Abstract
Microtubules, composed of α/β tubulin heterodimers, represent a validated target for cancer chemotherapy. Thus, tubulin- and microtubule-binding antimitotic drugs such as taxanes and vincas are widely employed for the chemotherapeutic management of various malignancies. Although quite successful in the clinic, these drugs are associated with severe toxicity and drug resistance problems. Noscapinoids represent an emerging class of microtubule-modulating anticancer agents based upon the parent molecule noscapine, a naturally-occurring non-toxic cough-suppressant opium alkaloid. Here we report in silico molecular modeling, chemical synthesis and biological evaluation of novel analogs derived by modification at position-7 of the benzofuranone ring system of noscapine. The synthesized analogs were evaluated for their tubulin polymerization activity and their biological activity was examined by their antiproliferative potential using representative cancer cell lines from varying tissue-origin [A549 (lung), CEM (lymphoma), MIA PaCa-2 (pancreatic), MCF-7 (breast) and PC-3 (prostate)]. Cell-cycle studies were performed to explore their ability to halt the cell-cycle and induce subsequent apoptosis. The varying biological activity of these analogs that differ in the nature and bulk of substituent at position-7 was rationalized utilizing predictive in silico molecular modeling.
Keywords: Noscapine, anticancer activity, tubulin-binding, cell cycle
1. Introduction
Composed of α/β tubulin heterodimers, microtubules are ubiquitous dynamic cytoskeletal polymers that have been long recognized as a validated pharmaceutical target in cancer chemotherapy [1–2]. Drugs that interfere with microtubule dynamic stability are widely employed in the clinic to treat a variety of cancers or are exploited as probes to gain insights into microtubule structure and function. Three major classes of drugs namely, taxanes, vinca alkaloids and colchicine analogs are well-recognized and the positions they occupy on the cellular target, tubulin, have been identified [1]. Traditionally, these three drug classes are categorized into stabilizers and destabilizers; the stabilizers predominantly causing overpolymerization of microtubules into bundles and sheets and the destabilizers resulting in depolymerization of microtubules into soluble tubulin.
Yet another emerging class of microtubule-modulating agents is based upon noscapine, a non-sedative naturally-occurring phthalideisoquinoline alkaloid from the opium poppy [3–4]. Noscapine, a long-known innocuous cough-suppressant was discovered for its tubulin-binding anticancer activity about a decade ago [3]. Essentially, the identif ication and discovery of noscapine was based upon its structural resemblance to commonly known microtubule poisons such as colchicine [3]. Ever since, noscapine has been successfully shown to inhibit various neoplasms in vitro as well as in vivo such as leukemia and lymphoma [3, 5–6], melanoma [7], ovarian [8], gliomas [9], breast [10], lung [11], and colon [12] cancer. Currently, noscapine is in Phase I/II clinical trials for the treatment of multiple myeloma.
