Abstract
Peripheral neuropathy is a common aging-related degenerative disorder that interferes with daily activities and leads to increased risk of falls and injury in the elderly. The etiology of most aging-related peripheral neuropathy is unknown. Inherited defects in several genome maintenance mechanisms cause tissue-specific accelerated aging, including neurodegeneration. We tested the hypothesis that a murine model of XFE progeroid syndrome, caused by reduced expression of ERCC1-XPF DNA repair endonuclease, develops peripheral neuropathy. Nerve conduction studies revealed normal nerve function in young adult (8 week) Ercc1−/Δ mice, but significant abnormalities in 20 week-old animals. Morphologic and ultrastructural analysis of the sciatic nerve from mutant mice revealed significant alterations at 20 but not 8 weeks of age. We conclude that Ercc1−/Δ mice have accelerated spontaneous peripheral neurodegeneration that mimics aging-related disease. This provides strong evidence that DNA damage can drive peripheral neuropathy and offers a rapid and novel model to test therapies.
Keywords: Xeroderma pigmentosum, progeria, neurodegeneration, DNA repair, nerve conduction, nerve morphology
Introduction
Aging-related peripheral neuropathy contributes significantly to morbidity in the elderly. The prevalence of idiopathic peripheral neuropathy, i.e. neuropathy that is not associated with some underlying disease process such as diabetes, has been reported to be between 19–22% in persons aged 60–74 years and up to 58% of people aged 85 and older (Mold et al., 2004; Richardson, 2002). Approximately 14% of adults 65 years or older, and 35% of adults 85 years or older, report difficulty with walking (Resnick et al., 2000). Loss of lower extremity sensory input is associated with impaired balance and falls in the elderly. Indeed, unintentional injury, mostly due to falls, is the sixth leading cause of death in those 65 years of age or older (Sattin, 1992).
Nerve conduction studies (NCS) in humans have shown a progressive decrease in nerve conduction velocities and an increase in the latency of onset of F-waves and provoked sensory nerve responses with advancing age (Bouche et al., 1993; Dorfman and Bosley, 1979; Olney, 1998; Taylor, 1984). Sensory nerve action potential amplitudes appear to decrease at a faster rate than conduction velocities. Morphologic analysis of the sural nerve revealed a progressive loss of both large and small myelinated fiber density with age (Tohgi et al., 1977).
Aging-related changes in peripheral nerves of mice reflect those that have been described in humans. Nerve conduction velocities remain unchanged during adulthood until the last third of life, after which they begin to decline (Verdu et al., 1996). Morphologic examination agrees with this finding as tibial nerves appear to be stable in the adult 6–12 month-old mouse. From 12–20 months, there is a gradual decline in the number and density of myelinated and unmyelinated nerve fibers. From 20 months on, there is marked attrition of fibers with approximately 50% loss of myelinated fibers and 35% loss of unmyelinated fibers. This results in a general disorganization of the endoneurium, coupled with an increase in the amount of collagen deposition (Ceballos et al., 1999; Verdu et al., 2000). Along with a loss of fibers, there is a decrease in myelin thickness and decrease in fiber diameter (Ceballos et al., 1999). Furthermore, the fraction of myelinated axons showing irregular shapes increases to 50% in aged mice. These large irregular fibers show abnormalities in the myelin sheath such as wide incisures, separation of lamellae, and myelin loops (Ceballos et al., 1999; Knox et al., 1989).
The molecular basis of aging and aging-related degenerative changes is not known (Kirkwood, 2005). However, it is generally accepted that aging is driven by time-dependent accumulation of stochastic molecular and cellular damage (Campisi and Vijg, 2009; Kirkwood, 2005; Vijg, 2008). Consistent with this, the majority of human progeroid syndromes, or diseases of segmental (tissue-specific) accelerated aging, are caused by defects in genome maintenance mechanisms, including Werner syndrome, ataxia telangiectasia, Cockayne syndrome and trichothiodystrophy (Hasty et al., 2003). This suggests that DNA damage is one type of stochastic molecular damage that promotes aging-related degenerative changes.
Nucleotide excision repair (NER) is an evolutionarily conserved mechanism that removes helix-distorting DNA lesions from the nuclear genome through the coordinated action of over 30 proteins including XPA through XPG, ERCC1, TFIIH and the replication machinery (Friedberg et al., 2006). Mutations in XPF can lead to XFE progeroid syndrome (Niedernhofer et al., 2006), a disease of systemic accelerated aging, or xeroderma pigmentosum (XP), which is primarily a cancer-predisposition syndrome but includes segmental aging (Niedernhofer, 2008a) including progressive peripheral neurodegeneration (Kraemer et al., 2007). The former was modeled in the mouse by creating a hypomorphic mutation in the mErcc1 locus, which encodes ERCC1 the essential binding partner of XPF (Dolle et al., 2006; Weeda et al., 1997). Together ERCC1-XPF form a nuclease that is required for numerous DNA repair mechanisms (Ahmad et al., 2008; Niedernhofer et al., 2004; Sijbers et al., 1996). Ercc1−/Δ mice harboring one knock-out and one mutant allele of Ercc1 express 10% of the normal level of the nuclease ERCC1-XPF. These mice demonstrate spontaneous premature onset of aging-related changes of the epidermal, hematopoietic, endocrine, hepatobiliary, renal, nervous and musculoskeletal identical to XFE progeroid syndrome (Niedernhofer, 2008a) and a maximum lifespan of 32 weeks. In this study we evaluated peripheral nerve structure and function, in Ercc1−/Δ mice to determine if they represent an accelerated model of aging-related peripheral neuropathy that could be used to identify the molecular mechanism of disease and rational strategies for prevention and/or treatment.
