Abstract
Apart from its role in axon guidance, netrin-1 is also known to be pro-angiogenic. The aim of this study is to determine whether adeno-associated viral (AAV) mediated overexpression of netrin-1 improves post-stroke neurovascular structure and recovery of function. AAV-Netrin-1 or AAV-LacZ of 1×1010 genome copies each was injected medial and posterior to ischemic lesion at one hour following reperfusion using the distal middle cerebral artery occlusion (MCAO) method. Quantitative RT-PCR revealed that the expression of netrin-1 transgene began as early as one day and increased dramatically about 3 weeks following vector injection. Western blot analysis and confocal microscopy suggested that both the endogenous and transduced netrin-1 were expressed in the neurons of the peri-infarct cortex after MCAO. AAV-mediated netrin-1 overexpression significantly increased vascular density in the peri-infarct cortex and promoted the migration of immature neurons into the peri-infarct white matter, but it did not significantly reduce infarct size. Netrin-1 overexpression also enhanced post-stroke locomotor activity, improved exploratory behavior, and reduced ischemia-induced motor asymmetry in forelimb usage. However, it had little effect on post-stroke spatial learning and memory. Our results suggest that AAV mediated netrin-1 overexpression improves peri-infarct vascular density and post stroke motor function.
Keywords: forelimb asymmetry, distal MCA occlusion, Barnes maze, microvessel density, doublecortin, angiogenesis
Introduction
Netrins are guidance proteins crucial for neural and vascular development (Freitas et al. 2008). Netrin-1, one of the three members in the mammalian netrin family, has a bidirectional role in axon guidance. It attracts or repels axons according to which netrin receptor is expressed on an individual axon (Metin et al. 1997; Serafini et al. 1996). Netrin-1 is essential in directing axons relative to the ventral midline of the developing nervous system. Mice deficient in netrin-1 exhibit disruption in the formation of major axon projections to the midline, resulting in the agenesis of corpus callosum and hippocampal commissure (Serafini 1996; Faseli 1997). Apart from its involvement in midline guidance, netrin-1 is also implicated in the axonal growth of the hippocampal formation (Steup et al. 2000), corticospinal tract (Finger et al. 2002), and thalamocortical projections (Braisted et al. 2000). Contrary to its attractive role during neural development, netrin-1 is described as a myelin-associated inhibitor in the adult spinal cord, contributing to axon growth failure after spinal cord injury (Low et al. 2008).
Netrin-1 also serves as a potent vascular mitogen, stimulating proliferation, inducing migration, and promoting adhesion of endothelial cells and vascular smooth muscle cells. Netrin-1 not only stimulates angiogenesis in vivo but also augments the response to vascular endothelial growth factor (VEGF) (Park et al. 2004). The angiogenic effect of netrin-1 offers unique therapeutic potentials in restoring blood circulation under pathological conditions. For example, netrin-1 accelerates neovascularization in an in vivo model of hindlimb ischemia and reverses neuropathy and vasculopathy in a murine model of diabetes (Wilson et al. 2006). Furthermore, netrin-1 introduced by adeno-associated viral (AAV) gene transfer induces neovascularization in the adult mouse brain (Fan et al. 2008), while recombinant netrin-1 protein increases the density of functionally competent blood vessels in the infarcted heart (Ahmed et al. 2010).
Some of the pro-angiogenic benefits of netrin-1 can be attributed to its anti-apoptotic potential. By blocking the pro-apoptotic effect of its receptor uncoordinated 5B (UNC5B), netrin-1 acts as a survival factor for developing endothelial cells (Castets et al. 2009). Besides the anti-apoptotic effect on vascular cells, netrin-1 is also a survival factor during hindbrain development (Marcos et al. 2009). In the adult tissue, netrin-1 reduces ischemia reperfusion injury in the kidneys and hearts by suppressing apoptosis (Wang et al. 2009a; Zhang and Cai 2010). In addition, intraventricularly-administered netrin-1 was shown to reduce infarct volume and apoptosis in a murine model of experimental stroke (Wu et al. 2008). Furthermore, netrin-1 receptors are implicated in the regulation of differentiation and migration of stem cells in the adult mouse and human subventricular zone (SVZ) (Bradford 2010), and in amphetamine- induced neuroplasticity (Yetnikoff et al. 2007). In light of the multiple effects of netrin-1 in inducing neovascularization and neuroplasticity in the adult brain, the current study set out to determine the therapeutic effect of netrin-1 via AAV gene transfer in experimental cortical stroke. Here, we show that virally-transduced netrin-1 gene was expressed in peri-infarct brain tissue and successfully produced netrin-1 protein. Although overexpression of netrin-1 via AAV gene transfer did not reduce the size of ischemic infarct, it improved post stroke motor function. In addition, functional restoration induced by netrin-1 gene therapy was associated with an increase in vascular density in the peri-infarct cortex.
Materials and methods
Experimental stroke model and adeno-associated virus gene delivery
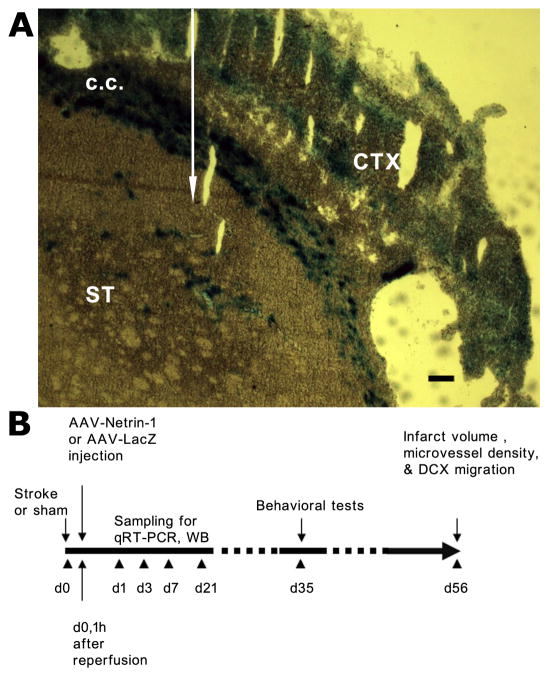
This study was conducted in accordance with the animal care guidelines issued by the National Institutes of Health and by the San Francisco Veterans Affairs Medical Center Animal Care and Use Committee. Stroke was induced unilaterally in rats by the distal middle cerebral artery occlusion (dMCAO) method under isoflurane (1.5%)/O2(30%)/N2O(68.5%) anesthesia as described previously (Matsumori et al. 2006a). In brief, the main trunk of the left MCA was ligated just underneath the rhinal fissure with a 10-0 suture, and the bilateral common carotid arteries (CCA) were occluded for 60 min with 4-0 sutures. The sutures were then removed to restore blood flow, and the cervical incision was closed. Sham-operated rats did not receive occlusion of either the MCA or the CCAs. Body temperature was maintained at 37±0.5 °C during the procedures. The engineering and production of the adeno-associated virus (AAV) vectors used in this study were previously described in detail (Fan et al. 2008). In brief, the recombinant plasmid pAAV-Netrin-1 was constructed by inserting a full-length chicken netrin-1 cDNA (about 2 kb) between the 1 inverted terminal repeats of AAV genome. CMV promoter was used to drive gene expression. Two helper plasmids, one containing adenoviral VA, E2A, and E4 regions and the other containing the AAV rep and AAV serotype one cap genes (kindly provided by Xiao Xiao, University of North Carolina) were co-transfected with pAAV-Netrin-1 into HEK 293 cells to package the AAV vector. Vectors were purified by CsCl density gradient their titers determined by dot blot analysis. AAV-LacZ was produced in a similar fashion and used as a control vector for this study. 1×1010 genome copies of AAV-Netrin-1 or AAV-LacZ were injected one hour following reperfusion in brain regions medial and posterior to the ischemic lesion in the ipsilateral striatum (as shown in Figure 1A), at the respective stereotaxic coordinates: (AP 0.5, LM 3.5, Depth -4 mm) and (AP -1.9, LM 4, Depth -4.5 mm). The extent of LacZ expression was visualized following incubation of formalin-fixed frozen sections with X-gal and buffers using the NovaUltra Special Stain Kit (IHC World, Woodstock, MD). To ensure complete dispersion of the virus into brain tissue, the needle remained at the injection site for at least 15 minutes before being pulled out. To determine the endogenous netrin-1 expression and time course following ischemic stroke, a separate group of rats underwent the same dMCAO procedures without vector injection. The schematic for experiments and corresponding time line is shown in Figure 1B.