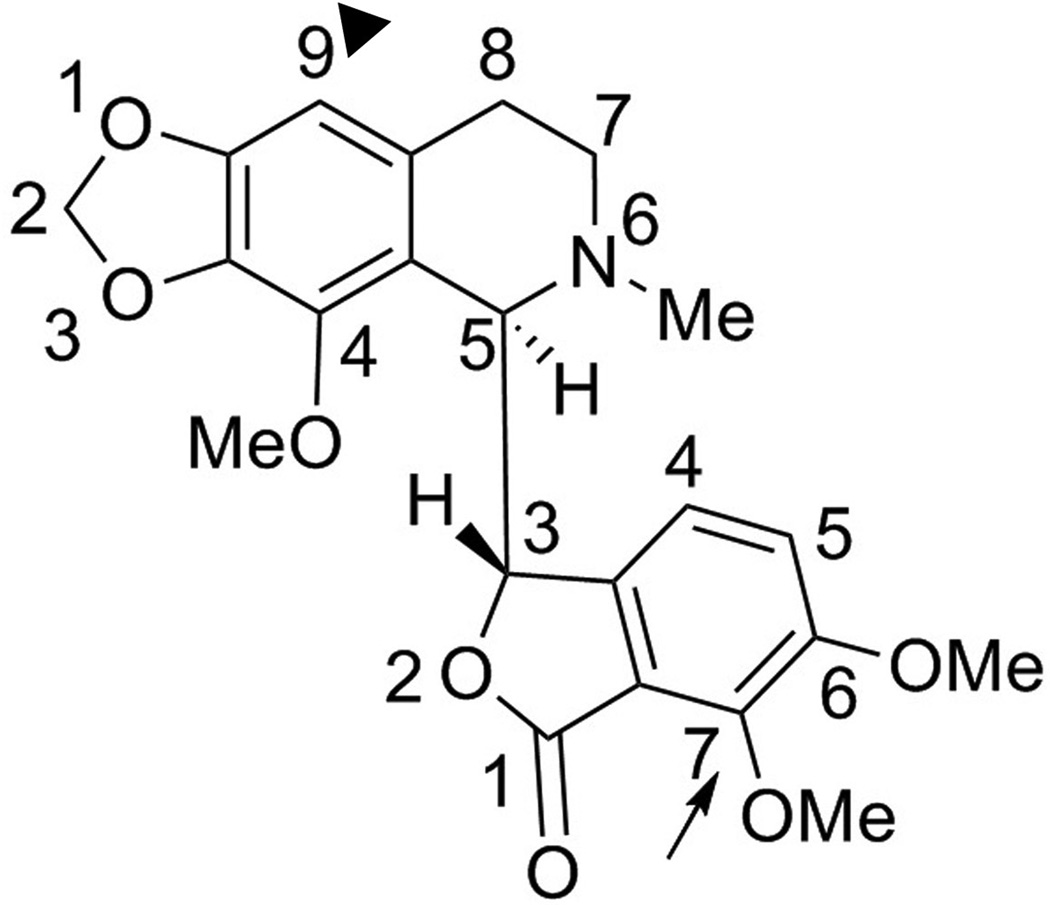
Our ongoing chemical synthetic efforts to improve the therapeutic efficacy and pharmacological properties of noscapine have yielded a battery of more potent first-generation noscapine analogs, collectively referred to as noscapinoids [10, 13–20]. Noscapinoids avoid the harsher effects of currently-available chemotherapeutic agents by leaving the total polymer mass of tubulin unaffected [3, 5, 21]. Noscapine analogs have been extensively shown to impede cell-cycle progression, inhibit cellular proliferation and induce apoptosis in a variety of cancer cells both in vitro and in xenograft models of human cancers implanted in nude mice [10, 12, 14, 16–17, 22–23]. From a synthetic perspective, most of these first-generation analogs were generated by the chemical manipulation of position-9 on the isoquinoline ring system of noscapine (Fig. 1). The position-9 substituted (black-arrowhead, Fig. 1) tubulin-binding analogs that displayed superior anticancer activity included 9-nitronoscapine as well as halogenated (fluoro, chloro, bromo, and iodo) analogs [13, 19]. In particular, the brominated analog of noscapine is most well-studied because of its effectiveness against drug-resistant xenograft tumors without any detectable toxicity [14, 16–17, 22–23]. It is therefore, no wonder that noscapine and its analogs have been described as ‘kinder and gentler’ microtubule-modulating agents that do not cause any apparent toxicity [24].
Fig. 1.
Molecular structure of noscapine ((S)-6,7-dimethoxy-3-((R)-4-methoxy-6-methyl-5,6,7,8-tetrahydro-[1,3]dioxolo[4,5-g]isoquinolin-5-yl)isobenzofuran-1(3H)-one).
More recently, chemical modifications of position-7 (arrow, Fig. 1) on the benzofuranone ring system of noscapine have been reported [25–26]. The regioselective O-demethylation at position-7 yielded the O-alkylated derivatives including the hydroxy compound which is about ~100 fold more potent than the parent noscapine [25–26]. This strongly suggested that chemical maneuvering of the benzofuranone ring had significant impact on the biological activity of the parent molecule. Based upon this impetus, we modeled noscapine in the colchicine binding site [27] to rationalize the exceptionally enhanced biological activity of the 7-hydroxy noscapine analog as well as aid future drug discovery by rational drug design.
Here we report the chemical synthesis of second generation 7-position benzofuranone noscapine analogs that differ in the steric bulk of the substituent. The analogs were examined for their anti-proliferative activity using representative cancer cell lines of lung, myeloma, breast, pancreas and prostate. In silico molecular modeling data were employed to rationalize and comprehend the observed biological activity patterns of these analogs. This has contributed to an enhanced understanding of structure-based drug design to facilitate drug discovery and development of the noscapinoid family, a novel class of tubulin-active, non-toxic agents.
