Abstract
Human studies are reviewed concerning whether “aging”-related mechanisms contribute to Alzheimer’s disease (AD) pathogenesis. AD is defined by specific neuropathology: neuritic amyloid plaques and neocortical neurofibrillary tangles. AD pathology is driven by genetic factors related not to aging per se, but instead to the amyloid precursor protein (APP). In contrast to genes involved in APP-related mechanisms, there is no firm connection between genes implicated in human “accelerated aging” diseases (progerias) and AD. The epidemiology of AD in advanced age is highly relevant but deceptively challenging to address given the low autopsy rates in most countries. In extreme old age, brain diseases other than AD approximate AD prevalence while the impact of AD pathology appears to peak by age 95 and decline thereafter. Many distinct brain diseases other than AD afflict older human brains and contribute to cognitive impairment. Additional prevalent pathologies include cerebrovascular disease and hippocampal sclerosis, both high-morbidity brain diseases that appear to peak in incidence later than AD chronologically. Because of these common brain diseases of extreme old age, the epidemiology differs between clinical “dementia” and the subset of dementia cases with AD pathology. Additional aging-associated mechanisms for cognitive decline such as diabetes and synapse loss have been linked to AD and these hypotheses are discussed. Criteria are proposed to define an “aging-linked” disease, and AD fails all of these criteria. In conclusion, it may be most fruitful to focus attention on specific pathways involved in AD rather than attributing it to an inevitable consequence of aging.
Introduction and definition of terms
This review examines the relationship between Alzheimer’s disease (AD) biology and human brain aging. AD mostly affects older individuals. There has been debate about whether AD is linked mechanistically to “brain aging” and to cellular senescence mechanisms [44, 90, 142, 183, 192, 232]. This is an important issue, especially since the number of very old individuals is predicted to increase dramatically in coming decades ([116, 174], Fig. 1). Although animal models provide a valuable context for experimental work, their direct correlation with human neurodegenerative diseases is still being characterized. The human aging trajectory and other aspects of Homo sapiens brain biology are also unique to our species. Hence, in the present literature review, we focus on data derived from human studies.
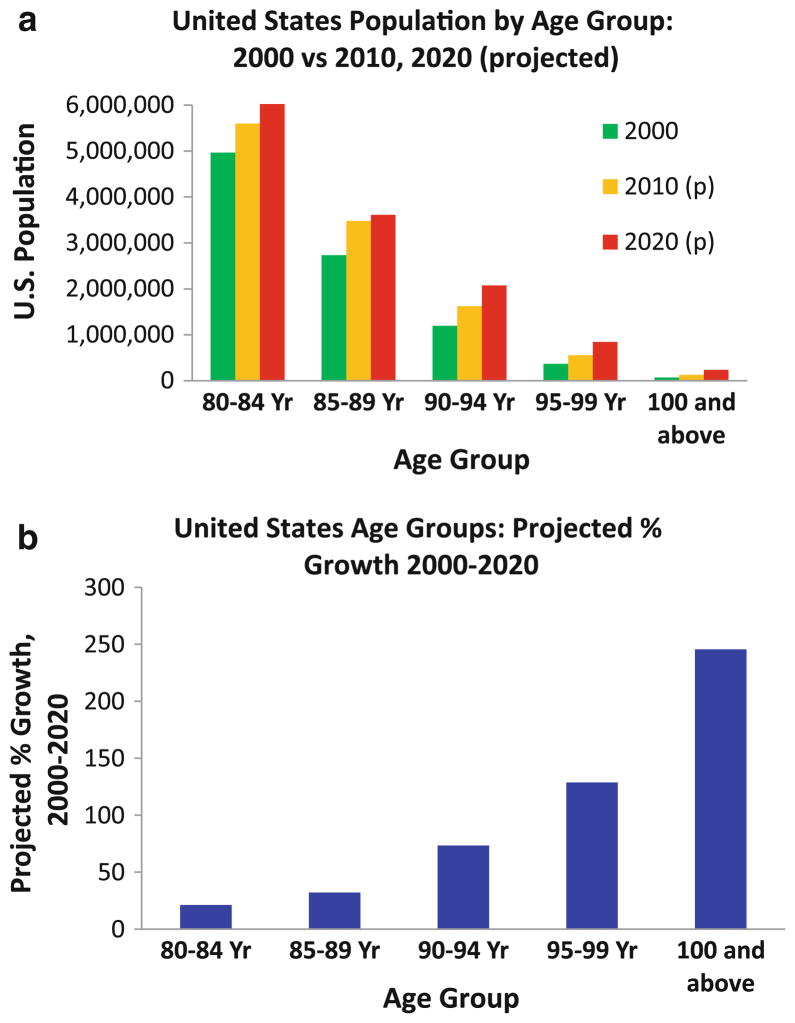
Fig. 1.
United States Census Bureau data and projections illustrate the practical need to understand the correlation between aging and Alzheimer’s disease (AD). Raw numbers for the United States population (2000) rand projected (2010 and 2020) are shown (a). Note that the demographic changes between 2000 and 2020 in the US are not projected to affect all age groups the same. Projected increases over time (2000–2020) are shown in b. The number of persons between ages 85 and 89 years of age will increase by approximately 30% in that time period, whereas the number of people between ages 95–99 years of age will increase by over 100%. If aging itself is linked mechanistically to AD, then Western cultures are at gravely increased AD risk. However, it may be a mistake to conflate AD with other causes of cognitive impairment. Source: US Census Bureau
Recent scholarship has called into question the idea that “age is the greatest risk factor for AD”. The current review focuses on the changes that occur in humans past 90 years of age since the central question we wish to address is whether AD is linked to aging. Both of these concepts—AD and aging—are incompletely understood, so our discussion begins with a definition of terms.
AD is defined by the presence of three different elements, each necessary (and together entirely sufficient) for definitive diagnosis [1]:
Clinical dementia (cognitive impairment with a memory component that impacts daily living skills);
Substantial numbers of neocortical neurofibrillary tangles (NFTs) at autopsy as quantified using Braak staging [37]; and
Substantial numbers of neuritic amyloid plaques as quantified using CERAD scores [139] at autopsy.
The neuropathology-based definition of AD is a key assumption of the present review. Despite controversy in this area [46], no rival method discriminates AD from other causes of cognitive decline in aging as accurately as does postmortem evaluation. AD neuropathology correlates well with cognitive impairment although the relationship is complex [151]. Like all diseases, there is an expected prodromal phase and imperfect clinical–pathological correlation [120, 189, 206]. As in other diseases such as cancer, increased patient age correlates with greater dissociation between clinical expectations and autopsy-proven pathology [36, 212].
A modicum of AD pathology may be present in “nondemented” aged brains [146, 147, 149, 175, 198]. NFTs are not disease-specific and may develop in hippocampi and elsewhere independently of the processes present in AD brains [38, 92, 137, 147, 149, 175, 222], which by definition contain numerous Aβ plaques surrounded by abnormal tau-containing neurites [1]. There are also many individuals whose brains harbor substantial diffuse amyloid plaques yet they do not meet criteria for MCI or AD clinically [77, 129, 148, 175]. It is important to acknowledge our incomplete understanding about how these cases relate to full-blown AD. However, given ADs well-documented multiyear prodromal period [45, 48, 51, 141, 182, 194, 197, 208], the observed AD-specific pathology (neuritic Aβ plaques combined with neocortical NFTs) closely matches expectations when one compares autopsy data and AD epidemiology [5, 149].
Alternative definitions of AD, emphasizing clinical and biomarker data, have been proposed recently that remove the need for postmortem (definitive) diagnosis in a practical attempt to support clinical trials and patient management [67]. Biomarkers assessed during life are only surrogates for pathology, not for cognitive symptoms, nor even for the prediction of future cognitive impairment (which often are due to factors other than AD). AD bio-markers are valid for the detection of preclinical AD inasmuch as they predict eventual cognitive impairment accompanied by plaques and tangles in the brain.
Whereas clinical–pathological correlation in AD defies simplistic interpretations, the concept of “aging” is even less completely understood. Multiple mechanisms have been described for decreased “molecular fidelity” in the mature epoch of a cell, organ, and organism [88, 93]. Senescence near the end of life has been attributed to mitochondrial dysfunction, telomerase activity, free radicals, oxidative stress, and other factors [47, 66, 71, 85, 88, 113, 114]. In addition, genes have been described that either augment natural aging processes deleteriously (see below) or that delay normal aging effects in some organisms (e.g., sirtuins).
One thing that is clear is that advanced human age is accompanied by characteristic medical conditions [221]. Diseases that affect 95-year olds are not identical to those that affect most 75-year olds [154]. In extreme old age, there is a lamentable combination of vascular pathologies accompanied by weaker regenerative capacity. The background of generalized infirmity, specific high-morbidity diseases, polypharmacy, mood and sleep disorders represent formidable challenges to determining if a specific process exacerbates AD per se, or contributes to impaired cognitive functioning through other mechanisms [13, 110, 136, 154, 190, 220, 227]. These are some of the complexities that may have led to conflation between cognitive impairment in advanced age (“dementia”), and AD. A key feature of aging is that it is universally linked to the physical time dimension. The question then is the universality of the AD-aging linkage: is AD a manifestation of aging or is it not?
We propose five testable criteria which, if supported by experimental data, would indicate that AD pathogenesis is linked specifically to aging-associated mechanisms (Table 1):
Table 1.
Criteria for whether “aging mechanisms” specifically are linked with Alzheimer’s disease (AD)
| Criteria for whether or not the mechanism of AD pathogenesis is specifically linked to aging | Criterion satisfied? | Directly relevant research |
|---|---|---|
| 1. There is not some other mechanism outside of aging that seems likely to cause AD | NO | Clinical trials, genetics, and cancer biology |
| 2. Human genetic aberrations that appear to hasten the aging process also increase incidence or prevalence of AD, and vice versa | NO | |
| 3. An elderly person should have a higher likelihood of dying with AD with every added year of life | NO | AD and dementia epidemiology |
| 4. As with wrinkled skin and subtle cognitive impairment relative to baseline, everyone will experience AD if they live long enough (and cognitive loss in aging relates specifically to AD) | NO | |
| 5. Other aging-linked mechanisms (diabetes and synapse loss) contribute specifically to AD | NO | Diabetes and synapse loss |
Criteria #1: No specific mechanism outside of aging causes most known AD cases;
Criteria #2: Human genetic aberrations that hasten the aging process also increase incidence of AD and vice versa;
Criteria #3: Everyone will develop AD if they live long enough;
Criteria #4: An aged individual should have a higher likelihood of AD with each additional year of life; and
Criteria #5: Other aging-linked mechanisms (diabetes and synapse loss) contribute specifically to AD.
The present review is organized to address critically the experimental data related to these testable hypotheses. Criteria #1 and #2 are discussed in the context of clinical trial data, AD genetics, and cancer biology. Criteria #3 and #4 can be assessed by focusing on the nexus of neurodegenerative disease epidemiology and neuropathology. Criterion #5 is discussed in connection with diabetes and synapse loss. The above criteria for “aging-linked” mechanisms are admittedly debatable. Whether or not one accepts these tenets, there are additional interesting observations relevant to the question of whether AD pathogenesis is linked specifically to “aging” mechanisms.
AD pathology: insights from clinical trials, genetics, and cancer
Significance of extant clinical trial data
No therapeutic strategy for AD has demonstrated long-term efficacy to date. However, the existing data on clinical trials need to be interpreted with caution. We briefly discuss clinical trials related to “anti-aging” or “anti-amyloid” mechanisms.
Extensive links have been made between reactive oxygen species (ROS), aging mechanisms, and AD pathologic changes [64, 127, 128, 157, 167, 216]. However, molecular interactions between aging-linked ROS mechanisms, amyloid protein processing (plaques, oligomers, etc.), and tau modification have not yet been clearly drawn [131, 215]. Beta-amyloid itself may promote excess oxidation species reactions, further complicating the relationships between aging, ROS and AD pathology [43]. Therapies oriented toward anti-oxidant or anti-aging strategies have not been clearly successful although future studies may prove otherwise [6, 32, 163, 164].
There have been a handful of reported cases where individuals with mid-to-late stage AD were administered anti-Aβ immunotherapy that apparently cleared a large proportion of brain amyloid plaques, but this clearance did not seem to alter the presence of NFTs or the inexorable course of the disease [94, 155]. These data have been interpreted to constitute a repudiation of the amyloid cascade hypothesis [214], and to indicate that it is “aging”, rather than Aβ-related mechanisms, that underlies AD progression [90]. However, the limitations of the conclusions that can be drawn from the few autopsied anti-Aβ immunotherapy cases have been acknowledged [34] and in fact some beneficial effects of the human immunotherapy studies have been seen in clinical trials [31, 35, 191, 202]. By far, the best clinical–pathological correlation in AD is between neocortical NFTs and cognitive impairment [14, 149] and no therapies that target cortical NFTs have been successfully tested in humans. The current clinical trial literature does not contradict the idea that there is a pathogenetic synergy in which amyloid plaques kindle an auto-propagating process related to cortical NFTs, as has been hypothesized [49, 58, 72, 82]. Future drug trials oriented toward earlier stages of disease progression may help explain and reconcile these phenomena. For now, clinical trials have not provided definitive answers either way.
Progerias and AD
A subset of human genetic aberrations cause well-characterized phenotypes with specific features of advanced human aging in individuals of younger ages. These diseases are called “progerias” [40, 117, 178]. It has been suggested that progerias provide insights about the aging process and the pathways that are involved in human aging [42, 177]. Clinical signs and symptoms that may exist even in pre-teens, include cognitive impairment, wrinkled skin, atherosclerosis, brittle bones, cataracts, and many other changes, although there is not a single disease that can be said to definitely cause “accelerated aging”.
