Abstract
It is not well known whether Alzheimer’s disease (AD) cerebrospinal fluid (CSF) biomarkers are associated with brain damage in cognitively normal elderly. The combined influence of CSF biomarkers and hypertension (HTN) on the gray matter (GM) is also not well described.
115 cognitively healthy subjects (mean age 62.6±9.5, 62% women) received clinical assessment, a high resolution MRI and a lumbar puncture. The CSF levels of total tau (t-tau), hyperphosphorylated tau (p-tau231), amyloid beta (Aβ42/Aβ40), p-tau231/Aβ42 and t-tau/Aβ42 were dichotomized as ‘high’ and ‘low’ based on accepted cut-off values. Statistical parametric mapping was used to examine MRI scans for regional GM density, studied as a function of the CSF markers, HTN and combination of both. Global and medial temporal lobe (MTL) GM was also assessed. Voxel based morphometry revealed that higher t-tau was associated with lower GM density in the precunei. Subjects with higher p-tau231 and p-tau231/Aβ42 had less GM in temporal lobes. Low Aβ42/Aβ40 was related to less GM in the thalami, caudate and midbrain. Subjects with hypertension showed more GM atrophy in the cerebellum, occipital and frontal regions. Simultaneous presence of elevated CSF AD biomarkers and HTN was associated with more GM atrophy than either marker individually, but no interaction effects were identified.
In conclusion, in normal elderly CSF tau markers were associated predominantly with lower GM estimates in structures typically affected early in the AD process. In this presymptomatic stage when no cognitive impairment is present, AD pathology and HTN have additive effects on gray matter damage.
Keywords: Aging, Biomarkers, MRI, Alzheimer’s Disease, Cerebrospinal Fluid, Hypertension
Alzheimer’s disease (AD) is characterized by a long clinically non-symptomatic period when detectable biological abnormalities are present, as evidenced by increased cerebrospinal fluid (CSF) levels of total tau (t-tau) and hyperphosphorylated tau (p-tau) and reductions in amyloid beta 1–42 (Aβ42) levels. These changes are thought to reflect cell loss, progressive neurofibrillary tangle and senile plaques accumulation (Blennow et al., 2006) and are predictive of cognitive decline already at normal stages of cognition (Fagan et al., 2007; Li et al., 2007; Glodzik et al., 2010).
Volume reductions of the medial temporal lobes (MTL) associated with neuronal loss and the intraneuronal accumulation of neurofibrillary tangles (Braak and Braak, 1991), constitute another typical feature of early AD (de Leon et al., 1989). The pathological involvement of other limbic (amygdale, cingulate gyri) and cortical (temporal, parietal) regions follows the MTL changes. This pattern has been observed with both MRI (Glodzik-Sobanska et al., 2005) and positron emission tomography (PET) (Mosconi, 2005).
Despite the growing recognition of CSF biomarkers and imaging in the early AD diagnosis, it is not clearly established whether abnormal concentrations of CSF markers are associated with regional or global brain volume reductions in normal elderly (Sluimer et al., 2008; Fagan et al., 2009; Loew et al., 2009). An evidence of such association would further corroborate the validity of CSF markers as early indices of AD pathology, particularly if their presence was related to atrophy in AD-specific brain regions.
Adding complexity to this picture is hypertension (HTN), also associated with brain atrophy and cognitive decline (Nagai et al., 2008; Firbank et al., 2007). It is not well known, however, how this highly prevalent condition interacts with abnormal CSF AD biomarkers to affect brain morphology or cognition in normal subjects, and whether AD pathology and HTN contribute in an additive or synergistic way to brain damage.
The current study examined whether 1) AD CSF biomarkers are associated with GM atrophy in regions known for their vulnerability to AD and 2) elevated AD CSF biomarkers and hypertension combine to further increase the GM damage. In addition, we also examined how abnormal CSF biomarkers and HTN, separately and combined, influence cognition.
METHODS
Participants
This cross-sectional study included 115 healthy, cognitively healthy individuals (age 62.6±9.5, range 46–86 years; education 16.8±2, range 10–20 years; 62% women) studied at the NYU School of Medicine, Center for Brain Health and Alzheimer’s Disease Center. All subjects signed an IRB approved informed consent. They constituted a consecutive group recruited for aging and memory studies, which involved MRI and CSF examinations. Subjects were recruited either through advertisements or they were the caregivers of subjects seeking treatment for cognitive impairments. They received medical, neurological, psychiatric, radiological, and laboratory examinations and underwent lumbar puncture and MRI examination (high resolution T1, T2 and FLAIR). Patients with confounding brain pathology (e.g. tumor, neocortical infarction) were excluded. The clinical assessment included a semi-structured interview based on the Brief Cognitive Rating Scale (BCRS) from which a Global Deterioration Scale score (GDS) was derived (Reisberg and Ferris, 1988). All subjects were diagnosed as normal: GDS 1 or 2 (Reisberg et al., 1993). GDS=1 indicated no subjective memory complaint, and GDS=2 indicated awareness of memory change over the lifespan, in the absence of objective evidence of memory or functional problems on clinical interview. Subjects scoring ≥16 on the 17-item Hamilton Depression Scale were excluded (Bech et al., 1986).
General cognitive abilities were tested with Mini Mental State Examination (MMSE) (Folstein et al., 1975). In addition, a neuropsychological test battery was administered in every case. The measures include subtests of the Guild Memory Scale (Gilbert et al., 1968) assessing immediate and delayed recall of orally presented paragraphs (initial: PARI, and delayed: PARD); verbal paired associates (initial: PRDI, and delayed: PRDD); visual/ verbal paired associates with numbers (DESN). Subtests of the Wechsler Intelligence Scale Revised (WAIS-R) (Wechsler, 1981) were used to assess working memory (digits forward: WAISDIG-F, and backward: WAISDIG-B), and attention (digit symbol substitution test: DSST).
The presence of hypertension was determined based on current antihypertensive treatment or systolic blood pressure ≥ 140 mmHg, or diastolic blood pressure ≥ 90 mmHg (Chobonian et al., 2003). If patient was not treated but high blood pressure was identified, we further verified whether it was high only at one occasion or also during other visits to our Center.
Lumbar puncture, CSF collection and assays
Using a 25G needle guided by fluoroscopy, 15 ml of clear CSF was collected into three polypropylene tubes. All CSF samples were kept on ice for a maximum of 1 hour until centrifuged for 10 min at 1500 g at 4 C. Samples were aliquoted to 0.25 ml polypropylene tubes and stored in at −80°C until the assay. All samples were blindly analyzed in batch mode. We determined the concentrations of total tau (t-tau) (Blennow et al., 1995), tau phosphorylated at threonine 231 (p-tau231) (Kohnken et al., 2000), Aβ40 and Aβ42 (Metha et al., 2000). The ratio of these two Aβ species Aβ42/Aβ40 was used in the analysis. We also analyzed p-tau231/ Aβ42 and t-tau/Aβ42 ratios.
Study groups
Biomarker groups
To examine the effects of CSF biomarkers on brain measures and cognition subjects were classified into high (+) and low (−) biomarkers groups based on the published diagnostic and predictive cut-off values: high t-tau > 350 pg/mL (Hansson et al., 2006), high p-tau231 > 18 pg/mL (Buerger et al., 2003), low Aβ42/Aβ40 < .11 (Lewczuk et al., 2004). For p-tau231/Aβ42 and t-tau/Aβ42 we used their respective medians since the cutoff values are not well established. For all the ratios (Aβ42/Aβ40, p-tau231/ Aβ42, t-tau/Aβ42) data were available for 86 subjects.
Hypertension (HTN) groups
As described above, subjects were classified as hypertensive or normotensive.
Biomarker-HTN groups
Dichotomization of CSF biomarkers and HTN was used to test the interaction between these 2 factors. To examine the interactions of the CSF biomarkers and HTN on brain measures and cognition, subjects were classified in the following four biomarker-HTN groups: normal biomarker level and HTN absent: (B−/H−); normal biomarker level and HTN present: (B−/H+); abnormal biomarker level and HTN absent: (B+/H−); abnormal biomarker level and HTN present: (B+/H+).
MRI and image analysis
T1 weighted MRI scans were uniformly acquired in the coronal orientation (slice thickness: 1.6mm FOV=25cm, NEX=1, matrix=256×192, TR=35ms, TE=9ms, FA=60°), using a 1.5T GE scanner (GE, Milwaukee, USA). Voxel based morphometry (VBM) was performed with MATLAB 7.1 and Statistical Parametric Mapping (SPM’2, Wellcome Department of Cognitive Neurology, London, UK) procedures (Ashburner and Friston, 2000;Good et al., 2002).
