Abstract
Interferon (IFN)-α treatment for infectious diseases and cancer is associated with significant depressive symptoms that can limit therapeutic efficacy. Multiple mechanisms have been implicated in IFN-α-induced depression including immune, neuroendocrine and neurotransmitter pathways. To further explore mechanisms of IFN-α-induced depression and establish associated genetic risk factors, single nucleotide polymorphisms in genes encoding proteins previously implicated in IFN-α-induced depression were explored in 2 self-reported ethnic groups, Caucasians (n=800) and African Americans (n=232), participating in a clinical trial on the impact of three pegylated IFN-α treatment regimens on sustained viral response in patients with chronic hepatitis C. Prior to treatment, all subjects were free of psychotropic medications and had a score ≤20 on the Center for Epidemiologic Studies Depression Scale (CES-D), which was used to assess depressive symptom severity throughout the study. In Caucasians, a polymorphism (rs9657182) in the promoter region of the gene encoding indoleamine-2,3-dioxygenase (IDO1) was found to be associated with moderate or severe IFN-α-induced depressive symptoms (CES-D >20) at 12 weeks of IFN-α treatment (p=0.0012, p<0.05 corrected). Similar results were obtained for treatment weeks 24, 36 and 48. In subjects homozygous for the risk allele (CC, n=150), the odds ratio for developing moderate or severe depressive symptoms at treatment week 12 was 2.91 (CI: 1.48–5.73) compared to TT homozygotes (n=270). rs9657182 did not predict depression in African Americans, who exhibited a markedly lower frequency of the risk allele at this locus. The findings in Caucasians further support the notion that indoleamine-2,3-dioxygenase plays an important role in cytokine-induced behavioral changes.
Keywords: interferon-α, indoleamine-2, 3-dioxygenase, cytokines, depression, genes, single nucleotide polymorphism
Introduction
The potential role of inflammatory cytokines in major depression has received increasing attention.1 To elucidate mechanisms by which inflammatory cytokines contribute to depression, a number of investigators have studied patients undergoing treatment with the innate immune cytokine, interferon (IFN)-α. While an effective therapy for certain infectious diseases and cancers, IFN-α has been shown in a multitude of studies to induce behavioral changes that markedly overlap with those used to make a diagnosis of major depression.6,10–16 Considerable progress has been made regarding the immunological and neurobiological pathways that are associated with depressive symptoms during IFN-α. In terms of immunological mechanisms, studies have shown that pretreatment concentrations of tumor necrosis factor (TNF)-α and interleukin (IL-6) and/or their soluble receptors predict IFN-α-induced depression,17–18 and both TNF-α and IL-6 in the peripheral blood and cerebrospinal fluid (CSF) have been shown to be associated with IFN-α-induced depressive symptoms and/or CSF changes in the serotonin metabolite, 5-hydroxyindoleacetic acid.9,19–20 In addition, a functional polymorphism in the IL-6 gene (IL-6) has been found to predict depressive symptoms in relationship with polymorphisms in the serotonin transporter gene (SLC6A4).21 Of note, IFN-α-induced increases in CSF IL-6 and soluble TNF receptor 2 were highly correlated with CSF concentrations of chemokine (C-C motif) ligand 2 (CCL2) (also known as monocyte chemoattractant protein-1), a chemokine which has been implicated in attracting monocytes to the brain during chronic inflammation.9,22
Regarding the impact of IFN-α on the neuroendocrine system, studies have shown that the initial response of the hypothalamic-pituitary-adrenal (HPA) axis to IFN-α administration including ACTH and cortisol is markedly increased in patients who subsequently develop depression compared to patients who do not.23 These data suggest that there may be increased sensitivity in HPA axis regulatory pathways including corticotropin releasing hormone (CRH) and/or genes involved in the synthesis of HPA axis hormones such as the pro-opiomelanocortin (POMC) gene, which regulates the production of ACTH. In addition to the predictive value of acute HPA axis responses to IFN-α, studies examining the long term effects of IFN-α on HPA axis function have revealed that IFN-α leads to flattening of the diurnal cortisol curve and increased evening cortisol concentrations.20 These neuroendocrine changes have been associated in unrelated studies with decreased sensitivity to glucocorticoids,24 which may be related to IFN-α’s inhibitory effects on both the function and the expression of the glucocorticoid receptor.25–26
IFN-α-induced changes in neurotransmitter metabolism have also been implicated in depression during IFN-α including effects on serotonin and dopamine. Previous studies have implicated polymorphisms in the serotonin transporter as a risk factor for the development of IFN-α induced depression.21,27–28 These findings are consistent with reports that inflammatory cytokines can increase serotonin transporter expression and function both in vitro and in vivo.29–31 Moreover, drugs that block serotonin transporter function can significantly reduce depression during IFN-α treatment.16,32 Relevant to the metabolism of serotonin, much attention has been paid to the enzyme, indoleamine-2,3-dioxygenase (IDO), which breaks down tryptophan (TRP), the primary amino acid precursor for serotonin, into kynurenine (KYN).33 Decreased peripheral blood TRP concentrations and increased peripheral blood KYN have been associated with IFN-α-induced depression in a number of studies.34–36 In addition, KYN can be converted in the brain into the neuroactive metabolites, quinolinic acid (QUIN) and kynurenic acid (KA).33 Finally, neuroimaging data from humans indicate that IFN-α alters basal ganglia glucose metabolism which may be related to alterations in dopamine metabolism.37 Indeed, rhesus monkeys administered IFN-α exhibit depressive-like huddling behavior, which has been associated with decreases in the dopamine metabolite, homovanillic acid.38 Of note, huddling behavior in monkeys was initially described in rhesus monkeys chronically administered the monoamine depleting drug, reserpine.39
Given the rich array of molecules and pathways that have been shown to play a role in IFN-α-induced depression, we endeavored to determine whether single nucleotide polymorphisms (SNPs) in the genes associated with these molecules/pathways may predict depression in patients receiving pegylated (Peg) IFN-α treatment for chronic hepatitis C. We thus examined SNPs in genes relevant to the immune (IFN-α, TNF-α, IL-1, IL-6, CCL2), neuroendocrine [CRH, POMC, glucocorticoid receptor (NR3C1)] and neurotransmitter (dopamine, IDO1) contributions to IFN-α-induced depressive symptoms in patients with hepatitis C virus (HCV) infection.
