Abstract
Modulation of TASK-3 (Kcnk9) potassium channels affect neurotransmitter release in thalamocortical centers and other sleep-related nuclei having the capacity to regulate arousal cycles and REM sleep changes associated with mood disorders and antidepressant action. Circumstantial evidence from this and previous studies suggest the potential for TASK-3 to be a novel antidepressant therapeutic target; TASK-3 knock-out mice display augmented circadian amplitude and exhibit sleep architecture characterized by suppressed REM activity. Detailed analysis of locomotor activity indicate that the amplitude of activity bout duration and bout number are augmented in TASK-3 mutants well beyond that seen wildtypes, findings substantiated by amplitude increases in body temperature and EEG recordings of sleep stage bouts. Polysomnographic analysis of TASK-3 mutants reveal increases in nocturnal active wake and suppressed REM sleep time while increased slow wave sleep typifies the inactive phase, findings that have implications for the cognitive impact of reduced TASK-3 activity. In direct measures of their resistance to despair behavior, TASK-3 knock-outs displayed significant decreases in immobility relative to wildtype controls in both tail suspension and forced swim tests. Treatment of wildtype animals with the antidepressant Fluoxetine markedly reduced REM sleep, while leaving active wake and slow wave sleep relatively intact. Remarkably, these effects were absent in TASK-3 mutants indicating that TASK-3 is either directly involved in the mechanism of this drug’s action, or participates in parallel pathways that achieve the same effect. Together, these results support the TASK-3 channel to act as a therapeutic target for antidepressant action.
Keywords: Kcnk9, TASK-3, sleep, polysomnography, Major Depressive Disorder, circadian
1. INTRODUCTION
Two-pore potassium channels have the capacity to regulate the activity of neuronal pathways by influencing the resting membrane potential of neurons on which they are expressed. TASK-3 is a member of the two-pore-domain K+ channel family (K2p, KCNK) that includes TWIK (tandem P-domain weak inward rectifying K+) and TASK (TWIK-related acid-sensitive K+) channels. TASK-1 (KCNK3 or K2P3.1) and TASK-3 (KCNK9 or K2P9.1) channels have the greatest homology within this family, sharing greater than 50% amino acid identity (Coetzee et al., 1999; Goldstein et al., 2001; Lesage, 2003; Talley et al., 2001). Decreased K+ conductance through these channels depolarize neuron resting potential resulting in an increase in neuronal excitability. An antagonist of these channels has the capacity to selectively activate a particular neuronal pathway. Because TASK channels are also inhibited by acidic pH within the physiological range (Duprat et al., 1997), they are also well positioned to mediate central responses to respiratory and metabolic changes (Bayliss et al., 2001; Buckler et al., 2000).
The CNS expression of TASK-3 suggests potential roles in mood disorders, sleep/wake control and cognition. It is particularly abundant in hippocampus, cerebellum and cortex and in specific nuclei including the Locus Coeruleus, Paraventricular nuclei of Thalamus and the Dorsal Raphe (Talley et al., 2001). TASK-3 channel activity has been found to regulate both neurotransmitter release and to mediate the effects of neurotransmitter activation. 5-HT mediated GABA release by entorhinal cortical neurons occurs through inhibition of TASK-3 (Deng and Lei, 2008). The channel has also been found to contribute to thalamocortical neuron activity involved in regulating sleep stage and cognition (Meuth et al., 2003; Pang et al., 2009), and regulates the activity of 5-HT releasing neurons of the Dorsal Raphe (Washburn et al., 2002).
Disruption of the gene encoding TASK-3 has been observed to be associated with behavioral and neurophysiological alterations. Probably the most salient phenotype is an increase in active phase locomotor activity that is not apparent in the inactive phase or during exploratory behavior associated with a novel environment (Linden et al., 2007). Deficits in cognitive behavior of TASK-3 mutants have also been seen in a T-maze spontaneous alternation task of working memory, and a slight but overall significant impairment in spatial memory as measured in the Morris water maze (Linden et al., 2007). These findings are consistent with the identification of a human KCNK9 gene mutation responsible for mental retardation associated with a rare maternally transmitted dysmorphism syndrome (Barel et al., 2008). The effects of some anesthetics such as halothane are also blunted in TASK-3 knock-outs (Linden et al., 2007) that appears to be due to a direct activation of TASK-3 channel activity (Meadows and Randall, 2001; Patel et al., 1999; Talley and Bayliss, 2002).
Many of these behavioral changes are associated with altered qEEG spectra and sleep architecture, particularly alterations in theta power and REM sleep. Type I, or “exploratory” theta oscillations (6–12 Hz) appear intact in TASK-3 mutant animals (Pang et al., 2009), consistent with normal exploratory responses to a novel environment. On the other hand, Type II theta activity (6–12 Hz), typically associated with sensory stimuli processing prior to motor induction and induced by inhalation anesthetics, is disrupted in animals lacking TASK-3 (Pang et al., 2009), directly correlating with altered anesthetic responses and cognitive performance. Further, both REM-associated theta oscillations and REM sleep time are suppressed for intervals of time in TASK-3 knock-outs in favor of increased active wake time (Pang et al., 2009).
In the present study, we further evaluated TASK-3 mutant animals, and identified a phenotype characterized by substantial circadian amplitude increases in locomotor activity parameters, body temperature and EEG activity, as well as extensive active phase REM suppression—responses characteristic of elevated mood and antidepressant action. Human depressive patients exhibit decreased amplitude in diurnal/nocturnal sleep/wake cycles associated with inactive phase sleep disturbances and active phase lethargy which are reflected by decreases in daily amplitude of core temperature as well as secretion of cortisol and melatonin, changes that normalize during remission and antidepressant action (Duval et al., 2006; Ford and Kamerow, 1989; Rubin et al., 1992; Szuba et al., 1997; Wirz-Justice, 2006). Electroconvulsive shock treatment of depressed patients and in animal models is also associated with increases in day/night circadian amplitude without affecting circadian timing (e.g. free running period or phase shifting responses to light) (Angles-Pujolras et al., 2009; Szuba et al., 1997). Another hallmark of major depressive disorder (MDD) in human patients is an elevated propensity toward REM sleep, including diminished latency to REM, increased REM density and increased mean time in REM (Steiger and Kimura, 2010), as well as coincident decreases in delta qEEG power occurring during the inactive phase (Borbely et al., 1984; Kupfer et al., 1986). These effects are largely recapitulated in animal models of depression where elevated REM sleep is associated with despair behavior in distinct genetic mouse strains (El Yacoubi et al., 2003), and with stress-induced depressive behavior that track with elevated levels of corticosterone (Dugovic et al., 1999; Dugovic et al., 2000; Touma et al., 2009). Conversely, REM suppression is commonly associated with antidepressant treatment, including monoamine reuptake inhibitors such as the selective serotonin reuptake inhibitor (SSRI), Fluoxetine (Pastel and Fernstrom, 1987; Slater et al., 1978), as well as non-pharmacological treatments including total sleep deprivation (Riemann and Berger, 1990) and electroconvulsive shock therapy (Grunhaus et al., 1997). Although REM suppression is an accepted biomarker for depression and does not necessarily underlie its etiology, selective REM deprivation for 3 weeks in human subjects is associated with the appearance of anti-depressive effects (Vogel et al., 1975).