Figure 1.
AAV injection site and the schematic of experimental design. A, A representative photomicrograph shows the extent of spreading of AAV-lacZ vector upon injection into striatum as indicated by the white arrow. LacZ staining was visualized as blue color following incubation with X-gal and buffers. C.C: corpus callosum; ST: striatum; CTX: cortex. Scale bar: 250 µm. B, AAV-Netrin-1 or AAV-LacZ was injected one hour following reperfusion in brain regions medial and posterior to the ischemic lesion in the ipsilateral striatum at the respective stereotaxic coordinates described in the materials and methods. Samples were collected at various time points as indicated for double immunofluorescence staining, qRT-PCR and western blotting analyses. Behavioral tests were conducted at 5 weeks after dMCAO and euthanized at 8 weeks after dMCAO.
Immunohistochemistry and double immunofluorescence staining
Rats were anesthetized and perfused transcardially with 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB), at pH 7.4. The brains were removed, post-fixed overnight in 4% PFA-PB and placed in a 20% sucrose solution for 48 hours. Forty-micron-thick coronal sections were cut on a microtome and collected serially. Endogenous netrin-1 expression was determined with chicken-anti-mouse netrin-1 antibody (1:3000), followed by goat-anti-chicken IgY (1:400) secondary antibody, ABC solution (Vector laboratories, Burlingame, CA) and 0.05% diaminobenzine-tetrachloride (DAB Fast; Sigma, St. Louis, MO). For double immunofluorescence staining, Streptavidine Alexa Fluor 488 and Alexa Fluor 594 goat anti-mouse IgG conjugate (5μg/ml, each, Molecular Probes, Eugene, OR) were used after the incubation with the following primary antibodies: mouse-anti-NeuN (1:1000), mouse-anti-GFAP (1:150), or mouse-anti-rat RECA1 (1:1000). Fluorescence signals were revealed and detected by Zeiss LSM 510 confocal image system (Zeiss, Thornwood, NY), with a sequential scanning model. Stacks of images (1024×1024 pixels) were obtained and processed with Adobe Photoshop as previously described (Adobe System, Mountain View, CA) (Liu et al. 2007).
Quantitative real-time RT-PCR
Cortex ipsilateral to the lesion at Bregma level +2.0 ~ −5.0 mm was dissected using the underlying white matter as the landmark, and processed for RNA extraction and purification. Reverse transcription was performed with SuperScript III First-Strand Synthesis Kit (Invitrogen, Carlsbad, CA). A SYBR Green Detection and Applied Biosystems Prism 7900HT detection system was used to amplify transcribed cDNA for 40 cycles. The real time thermal cycler program consists of 4 stages. Stage One: 50 °C for 2 minutes; Stage Two: 95 °C for 10 minutes; Stage Three: 95 °C for one second, followed by 66.5 °C for 20 seconds, and then 72 °C for 20 seconds (repeated 40 times); and Stage Four: 95 °C for 15 seconds, followed by 66.5 °C for 15 seconds, and then 95 °C for 15 seconds. The copy numbers of netrin-1 mRNA was normalized to that of the internal control β-actin. Rat- and chicken-specific primers were used in the qRT-PCR to detect the expression of endogenous and transduced netrin-1 gene expression, respectively. These primers were designed based on the non-homologous regions between rat and chicken netrin-1 cDNA. For endogenous netrin-1, the r2 primers were AAGCAGGGCACAAGTCGTA (upper) and CAGGCGAGTCCTCAGCAT (lower). For transduced netrin-1, the c3 primers were CTGTGGGTGCACGCCAAG (upper) and CGTGTCCCGCCATTGGAT (lower).
Western blotting
Peri-infarct cortex was dissected and processed as described previously (Matsumori et al. 2006b). Protein concentrations were determined by the Lowry method using a BioRad DC protein assay kit. 15–20 μg of proteins were loaded onto a 10% acrylamide gel for electrophoresis. A Bio-Rad Mini Trans-Blot® Electrophoretic Transfer Cell was used to transfer the proteins onto polyvinylidene fluoride membranes (Bio-Rad, Richmond, CA, USA). The membrane was then blocked with 5% non-fat milk in Tris-buffered saline, with 0.1% Tween-20 for 1 hour, followed by incubation with primary antibody overnight at room temperature. After washing, the membrane was incubated with secondary antibody, followed by ECL detection (Amersham) and film (GE Healthcare) exposure (Matsumori et al. 2006b). β-actin was used as the internal control. The primary antibodies were: rabbit-anti-mouse netrin-1 (1:500, Abcam) and goat-anti-actin (1:1000, Santa Cruz). The secondary antibodies were goat-anti-rabbit-HRP (1:1000, Santa Cruz) and donkey-anti-goat-HRP (1:1000, Santa Cruz).
Assessment of neurobehavioral outcomes
Five weeks following AAV gene transfer, rats were subjected to behavioral tests in the following order (Liu et al. 2007; Rapp et al. 2008) (n= 11 for AAV-LacZ; n= 10 for AAV-Netrin-1).
Open Field test
Rats were placed in a brightly lit, square Plexiglas enclosure (40 × 40 inches), surrounded by automated infrared photocells interfaced with a computer (Hamilton & Kinder, San Diego, CA) to record the data. On each of 3 consecutive days, open field activity was recorded for 10 minutes after an initial 1-minute adaptation period. For analysis of the exploratory behavior, the arena was divided on a zone map consisting of a center region (15 × 15 inches), four corner regions of 7.5 × 7.5 inches each, and a peripheral region (the remaining area).
Forelimb use asymmetry test
Forelimb use during explorative activity was analyzed in a 10-min videotaped session. Each behavior was expressed as a percentage in the independent use of either limb or the simultaneous use of both limbs during wall exploration.
Horizontal ladder test
Rats were videotaped while traversing a 30° ladder (60 cm in length with variable spacing between bars ranging from 1–4 cm). The percentages of footfalls (slipping through the bars) with the affected or unaffected forelimb were recorded and analyzed in 3 consecutive trials.
Rotor-rod test
Rats were tested on an accelerating rotor-rod (San Diego Instruments, San Diego, CA) with a one-min adaptation period before each trial and an acceleration of 5 rpm every 15 seconds (final speed capped at 40 rpm). The latency to fall from the rod in 3 consecutive trials was averaged.
Barnes Maze test
The Barnes Maze was used to measure spatial memory acquisition and retention. A black acrylic escape tunnel was placed under one of the holes on a circular platform (120 cm in diameter), with 18 holes (each 10 cm in diameter) along the platform perimeter (Hamilton Kinder, Poway, CA). To rule out spatial preference, rats from each treatment group were randomly assigned to locate the escape tunnel from one of the three pre-determined locations. A Noldus EthoVision video tracking system (Noldus, Leesburg, VA) was used to record and analyze the data. Rats were trained to locate the escape tunnel in 2 successive daily sessions (3 trials per session, 3 minutes per trial), with a 1-hour intersession interval from different counterbalanced starting positions. A 3-min probe trial was performed 24 hours after the last trial on day 6.
Infarct volume assessment
The same animals underwent behavioral tests were euthanized for histological analyses of infarct size, vessel density and DCX staining. Infarct volume was measured by subtracting the volume of intact tissue in the ipsilateral hemisphere from that in the contralateral hemisphere in NeuN-stained serial sections (480 μm apart), by unbiased stereology (StereoInvestigator, MicroBrightField, VA) (Matsumori et al. 2006a).
