Abstract
The great majority of tobacco addiction begins during adolescence. More heavily addicted smokers begin smoking earlier, but differentiating the neurobehavioral impact of nicotine self-administration during adolescence from self-selection bias (whereby people more prone to heavy addiction also begin earlier) cannot be ethically unconfounded in humans. The goals of this research were to determine the age threshold for the adult-like nicotine self-administration and determine sex differences. Male and female Sprague-Dawley rats were tested for nicotine self-administration starting at 4, 5, 6, 7, and 8 weeks of age in an operant FR1 schedule for IV nicotine (0.03 mg/kg/infusion) in 45-minute sessions for two weeks, with one week of enforced abstinence and one week of resumed access. This study replicated our earlier work that nicotine self-administration was increased in adolescent vs. adult rats and that the effect was more pronounced in adolescent males, but the increased nicotine self-administration was more persistent in adolescent-onset females. The age threshold for adult-like behavior was 6–7 weeks of age. Adolescent-onset nicotine self-administration had persisting effects of eggaurated increases of nicotine self-administration when fixed-ratio requirements for self-administration were lowered. Female rats that had begun nicotine self-administration during adolescence showed exaggerated increases in nicotine self-administration after a switch back to FR1 from FR8, indicating a lessened control over their self-administration. Adolescent-onset nicotine self-administration was not found to potentiate cocaine self-administration. Adolescent-onset nicotine self-administration causes persistent increases in nicotine self-administration in female rats even after they reach adulthood and disrupts control over self-administration behavior.
Keywords: Nicotinic, Nicotine, Self-administration, Adolescence
1. Introduction
The great majority of cigarette smokers begin using tobacco during adolescence [1, 2]. Approximately 75% of adult tobacco users report that their first tobacco use occurred 11–17 years of age and 60% before 14 years of age [3–5]. It is clear that those who begin smoking in adolescence develop greater dependence and higher rates of smoking throughout adult life [6, 7]. Adolescents progress into nicotine dependence very quickly [8]. Specifically, individuals who smoke their first cigarette before the age of 13 are more likely to have difficulty quitting [9]. Adolescent girls are increasing in their smoking at a rate greater rate than boys and tend to have a more difficult time stopping [10]. Understanding the mechanisms of nicotine actions on neurobehavioral function in adolescents and how those may differ from those in adults could help elucidate the foundations tobacco addiction and improved ways to combat it. However, with human studies it is difficult to differentiate the possibilities that nicotine exposure during adolescence has lasting impacts on late-stage neurobehavioral development resulting in greater liability to addiction vs. the alternative that those individuals who are prone to greater addiction because of genetic and/or early environmental reasons also are prone to start drug use earlier. Self-selection bias can confound discrimination of drug effects on adolescent development in human studies of early vs. later onset of nicotine use. In experimental animal studies there can be random assignment of subjects to early access vs. later access drug use conditions to determine the cause-effect relationship between age of initiation and the onset and persistence of drug use.
In humans, adolescence can be broadly defined as the beginning of the first signs of sexual development, which can be as early as ten years of age and continuing into full adulthood in one’s early 20s [11]. Adolescence is also characterized by significant late phase neurobehavioral development. These are also years characterized by the initiation of many risky behaviors including sampling drugs of abuse with nicotine as a primary example. Spear and Brake [12] in a seminal paper reviewed the data showing altered responsiveness to catecholaminergic drugs in adolescent rats. Adolescent rats were found to be hypersensitive to dopaminergic antagonists and hyposensitive to amphetamine in terms of motor function. This differential dopaminergic sensitivity during adolescence may be key to the impact of drugs of abuse during this period. Because the typical initiation of nicotine intake via tobacco use comes when the brain is still undergoing important development, the consequences of drug use during adolescence may be different from the consequences of drug use later in life. Adolescent nicotine effects may critically differ from those in adulthood because of important late phase neural development during adolescence [13]. In the rat the core period of adolescence begins at approximately four weeks of age when initial signs of sexual development begin and has been proposed to continue for the next two weeks [13]. However, the boundaries of adolescence in humans and animal models is a matter of debate and appears to depend on the function under consideration [13].
Adolescent rats behaviorally respond to nicotine as rewarding and less aversive, compared to adult response. This shift in the balance between the rewarding and aversive effects of nicotine may make adolescents more susceptible to continued nicotine use [14]. Conditioned place preference is more readily seen in adolescents than in adults [14–17]. Adolescent nicotine exposure retards the development of reward systems, thus, maintaining an adolescent state indefinitely, which could result in increased vulnerability to substance abuse problems throughout adulthood [18]. Adolescent mice show nicotine conditioned place preference at lower doses than adults and showed increased physical withdrawal signs in terms of changes in locomotion, anxiety response, nociception and hypothermia [19]. Early adolescent rats are more sensitive to nicotine’s rewarding effects and are in accord with studies showing a unique profile of neurobehavioral alterations following nicotine exposure when compared with adults. In contrast to mice, in rats there is evidence for diminished nicotine withdrawal symptoms in adolescence [20]. Adolescent rats show fewer acute somatic signs of nicotine withdrawal than adults [21]. This diminished negative consequence of intermittent nicotine use may also facilitate continued use. Adolescent rats undergoing nicotine withdrawal show diminished conditioned place aversion compared to adult rats even though there was no age difference in lithium chloride induced place aversion [22]. Adolescent nicotine exposure can have long-lasting adverse effects. Nicotine administered chronically to male adolescent rats produced over the next 7 weeks a persistent withdrawal syndrome characterized by increased arousal, anxiety and emotionality [23, 24]. Animals tested during adolescence responded to the nicotine challenge with less hypoactivity compared with adults [25, 26].
In general, the literature primarily using rodent models supports the idea that there is a biobehavioral basis for the greater susceptibility of adolescents to effects of drugs of abuse [13, 27], with greater and more persistent nicotinic α4β2 acetylcholine receptor upregulation than in adults, outright neuronal loss in neural systems responsible for learning, memory and mood, and more pronounced alterations in synaptic activity upon withdrawal of nicotine [28–35]. In the transition from adolescence to adulthood there was a complex pattern of functional maturation of nicotinic receptors in the ventral, but not dorsal striatum. In males, but not females, there are significant changes in nicotine potency and efficacy. These findings suggest that nicotinic receptors may play critical functional roles throughout dopamine neuronal maturation [36]. However, in contrast, Doura et al. found that adolescent rats showed a smaller increase in α4β2 nicotinic receptors than adults after chronic nicotine [37]. It is difficult to directly compare the two study sets because of differing methods used to quantify the receptor binding with Doura et al. using autoradiography and the Slotkin group using homonogenate binding and the fact that different time points of assessment. The findings of Doura et al. and the Slotkin group were similar insofar as they both found less upregulation of α4β2 binding in the hippocampus of adolescents than adults after chronic nicotine infusion.
Concerning nicotine self-administration, the importance of adolescent onset has been shown in our studies [38–40], as well as those of other laboratories [41–44]. When access to nicotine is started in rats during adolescence, there is a substantially higher level of self-administration than in adult rats. Adolescent rats acquire nicotine self-administration faster than adults, an effect robust in females [44]. We have found greater adolescent-onset nicotine self-administration in both males and females compared with same sexed adults with the males showing a greater increase during adolescence and the females showing greater persistence into adulthood after adolescent-onset [38, 40]. Interestingly, no increase in adolescent vulnerability vs. adults to nicotine self-administration has been seen in other studies [45, 46]. This points to the need for a detailed analysis of the effect of age of onset of nicotine self-administration over the course of adolescence from the juvenile period to adulthood.