Here, we substantiate TASK-3 as a target for antidepressant action by characterizing the phenotype of mice lacking the TASK-3 channel. In addition to increases in the amplitude of diurnal/nocturnal oscillations in locomotor activity and temperature, these mutant animals also display prolonged periods of REM suppression. Further, TASK-3 knock-outs also display resistance to despair behavior in tail suspension and forced swim tests. Remarkably, the REM suppressing effects of Fluoxetine observed in wildtype animals were greatly reduced in TASK-3 mutants indicating that the channel can mediate antidepressant action by either direct or parallel mechanisms.
2. RESULTS
2.1 Augmented Circadian Amplitude of TASK-3 Knock-outs
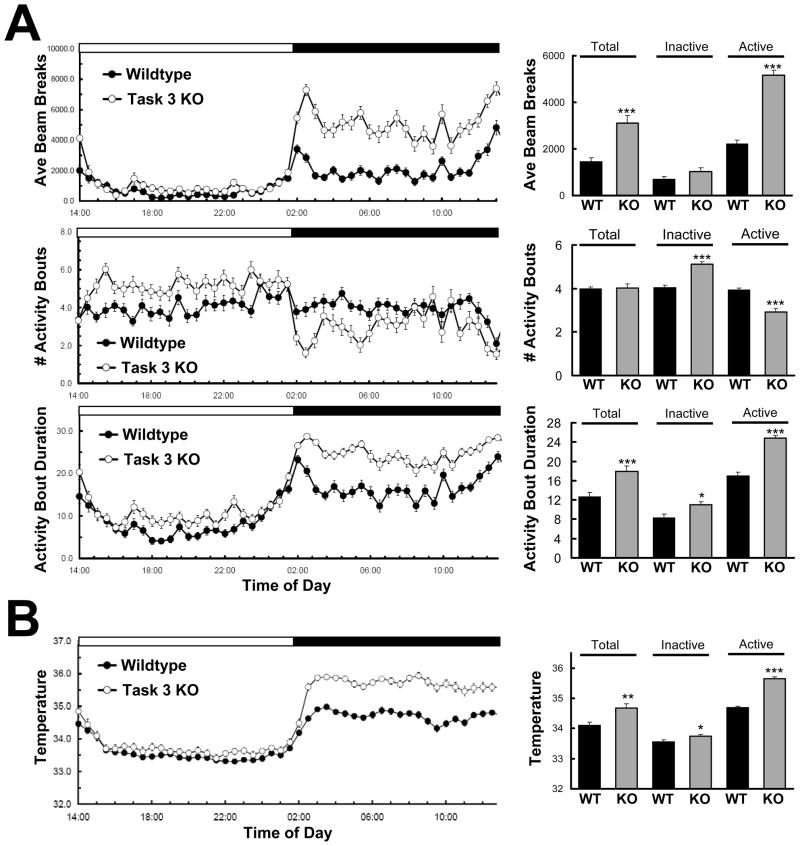
Detailed evaluation of the locomotor activity of TASK-3 mutants not only illustrates active phase hyperactivity, but an overall increase in circadian amplitude of these animals relative to wildtype animals. Home cage activity monitored continuously by infrared beam breaks over 5 days showed that TASK-3 mutants had a significant increase in average beam breaks relative to wildtype controls over a full 24 hour period (Fig. 1A, top panel) both in their time course of activity (F1,3666=162.8, P <0.001 [2-way ANOVA]) and in cumulative average beam breaks (p < 0.001, t test for repeated measures). This difference is largely due to enhanced nocturnal activity which was 2.3 fold higher in mutants (p < 0.001, t test for repeated measures), while inactive phase activity was similar to that of wildtype animals (p = 0.083, t test for repeated measures), consistent with previous observations (Linden et al., 2007). Activity bout analysis revealed further day/night differences. TASK-3 mutants show a clear distinction between the diurnal and nocturnal pattern of activity bout number, where short but numerous activity bouts mark the inactive phase while the active phase is characterized by fewer bouts of longer duration (Fig. 1A, lower panels). Although a difference in the time course of the number of activity bouts of wildtype versus TASK-3 knock-outs did not reach significance by 2-way ANOVA (F1,3666=0.05, P=0.8267), quantification of inactive and active phase periods revealed significant increases and decreases in bout number (P<0.001, t test for repeated measures), respectively.
Figure 1.
TASK-3 knock-outs display enhanced amplitude in diurnal locomotor activity and temperature. A. Locomotor assessment by infrared beam break of wildtype (filled symbols, n=8) and TASK-3 knock-outs (open symbols, n=8) averaged from 5 days of continuous monitoring under home cage conditions. Average beam breaks as well as the number and duration of activity bouts (± SEM) from 30 minute intervals are plotted over a 24 hour time course (traces, left). Activity bouts are defined as consecutive 30 second scoring epochs in which 5 beams were broken. Lights on occurred at 14:00 and lights off at 02:00 as indicated by open and dark filled horizontal bars above graphs. TASK-3 knock-outs differed from wildtypes in both total beams broken and activity bout duration (2-way ANOVA: F1,3666=162.8, P <0.0001, F1,3666 = 235.28, P <0.0001, respectively), but not in number of beams broken (F1,3666=0.05, P=0.8267). Average beam breaks/30 minute interval are quantified for total 24 hour period, inactive (light) phase only, or active (dark) phase only (bars, right). *, ***, p < 0.05, 0.001, respectively (unpaired t test for repeated measures). B. Subcutaneous temperature of wildtype (filled symbols, n=7) and TASK-3 knock-outs (open symbols, n=8) monitored continuously over 5 days of baseline home cage conditions. Wildtype and mutant animals exhibited significant differences in their time course of temperature variation (traces, left) over 24 hours (F1,3358=37.04, P < 0.0001) and during the active phase (F1,1606=3.04, P < 0.001), but not during the inactive phase (F1,1679=0.71, P<0.8379). Average temperatures also quantified for the total 24 hour period, inactive and active phases (bars, right). *, **, ***, p < 0.05, 0.01, 0.001, respectively (unpaired t test for repeated measures).
Increases in circadian amplitude were also observed in body temperature measurements, reflecting changes in locomotor activity and general metabolic rate (Fig. 1B). Once again, the greatest differences occurred in the active phase, where knock-out animals exhibited an average 0.96 °C increase over wild types (p < 0.001), while the average difference during the inactive phase was a mere 0.18 °C (p = 0.046).