Materials and Methods
Animals
All Ercc1−/Δ mice were generated by matings of heterozygous mice in two different inbred backgrounds to create f1 hybrids that are isogenic: (e.g., Ercc1+/Δ FVB/n X Ercc1+/minus; C57Bl/6). Genomic DNA was isolated from a 1mm ear plug of 10–14 day-old mice using a NucleoSpin® 96 Tissue DNA extraction system (Macherey-Nagel, Inc.). Genotyping of the Ercc1 null allele was done by PCR co-amplification of the 3′ end of exon 7 from the wild-type (wt) allele and the neomycin resistance marker cloned into exon 7 of the targeted allele using primers specific for exon 7, neor and intron 7 (5′-AGCCGACCTCCTTATGGAAA, 5′-TCGCCTTCTTGACGAGTTCT and 5′-ACAGATGCTGAGGGCAGACT, respectively). Wt (0.25-kb) and mutant (0.4-kb) fragments were separated by electrophoresis on a 2% agarose gel. The mutant allele of Ercc1 (Δ) was amplified by adding a fourth primer (5′-CTAGGTGGCAGCAGGTCATC) to amplify the neomycin cassette in the Δ allele (0.5-kb) (Ahmad et al., 2008). Wild type littermates were retained as aging-matched controls. All animal experiments were reviewed and approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Nerve conduction studies (NCS)
Mice were anesthetized with ketamine/xylazine (80/10 mg/kg IP). They were placed on a temperature-controlled angled platform that was maintained at 37°C and the hind limbs secured at an angle of 30–45 relative to the long axis of the body. Motor and sensory nerve amplitude and conduction velocities were measured using a VikingQuest NCS/EMG Portable System with Grass™ platinum subdermal needle electrode pairs for stimulation and recording. A ground electrode was placed in the tail. Compound muscle action potentials (CMAP) and motor nerve conduction velocities (MNCV) were recorded from the gastrocnemius muscle. A recording electrode was placed in the gastrocnemius muscle and a stimulating electrode was placed in the sciatic notch with reference electrodes placed approximately 10 mm distal from each. The proper placement of the stimulating electrode was confirmed by recording a maximum CMAP from the gastrocnemius muscle during minimal (0.8 mA) stimulation at the sciatic notch. The stimulating electrode was moved to the knee (popliteal fossa) and a CMAP recorded. The CMAP latencies and distance between the sciatic notch and knee were used to calculate the gastrocnemius MNCV. Following this, the needle electrode pair at the sciatic notch was used for recording. A stimulation electrode is placed at the first toe with a reference electrode at the fifth digit. Peak foot sensory nerve amplitudes (FSA) and conduction velocities (FSCV) were measured from several orthodromic stimulations (0.8 mA–15 mA current) and the best response was used for analysis. Caudal nerve evoked amplitudes (CNA) and conduction velocities (CNCV) were measured by recording from the base of the tail and stimulating near the distal tip. The electrophysiological measurements for all treatment groups were analyzed by ANOVA and Fisher’s PLSD post-hoc analysis (Statview; SAS Institute Inc., Cary, NC). All values are presented as mean ± standard error.
Nerve tissue processing
Following NCS, the mice were euthanized by CO2 inhalation followed by cervical dislocation then perfused with cold saline followed by 2.5% glutaraldehyde in PBS. Sciatic nerves were isolated, removed and immersed in fixative for an additional hour. Tissue was then dehydrated and embedded in resin for sectioning. Toluidine blue thick sections (300 nm) for light microscopy and ultrathin (60 nm) sections for transmission electron microscopy (TEM) were obtained.
Morphometric analysis
Toluidine blue thick sections were imaged with a Zeiss Axiovert 200 microscope and stitched together (if necessary) using Adobe Photoshop v10.0.1. Zeiss AxioVision v4.8.0 software was used to determine individual nerve fiber sizes and total area occupied by myelin. One or two sections per sciatic nerve were analyzed per animal by a person blinded to treatment or animal ID. All values are presented as mean ± standard error; significance was determined by ANOVA and Fisher’s PLSD post-hoc analysis (Statview; SAS Institute Inc., Cary, NC).
