(OH)2D3. The normal human bronchial epithelial (NHBE) cells were grown in air-liquid interface (ALI) and stimulated with 10−8 M 1,25(OH)2D3 or ethanol in the basolateral medium for increasing times from 0–12 h (A,B). Total mRNA was isolated and TREM-1 mRNA levels were observed by RT-PCR followed by gel electrophoresis (A). The experiment was repeated and the mRNA was quantified by QPCR (B). Results in B are shown as levels relative to ethanol-treated samples at each timepoint. The mRNA levels are relative to β-2 microglobulin (n = 3; bars indicate mean ± SD). The increase in TREM-1 mRNA in basolaterally stimulated cultures is significant at all timepoints, as determined by t-test (P <0.001). (C) RT-PCR analysis of TREM-2 and DAP 12 in cultures stimulated with either 10−8 M 1,25(OH)2D3 or 0.01% ethanol for 18 h. (D). The TREM-1 protein levels were visualized by western blot analysis of whole cell lysates of NHBE cells cultured in ALI, and stimulated with 1,25(OH)2D3 for 18 h. (E) Surface expression of TREM-1 was observed by stimulation of NHBE cells grown on chamber slides in the presence of 10−8 M 1,25(OH)2D3 or 0.01% ethanol for 24 h. Slides were fixed and incubated with anti-TREM-1 antibody, followed by an Alexa-Fluor conjugated secondary antibody, and visualized by fluorescence microscopy.
Induction of LL-37 in cystic fibrosis and normal cell lines by 1,25(OH)2D3
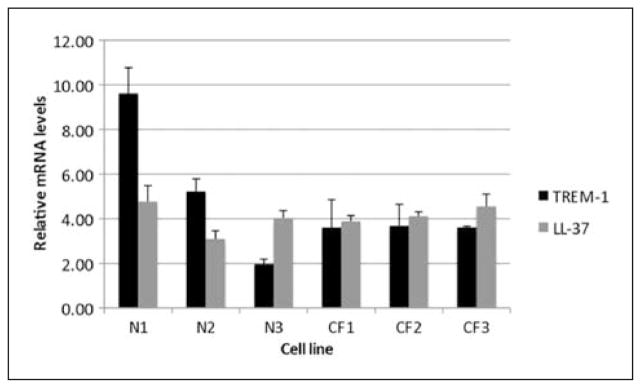
We previously demonstrated that LL-37 mRNA and antibacterial activity was induced by 1,25(OH)2D3 in primary cultures of AEC from healthy individuals. The response was similar in two undifferentiated cell lines, one from a CF individual and one normal individual.19 To confirm our results of LL-37 and TREM-1 with well-differentiated cultures, we examined six recently described cell lines, three from healthy individuals and three from CF airways, which grow in ALI, and are more representative of airway epithelium.22 After stimulation with 10−8 M 1,25(OH)2D3, we observed kinetics of induction of LL-37 and TREM-1, with maximal levels at 12 h (data not shown). When the six lines were compared for levels of induction at 12 h, we observed a similar level of LL-37 mRNA induced among the lines (Figure 2; gray bars). Surprisingly, a wide difference in TREM-1 mRNA levels were seen between the three normal lines, compared with the CF lines (Figure 2, black bars) and NHBE cells (Figure 1B). As there are numerous reports of associations between single nucleotide polymorphisms (SNPs) found in the vitamin D receptor (VDR) gene and a variety of disorders involving host defense against microbial pathogens,23 we determined the status of the four common SNPs in these six lines. All alleles of the different SNPs were seen with no suggestion of association between VDR genotype and response to 1,25(OH)2D3 (data not shown).
Figure 2.