2. Materials and methods
2.1. In silico modeling studies
Crystal structure coordinates of the tubulin heterodimer-colchicine models (PDB: 1sa0) [27] were used in this study. Theoretical binding sites of noscapinoids were generated by superimposing the drugs onto the colchicine molecule using Phase Flexible Ligand Superpositioning program from Schrodinger software [28] and then placing the resulting conformations into their respective 3D protein models. UCSF Chimera was used to determine hydrogen bonds and steric clashes of drugs docked to protein [29–30]. Hydrophobic protein surface was made using Tripos Sybyl (v. 8.1) to further visualize sites of potential steric clashes.
Chemical Synthesis
2.2. Synthesis of benzofuranone ring substituted noscapine analogs
1H NMR and 13C NMR spectra were measured in DMSO-d6 on Bruker 400 NMR spectrometer. All proton NMR spectra were recorded at 400 MHz and were referenced with residual DMSO (2.50 ppm). Carbon NMR spectra were recorded at 100 MHz and were referenced with 77.27 ppm (CDCl3) resonance of residual chloroform. Abbreviations for signal coupling are as follows: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet. High resolution mass spectra were collected on Waters Q-TOF micro mass spectrophotometer using 3-nitrobenzyl alcohol, in some cases with addition of LiI as a matrix. All reactions were conducted in oven-dried (125°C) glassware under nitrogen atmosphere. All common reagents and solvents were obtained from Sigma (St. Louis, MO) and used without further purification unless otherwise indicated. Solvents were dried by standard methods. The reactions were monitored by thin layer chromatography (TLC) using silica gel 60 F254 (Merck) pre-coated aluminum sheet. Flash chromatography was carried out on standard grade silica gel (230–400 mesh).
2.2.1. (S)-7-hydroxy-6-methoxy-3-((R)-4-methoxy-6-methyl-5,6,7,8-tetrahydro[1,3]dioxolo-[4,5-g]isoquinolin-5-yl)isobenzofuran-1(3H)-one (2)
Noscapine (2.0 g, 4.84 mmol), was dissolved in anhydrous DMF (5.0 mL) followed by the addition of sodium azide (0.63 g, 9.68 mmol) and sodium iodide (0.36 g, 2.42 mmol). The mixture was stirred vigorously at 140°C for 4 h. The mixture was concentrated under reduced pressure to yield a dark residue which was dissolved in EtOAc (50 mL). The insoluble material was filtered through celite and the filtrate was diluted with EtOAc (150 mL) followed by washing with water (2 × 25 mL) and brine (2 × 25 mL). The organic layer was dried over sodium sulfate and concentrated under reduced pressure to give crude product which was crystallized from methanol. The product 2 was isolated as off-white needles. (78 % yield): mp 142-143 °C; 1H NMR (DMSO-d6, 400 MHz): δ 9.73 (s, 1H), 7.11 (d, J = 8.0 Hz, 1H), 6.47 (s, 1H), 6.01 (m, 2H), 5.81 (d, J = 8.0 Hz, 1H), 5.48 (d, J = 4.0 Hz, 1H), 4.24 (d, J = 4.0 Hz, 1H), 3.96 (s, 3H), 3.79 (s, 3H), 2.48-2.34 (m, 2H) 2.43 (s, 3H), 2.31-2.18 (m, 1H), 1.95-1.83 (m, 1H): 13C NMR (CDCl3, 100 MHz): δ 174.6, 161.9, 151.6, 148.1, 140.7, 140.3, 133.9, 131.6, 116.8, 113.9, 111.8, 102.7, 102.4, 100.6, 80.9, 60.7, 59.3, 55.4, 48.2, 45.0, 25.76: HRMS: [M+H]+ [C21H21NO7+H]+, calcd: 400.1396, found: 400.1382.