Characteristic features of human progerias are listed in Table 2. There is no firm indication that individuals with progeria have any increase in dementia with AD pathology. One nondemented individual with Werner syndrome and apolipoprotein E (APOE)-ε4 genotype was reported to have early AD-type pathology upon death at age 55 [126]. Otherwise, the link between Werner syndrome (or any other progeria) and AD pathology has been queried and not found to exist [132, 133, 160] although some of the progerias do in fact involve cognitive impairment (Table 2). Mutations of the lamin gene (LMNA) cause a severe autosomal dominant progeria syndrome, muscular dystrophy, and Charcot–Marie–Tooth disease, but not AD [42]. In contrast, gene defects in presenilin-1 (PSEN1) produce early onset autosomal dominant AD and a wide range of associated neurologic deficits, but not a progeria syndrome (see below). Nor is there any firm association between AD and other known aging genes including the sirtuins (for example SIRT1 or SIRT3) although these have been exhaustively analyzed for AD-linked polymorphisms [11].
Table 2.
Progerias, human genetic diseases with “accelerated aging”: features and relationship to AD pathology
| Accelerated aging syndrome | Clinical features | Mutated gene(s) | Life expectancy | Definitive increase of AD pathology? |
|---|---|---|---|---|
| Werner syndrome | Short stature, gray hair, hoarse voice, cataracts, wrinkled skin, diabetes, osteoporosis, cancers | WRN | 40–50s | No but see [126] |
| Hutchinson–Gilford syndrome | Hair loss, thickened/wrinkled skin, atherosclerosis, premature frailty, usually die in teens | LMNA | Teens | No |
| Cockayne syndrome | Small stature, photophobia, small head, mental retardation, neurological symptoms (e.g. ataxia) | CSA/ERCC8, CSB/ERCC6 | Childhood-30s | No |
| Trichothio-dystrophy | Short stature, brittle/sparse hair and nails, intellectual impairment, photosensitivity, infections | XPB, XPD, TTDA/GTF2H5 | Childhood-40s | No |
In summary, genetic diseases provide important insights into human aging and senescence. The dissociation between premature aging-linked genetic aberrations and AD cannot by itself negate the hypothesis that AD is caused by aging mechanisms, but it is a pertinent clue. The lack of linkage between progerias and AD is all the more significant because there are indeed other genetic loci that strongly impact AD pathogenetically.
AD genetic risk factors
Human alleles that alter AD risk are critically relevant to any discussion of AD biology. Approximately 70% of a given individual’s risk for developing AD is conferred through her genetic repertoire [28, 75]. AD is thus like many predominantly genetic diseases, which are not linked to aging mechanisms, but which can manifest late in life with neurodegeneration—trinucleotide repeat diseases, familial prion diseases, familial motoneuron diseases, FTLDs, and dozens of other genetic diseases (see for example [7, 69, 84, 201, 230]).
The high-penetrant human gene loci that alter risk for developing AD are APP, PSEN1, PSEN2, and APOE. None of these genes are known to be directly related to the aging process, nor with aging-related processes such as combating oxidative stress. These AD-affecting genes all influence processing of the amyloid precursor protein (APP). The triplication of APP in the context of Down’s syndrome can induce AD-like pathology in the human brain as young as 8 years of age [124] with precursor lesions present even during infancy (Fig. 2). Even outside of Down’s syndrome, the focal duplication of the APP gene, and mutations in APP promoter regions that increase APP production, can cause AD [138, 207, 218]. AD-relevant mutations in the APP gene affect its proteolysis as do mutations in PSEN1 and PSEN2 [28].
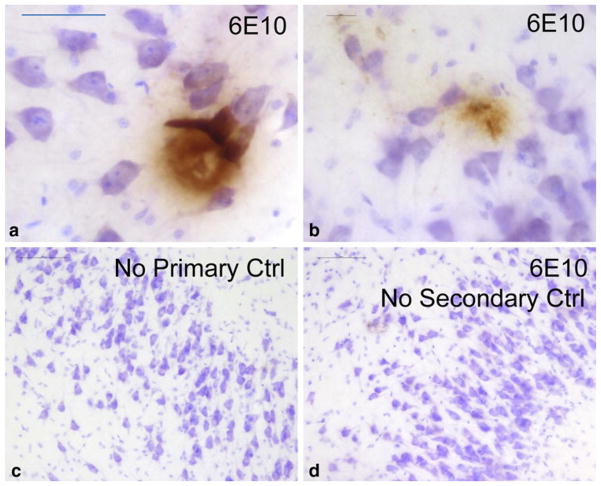
Fig. 2.
Aβ deposition in the hippocampus of a 5-month-old infant with Down syndrome (DS). DS is caused by triplication of chromosome 21 genes including the amyloid precursor protein (APP) locus. This Caucasian female died after 148 days due to complications of congenital heart disease. At autopsy, brain weight was 550 g with foreshortening of the anterior–posterior dimension as is characteristic of DS. Photomicrographs show hippocampus counterstained with Nissl stain (blue) and immunostained (brown) using anti-Aβ (6E10). Sections showed diffuse plaque-like structures (a) and smaller, more filamentous-looking Aβ deposits (b). Separate controls run in parallel without primary or secondary antibodies were completely negative (c, d). All persons with DS eventually develop Alzheimer’s disease (AD). This drives home the point that although much is still unknown about AD pathobiology, genetic risk factors related to APP are of paramount relevance. Scale bars 50 μM (a), 20 μM (b), and 100 μM (c, d)
By far, the strongest risk factor for late-onset AD is the ε4 allele of the APOE gene [52, 213]. The impact of APOE-ε4 allele in terms of boosting brain Aβ deposition is well established [29, 41, 111, 122]. APOE alleles alter Aβ plaque burden and cerebral amyloid angiopathy in dramatic fashion [26, 27, 180]. The strong genetic risk for AD in persons aged 50–80 leads to a survival bias in terms of individuals beyond that age range. Relatively few individuals with APOE-ε4 allele (much less with APP or PSEN mutations) survive AD-free past age 90 [78, 119, 144, 169, 187]. This survival bias has been discussed previously [107].
The fact that individuals without genetic risk tend to survive AD-free beyond the chronological peak for disease incidence (see below) indicates that aging and AD invoke discrete mechanisms of cerebral degeneration. High penetrance AD genetic loci all connect directly to APP mechanisms, but not aging; aging genes do not link to AD risk. Thus, criteria #1 and #2 (Table 1) are not met for AD pathogenesis being an aging-linked mechanism.
There are relatively recently discovered single nucleotide polymorphisms (SNPs) that alter AD risk and these include alleles in CLU, PICALM, SORL1, and BIN1 [25, 30, 109, 204]. The penetrance of these genes is far weaker (i.e. effect of the mutation on risk for the disease phenotype is less predictable) in comparison to mutations in APP, PSEN1, PSEN2, or APOE. SNP/GWAS data remain to be fully understood both in normal brain and in relation to AD manifestations. Aside from genetic polymorphism linked to this disease, some additional clues about what to expect with regard to AD expression in human aging may be gleaned by study of another aging-linked disease category, namely cancer.
Human aging and cancer
Other human diseases can provide valuable insights about AD and illustrate the need for pathology-based epidemiology. Cancer is one of the major aging-associated diseases [17, 20, 178, 192] that can be compared and contrasted with AD. We describe some features of cancer research with the caveat that human malignancies differ from each other in mechanisms and manifestations. Cancer biology diverges meaningfully from AD, but both AD and cancer mechanisms have been linked with cell maintenance pathways, oxidative stress, aberrant phosphorylation, immunity, and apoptosis [168].
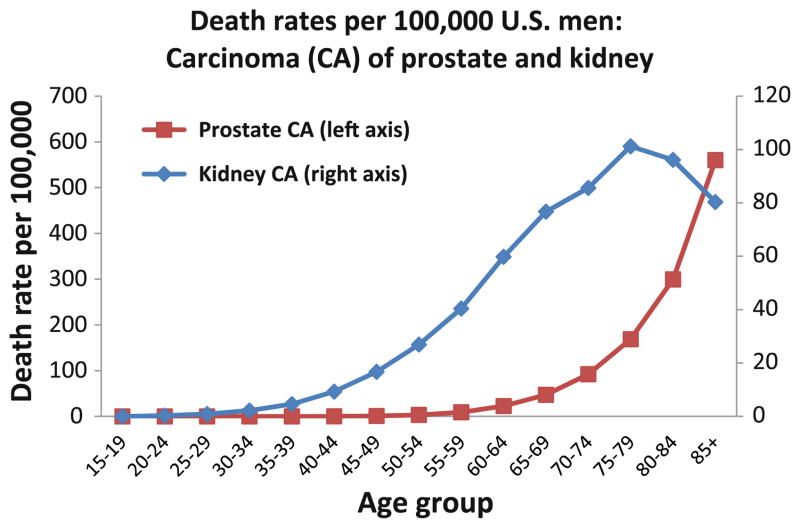
As with neurodegenerative diseases, particular cancer subtypes tend to affect humans of distinct age ranges. Pathology-based epidemiology can provide clues about which subtypes of cancer are more strongly associated with advanced aging. Figure 3, derived from the publicly accessible SEER database [87], demonstrates the difference in death rates from two types of human genitourinary carcinoma—kidney and prostate—in American males. Both cancers are “age-associated”; mortality of both is increased in old age. However, the natural histories of the two cancers differ. Kidney carcinoma incidence increases in age 50–80s unlike prostate cancer that surges later in the aging scale; these differences may be relevant to clinical diagnostics and drug trials. Affecting an older population, prostate cancer is more strongly linked to senescence pathways [205]. Although seemingly remote from AD pathobiology, these data provide insights into the stochastic tendencies of human disease to manifest in distinct ways, for reasons that are currently not well understood, and help establish the need for sophisticated pathology-based epidemiology.
Fig. 3.
Death rates for prostate and kidney carcinoma (CA) in American males according to the SEER database [87]. These data are presented to show the different epidemiology of two different urothelial-derived cancers in men. This is a case where the epidemiology can help guide important research questions related to the biology of the cancers and the impact of aging and senescence. Although cancer is considered an aging-associated disease, the particular subtypes of cancer tend to inhabit particular ranges of the human age spectrum. This paradigm also applies to human neurodegenerative diseases which (like kidney cancer) may peak prior to extreme old age
AD and dementia epidemiology
Dementia versus AD epidemiology: recent progress
The epidemiology of AD in advanced age is deceptively challenging to address [100, 123, 136, 229, 233]. Autopsy series are critical for understanding AD epidemiology for the simple reason that definitive AD diagnosis requires autopsy confirmation. This fact is important to acknowledge because there is no truly epidemiological study cohort with a 100% autopsy rate. With that caveat in mind, recent scientific progress has been achieved through analyses of large high-quality autopsy series. Excellent studies, which have included community-based cohorts, have provided important new insights [8, 70, 91, 97, 156, 171, 198, 233]. European datasets have provided notably rich contributions [10, 38, 104]. In the US, some of these data derive from NIH-sponsored Alzheimer’s Disease Centers (ADCs), which comprise many thousands of autopsies of clinically well-characterized patients, consolidated under the National Alzheimer’s Coordinating Center (NACC) [22].
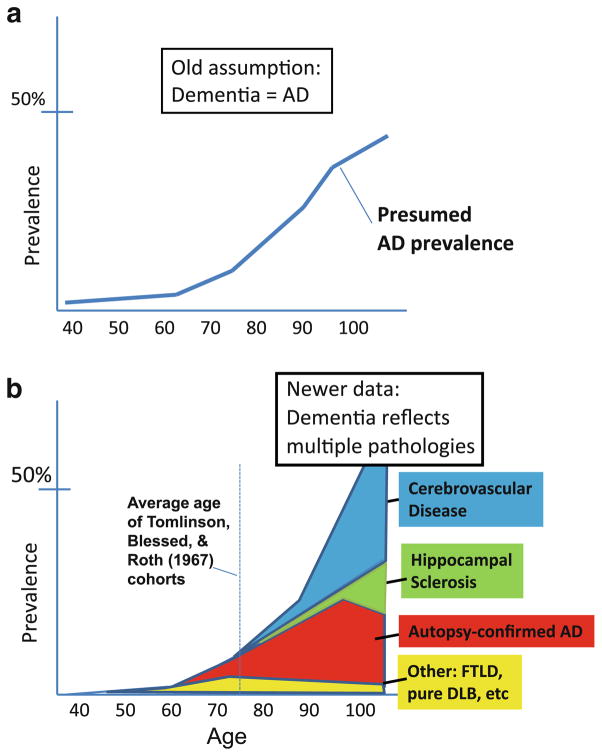
Recent autopsy series have established the central importance of previously ignored non-AD pathologies. The classic clinical–pathological studies of Tomlinson, Blessed, and Roth had substantial impact on researchers’ views of AD clinical–pathological correlation, despite being necessarily unacquainted with prevalent brain diseases, such as dementia with Lewy bodies (which often coexists with AD pathology), hippocampal sclerosis, frontotemporal lobar dementia (FTLD) subtypes, and other diseases. The mean age at death for demented subjects and control subjects in the seminal Tomlinson, Blessed, and Roth papers [188, 219] were 76.4 and 75.4 years, respectively. This is 2–5 years below the average life expectancies for Western countries [16], and a critically distinct cohort from the many individuals that live beyond their 90th year. The large majority of cognitively impaired subjects in their mid-70s is affected by advanced AD pathology, but this is less true in extreme old age (see below). In summary, early clinical–pathological studies were scientifically important, but also misleading if read without recourse to subsequent scholarship.