Expression and activity of LL-37 and TREM-1 in response to 1,25(OH)2D3 in differentiated normal and cystic fibrosis (CF) airway epithelial air-liquid interface (ALI) cell cultures. Three normal and three CF cell lines were grown in ALI, and stimulated with 10−8 M 1,25(OH)2D3 or 0.01% ethanol for 12 h. Total mRNA was isolated and LL-37 or TREM–1 mRNA levels were quantified by QPCR relative to ethanol–stimulated cells and normalized to β-2 microglobulin.
Mechanism of TREM-1 regulation by 1,25(OH)2D3
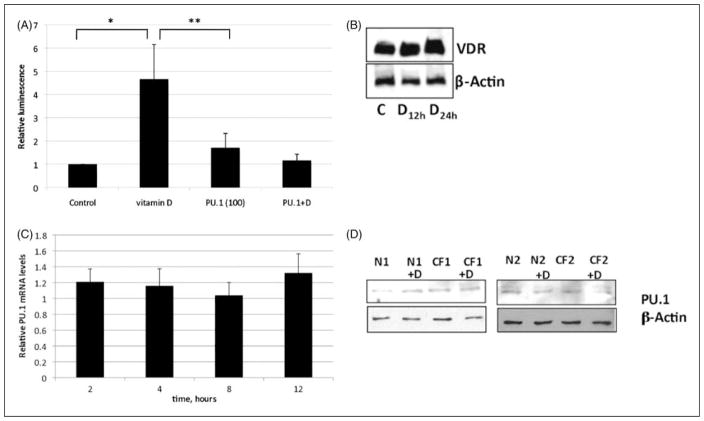
Computer-based analysis (Matinspector, www.genomatix.de) of the sequence upstream from the TREM-1 gene indicates VDREs in both mouse and human genomes. We obtained a luciferase TREM-1 promoter construct and transfected into A549 cells, which were then treated with 1,25(OH)2D3. We obtained a luciferase TREM-1 promoter construct and transfected into A549 cells, which have been shown to utilize the same mechanism of vitamin D gene induction. The transfected cultures20 were then treated with 1,25(OH)2D3. The results shown in Figure 3A indicate that 1,25(OH)2D3 stimulates TREM-1 promoter activity in these epithelial cells, supporting the hypothesis that 1,25(OH)2D3 induces TREM-1 transcription via a functional VDRE. Western blot analysis of nuclear extracts demonstrated the presence of VDR in these cells (Figure 3B).
Figure 3.
Mechanism of vitamin D induction. (A) A luciferase TREM-1 promoter construct was transfected into A549 cells, followed by incubation with 10−8 M 1,25(OH)2D3 or control. Luciferase levels were quantified as described. Thirteen co-transfections were also carried out with 50 ng/ml of PU.1 expression plasmid in the presence or absence of 10−8 M 1,25(OH)2D3 for 18 h. Luminescence of vitamin D-stimulated transfected cultures is significant over control (P = 0.02) and over vitamin D + PU.1 (P = 0.01). Data shown are the means of two independent experiments, each carried out in triplicate ± SEM. (B) Western blot analysis of vitamin D receptor (VDR) protein from cells stimulated with 0.01% ethanol for 24 h (labeled C) or 10−8 M 1,25(OH)2D3 or for 12 or 24 h. (C) The PU.1 mRNA levels were quantified by QPCR in normal human bronchial epithelial (NHBE) cells treated with 10−8 M 1,25(OH)2D3 or 0.01% ethanol for 2–12 h. (D) Nuclear extracts were isolated from UNC-N1, -N2, -CF1, and CF2 cells incubated with 10−8 M 1,25(OH)2D3 or 0.01% ethanol for 24 h, and subjected to western blot analysis for PU.1, and β-actin as a control.
