Abstract
Studies typically measure mood changes during smoking cessation treatment in two ways: (a) by tracking mean change in depression scores or (b) by tracking the incidence of major depression development using diagnostic assessments. However, tracking mean change does not capture variability in individual mood trajectories, and diagnosing participants at multiple time points is time and labor intensive. The current study proposes a method of assessing meaningful increases in depression without the use of diagnostic assessments by utilizing reliable and clinically significant change criteria. This method was applied to 212 participants in a smoking cessation trial to explore the relationship between smoking status and depressed mood, assessed at baseline, end-of-treatment, and 2-, 6-, and 12-month follow-ups. High rates of reliable (24–28%) and both reliable and clinically significant increases (23–24%) in depressed mood were observed across all participants, regardless of whether or not they achieved abstinence. However, when we calculated group mean change in depression during the trial, only decreases in depressed mood where observed across several intervals. Findings indicate that utilizing reliable and clinically significant change criteria to track symptoms of depression during smoking cessation treatment leads to different conclusions than simply tracking mean changes. We propose that a combination of reliable and clinically significant change criteria may serve as a useful proxy measure for the development of major depressive disorder during smoking cessation.
Keywords: Smoking, depression, reliable change, clinically significant change
1. Introduction
Smokers are more likely to have major depressive disorder (Wilhelm, Mitchell, Slade, Brownhill, & Andrews, 2003) and report more symptoms of depression than non-smokers (Anda et al., 1990). Over 30% of those with current depression are regular smokers, and 60% of individuals with a lifetime history of depression are current or past smokers (Grant, Hasin, Chou, Stinson, & Dawson, 2004; Lasser et al., 2000; Waxmonsky et al., 2005). Smokers with depressed mood are less likely to quit smoking (Anda et al., 1990), and even very low levels of depressed mood predict smoking relapse (Niaura et al., 2001). The causal link between depression and smoking is far from clear, although several hypotheses have been proposed, including self-medication (Breslau, Peterson, Schultz, Chilcoat, & Andreski, 1998) and common genetic risk factors (Lyons et al., 2008).
Research examining changes in depressed mood during smoking cessation has yielded mixed findings (Hughes, 2007a). Some studies report that smoking cessation either has no effect on or significantly decreases depressed mood (e.g., Brown et al., 2001), while others report that smoking cessation significantly increases depressed mood (e.g., Pomerleau, Namenek Brouwer, & Pomerleau, 2001). These incongruous findings do not appear to be related to the scale used to assess mood symptoms, level of nicotine dependence, or treatment setting (Hughes, 2007a).
One reason for these mixed findings might be that many of these studies measured mean changes in depressed mood. This approach has limited utility if there is high variability in individual patient response patterns. Burgess and colleagues (2002) modeled trajectories of depressive symptoms before and after counseling-supported attempts to quit smoking and identified five patterns of mood response. Two of these patterns involved overall increases in depressive symptoms, two involved overall decreases, and one pattern was characterized by an initial increase followed by a return to baseline. Thus, mood changes in response to smoking cessation attempts are highly varied, supporting the notion that the relationship between mood change and smoking cessation may be better understood using idiographic tracking rather than using group mean changes in mood.
Nine studies have utilized an idiographic approach by identifying specific individuals who developed a diagnosis of depression during or after smoking cessation treatment (Borrelli et al., 1996; Covey, Glassman, & Stetner, 1997; Glassman, 1993; Glassman, Covey, Stetner, & Rivelli, 2001; Kahler et al., 2002; Killen, Fortmann, Schatzberg, Hayward, & Varady, 2003; Patten, Rummans, Croghan, Hurt, & Hays, 1999; Torres et al., 2010; Tsoh et al., 2000). Collectively, these studies indicate that <1%–7% of smokers develop depression between pre- and post-treatment and that 2.1–18% develop depression in the months following cessation treatment.
Differences between these studies in diagnostic method (e.g., structured interview, self-report) and number and timing of assessments limit conclusions that can be made regarding the typical rate of depression development during smoking cessation treatment. In addition, the majority of these studies are anti-depressant medication trials, which could confound results (Hughes, 2007b).