AD pathology in persons past 90 years of age
Epidemiologic data initially appeared to support the concept that aging is a major risk factor for dementia, and therefore AD. According to many different studies, dementia prevalence in Western populations is approximately 2% at age 65 and then doubles every 5 years thereafter [73, 112, 118]. Dementia epidemiology is less thoroughly studied in extreme old age. Dementia incidence appears to level off after age 90 [83, 118, 123, 185, 186] although clinical dementia prevalence probably does keep increasing [54, 203]. Whereas dementia prevalence keeps increasing, this does not necessarily mean that AD has increased prevalence in the same cohorts. For example, ear infections can lead to hearing loss in children; elderly individuals often have hearing loss, but this does not necessarily indicate increased ear infections in elderly individuals.
Clinical data without rigorous pathological correlation can be misleading. In contrast to the increased prevalence of dementia with advanced age (a clinical observation), the appearance of neuritic amyloid plaques and NFTs by pathology seems to level off in older cognitively impaired individuals according to multiple autopsy series [2, 56, 86, 193, 222], with the caveat that not all studies agree (discussed in [96, 106]). Even in extreme old age, the presence of many neocortical NFTs correlates with antemortem cognitive decline [65, 148, 150, 228], the enigma relates to cognitively impaired individuals whose brains lack AD pathology at autopsy. Recall that AD is defined by the presence of neocortical neuritic amyloid plaques and neocortical NFTs [1]. If the prevalence of AD pathological hallmarks is leveling off, or even decreasing, after the ninth decade of life while the prevalence of dementia is still increasing, then there must be a scientific explanation. Three unproven—and not necessarily reconcilable—hypotheses could help explain the apparent paradox:
Hypothesis #1: All demented subjects have AD unless proven otherwise. Because extremely old individuals often suffer from dementia without excessive burden of plaques and tangles, then plaques and tangles are mere epiphenomena that are not biologically important;
Hypothesis #2: There is a survival bias, whereby individuals genetically predisposed to AD are less represented in cohorts of extreme old age; or
Hypothesis #3: AD is indeed a distinct disease but like many other diseases (e.g., kidney carcinoma) levels off in prevalence prior to extreme old age, and the increased prevalence of dementia past age 90 is due to specific diseases other than AD, in combination with the frequent, but not increasing, AD in that cohort.
We consider that the existing published data best support Hypotheses #2 and #3, but not Hypothesis #1. The survival bias in AD has been described above, related to genetic disease risk. However, why are so many individuals in extreme old age cognitively impaired and lacking AD pathology? There are prevalent brain diseases, besides AD, that contribute to dementia in the elderly. Some of these brain diseases are particularly germane to populations beyond 90 years of age.
Consider the following two statements:
Mechanism “X” contributes to AD.
Mechanism “X”: contributes to dementia.
These two statements are critically different, but seem to be frequently confused because, for a given patient, mechanism “X” could worsen cognitive symptoms in either case. Yet if AD is a discrete disease then merging it with other dementias (as an abstract idea or clinically for particular patients) can be seriously misleading both for researchers and clinicians. Ideally, each disease should be expected to be studied using different experimental designs, diagnosed using specific tests, and each disease would probably require separate treatments.
Many diseases other than AD affect the aged brain (Table 3). Prevalent brain diseases in advanced age, include cerebrovascular disease (CVD), hippocampal sclerosis (HS-Aging), synucleinopathies, FTLD, hematomas, normal pressure hydrocephalus, and numerous other neurological conditions. These are all directly relevant to a discussion of “dementia versus AD” because they may worsen cognition irrespective of AD status. We focus here on four different non-AD mechanisms: CVD, HS-Aging, diabetes, and synapse loss. While each can contribute to dementia, none of them has been proven to relate specifically to AD pathogenesis.
Table 3.
Sample of relatively prevalent conditions in advanced age that can adversely affect cognition or performance on cognitive tests independent of AD status
| Brain diseases in advanced age | Systemic illness that can affect cognition |
|---|---|
| Occlusive or embolic infarct(s), hemorrhagic infarct(s), frontotemporal lobar dementias, α-synucleinopathies, including Parkinson disease dementia and dementia with Lewy bodies, HS-Aging and other TDP-43 pathologies, tangle-predominant dementia, subdural or epidural hematomas, chronic traumatic encephalopathy, depression, anxiety, normal pressure hydrocephalus, and many others | Side effect of chemotherapy or other medication, side effect of other disease including cancers, heart failure and other causes of vascular insufficiency, hyperglycemia, hypoglycemia, renal dysfunction, liver dysfunction, pulmonary disease including emphysema and other causes of chronic hypoxia, metabolic fluxes (e.g. electrolyte imbalances), infection (CNS or outside CNS), vasculitis or other autoimmune/rheumatic disease, substance abuse, and many others |
| Number of the above that have been proven to specifically induce Alzheimer’s disease, as opposed to co-morbidly altering the cognitive symptoms? | |
| None | |
Hippocampal sclerosis and cerebrovascular disease
With advanced age, it is normal for individuals’ cognitive abilities to decline from previous levels although the brains of many aged individuals lack abundant AD pathology [56, 154]. As possible explanations for this phenomenon, we describe two brain diseases that, unlike AD, preferentially affect people in extreme old age: CVD and HS-Aging. Study of both diseases is still evolving, and rubrics are still being developed for optimal clinical–pathological correlation.
CVD, unlike AD, occurs through many distinct mechanisms, progresses in an unpredictable fashion, and currently lacks a universally applied nosology for pathological characterization. There are large vessel pathologies, small vessel pathologies, diseases related to the heart or kidney, and CVD related to other diseases and their therapies (including chemotherapies for cancer) that may manifest as CVD. Embolism, hemorrhage, neuroinflammation, impaired perfusion, hypertension, hypoxia, vascular malformations, glycemic fluxes, and other disease-inducing vascular mechanisms are all relatively common conditions that can induce adverse changes throughout the CNS [4].
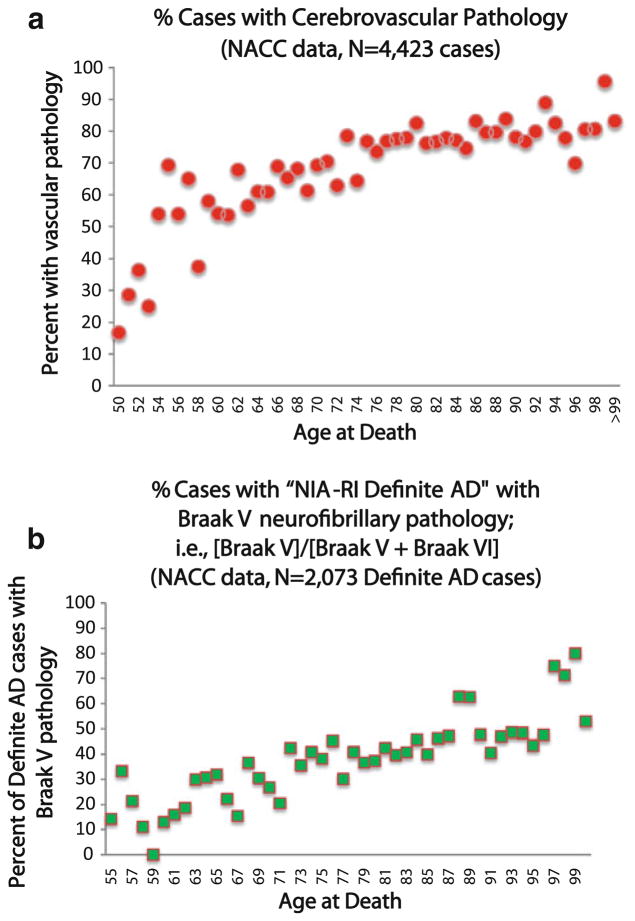
Whereas overt clinical strokes affect ~750,000 in America each year, over 11 million discrete, but clinically silent cerebrovascular events are thought to occur over the same interval [3, 12, 68]. Radiographically, discrete infarcts may be seen in only ~25% of octogenarians [60], yet MRI white matter hyperintensities are noted in over 80% of octagenarians [74, 226] and literally 100% of persons in extreme old age [172]. This is indicative of CVD pathology [39, 153] in advanced aging and a higher risk for clinical stroke [59]. On microscopic examination, at least subtle CVD pathology is detectable in 75–90% of persons over the age of 90 years [150, 224, 225]. The increased prevalence of CVD pathology with age is clearly seen in the NACC neuropathological dataset (Fig. 4a).
Fig. 4.
National Alzheimer’s Coordinating Center (NACC) Registry data provide important clues about the correlations between aging, Alzheimer’s disease (AD), and cerebrovascular disease (CVD). Data were obtained from 25 different Alzheimer’s Disease research centers as described previously [23]. For the assessment of CVD pathology (a), all cases in the relevant age range (N = 4,423) were included. These show that CVD is strongly increased in advanced age with ~90% of centenarians showing at least subtle CVD pathology. In assessing AD (b), we only evaluated individuals with Braak stages V or VI neurofibrillary pathology (N = 2,073). Note that among brains with “definite” AD pathology, there is an increasing tendency for Braak V (instead of VI) in older individuals
Why is the high prevalence of CVD directly and critically relevant to a discussion of AD? The frequency of cerebrovascular pathologies in advanced old age means that both cognition and other pathology need to be assessed entirely differently. An analogy can be made to a given patient’s lung function and pathological outcomes if he had both asthma and pneumonia; this would magnify the impact of each separate disease. Accordingly, CVD (not limited to cases of “vascular dementia”) often coexists, and synergizes, with AD along a broad spectrum of disease severity [103].
CVD causes more rapid cognitive deterioration in patients with coexisting AD pathology relative to patients without AD pathology [143, 181]. Subcortical and/or lacunar infarcts alter the detection threshold for dementia [81, 170, 171, 199, 200, 209]. The clinical impact of CVD—although difficult to predict confidently for any individual patient—is correlated with systematic changes in pathological outcomes related to AD. Persons with CVD pathology tend to have lower AD-related pathology with a given degree of cognitive impairment [150, 170, 234]. Correspondingly, as people become older (with more CVD), a larger percentage of AD cases die with more moderate AD pathology—i.e., Braak stage V rather than Braak stage VI (Fig. 4b).
The frequent presence of concomitant CVD dampens the association for other pathologies, such as neurofibrillary pathology, to the severity of antemortem cognitive decline. In summary, CVD alters autopsy results related to AD pathology without necessarily interacting with the disease-specific mechanisms. Some researchers have argued for a more direct link in pathogenetic mechanisms than can be supported by existing autopsy data, and this consideration warrants further investigation.
As with CVD but unlike AD, HS-Aging pathology appears to increase dramatically in extreme old age [53, 63, 101]. The presence of HS-Aging pathology is strongly correlated with antemortem cognitive impairment [148], which can be seen both with mental status assessment and, more specifically, with certain testing methods [152]. Pathogenetically, HS-Aging is probably not attributable to CVD, but is instead linked to aberrant TDP-43 pathology [108]. HS-Aging is a distinct brain disease in comparison to FTLD-TDP cases (which also have aberrant TDP-43 by definition) because of the far older age group affected in HS-Aging and the lack of frontotemporal dementia, progressive aphasia, or semantic dementia clinically [152]. Although by definition HS-Aging affects the hippocampal formation, evidence does not indicate any direct causative association with AD. The strongest genetic risk factor for AD (the APOE-ε4 allele) does not increase risk of HS-Aging [125, 165].
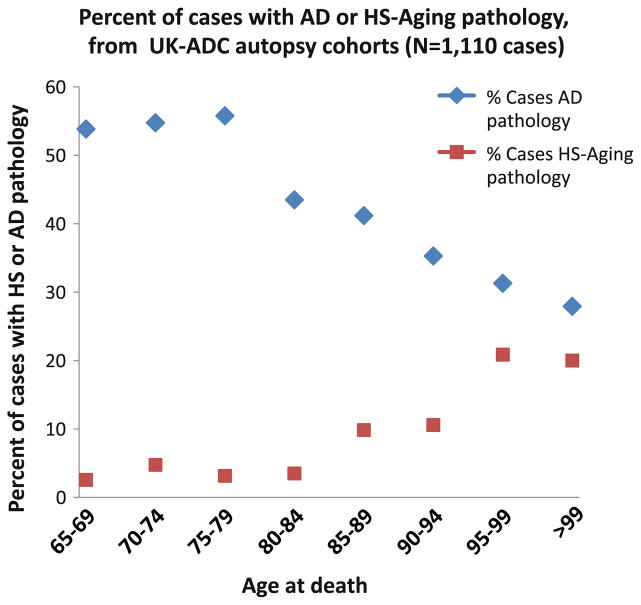
Based on the data from the largest series of HS-Aging cases to date (N = 106 pathologically confirmed HS-Aging cases compared with N = 1,004 controls), we consider it possible that prior low estimates of HS-Aging prevalence [9, 99] were partially biased by inclusion of younger patients. The average age at death for HS-Aging in this large cohort was 93.4 years at death [152] and in this age range HS-Aging pathology is seen in over one-fourth of cases. These results are concordant with a recent autopsy study, showing that 43% of aged individuals’ hippocampi harbored TDP-43 pathology [179]. In our large autopsy series, each year of life after age 95 brought higher risk for HS-Aging pathology, but lower risk for AD pathology (Fig. 5). HS-Aging shares a key biomarker with AD (shrunken hippocampi on MRI), so there is a high chance for confusion clinically [165].