The current studies were conducted to determine in the rat model the threshold of adult-like responding to the onset of nicotine access for self-administration. A fine-grained age effect function was tested with rats starting nicotine access at weekly intervals from one week post-weaning (4 weeks of age) until young adulthood (8 weeks of age). Because of the observed sex differences in human tobacco smoking and rat nicotine self-administration [47, 48] the full age-effect function was run for both males and females. The effect of the age of initiation on the longer-term persistence of nicotine self-administration was carried out over four weeks covering the period during which even the youngest group matured into adulthood. To test the effect of age of onset of nicotine self-administration on relapse to nicotine self-administration in adulthood, resumption of nicotine self-administration after a period of enforced abstinence was tested in all groups. The enforced abstinence and resumption of access paradigm was used to test relapse to nicotine use instead of the extinction and reinstatement paradigm because the most common way people attempt to quit smoking is simple cessation of cigarette use without extinction of smoking rather than extinction smoking nicotine free cigarettes. Effects of varying fixed ratio (FR) requirements for nicotine self-administration were tested in adolescent and adult-onset of nicotine self-administration rats to determine the dynamics of self-regulation of nicotine self-dosing when access was easy or difficult. Finally, the possible effect of adolescent nicotine self-administration experience on later cocaine self-administration was tested in adolescent vs. adult onset rats to test the gateway hypothesis that early nicotine use would predispose individuals to later use of other drugs. These studies were directed towards providing critical age-related nicotine self-administration information so that further studies could determine mechanisms of adolescent risk for tobacco addiction and its optimal treatment.
2. Methods
2.1. Subjects
This study was conducted in accordance with the regulations of the Duke University Animal Care and Use Committee, Ten cohorts of male and female Sprague-Dawley albino rats were used. One male and one female subject made up each age group of each cohort, and five different age groups (4, 5, 6, 7 and 8 weeks old) were tested. They were singly housed in approved standard laboratory conditions in a Duke University Vivarium facility near the testing room to minimize any stress induced by transporting the rats. They were kept on a 12:12 reverse day:night cycle so that they are in their active phase during behavioral testing. All of the rats in a cohort arrived at the lab at the same time to equalize the experience of all of the subjects. The rats were allowed access to water at all times and fed daily approximately 20–30 minutes after completing the sessions. The female rats were staged for estrus cycle throughout the study. For staging of estrus vaginal swabs were placed in microscopic slides and the cells examined using a microscope and cell morphology classified histologically to determine proestrus, estrus, metestrus and diestrus based on cytological characterization.
2.2. Drug Preparation
Solutions of nicotine ditartrate and cocaine HCl were prepared weekly in pyrogen-free glassware in sterilized isotonic saline. The pH of the solutions was adjusted to 7.0 first and the solutions were passed through a 0.22 μm Millipore filter for sterilization before use. All solutions were kept refrigerated in the dark between experiments.
2.3. Self-Administration Procedure
For behavioral training, rats were placed in dual lever operant chambers (Med Associates, Vermont, USA). Each chamber is equipped with a tone generator, house light, cue light above each lever, and a metal tether to cover the drug delivery line. A Pentium computer programmed with MED-PC software controlled experimental events and data collection. Each catheter was connected to a High Speed Micro-Liter Syringe Pump (MED-Associates) with Polyethylene tubing and is fitted with a blunt edged 23-gauge needle. During each session, the rats wear vascular access harnesses (Instech Solomon, Plymouth Meeting, PA, USA) to connect them to the tethers and prevent chewing of the delivery lines.
During tutorial sessions, each rat was placed in a dual level operant chamber and hand-trained for a total of 14 periods of 15 minutes. One of the two levers was designated the cue lever in each box. The experimenter controlled the release of a pellet in a FR1 schedule. The experimenter was trained to reward successive approximations for the early training sessions and the rats gradually learned to press the levers independently for food pellets. More specifically, at first the rat would be rewarded for approaching the lever, then for placing a paw on the lever and finally for pressing the lever. Once the tutorial sessions were completed, the rats had 3 pellet sessions lasting 45 minutes to confirm their ability to lever press before undergoing jugular catheterization surgery.
The chamber was equipped with two forms of secondary stimuli: a feedback tone and a cue light. The feedback tone sounded for 0.5 s upon the delivery of a reward. The cue light was located above the designated active lever. During the pellet sessions, the rats wore plastic harnesses to familiarize them for the nicotine sessions. The harness was tightened around the body with plastic tubing and attached to the drug delivery line. After completing three 45-minute pellet sessions, the rats were surgically implanted with jugular catheters and an administration port. Ketamine (70 mg/kg) and Domitor (0.7 mg/kg) were used as anesthesia.
During the pre-session, each chamber was flushed with 5 ml of sterile saline solution and loaded with the appropriate nicotine dosage. Approximately 0.5 ml of nicotine solution was run through the drug delivery lines. Each rat participated in one nicotine session daily lasting 45 min. Before the session, lidocaine was administered topically to limit the pain caused to the surgical area. Then, the catheters were flushed with approximately 0.2–0.3 ml of 100 units/ml heparinized sterile saline. To begin the session, the rats were secured in their harnesses with the drug delivery needles entering the ports on their backs. When the program was issued, the rats received 3 infusions of nicotine to fill their port to ensure that upon pressing the lever they would feel the effects of the drug. The rats received 0.03 mg/kg of nicotine per infusion in a volume of 50 μl of saline. Following the 45-min. session, the rats were removed from their chambers, and liquid was withdrawn from their ports. The presence of blood was noted. The catheters were flushed with 0.2–0.3 ml of a sterile lock consisting of 500units/ml heparinized sterile saline with 8 mg/ml Gentamicin, and antibiotic ointment was applied.
In Experiment 1, the male and female rats starting access to nicotine at 4, 5, 6, 7 and 8-weeks of age arrived to the lab on the same time in ten cohorts one week before their initial nicotine access (Final N’s Males Week 4=10, Week 5=9, Week 6=7, Week 7=7, Week 8=10; Females Week 4=9, Week 5=9, Week 6=10, Week 7=9, Week 8=9). The rats had four weeks of access to nicotine for five days per week and then underwent a period with no access to nicotine. During this period of enforced abstinence the rats were not put into the testing chamber. Finally, during the sixth week the rats were given resumed access to nicotine to test for relapse after the period of enforced abstinence.
Experiment 2 with a new set of rats focused on females given the results of experiment 1 showing clear persisting effects of adolescent nicotine in females but not males. This study compared females starting nicotine self-administration at 6 and 8 weeks of age (N=9 for Week 6, N=9 for Week 8). After initial training for nicotine self-administration for seven sessions, the rats were tested for the effects of shifting the FR requirement from FR1 to FR2, FR4, FR8 and back to FR1 on consecutive days. This was repeated in the same fashion for a second week.
Experiment 3 with a new set of female rats also starting nicotine access at 6 or 8 weeks of age in female rats (N’s Week 6=13, Week 8=11) focused on the effect of earlier or later initiation of nicotine self-administration for two weeks on subsequent self-administration of cocaine (FR1 at 0.32 mg/kg/infusion) for two weeks to test the gateway hypothesis. Then after a week of enforced abstinence the rats were given access to cocaine for an additional week. The effect of the phase of estrus cycle on nicotine and cocaine self-administration was analyzed.