One barrier to a broader investigation of depression development during smoking cessation is that traditional diagnostic measures (e.g., SCID-I) are rarely administered at multiple time points (cf. Kahler et al., 2002). One reason is that these measures are time intensive and require great use of resources (e.g., highly trained assessors). Thus, the development of low cost and valid methods to assess development of major depression on the individual level is needed. In the current paper, we apply reliable and clinically significant change criteria (Jacobson & Truax, 1991) to identify reliable increases in depressed mood and the likely development of major depressive disorder among individuals undergoing smoking cessation treatment.
Reliable and clinically significant change criteria are routinely applied to psychotherapy treatment outcome studies. To our knowledge, these criteria have never been applied to changes in mood during smoking cessation or any other addiction treatment. Reliable and clinically significant change criteria were developed in reaction to the over-reliance on statistically significant mean differences to determine the efficacy of clinical interventions. Reliable change criteria are used to assess whether an individual changed significantly more than would be expected given measurement error. Thus, the threshold for reliable change is calculated using the scale’s standard deviation and reliability, which are used to produce a range of change scores that includes 95% of the change scores that would occur by chance. Individuals whose change scores fall outside of this range are considered to have experienced reliable change. Given that reliable change has occurred, clinically significant change indicates whether an individual has transitioned from a functional to a dysfunctional population (or vice versa). For example, an individual experiencing severe depression may show reliable improvements in depressed mood but continue to have a score in the dysfunctional (but less severe) range.
The primary aim of the current study is to demonstrate the potential usefulness of using reliable and clinically significant change criteria as a proxy measure for the development of major depressive disorder during smoking cessation treatment. Specifically, we hypothesize that applying reliable and clinically significant change criteria will lead to different conclusions regarding the development of depression during smoking cessation treatment versus using mean changes in depressed mood over time. Unlike most previous studies, we will directly compare the rates of depression development between those who achieved abstinence and those who failed to achieve abstinence.
2. Methods
2.1. Participants
This study utilized 212 participants from a previously completed smoking cessation study targeting caregivers who smoke and have children with asthma (R01 Hl 62165, B. Borrelli, PI). A total of 241 smokers were enrolled, but 29 did not complete any follow-up assessments and were thus excluded from the current study. In the parent trial from which these data are extracted, participants were randomly assigned to receive one of two nurse-delivered smoking cessation interventions embedded in an in-home asthma education program: a motivational enhancement treatment based on the Precaution Adoption Model or a treatment based on the Behavioral Action Model (see Borrelli et al., 2002). Nicotine patches were provided to smokers who reported readiness to quit within 30 days. Participants did not have to want to quit smoking to be in the study. This study received approval from the Institutional Review Board at The Miriam Hospital.
Smokers were included if they (a) were over 18 years of age and a caregiver of a child with asthma under 18 years of age, (b) spoke English, and (c) were not receiving other smoking cessation treatment. Potential participants were not excluded on the basis of baseline depression scores. The sample in the current study consisted of 212 participants (Mage = 32.9, SD = 8.6, 89.6% female) who smoked an average of 15.1 cigarettes per day (SD = 8.6) and had an average score of 4.6 (SD = 1.4) on the Fagerstrom Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991). The majority of participants (84.8%) reported at least one 24-hour quit attempt in their lifetime, 43.8% were employed full or part time, 81.4% had an income below $30,000 per year, 70.8% had ≥ a high school education, and 35.8% were married or living with someone. The ethnic composition of the sample was 54.3% Caucasian, 19.5% Hispanic/Latino, 19.0% African American, and 7.1% other.
2.2. Measures and Procedure
Participants were assessed at baseline (pre-treatment); end-of-treatment (EOT, eight weeks after baseline); and 2, 6, and 12 months after EOT. Demographic information and the FTND (Heatherton et al., 1991) were assessed at baseline only. The Center for Epidemiological Studies Depression scale (CESD; Radloff, 1977) was used to assess depressed mood and was administered at all time-points. The CESD is a 20-item self-report measure with higher scores indicating greater depression (range 0–60). The CESD has demonstrated good reliability and validity across multiple populations (e.g., Lewinsohn, Hoberman, & Rosenbaum, 1988).
Abstainers were defined as those self-reporting ≥ 7 days of abstinence and achieving ≤ 10 ppm on an expired air Carbon Monoxide (CO) test. Those who self-reported 7 day point prevalence abstinence but had a CO > 10 ppm were recoded as smoking at that time point.