Methods
Patients and Study Design
Patients included in the study were a subset of the 3,070 participants in the Individualized Dosing Efficacy vs. Flat Dosing to Assess Optimal Pegylated Interferon Therapy (IDEAL) trial (Schering-Plough Research Institute), which enrolled subjects from 118 academic and community centers in the United States between March 2004 and June 2006.40 The primary outcome variable of the IDEAL trial was sustained viral response determined at 24 weeks post PegIFN-α treatment. Participants in the IDEAL trial were randomly assigned treatment in a 1:1:1 ratio and stratified according to HCV RNA level (≤600,000 IU/mL or >600,000 IU/mL) and race (African American or non–African American). Patients received one of three treatment regimens: Arm 1) PegIFN-alfa-2b 1.5 mcg/kg/wk or Arm 2) PegIFN-alfa-2b 1.0 mcg/kg/wk, both in combination with oral ribavirin (RBV) dosed by body weight (40–65 kg, 800 mg/day; >65–85 kg, 1000 mg/day; >85–105 kg, 1200 mg/day; >105–125 kg, 1400 mg/day), or Arm 3) PegIFN-alfa-2a 180 mcg/wk plus oral RBV 1000–1200 mg/day dosed by body weight (<75 kg, 1000 mg/day; ≥75 kg, 1200 mg/day). Patients received up to 48 weeks of treatment and 24 weeks of follow-up. HCV RNA levels were measured in a central laboratory using the COBAS TaqMan assay (Roche Diagnostics), with a lower limit of quantitation of 27 IU/mL. Measurement time points included screening visits 1 and 2 (baseline); weeks 2, 4, 12, 24, and 48; and follow-up weeks 4, 12, and 24. Per established treatment guidelines, patients with insufficient viral response at 12 weeks (detectable HCV RNA and <2−log10 decrease in HCV RNA from baseline) or 24 weeks (detectable HCV RNA) were discontinued from PegIFN-α treatment.
Inclusion criteria for the IDEAL trial were ≥18 years of age with compensated liver disease due to chronic HCV genotype 1 who were treatment-naïve. Hematologic requirements included absolute neutrophil count ≥1500/mm3, platelet count ≥80,000/mm3, and hemoglobin (Hgb) ≥12 g/dL for females and ≥13 g/dL for males. Liver biopsies were required within three years prior to screening with a blinded assessment of METAVIR fibrosis stage and steatosis by a central pathologist. Patients were excluded for human immunodeficiency virus (HIV) or hepatitis B co-infection, any other cause of significant liver disease, poorly controlled diabetes mellitus (HbA1C >8.5%), and morbid obesity (weight >125 kg). Psychiatric exclusion criteria (as determined by the treating gastroenterologist) included a history of severe depression or a history of severe psychiatric disorder such as psychosis, suicidal ideation, suicide attempt, and/or active substance abuse. Severe depression was defined as any depression that required hospitalization or electroconvulsive therapy or any depression that resulted in prolonged absence from work or significant disruption of daily functions. The psychiatric history of each patient was collected and coded using the Standardized Medical Dictionary for Regulatory Activities (MedDRA) version 10.1. Relevant MedDRA terms were categorized into “Psychiatric” and “Substance Abuse”.
In order to be included in the current genetic study, patients had to fulfill the following additional entry criteria: 1) willingness to provide a blood sample for DNA analysis, 2) be free of antidepressants, mood stabilizers, or antipsychotic medications prior to administration of IFN-α and 3) have a score of ≤20 on the Center for Epidemiologic Studies Depression Scale (CES-D) at baseline.
The study was approved by institutional review boards or ethics committees at participating centers and was conducted in accordance with provisions of the Declaration of Helsinki and Good Clinical Practice guidelines. All subjects provided written informed consent.
Assessment of Depression
Depressive symptom severity was assessed using the CES-D, a 20-item scale developed for use in studies of depression in the general population.41 The CES-D inquires about depressive symptoms including depressed mood, hopelessness, difficulty concentrating, impaired sleep, fatigue and reduced appetite with higher scores representing greater symptom severity. Based on general and psychiatric population samples, CES-D scores 21–25 are considered to represent moderate depression, while scores >25 are considered severe depression.42–44 Patients self-administered the CES-D via an automated telephone system at each center prior to being interviewed by center staff. Assessments occurred at baseline and treatment weeks 2, 4, 12, 24, 36, and 48, and follow-up weeks 4 and 24. Each question was scored and totaled, and scores were blinded. If a patient had a CES-D score >25, the center was immediately notified (although the actual score remain blinded), and a pre-established and approved protocol, which typically included consultation with a mental health professional, was initiated.
Independent of CES-D evaluation, adverse events including depression were graded by center investigators as mild, moderate, severe, or potentially life-threatening using a modified World Health Organization grading system. Per the protocol, mild depression was monitored weekly for 4–8 weeks; PegIFN dose reduction was added for moderate depression with psychiatric intervention instituted as needed; and treatment was discontinued with immediate psychiatric consultation for severe depression, suicidal ideation and/or a suicide attempt. Dosage reduction of PegIFN-α treatment and/or ribavirin was monitored along with use of psychotropic medications (including antidepressants, mood stabilizers and antipsychotics) and erythropoietin. In addition, Hgb and thyroid stimulating hormone (TSH) were measured at baseline and regular intervals throughout the study by a central laboratory.
Genotyping
DNA was extracted from EDTA-preserved blood at a central laboratory (Beckman Coulter Genomics, Morrisville NC) using the Puragene kit according to manufacturer’s recommendations (Gentra Systems, Minneapolis, MN). SNPs in 14 genes related to immune, neuroendocrine and neurotransmitter systems were selected for genotyping 1) from HapMap using the tagger algorithm (http://hapmap.ncbi.nlm.nih.gov/) to assess linkage disequilibrium (LD) blocks with a r2 of 0.8 and a minor allele frequency of 5% in the CEPH population or 2) from the literature. Specific genes and the number of SNPS evaluated are indicated in Table 1 and Supplementary Table 1. SNPs were genotyped using TaqMan genotyping kits (Applied Biosystems, Carlsbad, CA)
Table 1.