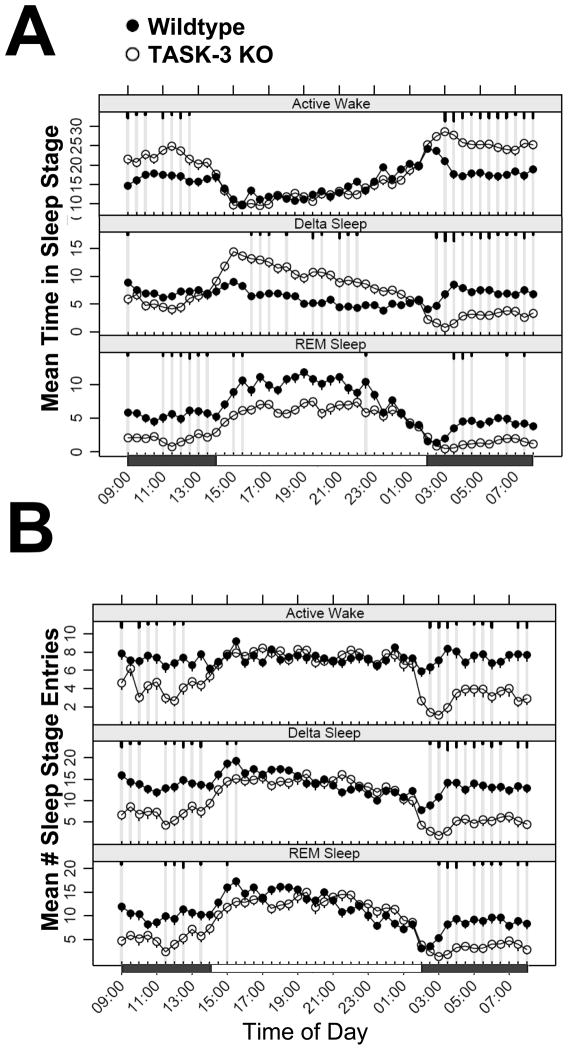
Polysomnographic comparison of wildtype and TASK-3 knock-outs substantiates the enhanced circadian amplitude observed in locomotor and temperature experiments and is consistent with that previously reported (Pang et al., 2009). Continuous ECoG/EMG recording revealed that TASK-3 knock-out mice spend more time in active wake accompanied by decreases in delta sleep time and REM suppression during the active phase relative to wildtype animals (Fig. 2A). Conversely, during the inactive phase, TASK-3 mutants display little difference with wildtype animals in active wake, but do exhibit increases in delta, or slow wave sleep (SWS) accompanied by variable decreases in REM. A striking difference between TASK-3 mutants and wildtype animals is an increase in the daily oscillation in the number of entries into all sleep states, the greatest differences occurring during the active phase where decreases are seen in knock-out animals relative to controls (Fig. 2B). These changes are indicative of an increase in sleep state bout duration and are consistent with the locomotor activity changes discussed above. Together these results demonstrate that animals lacking TASK-3 display an increase in diurnal/nocturnal behavioral amplitudes, which not only suggests TASK-3 as a therapeutic target for antidepressant action, but also has implications for other behavioral assays.
Figure 2.
TASK-3 knock-outs show increased amplitude in sleep EEG architecture. Baseline ECoG/EMG of Wildtype (closed symbols, n=5) and TASK-3 homozygous mutant (open symbols, n=4) mice was monitored continuously over 16 days, and scored for both mean time spent in Active Wake, Delta and REM sleep (upper panel) as well as the number of entries into these sleep stages during 30 minute intervals. Each value is the 16 day mean ± SEM plotted on a single 24 hour period. Time points exhibiting significant differences from wildtype are highlighted by grey lines with short, medium and long tics marks representing p values ≤ 0.05, 0.01, ≤ 0.001 (linear mixed effects model for repeated measures).
2.2 Altered Cognitive Performance of TASK-3 Mutants
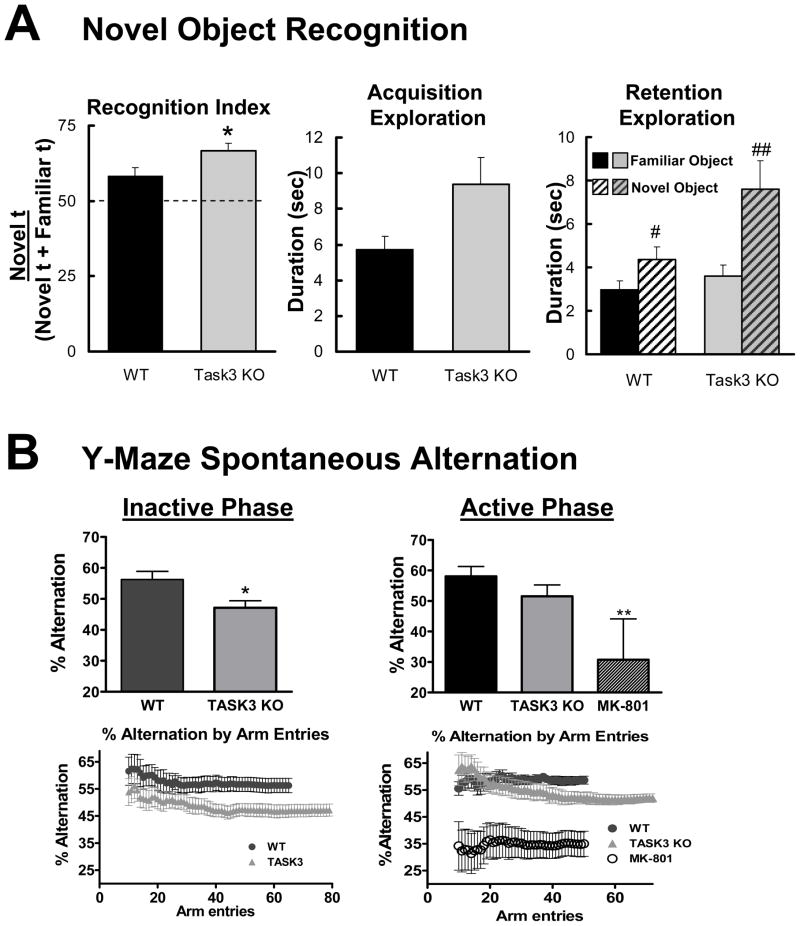
Previous genetic studies have suggested an association between cognitive impairment and loss of TASK-3 activity (Barel et al., 2008; Linden et al., 2007). The ability of wildtype and TASK-3 mutants to recall and discriminate a familiar object from a novel one was tested during the inactive or light phase, a time at which locomotor activity of these genotypes is comparable. Three hours after an initial acquisition trial, both groups explored a novel object significantly more than a familiar one, thus displaying recognition performance above the chance level (wildtype average, 58.3%, p = 0.014; knock-out average, 66.5% p < 0.0001, one tailed t test) (Fig. 3A, left, right panels). TASK-3 mutants, however, showed significantly improved recognition performance relative to wildtypes (p = 0.042). Such improvement of performance may be linked to a general increase in object exploration as TASK-3 mutants displayed a trend toward increased exploration during acquisition (p=0.059, Fig. 3A, center panel).
Figure 3.
TASK-3 knock-outs display changes in cognitive behavior. A. Novel object recognition assessment of wildtype (n=10) and TASK-3 mutants (n=12). Recognition index = (time spent exploring a novel object)/(total time spent exploring both novel and familiar objects). *, p < 0.05 (unpaired, two tailed t test) relative to wildtype; #, ##, p < 0.05, 0.01 (paired t test) relative to familiar object condition. B. Y-maze spontaneous alternation of wildtype (n=13) and TASK-3 mutants (n=13) during the Inactive phase (left panels) and Active phase (right panels). Upper panels show overall % correct alternation trios during the 7.5 minute assessment. Bottom panels plot the correct alternation % relative to arm entries during the 7.5 minute trial. Note that TASK-3 knock-outs exhibit an increased number of arm entries in the 7.5 minute assessment period. *, **, p < 0.05, 0.01 (unpaired t test) relative to wildtype condition.
In the Y-maze spontaneous alternation task performed during the inactive phase, TASK-3 knock-outs displayed decreased performance relative to wildtypes (Fig. 3B, left panel), consistent with a previous T-maze study performed at a similar time (Linden et al., 2007). During the current 7.5 minute test, wildtypes performed the correct trio alternation 56.2% of the time while mutants were significantly impaired, performing at 47.1% (p = 0.015, p < 0.05, unpaired t test). The increased circadian amplitude in locomotor activity, core temperature and polysomnography measures, however, suggest that these changes could be subject to differences in nocturnal versus diurnal performance. The same test performed during the active phase yielded different results, where TASK-3 mutants did not significantly differ from wildtype controls in overall performance during the 7.5 minute test (Fig. 3B, right panel; 51.6±1.6% vs 58.1±1.5%, respectively; p < 0.05, 1-way ANOVA, Tukey’s post-hoc test), while the positive control, MK-801 showed a significant decrease in performance (30.7±21.4%; p < 0.05, 1-way ANOVA, Tukey’s post-hoc test). Examination of performance relative to arm entries revealed that TASK-3 knock-outs display an early increase in successful alternation during the initial 10 to 20 arm entries of the assessment.