2.4. Statistical Analysis
The data from the behavioral studies were assessed for significance by an analysis of variance for between and within subjects factors. Between subjects factors were the age of the onset of nicotine self-administration and sex. The within subjects factors were weeks of access to nicotine and the FR ratio used. The principal dependent measure was the number of infusions per session. Other dependent measures were the number of active and inactive lever presses per session. Fisher’s protected least significant difference tests were used for planned comparisons between groups. An alpha level of p<0.05 was used as the cutoff for statistical significance.
3. Results
3.1. Experiment 1: Age-Effect Function for Onset of Nicotine Self-Administration
In pre-training of lever pressing for food pellets there was no significant effect of age for either the average of the three training sessions or for the final session. There was a significant effect of sex (F(1,90)=13.81, p<0.0005), with males self-administering significantly more pellets by the end of pre-training than females (males=100.2±6.0, females=71.6±5.0). The interaction of age × sex was not significant for food self-administration.
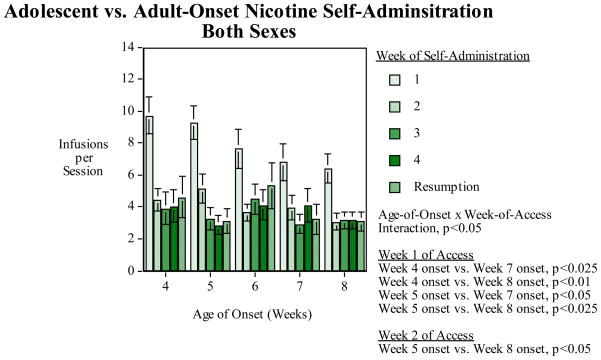
For nicotine-self-administration, we conducted a detailed analysis of sex and age differences in adolescent vs. adult-onset nicotine self-administration with male and female Sprague-Dawley rats given initial access to IV nicotine self-administration at 4, 5, 6, 7 and 8 weeks of age and continuing for four weeks and one week of resumption of access after a week of enforced abstinence. There was a significant (F(16,164)=1.83, p<0.05) interaction of age-of-onset × week-of-access. Follow-up comparisons of the effects of age-of-onset at each week-of-access were made using the Fisher’s Protected Least Significant Difference Test. During week 1 of nicotine access the 4 week age-of-age onset group self-administered significantly more than the week 7 (p<0.025) and week 8 (p<0.01) age-of-onset groups (Fig. 1). During week 1 of nicotine access the week 5 age-of-onset self-administered significantly more than the week 7 (p<0.05) and week 8 (p<0.025) age-of-onset groups. No significant effect of age was seen during weeks 3–4 of access. During week 2 of nicotine access the week 5 age-of-onset self-administered significantly more than the week 8 (p<0.05) age-of-onset group. No other significant effects of age averaged across sex were seen at the later week-of-access time points.
Figure 1.
The age-effect function of age of onset and nicotine self-administration averaged across sex, infusions (mean±sem) per 45-min. session for each of the four consecutive weeks of nicotine access as well as the week of nicotine access after one week of enforced abstinence. There was a significant Age-of-onset × Week-of-access interaction (p<0.05). Analysis of the simple main effects of age-of-onset during each week showed that during the first week of access the rats beginning access at four and five weeks of age self-administered significantly more nicotine than those beginning at seven or eight weeks of age (p<0.05-p<0.01). During the second week of access the rats beginning access at five weeks of age self-administered significantly more nicotine (p<0.05) than the rats that began access at eight weeks of age.
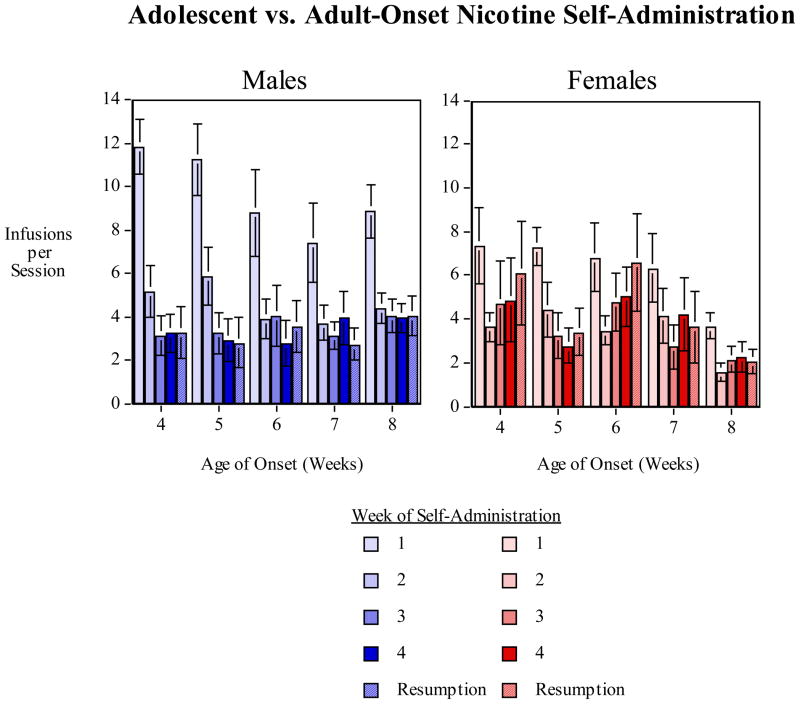
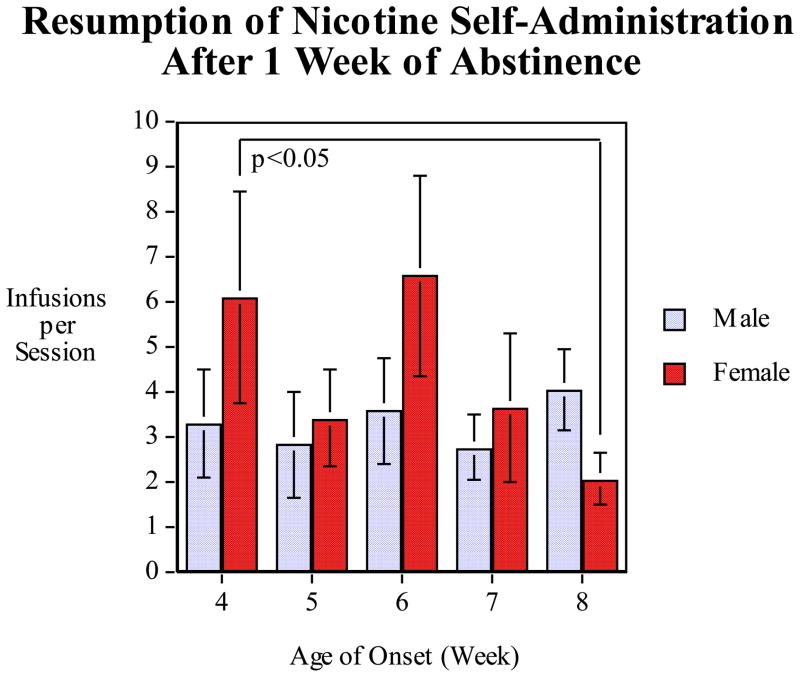
There was a significant (F(4,164)=9.25, p<0.0005) with males having greater nicotine self-administration during the first (p<0.0005) and second (p<0.05) week of access (Fig. 2). Males decreased their self-administration and did not have greater nicotine self-administration thereafter. Adolescent-onset female rats starting at 4 weeks of age showed a significantly (p<0.05) greater persistence of higher nicotine self-administration into adulthood than females starting as young adults (8 weeks of age) during the resumption of access week (Fig. 3). Females with an age of onset of nicotine self-administration of 6 weeks showed a trend for increased long-term self-administration vs. females starting at 8 weeks during the resumed access week, but this was not significant (p=0.056). The significantly greater nicotine self-administration in female rats which had onset of access at four-weeks of age relative to females which had onset of access at eight-weeks of age was seen at a point when all the rats were adults (9–13 weeks of age). Thus, even when all of the rats are adults during the resumption period, adolescent vs. adult onset is an important determinant in females of the amount of nicotine self-administered. Males did not show any such persistent effect of adolescent onset. Hormonal and neurobehavioral changes in adolescent females may underlie particular vulnerability to nicotine addiction.