Selected Genes and Number of Tagging SNPs
| Gene Name | Gene Symbol | # of SNPs |
|---|---|---|
| Chemokine (C-C motif) Ligand 2 | CCL2 | 3 |
| Catechol-O-Methyltransferase | COMT | 2 |
| Corticotropin Releasing Hormone | CRH | 3 |
| Corticotropin Releasing Hormone Receptor 1 | CRHR1 | 3 |
| Dopamine Beta-Hydroxylase | DBH | 3 |
| FK506 Binding Protein 5 | FKBP5 | 2 |
| Interferon (Alpha, Beta & Omega) Receptor 1 | IFNAR1 | 3 |
| Interleukin 1, Alpha | IL1A | 2 |
| Interleukin 1, Beta | IL1B | 4 |
| Interleukin 6 | IL6 | 3 |
| Indoleamine 2,3-Dioxygenase 1 | IDO1 | 4 |
| Proopiomelanocortin | POMC | 3 |
| Solute Carrier Family 6, Member 3 | SLC6A3 | 2 |
| Tumor Necrosis Factor | TNF | 4 |
SNP- single nucleotide polymorphism
Statistical Analysis
The association between demographic or clinical factors and moderate or severe depression (CES-D >20) at 12 weeks was assessed by applying a general linear model (GLM). As these nominally associated measures may be potentially correlated and may confound genetic association analysis, they were then entered into forward and reverse stepwise regression models. Each demographic or clinical factor that remained significant in the model at p<0.05 was included as a covariate in the analysis of each polymorphism.
All polymorphisms had call rates that exceeded 95%. Polymorphisms were assessed to determine if observed genotype frequencies were consistent with Hardy-Weinberg Equilibrium (HWE) using Chi-square tests. Markers which deviated from HWE or that were not polymorphic in the Caucasian or African American population were excluded from analysis (Supplementary Table 1). A final total of 36 SNPS were considered in the analyses. Linear regressions were used to test for associations between each polymorphism and moderate or severe depression at 12 weeks assuming an additive model incorporating covariates from the stepwise regression. The 12 week time point was chosen for the initial analysis, because, by study design, PegIFN-α was not discontinued until after 12 weeks in subjects with an insufficient viral response. P-values were based on two-tailed tests, and Bonferroni correction was applied for each SNP examined to determine experiment-wide significance (p<0.0014). Of note, this correction may be overly conservative as many of the polymorphisms are in linkage disequilibrium and therefore do not represent independent tests. Comparisons among specific genotypes within relevant SNPs were conducted using pair-wise comparisons within the GLM. Statistical and genetic analyses were performed using Statistical Analysis Software (SAS) (Cary, NC).
Results
Clinical and Demographic Predictors
Of the 3,070 patients enrolled in the IDEAL trial, 1,032 subjects met entry criteria for the current study (800 Caucasians and 232 African Americans). Of the 1,599 subjects with available DNA samples, 162 were excluded for not being either Caucasian or African American; 391 were excluded for using psychotropic medications prior to IFN-α administration, and 14 were excluded because their baseline depression score exceeded the cut-off for moderate or severe depression (CES-D>20). Excluded subjects were similar in age (p=0.90) but were more likely to be female (F=63.33; p<0.0001).
The demographic and clinical characteristics of the study population are presented in Table 2. Associations between clinical and demographic measures and development of moderate or severe depression (CESD >20) at 12 weeks were assessed. Variables considered in these analyses included age, gender, weight at baseline, Hgb at baseline and the study visit prior to week 12, TSH at baseline, viral genotype, viral load at baseline, degree of liver fibrosis, treatment group assignment, ribavirin dosage at baseline and the study visit prior to week 12, depressive symptoms at baseline, history of substance abuse and/or psychiatric disorder, and psychotropic medication usage prior to week 12.
Table 2.
Clinical and Demographic Characteristics of Study Participants.
| Caucasian N=800 | African American N=232 | p-value | |
|---|---|---|---|
|
| |||
| Age, Mean ± SD | 47.03 ± 7.90 | 50.03 ± 6.90 | <0.0001 |
|
| |||
| Weight (kg), Mean ± SD | 84.27 ± 15.79 | 88.94 ±14.62 | <0.0001 |
|
| |||
| Hemoglobin, (g/dl), Baseline, Mean ± SD | 15.25 ± 1.20 | 14.52 ±1.16 | <0.0001 |
|
| |||
| Gender, % Male | 68.3% | 59.5% | 0.0124 |
|
| |||
| Viral Genotype | |||
| 1A | 66.9% | 59.5% | 0.0374 |
| 1B | 33.1% | 40.5% | |
|
| |||
| Drug | 0.7339 | ||
| Peg2b 1.0/Ribavirin | 33.1% | 34.5% | |
| Peg2b 1.5/Ribavirin | 32.8% | 31.0% | |
| Peg2a/Ribavirin | 34.1% | 34.5% | |
|
| |||
| Medical & Psychiatric History | |||
|
| |||
| Fibrosis, Mean ± SD | 1.39 ± 0.85 | 1.38 ± 0.83 | 0.8107 |
|
| |||
| History of Substance Abuse | 45.4% | 33.6% | 0.0014 |
|
| |||
| History of Psychiatric Diagnosis | 12.9% | 7.3% | 0.0197 |
|
| |||
| Depression Scores (CES-D) | |||
|
| |||
| Baseline, Mean ± SD | 4.36 ± 4.41 | 6.31 ± 5.08 | <0.0001 |
|
| |||
| Moderate/Severe Depression at Week 12 | 15.5% | 17.7% | 0.1497 |
SD-Standard Deviation
CES-D-Center for Epidemiologic Studies Depression Scale
Interferon-induced Depression in Caucasians
Clinical Predictors
Depression score at baseline predicted 9.4% of the variance in Caucasian patients who developed moderate or severe depression at 12 weeks (t=9.06, p=<0.0001). History of substance abuse (t=2.81; p=0.005 and history of psychiatric disorder (t=4.69; p=<0.0001) also predicted 1% and 2.7% of the variance, respectively. Finally, the use of psychiatric medications prior to week 12 was also predicative of moderate or severe depression at 12 weeks (t=3.97; p<0.0001).