2.3 TASK-3 knock-outs Display Resistance to Despair Behavior
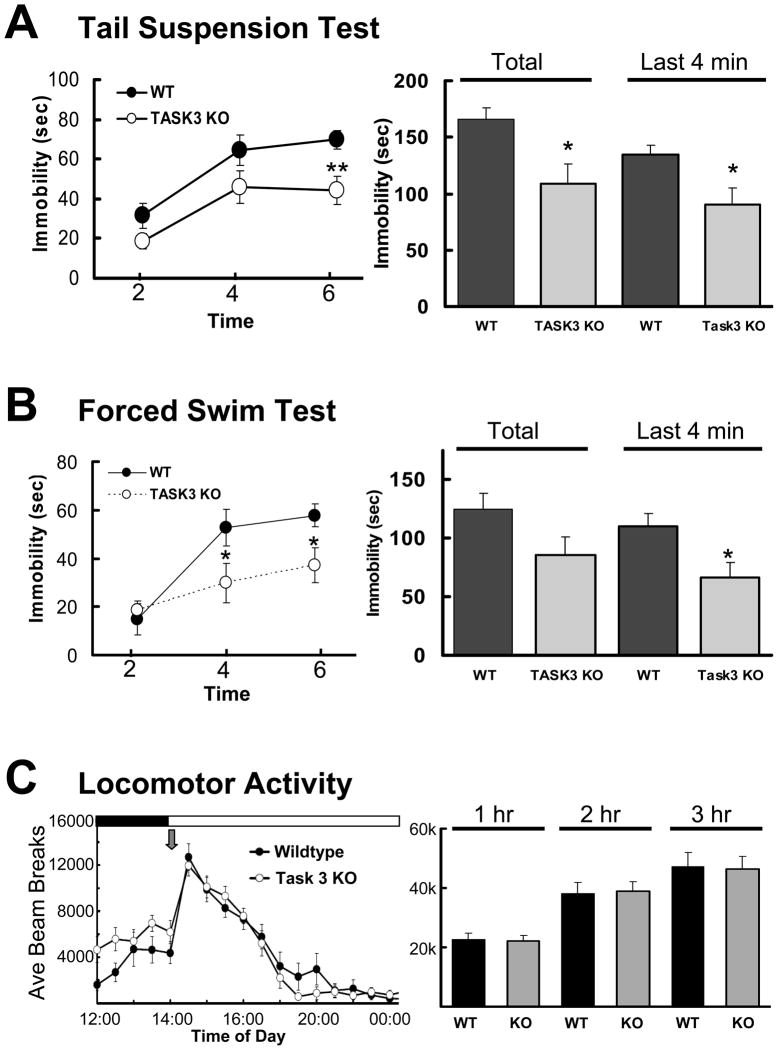
The increased circadian amplitude and REM suppression observed in the experiments above suggest the involvement of TASK-3 channel activity in depressive behavior. Resistance to despair behavior was evaluated in the tail suspension test administered during the inactive phase, a time at which locomotor differences between wildtype and mutants are minimal (Fig. 4A). Total immobility, indicative of despair behavior, was significantly reduced in TASK-3 mutants over the entire 6 minute period of the test as well as the last 4 minutes, where the average duration of immobility was reduced from 134.2±8.8 to 90.2±14.8 seconds (p = 0.025).
Figure 4.
TASK-3 mutants exhibit resistance to despair behavior. A. Tail suspension test assessment of wildtype (n=10) and TASK-3 knock-out (n=12) animals. Mice were suspended by their tails from motion-sensitive pressure transducers for an evaluation period of six minutes during the inactive phase. Mean time (in seconds) of immobility was calculated for each group and plotted at two minute intervals and the mean immobility for the total experiment and for the last 4 minutes of the experiment (right) (*, **, p < 0.05, 0.01, unpaired t test). B. Forced swim test evaluation of wildtype (n=10) and TASK-3 mutants (n=12) placed into a room temperature water bath for 6 minutes and visually scored for struggling versus minimal floating behavior. Mean time (in seconds) of immobility was calculated for each group and plotted at two minute intervals. The mean immobility for the total experiment and for the last 4 minutes of the experiment (right) are also shown (*, **, p < 0.05, 0.01, unpaired t test). C. Locomotor activity following introduction of novel environment (grey arrow). Wildtype (n=8) and Task 3 knock-out (n=8) animals were subjected to a fresh cage change during which locomotor activity was assessed by infrared beam break and averaged over 30 minute intervals (left panel) or quantified over 1, 2 or 3 hours following cage change. No statistical differences were observed at 30 minute intervals (2-way ANOVA) or during 1, 2 or 3 hours following treatment (t test).
TASK-3 knock-outs also displayed attenuated despair behavior in the forced swim test. In this case, both groups of animals struggled to a similar extent during the initial two minutes of the test, but by the fourth and six minutes, mutants were significantly less immobile such that TASK-3 knock-outs exhibited an average reduction in floating activity from 110.3±10.9 to 66.8±12.4 seconds (p = 0.018) over the last 4 minutes of the test (Fig. 4B). Reduced immobility in both tail suspension and forced swim tests does not appear to be due to a general increase in locomotor activity since these studies were done during the inactive phase, a time at which both genotypes exhibit similar activity, and given nearly identical behavior following the introduction of a novel environment (Fig. 4C). Overall, these behavioral studies indicate the potential for TASK-3 to be involved despair behavior and its potential as an antidepressant therapeutic target.
2.4 TASK-3 Mutants Exhibit Altered EEG responses to the Antidepressant, Fluoxetine
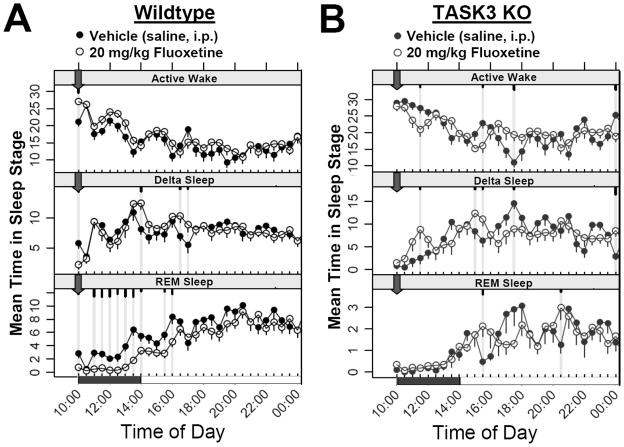
A hallmark of antidepressant interventions ranging from total sleep deprivation to pharmacological treatments is the suppression of REM sleep (Pastel and Fernstrom, 1987; Slater et al., 1978; Steiger and Kimura, 2010). In wildtype animals, treatment with 20 mg/kg intraperitoneal (i.p.) Fluoxetine four hours prior to the inactive phase (ZT 20:00) reduced REM sleep for up to 6 hours following administration (Fig. 5A). This effect was relatively specific for REM sleep since no sustained changes in active wake or slow wave sleep were observed in these animals. In TASK-3 knock-out animals, however, Fluoxetine was ineffective in suppressing REM even after the inactive phase onset when REM sleep began increasing to more elevated levels (Fig. 5B). These results suggest that either the TASK-3 channel is involved in the mechanism of the action of Fluoxetine in suppressing REM sleep, or that TASK-3 mutants exhibit minimal basal levels of REM sleep available for Fluoxetine to act. As seen previously, TASK-3 mutants do exhibit reduced REM activity relative to wildtype animals (see Fig. 2). Regardless of the direct or parallel mechanism, however, these results suggest that TASK-3 is an attractive target for antidepressant action.