The transcription factor PU.1 is known to regulate the expression of TREM-1.13 While previously thought to be restricted to myeloid cells, its expression has been observed in respiratory epithelial cells.24 To examine whether PU.1 plays a role in vitamin D-mediated TREM-1 gene expression in AEC, plasmid constructs expressing PU.1 were co-transfected with the TREM-1 reporter plasmid, followed by treatment with 1,25(OH)2D3 or ethanol. The results shown in Figure 3(A) demonstrate that the presence of PU.1 does not affect uninduced TREM-1 promoter activity, but inhibits its activity in the presence of 1,25(OH)2D3. A time course of induction by 1,25(OH)2D3 shows that PU.1 mRNA levels do not change in cultured airway epithelial cells (Figure 3C). As an increase in nuclear PU.1 levels was observed for CF monocytes,17 we performed a western blot on PU.1 in two CF and two normal cell lines in response to 1,25(OH)2D3. The results shown in Figure 3D illustrate no difference in the PU.1 protein levels, suggesting other mechanisms are responsible for this difference.
Activation of TREM-1-responsive genes in airway cells
The expression of DAP12 in the airway cells (Figure 1C) supported the hypothesis that the TREM-1 was functional. To determine the activity of induced TREM-1 on the AEC, NHBE cells were initially treated with 1,25(OH)2D3 or ethanol for 12 h to increase TREM-1 levels, followed by treatment with a cross-linking antibody to TREM-1 previously shown to activate it.5 We quantified hBD2 and tumor necrosis factor (TNF)-α mRNA levels by QPCR to determine the effect on the transcription of NF-κB-regulated genes. The results shown in Figure 4 demonstrate that TREM-1 is functional in these cells, as demonstrated by the induction of both hBD2 and TNF-α in cells stimulated with the specific antibody compared with the isotype control and cultures pre-treated with ethanol. No induction of either gene by the antibody was observed in cultures pre-treated with the ethanol control (not shown).
Figure 4.
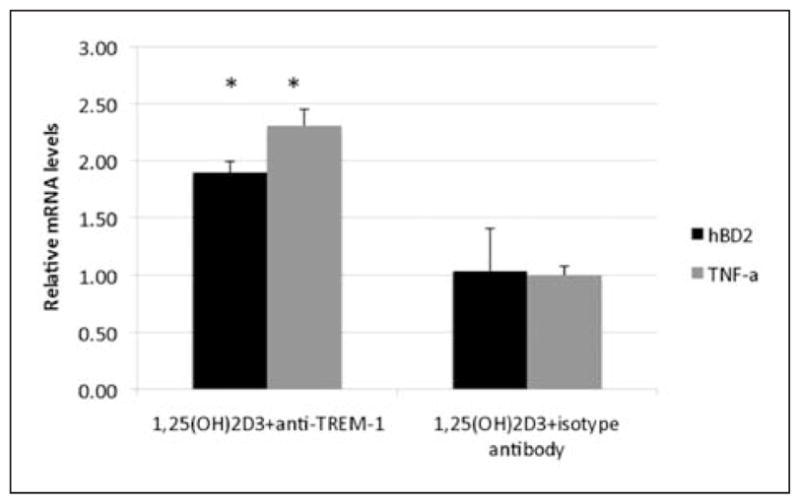
Activation of TREM-1 in airway cells. The normal human bronchial epithelial (NHBE) cells were grown in air-liquid interface (ALI), and incubated with 10−8 M 1,25(OH)2D3 in the presence of a 1:100 dilution of anti-TREM-1 antibody or isotype antibody for 24 h. Total mRNA was isolated and hBD2 and tumor necrosis factor (TNF)-α mRNA levels were quantified by QPCR, and shown as the mean of triplicates relative to cultures incubated with ethanol. Increase in hBD2 and TNF-α mRNA levels in treated cultures is significant as determined by t-test, P <0.01. Bars indicate mean ± SD.
Discussion
It is increasingly recognized that innate immunity in the respiratory tract includes not only macrophages and neutrophils, but also involves the recognition of pathogens by surface receptors expressed by epithelial cells, and the subsequent induction of host defense gene expression, secretion of cytokines and antimicrobial agents into the apical surface fluid.1 Our recent results that 1,25(OH)2D3 can regulate the expression of one component of this defense,19 together with a subsequent report of the expression of 1-α-hydroxylase and the cross-talk with TLRs in the epithelial cells,20 suggested that vitamin D could be useful in the exogenous regulation of host defense in the airway. This is especially important in a disease like CF, where the genetic defect appears to affect the innate immune response in the airway, leading to recurrent infections.