Genetic Predictors
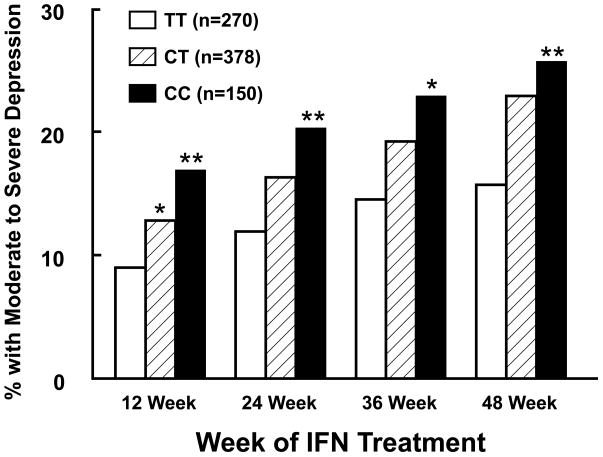
The association between each polymorphism and moderate or severe depression at 12 weeks was evaluated using a linear regression model incorporating the significant clinical covariates described above. Four polymorphisms were associated with depression at week 12 (p<0.05; Table 3). One polymorphism (rs9657182) in indoleamine 2,3-dioxygenase 1 (IDO1) gene (Figure 1) remained significant after Bonferroni correction for multiple tests (t=−3.25, p=0.0012, p corrected <0.05). As indicated in Figure 2, subjects with the CC genotype (n=150) were more likely to exhibit moderate or severe depression at week 12 compared to those with either the CT (n=378) or TT (n=270) genotypes (16.9% versus 12.8% versus 8.9%, respectively). The C allelle was also associated with an increased risk of moderate or severe depression at weeks 24, 36 and 48 of PegIFN-α therapy (all p≤0.01)(Figure 2). As expected, when compared to subjects with the TT genotype, the odds ratio (OR) for moderate or severe depression at 12 weeks increased progressively in those with a CT (OR, 1.61; CI: 0.89–2.90) or CC (OR, 2.91; CI: 1.48–5.73) genotype. When entered into a stepwise regression along with the clinical predictors identified previously, rs9657182 (IDO1) variants remained highly predictive, contributing 1.1% of the variance to moderate or severe depression at week 12 (Table 4).
Table 3.
Genotypic Association with Percent Development of Moderate or Severe Depression at Treatment Week 12 in Caucasians.
| Gene | SNP | Genotype | None/Mild, % | Moderate/Severe, % | t | p-value |
|---|---|---|---|---|---|---|
| IDO1 | rs9657182 | CC CT TT |
83.1 87.2 91.1 |
16.9 12.8 8.9 |
−3.25 | 0.0012 |
| IDO1 | rs7820268 | CC CT TT |
85.6 89.7 89.0 |
14.4 10.3 11.0 |
−2.63 | 0.0088 |
| CRHR1 | rs7209436 | CC CT TT |
84.4 89.3 89.8 |
15.7 10.7 10.2 |
−1.97 | 0.0489 |
| POMC | rs1866146 | AA AG GG |
85.4 88.7 92.9 |
14.6 11.3 7.14 |
−2.16 | 0.0311 |
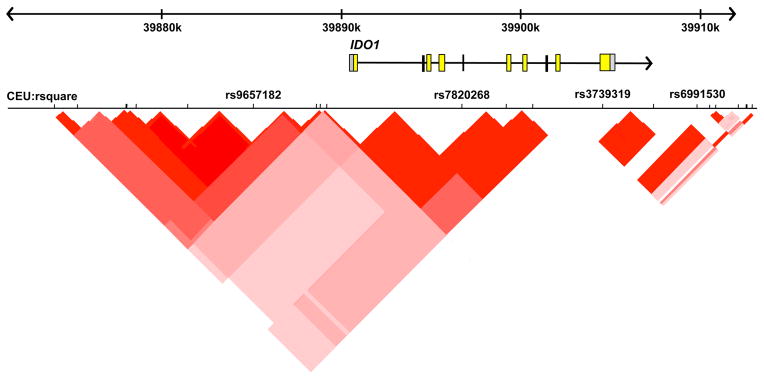
Figure 1. Genomic structure of IDO1 and the position of the polymorphisms examined in relation to linkage disequilibrium in a representative Caucasian sample (CEU).
Linkage disequilibrium in the population is indicated using r2 and visualized using haploview.
Figure 2. Development of Moderate or Severe Depression in Caucasians across the 48 week study stratified by TT, CT, and CC genotype at the rs9657182 locus.
Depressive symptom severity was assessed across 48 weeks of IFN-α therapy in 800 Caucasian subjects with hepatitis C using the Center for Epidemiologic Studies Depression Scale (CES-D). All subjects were free from psychotropic medications at baseline. Patients homozygous for the C allele (CC) in a polymorphism (rs9657182) in the promoter region of the gene encoding for indoleamine-2,3-dioxygenase (IDO1) exhibited a significantly higher percentage of moderate to severe depression (CES-D >20) compared to those homozygous for the T allele at all time points examined. **p<0.01, *p<0.05 compared to CC genotype.
Table 4.
Summary of Stepwise Regression Model for the Development of Moderate to Severe Depression (CES-D>20) at Treatment Week 12.
| Predictor | R2 | R2 Change | F-value | p-value |
|---|---|---|---|---|
|
| ||||
| Depression Scores at Baseline | 0.0921 | 0.0921 | 77.79 | <.0001 |
| History of Psychiatric Disorder | 0.1076 | 0.0155 | 13.28 | 0.0003 |
| rs9657182 | 0.1185 | 0.0109 | 9.50 | 0.0021 |
| Psychiatric Medication at Week 4 | 0.1256 | 0.0071 | 6.24 | 0.0127 |
| History of Substance Abuse | 0.1318 | 0.0061 | 5.39 | 0.0205 |
Interferon-induced Depression in African Americans
Clinical Predictors
Potential clinical covariates were evaluated in the same manner as for the Caucasian subjects. On average, at baseline African American subjects were older (50.0 vs. 47.0 years; t=5.23, p<0.0001) and heavier (88.9 kg versus 84.3 kg; t=4.04, p<0.0001) and had lower mean Hgb levels (14.52 vs. 15.25; t=−8.16; p=<0.0001) than Caucasians. The African American population also had a greater proportion of females (40.5 vs, 31.7%; F=6.28; p=0.012) and a greater proportion of subjects with HCV genotype 1B (p=0.038). Reported rates of history of substance abuse and history of psychiatric disorder were lower in African American subjects than Caucasians (33.6% vs. 45.4%; F=10.21; p=0.001 and 7.3% vs. 12.9%; F=5.45; p=0.02, respectively). Finally, baseline depression scores were significantly higher in the African American subjects than the Caucasian subjects (6.31 vs. 4.35; t=5.76, p<0.0001, respectively).
As with the Caucasian subjects, associations between clinical and demographic measures and moderate or severe depression at 12 weeks were assessed. Depression score at baseline predicted 13.9% of the variance in moderate/severe depression at 12 weeks (t=6.01; p<0.0001), and treatment arm also predicted 3.3% of the variance (t=−2.76; p=0.006).