Figure 5.
REM suppressing effects of the antidepressant Fluoxetine are altered in TASK-3 knock-out animals. Continuous ECoG/EMG recordings from radio telemetry implanted wildtype (n = 5) and TASK-3 mutants (n = 4) were used to evaluate the polysomnographic responses to either vehicle (saline, i.p.; closed circles) or 20 mg/kg Fluoxetine (open circles). A 5 day balanced cross-over paradigm was used to evaluate the response to daily treatment administered at 10:00 (ZT 20:00, block arrow). In each group, time points exhibiting significant differences between vehicle and Fluoxetine treatment conditions are highlighted by grey lines with short, medium and long tics marks representing p values ≤ 0.05, 0.01, ≤ 0.001 (linear mixed effects model for repeated measures).
3. DISCUSSION
The most salient indication that TASK-3 has the capacity to regulate mood is an increase in circadian amplitude of TASK-3 mutant animals. In the present studies, this is exemplified by active phase increases in arousal as measured by sleep EEG, locomotor activity, activity bout duration and body temperature. These changes are not simply a general increase in arousal as these measures were largely unchanged by the mutation during the inactive phase. One exception was an increase in the number of locomotor activity bouts, which in wildtype animals remains at a consistent level throughout the light and dark periods, but does oscillate with a significant amplitude in TASK-3 mutants. Substantiating these results is an increase in the nocturnal/diurnal amplitude in the number of sleep stage entries measured in sleep EEG of knock-outs relative to wildtype animals. These observations are consistent with a role for TASK-3 in regulating mood such that inhibition of the channel has the potential to have antidepressant effects, since both patients with MDD and despair-related animal models exhibit diminished amplitude in diurnal to nocturnal behavior, physiology and hormonal secretion that are reversed by antidepressant treatment (Duval et al., 2006; Ford and Kamerow, 1989; Rubin et al., 1992; Wirz-Justice, 2006). It is worth noting that changes in circadian amplitude of TASK-3 mutants were not associated with alterations in circadian timing. Under constant dim red light conditions, TASK-3 knock-outs were unchanged relative to wildtype animals in their free running period of running wheel activity and in their responses to phase delaying or advancing light pulses was unchanged relative to wildtype animals (not shown). This lack of an effect on bone fide circadian rhythms does not exclude a role for the channel as an antidepressant target since changes in circadian amplitude associated with changes in mood have not been observed to be accompanied by changes in circadian timing measured under constant lighting conditions (Angles-Pujolras et al., 2009; Szuba et al., 1997).
The increase in circadian amplitude exhibited by TASK-3 mutants has important implications for the interpretation of results obtained for behavioral models such as those testing despair behavior and cognitive performance. It is standard practice to administer and validate most all of these tests during the early to mid light phase during a nocturnal rodent’s inactive phase, but time-of-day differences in performance may be expected. The current study evaluated the resistance to despair behavior in tail suspension and forced swim tests administered during the inactive phase to 1) remain consistent with standard protocols and prior validation experiments and 2) to avoid a potential locomotor confound that might be expected of TASK-3 mutants during their hyperactive nocturnal period. Deficits in spatial and working memory tasks were previously observed in the Morris water maze and T-maze spontaneous alternation tasks administered during the inactive phase (Linden et al., 2007), consistent with the Y-maze deficit observed during the inactive phase of the present experiments. Although a comprehensive evaluation of the validity of cognition models throughout the circadian period as well as the cognitive potential of TASK-3 mutants is beyond the scope of the present study, the potentiated attention memory of TASK-3 animals in the novel object recognition task and the altered responses of mutants in the Y-maze spontaneous alternation task administered during the active phase indicate that constitutive genetic ablation of TASK-3 channel activity may not equally affect all cognition domains throughout the day, particularly during the active phase most relevant to cognition in humans. Acute pharmacological perturbation of TASK-3 with a targeted therapeutic, however, may not be expected to generate the same effects as a constitutive genetic mutation having the potential to disrupt neuronal development. Nevertheless, cognitive impairment is a potential contraindication that needs to be closely monitored for any antidepressant therapeutics targeting this channel. Our results suggest, however, that short half-life compounds (<12 hours) administered during the early active phase to restrict the activity of these therapeutics to waking hours may provide a mechanism to alleviate this potential contraindication.
The sleep architecture of animals lacking TASK-3 channels has several antidepressive characteristics. As might be expected from locomotor and temperature results, TASK-3 knock-outs exhibit far greater day/night variations in time spent in active wake and delta sleep as well as substantial oscillations in the number of entries into all sleep stages as suggested from locomotor activity bout analysis. Additionally, REM sleep time showed reductions across most of the circadian period with the greatest number of significant points occurring during the active phase. These active phase results are generally consistent with previous observations (Pang et al., 2009) and antidepressant potential. Human MDD patients exhibit a general disinhibition of REM, exemplified by increased mean time and transitions into REM as well as shortened REM latency (Steiger and Kimura, 2010), similar to animal models prone to despair behavior (Dugovic et al., 1999; Dugovic et al., 2000; El Yacoubi et al., 2003; Touma et al., 2009). In this regard, the loss of TASK-3 channel activity resembles REM suppression associated with both pharmacological, behavioral and electroconvulsive antidepressant treatments (Grunhaus et al., 1997; Pastel and Fernstrom, 1987; Riemann and Berger, 1990; Slater et al., 1978). TASK-3 mutants also exhibit elevated delta sleep during the inactive phase which is also consistent with antidepressant potential since MDD patients exhibit an opposite propensity toward decreased inactive phase delta power (Borbely et al., 1984; Kupfer et al., 1986). In the current studies, the SSRI Fluoxetine administered during the late active phase induced a specific suppression of REM time with limited effects on active wake or delta sleep in wildtype animals, consistent with previous reports in rats (Pastel and Fernstrom, 1987), while animals lacking TASK-3 exhibited no further reduction in REM relative to the vehicle treatment. Although tempting to interpret this result to indicate that TASK-3 is involved directly in the mechanism of Fluoxetine action, it remains possible that TASK-3 functions through a parallel pathway where loss of TASK-3 activity affects REM in a manner similar to the antidepressant, since baseline REM levels of TASK-3 knockouts are reduced even in the absence of the drug. It is also unlikely that Fluoxetine acts directly on the TASK-3 channel; the channel only exhibits 31.4% inhibition in the presence of 100 μM Fluoxetine in vitro while the related TREK-1 (Kcnk2) channel shows 84.1% inhibition under the same conditions (Kennard et al., 2005). Regardless of the specific mechanism, however, these results taken with observations of reduced despair susceptibility observed in tail suspension and forced swim tests of TASK-3 mutant animals illustrates the potential for this channel, and possibly other two-pore channels, as a target for antidepressant action.