Fig. 5.
In a large clinical–pathological study that incorporated the UK-ADC autopsy cohort, The Nun Study [209], and The Georgia Centenarian Study [173], we evaluated 106 cases with hippocampal sclerosis (HS-Aging) and 1,004 controls [152], including many with AD. Collectively, these cohorts may be enriched for individuals with cognitive impairment at death because of recruitment bias; however, N = 625/1,110 or 57% had neither HS-Aging nor AD. Beyond 95 years of age at death, the prevalence of AD pathology (defined by numerous neuritic amyloid plaques and Braak stages V or VI on autopsy) appeared to decline, whereas the prevalence of HS-Aging pathology became almost as high as AD pathology
Figure 5 provides empirical data to demonstrate that not all aged individuals have AD by pathology. Nor does each added year of life lead to increased prevalence of AD pathology (unlike HS-Aging and CVD). Thus, criteria #3 and #4 (Table 1) are not met. The high prevalence of non-AD pathologies in the “oldest old” helps account for increased dementia in this cohort. The “classic” epidemiology of AD and dementia, and a newer representation that reconciles these data, are presented in Fig. 6. This begins to explain some of the complex underpinnings of pathology-based epidemiology. However, we are also challenged to consider additional aging-associated mechanisms that also have been linked with AD pathogenesis.
Fig. 6.
Epidemiology of clinical dementia and specific pathologies that contribute to dementia. The epidemiology of dementia (a) is based on well-characterized clinical cohorts (for review see [112]). The epidemiology of the different brain diseases—with autopsy confirmation—is far harder to know for sure because the data are critically dependent on autopsy-derived information including the neuropathological practices used at autopsy. Most autopsy series lack large numbers of individuals of extreme advanced age, and each study has distinct biases in terms of recruitment, inclusion, and neuropathological assessments. The chart (b) is a subjective amalgamation of multiple published studies pertinent to this important topic [19, 21, 61, 76, 79, 80, 96, 102, 105, 106, 115, 140, 145, 150, 156, 158, 171, 176, 184, 193, 198, 211, 217, 228, 231]
Diabetes and synapse loss
Type 2 diabetes (T2DM) [57] and synapse loss [95] have been connected with both advanced age and with AD. If there were strong etiological connections between these aging-associated (but poorly understood) mechanisms, it would bolster the idea that aging and AD are linked with each other. However, how persuasive is the evidence that T2DM and synapse loss are specific, early contributors to AD, rather than parameters that correlate with dementia risk instead of AD risk?
T2DM is an aging-associated disease that has been linked to AD. A current Pubmed search of “Alzheimer’s and diabetes” retrieves 1,906 published papers, of which 789 (41.4%) are review papers. Many of these papers hypothesize that AD is mechanistically linked with T2DM [153]. Yet of the research papers related to AD and diabetes, only a relative handful involve human patients with assessment of antemortem glycemic status correlated with postmortem autopsy results, i.e. AD pathology [8, 15, 24, 89, 98, 135, 153, 166, 210]. These studies, comprising 1,843 autopsies from nine different research centers, are summarized briefly in Table 4. Note that most of the published work demonstrates that there is no positive correlation between diabetes and AD pathology. To the contrary, studies that have evaluated the question of whether antemortem diabetes correlates with AD pathology have shown that persons dying with a history of diabetes have less AD pathology (but more cerebrovascular disease pathology) than individuals without T2DM. These data highlight a key study design feature—namely, incorporating autopsy studies for a disease that requires neuropathologic evaluation for diagnosis—which has been regrettably ignored in meta-analyses discussing the correlation between T2DM and AD risk.
Table 4.
Clinical–pathological studies that correlate the presence of antemortem diabetes with autopsy-confirmed Alzheimer’s disease (AD) and cerebrovascular disease (CVD) pathology
| Study | Diabetics | Non-diabetics | In diabetics, versus nondiabetics
|
|
|---|---|---|---|---|
| N | N | Risk of AD pathology | Risk of CVD pathology | |
| Heitner and Dickson [89] | 49 | 52 | Not increased | Not evaluated |
| Peila et al. [166] | 216 total autopsies | Not increaseda | Increased | |
| Janson et al. [98] | 28 | 19 | Not increasedb | Not evaluated |
| Beeri et al. [24] | 61 | 324 | Decreased | Increased |
| Arvanitakis et al. [15] | 36 | 197 | Not increased | Increased |
| Nelson et al. [149] | 50 | 189 | Decreased | Increased |
| Sonnen et al. [210] | 59 | 137 | Decreasedc | Increased |
| Ahtiluoto et al. [8] | 70 | 221 | Decreased | Increased |
| Matsuzaki et al. [135] | 135 total autopsies | Not cleard | Not cleard | |
No effect of “diabetes only” on AD pathology overall, but a synergy was seen between APOE4 allele and diabetes that correlated with increased AD pathology for APOE4 + diabetics in this study
No effect of type 2 diabetes in terms of amyloid plaques (diffuse or neuritic) or neurofibrillary tangles. Duration of diabetes appeared linked to AD pathology (although the significance of this is not clear) and clinical AD diagnosis was linked to diabetes although this impression was not confirmed with pathology
Among individuals with dementia only; non-demented persons showed no difference
Authors focused on amyloid plaque and sub-threshold pathology and not diagnostic pathology or neurofibrillary pathology (Braak stages). Data were interpreted to suggest insulin resistance correlates with increased amyloid plaques with synergy between glycemic factors, APOE, and AD pathology. However, presented data could be interpreted differently
Although autopsy studies argue against the link between T2DM with AD pathology, published data have established that T2DM is a strong risk factor for impaired cognition [50, 159]. Diabetes leads to small vessel ischemic disease including CVD, not to mention glycemic fluxes and neuroinflammation that can be neurotoxic [153]. There are still many unknowns and potential confounders. T2DM associated clinical–biological mechanisms, including metabolic syndrome, adiposity, hormone changes including leptin and insulin, lifestyle (diet and exercise), and inflammation-related factors that may be eventually linked specifically to AD [18, 55, 130, 223, 235]. At this time, the best-supported hypothesis, based on the human studies that include autopsy confirmation, indicates that T2DM induces CVD pathology (and thus is likely to worsen brain function), but T2DM itself does not lead to increased AD pathology.
Another process at the ill-defined interface between human brain aging and cognitive impairment is “synapse elimination”. In a recent review article, the elimination of synapses was considered a likely connection-point between aging mechanisms and AD pathogenesis [90]. There are at least two key pertinent questions, only one of which has been adequately answered:
How specific is synapse elimination to AD?
Are synapses eliminated upstream, downstream, or in parallel with plaques and tangles?
In AD, there is a wide distribution of synapse loss in both hippocampus and neocortex relative to non-demented controls [134, 195]. It has been stated that decreased synapses is the pathological feature that best correlates with cognitive decline in AD. Even if this were to be taken at face value (see below), this does not imply that synapse loss is a specific pathological occurrence, much less a diagnostic feature, of AD.
Synapse loss occurs during the course of a broad variety of brain diseases: stroke, FTLD, synucleinopathy, traumatic brain injury and many other conditions. In fact, we know of no human condition with progressive neurodegeneration lacking synapse loss. In this sense, synapse loss is utterly nonspecific.
Nor is the relationship between synapse loss and “normal aging” well understood. There have been surprisingly few human studies that have adequately addressed the question about whether there is a “normal” age-related loss of synapses independent of brain disease (see [196] for review). Most of the well-controlled studies report no significant loss of synapses in non-pathological aged brains. Interestingly, there is also not a substantial loss of neocortical neurons in the absence of brain diseases of aging [161, 162]. Age-related changes in synapse numbers may be either region specific or not a common feature of brain aging.
There are published reports in which synaptic markers do not closely correlate with the numbers of amyloid plaques or NFTs on the same cases ([33, 121] although see [62]), which have indicated synapse loss may be a superior metric of AD severity. However, in these and related studies, there is the potential for spurious results since there is a tendency to correlate synapse loss in a particular brain region with AD pathology using either ordinal variables (e.g., Braak/CERAD stages) or else pathological metrics that are not relevant for clinical–pathological correlations (e.g., hippocampal NFTs, “amyloid plaques”, or “amyloid load”). Further, these reports may be confounded by mixed pathologies so frequently seen in aged individuals’ brains.
Unfortunately, whereas synapse changes clearly occur in a variety of conditions, we still do not know whether there are specific synaptic changes upstream of AD-type pathology (plaques and tangles). It is thus unknown where the synaptic changes belong in the AD pathogenetic schema. As with diabetes, the links to AD are as yet too weak to satisfy our criteria #5 (Table 1). The biology of synapse changes in AD is an active and fascinating area of AD research, yet it needs to be kept in mind that synapse elimination is not specific for AD or for “brain aging” and much remains to be learned in this area.
Summary and conclusions
A summary of reviewed topics is presented in Table 5. Data from genetics indicate that APP pathway genes and not aging-related genes are associated with AD risk. AD pathology is not inevitable in all humans, even in advanced age. In contrast, there are diseases that increase in prevalence in extreme old age (especially HS-Aging and CVD pathology). Neither diabetes, synapse loss, nor any other aging mechanism have been proven to be involved directly in AD pathogenesis although they may worsen cognitive symptoms independently of AD. Thus, none of the criteria for AD being linked specifically to aging mechanisms have been met (Table 1), so the null hypothesis is sustained, at least for now. The preponderance of data indicates that research aiming at diagnostic or therapeutic relevance will most productively be oriented toward those specific pathways known to be activated in AD, rather than focusing on aging-related mechanisms as a sole target for intervention in this devastating disease.
Table 5.
Summary
| Summary points |
|---|
| Persons with many NFTs in neocortex always have cognitive impairment; in the presence of neuritic plaques this is the definition of AD |
| Genetic diseases that lead to premature aging (“progeria”) do not induce AD phenotype |
| Highly penetrant genetic diseases that lead to AD phenotype have no known link to aging but instead to APP processing |
| Persons with cognitive impairment may lack neocortical NFTs, especially in extreme advanced old age; this is probably due to the brain diseases other than AD that affect aged persons’ brains including HS-Aging, CVD, and diabetes |
| HS-Aging and CVD, unlike AD, increase in aged persons’ brains every year after age 95 |
| Other human diseases including specific cancer subtypes have increased prevalence in seventh and eighth decade of life but lower prevalence thereafter |
| Aging-linked mechanisms, including HS-Aging, CVD, diabetes and synapse loss, have not been proven to induce AD pathology |
| There is no particular senescence-related biochemical mechanism that has been definitively linked to AD |
Acknowledgments
We are deeply grateful to all of the study participants. We also thank Leslie Phillips, MS and Sarah Monsell, MS for help with NACC data. Brain tissue from the DS autopsy case was obtained from NICHD Brain and Tissue Bank for Developmental Disorders at the University of Maryland, Baltimore, MD, was under contract HHSN275200900011C, reference number N01-HD-9-0011. Corresponding author: Dr. Nelson had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Conflict of interest This study was supported by NIH grants R01 NS061933, K08 NS050110, P30 AG028383, and U01 AG016976.
Contributor Information
Peter T. Nelson, Email: pnels2@email.uky.edu, Department of Pathology, University of Kentucky, Rm 311, Sanders-Brown Building, 800 S. Limestone, Lexington, KY 40536-0230, USA. Sanders-Brown Center on Aging, University of Kentucky, Lexington, KY 40536-0230, USA
Elizabeth Head, Sanders-Brown Center on Aging, University of Kentucky, Lexington, KY 40536-0230, USA. Department of Anatomy and Neurobiology, University of Kentucky, Lexington, KY 40536-0230, USA.