3. Calculation
The calculation of reliable change requires estimates of a scale’s internal consistency and standard deviation for a given population. The threshold for reliable change is calculated as 1.96 times the standard error of the difference between scores of a given measure administered on two occasions. Standard error of the difference (SEDiff) was calculated using the following formula (Jacobson & Truax, 1991)1:
SDpre represents standard deviation at pre-test and α represents the reliability of the measure. This sample is of adequate size (N = 212) for the standard deviation of total CESD score and internal consistency of items at baseline to provide reasonable estimates of these statistics in this population. Thus, a reliable change cut off was calculated using baseline data rather than general psychometric data. The standard deviation of the CESD at baseline (SDPre) was 12.187 and Cronbach’s alpha (α) was .914. A SEDiff of 5.05 and a reliable change cut-off of 9.91 (5.05 × 1.96) was computed for the current sample. Thus, any increase of ≥ 10 CESD points was considered a reliable increase in depressed mood.
The calculation of clinically significant change requires a cut point between functional and dysfunctional populations. There is a large empirical literature indicating that scores ≥ 16 on the CESD is indicative of significant depression symptoms (e.g., Weissman & Locke, 1975). However, as an indicator of major depressive disorder, a cut-off of ≥ 16 produces a high rate of false positives (Roberts & Vernon, 1983) and a higher cut-off of ≥ 23 has been shown to produce fewer false positives when cut-offs were compared to diagnoses based on structured clinical interviews (Husaini, Neff, Harrington, Hughes, & Stone, 1980). Other authors have argued that ≥ 23 is a more appropriate indicator of probable depression among women due to possible gender bias in CESD items (e.g., Ferketich, Schwartzbaum, Frid, & Moeschberger, 2000). Given that the purpose of the current study was to model the development of a depression diagnosis and almost 90% of the sample was female, we conservatively chose to use CESD scores 23 as the “dysfunctional range.” We propose that those patients who experience both a reliable (increase of ≥10 CESD points in this sample) and clinically significant increase (move from <23 to ≥23) in depressed mood likely develop major depressive disorder.
3.1. Analytic Plan
First, we examined the rates of reliable and both reliable and clinically significant increases in depression from baseline to any later time point. Specifically, for each participant, we examined whether their CESD score reached a point at EOT or 2, 6, or 12-month follow-ups that represented a reliable increase from the participant’s baseline CESD level. Those who experienced reliable change were further examined to determine if they also met criteria for clinically significant change (i.e., moved from <23 to ≥23). These rates were calculated for Any Point Abstainers (individuals who met abstinence criteria at one or more assessments) and Never Abstainers (individuals who failed to attain abstinence at any assessment).
We contrasted the rates at which participants experienced (a) reliable increases in depression and (b) both reliable and clinically significant increases in depression with mean CESD changes observed during the trial. Mean CESD change was calculated between baseline and each later assessment (i.e., baseline to end-of-treatment, baseline to 2 month follow-up, baseline to 6 month follow up and baseline to 12 month follow-up) and between baseline and last contact (i.e., participants last completed assessment). 2 In addition, for those who achieved abstinence we examined mean change in CESD between baseline and the assessment when abstinence was initially achieved. These intervals were chosen in order to approximate how mean change in depression is examined in smoking cessation trials.
4. Results
There were no significant differences between treatment conditions on rates of reliable or clinically significant increases or in mean CESD changes across all intervals tested below. Thus, all analyses were collapsed across treatment condition.
The first column of Table 1 presents the percentage of participants who experienced a reliable increase in depressed mood between baseline and any later assessment for Any Point Abstainers and Never Abstainers. Chi square analyses indicated no significant differences in the rate of reliable increases in depression between Any Point Abstainers and Never Abstainers.
Table 1.
Reliable and Clinically Significant Changes in Depression from Baseline Assessment to Any Later Assessment
| Reliable Increase in CESD from Baseline (n = 212) | Reliable & Clinically Significant Increase in CESD from Baseline (n = 137; Participants with CESD < 23 at Baseline) | |
|---|---|---|
| Any Point Abstainers | 16/57, 28.1 % | 9/38, 23.7% |
| Never Abstainers | 37/155, 23.9% | 23/99, 23.2% |
| Total | 53/212, 25.0% | 32/137, 23.4% |
The second column of Table 1 includes only those who were non-depressed (CESD < 23) at baseline and presents the percentage of participants who experienced both reliable and clinically significant increases in CESD from baseline to any later assessment. Chi square analyses indicated no significant differences in the rate of both reliable and clinically significant increases in depression between Any Point Abstainers and Never Abstainers.