Results
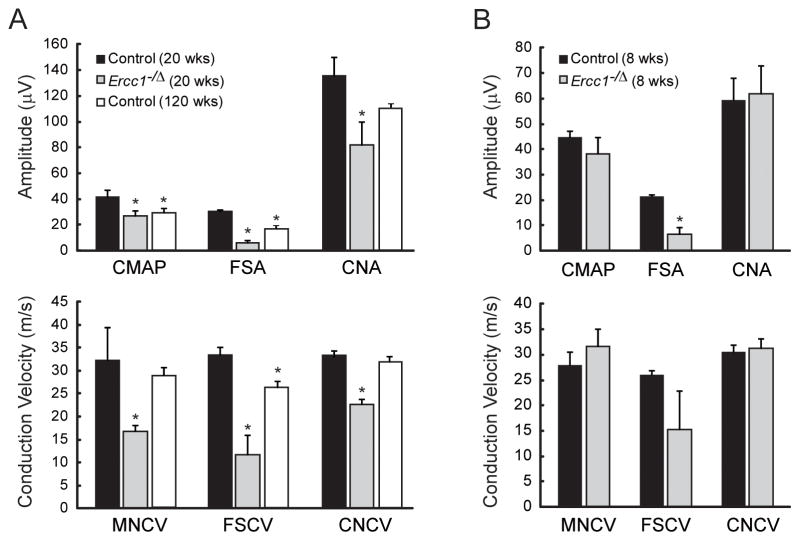
Ercc1−/Δ mice develop normally until 8 weeks of age, when they begin to spontaneously exhibit progressively worsening degenerative changes associated with old age, including multiple symptoms associated with neurodegeneration (dystonia, trembling and ataxia, at ~9, 12 and 15 weeks, respectively) (Ahmad et al., 2008; Dolle et al., 2006; Weeda et al., 1997). Nerve conduction studies in twenty week-old Ercc1−/Δ mice, which are symptomatic, demonstrated numerous functional deficits characteristic of peripheral neuropathy (Figure 1A, gray bars). Ercc1−/Δ mice had significantly lower compound muscle action potentials (CMAP) and conduction velocities in the sciatic nerve, foot sensory nerve and the caudal nerve (MNCV, FSCV, and CNCV) compared to normal sibling mice (Figure 1A, black bars). Ercc1−/Δ mice also had a significant reduction in the evoked response of the foot sensory nerve (FSA) and caudal nerve (CNA). These data demonstrate that Ercc1−/Δ mice spontaneously and prematurely develop peripheral sensory and motor neuropathy.
Figure 1.
Analysis of peripheral nerve function. (A) Nerve conduction studies performed on 20 week-old Ercc1−/Δ mice (gray bars, N=7), normal littermates (black bars, N=4), and 120 week-old controls (open bars, N=3). (B) Nerve conduction studies performed on 8 week-old Ercc1−/Δ mice (N=3) and normal littermates (N=3). Values represent mean ± SEM. CMAP - sciatic nerve compound muscle action potential, MNCV - sciatic nerve motor nerve conduction velocity, FSA - foot sensory nerve amplitude, FSCV - foot sensory nerve conduction velocity, CNA - caudal nerve amplitude, CNCV - caudal nerve conduction velocity. *p < 0.05 compared to 20 week-old controls.
To determine if the peripheral neuropathy in Ercc1−/Δ mice is due to degenerative changes that develop over time, as with normal aging, we performed NCS on 8 week-old Ercc1−/Δ mice that are not yet symptomatic. The 8 week-old Ercc1−/Δ mice weighed more than 20 week-old mutant animals (19 vs. 13 gm), illustrating that these mice are undergoing degenerative changes with age. The only difference found between the 8 week-old Ercc1−/Δ mice and their littermate controls was a significant decrease in the FSA in the Ercc1−/Δ mice (Figure 1B), which was the most distal site tested. This is similar to aging-related peripheral neuropathy that first occurs distally then proceeds proximally. The observation that 5 of 6 nerve function tests are normal at 8 weeks of age in Ercc1−/Δ mice, but all 6 are significantly impaired in 20 week-old Ercc1−/Δ mice, demonstrate that the peripheral nervous system of the mutant animals is for the most part normal into adulthood and that neuropathy is a consequence of degenerative changes that occur with increasing age.
To determine how closely the neuropathy that develops Ercc1−/Δ mice mirrors that observed in normal aging, we performed NCS in 120 week old control mice (Figure 1A, open bars). The aged control mice had significantly reduced CMAP, FSA and FSCV measurements compared to young adult controls; However, unlike the Ercc1−/Δ mice, the aged mice showed no changes in MNCV or CNCV (CNA was reduced but did not reach statistical significance). In addition, the decreases in FSA and FSCV in the aged mice were not as substantial as those observed in the Ercc1−/Δ mice. This data suggests the neuropathy that develops in the Ercc1−/Δ mice by 5 months of age is more advanced than that which develops over years with normal murine aging.