Frederick A. Schmitt, Division of Neuropathology, Department of Neurology, University of Kentucky, Lexington, KY 40536-0230, USA. Sanders-Brown Center on Aging, University of Kentucky, Lexington, KY 40536-0230, USA
Paulina R. Davis, Sanders-Brown Center on Aging, University of Kentucky, Lexington, KY 40536-0230, USA. Department of Anatomy and Neurobiology, University of Kentucky, Lexington, KY 40536-0230, USA
Janna H. Neltner, Department of Pathology, University of Kentucky, Rm 311, Sanders-Brown Building, 800 S. Limestone, Lexington, KY 40536-0230, USA
Gregory A. Jicha, Division of Neuropathology, Department of Neurology, University of Kentucky, Lexington, KY 40536-0230, USA. Sanders-Brown Center on Aging, University of Kentucky, Lexington, KY 40536-0230, USA
Erin L. Abner, Sanders-Brown Center on Aging, University of Kentucky, Lexington, KY 40536-0230, USA
Charles D. Smith, Division of Neuropathology, Department of Neurology, University of Kentucky, Lexington, KY 40536-0230, USA. Sanders-Brown Center on Aging, University of Kentucky, Lexington, KY 40536-0230, USA
Linda J. Van Eldik, Sanders-Brown Center on Aging, University of Kentucky, Lexington, KY 40536-0230, USA. Department of Anatomy and Neurobiology, University of Kentucky, Lexington, KY 40536-0230, USA
Richard J. Kryscio, Department of Statistics, University of Kentucky, Lexington, KY 40536-0230, USA. Sanders-Brown Center on Aging, University of Kentucky, Lexington, KY 40536-0230, USA
Stephen W. Scheff, Sanders-Brown Center on Aging, University of Kentucky, Lexington, KY 40536-0230, USA. Department of Anatomy and Neurobiology, University of Kentucky, Lexington, KY 40536-0230, USA
References
- 1.Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- 2.Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) Lancet. 2001;357:169–175. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- 3.Prevalence of stroke–United States, 2005. MMWR Morb Mortal Wkly Rep. 2007;56:469–474. [PubMed] [Google Scholar]
- 4.Sundt’s occlusive cerebrovascular disease. W.B. Saunders; Philadelphia: 1994. [Google Scholar]
- 5.Abner EL, Kryscio RJ, Schmitt FA, et al. “End-Stage” neurofibrillary tangle pathology in preclinical Alzheimer’s disease: fact or fiction? J Alzheimers Dis. 2011 doi: 10.3233/JAD-2011-101980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abner EL, Schmitt FA, Mendiondo MS, Marcum JL, Kryscio RJ. Vitamin E and all-cause mortality: a meta-analysis. Curr Aging Sci. 2011 doi: 10.2174/1874609811104020158. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adib-Samii P, Brice G, Martin RJ, Markus HS. Clinical spectrum of CADASIL and the effect of cardiovascular risk factors on phenotype: study in 200 consecutively recruited individuals. Stroke. 2010;41:630–634. doi: 10.1161/STROKEAHA.109.568402. [DOI] [PubMed] [Google Scholar]
- 8.Ahtiluoto S, Polvikoski T, Peltonen M, et al. Diabetes, Alzheimer disease, and vascular dementia: a population-based neuropathologic study. Neurology. 2010;75:1195–1202. doi: 10.1212/WNL.0b013e3181f4d7f8. [DOI] [PubMed] [Google Scholar]
- 9.Ala TA, Beh GO, Frey WH., 2nd Pure hippocampal sclerosis: a rare cause of dementia mimicking Alzheimer’s disease. Neurology. 2000;54:843–848. doi: 10.1212/wnl.54.4.843. [DOI] [PubMed] [Google Scholar]
- 10.Alafuzoff I, Arzberger T, Al-Sarraj S, et al. Staging of neurofibrillary pathology in Alzheimer’s disease: a study of the BrainNet Europe Consortium. Brain Pathol. 2008;18:484–496. doi: 10.1111/j.1750-3639.2008.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albani D, Polito L, Forloni G. Sirtuins as novel targets for Alzheimer’s disease and other neurodegenerative disorders: experimental and genetic evidence. J Alzheimers Dis. 2010;19:11–26. doi: 10.3233/JAD-2010-1215. [DOI] [PubMed] [Google Scholar]
- 12.Allen NB, Lichtman JH, Cohen HW, et al. Vascular disease among hospitalized multiple sclerosis patients. Neuroepidemiology. 2008;30:234–238. doi: 10.1159/000128103. [DOI] [PubMed] [Google Scholar]
- 13.Altena E, Ramautar JR, Van Der Werf YD, Van Someren EJ. Do sleep complaints contribute to age-related cognitive decline? Prog Brain Res. 2010;185:181–205. doi: 10.1016/B978-0-444-53702-7.00011-7. [DOI] [PubMed] [Google Scholar]
- 14.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 15.Arvanitakis Z, Schneider JA, Wilson RS, et al. Diabetes is related to cerebral infarction but not to AD pathology in older persons. Neurology. 2006;67:1960–1965. doi: 10.1212/01.wnl.0000247053.45483.4e. [DOI] [PubMed] [Google Scholar]
- 16.Asiskovitch S. Gender and health outcomes: the impact of healthcare systems and their financing on life expectancies of women and men. Soc Sci Med. 2010;70:886–895. doi: 10.1016/j.socscimed.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 17.Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88:557–579. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- 18.Baker LD, Cross DJ, Minoshima S, et al. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol. 2011;68:51–57. doi: 10.1001/archneurol.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barker WW, Luis CA, Kashuba A, et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord. 2002;16:203–212. doi: 10.1097/00002093-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Bassi P, Sacco E. Cancer and aging: the molecular pathways. Urol Oncol. 2009;27:620–627. doi: 10.1016/j.urolonc.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Beach TG, Sue LI, Walker DG, et al. The Sun Health Research Institute Brain Donation Program: description and experience, 1987–2007. Cell Tissue Bank. 2008;9:229–245. doi: 10.1007/s10561-008-9067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beekly DL, Ramos EM, Lee WW, et al. The National Alzheimer’s Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord. 2007;21:249–258. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- 23.Beekly DL, Ramos EM, van Belle G, et al. The National Alzheimer’s Coordinating Center (NACC) Database: an Alzheimer disease database. Alzheimer Dis Assoc Disord. 2004;18:270–277. [PubMed] [Google Scholar]
- 24.Beeri MS, Silverman JM, Davis KL, et al. Type 2 diabetes is negatively associated with Alzheimer’s disease neuropathology. J Gerontol A Biol Sci Med Sci. 2005;60:471–475. doi: 10.1093/gerona/60.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belbin O, Carrasquillo MM, Crump M, et al. Investigation of 15 of the top candidate genes for late-onset Alzheimer’s disease. Hum Genet. 2010 doi: 10.1007/s00439-010-0924-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennett DA, Schneider JA, Wilson RS, et al. Amyloid mediates the association of apolipoprotein E e4 allele to cognitive function in older people. J Neurol Neurosurg Psychiatry. 2005;76:1194–1199. doi: 10.1136/jnnp.2004.054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett DA, Wilson RS, Schneider JA, et al. Apolipoprotein E epsilon4 allele, AD pathology, and the clinical expression of Alzheimer’s disease. Neurology. 2003;60:246–252. doi: 10.1212/01.wnl.0000042478.08543.f7. [DOI] [PubMed] [Google Scholar]
- 28.Bertram L, Lill CM, Tanzi RE. The genetics of Alzheimer disease: back to the future. Neuron. 2010;68:270–281. doi: 10.1016/j.neuron.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Biere AL, Ostaszewski B, Zhao H, et al. Co-expression of beta-amyloid precursor protein (betaAPP) and apolipoprotein E in cell culture: analysis of betaAPP processing. Neurobiol Dis. 1995;2:177–187. doi: 10.1006/nbdi.1995.0019. [DOI] [PubMed] [Google Scholar]
- 30.Biffi A, Anderson CD, Desikan RS, et al. Genetic variation and neuroimaging measures in Alzheimer disease. Arch Neurol. 2010;67:677–685. doi: 10.1001/archneurol.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Black RS, Sperling RA, Safirstein B, et al. A single ascending dose study of bapineuzumab in patients with Alzheimer disease. Alzheimer Dis Assoc Disord. 2010;24:198–203. doi: 10.1097/WAD.0b013e3181c53b00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blagosklonny MV. Validation of anti-aging drugs by treating age-related diseases. Aging (Albany NY) 2009;1:281–288. doi: 10.18632/aging.100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blennow K, Bogdanovic N, Alafuzoff I, Ekman R, Davidsson P. Synaptic pathology in Alzheimer’s disease: relation to severity of dementia, but not to senile plaques, neurofibrillary tangles, or the ApoE4 allele. J Neural Transm. 1996;103:603–618. doi: 10.1007/BF01273157. [DOI] [PubMed] [Google Scholar]
- 34.Boche D, Denham N, Holmes C, Nicoll JA. Neuropathology after active Abeta42 immunotherapy: implications for Alzheimer’s disease pathogenesis. Acta Neuropathol. 2010;120:369–384. doi: 10.1007/s00401-010-0719-5. [DOI] [PubMed] [Google Scholar]
- 35.Boche D, Donald J, Love S, et al. Reduction of aggregated Tau in neuronal processes but not in the cell bodies after Abeta42 immunisation in Alzheimer’s disease. Acta Neuropathol. 2010;120:13–20. doi: 10.1007/s00401-010-0705-y. [DOI] [PubMed] [Google Scholar]
- 36.Bordin P, Da Col PG, Peruzzo P, et al. Causes of death and clinical diagnostic errors in extreme aged hospitalized people: a retrospective clinical–necropsy survey. J Gerontol A Biol Sci Med Sci. 1999;54:M554–M559. doi: 10.1093/gerona/54.11.m554. [DOI] [PubMed] [Google Scholar]
- 37.Braak H, Braak E, Bohl J. Staging of Alzheimer-related cortical destruction. Eur Neurol. 1993;33:403–408. doi: 10.1159/000116984. [DOI] [PubMed] [Google Scholar]
- 38.Braak H, Del Tredici K. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol. 2011;121:171–181. doi: 10.1007/s00401-010-0789-4. [DOI] [PubMed] [Google Scholar]
- 39.Braffman BH, Zimmerman RA, Trojanowski JQ, et al. Brain MR: pathologic correlation with gross and histopathology. 2. Hyperintense white-matter foci in the elderly. AJR Am J Roentgenol. 1988;151:559–566. doi: 10.2214/ajr.151.3.559. [DOI] [PubMed] [Google Scholar]
- 40.Brown WT, Kieras FJ, Houck GE, Jr, Dutkowski R, Jenkins EC. A comparison of adult and childhood progerias: Werner syndrome and Hutchinson–Gilford progeria syndrome. Adv Exp Med Biol. 1985;190:229–244. doi: 10.1007/978-1-4684-7853-2_10. [DOI] [PubMed] [Google Scholar]
- 41.Bu G. Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nat Rev Neurosci. 2009;10:333–344. doi: 10.1038/nrn2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burtner CR, Kennedy BK. Progeria syndromes and ageing: what is the connection? Nat Rev Mol Cell Biol. 2010;11:567–578. doi: 10.1038/nrm2944. [DOI] [PubMed] [Google Scholar]
- 43.Butterfield DA, Bush AI. Alzheimer’s amyloid beta-peptide (1–42): involvement of methionine residue 35 in the oxidative stress and neurotoxicity properties of this peptide. Neurobiol Aging. 2004;25:563–568. doi: 10.1016/j.neurobiolaging.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 44.Cacabelos R, Fernandez-Novoa L, Lombardi V, Kubota Y, Takeda M. Molecular genetics of Alzheimer’s disease and aging. Methods Find Exp Clin Pharmacol. 2005;27(Suppl A):1–573. [PubMed] [Google Scholar]
- 45.Caselli RJ, Graff-Radford NR, Reiman EM, et al. Pre-clinical memory decline in cognitively normal apolipoprotein E-epsilon4 homozygotes. Neurology. 1999;53:201–207. doi: 10.1212/wnl.53.1.201. [DOI] [PubMed] [Google Scholar]
- 46.Castellani RJ, Zhu X, Lee HG, et al. Neuropathology and treatment of Alzheimer disease: did we lose the forest for the trees? Expert Rev Neurother. 2007;7:473–485. doi: 10.1586/14737175.7.5.473. [DOI] [PubMed] [Google Scholar]
- 47.Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 48.Chong MS, Sahadevan S. Preclinical Alzheimer’s disease: diagnosis and prediction of progression. Lancet Neurol. 2005;4:576–579. doi: 10.1016/S1474-4422(05)70168-X. [DOI] [PubMed] [Google Scholar]
- 49.Clavaguera F, Bolmont T, Crowther RA, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11:909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coker LH, Shumaker SA. Type 2 diabetes mellitus and cognition: an understudied issue in women’s health. J Psychosom Res. 2003;54:129–139. doi: 10.1016/s0022-3999(02)00523-8. [DOI] [PubMed] [Google Scholar]
- 51.Convit A, De Leon MJ, Tarshish C, et al. Specific hippocampal volume reductions in individuals at risk for Alzheimer’s disease. Neurobiol Aging. 1997;18:131–138. doi: 10.1016/s0197-4580(97)00001-8. [DOI] [PubMed] [Google Scholar]
- 52.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science (New York, NY) 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 53.Corey-Bloom J, Sabbagh MN, Bondi MW, et al. Hippocampal sclerosis contributes to dementia in the elderly. Neurology. 1997;48:154–160. doi: 10.1212/wnl.48.1.154. [DOI] [PubMed] [Google Scholar]
- 54.Corrada MM, Brookmeyer R, Paganini-Hill A, Berlau D, Kawas CH. Dementia incidence continues to increase with age in the oldest old: the 90+ study. Ann Neurol. 2010;67:114–121. doi: 10.1002/ana.21915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Craft S. The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Arch Neurol. 2009;66:300–305. doi: 10.1001/archneurol.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crystal HA, Dickson D, Davies P, et al. The relative frequency of “dementia of unknown etiology” increases with age and is nearly 50% in nonagenarians. Arch Neurol. 2000;57:713–719. doi: 10.1001/archneur.57.5.713. [DOI] [PubMed] [Google Scholar]