All scans were realigned and spatially normalized. Normalization was done by estimating the optimal 12-parameter affine transformation (Freeborough et al., 1996). The spatially normalized images were resliced using sinc interpolation to a final voxel size of 1.5×1.5×1.5mm, and segmented into GM (gray matter), WM (white matter), and CSF images (Ashburner and Friston, 2000). The GM images re-normalized to an a priori GM templates(Good et al., 2002), by using the high dimensional normalization (optimal 12-parameter affine transformation, followed by an iterative estimate of local alignment based on a family of 7×8×7 discrete cosine functions, using the residual sum of squared differences as the matching criterion (Good et al., 2002; Freeborough et al., 1996) and smoothed with an 8-mm FWHM isotropic Gaussian kernel. To preserve the volume of a particular tissue compartment within each voxel, the images were modulated by the Jacobian determinants of the transformed matrix (Good et al., 2002). Anatomical location of areas showing GM effects was described using the Talairach and Tournoux coordinates (Talairach and Tournoux, 1988), after conversion of MNI coordinates to Talaraich space (Lancaster et al., 2000). In all analyses of GM density, age, gender and education were accounted for. Results were examined over the whole brain at p<0.001 with a minimum cluster size of 75 voxels. In the context of voxel based morphometry (VBM) “density” refers to the relative amount of gray matter in a voxel (Asburner and Friston, 2009) and does not refer to cell packing density measured by histology or term “density” as used in computed tomography.
To study global gray matter, native images were segmented into GM, WM and CSF using SPM’2 routines. Global GM volume was normalized to the total intracranial volume (ICV). The ICV was calculated as the integral of all three tissue compartments.
Finally, in an exploratory analysis, in addition to unbiased VBM we used automated medial temporal lobe (MTL) regions of interest (ROI) (Li et al., 2008) to extract GM density. Estimates were obtained separately for right and left hemisphere and the average was computed. Average GM density was compared across the high and low biomarkers group, subjects with and without HTN, and across biomarker-HTN groups.
FLAIR images were acquired to assess the extent of periventricular (PWMH) and deep white matter hyperintesities (DWMH). We used 0–3 Fazekas scale (Fazekas et al., 1987). For the final analyses a total WMH score was used, which was a sum of PWMH and DWMH scores.
Statistical analysis
Continuous demographic measures were examined using t-test or ANOVA. Categorical variables were examined with χ2 test. Between biomarker, HTN or biomarker-HTN groups comparisons were performed using analysis of covariance (ANCOVA) models with age, gender, education and WMH score accounted for. Linear contrast analyses were performed when group differences appeared to follow a gradual change in diagnostic category. Cognitive variables were compared using multivariate analysis of covariance (MANCOVA) models with age, gender and educations as covariates.
Normality was checked with the Shapiro-Wilk test. When the data did not meet the assumptions of normality, the Mann Whitney U test was used to 2 compare groups (Z values are given) or Kruskal-Wallis ANOVA, when more than 2 groups were analyzed. For non-normally distributed data in ANCOVA models statistical results were verified by using rank transformed values. Results are given for models with transformed (rank) variables, when transformation was necessary. Statistical analyses were performed with SPSS 16, Chicago IL, with p values declared statistically significant when <0.05.
To assess the impact of CSF biomarkers and HTN on regional GM SPM’2 was used. For the voxel-wise SPM analysis of the MRI scans, GM volumes were examined using the General Linear model (GLM)/univariate analysis (F contrast) followed by post-hoc t-tests, to compare high and low biomarker groups, and HTN+ vs. HTN− groups, at p<.001, with a minimum cluster size of 75 voxels, uncorrected for multiple comparisons. We then examined the interaction between HTN and biomarker groups on GM volumes. Biomarker- HTN interaction was defined as GM reduction beyond the one expected from mere summation of both effects. It was examined with F contrasts after controlling for the main effects of biomarkers and HTN groups on GM measures. In order to do this, the F contrast for the interaction was computed after inclusive masking (p<.001, uncorrected) with the main effects as implemented in the SPM. With this procedure, SPM tests for interaction effects only within the voxels showing significant main effects of Biomarker and HTN. Finally, a linear contrast was used to test whether the interaction was driven by the B+/H+ group (abnormal biomarker level and HTN present) showing GM reductions compared to the other 3 subgroups. For all analyses, results were examined at p<.001, uncorrected.
Since intra-individual variability of biomarkers’ concentration could lead to different group assignments and influence the observed associations between GM and AD CSF biomarkers, in addition to comparing high and low biomarker groups, we repeated the SPM analyses using a regression model where CSF biomarkers were used as continuous variables.
In all analyses of global brain volumes and regional GM densities age, gender and education were accounted for. Analyses were repeated after accounting for the WMH score.
RESULTS
Characteristics of the study groups
Table 1 presents general characteristics for the entire study group.
Table 1.
Main characteristics of the entire study group.
| Variable | |
|---|---|
|
t-tau pg/mL median (interquartile range) |
260. 0 (192.0) |
|
p-tau231 pg/mL median (interquartile range) |
7.25 (12.79) |
|
Aβ42/Aβ40 median (interquartile range) |
.1159 (.0394) |
|
t-tau/Aβ42 median (interquartile range) |
.2839 (.2067) |
|
p-tau231/Aβ42 median (interquartile range) |
.0069 (.0101) |
|
Total WMH median (interquartile range) |
1.0 (2.0) |
| Hypertension n (%) | 39 (34) |
|
Age mean (standard deviation) |
62.63 (9.48) |
| Female n (%) | 71 (62) |
WMH: white matter hyperintensity score expressed as a sum of Fazekas scores for deep and periventricular white matter hyperintensities; the number of subjects with WMH information available n=112.
Biomarker groups (Table 2)
Table 2.
Study variables by biomarkers (high/ low) and HTN (absent/ present) groups. Values are presented as mean ± standard deviation. For t-tau the cut-off of 350 pg/mL (Hansson et al., 2006) was used, for p-tau231 the cutoff of 18 pg/mL (Buerger et al., 2003), for Aβ42/Aβ40 the cut-off of .11 (Lewczuk et al., 2004). High and low p-tau231/Aβ42 and t-tau/Aβ42 groups were defined based on the median split. For GM comparisons p values are given for F tests, corrected for age, gender and education. For WMH comparisons p values are given for F tests corrected for age and gender; the number of subjects with WMH information available n=112. For MTL GM comparisons p values are given for t tests, as explained in the text.
| Age (years) |
Female (%) |
Education (years) |
HTN (%) |
GM volume (% ICV) |
WMH | MTL GM | ||
|---|---|---|---|---|---|---|---|---|
| t-tau | Low n=80 | 60.7±8.9 | 64 | 16.7 ± 2.0 | 31 | 47 ± 2 | 1.12 ± 1.08 | .80 ± .05 |
| High n=35 | 67.1±9.4 | 57 | 17.1 ± 2.1 | 40 | 46 ± 3 | 1.13 ± 1.26 | .78 ± .05 | |
| p | .001 | ns | ns | ns | ns | ns | .05 | |
| p-tau231 | Low n=91 | 61.0± 8.9 | 66 | 16.7 ± 1.9 | 33 | 47 ± 2 | .98 ± 1.04 | .80 ± .05 |
| High n=24 | 68.7± 9.3 | 46 | 17.5 ± 2.4 | 37 | 46 ± 2 | 1.75 ± 1.29 | .78 ± .05 | |
| p | .001 | .07 | .02 | ns | .10 | ns | ns | |
| Aβ42/Aβ40a | High n=48 | 60.6 ± 7.0 | 65 | 16.8 ± 1.9 | 33 | 47 ± 2 | .91 ± .97 | .79 ± .05 |
| Low n=38 | 63.8±11.0 | 53 | 16.6 ± 2.0 | 31 | 47 ± 2 | 1.26 ± 1.20 | .79 ± .05 | |
| p | ns | ns | ns | ns | ns | ns | ns | |
| t-tau/Aβ42a | Low n=43 | 60.2±7.3 | 58 | 16.7 ± 2.0 | 33 | 48 ± 2 | .78 ± .90 | .79 ± .05 |
| High n=43 | 63.8±10.3 | 60 | 16.7 ± 1.9 | 33 | 47 ± 2 | 1.35 ± 1.19 | .79 ± .05 | |
| p | .07 | ns | ns | ns | .07 | ns | ns | |
| p-tau/Aβ42a | Low n=43 | 60.2±7.4 | 63 | 16.5 ± 2.0 | 30 | 48 ± 2 | 1.05 ± 1.06 | .80 ± .05 |
| High n=43 | 64.0±10.2 | 56 | 16.9 ± 1.9 | 35 | 46 ± 2 | 1.10 ± 1.13 | .79 ± .05 | |
| p | .04 | ns | ns | ns | .005 | ns | ns | |
| HTN | Absent n=76 | 60.5 ± 9.1 | 72 | 16.7 ± 2.1 | NA | 47 ± 2 | .81 ± .86 | .80 ± .05 |
| Present n=39 | 66.7±8.9 | 41 | 17.2 ± 1.9 | NA | 46 ± 2 | 1.80 ±1.32 | .78 ± .05 | |
| p | .001 | .001 | ns | NA | .06 | .001 | .02 | |
results presented for 86 subjects and for WMH for 85 subjects.
HTN: hypertension, GM: gray matter, WMH: white matter hyperintensity score expressed as a sum of Fazekas scores for deep and periventricular white matter hyperintensities.
MTL GM: Medial temporal lobe gray matter, the values represents estimates (densities) extracted from processed GM images using MTL ROI (Li et al., 2008).
Subjects expressing more abnormal biomarker concentrations were older than those classified into groups with ‘normal’ levels. Statistics were: for t-tau groups (Z=− 3.2, p=.001), p-tau231 (Z=−3.4, p=.001), t-tau/Aβ42 (trend, t=[84]-1.8, p=.07) and p-tau231/Aβ42 (t=[84]-2.1, p=.04). There were more men than women among subjects with high p-tau231 than in the low p-tau231 group (χ2=3.2, p=.07) and the high p-tau231 group had more years of education (Z=−2.3, p=.02). The prevalence of hypertension did not differ between high and low biomarker groups. After accounting for age and gender the groups did not differ in the total WMH score.