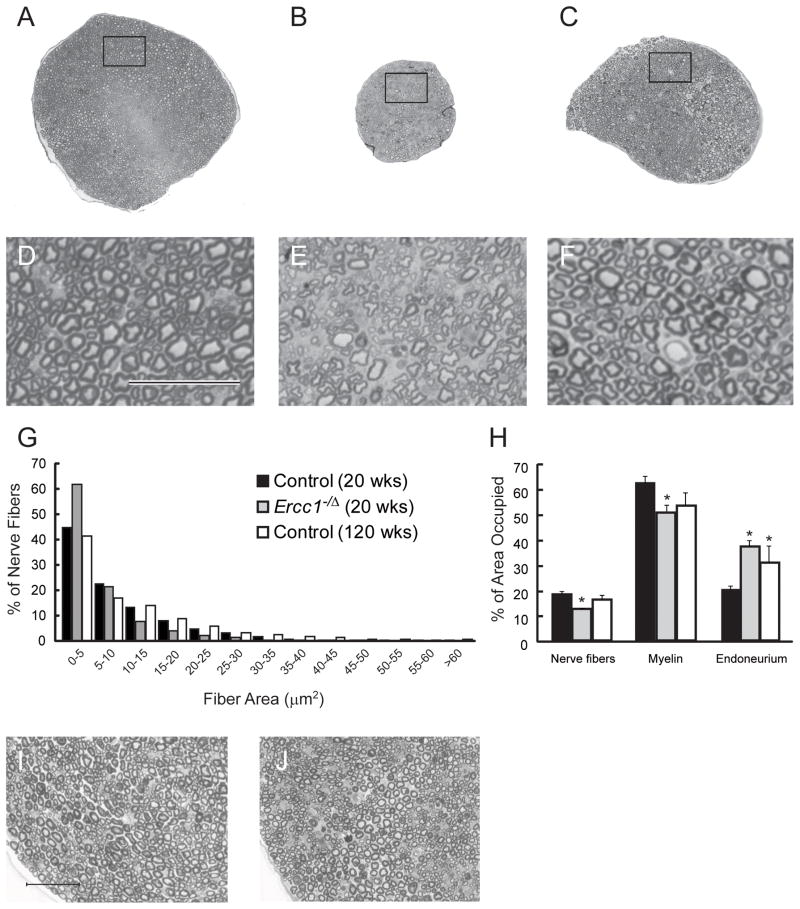
A reduction in the amplitude of an evoked action potential signifies axonal degeneration, whereas a loss of conductance velocity is typically caused by demyelination. Both were observed in Ercc1−/Δ mice relative to normal littermates. Thus we analyzed sciatic nerve cross sections to determine if the morphology supported both of these types of degenerative changes. Toluidine blue stained cross-sections of fascicles from 20 week-old normal mice appeared uniform with large and small fibers throughout the nerve bundle (Figure 2A, D). The large diameter fibers were well myelinated and the nerve bundle had a well organized appearance. In contrast, fascicles from 20 week-old Ercc1−/Δ mice were significantly smaller than those of the wild-type mice (95,000 μm2 vs. 213,000 μm2) and showed a disorganized appearance (Figure 2B, E). There was a loss of large fibers and most of the fibers that remain appear misshapen. Sciatic nerve fascicles from 120 week-old control mice (140,000 μm2) were smaller than those taken from 20 week old wild-type mice but larger than those of the Ercc1−/Δ mice. The nerve bundle in the old wild-type mice appeared organized with large and small fibers throughout (Figure 2C, F).
Figure 2.
Peripheral nerve morphology. Representative micrographs from cross sections of the sciatic nerve of a 20 week-old control mouse (A,D), a 20 week-old Ercc1−/Δ mouse (B,E), and a 120 week-old control mouse (C,F). Notice the well organized appearance and number of larger diameter nerve fibers in the control sections. Compare this with the disorganized and ‘empty’ appearing section from the 20 week-old Ercc1−/Δ mouse. Morphometric analysis of nerve fiber size distribution (G) and area occupied by nerve fibers, myelin, or endoneurium space (H) in 20 week-old Ercc1−/Δ mice (gray bars, N=5), 20 week-old control mice (black bars, N=5), and 120 week-old control mice (open bars, N=2). Representative micrographs from cross sections of the sciatic nerve of an 8 week-old Ercc1−/Δ mouse (J) and age-matched control mouse (I). Bars in (D) and (I) = 50 um. Values represent mean ± SEM. *p < 0.05 compared to 20 week-old controls.
Morphometric analysis revealed a shift in fiber size distribution between the control and Ercc1−/Δ mice (Figure 2G). Ercc1−/Δ mice had a much higher percentage of small fibers (<5 μm2) with a concomitant loss of larger fibers (>45 μm2). There were no discernable differences in fiber size distribution between the 20 week- and 120 week-old control mice. Analysis of the area occupied by nerve fibers, myelin, and endoneurium (extracellular space) showed clear differences between all three groups (Figure 2H). The percent of the total fascicle area occupied by nerve fibers was significantly less in the Ercc1−/Δ mice (12.5 ± 0.2%) compared to the 20 week-old control (18.1 ± 1.2%) or 120 week-old control (16.1 ± 1.7%) mice. Conversely, the 20 week-old control mice had significantly more area occupied by myelin (61.9 ± 2.8%) than either the Ercc1−/Δ mice (50.3 ± 2.7%) or 120 week-old control (53.3 ± 5.2%) mice and significantly less endoneurial space (19.9 ± 1.6% vs. 37.1 ± 2.7% and 30.6 ± 6.9%). These data strongly support the functional data and indicate that there is a loss of both nerve fibers and myelin in the Ercc1−/Δ mice.