Genetic predictors
After accounting for the clinical factors indicated above, only one polymorphism, rs1042571, in the POMC gene was nominally associated with the development of depression in African American subjects at treatment week 12 (t=2.12; p=0.035), but this association was not significant after correction for multiple testing. There was no association of rs9657182 in IDO1 with depression in African Americans (p>0.05), although the risk allele (C) frequency observed in African Americans was almost 50% of that observed in Caucasians (37% versus 66%, respectively) and only 9 African Americans exhibited the homozygous CC genotype, thus markedly limiting the power to detect a significant relationship.
Discussion
The present results indicate that a polymorphism in the promoter region of IDO1 gene (rs9657182) predicts the development of moderate or severe depressive symptoms during IFN-α therapy in Caucasian subjects undergoing treatment for chronic hepatitis C. This finding, which remained significant after adjustment for multiple comparisons, was not found in a significantly smaller sample of African Americans, who exhibited an almost 50% lower frequency of the risk allele (C) and few CC homozygotes (n=9). Taken together, the data are consistent with the notion that IDO activity may play an important role in the pathophysiology of IFN-α-induced depression as well as depression induced by other forms of immune stimulation.
IDO is expressed in multiple immune cells throughout the body including monocytes, macrophages, microglia and dendritic cells and can be induced by a multiple cytokines such as IFN-α, IFN-γ and TNF-α. As noted previously, IDO activation leads to the breakdown of TRP into KYN, thereby reducing the availability of TRP, which not only is involved in the regulation of T cells,49 but also is the primary precursor of serotonin, a prominent neurotransmitter in the neurobiology of mood disorders.50–51
Activation of IDO plays a critical role in depressive-like symptoms in laboratory animals following exposure to inflammatory stimuli such as lipopolysaccharide (LPS) and bacille Calmette-Guérin (BCG), an attenuated form of Mycobacterium bovis.52–53 Treatment of mice with LPS or BCG induces IDO mRNA in the brain, and administration of the specific IDO inhibitor, 1-methyltryptophan, blocks both LPS- and BCG-induced increases in immobility during the forced swim and tail suspension tests.52–53 In addition, mice genetically deficient in IDO are resistant to depressive-like behaviors following BCG inoculation, despite induction of proinflammatory cytokines.53 Evidence of IDO activation (decreases in peripheral blood TRP and increases in peripheral blood KYN) also have been associated with depressive symptoms in humans including patients undergoing IFN-α treatment34–36,54–55 as well as patients with cancer and patients infected with HIV.56–57
Of note, peripheral administration of KYN alone can induce depressive-like behavior in laboratory animals.52,58 Blood borne KYN, which is a primary source for central nervous system (CNS) kynurenines, is taken up into the brain through the large amino acid transporter, where it is further metabolized in microglia and astrocytes to QUIN and KA, respectively.33 QUIN is an excitotoxic neuromodulator which promotes glutamate release through direct stimulation of N-methyl-D-aspartate receptors, while also inducing oxidative stress including lipid peroxidation.33,60–63 Increased CNS QUIN has been associated with several neurodegenerative disorders such as Alzheimer’s Disease, amyotrophic lateral sclerosis, and Huntington’s Disease, as well as the dementia complex associated with HIV infection.64–68 In contrast to QUIN, KA has been shown to antagonize glutamate release and block excitatory neurotransmission.33 Of relevance to depression, when injected into the striatum of rats, KA significantly reduces extracellular dopamine, a key neurotransmitter involved in motivation and reward circuitry.69 Recent data indicate that following IFN-α administration, increases in peripheral blood KYN are associated with significant increases in CSF KYN.70 Moreover, increased CSF KYN was accompanied by significant increases in both CSF KA and QUIN. Both CSF KYN and QUIN were significantly correlated with IFN-α-induced depression.70 Taken together with the association of variants in IDO1 with IFN-α-induced depression, these data provide strong reason to believe that IDO is an important pathway by which cytokines influence behavior.
Strengths and weaknesses of the study warrant consideration. A large sample of patients free of psychotropic medications and without significant depressive symptoms at baseline was exposed to standardized doses of a chronic immune stimulus, thereby allowing significant control of the gene-environment interaction that was the focus of the study. Moreover, assessment of depressive symptom severity was conducted via an automated, centralized data collection system, limiting variability across sites. In addition, baseline factors that may have influenced the development of moderate to severe depressive symptoms were assessed and included as covariates in statistical analyses where appropriate. Further, a significant number of patients exhibited moderate or severe depressive symptoms (CES-D >20) at treatment week 12 (n=124), and the primary study outcome variable (development of moderate or severe depressive symptoms) represents a clinically relevant endpoint that warrants IFN-α dosage reduction or discontinuation according to established treatment guidelines. Finally, the risk allele at rs9657182 locus (C) is common in Caucasians (66%) with 19% of Caucasian subjects being CC homozygotes.
Regarding weaknesses, the study had significantly reduced power to detect the association between rs9657182 (IDO1) variants and IFN-α-induced depression in African Americans. In addition, it is unclear to what extent variants of rs9657182 may alter the function of IDO1. Blood samples were not obtained for the purpose of measuring tryptophan or the IDO metabolite kynurenine. Moreover, the alleles of rs9657182 have not been shown to alter any transcription factor binding sites. Nevertheless, the rs9657182 resides in a linkage disequilibrium block encompassing the 20 kilobases upstream of IDO1, which includes at least 6 additional polymorphisms (Figure 1). Thus, rs9657182 may promote a change in gene function on its own (independent of effects on transcription factor binding), or through association with one of the other polymorphisms tagged by rs9657182, several of which appear to alter consensus binding sites of factors involved in the transcription of a variety of genes (e.g. GATA1, GATA3 and ETS1). Of note, the second IDO polymorphism (rs7820268) that was found to be associated with IFN-alpha-induced depressive symptoms is in weak linkage disequilibrium with rs9657182 (r2=0.26)(Figure 1). These data provide further evidence of an impact of polymorphisms within this region of the IDO1 gene on IFN-alpha-induced depressive symptoms, warranting detailed functional assays to elucidate the potential importance of this region. Of note, the current study did not replicate previous findings regarding a SNP in the promoter region of the IL-6 gene (rs1800795) which predicted IFN-alpha-induced depressive symptoms.21 Discrepancies may relate to differences in study endpoints which were development of moderate to severe depression in the current study and change of depressive symptoms from baseline in the previous study. Finally, it should be mentioned that there were no standardized clinician-administered assessments to establish a diagnosis of major depression, the severity of depressive symptoms, or a past history of either substance abuse or psychiatric disorder. Nevertheless, despite these limitations, past history of psychiatric disorder as well as baseline depressive symptoms were significant predictors of depression during IFN-α therapy as has been reported previously.15
In summary, the association of a polymorphism in the promoter region of the IDO1 gene with significant depressive symptoms during chronic exposure to IFN-α provides further support for the role of IDO in cytokine-induced behavioral pathology. Moreover, the results suggest that IDO and its downstream products including KYN, KA and QUIN represent novel biomarkers and/or treatment targets for management of behavioral changes secondary to IFN-α and/or chronic immune stimulation.