Microvessel density quantification
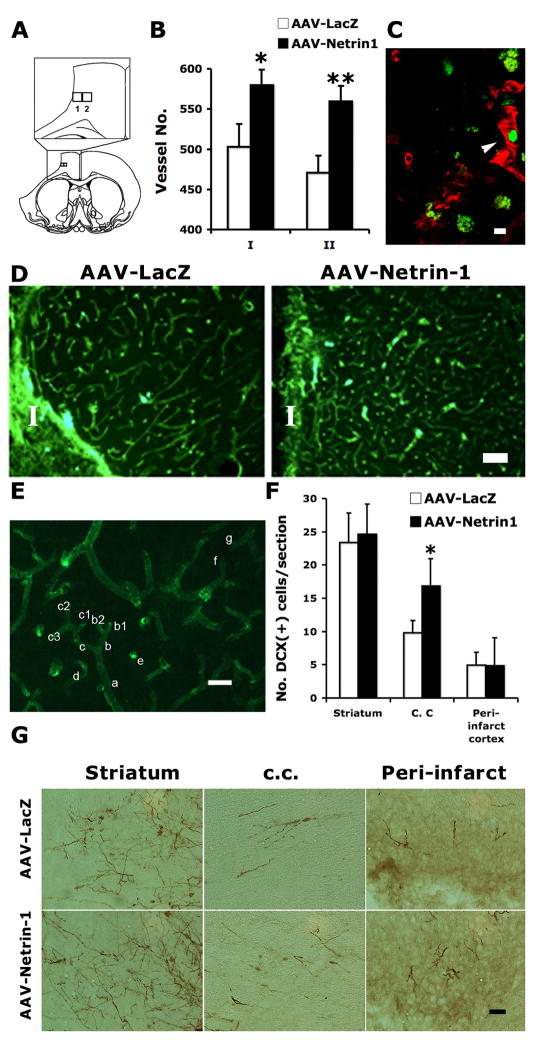
Microvessel density was quantified in the peri-infarct cortex as previously described with modifications (Weidner 1995). Briefly, the sections were incubated overnight with mouse-anti-rat RECA-1 antibody (1:1000 dilution, AbD Serotec, Oxford, UK). After washing, the sections were incubated with Cy2-conjugated goat-anti-mouse fluorescent antibody (1:50 dilution, Jackson ImmunoResearch, West Grove, PA). For each animal, four serial sections spanning AP −0.26 to 0.20 mm were imaged using a 40X objective. In each image, blood vessels were counted in two rectangular grids, each 280 × 250 μm in size. Grid 1 is directly adjacent to infarct margin. Grid 2 is medial to grid 1 as shown in Figure 8A. The rules for microvessel counting are elaborated below using Figure 8E as an example: (1) Only include the end terminals if bifurcations exist. For example, in the clusters of a-c, count only the final branches such as “b1”, “b2”, “c1”, “c2” and “c3”. “a”, “b” or “c” are not counted; (2) For vessel “d”, since there is no apparent evidence that it comes from any of the adjacent vessels (confirmed by adjusting the microscope focus up and down), it should be counted; (3) “e” is the cross section of a vessel (instead of background staining), thus should be counted; (4) “f” and “g”, although appear discontinuous in the image, are the same vessel with the same diameter and running direction (confirmed by adjusting the microscope focus up and down), thus should be counted only once.
Figure 8. AAV-Netrin-1 gene therapy increases microvessel density in the peri-infarct cortex and preferentially increases immature neuron migration into ipsilateral white matter.
A, Diagrams showing the location of grids 1 and 2 in relation to ischemic infarct. Grid 1 covers a rectangular area of 280 × 250 μm adjacent to the infarct border. Grid 2 is medial to grid 1, which is the same size as grid 1. B, Blood vessels were counted in both grids in 4 serial sections following immunostaining with mouse-anti-rat RECA-1 antibody. There was a significant increase in the density of microvessels in stroke rats that received AAV-Netrin-1, compared to those that received AAV-LacZ in both counting areas. *: p<0.05; **: p<0.01. C, Co-localization between Ki67 (green) and mouse-anti-rat RECA1 in the peri-infarct of a rat receiving AAV-Netrin-1 suggests the presence of newly formed blood vessels (arrowhead). Scale bar: 10 μm. D, Representative photomicrographs showing the blood vessels stained with mouse-anti-rat RECA-1 antibody in the peri-infarct cortex of animals receiving AAV-LacZ and AAV-Netrin-1. I: ischemic core. Scale bar: 100 μm. E, An image of RECA-1 staining showing the labeled microvessels. The rules for microvessel counting are described in detail in the material and method section. Scale bar: 15 μm. F, There was a significant increase in the number of DCX immunoreactive cells detected in the ipsilateral corpus callosum below the ischemic infarct (*: p<0.05). However, there was no difference in the number of DCX cells quantified in either the striatum or peri-infarct cortex. G, Representative photomicrographs showing DCX immunoreactive neuroblasts in the ipsilateral striatum, corpus callosum (c. c.), and peri-infarct cortex at 5 weeks after gene therapy. Scale bar: 50 μm.
Quantification of migrating neuroblasts
Migrating immature neurons were detected by immunohistochemistry with goat-anti-doublecortin (DCX) C-18 antibody (1:250 dilution, Santa Cruz Biotechnology, Santa Cruz, CA), followed by biotin-conjugated donkey-anti-goat secondary antibody (1:1000 dilution, Jackson ImmunoResearch, West Grove, PA) and visualized by DAB. For each brain, two sections at the injection level were chosen. Images of the whole sections were taken using Virtual Slice module of Stereo Investigator. DCX positive cells were counted in striatum, white matter (corpus callosum) and peri-infarct cortex. Cells in the subventricular zone and ventricular lining (within 50 μm from the ventricle wall) were too dense to be properly estimated in the 40-μm sections and, thus, were not counted.
Statistical Analysis
Data were expressed as mean ± SEM and analyzed by ANOVA or repeated measure ANOVA when appropriate, using the StatView software (SAS Institute, Cary NC). Differences between groups were considered significant when p<0.05.
Results
Focal cerebral ischemia induces the expression of Netrin-1 in peri-infarct neurons
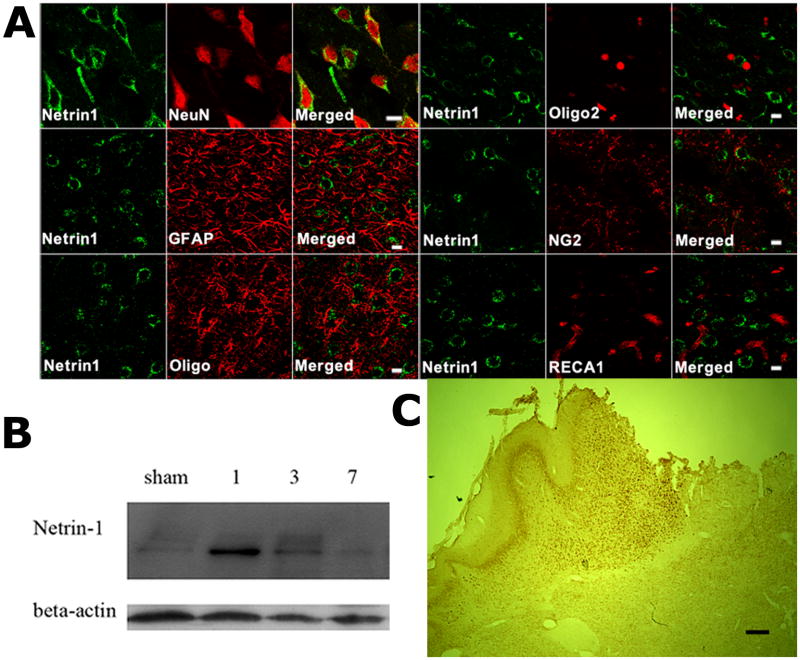
In stroke rats without viral vector injection, the expression of endogenous netrin-1 protein was detected mainly in neurons but not in astrocytes of the peri-infarct cortex one day after MCAO (Figure 2A&C). Unlike the vascular expression of netrin-4 reported after cerebral focal ischemia (Hoang et al. 2009), netrin-1 was not found in either rat endothelial cell antigen-1 (RECA1)-expressing blood vessel cells in the peri-infarct (Figure 2A) or other brain regions (not shown). Although a previous report suggests that netrin-1 is expressed by oligodendrocytes in the adult spinal cord (Manitt et al. 2001), we failed to detect co-localization between netrin-1 and 3 oligodendrocyte markers in the peri-infarct cortex by confocal microscopy (Figure 2A). The temporal pattern of netrin-1 protein expression following MCAO, as determined by western blotting, suggests that there was a robust but transient increase in netrin-1 expression after 1 day, waning 7 days following MCAO (Figure 2B).