HTN groups (Table 2)
HTN was found in 39 (34%) of subjects. Individuals with HTN were older (Z=−3.4, p=.001) and more likely to be men (χ2=10.7, p=.001). After correction for age and gender the mean systolic (134.1±16.5 vs. 118.1±10.0; F[3,111]=26.7, p<.001) and diastolic (80.2±12.3 vs. 71.6±8.3; F[3,110]=15.5, p<.001) blood pressures remained higher in the HTN group. Age and gender adjusted biomarkers concentrations did not differ between hypertensive and normotensive group. The means ± standard error were respectively: t-tau 299.5±24.7 (HTN+) vs. 302.2±17.3 (HTN-) pg/mL, p-tau231: 10.3±2.0=1 vs. 11.7±1.4 pg/mL, Aβ42/Aβ40: .12±.007 vs. .12±.005, tau/Aβ42: .40±.079 vs. .39±.054, and p-tau231/Aβ42: .016±.006 vs. .017±.004. The groups did differ, however, in total WMH score: hypertensive subjects had more white matter lesions (F[3,108]=10.8, p<.001). Smoking (8% of subjects) and hyperglycemia (9%) were infrequent in our group.
Biomarker - HTN groups (Table 3)
Table 3.
Study variables by biomarker-HTN groups. Values are presented as mean ± standard deviation. For GM volume comparisons p values are given for F tests, corrected for age, gender and education. For WMH comparisons p values are given for F tests corrected for age and gender; the number of subjects with WMH information available n=112. For MTL GM comparisons p values are given for F tests not corrected, as explained in the text.
B−/H−: normal biomarker level and HTN absent
B−/H+: normal biomarker level and HTN present
B+/H−: abnormal biomarker level and HTN absent
B+/H+: abnormal biomarker level and HTN present.
| Age | Female (%) |
Education (years) |
GM volume (% ICV) |
WMH | MTL GM | ||
|---|---|---|---|---|---|---|---|
| t-tau-HTN | B−/H− n=55 | 58.3± 7.5 | 75 | 16.6 ± 2.0 | 48 ± 2 | .75 ± .81 | .80 ± .05 |
| B−/H+ n=25 | 65.9±9.6 | 40 | 17.1 ± 2.0 | 46 ± 2 | 1.90 ± 1.20 | .79 ± .04 | |
| B+/H− n=21 | 66.4±10.5 | 66 | 16.9 ± 2.3 | 47 ± 3 | .95 ± .97 | .79 ± .05 | |
| B+/H+ n=14 | 68.1±7.7 | 43 | 17.4 ± 1.9 | 46 ± 3 | 1.64 ± 1.55 | .77 ± .06 | |
| p | <.001 | .01 | ns | ns | .003 | .04 | |
| p-tau231-HTN | B−/H− n=61 | 58.9±7.8 | 77 | 16.6 ± 1.9 | 48 ± 2 | .74 ± .81 | .80 ± .05 |
| B−/H+ n=30 | 65.3±9.6 | 43 | 16.7 ± 1.9 | 46 ± 2 | 1.47 ± 1.28 | .78 ± .04 | |
| B+/H− n=15 | 67.2±11.3 | 53 | 16.8 ± 2.7 | 46 ± 2 | 1.07 ±1.03 | .79 ± .06 | |
| B+/H+ n=9 | 71.3±4.1 | 33 | 18.8 ± 0.9 | 46 ± 2 | 2.89 ±.78 | .77 ± .05 | |
| p | <.001 | .003 | .01 | .04 | .001 | .07 | |
| Aβ42/Aβ40-HTNa | B−/H− n=32 | 58.0±6.0 | 78 | 16.5 ± 1.8 | 48 ± 2 | .61 ± .62 | .80 ± .04 |
| B−/H+ n=16 | 66.0±5.8 | 37 | 17.4 ± 2.1 | 46 ± 3 | 1.50 ± 1.26 | .78 ± .05 | |
| B+/H− n=26 | 63.3±11.2 | 58 | 16.5 ± 2.2 | 47± 2 | 1.04 ± 1.04 | .80 ± .05 | |
| B+/H+ n=12 | 64.7±10.7 | 42 | 16.7 ± 1.6 | 47 ± 3 | 1.75 ± 1.42 | .77 ± .04 | |
| p | .009 | .02 | ns | ns | .06 | ns | |
| t-tau/Aβ42-HTNa | B−/H− n=29 | 58.0±6.4 | 72 | 16.3 ± 2.0 | 48 ± 2 | .71 ± .76 | .80 ± .05 |
| B−/H+ n=14 | 64.9±7.0 | 29 | 17.5 ± 1.9 | 46 ± 3 | 1.71 ± 1.27 | .78 ± .04 | |
| B+/H− n=29 | 62.7±10.7 | 66 | 16.7 ± 2.0 | 47± 2 | .89 ± .94 | .80 ± .05 | |
| B+/H+ n=14 | 66.0±9.0 | 50 | 16.7 ± 1.8 | 47 ± 2 | 1.50 ± 1.40 | .77 ± .05 | |
| p | .02 | .04 | ns | ns | .06 | ns | |
| p-tau/Aβ42-HTNa | B−/H− n=30 | 57.9±6.5 | 70 | 16.3 ± 1.9 | 48 ± 2 | .55 ± .57 | .80 ± .05 |
| B−/H+ n=13 | 64.9±7.1 | 46 | 17.0 ± 2.2 | 47 ± 3 | 1.30 ± 1.25 | .78 ± .05 | |
| B+/H− n=28 | 63.0±10.7 | 68 | 16.7 ± 2.1 | 47± 2 | 1.07 ± 1.01 | .80 ± .05 | |
| B+/H+ n=15 | 65.9±9.2 | 33 | 17.2 ± 1.6 | 46 ± 2 | 1.87 ± 1.36 | .77 ± .04 | |
| p | .01 | .06 | ns | .02 | .03 | ns | |
results presented for 86 subjects and for WMH for 85 subjects.
HTN: hypertension, GM: gray matter, WMH: white matter hyperintensity score expressed as a sum of Fazekas scores for deep and periventricular white matter hyperintensities.
MTL GM: Medial temporal lobe gray matter, the values represents estimates (densities) extracted from processed GM images using MTL ROI (Li et al., 2008).
Across all biomarker-HTN groups age increased from the group without any risk markers (B−/H−) to the group with both risk factors (B+/H+). For the four t-tau-HTN groups: F[3,111]=9.04, p<.001; p-tau231-HTN groups: F=9.3[3,111], p<.001; Aβ42/Aβ40-HTN groups: F[3,82]=4.1, p=.009; t-tau/Aβ42-HTN groups: F[3,82]=3.7, p=.02 and p-tau231/Aβ42-HTN groups: F[3,82]=3.9, p=.01. Gender distribution was also different with more women in (B−/H−) groups. Statistics were: t-tau-HTN groups: χ2=11.2, p=.01; p-tau231-HTN: χ2=13.9, p=.003; Aβ42/Aβ40-HTN: χ2=9.4, p=.02; t-tau/Aβ42-HTN: χ2=8.5, p=.04; and p-tau231/Aβ42-HTN: χ2=7.4, p=.06, respectively. Subjects with HTN and high p-tau231 had more years of education than other p-tau231-HTN groups (Kruskal-Wallis χ2=11.0, p=.01). After accounting for age and gender total WMH score differed between the groups: t-tau-HTN groups: F[5,106]=4.90, p<.003; p-tau231-HTN groups: F=6.40[5,106], p<.001; Aβ42/Aβ40-HTN groups: F[5,79]=2.6, p=.06; t-tau/Aβ42-HTN groups: F[5,79]=2.6, p=.06 and p-tau231/Aβ42-HTN groups: F[5,79]=3.3, p=.03.