Acknowledgments
This study was supported by a grant from Schering Plough Research Institute as well as a Mentored Scientist Development Award from the National Institute of Health to JC (K01DA015766).
Footnotes
Supplementary Information is available at Molecular Psychiatry’s website.
Financial Disclosure
Alicia K. Smith has received research support from Schering-Plough Research Institute; Jason S. Simon is an employee of Eisai Incorporated; Eric L. Gustaffson, David J. Devlin, Ping Qiu, Janice K. Albrecht and Clifford A. Brass are employees of Merck Research Laboratories and are stockholders in this company; Stephanie Noviello is a consultant for Merck Research Laboratories; Joseph F. Cubells has served as a consultant for Barnes and Thornberg, LLP and has received research support from Seaside Therapeutics and Schering Plough Research Institute; Mark S. Sulkowski has served on the advisory board and received research support from Abbott Laboratories, Bristol Meyers Squibb, Boehringer Ingelheim Pharmaceuticals, Inc., F. Hoffmann-La Roche Ltd., Gilead Sciences Incorporated, Merck Research Laboratories, Tibotec, and Vertex Pharmaceuticals, has served on the advisory board of GlaxoSmithKline, has been a consultant for Biolex Therapeutics and Teva Pharmaceutical Industries Ltd and served on a study steering committee for Pfizer Inc.; John G. McHutchinson is an employee of Gilead Sciences Incorporated; Andrew H. Miller has served as a consultant for Abbott Laboratories, AstraZeneca, GlaxoSmithKline, Lunbeck Research USA, F. Hoffmann-La Roche Ltd., Schering-Plough Research Institute and Wyeth/Pfizer Inc. and has received research support from Centocor Inc., GlaxoSmithKline, and Schering-Plough Research Institute. Michael P. Epstein has nothing to disclose.
Bibliography
- 1.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maes M. Evidence for an immune response in major depression: a review and hypothesis. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:11–38. doi: 10.1016/0278-5846(94)00101-m. [DOI] [PubMed] [Google Scholar]
- 3.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 4.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 5.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- 8.Muller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Muller B, et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry. 2006;11:680–684. doi: 10.1038/sj.mp.4001805. [DOI] [PubMed] [Google Scholar]
- 9.Raison CL, Borisov AS, Majer M, Drake DF, Pagnoni G, Woolwine BJ, et al. Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol Psychiatry. 2009;65:296–303. doi: 10.1016/j.biopsych.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capuron L, Miller AH. Cytokines and psychopathology: lessons from interferon-alpha. Biol Psychiatry. 2004;56:819–824. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Capuron L, Fornwalt FB, Knight BT, Harvey PD, Ninan PT, Miller AH. Does cytokine-induced depression differ from idiopathic major depression in medically healthy individuals? J Affect Disord. 2009;119:181–185. doi: 10.1016/j.jad.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loftis JM, Hauser P. The phenomenology and treatment of interferon-induced depression. J Affect Disord. 2004;82:175–190. doi: 10.1016/j.jad.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Kraus MR, Schafer A, Faller H, Csef H, Scheurlen M. Psychiatric symptoms in patients with chronic hepatitis C receiving interferon alfa-2b therapy. J Clin Psychiatry. 2003;64:708–714. doi: 10.4088/jcp.v64n0614. [DOI] [PubMed] [Google Scholar]
- 14.Schaefer M, Engelbrecht MA, Gut O, Fiebich BL, Bauer J, Schmidt F, et al. Interferon alpha (IFNalpha) and psychiatric syndromes: a review. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:731–746. doi: 10.1016/s0278-5846(01)00324-4. [DOI] [PubMed] [Google Scholar]
- 15.Raison CL, Borisov AS, Broadwell SD, Capuron L, Woolwine BJ, Jacobson IM, et al. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J Clin Psychiatry. 2005;66:41–48. doi: 10.4088/jcp.v66n0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, et al. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med. 2001;344:961–966. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- 17.Wichers MC, Kenis G, Koek GH, Robaeys G, Nicolson NA, Maes M. Interferon-alpha-induced depressive symptoms are related to changes in the cytokine network but not to cortisol. J Psychosom Res. 2007;62:207–214. doi: 10.1016/j.jpsychores.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Friebe A, Schwarz MJ, Schmid-Wendtner M, Volkenandt M, Schmidt F, Horn M, et al. Pretreatment levels of sTNF-R1 and sIL-6R are associated with a higher vulnerability for IFN-alpha-induced depressive symptoms in patients with malignant melanoma. J Immunother (1997) 2007;30:333–337. doi: 10.1097/01.cji.0000211346.19330.c9. [DOI] [PubMed] [Google Scholar]
- 19.Prather AA, Rabinovitz M, Pollock BG, Lotrich FE. Cytokine-induced depression during IFN-alpha treatment: the role of IL-6 and sleep quality. Brain Behav Immun. 2009;23:1109–1116. doi: 10.1016/j.bbi.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raison CL, Borisov AS, Woolwine BJ, Massung B, Vogt G, Miller AH. Interferon-alpha effects on diurnal hypothalamic-pituitary-adrenal axis activity: relationship with proinflammatory cytokines and behavior. Mol Psychiatry. 2010;15:535–547. doi: 10.1038/mp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bull SJ, Huezo-Diaz P, Binder EB, Cubells JF, Ranjith G, Maddock C, et al. Functional polymorphisms in the interleukin-6 and serotonin transporter genes, and depression and fatigue induced by interferon-alpha and ribavirin treatment. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Mello C, Le T, Swain MG. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J Neurosci. 2009;29:2089–2102. doi: 10.1523/JNEUROSCI.3567-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Capuron L, Raison CL, Musselman DL, Lawson DH, Nemeroff CB, Miller AH. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am J Psychiatry. 2003;160:1342–1345. doi: 10.1176/appi.ajp.160.7.1342. [DOI] [PubMed] [Google Scholar]