Figure 2. Early induction of endogenous netrin-1 in the neurons of the peri-infarct cortex following MCAO.
A, Confocal images showing double immunofluorescence staining of Netrin-1 (green) with cell type specific markers (red) including NeuN, GFAP, Oligo, Oligo2, NG2 or RECA at one day after MCAO. The results suggest that post-stroke endogenous netrin-1 was predominantly expressed by neurons. There was no netrin-1 immunoreactivity detected in GFAP-expressing astrocytes, oligodendrocytes, or RECA1-expressing blood vessels (n=3 per group). Scale bars: 10 μm. B, Western blots showing endogenous netrin-1 expression (rabbit-anti-mouse netrin-1) in sham, 1, 3, or 7 days after dMCAO. A robust induction of netrin-1 began as early as one day after MCAO, and lasted for one week. (n=3 per group). C, A low magnification view of endogenous netrin-1 expression suggests that it is mainly expressed in the peri-infarct cortex. Scale bar: 200 μm.
AAV-Netrin-1 gene transfer induces an increase in transduced Netrin-1 mRNA and protein in the peri-infarct cortex
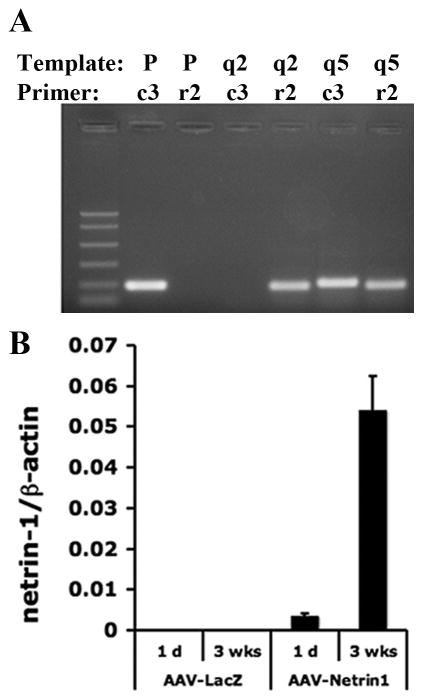
The AAV-Netrin-1 vector was capable of producing RNA transcripts and protein when transfected into HEK293 cells (Fan et al. 2008), so in order to determine the functional efficacy of AAV-Netrin-1 for the treatment of ischemic stroke, we first investigated the transgene expression in AAV-Netrin-1 injected brain. Following distal MCAO, vectors containing AAV-Netrin-1 or AAV-LacZ were injected into peri-infarct at one hour after reperfusion. In addition to the peri-infarct cortex, AAV were found in the ipsilateral corpus callosum (cc) and dorsolateral striatum underneath the cc (Figure 1A), but never found in the contralateral hemisphere (Shen et al. 2006a; Shen et al. 2006b). In order to differentiate the transduced netrin-1 mRNA from the endogenous counterpart, primers targeting the non-homologous regions of rat and chicken netrin-1 cDNA were designed, with specificity validated by PCR using corresponding templates (Figure 3A). Expression of chicken netrin-1 was detected as early as one day after intracerebral injection of AAV-Netrin-1 and significantly increased at 3 weeks following gene transfer (Figure 3B). Thus, the expression of transduced netrin-1 messenger RNA corroborated a strong expression of transduced netrin-1 protein in the ipsilateral cortex at 3 weeks following gene transfer (Figure 4A). Similar to endogenous netrin-1, transduced netrin-1 was mainly found in neurons of the peri-infarct cortex with a colocalization rate of 92.8±2.6% (Figure 4B), consistent with the neurotrophic nature of AAV1 (Passini et al. 2003) (de Backer et al. 2010).
Figure 3. AAV-Netrin-1 vector produces netrin-1 gene transcript in the peri-infacrt cortex.
A, Validation of the specificity of rat and chicken primers by PCR. Rat-specific (r2) and chicken-specific (c3) primer sets were used in the PCR reactions with chicken netrin-1 containing plasmid (P), cDNA from the ipsilateral cortex of a dMCAO rat injected with AAV-Netrin-1 (q5) or AAV-LacZ (q2) as the templates (labeled as Temp) at annealing temperature 66.5°C. The chicken specific primers c3 generated PCR products only when P and q5 were used as templates. Conversely, the rat-specific primers r2 only produced PCR products in cDNA templates derived from q2 and q5, both of which expressed endogenous rat netrin-1 gene. B, Transduced netrin-1 containing vector survived in the peri-infarct cortex and produced netrin-1 mRNA. The relative amount of chicken netrin-1 expression was determined by quantitative real-time RT-PCR using primers specific to chicken netrin-1 cDNA sequence (c3). The level of chicken Netrin-1 mRNA expression was reflected by the ratio of copy numbers, relative to that of the internal control (β-actin). Transduced netrin-1 message was detected one day after viral transduction and maintained at a high level of expression 3 weeks later. (n=3 per group).
Figure 4. AAV-Netrin-1 gene transfer leads to an increase in transduced Netrin-1 protein.
A, AAV-Netrin-1 gene therapy resulted in a significant increase in transduced Netrin-1 protein (detected by goat-anti-chicken netrin-1) in the peri-infarct cortex of dMCAO rats by western blotting 3 weeks following AAV-LacZ or AAV-Netrin-1 injection. (n=3 per group). B, Confocal images showing double immunofluorescence staining of chicken netrin-1 (green) with cell type specific markers (red) including NeuN, GFAP, Oligo, Oligo2, NG2 or RECA. The results suggest that the transduced netrin-1 following AAV gene transfer was predominantly expressed by neurons. (n=3 per group). Scale bars: 10 μm.
AAV-Netrin-1 gene therapy enhances post-stroke locomotor activity and improves exploratory behavior
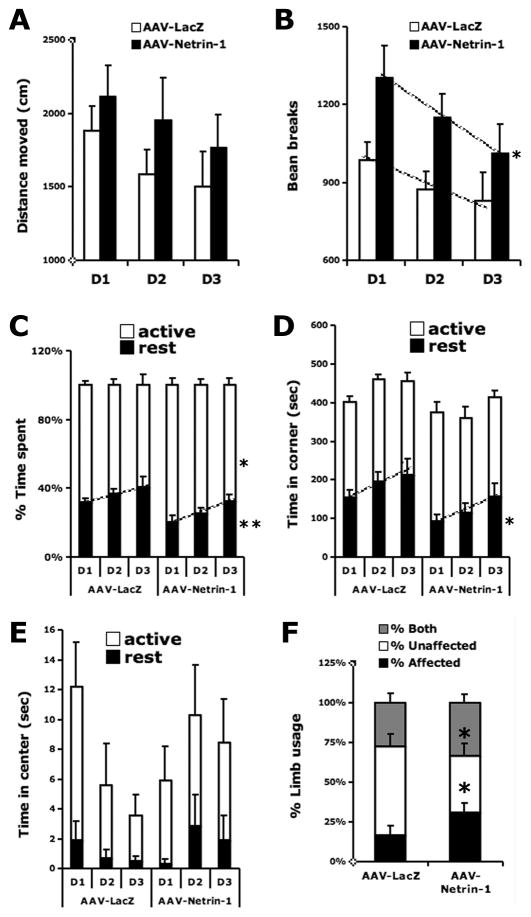
We next determined whether the increased netrin-1 protein following AAV-Netrin-1 transduction led to a recovery of locomotor activity following MCA stroke. Stroke rats that received AAV-Netrin-1 gene therapy displayed significantly increased movement (F1,38= 6.2, p<0.05), spent more time being active (F1,38= 6.3, p<0.05) and less time resting (F1,38= 9.6, p<0.01) while exploring the open arena over 3 days, compared to those that received control viral vector (Figure 5A-C). Because the novel environment in the open field concurrently evokes both anxiety and exploration (Dulawa et al. 1999; Prut and Belzung 2003), an increase in time spent in the center of the open field suggests reductions in anxiety and/or increases in exploration (Dulawa et al. 1999). We found that stroke rats treated with AAV-LacZ spent more time resting in the corner zone of an open arena, compared to those injected with AAV-Netrin-1 (F1,38= 6.8, p<0.05; Figure 5D). AAV-Netrin-1 gene therapy also increased the tendency of stroke rats to rest in the center zone during open field test, which indicates a reduction in anxiety-like behavior (Interaction: day × rest time center zone: F1,38= 2.9, p= 0.06; Figure 5E).