Figure 2.
Sex differences in the age-effect function of age of onset and nicotine self-administration, infusions (mean±sem) per 45-min. session for each of the four consecutive weeks of nicotine access as well as the week of nicotine access after one week of enforced abstinence.
Figure 3.
The age-effect function of age of the resumption of nicotine self-administration after a one-week period of enforced abstinence, infusions (mean±sem) per 45-min. session. The female rats that had begun nicotine access at 4 weeks of age self-administered significantly (p<0.05) more nicotine than the female rats that had begun nicotine access at eight weeks of age.
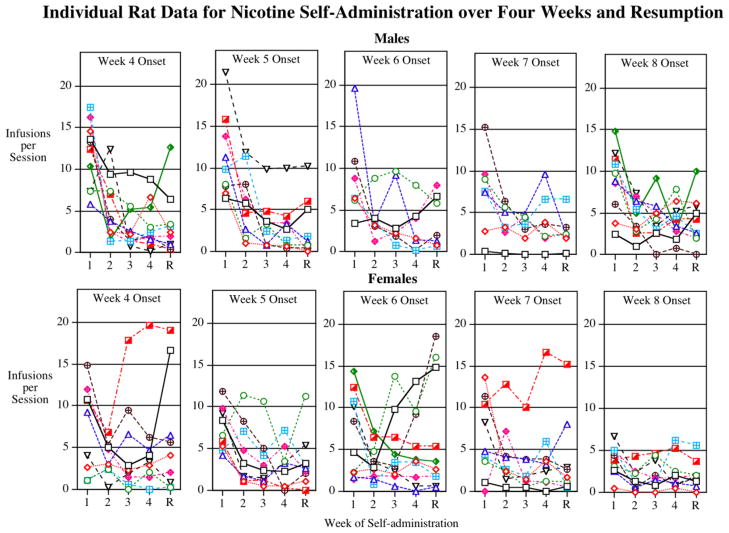
The individual animal data are shown in figure 4. This shows the heterogeneity of response with a subset of the adolescent onset females showing a long-term elevation in nicotine self-administration during the resumption period after enforced abstinence. There were 2 out of 9 rats that started at 4 weeks of age that averaged 15 or more nicotine infusions per session during the resumption period. Three out of 10 females that started at 6 weeks of age and 1 of 9 that started at 7 weeks also showed high levels of nicotine self-administration (15 or more nicotine infusions in the 45-minute session) during the resumption period. None of the females that started nicotine access in young adulthood at 8 weeks of age showed elevated nicotine self-administration during the resumption period. Curiously, none of those starting at 5 weeks of age showed a persistent increase, but with a heterogeneous effect this sort of outcome can be expected. As can be seen none of the males of any age showed such persistent increases in nicotine self-administration. There were substantial individual differences in adolescent-onset nicotine self-administration in females. The rate of high nicotine self-administration among adolescent-onset female rats was approximately 30%, quite similar to the rate of progression to regular smoking after initial experimentation with cigarettes in humans.
Figure 4.
Individual animal data for the age-effect function of age of the resumption of nicotine self-administration after a one-week period of enforced abstinence, infusions per 45-min. session. X-axis: Weeks 1, 2, 3, 4 of nicotine access, resumption (R) of nicotine access after 1 week of enforced abstinence.
3.2. Experiment 2: FR Shifts in Adolescent vs. Adult-Onset Nicotine Self-Administration
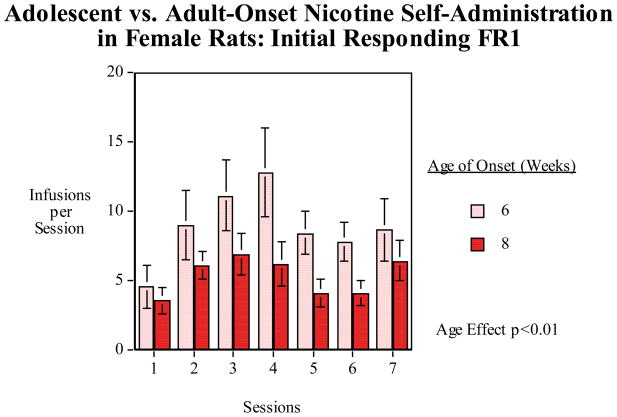
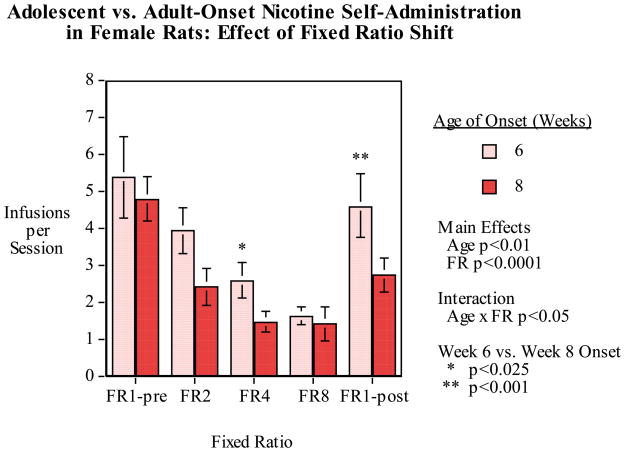
Female rats that started nicotine self-administration at 6 or 8 weeks of age were tested for the effects of day to day changes in FR requirement to self-administer nicotine. We trained female rats 6 and 8 weeks of age (N=9/age group) as previously on nicotine self-administration. As shown in figure 5 there was a significant main effect of age (F(1,8)=12.58, p<0.01) with the rats that began nicotine self-administration at 6 weeks of age showing a greater number of infusions over sessions of training than those that started at 8 weeks (6-week group=8.91±1.79 and 8-week group=5.35±0.72 infusions per session) over the seven sessions of pretraining. Then, the rats went through a sequence of doubling fixed-ratio requirements over sessions each day from FR1 to FR2, FR4 and FR8 and then a sudden return the without any intervening steps to FR1 the next day. The most interesting result is that the female rats that began nicotine self-administration during mid-adolescence showed a much greater rebound increase in nicotine self-administration when returning to FR1 after the progressive increase in response requirement to FR8 (Fig. 6).
Figure 5.
Six vs. eight-week old age of onset of nicotine self-administration initial training for nicotine self-administration fixed-ratio-1 (FR1), infusions (mean±sem) per 45-min. session in female rats. There was a significant (p<0.01) main effect of age of onset with the rats that had begun access at six weeks of age self-administering more nicotine than the rats that had begun access at eight weeks of age.