Brain effects
Biomarker groups
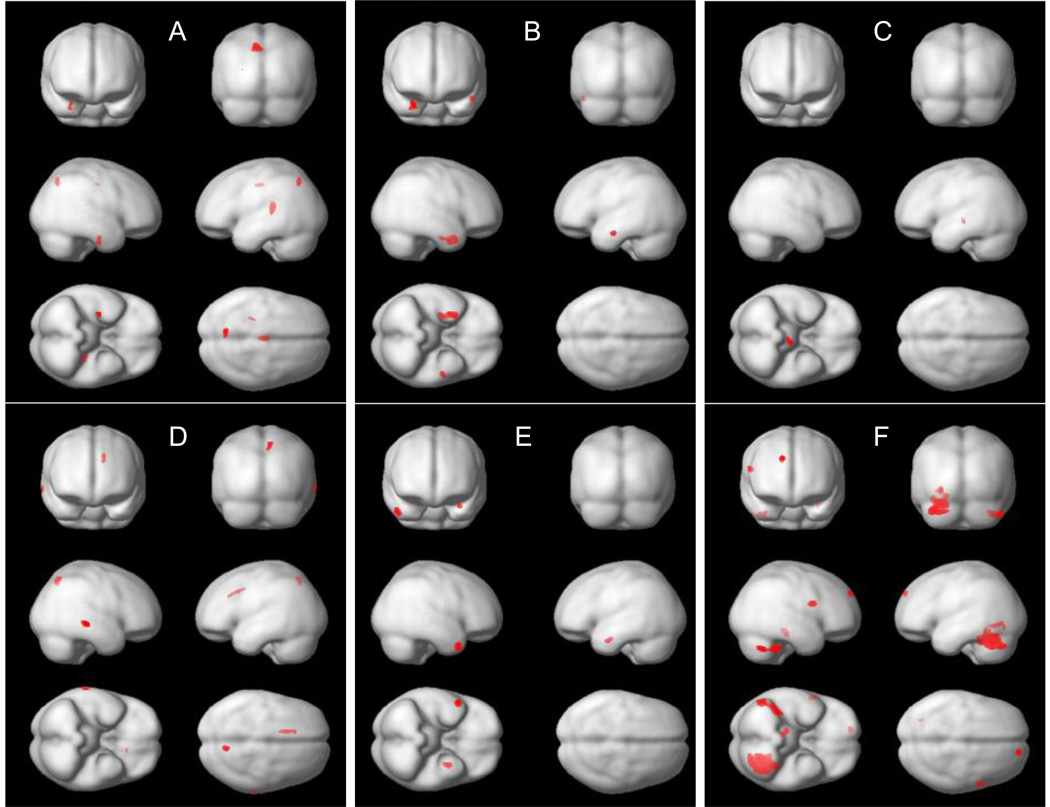
Global effects. The group with high p-tau231/Aβ42 had a smaller global GM volume than the group with low ratio (F[4, 81]=8.3, p=.005). Similar trends were seen for tau/Aβ42 (F[4, 81]=3.4, p=.07) and p-tau231 groups (F[4, 110]=2.3, p=.1) (Table 2). Results remained the same after accounting for the total WMH score. Regional effects. The high t-tau group had less GM in the right hippocampus, left precuneus, anterior cingulate and insula than the low t-tau group. The high p-tau231 group had less GM bilaterally in the temporal lobes: in the right limbic lobe (entorhinal cortex) and the left inferior temporal gyrus. The low Aβ42/Aβ40 group had less GM in the midbrain. The high t-tau/Aβ42 had less GM in the right parietal lobule, left temporal cortex and anterior cingulate. The high p-tau231/Aβ42 group had less GM bilaterally in the temporal gyri (Table 4, Fig. 1A–E). After accounting for a total WMH score the results remained similar: The high t-tau group had less GM in the precunei and insula. The high p-tau231 group had less GM in the right limbic lobe and the temporal cortex. The high t-tau/Aβ42 had less GM in the right parietal cortex and left cingulate. The high p-tau231/Aβ42 group had less GM bilaterally in the temporal cortex. Aβ42/Aβ40 groups did not differ from each other.
Table 4.
SPM analysis. Regions showing significant differences between groups with high and low biomarkers levels. For t-tau the cut-off of 350 pg/mL (Hansson et al., 2006) was used, for p-tau231 the cutoff of 18 pg/mL (Buerger et al., 2003), for Aβ42/Aβ40 the cut-off of .11 (Lewczuk et al., 2004). High and low t-tau/Aβ42 and p-tau231/Aβ42 groups were defined based on the median split. All analyses accounted for age, gender and education.
| Cluster extent |
Brain region | Talaraich coordinates x,y,z |
F value |
|
|---|---|---|---|---|
| t-tau+ < t-tau− | 75 | R Hippocampus | 30, −12, −24 | 3.52 |
| 131 | L Parietal Lobe, Precuneus, BA 7 | −9, −64, 45 | 4.16 | |
| 112 | L Insula, BA 13 | −26, −30, 14 | 4.37 | |
| 105 | L Anterior Cingulate, BA 24 | 0, −13, 40 | 3.82 | |
| p-tau231+ < p-tau231− | 317 | R Limbic Lobe, BA 28 | 29, −10, −25 | 3.84 |
| R Inferior Temporal Gyrus, BA 20 | 33, 0, −32 | 3.54 | ||
| 75 | L Inferior Temporal Gyrus, BA 20 | −50, −10, −20 | 3.84 | |
| Aβ42/Aβ40- < Aβ42/Aβ40+a | 108 | L Midbrain | −6, −23, −4 | 3.59 |
| t-tau/Aβ42+ < t-tau/Aβ42−a | 86 | R Superior Parietal Lobule, BA 7 | 11, −61, 53 | 3.95 |
| 94 | L Middle Temporal Gyrus, BA 21 | 71, −30, −4 | 4.02 | |
| 85 | L Anterior Cingulate, BA 32 | −14, 15, 34 | 3.77 | |
| p-tau231/Aβ42+ < p-tau231/Aβ42−a | 166 | R Middle Temporal Gyrus, BA 21 | 53, 10, −31 | 4.51 |
| 96 | L Middle Temporal Gyrus, BA 21 | −35, −4, −25 | 3.58 | |
| HTN+ < HTN− | 447 | R Cerebellum, Posterior Lobe | 53, −60, −30 | 4.32 |
| 83 | R Superior Frontal Gyrus, BA 9 | 15, 57, 30 | 4.26 | |
| 142 | R Cerebellum, Anterior Lobe | 12, −29, −15 | 4.17 | |
| 88 | R Inferior Frontal Gyrus, BA 44 | 57, 7, 19 | 4.15 | |
| 2435 | L Cerebellum, Posterior Lobe | −27, −61, −21 | 4.46 | |
| 131 | L Lingual Gyrus, BA 19 | −30, −61, −4 | 3.66 |
results presented for 86 subjects.
p <.001, minimal cluster size=75
t-tau+: high t-tau group; t-tau-: low t-tau group,
p-tau231+: high p-tau231 group; p-tau231-: low p-tau231 group,
Aβ42/Aβ40+: high Aβ42/Aβ40 group; Aβ42/Aβ40-: low Aβ42/Aβ40 group
t-tau/Aβ42+: high t-tau/Aβ42 group; t-tau/Aβ42-: low t-tau/Aβ42 group
p- tau231/Aβ42+: high p-tau231/Aβ42 group; p-tau231/Aβ42-: low p-tau231/Aβ42 group
HTN+: group with hypertension, HTN-: group without hypertension
BA: Brodman area
Fig. 1.
Regions showing significant gray matter density differences (as detected with SPM) between groups with: (A) high and low t-tau, (B) high and low p-tau231, (C) high and low with Aβ42/Aβ40, (D) high and low t-tau/Aβ42, (E) high and low p-tau231/Aβ42 and (F) with and without hypertension. All analyses accounted for age, gender and education.
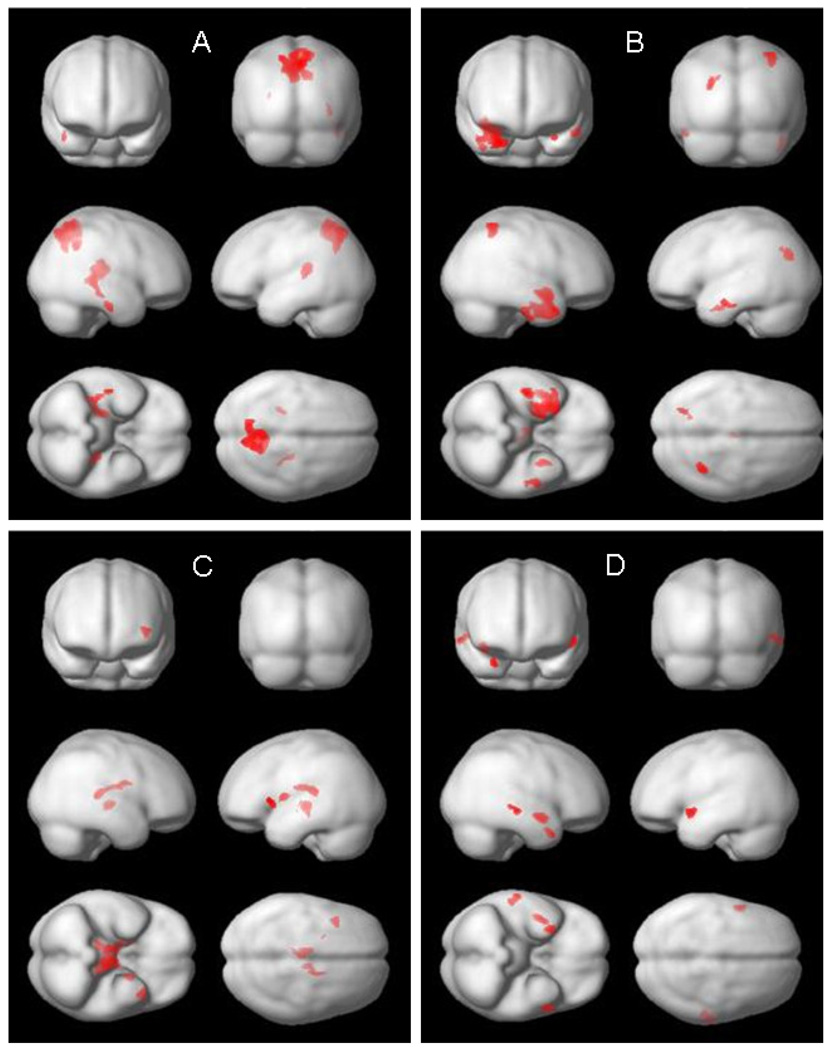
In the regression analyses (Table 5, Fig. 2) the regions of negative correlations between GM and t-tau were found in both precunei, left insula, right caudate and thalamus as well as the right inferior temporal gyrus. Thus precunei and insula were consistently identified in both analyses, but in the regression model the clusters were considerably larger. Similarly, for p-tau231 the regions consistently identified in both analyses, but with larger clusters in the regression model were located in the inferior and medial temporal lobe (comprising entorhinal cortex: BA 28). For Aβ42/Aβ40 the region showing positive correlation and identified in both analyses comprised left midbrain, but in the regression model the cluster extended to both thalami and caudate. No regions of positive correlation between GM and t-tau or p-tau231 and no regions of negative correlation between GM and Aβ42/Aβ40 were found. For t-tau/Aβ42 no region was identified in the regression model. For p-tau231/Aβ42 both analyses pointed to the temporal cortex. After accounting for a total WMH score the results remained comparable.