The sciatic nerve fascicles from the younger, 8 week-old Ercc1−/Δ mice were substantially more organized than those of the older mutant animals with little evidence of degeneration (Figure 2J) and looked similar to aging-matched control mice (Figure 2I). The fascicle size of the 8 week old Ercc1−/Δ mice was 20% smaller than that of littermate controls (120,000 μm2 vs. 147,000 μm2). But this is not unexpected, since Ercc1−/Δ mice weigh 40% less than their normal littermates. Remarkably, the fascicle size of 8 week-old Ercc1−/Δ mice is larger than that of 20 week-old mutant animals (120,000 μm2 vs. 95,000 μm2). These morphologic data emphasize that Ercc1−/Δ mice rapidly undergo peripheral neurodegeneration as adults rather than have developmental abnormalities.
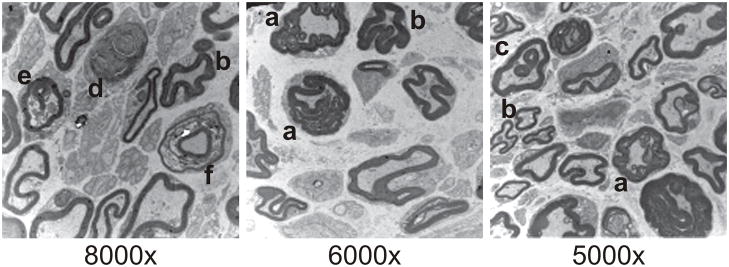
Finally, transmission electron micrographs of sciatic nerve from 20 week-old Ercc1−/Δ mice revealed abundant redundant myelin, crenated myelin sheaths, paranodal loops of myelin, myelin droplets and ovoids (Figure 3), all characteristic of axonal atrophy with secondary myelin degeneration (Crisci and Ferreira, 2002). These ultrastructural data confirm the results of the functional studies, demonstrating that DNA repair deficient Ercc1−/Δ mice spontaneous undergo premature peripheral neurodegeneration similar to what occurs in mice and humans with aging.
Figure 3.
Representative transmission electron micrographs of the sciatic nerve of 20 week-old Ercc1−/Δ mice. Hallmarks of axonal atrophy can be observed including redundant myelin (a), crenated myelin sheaths (b), myelin droplets (c), myelin ovoid (d), and degenerating axon profiles (e), leading to secondary myeline degeneration evidenced by wide myelin incisures or paranodal loops (f). Bars = 2 μm.
Discussion
Aging-related peripheral neuropathy is associated with reductions in both conduction velocity and evoked amplitude response (Verdu et al., 1996). Similar findings were observed in 20 week-old Ercc1−/Δ mice, which are chronologically still young adults (Figure 1). The nerve conduction studies are supported by morphological evidence of loss of peripheral nerve fibers, in particular large ones (Figure 2) and abnormal myelin structures indicative of axonal atrophy and myelin degeneration (Figure 3). These functional and histopathologic data provide strong evidence that the neuropathy in these DNA repair deficient Ercc1−/Δ mice is the same, or at least very similar, to the neuropathy that occurs with normal aging. In fact, the morphology that we observed in the 20 week old Ercc1−/Δ mice look remarkably similar to that previously reported in 27–33 month-old wild-type Swiss OF1 mice (Ceballos et al., 1999; Verdu et al., 2000). Interestingly, the nerve morphology of the 30 month-old control mice in our study, which were in a mixed C57Bl/6;FVB/n genetic background, appeared healthier than that of the aged mice in the previous reports. However, we did observe nerve conduction impairments, typical of aged rodents (Verdu et al., 1996). Given that foot sensory nerve measurements were more severely affected in the old wild-type mice than the sciatic nerve measurements, it is likely that more severe morphological alterations would be uncovered in more distal nerves.
The observations that both the nerve function and morphology are largely normal in 8 week-old Ercc1−/Δ mice, but deteriorate by age 20 week of age, demonstrate that the peripheral neuropathy in these DNA repair deficient mice arises as a consequence of rapid degenerative changes in adult animals. The implications of this are two-fold. First, the Ercc1−/Δ mice offer a novel, accurate and rapid model of aging-related peripheral neuropathy that can be used to discover the molecular mechanism of peripheral neurodegeneration and to screen therapeutic modalities for their ability to prevent, delay or reverse peripheral neuropathy. Second, the data provide evidence that DNA repair mechanisms play a crucial role in protecting against peripheral nerve degeneration.