- 57.De Berardis G, D’Ettorre A, Graziano G, et al. The burden of hospitalization related to diabetes mellitus: a population-based study. Nutr Metab Cardiovasc Dis. 2011 doi: 10.1016/j.numecd.2010.10.016. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 58.de Calignon A, Fox LM, Pitstick R, et al. Caspase activation precedes and leads to tangles. Nature. 2010;464:1201–1204. doi: 10.1038/nature08890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. Br Med J. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the Framingham heart study: establishing what is normal. Neurobiol Aging. 2005;26:491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 61.Del Tredici K, Braak H. Neurofibrillary changes of the Alzheimer type in very elderly individuals: neither inevitable nor benign: Commentary on “No disease in the brain of a 115-year-old woman”. Neurobiol Aging. 2008;29:1133–1136. doi: 10.1016/j.neurobiolaging.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 62.Dickson DW, Crystal HA, Bevona C, et al. Correlations of synaptic and pathological markers with cognition of the elderly. Neurobiol Aging. 1995;16:285–298. doi: 10.1016/0197-4580(95)00013-5. (discussion 298–304) [DOI] [PubMed] [Google Scholar]
- 63.Dickson DW, Davies P, Bevona C, et al. Hippocampal sclerosis: a common pathological feature of dementia in very old (> or =80 years of age) humans. Acta Neuropathol. 1994;88:212–221. doi: 10.1007/BF00293396. [DOI] [PubMed] [Google Scholar]
- 64.Ding Q, Dimayuga E, Keller JN. Oxidative stress alters neuronal RNA- and protein-synthesis: implications for neural viability. Free Radic Res. 2007;41:903–910. doi: 10.1080/10715760701416996. [DOI] [PubMed] [Google Scholar]
- 65.Dolan D, Troncoso J, Resnick SM, et al. Age, Alzheimer’s disease and dementia in the Baltimore Longitudinal Study of Ageing. Brain. 2010;133:2225–2231. doi: 10.1093/brain/awq141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Droge W. Oxidative stress and aging. Adv Exp Med Biol. 2003;543:191–200. doi: 10.1007/978-1-4419-8997-0_14. [DOI] [PubMed] [Google Scholar]
- 67.Dubois B, Feldman HH, Jacova C, et al. Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol. 2010;9:1118–1127. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- 68.Fang J, Alderman MH. Trend of stroke hospitalization, United States, 1988–1997. Stroke. 2001;32:2221–2226. doi: 10.1161/hs1001.096193. [DOI] [PubMed] [Google Scholar]
- 69.Ferenci P, Czlonkowska A, Merle U, et al. Late-onset Wilson’s disease. Gastroenterology. 2007;132:1294–1298. doi: 10.1053/j.gastro.2007.02.057. [DOI] [PubMed] [Google Scholar]
- 70.Fernando MS, Ince PG. Vascular pathologies and cognition in a population-based cohort of elderly people. J Neurol Sci. 2004;226:13–17. doi: 10.1016/j.jns.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 71.Finch CE. The biology of aging in model organisms. Alzheimer Dis Assoc Disord. 2003;17(Suppl 2):S39–S41. doi: 10.1097/00002093-200304002-00002. [DOI] [PubMed] [Google Scholar]
- 72.Frost B, Jacks RL, Diamond MI. Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem. 2009;284:12845–12852. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gao S, Hendrie HC, Hall KS, Hui S. The relationships between age, sex, and the incidence of dementia and Alzheimer disease: a meta-analysis. Arch Gen Psychiatry. 1998;55:809–815. doi: 10.1001/archpsyc.55.9.809. [DOI] [PubMed] [Google Scholar]
- 74.Garde E, Mortensen EL, Krabbe K, Rostrup E, Larsson HB. Relation between age-related decline in intelligence and cerebral white-matter hyperintensities in healthy octogenarians: a longitudinal study. Lancet. 2000;356:628–634. doi: 10.1016/S0140-6736(00)02604-0. [DOI] [PubMed] [Google Scholar]
- 75.Gatz M, Reynolds CA, Fratiglioni L, et al. Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry. 2006;63:168–174. doi: 10.1001/archpsyc.63.2.168. [DOI] [PubMed] [Google Scholar]
- 76.Giannakopoulos P, Gold G, Kovari E, et al. Assessing the cognitive impact of Alzheimer disease pathology and vascular burden in the aging brain: the Geneva experience. Acta Neuropathol. 2007;113:1–12. doi: 10.1007/s00401-006-0144-y. [DOI] [PubMed] [Google Scholar]
- 77.Giannakopoulos P, Hof PR, Michel JP, Guimon J, Bouras C. Cerebral cortex pathology in aging and Alzheimer’s disease: a quantitative survey of large hospital-based geriatric and psychiatric cohorts. Brain Res Brain Res Rev. 1997;25:217–245. doi: 10.1016/s0165-0173(97)00023-4. [DOI] [PubMed] [Google Scholar]
- 78.Giannattasio C, Poleggi A, Puopolo M, et al. Survival in Alzheimer’s disease is shorter in women carrying heterozygosity at codon 129 of the PRNP gene and no APOE epsilon 4 allele. Dement Geriatr Cogn Disord. 2008;25:354–358. doi: 10.1159/000119730. [DOI] [PubMed] [Google Scholar]
- 79.Gold G, Bouras C, Kovari E, et al. Clinical validity of Braak neuropathological staging in the oldest-old. Acta Neuropathol. 2000;99:579–582. doi: 10.1007/s004010051163. (discussion 583–574) [DOI] [PubMed] [Google Scholar]
- 80.Gold G, Giannakopoulos P, Herrmann FR, Bouras C, Kovari E. Identification of Alzheimer and vascular lesion thresholds for mixed dementia. Brain. 2007;130:2830–2836. doi: 10.1093/brain/awm228. [DOI] [PubMed] [Google Scholar]
- 81.Gold G, Kovari E, Herrmann FR, et al. Cognitive consequences of thalamic, basal ganglia, and deep white matter lacunes in brain aging and dementia. Stroke. 2005;36:1184–1188. doi: 10.1161/01.STR.0000166052.89772.b5. [DOI] [PubMed] [Google Scholar]
- 82.Guo JL, Lee VM. Seeding of normal tau by pathological tau conformers drives pathogenesis of Alzheimer-like tangles. J Biol Chem. 2011 doi: 10.1074/jbc.M110.209296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hall CB, Verghese J, Sliwinski M, et al. Dementia incidence may increase more slowly after age 90: results from the Bronx Aging Study. Neurology. 2005;65:882–886. doi: 10.1212/01.wnl.0000176053.98907.3f. [DOI] [PubMed] [Google Scholar]
- 84.Harder A, Jendroska K, Kreuz F, et al. Novel twelve-generation kindred of fatal familial insomnia from Germany representing the entire spectrum of disease expression. Am J Med Genet. 1999;87:311–316. doi: 10.1002/(sici)1096-8628(19991203)87:4<311::aid-ajmg6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 85.Harman D. Aging: overview. Ann N Y Acad Sci. 2001;928:1–21. doi: 10.1111/j.1749-6632.2001.tb05631.x. [DOI] [PubMed] [Google Scholar]
- 86.Haroutunian V, Schnaider-Beeri M, Schmeidler J, et al. Role of the neuropathology of Alzheimer disease in dementia in the oldest-old. Arch Neurol. 2008;65:1211–1217. doi: 10.1001/archneur.65.9.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Harras A. Tapping the SEER database. Cancer Pract. 1995;3:184–186. [PubMed] [Google Scholar]
- 88.Hayflick L. Biological aging is no longer an unsolved problem. Ann N Y Acad Sci. 2007;1100:1–13. doi: 10.1196/annals.1395.001. [DOI] [PubMed] [Google Scholar]
- 89.Heitner J, Dickson D. Diabetics do not have increased Alzheimer-type pathology compared with age-matched control subjects. A retrospective postmortem immunocytochemical and histofluorescent study. Neurology. 1997;49:1306–1311. doi: 10.1212/wnl.49.5.1306. [DOI] [PubMed] [Google Scholar]
- 90.Herrup K. Reimagining Alzheimer’s disease—an age-based hypothesis. J Neurosci. 2010;30:16755–16762. doi: 10.1523/JNEUROSCI.4521-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hof PR, Bussiere T, Gold G, et al. Stereologic evidence for persistence of viable neurons in layer II of the entorhinal cortex and the CA1 field in Alzheimer disease. J Neuropathol Exp Neurol. 2003;62:55–67. doi: 10.1093/jnen/62.1.55. [DOI] [PubMed] [Google Scholar]
- 92.Hof PR, Glannakopoulos P, Bouras C. The neuropathological changes associated with normal brain aging. Histol Histopathol. 1996;11:1075–1088. [PubMed] [Google Scholar]
- 93.Holliday R. Understanding ageing. Philos Trans R Soc Lond B Biol Sci. 1997;352:1793–1797. doi: 10.1098/rstb.1997.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Holmes C, Boche D, Wilkinson D, et al. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 95.Huttenlocher PR. Synaptic density in human frontal cortex—developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 96.Imhof A, Kovari E, von Gunten A, et al. Morphological substrates of cognitive decline in nonagenarians and centenarians: a new paradigm? J Neurol Sci. 2007 doi: 10.1016/j.jns.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 97.Iseki E, Tsunoda S, Suzuki K, et al. Regional quantitative analysis of NFT in brains of non-demented elderly persons: comparisons with findings in brains of late-onset Alzheimer’s disease and limbic NFT dementia. Neuropathology. 2002;22:34–39. doi: 10.1046/j.0919-6544.2001.00425.x. [DOI] [PubMed] [Google Scholar]
- 98.Janson J, Laedtke T, Parisi JE, et al. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 2004;53:474–481. doi: 10.2337/diabetes.53.2.474. [DOI] [PubMed] [Google Scholar]
- 99.Jellinger K. Pure hippocampal sclerosis: a rare cause of dementia mimicking Alzheimer’s disease. Neurology. 2000;55:739–740. doi: 10.1212/wnl.55.5.735-d. [DOI] [PubMed] [Google Scholar]
- 100.Jellinger KA. Criteria for the neuropathological diagnosis of dementing disorders: routes out of the swamp? Acta Neuropathol. 2009;117:101–110. doi: 10.1007/s00401-008-0466-z. [DOI] [PubMed] [Google Scholar]
- 101.Jellinger KA. Hippocampal sclerosis: a common pathological feature of dementia in very old humans. Acta Neuropathol. 1994;88:599. doi: 10.1007/BF00296500. [DOI] [PubMed] [Google Scholar]
- 102.Jellinger KA. The pathology of “vascular dementia”: a critical update. J Alzheimers Dis. 2008;14:107–123. doi: 10.3233/jad-2008-14110. [DOI] [PubMed] [Google Scholar]
- 103.Jellinger KA, Attems J. Is there pure vascular dementia in old age? J Neurol Sci. 2010;299:150–154. doi: 10.1016/j.jns.2010.08.038. [DOI] [PubMed] [Google Scholar]
- 104.Jellinger KA, Attems J. Neuropathological evaluation of mixed dementia. J Neurol Sci. 2007;257:80–87. doi: 10.1016/j.jns.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 105.Jellinger KA, Attems J. Prevalence and pathology of vascular dementia in the oldest-old. J Alzheimers Dis. 2010;21:1283–1293. doi: 10.3233/jad-2010-100603. [DOI] [PubMed] [Google Scholar]
- 106.Jellinger KA, Attems J. Prevalence of dementia disorders in the oldest-old: an autopsy study. Acta Neuropathol. 2010;119:421–433. doi: 10.1007/s00401-010-0654-5. [DOI] [PubMed] [Google Scholar]
- 107.Jicha GA, Parisi JE, Dickson DW, et al. Age and apoE associations with complex pathologic features in Alzheimer’s disease. J Neurol Sci. 2008;273:34–39. doi: 10.1016/j.jns.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Josephs KA, Whitwell JL, Knopman DS, et al. Abnormal TDP-43 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology. 2008;71:743–749. doi: 10.1212/01.wnl.0000304041.09418.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jun G, Naj AC, Beecham GW, et al. Meta-analysis confirms CR1, CLU, and PICALM as alzheimer disease risk loci and reveals interactions with APOE genotypes. Arch Neurol. 2010;67:1473–1484. doi: 10.1001/archneurol.2010.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jyrkka J, Enlund H, Lavikainen P, Sulkava R, Hartikainen S. Association of polypharmacy with nutritional status, functional ability and cognitive capacity over a three-year period in an elderly population. Pharmacoepidemiol Drug Saf. 2011 doi: 10.1002/pds.2116. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 111.Kang DE, Pietrzik CU, Baum L, et al. Modulation of amyloid beta-protein clearance and Alzheimer’s disease susceptibility by the LDL receptor-related protein pathway. J Clin Invest. 2000;106:1159–1166. doi: 10.1172/JCI11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Katzman R, Kawas C. The epidemiology of dementia and Alzheimer disease. In: Terry RD, editor. Alzheimer disease. Raven Press; New York: 1994. pp. 105–122. [Google Scholar]
- 113.Kirkwood TB. Systems biology of ageing and longevity. Philos Trans R Soc Lond B Biol Sci. 2011;366:64–70. doi: 10.1098/rstb.2010.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Knight JA. The biochemistry of aging. Adv Clin Chem. 2000;35:1–62. doi: 10.1016/s0065-2423(01)35014-x. [DOI] [PubMed] [Google Scholar]
- 115.Knopman DS, Parisi JE, Salviati A, et al. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003;62:1087–1095. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- 116.Krach C, Velkoff VA. Current Population Reports, vol. Series P23-199RV. US Dept of Health and Human Services/NIH, US Dept of Commerce/Census Bureau; Washington, DC: 1999. Centenarians in the United States. [Google Scholar]