- 24.Spiegel D, Giese-Davis J, Taylor CB, Kraemer H. Stress sensitivity in metastatic breast cancer: analysis of hypothalamic-pituitary-adrenal axis function. Psychoneuroendocrinology. 2006;31:1231–1244. doi: 10.1016/j.psyneuen.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu F, Pace TW, Miller AH. Interferon-alpha inhibits glucocorticoid receptor-mediated gene transcription via STAT5 activation in mouse HT22 cells. Brain Behav Immun. 2009;23:455–463. doi: 10.1016/j.bbi.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai W, Khaoustov VI, Xie Q, Pan T, Le W, Yoffe B. Interferon-alpha-induced modulation of glucocorticoid and serotonin receptors as a mechanism of depression. J Hepatol. 2005;42:880–887. doi: 10.1016/j.jhep.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 27.Pierucci-Lagha A, Covault J, Bonkovsky HL, Feinn R, Abreu C, Sterling RK, et al. A functional serotonin transporter gene polymorphism and depressive effects associated with interferon-alpha treatment. Psychosomatics. 2010;51:137–148. doi: 10.1176/appi.psy.51.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lotrich FE, Ferrell RE, Rabinovitz M, Pollock BG. Risk for depression during interferon-alpha treatment is affected by the serotonin transporter polymorphism. Biol Psychiatry. 2009;65:344–348. doi: 10.1016/j.biopsych.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu CB, Blakely RD, Hewlett WA. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology. 2006;31:2121–2131. doi: 10.1038/sj.npp.1301029. [DOI] [PubMed] [Google Scholar]
- 30.Zhu CB, Carneiro AM, Dostmann WR, Hewlett WA, Blakely RD. p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2A-dependent process. J Biol Chem. 2005;280:15649–15658. doi: 10.1074/jbc.M410858200. [DOI] [PubMed] [Google Scholar]
- 31.Zhu CB, Lindler KM, Owens AW, Daws LC, Blakely RD, Hewlett WA. Interleukin-1 Receptor Activation by Systemic Lipopolysaccharide Induces Behavioral Despair Linked to MAPK Regulation of CNS Serotonin Transporters. Neuropsychopharmacology. 2010 doi: 10.1038/npp.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raison CL, Woolwine BJ, Demetrashvili MF, Borisov AS, Weinreib R, Staab JP, et al. Paroxetine for prevention of depressive symptoms induced by interferon-alpha and ribavirin for hepatitis C. Aliment Pharmacol Ther. 2007;25:1163–1174. doi: 10.1111/j.1365-2036.2007.03316.x. [DOI] [PubMed] [Google Scholar]
- 33.Schwarcz R, Pellicciari R. Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J Pharmacol Exp Ther. 2002;303:1–10. doi: 10.1124/jpet.102.034439. [DOI] [PubMed] [Google Scholar]
- 34.Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D, et al. Interferon-alpha-induced changes in tryptophan metabolism. relationship to depression and paroxetine treatment. Biol Psychiatry. 2003;54:906–914. doi: 10.1016/s0006-3223(03)00173-2. [DOI] [PubMed] [Google Scholar]
- 35.Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, Artini M, et al. Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alpha-induced changes in the serotonergic system. J Clin Psychopharmacol. 2002;22:86–90. doi: 10.1097/00004714-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 36.Wichers MC, Koek GH, Robaeys G, Verkerk R, Scharpe S, Maes M. IDO and interferon-alpha-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicity. Mol Psychiatry. 2005;10:538–544. doi: 10.1038/sj.mp.4001600. [DOI] [PubMed] [Google Scholar]
- 37.Capuron L, Pagnoni G, Demetrashvili MF, Lawson DH, Fornwalt FB, Woolwine B, et al. Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacology. 2007;32:2384–2392. doi: 10.1038/sj.npp.1301362. [DOI] [PubMed] [Google Scholar]
- 38.Felger JC, Alagbe O, Hu F, Mook D, Freeman AA, Sanchez MM, et al. Effects of interferon-alpha on rhesus monkeys: a nonhuman primate model of cytokine-induced depression. Biol Psychiatry. 2007;62:1324–1333. doi: 10.1016/j.biopsych.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKinney WT, Jr, Eising RG, Moran EC, Suomi SJ, Harlow HF. Effects of reserpine on the social behavior of rhesus monkeys. Dis Nerv Syst. 1971;32:735–741. [PubMed] [Google Scholar]
- 40.McHutchison JG, Lawitz EJ, Shiffman ML, Muir AJ, Galler GW, McCone J, et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009;361:580–593. doi: 10.1056/NEJMoa0808010. [DOI] [PubMed] [Google Scholar]
- 41.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. J Ap[plied Psychol Measurement. 1977;1:385–401. [Google Scholar]
- 42.Unutzer J, Patrick DL, Marmon T, Simon GE, Katon WJ. Depressive symptoms and mortality in a prospective study of 2,558 older adults. Am J Geriatr Psychiatry. 2002;10:521–530. doi: 10.1097/00019442-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Chwastiak L, Ehde DM, Gibbons LE, Sullivan M, Bowen JD, Kraft GH. Depressive symptoms and severity of illness in multiple sclerosis: epidemiologic study of a large community sample. Am J Psychiatry. 2002;159:1862–1868. doi: 10.1176/appi.ajp.159.11.1862. [DOI] [PubMed] [Google Scholar]
- 44.Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol. 1977;106:203–214. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- 45.Robinson CM, Shirey KA, Carlin JM. Synergistic transcriptional activation of indoleamine dioxygenase by IFN-gamma and tumor necrosis factor-alpha. J Interferon Cytokine Res. 2003;23:413–421. doi: 10.1089/107999003322277829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robinson CM, Hale PT, Carlin JM. NF-kappa B activation contributes to indoleamine dioxygenase transcriptional synergy induced by IFN-gamma and tumor necrosis factor-alpha. Cytokine. 2006;35:53–61. doi: 10.1016/j.cyto.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 47.Pemberton LA, Kerr SJ, Smythe G, Brew BJ. Quinolinic acid production by macrophages stimulated with IFN-gamma, TNF-alpha, and IFN-alpha. J Interferon Cytokine Res. 1997;17:589–595. doi: 10.1089/jir.1997.17.589. [DOI] [PubMed] [Google Scholar]