Figure 5. Netrin-1 gene transfer enhances post-stroke locomotor activity, improves exploratory behavior and reduces ischemia-induced motor asymmetry in limb usage.
MCAO rats that received AAV-Netrin-1 gene transfer displayed increased movement, compared to those that received AAV-LacZ (B) over 3 days in the novel open field test (*: p<0.05). The AAV-Netrin-1 MCAO rats also had a tendency to move greater distances (p=0.13) (A), spend more time being active (*: p<0.05), and spend less time resting (**: p<0.01) (C). MCAO rats that received AAV-Netrin-1 gene transfer spent less rest time in the corner zone compared to those that received AAV-LacZ (D) (*: p<0.05). The AAV-Netrin-1 group spent more time resting in the center zone as the days progressed, a sign of being less anxious (Z2 rest: treatment × zone interaction: p=0.06) (E). Netrin-1 gene transfer decreased the favoring use of the unaffected limb after MCAO (*: p<0.05) and increased the use of the affected limb (*: p<0.05) during exploratory activity in a cylinder (F). There was no significant difference in the simultaneous use of the forelimbs between groups (p>0.2). D1, D2 and D3 in X-axis represent consecutive days of testing.
Netrin-1 gene transfer reduces ischemia-induced motor asymmetry in limb usage
Following AAV-Netrin-1 gene transfer, the favoring use of the unaffected limb resulting from stroke-induced asymmetry was reduced (p<0.05; Figure 5F), while the use of the affected limb was increased (p<0.05, Figure 5F) during exploratory activity in a cylinder, compared to that of the group that received AAV-LacZ. There was no significant difference observed in the simultaneous use of the forelimbs between groups (p>0.05, Figure 5F).
AAV-Netrin-1 gene therapy does not affect spatial learning and memory
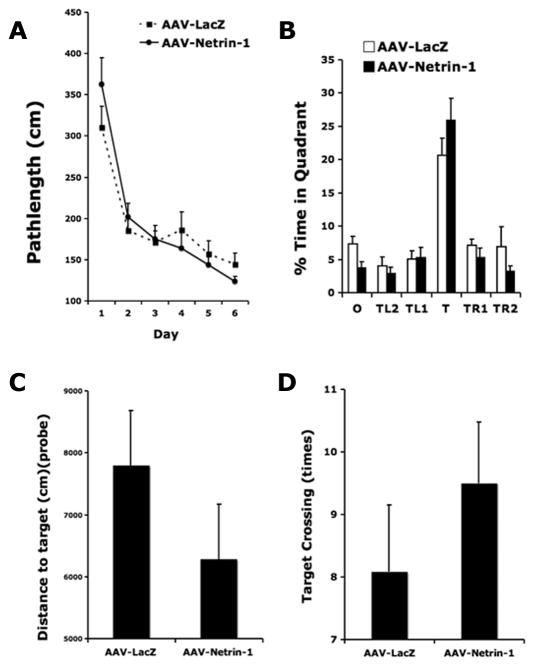
Previously we found that distal MCA occlusion produces mild impairment in spatial learning and memory (Wang et al. 2009b). Here we determined whether AAV-Netrin-1 gene therapy also improved post stroke cognitive function in the hippocampal dependent Barnes maze test. Over the 6 days of training in the Barnes maze acquisition test, both groups of rats learned the spatial task as evidenced by progressively less distance traveled to reach the escape tunnel (F5, 95=23.9; p<0.0001). However, there was no significant difference in pathlengh to find target between stroke rats that received AAV-Netrin-1 and those that received AAV-LacZ (F1, 95=0.16, p>0.6). (Figure 6A). Despite a tendency to spend more time searching the target quadrant during the probe trial (p=0.069), netrin-1 gene therapy did not significantly improve memory retention in the Barnes maze probe trial (Figure 6B). This observation was consistent with the notion that there was no significant difference in proximity to the target during search or the frequency of target-crossing during the retention trial between treatment groups (Figure 6C&D).
Figure 6. AAV-Netrin-1 gene therapy does not affect learning and memory after stroke.
Netrin-1 gene therapy did not significantly improve spatial learning and memory in the Barnes maze test, as there was no significant difference in path length traveled to find the target during Barnes maze acquisition (A). Despite a tendency to spend more time searching the target quadrant during the probe trial (p=0.069), there was no significant difference between groups in the memory retention test (B). O: opposite quadrant; T: target quadrant; TL1: quadrant left to T; TL2: quadrant left to TL1; TR1: quadrant right to T; TR2: quadrant right to TR1. Note that the MCAO rats receiving AAV-Netrin-1 also had a tendency to be closer to the target throughout the probe trial (C) (p=0.25) and made more target crossings (D) (p=0.34) during the retention memory test.
AAV-Netrin-1 gene transfer following dMCAO does not significantly reduce infarct size
There was no significant difference in lesion sizes between rats that received AAV-Netrin-1 and those that received AAV-LacZ (p=0.22), suggesting that AAV-Netrin-1 gene therapy-induced partial functional restoration after cerebral ischemia did not depend on the reduction of infarct size (Figure 7).
Figure 7. AAV-Netrin-1 gene therapy does not significantly reduce the size of ischemic infarct.
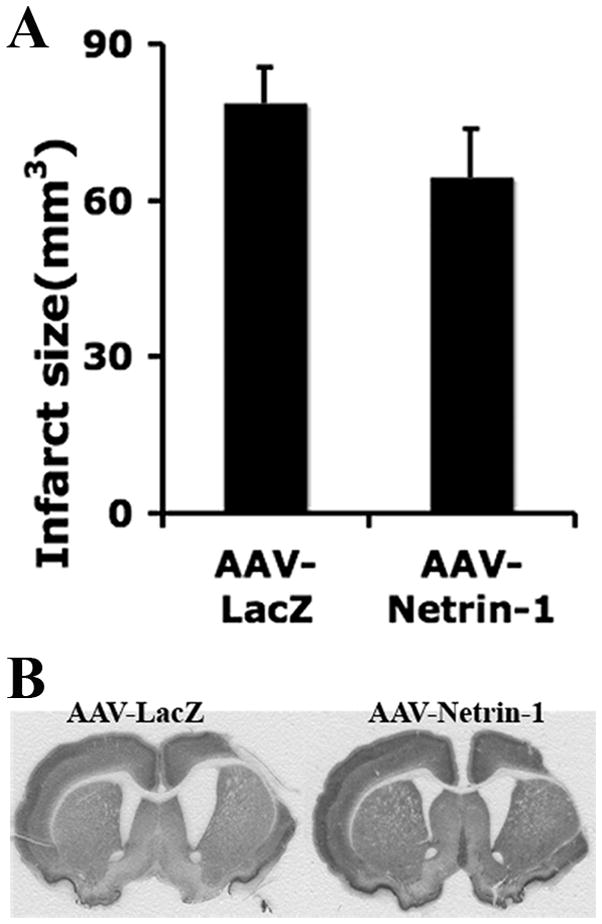
AAV-Netrin1 or AAV-LacZ was delivered at one hour following MCAO and the infarct volume was determined at 8 weeks following gene transfer. A, Although rats that received AAV-Netrin-1 gene therapy in general had a smaller infarct volume as compared to those that received AAV-LacZ, the difference was not statistically significant (p=0.22). B, Representative images of NeuN stained coronal sections showing the regions of infarct.