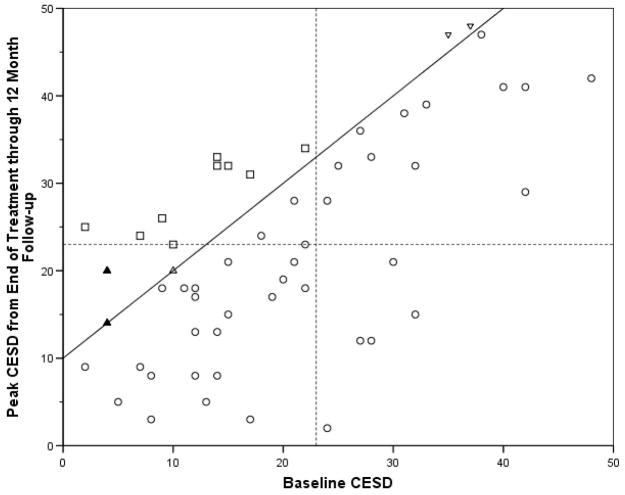
To visually clarify how reliable and clinically significant change was determined, Figure 1 presents a scatter plot of baseline CESD by peak CESD from end-of-treatment through follow-up (i.e., highest CESD after baseline) for all 57 Any Point Abstainers. The 16 Any Point Abstainers who experienced a reliable increase and the 9 who experienced both a reliable and clinically significant change are labeled. The solid diagonal line divides those who experienced a reliable increase in CESD (at least a 10 point increase) during the trial from those who did not experience such an increase. The dashed lines at 23 CESD points on the x and y axis represent the cut-off between dysfunctional (i.e., likely have major depressive disorder) and functional populations (i.e., likely do not have major depressive disorder).
Figure 1.
Scatter plot of Baseline CESD by peak CESD end-of-treatment through follow-up
○ = Participants who did not experience a reliable increase in depression
△ = Participants who experienced a reliable increase in depression but never scored ≥ 23 on the CESD (i.e., reliable but not clinically significant increase).
▽ = Participants who experienced a reliable increase in depression but had a CESD ≥ 23 at baseline (i.e., reliable but not clinically significant increase).
□ = Participants who experienced a both reliable and clinically significant increase in depression
Note: Solid shapes (i.e., ▲) indicate that there are two points at this location.
Any point abstainers (M = 19.09, SD = 11.58) and never abstainers (M = 18.21, SD = 12.43) had similar CESD scores at baseline. As seen in Table 2, there were no mean increases in CESD between baseline and any later assessment or between baseline and last contact for either group. In fact, several significant mean decreases in depressed mood were observed across these intervals, most consistently among any point abstainers.
Table 2.
Mean change in CESD between baseline and later time points for Any Point Abstainers and Never Abstainers
| Change in mean CESD scores from baseline to later assessments
|
||
|---|---|---|
| Any Point Abstainers | Never Abstainers | |
| Baseline to End-of-Treatment | −3.69 (9.01), n = 55*** | −1.81 (9.96), n = 147 ** |
| Baseline to 2 month follow-up | −4.66, (10.07), n = 51*** | −.71 (12.42), n = 122 |
| Baseline to 6 month follow-up | −1.16 (10.17), n =55 | −.48 (11.61), n = 111 |
| Baseline to 12 month follow up | −4.68 (13.19), n = 43** | −1.90 (12.13), n = 88 |
| Baseline to Last contact | −3.22 (12.85), n = 55* | −.90 (12.18), n = 155 |
| Baseline to initial abstinence | −4.80 (10.23), n = 57*** | NA |
Paired samples t tests:
p < .10,
p < .05,
p < .01
5. Discussion
Our results indicate that utilizing reliable and clinically significant change criteria to track symptoms of depression during smoking cessation treatment leads to different conclusions than tracking mean changes in depressed mood over time. Specifically, when we examined group mean change we found no evidence for significant increases in mean levels of depressed mood during the trial. When we utilized reliable change on the individual level, we found that a quarter of participants experienced a reliable increase in depressed mood. Similarly, a quarter of individuals who were not depressed at baseline likely developed a depression diagnosis at some point during the trial (i.e., experienced both reliable and clinically significant increase in CESD score).