Substantial evidence has also linked TREK-1 to a role in MDD, antidepressant action and resistance to despair. This mechano-activated two-pore K+ channel is also expressed in dorsal raphe nuclei, amygdalo-hypothalamic area and paraventricular nuclei where it modulates 5-HT signaling, influencing stress responses, cognition and resistance to despair behavior (Heurteaux et al., 2006; Honore, 2007). Analysis of single nucleotide polymorphisms in the vicinity of the human KCNK2 gene identified significant associations not only with MDD, but also resistance to antidepressant treatment and potentially responses to rewarding stimuli (Dillon et al., 2010; Liou et al., 2009; Perlis et al., 2008). TREK-1 knock-outs show decreased stress-induced plasma corticosterone levels, potentiated 5-HT responses, resistance to despair behavior in forced swim, tail suspension and conditioned suppression of motility tests, and exhibit altered Fluoxetine responses (Heurteaux et al., 2006; Kennard et al., 2005). These findings substantiate the role of two-pore channels to mediate stress and reward pathways affecting mood.
4. Conclusions
TASK-3 channels are well positioned to regulate the membrane excitability and neurotransmitter responses in key nuclei to modulate sleep wake cycles, cognition and mood. Here we demonstrate that animals lacking functional TASK-3 channels display substantially elevated circadian amplitude in locomotor activity and activity bout number and duration, body temperature and sleep architecture. These day/night alterations in mutant animals are not only changes in cognitive measures, but also resistance to despair behavior. Further, the REM suppressing effects of Fluoxetine are absent in TASK-3 knock-outs. Together with previous studies substantiating the potential of specific two-pore K+ channels to affect mood, cognition and reward pathways, these studies identify TASK-3 as a novel target for anti-depressant treatment.
5. EXPERIMENTAL PROCEDURES
5.1 Animal Subjects
The generation of mice with targeted ablation of the Kcnk9 gene used in the present study has been described previously (Mulkey et al., 2007). Homozygous mutants harboring a cre-mediated deletion of the 2nd exon encoding the M2, M3 and M4 transmembrane segments of the channel were backcrossed over 11 generations onto a C57BL/6J background. Wildtype age-matched C57BL/6J animals were used as controls for experiments described here. Mice were housed in 12:12 light:dark cycles under controlled temperature and humidity conditions with food and water available ad libitum. Prior to testing, animals were allowed a two week acclimation period prior to evaluation. Tail Suspension, Forced Swim and Novel Object Recognition tests were carried out in accordance with the European Communities Council Directive of 24 November 1986. All other studies and animal husbandry was approved by, and conducted in accordance with, Merck Institutional Animal Care and Use Committee (IACUC) standards.
5.2 Locomotor Activity Assessment
Home cage locomotor activity of individually housed wildtype (n=8) and TASK-3 mutant animals (n=8) was assessed by infrared beam break analysis utilizing arrays of photobeams spaced 0.5 inches apart and situated to measure movements in a horizontal plane in both x and y directions (Columbus Instruments, Columbus, OH). For the experiments of Fig. 1, animals were monitored continuously for 5 consecutive days in a 12:12 light:dark cycle (lights on: 14:00; lights off: 02:00) after a two week acclimation period. For basic locomotor analysis, average total number of beams broken by an individual animal at specific 30 minute interval of all 5 days was averaged with that of all other wildtype or knock-out animals. Activity bouts were defined as consecutive 30 second periods during which an animal broke 5 or more individual beams. Average activity bout results for individual animals were determined for specific 30 minute intervals during the 24 hour period and averaged with other animals in the experimental group. Overall active phase, inactive phase and total 24 hour data for wildtype and mutant animals were compared either by 2-way ANOVA analysis to compare differences between the time courses of both groups, or by t test to compare the cumulative averages during these periods.
5.3 Tail Suspension Test
After a 2 week acclimation period during which mice were housed 3–4 animals per cage in a 12:12 light:dark cycle (lights on: 07:00, lights off: 19:00), mice were tested during mid-inactive phase by suspending from their tails in an automated tail suspension device (MED associates Inc, St Albans, Vermont, USA). Immobility time quantified in 2 minute intervals over the 6 minute duration of the test and latency to the first immobilization were used as indices of despair behavior.
5.4 Forced Swim Test
In the forced swim procedure, mice are forced to swim in an inescapable situation (Porsolt et al., 1977). During the inactive period, mice were individually placed into glass chambers containing water at 22–23°C in which the animal can neither touch the bottom nor reach the top of the chamber. Using video camera monitoring, the duration of struggling or floating behavior was visually scored. After a period of vigorous struggling, the animal becomes immobile, or makes only those movements necessary to keep its head above the water. The immobility observed in this test is considered to reflect a state of despair. Animals were evaluated over a 6 minute trial during which struggling and floating behavior was quantified in 2 minute intervals.
5.5 Novel Object Recognition
The Novel Object Recognition task was performed in automated open field arena (Panlab, Barcelona, Spain). The open-fields were placed in a room homogeneously illuminated at 70 Lux at the level of each open field. The objects to be discriminated were a glass marble (2.5 cm diameter) and a plastic dice (2 cm). Animals were first habituated to the open-field for 45 min. The next day, they were submitted to a 10-minute acquisition trial during which they were placed in the open-field in presence of an object A (marble or dice). The time the animal took to explore the object A (when the animal’s snout was directed towards the object at a distance ≤ 1 cm) was manually recorded. A 10-minute retention trial was then performed 3 hours later. During the retention trial, object A and another object B were placed in the open-field, and the times (tA and tB) the animal took to explore the two objects were recorded. The recognition index calculated in the present experiments was defined as (tB/(tA + tB)) ×100.
5.6 Y-Maze Spontaneous Alternation
Male C57B6 wild type and TASK3-KO mice were run in an exploratory Y maze to determine differences in spontaneous alternation, a measure of working memory. The animals were tested in both the active and inactive phases, separated by two weeks. The animals were allowed to acclimate for 30 minutes in an adjacent holding room prior to testing. As a positive control, MK-801 (0.25 mg/kg, i.p.) was administered to C57BL6 mice 30 minutes prior to testing to demonstrate a known deficit in the assay. The three arm, Y shaped maze was made from opaque, solid plastic arms situated at 120° angles to one another. Mice were placed in the center of the maze at the start of the assay and allowed to freely explore for 7.5 minutes. Their movements were video taped and tracked by TopScan, software (Clever Sys Inc, Reston VA). The number and sequence of arm entries was recorded. Arm entries were scored only when the entire animal, excluding the tail, entered the arm. Three consecutive entries into three different arms were scored as a single successful alternation. Percent spontaneous alternation is a measure of the frequency the animal entered the new arm of the maze and was calculated by taking the number of correct alternations divided by the total number of entries minus two.
5.7 Sleep Architecture and Temperature
Electrocorticogram/electroencephalogram (ECoG/EEG) and electromyogram (EMG) were continuously monitored in single-housed male wildtype C57BL/6J and TASK-3 mutants held in a 12:12 light:dark cycle (lights on: 14:00, off: 02:00), and surgically implanted with TL11M2-F20-EET radio telemetry transmitters (Data Sciences International, Arden Hills, MN). Automated sleep stage analysis was performed as described previously (Renger et al., 2004) with modifications for mice (Kraus et al., 2010). Current studies employed either a 16 day baseline comparison between wildtype and mutant animals, or for studies evaluating the effect of Fluoxetine, a counterbalanced cross-over design in which all animals were alternatively treated with drug and vehicle daily for 5 consecutive days: A 5 day arm of drug or vehicle followed by 2 days of washout (no treatment) followed by 5 days of conditional crossover. Effects of 20 mg/kg Fluoxetine relative to vehicle (saline, i.p.) administered in the active phase (zeitgeber time (ZT) 20:00 [10:00 EST]), were evaluated simultaneously in wildtype and TASK-3 mutants in adjacent cages under identical conditions. Automated scoring and analyses were performed as previously described (Winrow et al., 2011). Averages of each 30 minute interval for each condition over 16 days for baseline studies and 5 days for vehicle/compound studies were statistically compared using a two-tailed Student’s t-test applied in a Linear Mixed Effects Model for Repeated Measures. Results were plotted on a single 24 hour time course (mixed models were fit in the R statistical computing environment [cran.us.r-project.org; the R Foundation for Statistical Computing, Vienna, Austria) with the nlme package (Pinheiro and Bates, 2002)). Implant temperature recorded subcutaneously from implanted TL11M2-F20-EET transmitters, was also measured and analyzed from baseline studies. Mean temperature over each 30 minute interval from wildtype or knock-out animals was averaged and plotted on a 24 hour time course. Overall active phase, inactive phase and total 24 hour data for wildtype and mutant animals were compared either by 2-way ANOVA analysis to compare differences between the time courses, or by unpaired t test for repeated measures in order to compare the cumulative average temperature during these periods.