AAV-Netrin-1 gene transfer increases microvessel density in peri-infarct area
It was shown previously that netrin-1 protein induced capillary-like tube formation in the human endothelial cell culture, and AAV-Netrin-1 gene transfer increased microvessels in the normal mouse brain in vivo (Fan et al. 2008). To determine whether post-stroke AAV-Netrin-1 gene therapy provided a permissive environment for angiogenesis, we quantified microvessel density in the peri-infarct cortex. Our results showed that there was a significant enhancement in the microvessel density in the ischemic peri-infarct cortex of AAV-Netrin-1-treated brains, compared to control-vector-treated ones (Figure 8A-E). Some blood vessels in the peri-infarct cortex were newly formed since they colocalized with Ki67, a cellular marker for proliferation (Figure 8C).
AAV-Netrin-1 gene transfer increases the migration of immature neurons into ipsilateral white matter
It was previously shown that post-stroke newly born neurons migrated into a unique neurovascular niche in the peri-infarct cortex (Ohab et al. 2006). We next determined whether the enhanced microvessel density in the peri-infarct tissue provided a better microenvironment for neuronal regeneration and migration. Following injection of AAV-Netrin-1 and AAV-LacZ vectors, we analyzed long-distance migration of newly born neurons from the subventricular zone in various regions en route to the peri-infarct cortex. We found that there was a significant increase in the number of migrating neuroblasts in the white matter bordering infarcted cortex following the injection of AAV-Netrin-1, compared to the control vector (p<0.05; Figure 8F&G), while the number of neuroblasts remained similar in the striatum between two treatment groups. There were very few DCX immunoreactive cells that reached the peri-infarct cortex in both groups, and there was no significant difference in their numbers.
Discussion
Due to the pleotrophic actions of netrins, we investigated the therapeutic potential of netrin-1 via AAV gene transfer in an experimental model of cortical stroke. Our results showed that AAV mediated netrin-1 transcript can be detected as early as one day after gene transfer and substantially increased at three weeks following vector injection. Both the transduced and endogenous netrin-1 protein was mainly found in the peri-infarct neurons, but not in the blood vessels. Administration of AAV-Netrin-1 in the peri-infarct areas at one hour following MCAO did not significantly reduce the infarct size. AAV-Netrin-1 gene transfer reversed post-stroke motor asymmetry in forelimb usage and improved exploratory behavior, but contributed little to the improvement of learning and memory. A significant enhancement in the microvessel density in the ischemic peri-infarct and a significant increase in the number of migrating immature neurons in the white matter bordering infarcted cortex were also observed following AAV-Netrin-1 gene transfer. Our data suggest that overexpression of netrin-1 via AAV mediated gene transfer improves the neurovascular environment in the peri-infarct cortex and contributes to the recovery of motor function following ischemic stroke.
Consistent with a previous study using the intraluminal thread model of MCA stroke (Tsuchiya et al. 2007), we detected an increase of endogenous netrin-1 in the peri-infarct by the distal occlusion method, suggesting that netrin-1 is likely induced by hypoxia related mechanisms. Indeed a recent report demonstrated that hypoxia robustly increased the expression of netrin-1 in epithelial cells by inducing binding of HIF-1α to the putative HRE site in the netrin-1 gene promoter (Rosenberger et al. 2009). HIF-1α loss- and gain-of function studies further supported its role in the induction of netrin-1 by hypoxia (Rosenberger et al. 2009). Interestingly, HIF-1α also mediates the induction of VEGF following hypoxia. Our data indicate that similar to VEGF, netrin-1 is an angiogenic factor induced by hypoxia/ischemia. However, unlike the wide expression profile of VEGF in many cell types, netrin-1 is predominantly expressed by neurons after cerebral ischemia. In addition to the ligand, hypoxia/ischemia also affects netrin-1 receptors in the brain. In response to MCA stroke, increased expression of netrins’ chemoattractive receptor, deleted in colorectal cancer (DCC), was found in the neurons and astrocyte feet, while the other chemoattractive receptor neogenin was induced in endothelial cells (Tsuchiya et al. 2007). The repulsion mediating netrin-1 uncoordinated-5 (UNC) receptors UNC5A and UNC5B were found in the peri-infarct neurons, although their expression remained unchanged after MCAO (Hoang et al. 2009). It is conceivable that as a secreted protein, the transduced netrin-1 binds to neogenin, leading to an increase in blood vessel density in the peri-infarct cortex more effectively than the transient endogenous netrin-1. It is also possible that netrin1 induction of angiogenesis could be mediated by an increase in endothelial nitric oxide production (Nguyen and Cai 2006). Despite the unrestricted nature of CMV promoter in directing netrin-1 transgene expression, the exclusive neuronal expression following gene transfer in the current study suggests that AAV1 is also a neurotrophic viral vector, similar to AAV2 (de Backer et al. 2010; Passini et al. 2003).
The increase in blood vessel density observed in the peri-infarct cortex following AAV-Netrin-1 gene transfer in our study is in line with the angiogenic effect of netrin proteins. A recent report demonstrated that cerebral ischemia increased the expression of another member of the netrin family, namely netrin-4, in the ischemic core and in astrocyte endfeet of the peri-infarct (Hoang et al. 2009). Similar to the proangiogenic effect of netrin-4 administered intracerebrally, we found that AAV-Netrin-1 increased microvessel density in the peri-infarct cortex. Although we did not observe a significant decrease in infarct size following AAV-Netrin-1 gene transfer due to a delayed transgene expression, a previous report showed that intracerebral delivery of netrin-1 peptide activated UNC5B, leading to the inhibition of p-53 mediated apoptosis in the peri-infarct (Wu et al. 2008). The anti-apoptotic function of netrin-1 is also supported by another study in which transgenic mice overexpressing chicken netrin-1 protected the kidney from renal ischemia and reperfusion injury, and reduced caspase-3 activation and apoptosis (Wang et al. 2009a). In considering the apoptotic effect of netrin-1 and without a direct comparison of new versus total microvessels in the peri-infarct cortex at various time points, we cannot rule out the possibility that the observed increase in vessel density after AAV-Netrin-1 gene transfer was due to an increased survival of existing vasculature.
The role of netrin-1 in axon guidance following CNS injury remains unclear. A correlation between UNC5 expression and poor axonal regeneration following lesion in lamprey suggests that netrins might restrain inappropriate axonal sprouting after injury. During striatal development, netrin-1 exerts a repulsive action on migrating SVZ cells, dispersing the matrix neurons into the striatum (Hamasaki et al. 2001). Netrin-1 receptor neogenin is expressed by cells displaying stem cell (type B cell) characteristics within the adult mouse and human SVZ and rostral migratory stream, suggesting that neogenin regulates signals important for neurogenesis in the adult forebrain (Bradford et al. 2010). Interestingly, when AAV-Netrin-1 was administered into the striatum beneath the corpus callosum, there were significantly more DCX expressing immature neurons migrated into the corpus callosum compared to those received control vector. However, the number of immature neurons in the striatum remained similar between the AAV-Netrin-1- and AAV-LacZ treated groups. It is unclear why netrin-1 overexpression increased immature neuronal migration preferentially into the white matter and whether it led to functional consequence. Although post-stroke newly born neurons are known to migrate into a unique neurovascular niche in the peri-infarct cortex (Ohab et al. 2006), there were very few DCX-expressing immature neurons found in the peri-infarct cortex of animals treated with either AAV-Netrin-1 or the control vector in our current ischemic model. It is plausible that the newborn neurons have adopted a more mature phenotype when reaching the peri-infarct cortex, thus no longer expressing DCX marker, a relatively early marker for immature neurons. However, several studies including our own detected very few BrdU/NeuN expressing new neurons in the peri-infarct cortex argued against such possibility (Arvidsson et al. 2002; Hoehn et al. 2005).