Figure 6.
Six vs. eight-week old age of onset of nicotine self-administration fixed-ratio (FR) progression and reversal, infusions (mean±sem) per 45-min. session in female rats. There were significant main effects of age of onset (p<0.05) and FR (p<0.0001) with younger rats and lower FR resulting in higher levels of nicotine self-administration. There was also a significant age-of-onset × FR interaction (p<0.05) and analysis of the simple main effects at each FR condition showed that the rats that had onset of nicotine access at six weeks of age self-administered significantly more nicotine at FR4 (p<0.025) and the FR1-post conditions (p<0.001).
3.3. Adolescent Nicotine and Later Cocaine Self-Administration
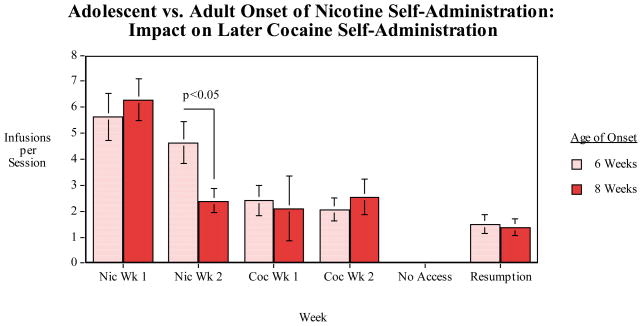
These procedures were conducted to test the hypothesis that adolescent onset nicotine self-administration would potentiate later self-administration of other drugs such as cocaine, commonly referred to as the “gateway hypothesis”. This was tested with adolescent vs. adult onset nicotine self-administration followed by a switch to cocaine. As seen before, adolescent onset did increase nicotine self-administration relative to the adult-onset group. In this experiment this significant effect (p<0.05) was seen during the second week of nicotine access. As shown in figure 7, beginning nicotine self-administration at 6 weeks of age was not found to significantly alter later total cocaine self-administration, but there were changes in the dynamics of cocaine self-administration over the estrus cycle.
Figure 7.
Six vs. eight-week old age of onset of nicotine self-administration effect on later cocaine self-administration to estrus phase, infusions (mean±sem) per 45-min. session in female rats. The rats that had begun nicotine access at six weeks self-administered significantly (p<0.05) more nicotine during the second week of access compared with rats that began nicotine access at eight weeks of age. No significant differences were seen with the subsequent switch to cocaine self-administration.
3.4. Estrus Cycle
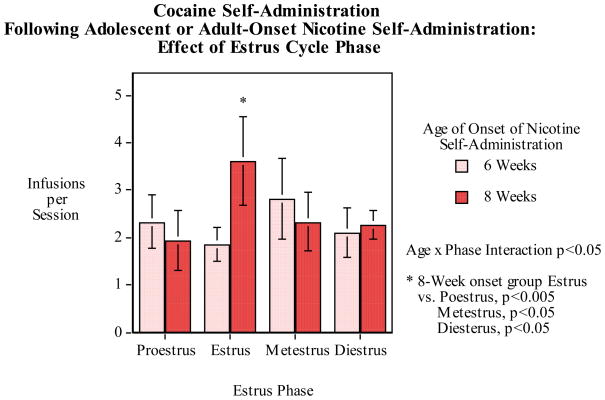
Relationship of nicotine self-administration to estrus cycle in females was assessed. As we and others have previously seen [49, 50] there was no detected significant relationship between estrus phase and nicotine self-administration. With regard to cocaine self-administration, there was a complex effect. There was a significant (F(3,72)=3.00, p<0.05) interaction of estrus phase and age of nicotine self-administration onset with regard to later cocaine self-administration. As shown in figure 8, the rats that began nicotine self-administration as adults (8 weeks of age) had significantly higher cocaine self-administration during estrus than any of the other phases (vs. proestrus p<0005, metestrus p<0.05, diestrus p<0.05), whereas there was no significant relationship in rats that began nicotine self-administration during adolescence (6 weeks of age).
Figure 8.
Six vs. eight-week old age of onset of nicotine self-administration effect on the relationship of later cocaine self-administration to estrus phase, infusions (mean±sem) per 45-min. session in female rats. The rats that had begun nicotine access at six weeks did not show any effect of estrus phase on subsequent cocaine self-administration. In contrast rats that had begun nicotine access at eight weeks of age did show a significantly (p<0.05-0.005) higher cocaine self-administration during the estrus phase than during the other phases of the cycle.
4. Discussion
This study investigated the threshold for adult-like nicotine self-administration in male and female rats. Analysis of the significant interaction of age-of-onset by week-of-access showed that rats that started to self-administer nicotine at either four or five weeks of age had during the first week of access significantly higher rates of nicotine self-administration than rats that started at either seven or eight weeks of age. Males self-administered significantly more nicotine than females for the first two weeks of access to nicotine. Importantly, as adolescent onset male rats entered adulthood they assumed the adult-onset like pattern of self-administration, whereas the adolescent-onset female rats continue to self-administer significantly greater amounts of nicotine even when they become adults. Adolescent-onset female rats had significant elevations of nicotine-self-administration continuing into adulthood. This was particularly evident in the face of changing fixed ratio (FR) for nicotine delivery. This is relevant to human adolescent smoking because of smoking restrictions, the pattern of smoking in human adolescents is quite variable. The results of this study support the hypothesis that adolescents do not regulate their nicotine as well as adults with changes in availability and that when nicotine suddenly becomes easier to have, adolescents do not adjust their intake patterns and take in more than those that start nicotine in adulthood.
In the current study, as well as previous ones [38, 40], we found that with the standard dose of 0.03 mg/kg/infusion adolescent males self-administered more nicotine than females, but that they quickly decreased their self-administration as they grew into adulthood, while adolescent-onset females showed a greater persistence on higher self-administration rates into adulthood. In a similar study with lower nicotine unit doses (0.005 or 0.01 mg/kg/infusion) Lynch et al. [51] also found that adolescent female rats acquired nicotine self-administration more avidly than adolescent males.
The age-effect function of higher nicotine self-administration did not appear to be merely a result of carryover from higher rates of the short three-session pre-training of food pellet self-administration. There were no significant age-related effects on food self-administration. There were significant sex effects on food self-administration during the pre-training with males in general self-administering more food, but there was no interaction of age × sex with food-self-administration.
Age related differences in nicotine self-administration did not seem to be due to the functional impact of body size on ability to press the lever. There were no significant age differences in responding for food as described above. In addition any increased difficulty in pressing the lever by younger rats would have biased increased lever pressing to older rats, the opposite to what was observed.
The difference between the initial nicotine self-administration of six and eight-week old female rats was significant in one study but not another in the current series. The data from week 1 of access for six and eight week old females from the first experiment showed that the six week olds averaged 6.82±1.57 and the eight week olds 4.74±1.18 infusions per session. If these were analyzed in isolation from the rest of the experiment the p-value would be p=0.19. The data from initial training for six and eight week old females from the second experiment showed that the six week olds averaged 8.90±1.79 and the eight week olds 5.35±0.71 infusions per session (p<0.01). One study was significant and the other missed significance but having a mean difference in the same direction. Given heterogeneity in response with age effects on nicotine self-administration this is not surprising.