2.2.2. (S)-5-methoxy-1-((R)-4-methoxy-6-methyl-5,6,7,8-tetrahydro-[1,3]dioxolo[4,5-g]isoquinolin-5-yl)-3-oxo-1,3-dihydroisobenzofuran-4-yl acetate (3)
Compound 2 (0.2 g, 0.5 mmol), was dissolved in anhydrous THF (5.0 mL) followed by the addition of acetic anhydride (57 µL, 0.6 mmol) and the dimethylamino pyridine (12 mg, 0.1 mmol). The mixture was stirred at ambient temperature for 4 h, the reaction progress was monitored by TLC, solvent was removed in vacuo and the residue thus obtained was dissolved in EtOAc (25 mL) followed by washing with water (2 × 25 mL). The organic layer was dried over sodium sulfate and concentrated under reduced pressure to give crude product which was separated over flash silica using methanol in chloroform as eluent (1:99) to afford compound 3 which was crystallized using methanol to yield pinkish crystals. (76 % yield): mp 111-113°C; 1H NMR (DMSO-d6, 400 MHz): δ 7.40 (d, J = 8.4 Hz, 1H), 6.48 (s, 1H), 6.40 (d, J = 8.4 Hz, 1H), 6.01 (s, 2H), 5.62 (d, J = 4.4 Hz, 1H), 4.25 (d, J = 4.4 Hz, 1H), 3.95 (s, 3H), 3.81 (s, 3H), 2.67-2.42 (m, 3H), 2.42 (s, 3H) 2.32 (s, 3H), 1.95-1.85 (m, 1H); 13C NMR (DMSO-d6, 100 MHz): δ 168.5, 167.2, 151.7, 148.6, 140.5, 136.0, 134.3, 131.7, 121.4, 120.5, 119.5, 102.9, 101.3, 81.8, 60.8, 59.7, 57.1, 49.3, 45.9, 27.2, 20.6: HRMS: [M+H]+ [C23H22NO8+H]+, calcd: 442.1502, found: 442.1505.
2.2.3. (S)-5-methoxy-1-((R)-4-methoxy-6-methyl-5,6,7,8-tetrahydro-[1,3]dioxolo[4,5-g]isoquinolin-5-yl)-3-oxo-1,3-dihydroisobenzofuran-4-yl benzoate (4)
Compound 2 (0.2 g, 0.5 mmol), was dissolved in anhydrous THF (5 mL), potassium carbonate (0.1 g) was added and the mixture was cooled over an ice bath (0–4°C). Benzoyl chloride (76 µl, 0.65 mmol) was added drop-wise and stirred vigorously at 0°C then warmed to RT for overnight. Solvent was removed in vacuo and the residue thus obtained was dissolved in ethyl acetate (25 mL) and washed with water (2 × 25 mL). The combined organic layers were dried over sodium sulfate and concentrated under reduced pressure to give crude product which was separated over flash silica using methanol in chloroform as eluent (1:99) to obtain compound 4 which was crystallized with methanol to yield dark yellow crystals. (92% yield): mp 152°C; 1H NMR (DMSO-d6, 400 MHz): δ 8.14 (m, 2H), 7.79 (m, 1H), 6.40 (d, J = 8.4 Hz, 1H), 7.64 (m, 2H), 7.47 (d, J = 8.4 Hz, 1H), 6.50 (s, 1H), 6.44 (bs, 1H), 6.02 (s, 2H), 5.67 (d, J = 4.4 Hz, 1H), 4.29 (d, J = 4.4 Hz, 1H), 3.98 (s, 3H), 3.82 (s, 3H) 2.62 (m, 1H), 2.54 (m, 1H) 2.44 (s, 1H), 2.33 (m, 1H), 1.93 (m, 1H); 13C NMR (CDCl3, 100 MHz), δ 170.6, 166.7, 164.2, 152.1, 149.2, 140.4, 140.1, 136.9, 133.9, 132.7, 131.4, 130.5, 129.9, 128.9, 128.3, 121.5, 120.4, 118.5, 102.4, 100.9, 81.0, 61.2, 59.0, 56.9, 47.8, 45.0, 25.2.: HRMS: [M+H]+ [C28H25NO8+H]+, calcd: 504.1658, found: 504.1668.
General synthesis procedure for carbamate derivatives 5–7
Compound 2 (0.25 g, 0.626 mmol), was dissolved in anhydrous dichloromethane (5 mL) followed by the addition of ethyl isocyanate (54 µl, 0.689 mmol) and the dimethylamino pyridine (8 mg, 0.065 mmol). The mixture was stirred vigorously at ambient temperature for 4 h. The mixture was condensed under reduced pressure to dryness. The residue was dissolved in ethyl acetate (25 mL) and washed with water (2 × 10 mL). The organic layer was dried over sodium sulfate and concentrated under reduced pressure to give crude product which was chromatographed over flash silica using methanol in chloroform as eluent (2:98) to yield carbamate derivatives 5–7.