Similar to the beneficial effect of netrin-4 recombinant protein on improving neurological score (Hoang et al. 2009), we found that AAV-mediated netrin-1 overexpression also enhances post-stroke motor function. In the current study, we have conducted an extensive investigation to determine not only motor but also memory functions using a battery of behavioral tests. As one of the most informative tests in revealing unilateral brain injury-induced asymmetry in forelimb usage, the Schallert cylinder test detected a significant reduction in the preferential use of the unaffected limb following AAV-Netrin-1 gene therapy. In addition to motor asymmetry and overall coordination, we also employed the novel open field test to examine locomotor activity and exploratory behavior. The 3-day open field test revealed that AAV-Netrin-1 gene therapy significantly increased post-stroke activity and reduced anxiety-like behavior. Following distal MCAO, tissue perfusion in the peri-infarct cortex improved gradually in a distance-dependent manner, although never fully restored even after 3 months. Besides, the recovery of local hemodynamics also affected the recovery of spine density (Mostany et al. 2010). Our data suggest that a reduction of infarct size is not necessary for the behavioral benefit of netrin-1, while subtle improvement in neurovascular integrity such as increased peri-infarct vascular density could contribute to the recovery of motor function. It is well recognized that the distal MCA stroke model produces very subtle memory impairment that cannot be detected by the conventional water maze test (Matsumori et al. 2006a). We have successfully adapted a training paradigm that can detect memory impairment of rats subjected to distal MCAO with the Barnes maze test (Wang et al. 2009b). However, we did not observe any significant difference in the acquisition of Barnes maze test between the stroke rats treated with AAV-Netrin-1 and AAV-LacZ vectors. Nevertheless, stroke rats receiving AAV-Netrin-1 gravitated towards a better search behavior in the quadrant that used to contain the escape tunnel during memory retrieval.
In spite of the pitfalls, gene therapy has been proven a viable option for the treatment of a wide spectrum of diseases. Among a variety of gene therapy vectors, AAV-based vectors offer several advantages including their chemical and physical stability, low immunogeneicity of capsid proteins, high safety profile in the host due to the predominant episomal state of AAV vectors following infection, and the feasibility of clinical-scale production (Heilbronn and Weger). The most attractive feature of AAV vectors lies in the sustained transgene expression in postmitotic cells following transduction, making them excellent candidates for CNS applications. However, the most limiting aspect of AAV-based vector application is the relatively slow onset of transgene expression. As a single-stranded DNA virus, the AAV genome requires the host DNA replication machinery to generate double-stranded DNA template for mRNA transcription. According to our data obtained by qRT-PCR, AAV serotype 1 mediated netrin-1 gene expression in post mitotic neurons probably did not reach a critical level to exert structural or functional effect until days after transduction. When a hypoxia-inducible AAV-H9-VEGF vector was delivered 5 days prior to MCAO, it effectively reduced the infarct volume by 55% and increased the peri-infarct microvessel density by 1.5 folds (Shen et al. 2008; Shen et al. 2006). Thus, the delayed overexpression might be the main contributor to the relatively small functional efficacy observed in our study. To circumvent the problem in delayed transgene expression, a self-complementary, or a dimeric AAV vector can be used to ensure an early transgene and protein expression. Alternatively, a lentiviral vector carrying the same netrin-1 sequence should be compared for the kinetics of netrin-1 protein expression and functional efficacy, in order to assess the therapeutic potential of netrin-1 overexpression using gene transfer. Due to the high sequence homology between chicken and rat netrin-1 and the demonstrated biological effect of transduced protein in previous in vitro and in vivo studies (Fan et al. 2008), the observed small functional efficacy is unlikely to be related to the species specificity. Our findings that AAV mediated netrin-1 overexpression enhanced peri-infarct blood vessel density and post-stroke motor function recovery further support the role of netrins in therapeutic angiogenesis. Our results also point to a beneficial effect of sustained netrin-1 expression conferred by AAV gene transfer, outlasting the transient expression of the endogenous netrin-1 induced by ischemic stroke.
Research highlights.
AAV mediated netrin-1 gene transfer improves post stroke motor function recovery
AAV-Netrin-1 gene transfer enhances peri-infarct blood vessel density
AAV-Netrin-1 gene transfer increases neuroblast migration
Sustained netrin-1 overexpression via AAV gene transfer is beneficial after stroke
Acknowledgments
This work was supported by the American Heart Association EIA 0940065N (JL), a VA Merit award from BLR&D (JL) and P01 NS44155C (WLY; HS).
Footnotes
Disclosure/Conflict of Interest
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed RP, Haider KH, Shujia J, Afzal MR, Ashraf M. Sonic Hedgehog gene delivery to the rodent heart promotes angiogenesis via iNOS/netrin-1/PKC pathway. PLoS One. 2010;5:e8576. doi: 10.1371/journal.pone.0008576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Bradford D, Faull RL, Curtis MA, Cooper HM. Characterization of the netrin/RGMa receptor neogenin in neurogenic regions of the mouse and human adult forebrain. J Comp Neurol. 2010;518:3237–3253. doi: 10.1002/cne.22397. [DOI] [PubMed] [Google Scholar]
- Braisted JE, Catalano SM, Stimac R, Kennedy TE, Tessier-Lavigne M, Shatz CJ, O'Leary DD. Netrin-1 promotes thalamic axon growth and is required for proper development of the thalamocortical projection. J Neurosci. 2000;20:5792–5801. doi: 10.1523/JNEUROSCI.20-15-05792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castets M, Coissieux MM, Delloye-Bourgeois C, Bernard L, Delcros JG, Bernet A, Laudet V, Mehlen P. Inhibition of endothelial cell apoptosis by netrin-1 during angiogenesis. Dev Cell. 2009;16:614–620. doi: 10.1016/j.devcel.2009.02.006. [DOI] [PubMed] [Google Scholar]
- de Backer MW, Fitzsimons CP, Brans MA, Luijendijk MC, Garner KM, Vreugdenhil E, Adan RA. An adeno-associated viral vector transduces the rat hypothalamus and amygdala more efficient than a lentiviral vector. BMC Neurosci. 2010;11:81. doi: 10.1186/1471-2202-11-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulawa SC, Grandy DK, Low MJ, Paulus MP, Geyer MA. Dopamine D4 receptor-knock-out mice exhibit reduced exploration of novel stimuli. J Neurosci. 1999;19:9550–9556. doi: 10.1523/JNEUROSCI.19-21-09550.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Shen F, Chen Y, Hao Q, Liu W, Su H, Young WL, Yang GY. Overexpression of netrin-1 induces neovascularization in the adult mouse brain. J Cereb Blood Flow Metab. 2008;28:1543–1551. doi: 10.1038/jcbfm.2008.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger JH, Bronson RT, Harris B, Johnson K, Przyborski SA, Ackerman SL. The netrin 1 receptors Unc5h3 and Dcc are necessary at multiple choice points for the guidance of corticospinal tract axons. J Neurosci. 2002;22:10346–10356. doi: 10.1523/JNEUROSCI.22-23-10346.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas C, Larrivee B, Eichmann A. Netrins and UNC5 receptors in angiogenesis. Angiogenesis. 2008;11:23–29. doi: 10.1007/s10456-008-9096-2. [DOI] [PubMed] [Google Scholar]