This latter point is further supported by the fact that DNA damaging agents such as the chemotherapeutic agent cisplatin, cause peripheral neuropathy (Chaudhary et al., 1994; LoMonaco et al., 1992). Cisplatin and related platinum drugs are first-line chemotherapeutic agents for treatment of lung, colorectal, pancreatic and genitourinary cancer (Abbas et al.; Geiger et al.; Nakamura and Miki; Sanchez and Trevino, 2008; Zeimet et al., 2009). The primary mechanism of cytotoxicity of this class of drugs is via formation of DNA interstrand crosslink lesions (McHugh et al., 2001). These drugs cause a dose-dependent sensory neuropathy (Krarup-Hansen et al., 1993). Thus the prediction is that the spontaneous neuropathy observed in ERCC1-deficient mice, which have an impaired ability to repair DNA interstrand crosslinks, should mimic neuropathy caused by crosslinking agents like cisplatin. Indeed in mice, chronic cisplatin treatment leads to a significant reduction in caudal nerve conduction velocity, axonal degeneration of myelinated fibers in the sciatic nerve (Carozzi et al.) and damage to myelin sheaths (Yoon et al., 2009). In rats, chronic cisplatin treatment leads to minor loss of motor function, decreased peripheral nerve conduction velocity (Screnci et al., 2000) and axonal degeneration of the sciatic nerve (Carozzi et al., 2009) with large, myelinated fibers being more affected than small or unmyelinated fibers (Authier et al., 2003). In humans treated with cisplatin, the amplitude of electrically evoked sensory action potentials are also reduced in a dose-dependent manner and there is a preferential loss of large fibers (Krarup-Hansen et al., 1993). Thus all of the functional and morphologic changes that spontaneously occur in the peripheral nerves of DNA repair-deficient Ercc1−/Δ mice also occur in rodents and humans chronically exposed to crosslinking agents. This strongly supports the conclusion that the pathologies observed in the Ercc1−/Δ mice are driven by failure to repair endogenous crosslinks. It also illustrates that Ercc1−/Δ mice offer a rapid and accurate model for screening treatments to prevent the neurotoxic side-effects of platinum-based therapy.
The novelty of this model is emphasized by the fact that the majority of murine models of human progerias or genome instability disorders have a much milder phenotype than the disease they mimic (Niedernhofer, 2008b). This includes Werner syndrome, Bloom syndrome and Fanconi anemia (Chang, 2005; Parmar et al., 2009). This appears to be particularly true of neurodegeneration, which is very prominent in patients with xeroderma pigmentosum, ataxia telangiectasia and many other genome instability disorders, but not in corresponding mouse models (Frappart and McKinnon, 2008; Kraemer et al., 2007; Niedernhofer, 2008b). This discrepancy has been attributed to reduced levels of DNA damage in mice compared to humans either because of less endogenous damage in mice or the fact that laboratory animals live in a highly controlled and simple environment (Niedernhofer, 2008b). Because Ercc1−/Δ mice are missing multiple DNA repair pathways (nucleotide excision repair, DNA interstrand crosslink repair and repair of some double-strand breaks), they presumably have a greater burden of DNA damage than mice missing a single pathway, and therefore more dramatic disease. The Ercc1−/Δ mice therefore offer not only a unique opportunity to probe the mechanism of aging-related neurodegeneration, but also to identify the types of DNA damage that promotes neurodegeneration.
Research Highlights.
Aging related idiopathic peripheral neuropathy is a common disease of unknown etiology.
DNA repair-deficient Ercc1−/Δ mice spontaneous develop peripheral neuropathy by 5 mths.
Functional and structural changes in their peripheral nerves mimic those that occur with old age.
This yields new insight on the cause of peripheral neurodegeneration.
This validates Ercc1−/Δ mice as a rapid and accurate model for screening therapies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbas A, Yang G, Fakih M. Management of anal cancer in 2010. Part 2: current treatment standards and future directions. Oncology (Williston Park) 24:417–24. [PubMed] [Google Scholar]
- Ahmad A, Robinson AR, Duensing A, van Drunen E, Beverloo HB, Weisberg DB, Hasty P, Hoeijmakers JH, Niedernhofer LJ. ERCC1-XPF endonuclease facilitates DNA double-strand break repair. Mol Cell Biol. 2008;28:5082–92. doi: 10.1128/MCB.00293-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Authier N, Gillet JP, Fialip J, Eschalier A, Coudore F. An animal model of nociceptive peripheral neuropathy following repeated cisplatin injections. Exp Neurol. 2003;182:12–20. doi: 10.1016/s0014-4886(03)00003-7. [DOI] [PubMed] [Google Scholar]
- Bouche P, Cattelin F, Saint-Jean O, Leger JM, Queslati S, Guez D, Moulonguet A, Brault Y, Aquino JP, Simunek P. Clinical and electrophysiological study of the peripheral nervous system in the elderly. J Neurol. 1993;240:263–8. doi: 10.1007/BF00838158. [DOI] [PubMed] [Google Scholar]