The present investigation shows that the routine practice of reporting mean mood changes in smoking cessation trials may overlook important, clinically relevant information that could guide treatment planning and research hypotheses. Specifically, these results suggest that in trials where only small mean changes in depression are observed, it is possible that a significant proportion of these participants actually developed a depression diagnosis at some point during the trial. The high rates for depression development observed in this study suggest that the development of depression symptoms during cessation treatment may be more common than other investigations have suggested (e.g., Patten et al., 1999). Thus, it may be useful for providers to couple cessation techniques with prophylactic mood management strategies and psychoeducation regarding the likelihood of mood disturbance when attempting to quit.
Unlike past investigations of the development of depression during cessation, the current dataset was drawn from a cessation induction trial (i.e., included smokers who were not motivated to quit). This allowed for a large comparison group of smokers who where smoking at every time point throughout the trial. There were no significant differences in the rates of reliable or clinically significant increases in depression among Any Point Abstainers and Never Abstainers, and high rates of depression development were found in both groups. This suggests that individuals engaging in smoking cessation treatment may be at risk for (at least temporary) increases in depressed mood regardless of smoking status. Although, these findings are at odds with the assertion that smoking cessation can increase symptoms of depression (Glassman et al., 2001), they comports with other recent findings (Kahler et al., 2002; Kahler, Spillane, Busch, & Leventhal, in press; Torres et al., 2010; Tsoh et al., 2000).
It is notable that most Never Abstainers made multiple failed attempts to quit smoking during the course of this trial. We speculate that this context of repeated failure may prompt the development of depression or that simply attempting to quit smoking (whether or not sustained abstinence is achieved) may trigger a period of high mood variability. These hypotheses will be explored in future investigations.
We propose that a reliable and clinically significant increase in self-reported depression is a useful and user-friendly proxy for measurement of the development of major depression. The greatest advantage to utilizing this method is that it can easily be applied to archival smoking cessation trials that tracked depressed mood using only self-report scales. It is not required that these trials have administered the CESD or that sample characteristics are similar to the current sample, as the calculation of reliable change criteria are population and scale specific. More statistically complex methods to examine mood change during smoking cessation on the level of the individual smoker have recently been proposed (see Kahler, et al., in press). However, examining reliable and clinically significant increases requires little statistical expertise and allows for an estimate of the proportion of smokers who develop depression.
It is notable that the rate of clinically significant change in the current study is higher than the rates of depression development reported in past studies. However, there are several variables distinguishing the current study from past investigations that help explain this discrepancy. First, this study was a trial utilizing a psychosocial treatment rather than antidepressant medication; medication could have prevented the development of depression in previous trials. Second, our study assessed for increases in depressed mood at four time points over the course of one year. Other studies have observed much shorter time frames and conducted fewer assessments. For example, Borrelli and colleagues (1996) found that 7% of participants developed a diagnosis of major depression in 10 weeks. Third, the majority (90%) of the sample in our study was female. Given that women suffer from depression at a much higher rate (Kessler, Berglund, Demler, Jin, & Walters, 2005) and more often use smoking to cope with negative mood states (Yong & Borland, 2008), rates of depression development during smoking cessation may be greater among women. Finally, participants in this trial were not required to be non-depressed at baseline. Thus, this trial included many mildly depressed participants who may have been more vulnerable to additional spikes in depression.
Future research should continue to explore the application of reliable and clinically significant change criteria to increases in depression during smoking cessation attempts. This method could be further validated by applying the current clinically significant change technique to datasets that administered both a psychiatric clinical interview and a self-report depression inventory at multiple time points. Then, the concordance of the two techniques using various functional/dysfunctional cut-offs for the calculation of clinically significant change could be explored. Future studies should also explore the potential moderating effect of a history of a depression diagnosis on the effects observed in this study3.
Research Highlights.
Major depression may develop during smoking cessation
We propose change criteria that may capture the development of depression
A combination of reliable and clinically significant change criteria was utilized
Abstinence did not affect the likelihood of developing depression
This method may serve as a useful proxy measure for the development of depression
Acknowledgments
Role of Funding Sources
This study was supported by NIH grant R01 HL-062165 to Belinda Borrelli. NIH had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Footnotes
This formula is a combination of the formulas for standard error and standard error of the difference presented by Jacobson and Truax (1991, p. 14).