The duration of adolescence is not clear and appears to vary across different functions. The ability to reproduce is just one measure of maturation during adolescence. Attainment of full adult body size is another. Spear [13] gave PND 28–42 as the core adolescent period for the rat. However, in the same article she mentioned that some developmental changes of adolescence persist until PND 55. This set of studies was conducted to determine the threshold for adult responding for nicotine self-administration. The results showed that six-week old rats (particularly females) do not yet respond like adults with regard to the initiation of nicotine self-administration. With regard to nicotine-self-administration it appears that the period between six and seven weeks of age is the threshold for adult-like responding.
Given the variable availability of nicotine via smoking in human adolescents this decreased ability to control intake when suddenly plenty of drug is easily available may be a key point of vulnerability to addiction. In humans the difference between adolescents and adults in this regard is often attributed to differential experience with the drug, but in our study the rats of the different ages had the same experience with the drug nicotine. This paradigm of variable response requirement self-administration with rats may better model the human situation with constraints on drug availability. Increasing FR has typically been used to determine the need and desire of the subject for the drug. That is important information but perhaps it is the reaction to easy availability after constraint that gives the clearer insight into addiction vulnerability.
A series of differential age of onset studies has been conducted to determine the threshold for adult-like responding in both sexes. The particular mid-adolescent vulnerability of female rats to higher nicotine self-administration continuing into adulthood is being studied for the relationship to the onset of estrous cycling. This includes examining the role of varying the fixed ratio (FR) demand for nicotine self-administration. The adolescent-onset rats have a significantly greater jump in nicotine self-administration when the FR demand goes from difficult (FR8) to easy (FR1). That is, the adolescent onset rats seem to not adjust their response rates to keep a fixed dose of nicotine. This would also fit into the idea that their perception of nicotine-induced reward is less and that they have greater tendency to keep with a fixed motor habit of self-administration.
The effect of estrus cycle on drug self-administration by female rats was examined. No relationship between estrus phase and nicotine self-administration was seen in either adolescent or adult self-administration onset rats. This matches our earlier finding [49] as well as that of Donney et al. [50]. Lynch et al did find a relationship but this was with a progressive ratio schedule [51]. With regard to the relationship of estrus cycle to cocaine self-administration the results showed an interesting effect. With the rats that started nicotine self-administration as young adults (8 weeks of age), self-administered nicotine for two weeks and then switched to cocaine for two weeks cocaine self-administration was significantly higher during estrus than during the other phases. This replicates what has been seen in other studies [52–55]. In contrast, the rats that started nicotine self-administration during adolescence (6 weeks of age), self-administered nicotine for two weeks and then self-administered cocaine for two weeks did not show this effect of estrus on cocaine self-administration. This differential relationship between estrus and cocaine self-administration in the adolescent and adult-onset nicotine self-administration groups was seen when both groups of rats were adults (8–9 weeks and 10–11 weeks of age). This suggests that adolescent nicotine exposure caused a persisting disruption of the relationship between female hormone cycle and drug reinforcement. The lowest cocaine seeking in proestrus and the highest in estrus corresponds to the highest progesterone levels in proestrus and the lowest levels during estrus [52]. Progesterone reduced cocaine seeking behavior in rats [56].
Important sex differences exist in adolescent-onset nicotine self-administration. Adolescent males self-administer more nicotine than adolescent females, but upon aging into adulthood they rapidly decreased self-administration levels to adult-onset levels. Adolescent-onset females self-administer significantly more nicotine than adult-onset females but less than adolescent-onset males. In contrast to the adolescent-onset males, the females that started during adolescence showed a persistence of greater self-administration into adulthood. Others have also seen sex differences in adolescent nicotine effects. Adolescent female rats are more sensitive than males, to the anxiolytic effects of nicotine [57] as well as to the persisting effects of nicotine on decreased cell number in the brain [32]. Adolescent males are more sensitive than adults or adolescent females to the stimulant effects of amphetamine after exposure to nicotine, and this effect is long-lasting, persisting into adulthood [58]. The age of maximal gene expression response to nicotine in female rats corresponds to puberty and the age of maximal behavioral response [59]. These data suggest that adolescent nicotine use may carry a greater risk than adult nicotine and that there are important sex differences [58]. Animals initially exposed to nicotine in adolescence exhibited greater sensitivity to nicotine’s activity-increasing effects than did females initially exposed to nicotine in adulthood [60]. The male-female differences may be related to the hormonal effects of progesterone and estrogen in females [61].
It is important to note that the rats in the current study were individually housed throughout the studies. This is necessary to prevent other rats from chewing on the infusion catheters and is common to all IV self-administration studies. It has been repeatedly shown that individual housing is a stressor in rats and increases reactivity of dopamine systems in the nucleus accumbens [62] and this may have affected self-administration patterns. Interestingly, sex-differences have been noted in the long term effects of single housing in rats [63]. The increased stress response seen in females but not males may have contributed to the observed sex differences in the current study.
As seen in the individual response data (Fig. 4) there was considerable heterogeneity of response with the access to nicotine. This reflects the human experience. It has been found in epidemiological studies that somewhat more than a third of high school students who have ever tried smoking eventually became daily smokers [64]. Thus, nearly two-thirds of those who have access to nicotine and sample it do not fall into regular daily use and addiction. Identification of individual differences in adolescent onset nicotine self-administration particularly in the females of the current study provides a good start for further research to determine the sources for individual vulnerability for high levels of nicotine self-administration.
Adolescent onset nicotine self-administration was not found in the current study to potentiate subsequent cocaine self-administration. Previously, McQuown et al. [65] found increased cocaine reinforced responding after adolescent exposure to low levels of nicotine during adolescence. The principal difference between the studies was that nicotine in the McQuown et al. study was administered passively and then the opportunity for cocaine self-administration was presented, while in the current study, the opportunity for cocaine self-administration came after nicotine self-administration in the same environment. Perhaps the rats became adapted to the low reinforcing value of nicotine, which carried over to the cocaine sessions. However, more recently the same group did a more detailed assessment of the age-effect function of adolescent nicotine effects [66]. They found that the potentiating effect of adolescent nicotine exposure on cocaine self-administration was seen only with early adolescent exposure during 28–31 days of age, but not with nicotine exposure during later adolescence during 38–41 days of age. This later adolescent was similar to our 6-week old adolescent group. Thus, the age of adolescent nicotine exposure seemed to be a critical determinant in the potentiation of later cocaine self-administration.
5. Conclusions
The great majority of tobacco addiction begins with onset of use during adolescence. This study showed in rats that early to mid-adolescent onset of nicotine access results in significantly higher nicotine self-administration during the initial period of access compared to initiation of nicotine access in young adulthood. The horizon for adult-like nicotine self-administration in this study was between six and seven weeks of age. There were sex differences in the magnitude nicotine self-administration. Males had a greater level of nicotine self-administration than females. However, female rats that start nicotine access during adolescence have more persistent increases in nicotine self-administration than adolescent-onset males. Adolescent-onset females self-administer significantly more than adult-onset females even after they mature into adulthood. Adolescent-onset female rats also show significantly greater nicotine self-administration in response to changes in FR requirement indicative of lessened control over the amount of nicotine they self-administer. This rat model of adolescent-onset nicotine self-administration could be useful in determining the mechanisms of nicotine impacts on adolescent neurobehavioral development that underlie persistent nicotine dependence.
Research Highlights.