Table 5.
SPM analysis. Regions showing regions of significant correlations between CSF AD biomarkers and gray matter density. All correlations were negative except for these presented for Aβ42/Aβ40, which were positive. All analyses accounted for age, gender and education.
| Clust er extent |
Brain region | Talaraich coordinates x,y,z |
F value |
|
|---|---|---|---|---|
| t-tau | 2349 | R Parietal Lobe, Precuneus, BA 7 | 8, −65, 48 | 5.31 |
| L Parietal Lobe, Precuneus, BA 7 | −8, −64, 44 | 4.92 | ||
| 230 | L Insula, BA 13 | −26, −31, 14 | 4.84 | |
| 611 | R Caudate Tail | 36, −34, 2 | 4.18 | |
| R Thalamus | 27, −31, 9 | 3.84 | ||
| 92 | R Inferior Temporal Gyrus, BA 20 | 47, −18, −24 | 3.70 | |
| p-tau231 | 1788 | R Superior Temporal Gyrus, BA 38 | 30, 8, −26 | 5.56 |
| R Superior Temporal Gyrus, BA 38 | 39, 1, −15 | 5.01 | ||
| R Limbic Lobe, BA 36 | 23, −4, −30 | 4.39 | ||
| R Limbic Lobe, BA 28 | 29, −10, −25 | 4.02 | ||
| 201 | R Inferior Temporal Gyrus, BA 20 | 47, −19, −27 | 4.13 | |
| 163 | L Parietal Lobe, Precuneus, BA 7 | −26, −69, 28 | 4.12 | |
| 249 | R Inferior Parietal Lobule, BA 40 | 36, −49, 55 | 3.99 | |
| 151 | L Inferior Temporal Gyrus, BA 20 | −51, −9, −20 | 3.87 | |
| 151 | R Thalamus | 3, −19, 15 | 3.70 | |
| 91 | L Amygdala | −30, −4, −24 | 3.62 | |
| Aβ42/Aβ40a | 685 | L Thalamus | −6, −22, −1 | 4.37 |
| R Thalamus | 8, −17, 1 | 3.75 | ||
| L Midbrain | −3, −15, −6 | 3.70 | ||
| 386 | L Thalamus | −11, −28, 14 | 4.26 | |
| 293 | R Thalamus | 12, −29, 12 | 3.98 | |
| R Caudate | 15, −8, 17 | 3.53 | ||
| 121 | L Insula, BA 13 | −41, 17, 2 | 3.87 | |
| 77 | L Lentiform Nucleus | −20, 0, 7 | 3.73 | |
| t-tau/Aβ42a | None | |||
| p-tau231/Aβ42a | 111 | L Superior Temporal Gyrus, BA 38 | −54, 5, −8 | 5.52 |
| 157 | R Temporal Lobe, BA 21 | 41, −3, −12 | 4.53 | |
| 102 | R Superior Temporal Gyrus, BA 38 | 30, 9, −27 | 4.10 | |
| 94 | R Middle Temporal Gyrus, BA 21 | 64, −24, −6 | 3.68 |
results presented for 86 subjects.
p <.001, minimal cluster size=75
BA: Brodman area
Fig. 2.
Regions showing significant correlations (as detected with SPM) between gray matter density and CSF AD biomarkers: (A) t-tau, (B) p-tau231, (C) Aβ42/Aβ40, (D) p-tau231/Aβ42. All analyses accounted for age, gender and education.
HTN groups
Global effects. Subjects with HTN tended to have smaller global GM volumes than subjects without HTN (F[4,110])=3.6, p=.06) (Table 2). After accounting for total WMH score the results were: F[5,106]=1.9, p=.17. Regional effects. Subjects with HTN showed significantly less GM in the cerebellum, occipital and frontal regions (Table 4, Fig. 1F). After accounting for a total WMH score GM reductions were still seen in the cerebellum.
Biomarker - HTN groups
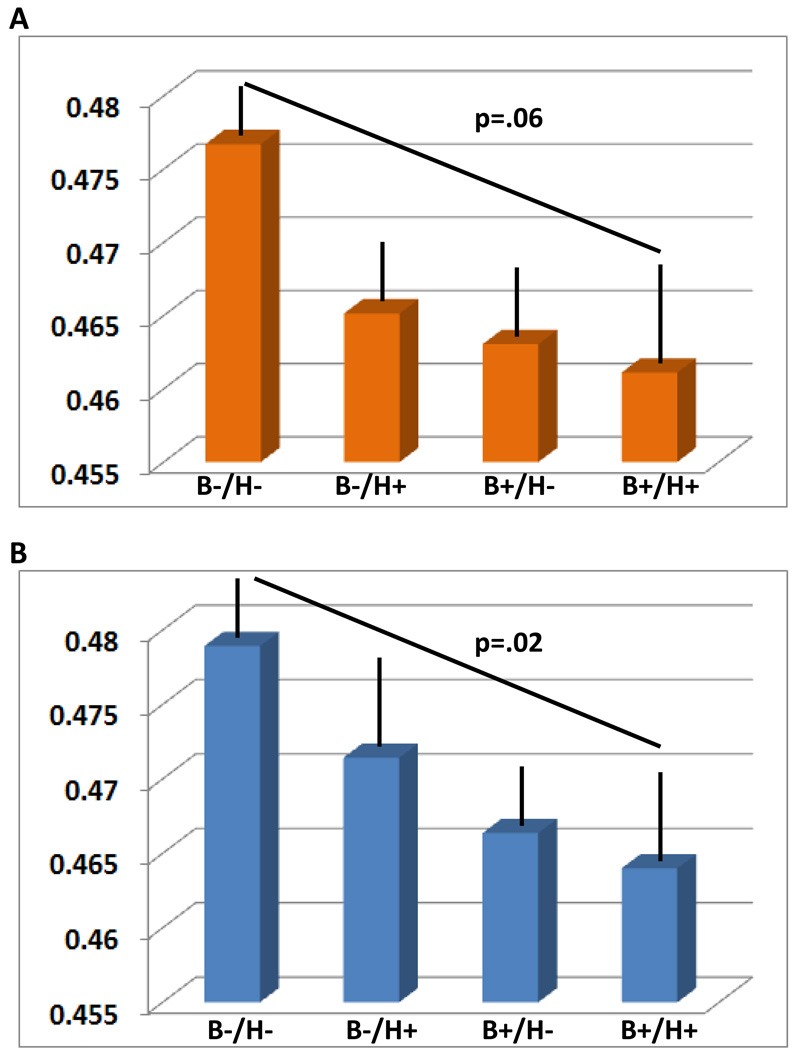
Global effects. Global GM volumes were significantly different across p-tau231-HTN groups (F[6,108]=2.8, p=.04), and p-tau231/Aβ42-HTN groups (F[6,79]=3.4, p=.02) (Table 3). Linear contrasts analyses revealed decreasing GM volumes from the group without any risk markers (B−/H−) to the group with both risk factors (B+/H+) both for p-tau231-HTN (trend: p=.06) and p-tau231/Aβ42-HTN groups (p=.02). The groups expressing only one risk marker had intermediate values (Fig. 3). Accounting for the WMH did not change the results substantially: p-tau231-HTN groups (F[7,104]=2.2, p=.09); p-tau231/Aβ42-HTN groups (F[7,77]=2.7, p=.05). Regional effects. No interactions at the regional level were found with VBM.
Fig. 3.
Estimated means for global GM volume (% of ICV) after accounting for age, gender and education, as a function of p-tau231-HTN groups (A) and p-tau231/Aβ42-HTN groups (B). Please note a linear decrease in GM volumes from the group without any risk markers (B−/H−) to the group with both risk factors (B+/H+). The groups expressing only one risk marker show intermediate values. Bars represent standard errors.
B−/H−: normal biomarker level and HTN absent
B−/H+: normal biomarker level and HTN present
B+/H−: abnormal biomarker level and HTN absent
B+/H+: abnormal biomarker level and HTN present.
Medial temporal lobe ROIs analyses
Biomarker groups (Table 2). Subjects with high t-tau tended to have lower MTL GM density (t[113]=1.9, p=.05). A similar trend was found for p-tau231 (t[113]=2.3, p=.1). HTN groups (Table 2). Subjects with HTN had lower MTL GM density (t[113]=2.3, p=.02) than normotensive individuals. Biomarker - HTN groups (Table 3). Significant differences were found between t-tau-HTN groups (F[3,111]=2.9, p=.04) and a trend for p-tau231-HTN groups (F[3,111]=2.4, p=.07) groups. Linear contrasts analyses both for t-tau-HTN and p-tau231-HTN groups revealed decreasing MTL GM volumes from the group without any risk markers (B−/H−) to the group with both risk factors (B+/H+) (p<.05 for both). Originally all the ROIs analyses were performed after accounting for age, gender and education. However more parsimonious models (without covariates) had the best fit and are presented here.