- Campisi J, Vijg J. Does damage to DNA and other macromolecules play a role in aging? If so, how? J Gerontol A Biol Sci Med Sci. 2009;64:175–8. doi: 10.1093/gerona/gln065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carozzi V, Chiorazzi A, Canta A, Oggioni N, Gilardini A, Rodriguez-Menendez V, Avezza F, Crippa L, Ceresa C, Nicolini G, Bossi M, Cavaletti G. Effect of the chronic combined administration of cisplatin and paclitaxel in a rat model of peripheral neurotoxicity. Eur J Cancer. 2009;45:656–65. doi: 10.1016/j.ejca.2008.10.038. [DOI] [PubMed] [Google Scholar]
- Carozzi VA, Canta A, Oggioni N, Sala B, Chiorazzi A, Meregalli C, Bossi M, Marmiroli P, Cavaletti G. Neurophysiological and neuropathological characterization of new murine models of chemotherapy-induced chronic peripheral neuropathies. Exp Neurol. 226:301–9. doi: 10.1016/j.expneurol.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Ceballos D, Cuadras J, Verdu E, Navarro X. Morphometric and ultrastructural changes with ageing in mouse peripheral nerve. J Anat. 1999;195(Pt 4):563–76. doi: 10.1046/j.1469-7580.1999.19540563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. A mouse model of Werner Syndrome: what can it tell us about aging and cancer? Int J Biochem Cell Biol. 2005;37:991–9. doi: 10.1016/j.biocel.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Chaudhry V, Rowinsky EK, Sartorius SE, Donehower RC, Cornblath DR. Peripheral neuropathy from taxol and cisplatin conbination chemotherapy: clinical and electrophysiological studies. Annals of Neurology. 1994;35:304–311. doi: 10.1002/ana.410350310. [DOI] [PubMed] [Google Scholar]
- Crisci AR, Ferreira AL. Low-intensity pulsed ultrasound accelerates the regeneration of the sciatic nerve after neurotomy in rats. Ultrasound Med Biol. 2002;28:1335–41. doi: 10.1016/s0301-5629(02)00576-8. [DOI] [PubMed] [Google Scholar]
- Dolle ME, Busuttil RA, Garcia AM, Wijnhoven S, van Drunen E, Niedernhofer LJ, van der Horst G, Hoeijmakers JH, van Steeg H, Vijg J. Increased genomic instability is not a prerequisite for shortened lifespan in DNA repair deficient mice. Mutat Res. 2006;596:22–35. doi: 10.1016/j.mrfmmm.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Dorfman LJ, Bosley TM. Age-related changes in peripheral and central nerve conduction in man. Neurology. 1979;29:38–44. doi: 10.1212/wnl.29.1.38. [DOI] [PubMed] [Google Scholar]
- Frappart PO, McKinnon PJ. Mouse models of DNA double-strand break repair and neurological disease. DNA Repair (Amst) 2008;7:1051–60. doi: 10.1016/j.dnarep.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T. DNA Repair and Mutagenesis. American Society for Microbiology; Washington, D.C.: 2006. [Google Scholar]
- Geiger S, Schlemmer M, Heinemann V, Stemmler HJ. Adjuvant cisplatin-based chemotherapy for resected NSCLC: one size fits all? Anticancer Drugs. 21:799–804. doi: 10.1097/CAD.0b013e32833dbbfe. [DOI] [PubMed] [Google Scholar]
- Hasty P, Campisi J, Hoeijmakers J, van Steeg H, Vijg J. Aging and genome maintenance: lessons from the mouse? Science. 2003;299:1355–9. doi: 10.1126/science.1079161. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–47. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Knox CA, Kokmen E, Dyck PJ. Morphometric alteration of rat myelinated fibers with aging. J Neuropathol Exp Neurol. 1989;48:119–39. doi: 10.1097/00005072-198903000-00001. [DOI] [PubMed] [Google Scholar]
- Kraemer KH, Patronas NJ, Schiffmann R, Brooks BP, Tamura D, DiGiovanna JJ. Xeroderma pigmentosum, trichothiodystrophy and Cockayne syndrome: a complex genotype-phenotype relationship. Neuroscience. 2007;145:1388–96. doi: 10.1016/j.neuroscience.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krarup-Hansen A, Fugleholm K, Helweg-Larsen S, Hauge EN, Schmalbruch H, Trojaborg W, Krarup C. Examination of distal involvement in cisplatin-induced neuropathy in man. An electrophysiological and histological study with particular reference to touch receptor function. Brain. 1993;116(Pt 5):1017–41. doi: 10.1093/brain/116.5.1017. [DOI] [PubMed] [Google Scholar]
- LoMonaco M, Milone M, Batocchi AP, Padua L, Restuccia D, Tonali P. Cisplatin neuropathy: clinical course and neurophysiological findings. Neurology. 1992;239:199–204. doi: 10.1007/BF00839140. [DOI] [PubMed] [Google Scholar]
- McHugh PJ, Spanswick VJ, Hartley JA. Repair of DNA interstrand crosslinks: molecular mechanisms and clinical relevance. Lancet Oncol. 2001;2:483–90. doi: 10.1016/S1470-2045(01)00454-5. [DOI] [PubMed] [Google Scholar]
- Mold JW, Vesely SK, Keyl BA, Schenk JB, Roberts M. The prevalence, predictors, and consequences of peripheral sensory neuropathy in older patients. J Am Board Fam Pract. 2004;17:309–18. doi: 10.3122/jabfm.17.5.309. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Miki T. Recent strategy for the management of advanced testicular cancer. Int J Urol. 17:148–57. doi: 10.1111/j.1442-2042.2009.02431.x. [DOI] [PubMed] [Google Scholar]