Adolescent rats self-administer more nicotine than young adults
Adolescent male rats self-administer more nicotine than adolescent females
As adolescent rats aged males declined to adult levels of self-administration
As adolescent rats aged females persisted in higher levels of self-administration
Adolescent-onset females had less control of self-administration with varying FR
Acknowledgments
This research was supported by the by NIDA grant DA015756.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control and Prevention. Differences in the age of smoking initiation between blacks and whites, United States. Mortality and Morbidity Weekly Report. 1991;40:754–757. [PubMed] [Google Scholar]
- 2.Rigotti N. How can we help the remaining smokers to quit? American Journal of Preventative Medicine. 1990;6:249–250. [PubMed] [Google Scholar]
- 3.Eissenberg T, Balster RL. Initial tobacco use episodes in children and adolescents: current knowledge, future directions. Drug & Alcohol Dependence. 2000;59(Suppl 1):S41–60. doi: 10.1016/s0376-8716(99)00164-7. [DOI] [PubMed] [Google Scholar]
- 4.Glynn T, et al. Youth tobacco use in the United States: Problems, progress, goals, and potential solutions. Preventative Medicine. 1993;22:568–575. doi: 10.1006/pmed.1993.1049. [DOI] [PubMed] [Google Scholar]
- 5.US Public Health Service. US Department of Health, Education and Welfare. Washington, DC: US Government Printing Office; 1994. Preventing tobacco use among young people: A report of the Surgeon General. [Google Scholar]
- 6.Chassin L, et al. The natural history of cigarette smoking from adolescence to adulthood: Demographic predictors of continuity and change. Health Psychology. 1996;15:478–484. doi: 10.1037//0278-6133.15.6.478. [DOI] [PubMed] [Google Scholar]
- 7.Pomerleau C, Pomerleau O. Society for Research on Nicotine and Tobacco. Arlington, VA: 2000. Characteristics of early- and late-onset smokers. [Google Scholar]
- 8.Lloyd E, Niaura R, Kazura A. Society for Research on Nicotine and Tobacco. Arlington, VA: 2000. The progression of nicotine dependence in adolescents. [Google Scholar]
- 9.Breslau N, Peterson EL. Smoking cessation in young adults: age at initiation of cigarette smoking and other suspected influences. Am J Public Health. 1996;86:214–220. doi: 10.2105/ajph.86.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brownson R, et al. Patterns of cigarette and smokeless tobacco use among children and adolescents. Preventative Medicine. 1991;19:170–180. doi: 10.1016/0091-7435(90)90018-f. [DOI] [PubMed] [Google Scholar]
- 11.Le Foll B, et al. Genetics of dopamine receptors and drug addiction: a comprehensive review. Behavioural Pharmacology. 2009;20(1):1–17. doi: 10.1097/FBP.0b013e3283242f05. [DOI] [PubMed] [Google Scholar]
- 12.Spear LP, Brake SC. Periadolescence: Age-dependent behavior and psychopharmacological responsivity in rats. Develop Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- 13.Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 14.Shram MJ, et al. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology. 2006;186(2):201–208. doi: 10.1007/s00213-006-0373-8. [DOI] [PubMed] [Google Scholar]
- 15.Vastola B, et al. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiology & Behavior. 2002;77:107–114. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- 16.Belluzzi J, et al. Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology. 2004;174:389–395. doi: 10.1007/s00213-003-1758-6. [DOI] [PubMed] [Google Scholar]
- 17.Shram MJ, Le AD. Adolescent male Wistar rats are more responsive than adult rats to the conditioned rewarding effects of intravenously administered nicotine in the place conditioning procedure. Behavioural Brain Research. 2010;206(2):240–244. doi: 10.1016/j.bbr.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Nolley EP, Kelley BM. Adolescent reward system perseveration due to nicotine: studies with methylphenidate. Neurotoxicology & Teratology. 2007;29(1):47–56. doi: 10.1016/j.ntt.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 19.Kota D, Martin BR, Damaj MI. Age-dependent differences in nicotine reward and withdrawal in female mice. Psychopharmacology. 2008;198:201–210. doi: 10.1007/s00213-008-1117-8. [DOI] [PubMed] [Google Scholar]
- 20.O’Dell LE, et al. Diminished nicotine withdrawal in adolescent rats: implications for vulnerability to addiction. Psychopharmacology. 2006;186(4):612–9. doi: 10.1007/s00213-006-0383-6. [DOI] [PubMed] [Google Scholar]
- 21.O’Dell LE, et al. Nicotine withdrawal in adolescent and adult rats. Annals of the New York Academy of Sciences. 2004;1021:167–174. doi: 10.1196/annals.1308.022. [DOI] [PubMed] [Google Scholar]
- 22.O’Dell LE, et al. Adolescent nicotine exposure produces less affective measures of withdrawal relative to adult nicotine exposure in male rats. Neurotoxicology & Teratology. 2007;29(1):17–22. doi: 10.1016/j.ntt.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slawecki CJ, Ehlers CL. Lasting effects of adolescent nicotine exposure on the electroencephalogram, event related potentials, and locomotor activity in the rat. Brain Research Developmental Brain Research. 2002;138(1):15–25. doi: 10.1016/s0165-3806(02)00455-8. [DOI] [PubMed] [Google Scholar]
- 24.Slawecki CJ, et al. Increased anxiety-like behavior in adult rats exposed to nicotine as adolescents. Pharmacology, Biochemistry & Behavior. 2003;75(2):355–361. doi: 10.1016/s0091-3057(03)00093-5. [DOI] [PubMed] [Google Scholar]
- 25.Rezvani AH, Levin ED. Adolescent and adult rats respond differently to nicotine and alcohol: Motor activity and body temperature. International Journal of Developmental Neuroscience. 2004;22(5–6):349–354. doi: 10.1016/j.ijdevneu.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Brielmaier JM, McDonald CG, Smith RF. Immediate and long-term behavioral effects of a single nicotine injection in adolescent and adult rats. Neurotoxicology & Teratology. 2007;29(1):74–80. doi: 10.1016/j.ntt.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 27.Laviola G, et al. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neuroscience & Biobehavioral Reviews. 2003;27(1–2):19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- 28.Trauth JA, et al. Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Research. 1999;851(1–2):9–19. doi: 10.1016/s0006-8993(99)01994-0. [DOI] [PubMed] [Google Scholar]
- 29.Trauth JA, et al. Modeling adolescent nicotine exposure: Effects on cholinergic systems in rat brain regions. Brain Research. 2000;873(1):18–25. doi: 10.1016/s0006-8993(00)02465-3. [DOI] [PubMed] [Google Scholar]
- 30.Trauth JA, et al. Adolescent nicotine exposure produces immediate and long-term changes in CNS noradrenergic and dopaminergic function. Brain Research. 2001;892(2):269–80. doi: 10.1016/s0006-8993(00)03227-3. [DOI] [PubMed] [Google Scholar]
- 31.Abreu-Villaca Y, et al. Short-term adolescent nicotine exposure has immediate and persistent effects on cholinergic systems: critical periods, patterns of exposure, dose thresholds. Neuropsychopharmacology. 2003;28(11):1935–49. doi: 10.1038/sj.npp.1300221. [DOI] [PubMed] [Google Scholar]
- 32.Abreu-Villaca Y, et al. Nicotine is a neurotoxin in the adolescent brain: critical periods, patterns of exposure, regional selectivity, and dose thresholds for macromolecular alterations. Brain Research. 2003;979(1–2):114–28. doi: 10.1016/s0006-8993(03)02885-3. [DOI] [PubMed] [Google Scholar]
- 33.Slotkin TA. Nicotine and the adolescent brain: insights from an animal model. Neurotoxicology & Teratology. 2002;24(3):369–84. doi: 10.1016/s0892-0362(02)00199-x. [DOI] [PubMed] [Google Scholar]