Cognitive effects
No differences were found in cognitive measures between high and low biomarker groups, nor between subjects with and without HTN. Similarly, biomarkers-HTN groups were not different.
Discussion
In VBM analyses positive AD CSF biomarkers and HTN were associated with different patterns of regional GM atrophy. Elevated p-tau231 and t-tau were related to lower GM estimates in regions implicated in AD pathology. Hypertension was associated with GM loss in the cerebellum and frontal regions. No regional biomarker- HTN interactions, defined as GM reduction beyond the one expected from mere summation of both effects, were found.
Higher p-tau231 levels were associated with lower GM density in temporal lobes, encompassing entorhinal regions (BA28). High t-tau correlated with atrophy in the precunei. This, we believe, concurs with the observation that the medial temporal region is an early site of neurofibrillary pathology (Braak and Braak, 1991). The atrophy of the cingulate (Pennanen et al., 2005) and precunei (Fennnema-Notestine et al., 2009) has been shown in subjects with mild cognitive impairment (MCI), and hypometabolism in limbic and parietal/precuneus areas occurs early in the disease (Mosconi, 2005). We are not aware of any previous report showing the inverse relationship between t-tau and GM density in the precunei in normal individuals, but we believe this is consistent with the known progression of the disease. It is also in agreement with a recent study of healthy controls, showing a negative correlation between t-tau and p-tau and glucose metabolism in posterior cingulate/ precuneus and parahippocampal regions (Petrie et al., 2009). The above regions were identified both by between group comparisons and regression analyses. Importantly no regions of positive correlation between GM and t-tau or p-tau231 were found. This, we believe, increases the reliability of our findings. Our results conflict with earlier negative reports for the association between tau markers and MRI atrophy in cognitively healthy subjects (Loew et al., 2009; Sluimer et al., 2008). Since our sample was considerably larger than the others, we offer that they might have been underpowered to detect associations which among normal subjects are most likely subtle.
The lack of association between global or MTL GM and Aβ42/Aβ40 is in contrast to an earlier study where normal subjects with low Aβ42 had lower whole brain and hippocampal volumes (Fagan et al., 2009). This discrepancy might be due to different levels of AD pathology in the groups studied. CSF Aβ42 has been found to correlate with fibrillar amyloid in the brain (Fagan et al., 2006). However, in the very early stages of AD diffuse plaques, which are not associated with marked neuronal damage or loss, dominate. Second, at autopsy the neurofibrillary stage but not the amyloid burden correlates better with brain atrophy (Josephs et al., 2008). Aβ42/Aβ40 positively correlated with GM density in both thalami, caudate and left midbrain. Interestingly, Pittsburgh Compound B (PiB) retention was found in thalami and striatum both by us (Mosconi et al., 2010) and others (Klunk et al., 2008; Koivunen et al., 2008; Scholl et al., 2009) in subjects at risk or with AD. Even though these amyloid deposits are not believed to be related to cell loss (Klunk et al., 2008) and the relationships between amyloid burden and atrophy are less consistent than relationships between neurofibrally pathology and atrophy (Josephs et al., 2008; Fagan et al., 2009; Driscoll et al., 2010; Glodzik et al., 2010), a recent report demonstrated an association between PiB retention and gray matter atrophy on a region by region basis (Chetelat et al., 2010). Since CSF Aβ42 correlates with PiB retention in subjects without dementia (Fagan et al., 2006), we speculate that the observed correlations between CSF Aβ42/Aβ40 and GM may reflect a subtle regional atrophy. This explanation is, however, only tentative.
As for other ratios: high p-tau231/Aβ42 effects were seen in temporal lobes, high t-tau/Aβ42 was associated with GM damage in parietal and cingulate areas, although since regression analysis did not confirm these last results, they have to be treated with caution. High ratios of t-tau or p-tau231 to Aβ42 were also associated with less global GM. This, we speculate, corroborates the utility of tau to Aβ42 ratio concept. As in our previous work we used Aβ42/Aβ40 ratio instead of Aβ42 alone (Brys et al., 2008; Glodzik et al., 2010). In our experience ratio better reflects possible AD, since the concentration of Aβ42 is determined not only by ongoing pathology but also by the total CSF Aβ peptide concentration which is individually different (Wiltfang et al., 2007).
In agreement with previous reports (Firbank et al., 2007; Nagai et al., 2008), hypertension was related to global GM atrophy. Similarly, cerebellar involvement was not surprising, based on earlier volumetric study (Strassburger et al., 1997) and, we believe, also supports the notion that cerebellar Purkinje cells are particularly susceptible to ischaemia (Cervos-Navarro and Diemer, 1991). Yet again, this observation requires replication since signal differences in the cerebellum are often observed with SPM due to the registration difficulties in this region. We also found regional effects of HTN in both occipital and frontal regions. This replicates former findings of an increased atrophy in these areas in hypertensive subjects (Gianaros et al., 2006; Strassburger et al., 1997; den Heijer et al., 2005). We did not find an evidence for MTL atrophy with VBM approach, possibly it can be seen only in more advanced HTN. White matter hyperintesities are common in aging and are associated with brain atrophy (Godin et al., 2009); not surprisingly they were strongly related to HTN on our study. For these reasons we repeated our analyses after accounting for their burden. It did not change the association between AD biomarkers and GM, but somewhat lessened the relationship between HTN and brain atrophy, confirming that they can mediate HTN effects on the brain.
In addition to VBM analyses we performed direct comparisons of MTL GM density extracted with automated MTL ROI (Li et al., 2008). These revealed the effects of tau markers and lower MTL GM density in HTN subjects, in agreement with previous observations of Korf et al., 2004. The discrepancies between VBM and ROI approach came from different significance threshold. In unbiased VBM it was set up to .001 while for ROIs analyses we used a more liberal, 50 times higher threshold of .05. Altogether ROI analyses confirmed that markers of tau pathology are related to MTL damage, pointed out to HTN as another factor possibly implicated in MTL atrophy and indicated additive effects of abnormal CSF tau markers and HTN on MTL GM. Nonetheless, due to less stringent statistical criteria they require further replication.
In support of the additive effects of AD CSF biomarkers and HTN on the brain, we found their incremental effects on global GM volume. The simultaneous presence of both high p-tau231 or high p-tau231/Aβ42 and HTN were associated with more global GM atrophy than either marker individually. Similar effects were seen in MTL ROIs analyses. The presence of both high t-tau or p-tau231 and HTN was associated with more MTL GM atrophy than either marker alone. In all analyses group differences followed a gradual change in global GM volume or MTL GM density, suggesting rather additive than interaction effects.
The prevalence of HTN across biomarker groups was not different and HTN groups did not differ in their biomarker levels. Pathology reports are contradictory. Some studies, consistent with our results, show a lack of association between AD neuropathology and HTN (Wang et al., 2009) or atherosclerosis (Luoto et al., 2009), while others report increased numbers of hippocampal neurofibrillary tangles and senile plaques in HTN patients (Sparks et al., 1995; Petrovitch et al., 2000). Possibly in our group HTN impact was not yet severe enough to cause AD-related abnormalities, detectable in CSF. The eligibility criteria precluded inclusion of subjects with substantial vascular pathology (i.e. uncontrolled hypertension). Notably, the mean systolic and diastolic blood pressures, although different, were within normal ranges for both subjects with and without HTN. Also other risk factors like smoking of hyperglycemia were uncommon. This relatively low HTN burden, may also help explain the lack of regional biomarker and HTN interactions. Possibly, these interactions can be seen only with more severe hypertension and/ or in later stages of cognitive deterioration, or in cognitively normal population only with functional but not structural imaging.
We did not find differences in cognitive performance between the biomarker groups, the HTN groups, or biomarker- HTN groups, which may weaken the importance of our results. This unexpected finding is perhaps due to the entry criteria for the study. All the participants were cognitively healthy, and subjects with MCI were rigorously excluded. Thus, the differences in performance must have been subtle or else the subject would not have been included. Finally, it is possible that MRI changes are more sensitive and precede changes in cognitive measures. Only longitudinal study can confirm risk for decline among these biomarker and HTN groups.
The prevalence of “abnormal” biomarker’s levels in clinically healthy subjects merits a discussion. 30% of our subjects were classified as having high t-tau, 21% as having high p-tau231 and more than 40% as having low Aβ42/Aβ40. Arguably, such high prevalence can put in question their validity as indicators of pathology. Alternatively, maybe they should be seen more as trait not state dependent. Finally, the characteristic of our cohort can explain these numbers: despite all efforts, a selection bias associated with recruitment to memory and aging studies cannot be avoided. Many participants, although healthy, come because of the concern related to subjective memory impairment or having a family member with AD. Thus our sample may suffer from “self-enrichment”.
The study had several limitations. Our population was highly educated, predominantly Caucasian, with moderate levels of vascular risk, thus further studies of more heterogeneous populations are needed. The present VBM and automated ROI findings need to be replicated with manually traced regions of interest. We believe that evaluation of brain amyloid deposits with PiB, would be also a better indicator of pathology than CSF amyloid levels. Also perfusion studies would be warranted for better assessment of HTN influence on brain. It would be interesting to examine how both HTN and CSF AD biomarkers affect cerebral blood flow, since functional changes associated with them may be more prominent than structural changes.