- 117.Kudlow BA, Kennedy BK. Aging: progeria and the lamin connection. Curr Biol. 2006;16:R652–R654. doi: 10.1016/j.cub.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 118.Kukull WA, Higdon R, Bowen JD, et al. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol. 2002;59:1737–1746. doi: 10.1001/archneur.59.11.1737. [DOI] [PubMed] [Google Scholar]
- 119.Kulminski AM, Culminskaya I, Ukraintseva SV, et al. Trade-off in the effects of the apolipoprotein E polymorphism on the ages at onset of CVD and cancer influences human lifespan. Aging Cell. 2011 doi: 10.1111/j.1474-9726.2011.00689.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Langlois NE. The use of histology in 638 coronial postmortem examinations of adults: an audit. Med Sci Law. 2006;46:310–320. doi: 10.1258/rsmmsl.46.4.310. [DOI] [PubMed] [Google Scholar]
- 121.Lassmann H, Fischer P, Jellinger K. Synaptic pathology of Alzheimer’s disease. Ann N Y Acad Sci. 1993;695:59–64. doi: 10.1111/j.1749-6632.1993.tb23028.x. [DOI] [PubMed] [Google Scholar]
- 122.Lauderback CM, Kanski J, Hackett JM, et al. Apolipoprotein E modulates Alzheimer’s Abeta(1–42)-induced oxidative damage to synaptosomes in an allele-specific manner. Brain Res. 2002;924:90–97. doi: 10.1016/s0006-8993(01)03228-0. [DOI] [PubMed] [Google Scholar]
- 123.Launer LJ. Counting dementia: there is no one “best” way. Alzheimers Dement. 2011;7:10–14. doi: 10.1016/j.jalz.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lemere CA, Blusztajn JK, Yamaguchi H, et al. Sequence of deposition of heterogeneous amyloid beta-peptides and APO E in Down syndrome: implications for initial events in amyloid plaque formation. Neurobiol Dis. 1996;3:16–32. doi: 10.1006/nbdi.1996.0003. [DOI] [PubMed] [Google Scholar]
- 125.Leverenz JB, Agustin CM, Tsuang D, et al. Clinical and neuropathological characteristics of hippocampal sclerosis: a community-based study. Arch Neurol. 2002;59:1099–1106. doi: 10.1001/archneur.59.7.1099. [DOI] [PubMed] [Google Scholar]
- 126.Leverenz JB, Yu CE, Schellenberg GD. Aging-associated neuropathology in Werner syndrome. Acta Neuropathol. 1998;96:421–424. doi: 10.1007/s004010050914. [DOI] [PubMed] [Google Scholar]
- 127.Lovell MA, Markesbery WR. Oxidative damage in mild cognitive impairment and early Alzheimer’s disease. J Neurosci Res. 2007;85:3036–3040. doi: 10.1002/jnr.21346. [DOI] [PubMed] [Google Scholar]
- 128.Lovell MA, Markesbery WR. Oxidatively modified RNA in mild cognitive impairment. Neurobiol Dis. 2008;29:169–175. doi: 10.1016/j.nbd.2007.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lowe VJ, Kemp BJ, Jack CR, Jr, et al. Comparison of 18F-FDG and PiB PET in cognitive impairment. J Nucl Med. 2009;50:878–886. doi: 10.2967/jnumed.108.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Luchsinger JA. Diabetes, related conditions, and dementia. J Neurol Sci. 2010;299:35–38. doi: 10.1016/j.jns.2010.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mancuso C, Scapagini G, Curro D, et al. Mitochondrial dysfunction, free radical generation and cellular stress response in neurodegenerative disorders. Front Biosci. 2007;12:1107–1123. doi: 10.2741/2130. [DOI] [PubMed] [Google Scholar]
- 132.Martin GM. Genetic modulation of senescent phenotypes in Homo sapiens. Cell. 2005;120:523–532. doi: 10.1016/j.cell.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 133.Martin GM. Genetic modulation of the senescent phenotype in Homo sapiens. Genome. 1989;31:390–397. doi: 10.1139/g89-059. [DOI] [PubMed] [Google Scholar]
- 134.Masliah E. Mechanisms of synaptic dysfunction in Alzheimer’s disease. Histol Histopathol. 1995;10:509–519. [PubMed] [Google Scholar]
- 135.Matsuzaki T, Sasaki K, Tanizaki Y, et al. Insulin resistance is associated with the pathology of Alzheimer disease: the Hisayama study. Neurology. 2010;75:764–770. doi: 10.1212/WNL.0b013e3181eee25f. [DOI] [PubMed] [Google Scholar]
- 136.Mayeux R, Reitz C, Brickman AM, et al. Operationalizing diagnostic criteria for Alzheimer’s disease and other age-related cognitive impairment—Part 1. Alzheimers Dement. 2011;7:15–34. doi: 10.1016/j.jalz.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68:709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.McNaughton D, Knight W, Guerreiro R, et al. Duplication of amyloid precursor protein (APP), but not prion protein (PRNP) gene is a significant cause of early onset dementia in a large UK series. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.10.010. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 140.Mizutani T, Shimada H. Neuropathological background of twenty-seven centenarian brains. J Neurol Sci. 1992;108:168–177. doi: 10.1016/0022-510x(92)90047-o. [DOI] [PubMed] [Google Scholar]
- 141.Montine TJ. Prevalence estimates for latent neurodegenerative disease. Toxicol Pathol. 2011;39:99–102. doi: 10.1177/0192623310391481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Morrison JH, Hof PR. Life and death of neurons in the aging cerebral cortex. Int Rev Neurobiol. 2007;81:41–57. doi: 10.1016/S0074-7742(06)81004-4. [DOI] [PubMed] [Google Scholar]
- 143.Mungas D, Reed BR, Ellis WG, Jagust WJ. The effects of age on rate of progression of Alzheimer disease and dementia with associated cerebrovascular disease. Arch Neurol. 2001;58:1243–1247. doi: 10.1001/archneur.58.8.1243. [DOI] [PubMed] [Google Scholar]
- 144.Myers RH, Schaefer EJ, Wilson PW, et al. Apolipoprotein E epsilon4 association with dementia in a population-based study: the Framingham study. Neurology. 1996;46:673–677. doi: 10.1212/wnl.46.3.673. [DOI] [PubMed] [Google Scholar]
- 145.Nagy Z, Hindley NJ, Braak H, et al. The progression of Alzheimer’s disease from limbic regions to the neocortex: clinical, radiological and pathological relationships. Dement Geriatr Cogn Disord. 1999;10:115–120. doi: 10.1159/000017111. [DOI] [PubMed] [Google Scholar]
- 146.Nelson PT, Abner EL, Scheff SW, et al. Alzheimer’s-type neuropathology in the precuneus is not increased relative to other areas of neocortex across a range of cognitive impairment. Neurosci Lett. 2009;450:336–339. doi: 10.1016/j.neulet.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nelson PT, Abner EL, Schmitt FA, et al. Brains with medial temporal lobe neurofibrillary tangles but no neuritic amyloid plaques are a diagnostic dilemma but may have pathogenetic aspects distinct from Alzheimer disease. J Neuropathol Exp Neurol. 2009;68:774–784. doi: 10.1097/NEN.0b013e3181aacbe9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nelson PT, Abner EL, Schmitt FA, et al. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol. 2010;20:66–79. doi: 10.1111/j.1750-3639.2008.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nelson PT, Braak H, Markesbery WR. Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J Neuropathol Exp Neurol. 2009;68:1–14. doi: 10.1097/NEN.0b013e3181919a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nelson PT, Jicha GA, Schmitt FA, et al. Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles “do count” when staging disease severity. J Neuropathol Exp Neurol. 2007;66:1136–1146. doi: 10.1097/nen.0b013e31815c5efb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nelson PT, Kukull WA, Frosch MP. Thinking outside the box: Alzheimer-type neuropathology that does not map directly onto current consensus recommendations. J Neuropathol Exp Neurol. 2010;69:449–454. doi: 10.1097/NEN.0b013e3181d8db07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nelson PT, Schmitt FA, Lin Y, et al. Hippocampal sclerosis in advanced age: clinical and pathological features in 106 cases. Brain. 2010 doi: 10.1093/brain/awr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nelson PT, Smith CD, Abner EA, et al. Human cerebral neuropathology of type 2 diabetes mellitus. Biochim Biophys Acta. 2009;1792:454–469. doi: 10.1016/j.bbadis.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Newman AB, Glynn NW, Taylor CA, et al. Health and function of participants in the Long Life Family Study: a comparison with other cohorts. Aging (Albany NY) 2011 doi: 10.18632/aging.100242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nicoll JA, Barton E, Boche D, et al. Abeta species removal after abeta42 immunization. J Neuropathol Exp Neurol. 2006;65:1040–1048. doi: 10.1097/01.jnen.0000240466.10758.ce. [DOI] [PubMed] [Google Scholar]
- 156.Noda K, Sasaki K, Fujimi K, et al. Quantitative analysis of neurofibrillary pathology in a general population to reappraise neuropathological criteria for senile dementia of the neurofibrillary tangle type (tangle-only dementia): the Hisayama Study. Neuropathology. 2006;26:508–518. doi: 10.1111/j.1440-1789.2006.00722.x. [DOI] [PubMed] [Google Scholar]
- 157.Nunomura A, Castellani RJ, Zhu X, et al. Involvement of oxidative stress in Alzheimer disease. J Neuropathol Exp Neurol. 2006;65:631–641. doi: 10.1097/01.jnen.0000228136.58062.bf. [DOI] [PubMed] [Google Scholar]
- 158.O’Brien RJ, Resnick SM, Zonderman AB, et al. Neuropathologic studies of the Baltimore Longitudinal Study of Aging (BLSA) J Alzheimers Dis. 2009;18:665–675. doi: 10.3233/JAD-2009-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Okereke OI, Kang JH, Cook NR, et al. Type 2 diabetes mellitus and cognitive decline in two large cohorts of community-dwelling older adults. J Am Geriatr Soc. 2008;56:1028–1036. doi: 10.1111/j.1532-5415.2008.01686.x. [DOI] [PubMed] [Google Scholar]
- 160.Olovnikov AM. Hypothesis: lifespan is regulated by chronomere DNA of the hypothalamus. J Alzheimers Dis. 2007;11:241–252. doi: 10.3233/jad-2007-11211. [DOI] [PubMed] [Google Scholar]
- 161.Pakkenberg B, Gundersen HJ. Neocortical neuron number in humans: effect of sex and age. J Comp Neurol. 1997;384:312–320. [PubMed] [Google Scholar]
- 162.Pakkenberg B, Pelvig D, Marner L, et al. Aging and the human neocortex. Exp Gerontol. 2003;38:95–99. doi: 10.1016/s0531-5565(02)00151-1. [DOI] [PubMed] [Google Scholar]
- 163.Pallas M, Casadesus G, Smith MA, et al. Resveratrol and neurodegenerative diseases: activation of SIRT1 as the potential pathway towards neuroprotection. Curr Neurovasc Res. 2009;6:70–81. doi: 10.2174/156720209787466019. [DOI] [PubMed] [Google Scholar]
- 164.Pallas M, Verdaguer E, Tajes M, Gutierrez-Cuesta J, Camins A. Modulation of sirtuins: new targets for antiageing. Recent Pat CNS Drug Discov. 2008;3:61–69. doi: 10.2174/157488908783421492. [DOI] [PubMed] [Google Scholar]
- 165.Pao WC, Dickson DW, Crook JE, et al. Hippocampal sclerosis in the elderly: genetic and pathologic findings, some mimicking Alzheimer disease clinically. Alzheimer Dis Assoc Disord. 2011 doi: 10.1097/WAD.0b013e31820f8f50. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: the Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 167.Perry G, Nunomura A, Hirai K, et al. Is oxidative damage the fundamental pathogenic mechanism of Alzheimer’s and other neurodegenerative diseases? Free Radic Biol Med. 2002;33:1475–1479. doi: 10.1016/s0891-5849(02)01113-9. [DOI] [PubMed] [Google Scholar]
- 168.Perry JJ, Fan L, Tainer JA. Developing master keys to brain pathology, cancer and aging from the structural biology of proteins controlling reactive oxygen species and DNA repair. Neuroscience. 2007;145:1280–1299. doi: 10.1016/j.neuroscience.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pertovaara M, Lehtimaki T, Rontu R, et al. Presence of apolipoprotein E epsilon4 allele predisposes to early onset of primary Sjogren’s syndrome. Rheumatology (Oxford) 2004;43:1484–1487. doi: 10.1093/rheumatology/keh383. [DOI] [PubMed] [Google Scholar]
- 170.Petrovitch H, Ross GW, He Q, et al. Characterization of Japanese–American men with a single neocortical AD lesion type. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.03.026. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Petrovitch H, Ross GW, Steinhorn SC, et al. AD lesions and infarcts in demented and non-demented Japanese–American men. Ann Neurol. 2005;57:98–103. doi: 10.1002/ana.20318. [DOI] [PubMed] [Google Scholar]
- 172.Polvikoski TM, van Straaten EC, Barkhof F, et al. Frontal lobe white matter hyperintensities and neurofibrillary pathology in the oldest old. Neurology. 2010;75:2071–2078. doi: 10.1212/WNL.0b013e318200d6f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Poon LW, Clayton GM, Martin P, et al. The Georgia Centenarian Study. Int J Aging Hum Dev. 1992;34:1–17. doi: 10.2190/8M7H-CJL7-6K5T-UMFV. [DOI] [PubMed] [Google Scholar]
- 174.DoEaSA. Population Division, United Nations Secretariat, World Population Ageing 1950–2050. United Nations; New York: 2002. [Google Scholar]
- 175.Price JL, McKeel DW, Jr, Buckles VD, et al. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging. 2009;30:1026–1036. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Prohovnik I, Perl DP, Davis KL, et al. Dissociation of neuropathology from severity of dementia in late-onset Alzheimer disease. Neurology. 2006;66:49–55. doi: 10.1212/01.wnl.0000191298.68045.50. [DOI] [PubMed] [Google Scholar]