- 34.Abreu-Villaca Y, Seidler FJ, Slotkin TA. Impact of adolescent nicotine exposure on adenylyl cyclase-mediated cell signaling: enzyme induction, neurotransmitter-specific effects, regional selectivities, and the role of withdrawal. Brain Research. 2003;988(1–2):164–72. doi: 10.1016/s0006-8993(03)03368-7. [DOI] [PubMed] [Google Scholar]
- 35.Slotkin TA, et al. Lasting effects of nicotine treatment and withdrawal on serotonergic systems and cell signaling in rat brain regions: separate or sequential exposure during fetal development and adulthood. Brain Research Bulletin. 2007;73(4–6):259–72. doi: 10.1016/j.brainresbull.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 36.Azam L, Chen Y, Leslie FM. Developmental regulation of nicotinic acetylcholine receptors within midbrain dopamine neurons. Neuroscience. 2007;144(4):1347–1360. doi: 10.1016/j.neuroscience.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doura MB, et al. Adult and periadolescent rats differ in expression of nicotinic cholinergic receptor subtypes and in the response of these subtypes to chronic nicotine exposure. Brain Reserch. 2008;1215:40–52. doi: 10.1016/j.brainres.2008.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levin ED, et al. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology. 2003;169:141–149. doi: 10.1007/s00213-003-1486-y. [DOI] [PubMed] [Google Scholar]
- 39.Levin ED, et al. Increased nicotine self-administration following prenatal exposure in female rats. Pharmacology, Biochemistry and Behavior. 2006;85:669–674. doi: 10.1016/j.pbb.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levin ED, et al. Adolescent vs. adult-onset nicotine self-administration in male rats: duration of effect and differential nicotinic receptor correlates. Neurotoxicology and Teratology. 2007;29:458–465. doi: 10.1016/j.ntt.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30(4):705–712. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- 42.Adriani W, et al. Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. Journal of Neuroscience. 2003;23(11):4712–6. doi: 10.1523/JNEUROSCI.23-11-04712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klein LC, et al. Sex differences in voluntary oral nicotine consumption by adolescent mice: a dose-response experiment. Pharmacology, Biochemistry & Behavior. 2004;78(1):13–25. doi: 10.1016/j.pbb.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 44.Chen H, Matta SG, Sharp BM. Acquisition of nicotine self-administration in adolescent rats given prolonged access to the drug. Neuropsychopharmacology. 2007;32(3):700–709. doi: 10.1038/sj.npp.1301135. [DOI] [PubMed] [Google Scholar]
- 45.Shram MJ, Li Z, Le AD. Age differences in the spontaneous acquisition of nicotine self-administration in male Wistar and Long-Evans rats. Psychopharmacology. 2008;197(1):45–58. doi: 10.1007/s00213-007-1003-9. [DOI] [PubMed] [Google Scholar]
- 46.Shram MJ, et al. Nicotine self-administration, extinction responding and reinstatement in adolescent and adult male rats: evidence against a biological vulnerability to nicotine addiction during adolescence. Neuropsychopharmacology. 2008;33(4):739–48. doi: 10.1038/sj.npp.1301454. [DOI] [PubMed] [Google Scholar]
- 47.Chaudhri N, et al. Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology. 2005;180(2):258–66. doi: 10.1007/s00213-005-2152-3. [DOI] [PubMed] [Google Scholar]
- 48.Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine & Tobacco Research. 1999;1(4):301–315. doi: 10.1080/14622299050011431. [DOI] [PubMed] [Google Scholar]
- 49.Rezvani AH, et al. Neonatal 6-hydroxydopmine lesions of the frontal cortex in rats: Persisting effects on locomotor activity, learning and nicotine self-administration. Neuroscience. 2008;154:885–897. doi: 10.1016/j.neuroscience.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donny EC, et al. Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology. 2000;151(4):392–405. doi: 10.1007/s002130000497. [DOI] [PubMed] [Google Scholar]
- 51.Lynch WJ. Sex and ovarian hormones influence vulnerability and motivation for nicotine during adolescence in rats. Pharmacology, Biochemistry & Behavior. 2009;94(1):43–50. doi: 10.1016/j.pbb.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feltenstein MW, See RE. Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug & Alcohol Dependence. 2007;89(2–3):183–9. doi: 10.1016/j.drugalcdep.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lynch WJ, Arizzi MN, Carroll ME. Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology. 2000;152(2):132–9. doi: 10.1007/s002130000488. [DOI] [PubMed] [Google Scholar]
- 54.Lynch WJ. Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology. 2008;197(2):237–46. doi: 10.1007/s00213-007-1028-0. [DOI] [PubMed] [Google Scholar]
- 55.Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology. 1989;98(3):408–11. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- 56.Feltenstein MW, et al. Attenuation of cocaine-seeking by progesterone treatment in female rats. Psychoneuroendocrinology. 2009;34(3):343–52. doi: 10.1016/j.psyneuen.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheeta S, et al. In adolescence, female rats are more sensitive to the anxiolytic effect of nicotine than are male rats. Neuropsychopharmacology. 2001;25(4):601–607. doi: 10.1016/S0893-133X(01)00258-5. [DOI] [PubMed] [Google Scholar]
- 58.Collins SL, Montano R, Izenwasser S. Nicotine treatment produces persistent increases in amphetamine-stimulated locomotor activity in periadolescent male but not female or adult male rats. Brain Research Developmental Brain Research. 2004;153(2):175–87. doi: 10.1016/j.devbrainres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 59.Polesskaya OO, et al. Nicotine causes age-dependent changes in gene expression in the adolescent female rat brain. Neurotoxicology & Teratology. 2007;29(1):126–40. doi: 10.1016/j.ntt.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 60.Elliott BM, et al. Adolescent and adult female rats differ in sensitivity to nicotine’s activity effects. Pharmacology, Biochemistry & Behavior. 2005;80(4):567–75. doi: 10.1016/j.pbb.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 61.Lynch WJ, Sofuoglu M. Role of progesterone in nicotine addiction: evidence from initiation to relapse. Experimental & Clinical Psychopharmacology. 2010;18(6):451–461. doi: 10.1037/a0021265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fone KC, Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev. 2008;32:1087–1102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 63.Weintraub A, Singaravelu J, Bhatnagar S. Enduring and sex-specific effects of adolescent social isolation in rats on adult stress reactivity. Brain Res. 2010;1343:83–92. doi: 10.1016/j.brainres.2010.04.068. [DOI] [PubMed] [Google Scholar]
- 64.Centers for Disease Control and Prevention. Selected Cigarette Smoking Initiation And Quitting Behaviors Among High School Students - United States, 1997. Mortality and Morbidity Weekly Report. 1998;47:386. [PubMed] [Google Scholar]
- 65.McQuown SC, Belluzzi JD, Leslie FM. Low dose nicotine treatment during early adolescence increases subsequent cocaine reward. Neurotoxicology & Teratology. 2007;29(1):66–73. doi: 10.1016/j.ntt.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dao JM, et al. Nicotine alters limbic function in adolescent rat by a 5-HT1A receptor mechanism. Neuropsychopharmacology. 2011;36:1–13. doi: 10.1038/npp.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]