Highlights.
TASK-3 mutants display augmented circadian amplitude of activity, temperature and EEG signal.
Circadian amplitude consistent with despair resistance also impacts cognitive measures.
REM suppression of TASK-3 KOs also suggests antidepressant potential.
Altered Fluoxetine-induced EEG responses and reduced immobility in despair models is indicative of antidepressant potential.
Acknowledgments
Assistance from Merck’s Laboratory Animal Resources Staff and Alan T. Savitz (Central Pharmacology, Merck Research Laboratories) for expert animal handling and treatments are much appreciated. A.L.G., V.T.S., S.M.D, P.L.T., R.L.K., T.W.R., D.R.R., K.W., A.M., J.S., J.B. S.V.F., V.N.U., C.J.W, J.J.R. are or have been employed by Merck & Co., Inc. (USA) and potentially own stock and/or stock options in the company.
Abbreviations
- TWIK
Tandem P-domain weak inward rectifying K+
- TASK
TWIK related acid sensitive K+
- MDD
major depressive disorder
- ECoG/EEG
electrocorticogram/electroencephalogram
- EMG
electromyogram
- ZT
zeitgeber time
- i.p
intraperitoneal
- SWS
slow wave sleep
- SSRI
serotonin reuptake inhibitor
- tA, tB
time to explore object A, object B
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angles-Pujolras M, Diez-Noguera A, Soria V, Urretavizcaya M, Menchon JM, Cambras T. Electroconvulsive shock alters the rat overt rhythms of motor activity and temperature without altering the circadian pacemaker. Behav Brain Res. 2009;196:37–43. doi: 10.1016/j.bbr.2008.07.021. [DOI] [PubMed] [Google Scholar]
- Barel O, Shalev SA, Ofir R, Cohen A, Zlotogora J, Shorer Z, Mazor G, Finer G, Khateeb S, Zilberberg N, Birk OS. Maternally inherited Birk Barel mental retardation dysmorphism syndrome caused by a mutation in the genomically imprinted potassium channel KCNK9. Am J Hum Genet. 2008;83:193–199. doi: 10.1016/j.ajhg.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss DA, Talley EM, Sirois JE, Lei Q. TASK-1 is a highly modulated pH-sensitive ‘leak’ K(+) channel expressed in brainstem respiratory neurons. Respir Physiol. 2001;129:159–174. doi: 10.1016/s0034-5687(01)00288-2. [DOI] [PubMed] [Google Scholar]
- Borbely AA, Tobler I, Loepfe M, Kupfer DJ, Ulrich RF, Grochocinski V, Doman J, Matthews G. All-night spectral analysis of the sleep EEG in untreated depressives and normal controls. Psychiatry Res. 1984;12:27–33. doi: 10.1016/0165-1781(84)90135-5. [DOI] [PubMed] [Google Scholar]
- Buckler KJ, Williams BA, Honore E. An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J Physiol. 2000;525(Pt 1):135–142. doi: 10.1111/j.1469-7793.2000.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz dM, Rudy B. Molecular diversity of K+ channels. Ann N Y Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- Deng PY, Lei S. Serotonin increases GABA release in rat entorhinal cortex by inhibiting interneuron TASK-3 K+ channels. Mol Cell Neurosci. 2008;39:273–284. doi: 10.1016/j.mcn.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon DG, Bogdan R, Fagerness J, Holmes AJ, Perlis RH, Pizzagalli DA. Variation in TREK1 gene linked to depression-resistant phenotype is associated with potentiated neural responses to rewards in humans. Hum Brain Mapp. 2010;31:210–221. doi: 10.1002/hbm.20858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugovic C, Maccari S, Weibel L, Turek FW, Van Reeth O. High corticosterone levels in prenatally stressed rats predict persistent paradoxical sleep alterations. J Neurosci. 1999;19:8656–8664. doi: 10.1523/JNEUROSCI.19-19-08656.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugovic C, Solberg LC, Redei E, Van Reeth O, Turek FW. Sleep in the Wistar-Kyoto rat, a putative genetic animal model for depression. Neuroreport. 2000;11:627–631. doi: 10.1097/00001756-200002280-00038. [DOI] [PubMed] [Google Scholar]
- Duprat F, Lesage F, Fink M, Reyes R, Heurteaux C, Lazdunski M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J. 1997;16:5464–5471. doi: 10.1093/emboj/16.17.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval F, Lebowitz BD, Macher JP. Treatments in depression. Dialogues Clin Neurosci. 2006;8:191–206. doi: 10.31887/DCNS.2006.8.2/fduval. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Yacoubi M, Bouali S, Popa D, Naudon L, Leroux-Nicollet I, Hamon M, Costentin J, Adrien J, Vaugeois JM. Behavioral, neurochemical, and electrophysiological characterization of a genetic mouse model of depression. Proc Natl Acad Sci U S A. 2003;100:6227–6232. doi: 10.1073/pnas.1034823100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262:1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- Goldstein SA, Bockenhauer D, O’Kelly I, Zilberberg N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci. 2001;2:175–184. doi: 10.1038/35058574. [DOI] [PubMed] [Google Scholar]
- Grunhaus L, Shipley JE, Eiser A, Pande AC, Tandon R, Krahn DD, Demitrack MA, Remen A, Hirschmann S, Greden JF. Sleep-onset rapid eye movement after electroconvulsive therapy is more frequent in patients who respond less well to electroconvulsive therapy. Biol Psychiatry. 1997;42:191–200. doi: 10.1016/S0006-3223(96)00333-2. [DOI] [PubMed] [Google Scholar]
- Heurteaux C, Lucas G, Guy N, El Yacoubi M, Thummler S, Peng XD, Noble F, Blondeau N, Widmann C, Borsotto M, Gobbi G, Vaugeois JM, Debonnel G, Lazdunski M. Deletion of the background potassium channel TREK-1 results in a depression-resistant phenotype. Nat Neurosci. 2006;9:1134–1141. doi: 10.1038/nn1749. [DOI] [PubMed] [Google Scholar]
- Honore E. The neuronal background K2P channels: focus on TREK1. Nat Rev Neurosci. 2007;8:251–261. doi: 10.1038/nrn2117. [DOI] [PubMed] [Google Scholar]
- Kennard LE, Chumbley JR, Ranatunga KM, Armstrong SJ, Veale EL, Mathie A. Inhibition of the human two-pore domain potassium channel, TREK-1, by fluoxetine and its metabolite norfluoxetine. Br J Pharmacol. 2005;144:821–829. doi: 10.1038/sj.bjp.0706068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus RL, Li Y, Gregan Y, Gotter AL, Uebele VN, Fox SV, Doran SM, Barrow JC, Yang ZQ, Reger TS, Koblan KS, Renger JJ. In vitro characterization of T-type calcium channel antagonist TTA-A2 and in vivo effects on arousal in mice. J Pharmacol Exp Ther. 2010;335:409–417. doi: 10.1124/jpet.110.171058. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Reynolds CF, III, Ulrich RF, Grochocinski VJ. Comparison of automated REM and slow-wave sleep analysis in young and middle-aged depressed subjects. Biol Psychiatry. 1986;21:189–200. doi: 10.1016/0006-3223(86)90146-0. [DOI] [PubMed] [Google Scholar]