For the majority of the sample (131/212, 61.8%), last contact was the 12 month follow-up assessment.
Multiple previous studies have found that a history of major depression is a significant predictor of depression development during smoking cessation. However, this information was not collected in the present trial and thus we were unable to consider history of major depression as a moderator.
Contributors
All authors wrote sections of the current manuscript. The first author conducted statistical analyses. The forth author provided literature reviews. The fifth author provided mentorship and wrote the treatment protocols. All authors contributed to and have approved the final manuscript.
Conflict of Interest
All authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anda RF, Williamson DF, Escobedo LG, Mast EE, Giovino GA, Remington PL. Depression and the dynamics of smoking: A national perspective. Journal of the American Medical Association. 1990;264:1541–1545. doi: 10.1001/jama.264.12.1541. [DOI] [PubMed] [Google Scholar]
- Borrelli B, McQuaid EL, Becker B, Hammond K, Papandonatos G, Fritz G, et al. Motivating parents of kids with asthma to quit smoking: The PAQS project. Health Education Research. 2002;17:659–669. doi: 10.1093/her/17.5.659. [DOI] [PubMed] [Google Scholar]
- Borrelli B, Niaura R, Keuthen NJ, Goldstein MG, DePue JD, Murphy C, et al. Development of major depressive disorder during smoking-cessation treatment. Journal of Clinical Psychiatry. 1996;57:534–538. doi: 10.4088/jcp.v57n1106. [DOI] [PubMed] [Google Scholar]
- Breslau N, Peterson EL, Schultz LR, Chilcoat HD, Andreski P. Major depression and stages of smoking: A longitudinal investigation. Archives of General Psychiatry. 1998;55:161–166. doi: 10.1001/archpsyc.55.2.161. [DOI] [PubMed] [Google Scholar]
- Brown RA, Kahler CW, Niaura R, Abrams DB, Sales SD, Ramsey SE, et al. Cognitive–behavioral treatment for depression in smoking cessation. Journal of Consulting and Clinical Psychology. 2001;69:471–480. doi: 10.1037/0022-006X.69.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess ES, Brown RA, Kahler CW, Niaura R, Abrams DB, Goldstein MG, et al. Patterns of change in depressive symptoms during smoking cessation: Who's at risk for relapse? Journal of Consulting and Clinical Psychology. 2002;70:356–361. doi: 10.1037/0022-006X.70.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey LS, Glassman AH, Stetner F. Major depression following smoking cessation. American Journal of Psychiatry. 1997;154:263–265. doi: 10.1176/ajp.154.2.263. [DOI] [PubMed] [Google Scholar]
- Ferketich AK, Schwartzbaum JA, Frid DJ, Moeschberger ML. Depression as an antecedent to heart disease among women and men in the NHANES I study. Archives of Internal Medicine. 2000;160:1261–1268. doi: 10.1001/archinte.160.9.1261. [DOI] [PubMed] [Google Scholar]
- Glassman AH. Cigarette smoking: Implications for psychiatric illness. American Journal of Psychiatry. 1993;150:546–553. doi: 10.1176/ajp.150.4.546. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Covey LS, Stetner F, Rivelli S. Smoking cessation and the course of major depression: A follow-up study. The Lancet. 2001;357:1929–1932. doi: 10.1016/S0140-6736(00)05064-9. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States. Archives of General Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: Valid symptoms and time course. Nicotine & Tobacco Research. 2007a;9:315–327. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Depression during tobacco abstinence. Nicotine & Tobacco Research. 2007b;9:443–446. doi: 10.1080/14622200701243185. [DOI] [PubMed] [Google Scholar]
- Husaini BA, Neff JA, Harrington JB, Hughes MD, Stone RH. Depression in rural communities: Validating the CES-D Scale. Journal of Community Psychology. 1980;8:20–27. doi: 10.1002/1520-6629(198001)8:1<20::AID-JCOP2290080105>3.0.CO;2-Y. [DOI] [Google Scholar]
- Jacobson NS, Truax P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. Journal of Consulting and Clinical Psychology. 1991;59:12–19. doi: 10.1037/0022-006X.59.1.12. [DOI] [PubMed] [Google Scholar]