- 177.Prolla TA. Multiple roads to the aging phenotype: insights from the molecular dissection of progerias through DNA microarray analysis. Mech Ageing Dev. 2005;126:461–465. doi: 10.1016/j.mad.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 178.Puzianowska-Kuznicka M, Kuznicki J. Genetic alterations in accelerated ageing syndromes. Do they play a role in natural ageing? Int J Biochem Cell Biol. 2005;37:947–960. doi: 10.1016/j.biocel.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 179.Rauramaa T, Pikkarainen M, Englund E, et al. TAR-DNA binding protein-43 and alterations in the hippocampus. J Neural Transm. 2011 doi: 10.1007/s00702-010-0574-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 180.Rebeck GW, Cho HS, Grabowski TJ, Greenberg SM. The effects of AbetaPP mutations and APOE polymorphisms on cerebral amyloid angiopathy. Amyloid. 2001;8(Suppl 1):43–47. [PubMed] [Google Scholar]
- 181.Regan C, Katona C, Walker Z, et al. Relationship of vascular risk to the progression of Alzheimer disease. Neurology. 2006;67:1357–1362. doi: 10.1212/01.wnl.0000240129.46080.53. [DOI] [PubMed] [Google Scholar]
- 182.Reiman EM, Caselli RJ, Yun LS, et al. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 183.Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011 doi: 10.1038/nrneurol.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Riley KP, Snowdon DA, Markesbery WR. Alzheimer’s neurofibrillary pathology and the spectrum of cognitive function: findings from the Nun Study. Ann Neurol. 2002;51:567–577. doi: 10.1002/ana.10161. [DOI] [PubMed] [Google Scholar]
- 185.Rocca WA, Cha RH, Waring SC, Kokmen E. Incidence of dementia and Alzheimer’s disease: a reanalysis of data from Rochester, Minnesota, 1975–1984. Am J Epidemiol. 1998;148:51–62. doi: 10.1093/oxfordjournals.aje.a009560. [DOI] [PubMed] [Google Scholar]
- 186.Rocca WA, Petersen RC, Knopman DS, et al. Trends in the incidence and prevalence of Alzheimer’s disease, dementia, and cognitive impairment in the United States. Alzheimers Dement. 2011;7:80–93. doi: 10.1016/j.jalz.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rosvall L, Rizzuto D, Wang HX, et al. APOE-related mortality: effect of dementia, cardiovascular disease and gender. Neurobiol Aging. 2009;30:1545–1551. doi: 10.1016/j.neurobiolaging.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 188.Roth M, Tomlinson BE, Blessed G. Correlation between scores for dementia and counts of ‘senile plaques’ in cerebral grey matter of elderly subjects. Nature. 1966;209:109–110. doi: 10.1038/209109a0. [DOI] [PubMed] [Google Scholar]
- 189.Roulson J, Benbow EW, Hasleton PS. Discrepancies between clinical and autopsy diagnosis and the value of post mortem histology; a meta-analysis and review. Histopathology. 2005;47:551–559. doi: 10.1111/j.1365-2559.2005.02243.x. [DOI] [PubMed] [Google Scholar]
- 190.Rubin EH, Kinscherf DA, Grant EA, Storandt M. The influence of major depression on clinical and psychometric assessment of senile dementia of the Alzheimer type. Am J Psychiatry. 1991;148:1164–1171. doi: 10.1176/ajp.148.9.1164. [DOI] [PubMed] [Google Scholar]
- 191.Salloway S, Sperling R, Gilman S, et al. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73:2061–2070. doi: 10.1212/WNL.0b013e3181c67808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sarkar D, Fisher PB. Molecular mechanisms of aging-associated inflammation. Cancer Lett. 2006;236:13–23. doi: 10.1016/j.canlet.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 193.Savva GM, Wharton SB, Ince PG, et al. Age, neuropathology, and dementia. N Engl J Med. 2009;360:2302–2309. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- 194.Scarmeas N, Habeck CG, Hilton J, et al. APOE related alterations in cerebral activation even at college age. J Neurol Neurosurg Psychiatry. 2005;76:1440–1444. doi: 10.1136/jnnp.2004.053645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Scheff SW, Price DA. Alzheimer’s disease-related alterations in synaptic density: neocortex and hippocampus. J Alzheimers Dis. 2006;9:101–115. doi: 10.3233/jad-2006-9s312. [DOI] [PubMed] [Google Scholar]
- 196.Scheff SW, Price DA, Sparks DL. Quantitative assessment of possible age-related change in synaptic numbers in the human frontal cortex. Neurobiol Aging. 2001;22:355–365. doi: 10.1016/s0197-4580(01)00222-6. [DOI] [PubMed] [Google Scholar]
- 197.Schmitt FA, Davis DG, Wekstein DR, et al. “Preclinical” AD revisited: neuropathology of cognitively normal older adults. Neurology. 2000;55:370–376. doi: 10.1212/wnl.55.3.370. [DOI] [PubMed] [Google Scholar]
- 198.Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA. The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis. 2009;18:691–701. doi: 10.3233/JAD-2009-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Schneider JA, Boyle PA, Arvanitakis Z, Bienias JL, Bennett DA. Subcortical infarcts, Alzheimer’s disease pathology, and memory function in older persons. Ann Neurol. 2007;62:59–66. doi: 10.1002/ana.21142. [DOI] [PubMed] [Google Scholar]
- 200.Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004;62:1148–1155. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- 201.Sedel F, Tourbah A, Fontaine B, et al. Leukoencephalopathies associated with inborn errors of metabolism in adults. J Inherit Metab Dis. 2008;31:295–307. doi: 10.1007/s10545-008-0778-0. [DOI] [PubMed] [Google Scholar]
- 202.Serrano-Pozo A, William CM, Ferrer I, et al. Beneficial effect of human anti-amyloid-beta active immunization on neurite morphology and tau pathology. Brain. 2010;133:1312–1327. doi: 10.1093/brain/awq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Seshadri S, Beiser A, Au R, et al. Operationalizing diagnostic criteria for Alzheimer’s disease and other age-related cognitive impairment—part 2. Alzheimers Dement. 2011;7:35–52. doi: 10.1016/j.jalz.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Seshadri S, Fitzpatrick AL, Ikram MA, et al. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA. 2010;303:1832–1840. doi: 10.1001/jama.2010.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sharma S, Shin JS, Grimshaw M, Clarke RA, Lee CS. The senescence pathway in prostatic carcinogenesis. Pathology. 2010;42:507–511. doi: 10.3109/00313025.2010.508791. [DOI] [PubMed] [Google Scholar]
- 206.Shojania KG, Burton EC, McDonald KM, Goldman L. Overestimation of clinical diagnostic performance caused by low necropsy rates. Qual Saf Health Care. 2005;14:408–413. doi: 10.1136/qshc.2004.011973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sleegers K, Brouwers N, Gijselinck I, et al. APP duplication is sufficient to cause early onset Alzheimer’s dementia with cerebral amyloid angiopathy. Brain. 2006;129:2977–2983. doi: 10.1093/brain/awl203. [DOI] [PubMed] [Google Scholar]
- 208.Small BJ, Mobly JL, Laukka EJ, Jones S, Backman L. Cognitive deficits in preclinical Alzheimer’s disease. Acta Neurol Scand Suppl. 2003;179:29–33. doi: 10.1034/j.1600-0404.107.s179.6.x. [DOI] [PubMed] [Google Scholar]
- 209.Snowdon DA, Greiner LH, Mortimer JA, et al. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277:813–817. [PubMed] [Google Scholar]
- 210.Sonnen JA, Larson EB, Brickell K, et al. Different patterns of cerebral injury in dementia with or without diabetes. Arch Neurol. 2009;66:315–322. doi: 10.1001/archneurol.2008.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sonnen JA, Larson EB, Crane PK, et al. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol. 2007;62:406–413. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- 212.Stanta G, Campagner L, Cavallieri F, Giarelli L. Cancer of the oldest old. What we have learned from autopsy studies. Clin Geriatr Med. 1997;13:55–68. [PubMed] [Google Scholar]
- 213.Strittmatter WJ, Saunders AM, Schmechel D, et al. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Struble RG, Ala T, Patrylo PR, Brewer GJ, Yan XX. Is brain amyloid production a cause or a result of dementia of the Alzheimer’s type? J Alzheimers Dis. 2010;22:393–399. doi: 10.3233/JAD-2010-100846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sultana R, Boyd-Kimball D, Poon HF, et al. Redox proteomics identification of oxidized proteins in Alzheimer’s disease hippocampus and cerebellum: an approach to understand pathological and biochemical alterations in AD. Neurobiol Aging. 2006;27:1564–1576. doi: 10.1016/j.neurobiolaging.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 216.Sultana R, Butterfield DA. Role of oxidative stress in the progression of Alzheimer’s disease. J Alzheimers Dis. 2010;19:341–353. doi: 10.3233/JAD-2010-1222. [DOI] [PubMed] [Google Scholar]
- 217.Thal DR, Del Tredici K, Braak H. Neurodegeneration in normal brain aging and disease. Sci Aging Knowledge Environ. 2004:pe26. doi: 10.1126/sageke.2004.23.pe26. [DOI] [PubMed] [Google Scholar]
- 218.Theuns J, Brouwers N, Engelborghs S, et al. Promoter mutations that increase amyloid precursor-protein expression are associated with Alzheimer disease. Am J Hum Genet. 2006;78:936–946. doi: 10.1086/504044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Tomlinson BE, Blessed G, Roth M. Observations on the brains of demented old people. J Neurol Sci. 1970;11:205–242. doi: 10.1016/0022-510x(70)90063-8. [DOI] [PubMed] [Google Scholar]
- 220.Tractenberg RE, Singer CM, Kaye JA. Characterizing sleep problems in persons with Alzheimer’s disease and normal elderly. J Sleep Res. 2006;15:97–103. doi: 10.1111/j.1365-2869.2006.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Vaillancourt DE, Newell KM. Changing complexity in human behavior and physiology through aging and disease. Neurobiol Aging. 2002;23:1–11. doi: 10.1016/s0197-4580(01)00247-0. [DOI] [PubMed] [Google Scholar]
- 222.von Gunten A, Ebbing K, Imhof A, Giannakopoulos P, Kovari E. Brain aging in the oldest-old. Curr Gerontol Geriatr Res. 2010 doi: 10.1155/2010/358531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Watson GS, Craft S. Insulin resistance, inflammation, and cognition in Alzheimer’s disease: lessons for multiple sclerosis. J Neurol Sci. 2006;245:21–33. doi: 10.1016/j.jns.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 224.White L, Petrovitch H, Hardman J, et al. Cerebrovascular pathology and dementia in autopsied Honolulu-Asia Aging Study participants. Ann N Y Acad Sci. 2002;977:9–23. doi: 10.1111/j.1749-6632.2002.tb04794.x. [DOI] [PubMed] [Google Scholar]
- 225.White L, Small BJ, Petrovitch H, et al. Recent clinical–pathologic research on the causes of dementia in late life: update from the Honolulu-Asia Aging Study. J Geriatr Psychiatry Neurol. 2005;18:224–227. doi: 10.1177/0891988705281872. [DOI] [PubMed] [Google Scholar]
- 226.Williams LR, Hutchinson CE, Jackson A, et al. Clinical correlates of cerebral white matter hyperintensities in cognitively normal older adults. Arch Gerontol Geriatr. 2010;50:127–131. doi: 10.1016/j.archger.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 227.Wilson RS, Barnes LL, Mendes de Leon CF, et al. Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology. 2002;59:364–370. doi: 10.1212/wnl.59.3.364. [DOI] [PubMed] [Google Scholar]
- 228.Wilson RS, Leurgans SE, Boyle PA, Schneider JA, Bennett DA. Neurodegenerative basis of age-related cognitive decline. Neurology. 2010;75:1070–1078. doi: 10.1212/WNL.0b013e3181f39adc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wilson RS, Weir DR, Leurgans SE, et al. Sources of variability in estimates of the prevalence of Alzheimer’s disease in the United States. Alzheimers Dement. 2011;7:74–79. doi: 10.1016/j.jalz.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wong K, Sidransky E, Verma A, et al. Neuropathology provides clues to the pathophysiology of Gaucher disease. Mol Genet Metab. 2004;82:192–207. doi: 10.1016/j.ymgme.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 231.Xuereb JH, Brayne C, Dufouil C, et al. Neuropathological findings in the very old. Results from the first 101 brains of a population-based longitudinal study of dementing disorders. Ann N Y Acad Sci. 2000;903:490–496. doi: 10.1111/j.1749-6632.2000.tb06404.x. [DOI] [PubMed] [Google Scholar]
- 232.Yankner BA, Lu T, Loerch P. The aging brain. Annu Rev Pathol. 2008;3:41–66. doi: 10.1146/annurev.pathmechdis.2.010506.092044. [DOI] [PubMed] [Google Scholar]
- 233.Zaccai J, Ince P, Brayne C. Population-based neuropathological studies of dementia: design, methods and areas of investigation—a systematic review. BMC Neurol. 2006;6:2. doi: 10.1186/1471-2377-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zekry D, Duyckaerts C, Moulias R, et al. Degenerative and vascular lesions of the brain have synergistic effects in dementia of the elderly. Acta Neuropathol. 2002;103:481–487. doi: 10.1007/s00401-001-0493-5. [DOI] [PubMed] [Google Scholar]
- 235.Zhang L, Bruce-Keller AJ, Dasuri K, et al. Diet-induced metabolic disturbances as modulators of brain homeostasis. Biochim Biophys Acta. 2009;1792:417–422. doi: 10.1016/j.bbadis.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]