- Lesage F. Pharmacology of neuronal background potassium channels. Neuropharmacology. 2003;44:1–7. doi: 10.1016/s0028-3908(02)00339-8. [DOI] [PubMed] [Google Scholar]
- Linden AM, Sandu C, Aller MI, Vekovischeva OY, Rosenberg PH, Wisden W, Korpi ER. TASK-3 knockout mice exhibit exaggerated nocturnal activity, impairments in cognitive functions, and reduced sensitivity to inhalation anesthetics. J Pharmacol Exp Ther. 2007;323:924–934. doi: 10.1124/jpet.107.129544. [DOI] [PubMed] [Google Scholar]
- Liou YJ, Chen TJ, Tsai SJ, Yu YW, Cheng CY, Hong CJ. Support for the involvement of the KCNK2 gene in major depressive disorder and response to antidepressant treatment. Pharmacogenet Genomics. 2009;19:735–741. doi: 10.1097/FPC.0b013e32832cbe61. [DOI] [PubMed] [Google Scholar]
- Meadows HJ, Randall AD. Functional characterisation of human TASK-3, an acid-sensitive two-pore domain potassium channel. Neuropharmacology. 2001;40:551–559. doi: 10.1016/s0028-3908(00)00189-1. [DOI] [PubMed] [Google Scholar]
- Meuth SG, Budde T, Kanyshkova T, Broicher T, Munsch T, Pape HC. Contribution of TWIK-related acid-sensitive K+ channel 1 (TASK1) and TASK3 channels to the control of activity modes in thalamocortical neurons. J Neurosci. 2003;23:6460–6469. doi: 10.1523/JNEUROSCI.23-16-06460.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, Sen N, Mistry AM, Guyenet PG, Bayliss DA. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J Neurosci. 2007;27:14049–14058. doi: 10.1523/JNEUROSCI.4254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang DS, Robledo CJ, Carr DR, Gent TC, Vyssotski AL, Caley A, Zecharia AY, Wisden W, Brickley SG, Franks NP. An unexpected role for TASK-3 potassium channels in network oscillations with implications for sleep mechanisms and anesthetic action. Proc Natl Acad Sci U S A. 2009;106:17546–17551. doi: 10.1073/pnas.0907228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastel RH, Fernstrom JD. Short-term effects of fluoxetine and trifluoromethylphenylpiperazine on electroencephalographic sleep in the rat. Brain Res. 1987;436:92–102. doi: 10.1016/0006-8993(87)91560-5. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honore E, Lesage F, Fink M, Romey G, Lazdunski M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat Neurosci. 1999;2:422–426. doi: 10.1038/8084. [DOI] [PubMed] [Google Scholar]
- Perlis RH, Moorjani P, Fagerness J, Purcell S, Trivedi MH, Fava M, Rush AJ, Smoller JW. Pharmacogenetic analysis of genes implicated in rodent models of antidepressant response: association of TREK1 and treatment resistance in the STAR(*)D study. Neuropsychopharmacology. 2008;33:2810–2819. doi: 10.1038/npp.2008.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM. Mixed-effects models in S and S-Pluse. Springer; New York, New York: 2002. [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- Renger JJ, Dunn SL, Motzel SL, Johnson C, Koblan KS. Sub-chronic administration of zolpidem affects modifications to rat sleep architecture. Brain Res. 2004;1010:45–54. doi: 10.1016/j.brainres.2004.02.067. [DOI] [PubMed] [Google Scholar]
- Riemann D, Berger M. The effects of total sleep deprivation and subsequent treatment with clomipramine on depressive symptoms and sleep electroencephalography in patients with a major depressive disorder. Acta Psychiatr Scand. 1990;81:24–31. doi: 10.1111/j.1600-0447.1990.tb06444.x. [DOI] [PubMed] [Google Scholar]
- Rubin RT, Heist EK, McGeoy SS, Hanada K, Lesser IM. Neuroendocrine aspects of primary endogenous depression. XI. Serum melatonin measures in patients and matched control subjects. Arch Gen Psychiatry. 1992;49:558–567. doi: 10.1001/archpsyc.1992.01820070052008. [DOI] [PubMed] [Google Scholar]
- Slater IH, Jones GT, Moore RA. Inhibition of REM sleep by fluoxetine, a specific inhibitor of serotonin uptake. Neuropharmacology. 1978;17:383–389. doi: 10.1016/0028-3908(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Steiger A, Kimura M. Wake and sleep EEG provide biomarkers in depression. J Psychiatr Res. 2010;44:242–252. doi: 10.1016/j.jpsychires.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Szuba MP, Guze BH, Baxter LR., Jr Electroconvulsive therapy increases circadian amplitude and lowers core body temperature in depressed subjects. Biol Psychiatry. 1997;42:1130–1137. doi: 10.1016/s0006-3223(97)00046-2. [DOI] [PubMed] [Google Scholar]
- Talley EM, Bayliss DA. Modulation of TASK-1 (Kcnk3) and TASK-3 (Kcnk9) potassium channels: volatile anesthetics and neurotransmitters share a molecular site of action. J Biol Chem. 2002;277:17733–17742. doi: 10.1074/jbc.M200502200. [DOI] [PubMed] [Google Scholar]
- Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. Cns distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci. 2001;21:7491–7505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touma C, Fenzl T, Ruschel J, Palme R, Holsboer F, Kimura M, Landgraf R. Rhythmicity in mice selected for extremes in stress reactivity: behavioural, endocrine and sleep changes resembling endophenotypes of major depression. PLoS One. 2009;4:e4325. doi: 10.1371/journal.pone.0004325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel GW, Thurmond A, Gibbons P, Sloan K, Walker M. REM sleep reduction effects on depression syndromes. Arch Gen Psychiatry. 1975;32:765–777. doi: 10.1001/archpsyc.1975.01760240093007. [DOI] [PubMed] [Google Scholar]
- Washburn CP, Sirois JE, Talley EM, Guyenet PG, Bayliss DA. Serotonergic raphe neurons express TASK channel transcripts and a TASK-like pH- and halothane-sensitive K+ conductance. J Neurosci. 2002;22:1256–1265. doi: 10.1523/JNEUROSCI.22-04-01256.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winrow CJ, Gotter AL, Cox CD, Doran SM, Tannenbaum PL, Breslin MJ, Garson SL, Fox SV, Harrell CM, Stevens J, Reiss DR, Cui D, Coleman PJ, Renger JJ. Promotion of Sleep by Suvorexant-A Novel Dual Orexin Receptor Antagonist. J Neurogenet. 2011 doi: 10.3109/01677063.2011.566953. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A. Biological rhythm disturbances in mood disorders. Int Clin Psychopharmacol. 2006;21(Suppl 1):S11–S15. doi: 10.1097/01.yic.0000195660.37267.cf. [DOI] [PubMed] [Google Scholar]