In order to best assess the potential for vitamin D as a therapeutic agent in bolstering host defense of the airway, we report here a characterization of the effect of 1,25(OH)2D3 on the innate immune response. We uncovered the inducible expression of TREM-1 in ALI cultures of AECs. Most reports of TREM-1 focus on its expression in myeloid cells, although one report of its expression in gastric epithelial cells has been found.25 Our study thus demonstrates that TREM-1 is more widely expressed than previously thought. We further observed that the induction of TREM-1 differs somewhat between cells from different individuals, suggesting an inter-individual variability in the innate immune response to vitamin D. Furthermore, our results that activation of this receptor via antibody ligation can lead to an induction of innate immune defenses in AECs add another dimension to the activity of the airway epithelium in regulating the innate immune response. That we did not observe a significant response of TREM-1 induction in the absence of pre-stimulation with 1,25(OH)2D3, suggests that sufficient surface expression of TREM-1 is necessary for its utilization as an innate immune regulatory molecule. TREM-1 is considered an amplifier of the inflammatory response, and a molecule that fine-tunes the innate immune defense by secreting inflammatory mediators in synergy with TLR stimulation.3 Much of this is based on experiments that demonstrate that partial inhibition of TREM-1 can protect against polymicrobial sepsis, but complete inhibition with high concentrations of siRNA increased mortality.13 The role of TREM-1 in epithelial defense against microbial pathogens remains to be defined. A recent study demonstrating the role of LL-37 in regulation of TREM-1 in neutrophils,26 suggests a complex network of innate immune responses that may include those observed in epithelial cells as well.1
Expression of TREM-1 is induced in macrophages and neutrophils by bacteria, but not in inflammation where no colonization is seen, such as psoriasis and ulcerative colitis.27 Further studies have demonstrated that this induction occurs through TLR-mediated pathways.6 Our results demonstrate that TREM-1 appears to be induced by 1,25(OH)2D3 through a VDRE-mediated pathway, as indicated by the observation of VDR in the airway cells, and by the functional activation of expression in the presence of a VDRE-containing promoter. In contrast, PU.1 has been shown to suppress the expression of TREM-1.13 We demonstrate here that PU.1 is expressed in bronchial epithelial cells in addition to type II alveolar epithelial cells,24 and its over expression can bring TREM-1 mRNA levels to basal levels, but levels of PU.1 are not affected by 1,25(OH)2D3 treatment. This indicates that PU.1-mediated suppression and VDR-mediated induction of TREM-1 are independent, similar to what was observed for PU.1 and NF-κB regulation of TREM-1 in macrophages.13
Our results further demonstrate that LL-37 gene expression is increased in both normal and CF airway cells. This leads to the secretion of the mature form of the peptide into the ASF, increasing its antibacterial capability. The fact that this induction can occur even when the 1,25(OH)2D3 is added to the apical surface of ASF cultures suggests that it may be possible to apply it as an aerosol to the airways. This is important as many CF patients are not only deficient in serum vitamin D levels, but are often unable to absorb vitamin D through nutritional pathways as a result of deficient absorption of fat-soluble vitamins.18,28 Together, the results may suggest an avenue for further study on the mechanism leading to the increased inflammation seen in CF lungs.
In summary, our results show that 1,25(OH)2D3 not only increases the expression of LL-37 in AECs, but also induces the expression of TREM-1, which was previously thought to regulate inflammation in circulating cells of the myeloid lineage. This induction can be carried out even at the apical surface of the epithelium, supporting the potential use of inhaled vitamin D therapy to modulate the innate immune defenses of the respiratory tract.