- 48.Fujigaki H, Saito K, Fujigaki S, Takemura M, Sudo K, Ishiguro H, et al. The signal transducer and activator of transcription 1alpha and interferon regulatory factor 1 are not essential for the induction of indoleamine 2,3-dioxygenase by lipopolysaccharide: involvement of p38 mitogen-activated protein kinase and nuclear factor-kappaB pathways, and synergistic effect of several proinflammatory cytokines. J Biochem. 2006;139:655–662. doi: 10.1093/jb/mvj072. [DOI] [PubMed] [Google Scholar]
- 49.Mellor AL, Munn D, Chandler P, Keskin D, Johnson T, Marshall B, et al. Tryptophan catabolism and T cell responses. Adv Exp Med Biol. 2003;527:27–35. doi: 10.1007/978-1-4615-0135-0_3. [DOI] [PubMed] [Google Scholar]
- 50.Owens MJ, Nemeroff CB. Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin Chem. 1994;40:288–295. [PubMed] [Google Scholar]
- 51.Flores BH, Musselman DL, DeBattista C, Garlow SJ, Schatzberg AF, Nemeroff CB. Biology of Mood Disorders. In: Schatzberg AF, Nemeroff CB, editors. Textbook of Psychopharmacology. 3. America Psychiatric Publishing, Inc; Washington DC: 2004. pp. 717–763. [Google Scholar]
- 52.O’Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2008 doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Connor JC, Lawson MA, Andre C, Briley EM, Szegedi SS, Lestage J, et al. Induction of IDO by bacille Calmette-Guerin is responsible for development of murine depressive-like behavior. J Immunol. 2009;182:3202–3212. doi: 10.4049/jimmunol.0802722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maes M, Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, et al. Treatment with interferon-alpha (IFN alpha) of hepatitis C patients induces lower serum dipeptidyl peptidase IV activity, which is related to IFN alpha-induced depressive and anxiety symptoms and immune activation. Mol Psychiatry. 2001;6:475–480. doi: 10.1038/sj.mp.4000872. [DOI] [PubMed] [Google Scholar]
- 55.Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry. 2002;7:468–473. doi: 10.1038/sj.mp.4000995. [DOI] [PubMed] [Google Scholar]
- 56.Schroecksnadel K, Fiegl M, Prassl K, Winkler C, Denz HA, Fuchs D. Diminished quality of life in patients with cancer correlates with tryptophan degradation. J Cancer Res Clin Oncol. 2007;133:477–485. doi: 10.1007/s00432-007-0191-3. [DOI] [PubMed] [Google Scholar]
- 57.Fuchs D, Moller AA, Reibnegger G, Stockle E, Werner ER, Wachter H. Decreased serum tryptophan in patients with HIV-1 infection correlates with increased serum neopterin and with neurologic/psychiatric symptoms. J Acquir Immune Defic Syndr. 1990;3:873–876. [PubMed] [Google Scholar]
- 58.Vecsei L, Beal MF. Influence of kynurenine treatment on open-field activity, elevated plus-maze, avoidance behaviors and seizures in rats. Pharmacol Biochem Behav. 1990;37:71–76. doi: 10.1016/0091-3057(90)90043-h. [DOI] [PubMed] [Google Scholar]
- 59.Widner B, Laich A, Sperner-Unterweger B, Ledochowski M, Fuchs D. Neopterin production, tryptophan degradation, and mental depression--what is the link? Brain Behav Immun. 2002;16:590–595. doi: 10.1016/s0889-1591(02)00006-5. [DOI] [PubMed] [Google Scholar]
- 60.Saito K, Crowley JS, Markey SP, Heyes MP. A mechanism for increased quinolinic acid formation following acute systemic immune stimulation. J Biol Chem. 1993;268:15496–15503. [PubMed] [Google Scholar]
- 61.Guillemin GJ, Smythe G, Takikawa O, Brew BJ. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia. 2005;49:15–23. doi: 10.1002/glia.20090. [DOI] [PubMed] [Google Scholar]
- 62.Guillemin GJ, Smith DG, Smythe GA, Armati PJ, Brew BJ. Expression of the kynurenine pathway enzymes in human microglia and macrophages. Adv Exp Med Biol. 2003;527:105–112. doi: 10.1007/978-1-4615-0135-0_12. [DOI] [PubMed] [Google Scholar]
- 63.Rios C, Santamaria A. Quinolinic acid is a potent lipid peroxidant in rat brain homogenates. Neurochem Res. 1991;16:1139–1143. doi: 10.1007/BF00966592. [DOI] [PubMed] [Google Scholar]
- 64.Guillemin GJ, Wang L, Brew BJ. Quinolinic acid selectively induces apoptosis of human astrocytes: potential role in AIDS dementia complex. J Neuroinflammation. 2005;2:16. doi: 10.1186/1742-2094-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guillemin GJ, Brew BJ, Noonan CE, Takikawa O, Cullen KM. Indoleamine 2,3 dioxygenase and quinolinic acid Immunoreactivity in Alzheimer’s disease hippocampus. Neuropathol Appl Neurobiol. 2005;31:395–404. doi: 10.1111/j.1365-2990.2005.00655.x. [DOI] [PubMed] [Google Scholar]
- 66.Guillemin GJ, Meininger V, Brew BJ. Implications for the kynurenine pathway and quinolinic acid in amyotrophic lateral sclerosis. Neurodegener Dis. 2005;2:166–176. doi: 10.1159/000089622. [DOI] [PubMed] [Google Scholar]
- 67.Guidetti P, Schwarcz R. 3-Hydroxykynurenine and quinolinate: pathogenic synergism in early grade Huntington’s disease? Adv Exp Med Biol. 2003;527:137–145. doi: 10.1007/978-1-4615-0135-0_16. [DOI] [PubMed] [Google Scholar]
- 68.Heyes MP, Saito K, Crowley JS, Davis LE, Demitrack MA, Der M, et al. Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain. 1992;115 (Pt 5):1249–1273. doi: 10.1093/brain/115.5.1249. [DOI] [PubMed] [Google Scholar]
- 69.Wu HQ, Rassoulpour A, Schwarcz R. Kynurenic acid leads, dopamine follows: a new case of volume transmission in the brain? J Neural Transm. 2007;114:33–41. doi: 10.1007/s00702-006-0562-y. [DOI] [PubMed] [Google Scholar]
- 70.Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, et al. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry. 2010;15:393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]