- Hamasaki T, Goto S, Nishikawa S, Ushio Y. A role of netrin-1 in the formation of the subcortical structure striatum: repulsive action on the migration of late-born striatal neurons. J Neurosci. 2001;21:4272–4280. doi: 10.1523/JNEUROSCI.21-12-04272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn R, Weger S. Viral vectors for gene transfer: current status of gene therapeutics. Handb Exp Pharmacol. :143–170. doi: 10.1007/978-3-642-00477-3_5. [DOI] [PubMed] [Google Scholar]
- Hoang S, Liauw J, Choi M, Choi M, Guzman RG, Steinberg GK. Netrin-4 enhances angiogenesis and neurologic outcome after cerebral ischemia. J Cereb Blood Flow Metab. 2009;29:385–397. doi: 10.1038/jcbfm.2008.128. [DOI] [PubMed] [Google Scholar]
- Hoehn BD, Palmer TD, Steinberg GK. Neurogenesis in rats after focal cerebral ischemia is enhanced by indomethacin. Stroke. 2005;36:2718–2724. doi: 10.1161/01.STR.0000190020.30282.cc. [DOI] [PubMed] [Google Scholar]
- Liu Z, Fan Y, Won SJ, Neumann M, Hu D, Zhou L, Weinstein PR, Liu J. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke. 2007;38:146–152. doi: 10.1161/01.STR.0000251791.64910.cd. [DOI] [PubMed] [Google Scholar]
- Low K, Culbertson M, Bradke F, Tessier-Lavigne M, Tuszynski MH. Netrin-1 is a novel myelin-associated inhibitor to axon growth. J Neurosci. 2008;28:1099–1108. doi: 10.1523/JNEUROSCI.4906-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manitt C, Colicos MA, Thompson KM, Rousselle E, Peterson AC, Kennedy TE. Widespread expression of netrin-1 by neurons and oligodendrocytes in the adult mammalian spinal cord. J Neurosci. 2001;21:3911–392. doi: 10.1523/JNEUROSCI.21-11-03911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcos S, Backer S, Causeret F, Tessier-Lavigne M, Bloch-Gallego E. Differential roles of Netrin-1 and its receptor DCC in inferior olivary neuron migration. Mol Cell Neurosci. 2009;41:429–439. doi: 10.1016/j.mcn.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Matsumori Y, Hong SM, Fan Y, Kayama T, Hsu CY, Weinstein PR, Liu J. Enriched environment and spatial learning enhance hippocampal neurogenesis and salvages ischemic penumbra after focal cerebral ischemia. Neurobiol Dis. 2006a;22:187–198. doi: 10.1016/j.nbd.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Matsumori Y, Northington FJ, Hong SM, Kayama T, Sheldon RA, Vexler ZS, Ferriero DM, Weinstein PR, Liu J. Reduction of caspase-8 and -9 cleavage is associated with increased c-FLIP and increased binding of Apaf-1 and Hsp70 after neonatal hypoxic/ischemic injury in mice overexpressing Hsp70. Stroke. 2006b;37:507–512. doi: 10.1161/01.STR.0000199057.00365.20. [DOI] [PubMed] [Google Scholar]
- Metin C, Deleglise D, Serafini T, Kennedy TE, Tessier-Lavigne M. A role for netrin-1 in the guidance of cortical efferents. Development. 1997;124:5063–5074. doi: 10.1242/dev.124.24.5063. [DOI] [PubMed] [Google Scholar]
- Mostany R, Chowdhury TG, Johnston DG, Portonovo SA, Carmichael ST, Portera-Cailliau C. Local hemodynamics dictate long-term dendritic plasticity in peri-infarct cortex. J Neurosci. 2010;30:14116–14126. doi: 10.1523/JNEUROSCI.3908-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen A, Cai H. Netrin-1 induces angiogenesis via a DCC-dependent ERK1/2-eNOS feed-forward mechanism. Proc Natl Acad Sci U S A. 2006;103:6530–6535. doi: 10.1073/pnas.0511011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KW, Crouse D, Lee M, Karnik SK, Sorensen LK, Murphy KJ, Kuo CJ, Li DY. The axonal attractant Netrin–1 is an angiogenic factor. Proc Natl Acad Sci U S A. 2004;101:16210–16215. doi: 10.1073/pnas.0405984101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passini MA, Watson DJ, Vite CH, Landsburg DJ, Feigenbaum AL, Wolfe JH. Intraventricular brain injection of adeno-associated virus type 1 (AAV1) in neonatal mice results in complementary patterns of neuronal transduction to AAV2 and total long-term correction of storage lesions in the brains of beta-glucuronidase-deficient mice. J Virol. 2003;77:7034–7040. doi: 10.1128/JVI.77.12.7034-7040.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Rapp JH, Pan XM, Neumann M, Hong M, Hollenbeck K, Liu J. Microemboli composed of cholesterol crystals disrupt the blood-brain barrier and reduce cognition. Stroke. 2008;39:2354–2361. doi: 10.1161/STROKEAHA.107.496737. [DOI] [PubMed] [Google Scholar]
- Rosenberger P, Schwab JM, Mirakaj V, Masekowsky E, Mager A, Morote-Garcia JC, Unertl K, Eltzschig HK. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol. 2009;10:195–202. doi: 10.1038/ni.1683. [DOI] [PubMed] [Google Scholar]
- Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- Shen F, Fan Y, Su H, Zhu Y, Chen Y, Liu W, Young WL, Yang GY. Adeno-associated viral vector-mediated hypoxia-regulated VEGF gene transfer promotes angiogenesis following focal cerebral ischemia in mice. Gene Ther. 2008;15:30–39. doi: 10.1038/sj.gt.3303048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen F, Su H, Fan Y, Chen Y, Zhu Y, Liu W, Young WL, Yang GY. Adeno-associated viral-vector-mediated hypoxia-inducible vascular endothelial growth factor gene expression attenuates ischemic brain injury after focal cerebral ischemia in mice. Stroke. 2006a;37:2601–2606. doi: 10.1161/01.STR.0000240407.14765.e8. [DOI] [PubMed] [Google Scholar]
- Shen F, Su H, Liu W, Kan YW, Young WL, Yang GY. Recombinant adeno-associated viral vector encoding human VEGF165 induces neomicrovessel formation in the adult mouse brain. Front Biosci. 2006b;11:3190–3198. doi: 10.2741/2042. [DOI] [PubMed] [Google Scholar]
- Steup A, Lohrum M, Hamscho N, Savaskan NE, Ninnemann O, Nitsch R, Fujisawa H, Puschel AW, Skutella T. Sema3C and netrin-1 differentially affect axon growth in the hippocampal formation. Mol Cell Neurosci. 2000;15:141–155. doi: 10.1006/mcne.1999.0818. [DOI] [PubMed] [Google Scholar]
- Tsuchiya A, Hayashi T, Deguchi K, Sehara Y, Yamashita T, Zhang H, Lukic V, Nagai M, Kamiya T, Abe K. Expression of netrin-1 and its receptors DCC and neogenin in rat brain after ischemia. Brain Res. 2007;1159:1–7. doi: 10.1016/j.brainres.2006.12.096. [DOI] [PubMed] [Google Scholar]
- Wang W, Reeves WB, Pays L, Mehlen P, Ramesh G. Netrin-1 overexpression protects kidney from ischemia reperfusion injury by suppressing apoptosis. Am J Pathol. 2009a;175:1010–1018. doi: 10.2353/ajpath.2009.090224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Bontempi B, Leinekugel X, Weinstein PR, Liu J. Environmental enrichment promotes the resolution of post stroke mild cognitive impariment in rats. 9th International Symposium on Cerebral Blood Flow, Metabolism and Function; Chicago, IL, USA. International Society for Cerebral Blood Flow & Metabolism; 2009b. p. Abstract #861. [Google Scholar]
- Weidner N. Intratumor microvessel density as a prognostic factor in cancer. Am J Pathol. 1995;147:9–19. [PMC free article] [PubMed] [Google Scholar]
- Wilson BD, Ii M, Park KW, Suli A, Sorensen LK, Larrieu-Lahargue F, Urness LD, Suh W, Asai J, Kock GA, Thorne T, Silver M, Thomas KR, Chien CB, Losordo DW, Li DY. Netrins promote developmental and therapeutic angiogenesis. Science. 2006;313:640–644. doi: 10.1126/science.1124704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TW, Li WW, Li H. Netrin-1 attenuates ischemic stroke-induced apoptosis. Neuroscience. 2008;156:475–482. doi: 10.1016/j.neuroscience.2008.08.015. [DOI] [PubMed] [Google Scholar]
- Yetnikoff L, Labelle-Dumais C, Flores C. Regulation of netrin-1 receptors by amphetamine in the adult brain. Neuroscience. 2007;150:764–773. doi: 10.1016/j.neuroscience.2007.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Cai H. Netrin-1 prevents ischemia/reperfusion-induced myocardial infarction via a DCC/ERK1/2/eNOS s1177/NO/DCC feed-forward mechanism. J Mol Cell Cardiol. 2010;48:1060–1070. doi: 10.1016/j.yjmcc.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]