Acknowledgments
This work was supported by grants from the Cystic Fibrosis Foundation [DIAMON08G0] (GD) and the NIH [RO1 DK-38961-22] (SC), and [AI072247A1] (LKR). The authors wish to thank Dr Scott Randell for the UNC cell lines, and Urvashi Pandit and Kyell Schwartz for technical assistance.
References
- 1.Diamond G, Legarda D, Ryan LK. The innate immune response of the respiratory epithelium. Immunol Rev. 2000;173:27–38. doi: 10.1034/j.1600-065x.2000.917304.x. [DOI] [PubMed] [Google Scholar]
- 2.Singh PK, Tack BF, McCray PB, Jr, Welsh MJ. Synergistic and additive killing by antimicrobial factors found in human airway surface liquid. Am J Physiol Lung Cell Mol Physiol. 2000;279:L799–L805. doi: 10.1152/ajplung.2000.279.5.L799. [DOI] [PubMed] [Google Scholar]
- 3.Ford JW, McVicar DW. TREM and TREM-like receptors in inflammation and disease. Curr Opin Immunol. 2009;21:38–46. doi: 10.1016/j.coi.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klesney-Tait J, Turnbull IR, Colonna M. The TREM receptor family and signal integration. Nat Immunol. 2006;7:1266–1273. doi: 10.1038/ni1411. [DOI] [PubMed] [Google Scholar]
- 5.Bouchon A, Dietrich J, Colonna M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol. 2000;164:4991–4995. doi: 10.4049/jimmunol.164.10.4991. [DOI] [PubMed] [Google Scholar]
- 6.Bleharski JR, Kiessler V, Buonsanti C, et al. A role for triggering receptor expressed on myeloid cells-1 in host defense during the early-induced and adaptive phases of the immune response. J Immunol. 2003;170:3812–3818. doi: 10.4049/jimmunol.170.7.3812. [DOI] [PubMed] [Google Scholar]
- 7.Netea MG, Azam T, Ferwerda G, Girardin SE, Kim SH, Dinarello CA. Triggering receptor expressed on myeloid cells-1 (TREM-1) amplifies the signals induced by the NACHT-LRR (NLR) pattern recognition receptors. J Leukoc Biol. 2006;80:1454–1461. doi: 10.1189/jlb.1205758. [DOI] [PubMed] [Google Scholar]
- 8.Radsak MP, Salih HR, Rammensee HG, Schild H. Triggering receptor expressed on myeloid cells-1 in neutrophil inflammatory responses: differential regulation of activation and survival. J Immunol. 2004;172:4956–4963. doi: 10.4049/jimmunol.172.8.4956. [DOI] [PubMed] [Google Scholar]
- 9.Gibot S, Massin F, Marcou M, et al. TREM-1 promotes survival during septic shock in mice. Eur J Immunol. 2007;37:456–466. doi: 10.1002/eji.200636387. [DOI] [PubMed] [Google Scholar]
- 10.Dower K, Ellis DK, Saraf K, Jelinsky SA, Lin LL. Innate immune responses to TREM-1 activation: overlap, divergence, and positive and negative cross-talk with bacterial lipopolysaccharide. J Immunol. 2008;180:3520–3534. doi: 10.4049/jimmunol.180.5.3520. [DOI] [PubMed] [Google Scholar]
- 11.Cella M, Buonsanti C, Strader C, Kondo T, Salmaggi A, Colonna M. Impaired differentiation of osteoclasts in TREM-2-deficient individuals. J Exp Med. 2003;198:645–651. doi: 10.1084/jem.20022220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.N’Diaye EN, Branda CS, Branda SS, et al. TREM-2 (triggering receptor expressed on myeloid cells 2) is a phagocytic receptor for bacteria. J Cell Biol. 2009;184:215–223. doi: 10.1083/jcb.200808080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng H, Ornatowska M, Joo MS, Sadikot RT. TREM-1 expression in macrophages is regulated at transcriptional level by NF-kappaB and PU.1. Eur J Immunol. 2007;37:2300–2308. doi: 10.1002/eji.200737270. [DOI] [PubMed] [Google Scholar]