In conclusion, in cognitively healthy subjects CSF tau markers were associated predominantly with lower GM estimates in structures typically affected in early AD, highlighting their validity as indicators of pathology at the presymptomatic stage. These associations seem to be present early, when no clear cognitive abnormalities can be detected. The simultaneous presence of hypertension and abnormal biomarkers was associated with more pronounced GM atrophy. Neurofibrillary pathology and HTN both seem to contribute to brain damage before cognitive impairment is present. Lack of regional interactions in VBM and gradual increase in atrophy across biomarker-HTN groups indicates rather additive than interaction effects. Longitudinal studies are warranted to assess the risk for cognitive decline.
Acknowledgement
This study was supported by the following grants: NIH-NIA AG12101, AG08051, AG022374.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
Dr. Zinkowski is an employee of Applied NeuroSolutions, Inc and has stock options and owns stock in Applied NeuroSolutions, Inc.
Dr. Blennow has served at an Advisory Board for Innogenetics, Ghent, Belgium.
Other authors have nothing to disclose in connection with this manuscript.
Reference List
- Ashburner J, Friston KJ. Voxel-Based Morphometry--the Methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Imaging techniques: Voxel based morphometry, Chap 306. In: Squire LR, editor. In the Encyclopedia of Neuroscience. Elsevier; 2009. [Google Scholar]
- Bech P, Kastrup M, Rafaelsen OJ. Mini-compendium of rating scales for states of anxiety depression mania schizophrenia with corresponding dsm-iii syndromes. Acta Psychiatr Scand Suppl. 1986;326:1–37. [PubMed] [Google Scholar]
- Blennow K, de Leon MJ, Zetterberg H. Alzheimer's Disease. Lancet Neurology. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- Blennow K, Wallin A, Agren H, Spenger C, Siegfried J, Vanmechelen E. Tau protein in cerebrospinal fluid: a biochemical marker for axonal degeneration in alzheimer disease? Molecular & Chemical Neuropathology. 1995;26:231–245. doi: 10.1007/BF02815140. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brys M, Glodzik-Sobanska L, Switalski R, Rich K, Pirraglia E, Mosconi L, De Santi S, Javier E, Mehta P, Zinkowski R, Pratico D, Wallin A, Blennow K, Sadowski M, Rusinek H, de Leon MJ. MRI improves CSF biomarkers in the early detection of Alzheimer's Disease. Journal of Alzheimer's Disease. 2008;16:351–362. doi: 10.3233/JAD-2009-0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerger K, Zinkowski R, Teipel SJ, Arai H, DeBernardis J, Kerkman D, McCulloch C, Padberg F, Faltraco F, Goernitz A, Tapiola T, Rapoport SI, Pirttila T, Moller HJ, Hampel H. Differentiation of geriatric major depression from Alzheimer's disease with CSF tau protein phosphorylated at threonine 231. Am J Psychiat. 2003;160:376–379. doi: 10.1176/appi.ajp.160.2.376. [DOI] [PubMed] [Google Scholar]
- Cervos-Navarro J, Diemer NH. Selective vulnerability in brain hypoxia. Critical Reviews in Neurobiology. 1991;6:149–182. [PubMed] [Google Scholar]
- Chetelat G, Villemagne VL, Bourgeat P, Pike KE, Jones G, Ames D, Ellis KA, Szoeke C, Martins RN, O’Keefe GJ, Salvado O, Masters CL, Rowe CC the Australian Imaging Biomarkers and Lifestyle Research Group. Relationship between atrophy and beta -amyloid deposition in Alzheimer Disease. Ann. Neurol. 2010;67:317–324. doi: 10.1002/ana.21955. [DOI] [PubMed] [Google Scholar]
- Chobonian A, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Matterson BJ, Oparil S, Wright JT, Jr, Rocella EJ. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- de Leon MJ, George AE, Stylopoulos A, Smith G, Miller DC. Early marker for Alzheimer's Disease: The Atrophic Hippocampus. Lancet. 1989;2:672–673. doi: 10.1016/s0140-6736(89)90911-2. [DOI] [PubMed] [Google Scholar]
- den Heijer T, Launer LJ, Prins ND, van Dijk EJ, Vermeer SE, Hofman A, Koudstaal PJ, Breteler MM. Association between blood pressure, white matter lesions, and atrophy of the medial temporal lobe. Neurol. 2005;64:263–267. doi: 10.1212/01.WNL.0000149641.55751.2E. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Zhou Y, An Y, Sojkova J, Davatzikos C, Kraut MA, Ye W, Ferrucci L, Mathis CA, Klunk WE, Wong DF, Resnick SM. Lack of association between 11C–PiB and longitudinal brain atrophy in non-demented older individuals. Neurobiol. Aging. 2010 doi: 10.1016/j.neurobiolaging.2009.12.008. doi:10.1016/j.neurobiolaging.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Head D, Shah AR, Marcus D, Mintun M, Morris JC, Holtzman DM. Decreased cerebrospinal fluid a42 correlates with brain atrophy in cognitively normal elderly. Ann. Neurol. 2009;65:176–183. doi: 10.1002/ana.21559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Mintun MA, Mach RH, Lee S, Dence CS, Shah AR, LaRossa GN, Spinner ML, Klunk WE, Mathis C, DeKosky ST, Morris JC, Holtzman DM. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid. Ann. Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Roe CM, Xiong C, Mintun M, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid42 ratio as a prediction of cognitive decline in nondemented older adults. Arch. Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR Signal Abnormalities at 1.5 T in Alzheimer's Dementia and Normal Aging. Am. J. Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- Fennnema-Notestine C, Hagler DJJ, McEvoy LK, Fleisher AS, Wu EH, Karow DS, Dale AMand the Alzheimer's Disease Neuroimaging Initiative. Structural MRI Biomarkers for preclinical and mild Alzheimer's Disease. Hum. Brain Mapp. 2009;30:3238–3253. doi: 10.1002/hbm.20744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firbank MJ, Wiseman RM, Burton EJ, Saxby BK, O'Brien JT, Ford GA. Brain atrophy and white matter hyperintensity change in older adults and relationship to blood pressure. Brain Atrophy, WMH Change and Blood Pressure. J. Neurol. 2007;254:713–721. doi: 10.1007/s00415-006-0238-4. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. J. Psychiat. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Freeborough PA, Woods RP, Fox NC. Accurate registration of serial 3d mr brain images and its application to visualizing change in neurodegenerative disorders. Journal of Computer Assisted Tomography. 1996;20:1012–1022. doi: 10.1097/00004728-199611000-00030. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Greer PJ, Ryan CM, Jennings JR. Higher blood pressure predicts lower regional grey matter volume: consequences on short-term information processing. Neuroimage. 2006;31:754–765. doi: 10.1016/j.neuroimage.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert JG, Levee RF, Catalano FL. A preliminary report on a new memory scale. Perceptual and Motor Skills. 1968;27:277–278. doi: 10.2466/pms.1968.27.1.277. [DOI] [PubMed] [Google Scholar]
- Glodzik-Sobanska L, Rusinek H, Mosconi L, Li Y, Zhan J, De Santi S, Convit A, Rich KE, Brys M, de Leon M. The role of quantitative structural imaging in the early diagnosis of Alzheimer's Disease. Neuroimaging Clinics of North America. 2005;15:803–826. doi: 10.1016/j.nic.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Glodzik L, De Santi S, Tsui WH, Mosconi L, Zinkowski R, Pirraglia E, Wang H, Li Y, Rich KE, Zetterberg H, Blennow K, Mehta P, de Leon MJ. Phosphorylated Tau 231, memory decline and medial temporal atrophy in normal elders. Neurobiology of Aging Epub ahead of print. 2010 doi: 10.1016/j.neurobiolaging.2009.12.026. doi:10.1016/j.neurobiolaging.2009.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin O, Maillard P, Crivello F, Alperovitch A, Mazoyer B, Tzourio C, Dufouil C. Association of white matter lesions with brain atrophy markers: The Three-City Dijon MRI Study. Cerebrovasc Dis. 2009;28:177–184. doi: 10.1159/000226117. [DOI] [PubMed] [Google Scholar]
- Good CD, Scahill RI, Fox NC, Ashburner J, Friston KJ, Chan D, Crum WR, Rossor MN, Frackowiak RSJ. Automatic differentiation of anatomical patterns in the human brain: validation with studies of degenerative dementias. Neuroimage. 2002;17:29–46. doi: 10.1006/nimg.2002.1202. [DOI] [PubMed] [Google Scholar]
- Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between csf biomarkers and incipient alzheimer's disease in patients with mild cognitive impairment: a follow-up study. The Lancet Neurology. 2006;5:228–234. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Ahmed Z, Shiung MM, Weigand SD, Knopman DS, Boeve BF, Parisi JE, Petersen RC, Dickson DW, Jack CRJr. Beta-amyloid burden is not associated with rates of brain atrophy. Ann. Neurol. 2008;63:204–212. doi: 10.1002/ana.21223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk WE, Price JC, Mathis CA, Tsopelas ND, Lopresti BJ, Ziolko SK, Bi W, Hoge JA, Cohen AD, Ikonomivic MD, Saxton JA, Snitz BE, Pollen DA, Moonis M, Lippa CF, Swearer JM, Johnson KA, Rentz DM, Fischman AJ, Aizenstein HJ, DeKosky ST. Amyloid deposition begins in the striatum of presenilin-1 mutation carriers from two unrelated pedigrees. J Neurosci. 2007;27:6174–6184. doi: 10.1523/JNEUROSCI.0730-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohnken R, Buerger K, Zinkowski R, Miller C, Kerkman D, DeBernardis J, Shen J, Moller HJ, Davies P, Hampel H. Detection of tau phosphorylated at threonine 231 in cerebrospinal fluid of Alzheimer's disease patients. Neurosci. Lett. 2000;287:187–190. doi: 10.1016/s0304-3940(00)01178-2. [DOI] [PubMed] [Google Scholar]
- Koivunen J, Verkkoniemi A, Aalto S, Paetau A, Ahonen J-P, Viitanen M, Negren K, Rokka J, Haaparanta M, Kalimo H, Rinne JO. PET amyloid ligand [11C]PIB uptake shows predominantly striatal increase in variant Alzheimer’s disease. Brain. 2008;131:1845–1853. doi: 10.1093/brain/awn107. [DOI] [PubMed] [Google Scholar]
- Korf ESC, White LR, Scheltens P, Launer LJ. Midlife blood pressure and the risk of hippocampal atrophy: The Honolulu Asia Aging Study. Hypertension. 2004;44:29–34. doi: 10.1161/01.HYP.0000132475.32317.bb. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox NC. Automated Talairach atlas labels for functional brain mapping. Hum. Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewczuk P, Esselmann H, Otto M, Maler JM, Henkel AW, Henkel MK, Eikenberg O, Antz C, Krause WR, Reulbach U. Neurochemical Ddagnosis of Alzheimer's Dementia by CSF A[Beta]42, A[Beta]42/A[Beta]40 ratio and total Tau. Neurobiology of Aging. 2004;25:273–281. doi: 10.1016/S0197-4580(03)00086-1. [DOI] [PubMed] [Google Scholar]
- Li G, Sokal I, Quinn JF, Leverenz B, Brodey M, Schellenberg GD, Kaye JA, Raskind MA, Zhang J, Peskind ER, Montine TJ. CSF Tau/A{Beta}42 ratio for increased risk of mild cognitive impairment: a follow-up study. Neurol. 2007;69:631–639. doi: 10.1212/01.wnl.0000267428.62582.aa. [DOI] [PubMed] [Google Scholar]
- Li Y, Rinne JO, Mosconi L, Pirraglia E, Rusinek H, De Santi S, Kemppainen N, Nagren K, Kim BC, Tsui W, de Leon MJ. Regional analysis of FDG and PIB-PET images in normal aging, mild cognitive impairment and Alzheimer's disease. Eur J Nucl Med Mol Imag. 2008;35:2169–2181. doi: 10.1007/s00259-008-0833-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loew AD, Yanowsky I, Parikshak N, Hua X, Lee S, Toga AW, Jack CRJ, Bernstein MA, Briston PJ, Gunter JL, Ward CP, Borowski BJ, Shaw LM, Trojanowski JQ, Fleisher AS, Harvey D, Kornak J, Schuff N, Alexander GE, Weiner MW, Thompson PM the Alzheimer's Disease Neuroimaging Initiative. Alzheimer's Disease Neuroimaging Initiative: a one-year follow up study using tensor-based morphometry correlating degenerative rates, biomarkers and cognition. Neuroimage. 2009;45:645–655. doi: 10.1016/j.neuroimage.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luoto T, Haikonen S, Haapasalo H, Goebeler S, Huhtala H, Erkinjunti T, Karhunen PJ. Large vessel cerebral atherosclerosis is not in direct association with neuropathological lesions of Alzheimer's Disease. European Neurology. 2009;62:93–98. doi: 10.1159/000222779. [DOI] [PubMed] [Google Scholar]
- Metha PD, Pirttila T, Metha SP, Sersen E, Aisen PS, Wisnewski HM. Plasma and cerebrospinal fluids levels of amyloid beta proteins 1–40 and 1–42 in Alzheimer's Disease. Arch. Neurol. 2000;57:100–105. doi: 10.1001/archneur.57.1.100. [DOI] [PubMed] [Google Scholar]
- Mosconi L. Brain Glucose Metabolism in the Early and Specific Diagnosis of Alzheimer's Disease. Eur. J. Nucl. Med. 2005;32:486–510. doi: 10.1007/s00259-005-1762-7. [DOI] [PubMed] [Google Scholar]
- Mosconi L, Rinne JO, Tsui WH, Berti V, Li Y, Wang H, Murray J, Scheini N, Någren K, Williams S, Glodzik L, De Santia S, Vallabhajosula S, de Leon MJ. Increased fibrillar amyloid-β burden in normal individuals with a family history of late onset Alzheimer’s. PNAS. 2010;107:5949–5954. doi: 10.1073/pnas.0914141107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K. Ambulatory blood pressure as an independent determinant of brain atrophy and cognitive function in elderly hypertension. J. Hypertens. 2008;26:1636–1641. doi: 10.1097/HJH.0b013e3283018333. [DOI] [PubMed] [Google Scholar]
- Pennanen C, Testa C, Laakso MP, Hallikainen M, Helkala E-L, Haninen T, Kivipelto M, Kononen M, Nissinen A, Tervo S, Vanhanen M, Vanninen R, Frisoni GB, Soininen H. A Voxel based morphometry study on mild cognitive impairment. J. Neurol. Neurosurg. Psychiatry. 2005;76:11–14. doi: 10.1136/jnnp.2004.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie EC, Cross DJ, Galasko D, Schellenberg GD, Raskind MA, Peskind ER, Minoshima S. Preclinical Evidence of Alzheimer Changes. Convergent cerebrospinal fluid biomarker and fluorodeoxyglucosepositron emission tomography findings. Arch. Neurol. 2009;66:632–637. doi: 10.1001/archneurol.2009.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovitch H, White LR, Izmirilian G, Ross GW, Havlik RJ, Markesbery WR, Nelson J, Davis DG, Hardman J, Foley DJ, Launer LJ. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS. Neurobiology of Aging. 2000;21:57–62. doi: 10.1016/s0197-4580(00)00106-8. [DOI] [PubMed] [Google Scholar]
- Reisberg B, Ferris SH. The Brief Cognitive Rating Scale (BCRS) Psychopharmacology Bulletin. 1988;24:629–636. [PubMed] [Google Scholar]
- Reisberg B, Sclan SG, Franssen EH, de Leon MJ, Kluger A, Torossian CL, Shulman E, Steinberg G, Monteiro I, McRae T, Boksay I, Mackell JA, Ferris SH. Clinical Stages of Normal Aging and Alzheimer's Disease: The GDS Staging System. Neurosci. Res. Communications. 1993;13(Suppl. 1):551–554. [Google Scholar]
- Schöll M, Almkvist O, Axelman K, Stefanova E, Wall A, Westman E, Långström B, Lannfelt L, Graff C, Nordberg A. Glucose metabolism and PIB binding in carriers of a His163Tyr presenilin 1 mutation. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.08.016. doi:10.1016/j.neurobiolaging.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Sluimer J, Bouwman FH, Vrenken H, Blankenstein MA, Barkhof F, van der Flier WM, Scheltens P. Whole-brain atrophy rate and csf biomarker levels in mci and ad: a longitudinal study. Neurobiol. Aging. 2010;31:492–498. doi: 10.1016/j.neurobiolaging.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Scheff SW, Liu H, Landers TM, Coyne CM, Hunsaker JCI. Increased incidence of neurofibrillary tangles (nft) in non-demented individuals with hypertension. J. Neurol. Sci. 1995;131:162–169. doi: 10.1016/0022-510x(95)00105-b. [DOI] [PubMed] [Google Scholar]
- Strassburger TL, Lee HC, Daly EM, Szczepanik J, Krasuski JS, Mentis MJ, Salerno JA, DeCarli C, Schapiro MB, Alexander GE. Interactive effects of age and hypertension on volumes of brain structures. Stroke. 1997;28:1410–1417. doi: 10.1161/01.str.28.7.1410. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Stuttgart: Thieme; 1988. [Google Scholar]
- Wang L, Larson EB, Sonnen JA, Shofer JB, McCormick W, Bowen JD, Montine TJ, Li G. Blood pressure and brain injury in older adults: findings from a community-based autopsy study. J. Am. Geriatr. Soc. 2009;57:1975–1981. doi: 10.1111/j.1532-5415.2009.02493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. WAIS-R Manual. New York: Psychological Corporation; 1981. [Google Scholar]
- Wiltfang J, Esselmann H, Bibl M, Hull M, Hampel H, Kessler H, Frolich L, Schroder J, Peters O, Jessen F, Luckhaus C, Perneczky R, Jahn H, Fiszer M, Maler JM, Zimmermann R, Bruckmoser R, Kornhuber J, Lewczuk P. Amyloid Beta peptide ratio 42/40 but not A Beta 42 correlates with phospho-tau in patients with low- and high-CSF A Beta 40 load. J. Neurochem. 2007;101:1053–1059. doi: 10.1111/j.1471-4159.2006.04404.x. [DOI] [PubMed] [Google Scholar]