- Niedernhofer LJ, Odijk H, Budzowska M, van Drunen E, Maas A, Theil AF, de Wit J, Jaspers NG, Beverloo HB, Hoeijmakers JH, Kanaar R. The structure-specific endonuclease Ercc1-Xpf is required to resolve DNA interstrand cross-link-induced double-strand breaks. Mol Cell Biol. 2004;24:5776–87. doi: 10.1128/MCB.24.13.5776-5787.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedernhofer LJ, Garinis GA, Raams A, Lalai AS, Robinson AR, Appeldoorn E, Odijk H, Oostendorp R, Ahmad A, van Leeuwen W, Theil AF, Vermeulen W, van der Horst GT, Meinecke P, Kleijer WJ, Vijg J, Jaspers NG, Hoeijmakers JH. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature. 2006;444:1038–43. doi: 10.1038/nature05456. [DOI] [PubMed] [Google Scholar]
- Niedernhofer LJ. Tissue-specific accelerated aging in nucleotide excision repair deficiency. Mech Ageing Dev. 2008a;129:408–15. doi: 10.1016/j.mad.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedernhofer LJ. Nucleotide excision repair deficient mouse models and neurological disease. DNA Repair (Amst) 2008b;7:1180–9. doi: 10.1016/j.dnarep.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney RK. Clinical trials for polyneuropathy: the role of nerve conduction studies, quantitative sensory testing, and autonomic function testing. J Clin Neurophysiol. 1998;15:129–37. doi: 10.1097/00004691-199803000-00005. [DOI] [PubMed] [Google Scholar]
- Parmar K, D’Andrea A, Niedernhofer LJ. Mouse models of Fanconi anemia. Mutat Res. 2009;668:133–40. doi: 10.1016/j.mrfmmm.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick HE, Vinik AI, Schwartz AV, Leveille SG, Brancati FL, Balfour J, Guralnik JM. Independent effects of peripheral nerve dysfunction on lower-extremity physical function in old age: the Women’s Health and Aging Study. Diabetes Care. 2000;23:1642–7. doi: 10.2337/diacare.23.11.1642. [DOI] [PubMed] [Google Scholar]
- Richardson JK. The clinical identification of peripheral neuropathy among older persons. Arch Phys Med Rehabil. 2002;83:1553–8. doi: 10.1053/apmr.2002.35656. [DOI] [PubMed] [Google Scholar]
- Sanchez SE, Trevino JG. Current adjuvant and targeted therapies for pancreatic adenocarcinoma. Curr Med Chem. 2008;15:1674–83. doi: 10.2174/092986708784872348. [DOI] [PubMed] [Google Scholar]
- Sattin RW. Falls among older persons: a public health perspective. Annu Rev Public Health. 1992;13:489–508. doi: 10.1146/annurev.pu.13.050192.002421. [DOI] [PubMed] [Google Scholar]
- Screnci D, McKeage MJ, Galettis P, Hambley TW, Palmer BD, Baguley BC. Relationships between hydrophobicity, reactivity, accumulation and peripheral nerve toxicity of a series of platinum drugs. Br J Cancer. 2000;82:966–72. doi: 10.1054/bjoc.1999.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijbers AM, de Laat WL, Ariza RR, Biggerstaff M, Wei YF, Moggs JG, Carter KC, Shell BK, Evans E, de Jong MC, Rademakers S, de Rooij J, Jaspers NG, Hoeijmakers JH, Wood RD. Xeroderma pigmentosum group F caused by a defect in a structure-specific DNA repair endonuclease. Cell. 1996;86:811–22. doi: 10.1016/s0092-8674(00)80155-5. [DOI] [PubMed] [Google Scholar]
- Taylor PK. Non-linear effects of age on nerve conduction in adults. J Neurol Sci. 1984;66:223–34. doi: 10.1016/0022-510x(84)90011-x. [DOI] [PubMed] [Google Scholar]
- Tohgi H, Tsukagoshi H, Toyokura Y. Quantitative changes with age in normal sural nerves. Acta Neuropathol. 1977;38:213–20. doi: 10.1007/BF00688067. [DOI] [PubMed] [Google Scholar]
- Verdu E, Buti M, Navarro X. Functional changes of the peripheral nervous system with aging in the mouse. Neurobiol Aging. 1996;17:73–7. doi: 10.1016/0197-4580(95)02010-1. [DOI] [PubMed] [Google Scholar]
- Verdu E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst. 2000;5:191–208. doi: 10.1046/j.1529-8027.2000.00026.x. [DOI] [PubMed] [Google Scholar]
- Vijg J. The role of DNA damage and repair in aging: new approaches to an old problem. Mech Ageing Dev. 2008;129:498–502. doi: 10.1016/j.mad.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Weeda G, Donker I, de Wit J, Morreau H, Janssens R, Vissers CJ, Nigg A, van Steeg H, Bootsma D, Hoeijmakers JH. Disruption of mouse ERCC1 results in a novel repair syndrome with growth failure, nuclear abnormalities and senescence. Curr Biol. 1997;7:427–39. doi: 10.1016/s0960-9822(06)00190-4. [DOI] [PubMed] [Google Scholar]
- Yoon MS, Katsarava Z, Obermann M, Schafers M, Liedert B, Dzagnidze A, Kribben A, Egensperger R, Limmroth V, Diener HC, Thomale J. Erythropoietin overrides the triggering effect of DNA platination products in a mouse model of cisplatin-induced neuropathy. BMC Neurosci. 2009;10:77. doi: 10.1186/1471-2202-10-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeimet AG, Reimer D, Radl AC, Reinthaller A, Schauer C, Petru E, Concin N, Braun S, Marth C. Pros and cons of intraperitoneal chemotherapy in the treatment of epithelial ovarian cancer. Anticancer Res. 2009;29:2803–8. [PubMed] [Google Scholar]