- 14.Colonna M, Facchetti F. TREM-1 (triggering receptor expressed on myeloid cells): a new player in acute inflammatory responses. J Infect Dis. 2003;187(Suppl 2):S397–S401. doi: 10.1086/374754. [DOI] [PubMed] [Google Scholar]
- 15.Mohamadzadeh M, Coberley SS, Olinger GG, et al. Activation of triggering receptor expressed on myeloid cells-1 on human neutrophils by marburg and ebola viruses. J Virol. 2006;80:7235–7244. doi: 10.1128/JVI.00543-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagler H, Sharif O, Haslinger I, et al. TREM-1 activation alters the dynamics of pulmonary IRAK-M expression in vivo and improves host defense during pneumococcal pneumonia. J Immunol. 2009;183:2027–2036. doi: 10.4049/jimmunol.0803862. [DOI] [PubMed] [Google Scholar]
- 17.del Fresno C, Gomez-Pina V, Lores V, et al. Monocytes from cystic fibrosis patients are locked in an LPS tolerance state: down-regulation of TREM-1 as putative underlying mechanism. PLoS One. 2008;3:e2667. doi: 10.1371/journal.pone.0002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rovner AJ, Stallings VA, Schall JI, Leonard MB, Zemel BS. Vitamin D insufficiency in children, adolescents, and young adults with cystic fibrosis despite routine oral supplementation. Am J Clin Nutr. 2007;86:1694–1699. doi: 10.1093/ajcn/86.5.1694. [DOI] [PubMed] [Google Scholar]
- 19.Yim S, Dhawan P, Ragunath C, Christakos S, Diamond G. Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D3. J Cystic Fibrosis. 2007;6:403–410. doi: 10.1016/j.jcf.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol. 2008;181:7090–7099. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway epithelial cell cultures. Methods Mol Med. 2005;107:183–206. doi: 10.1385/1-59259-861-7:183. [DOI] [PubMed] [Google Scholar]
- 22.Fulcher ML, Gabriel SE, Olsen JC, et al. Novel human bronchial epithelial cell lines for cystic fibrosis research. Am J Physiol Lung Cell Mol Physiol. 2009;296:L82–L91. doi: 10.1152/ajplung.90314.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uitterlinden AG, Fang Y, Van Meurs JB, Pols HA, Van Leeuwen JP. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;338:143–156. doi: 10.1016/j.gene.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Joshi PC, Applewhite L, Mitchell PO, et al. GM-CSF receptor expression and signaling is decreased in lungs of ethanol-fed rats. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1150–L1158. doi: 10.1152/ajplung.00150.2006. [DOI] [PubMed] [Google Scholar]
- 25.Schmausser B, Endrich S, Beier D, et al. Triggering receptor expressed on myeloid cells-1 (TREM-1) expression on gastric epithelium: implication for a role of TREM-1 in Helicobacter pylori infection. Clin Exp Immunol. 2008;152:88–94. doi: 10.1111/j.1365-2249.2008.03608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amatngalim GD, Nijnik A, Hiemstra PS, Hancock RE. Cathelicidin peptide LL-37 modulates TREM-1 expression and inflammatory responses to microbial compounds. Inflammation. doi: 10.1007/s10753-010-9248-6. [DOI] [PubMed] [Google Scholar]
- 27.Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410:1103–1107. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- 28.Green DM, Leonard AR, Paranjape SM, Rosenstein BJ, Zeitlin PL, Mogayzel PJ., Jr Transient effectiveness of vitamin D2 therapy in pediatric cystic fibrosis patients. J Cyst Fibros. 9:143–149. doi: 10.1016/j.jcf.2010.01.002. [DOI] [PubMed] [Google Scholar]