- Kahler CW, Brown RA, Ramsey SE, Niaura R, Abrams DB, Goldstein MG, et al. Negative mood, depressive symptoms, and major depression after smoking cessation treatment in smokers with a history of major depressive disorder. Journal of Abnormal Psychology. 2002;111:670–675. doi: 10.1037/0021-843X.111.4.670\. [DOI] [PubMed] [Google Scholar]
- Kahler CW, Spillane NS, Busch AM, Leventhal AM. Time-varying smoking abstinence predicts lower depressive symptoms following smoking cessation treatment. Nicotine & Tobacco Research. doi: 10.1093/ntr/ntq213. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Schatzberg A, Hayward C, Varady A. Onset of major depression during treatment for nicotine dependence. Addictive Behaviors. 2003;28:461–470. doi: 10.1016/S0306-4603(01)00266-0. [DOI] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. Journal of the American Medical Association. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Hoberman HM, Rosenbaum M. A prospective study of risk factors for unipolar depression. Journal of Abnormal Psychology. 1988;97:251–264. doi: 10.1037/0021-843X.97.3.251. [DOI] [PubMed] [Google Scholar]
- Lyons M, Hitsman B, Xian H, Panizzon MS, Jerskey BA, Santangelo S, et al. A twin study of smoking, nicotine dependence, and major depression in men. Nicotine & Tobacco Research. 2008;10:97–108. doi: 10.1080/14622200701705332. [DOI] [PubMed] [Google Scholar]
- Niaura R, Britt DM, Shadel WG, Goldstein M, Abrams D, Brown R. Symptoms of depression and survival experience among three samples of smokers trying to quit. Psychology of Addictive Behaviors. 2001;15:13–17. doi: 10.1037/0893-164X.15.1.13. [DOI] [PubMed] [Google Scholar]
- Patten CA, Rummans TA, Croghan IT, Hurt RD, Hays JT. Development of depression during placebo-controlled trials of bupropion for smoking cessation: Case reports. Journal of Clinical Psychiatry. 1999;60:436–441. doi: 10.4088/jcp.v60n0703. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Namenek Brouwer RJ, Pomerleau OF. Emergence of depression during early abstinence in depressed and non-depressed women smokers. Journal of Addictive Diseases. 2001;20:73–80. doi: 10.1300/J069v20n01_07. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Roberts RE, Vernon SW. The Center for Epidemiologic Studies Depression Scale: Its use in a community sample. American Journal of Psychiatry. 1983;140:41–46. doi: 10.1176/ajp.140.1.41. [DOI] [PubMed] [Google Scholar]
- Torres LD, Barrera AZ, Delucchi K, Penilla C, Pérez-Stable EJ, Muñoz RF. Quitting smoking does not increase the risk of major depressive episodes among users of Internet smoking cessation interventions. Psychological Medicine. 2010;40:441–449. doi: 10.1017/S0033291709990560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoh JY, Humfleet GL, Muñoz RF, Reus VI, Hartz DT, Hall SM. Development of major depression after treatment for smoking cessation. American Journal of Psychiatry. 2000;157:368–374. doi: 10.1176/appi.ajp.157.3.368. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Locke HZ. Comparison of a self-report symptom rating scale (CES-D) with standardized depression rating scales in psychiatric populations. American Journal of Epidemiology. 1975;102:430–431. [Google Scholar]
- Waxmonsky JA, Thomas MR, Miklowitz DL, Allen MH, Wisniewski SR, Zhang H, et al. Prevalence and correlates of tobacco use in bipolar disorder: data from the first 2000 participants in the Systematic Treatment Enhancement Program. General Hospital Psychiatry. 2005;27:321–328. doi: 10.1016/j.genhosppsych.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Wilhelm K, Mitchell P, Slade T, Brownhill S, Andrews G. Prevalence and correlates of DSM-IV major depression in an Australian national survey. Journal of Affective Disorders. 2003;75:155–62. doi: 10.1016/S0165-0327(02)00040-X. [DOI] [PubMed] [Google Scholar]
- Yong HH, Borland R. Functional beliefs about smoking and quitting activity among adult smokers in four countries: Findings from the International Tobacco Control Four-Country Survey. Health Psychology. 2008;27:S216–223. doi: 10.1037/0278-6133.27.3(Suppl.).S216. [DOI] [PMC free article] [PubMed] [Google Scholar]