2.2.4. (S)-5-methoxy-1-((R)-4-methoxy-6-methyl-5,6,7,8-tetrahydro-[1,3]dioxolo[4,5-g]isoquinolin-5-yl)-3-oxo-1,3-dihydroisobenzofuran-4-yl ethylcarbamate (5)
Yellow solid. (64% yield): mp 133-135°C; 1H NMR (DMSO-d6, 400 MHz): δ 7.81 (t, J = 5.2 Hz, 1H), 7.34 (d, J = 8.4 Hz, 1H), 6.48 (s, 1H), 6.35 (d, J = 8.4 Hz, 1H), 6.00 (s, 2H), 5.58 (d, J = 4.4 Hz, 1H), 4.25 (d, J = 4.4 Hz, 1H), 3.95 (s, 3H), 3.79 (s, 3H), 3.09 (q, J = 7.2 Hz, 2H), 2.67-2.42 (m, 3H), 2.43 (s, 3H), 1.94-1.92 (m, 1H), 1.09 (t, J = 7.2 Hz, 3H); 13C NMR (DMSO-d6, 100 MHz): δ 167.2, 153.4, 152.5, 148.6, 140.6, 137.2, 134.4, 131.8, 121.2, 120.5, 119.2, 116.9, 102.9, 101.3, 81.6, 79.7, 60.9, 59.7, 57.04, 45.89, 49.3, 35.9, 27.1, 15.3: HRMS: [M+H]+ [C24H26N2O8+H]+, calcd: 471.1767, found: 471.1761.
2.2.5. (S)-5-methoxy-1-((R)-4-methoxy-6-methyl-5,6,7,8-tetrahydro-[1,3]dioxolo[4,5-g]isoquinolin-5-yl)-3-oxo-1,3-dihydroisobenzofuran-4-yl phenylcarbamate (6)
Light yellow solid. (52 % yield): mp 159-161°C. 1H NMR (DMSO-d6, 400 MHz): δ 7.49-7.39 (m, 6H), 7.24 (t, J = 5.2 Hz, 1H), 6.48 (s, 1H), 6.35 (d, J = 8.4 Hz, 1H), 6.01 (s, 2H), 5.64 (d, J = 4.4 Hz, 1H), 4.32 (d, J = 4.4 Hz, 1H), 3.95 (s, 3H), 3.82 (s, 3H), 2.73 (m, 3H), 2.45 (s, 3H), 1.94 (m, 1H); 13C NMR (DMSO-d6, 100 MHz): δ 167.2, 153.4, 152.5, 148.6, 144.3, 140.6, 137.2, 134.4, 131.8, 124.6, 121.2, 120.5, 119.2, 116.9, 102.9, 101.3, 81.6, 79.7, 60.9, 59.7, 46.4, 35.9, 27.1: HRMS: [M+H]+ [C28H26N2O8+H]+, calcd: 519.1767, found: 519.1762.
2.2.6. (S)-5-methoxy-1-((R)-4-methoxy-6-methyl-5,6,7,8-tetrahydro-[1,3]dioxolo[4,5-g]isoquinolin-5-yl)-3-oxo-1,3-dihydroisobenzofuran-4-yl benzylcarbamate (7)
Yellow solid. (78 % yield): mp 122-123°C; 1H NMR (DMSO-d6, 400 MHz): δ 8.39 (t, J = 5.2 Hz, 1H), 7.38-7.27 (m, 6H), 6.48 (s, 1H), 6.37 (d, J = 8.4 Hz, 1H), 6.01 (s, 2H), 5.60 (d, J = 4.4 Hz, 1H), 4.31-4.27 (m, 2H), 4.24 (d, J = 4.4 Hz, 1H), 3.95 (s, 3H), 3.81 (s, 3H), 2.72-2.48 (m, 2H), 2.44 (s, 3H), 2.32-2.28 (m, 1H), 1.91-1.86 (m, 1H); 13C NMR (DMSO-d6, 100 MHz): δ 158.6, 154.1, 152.5, 148.6, 140.6, 137.2, 134.4, 131.8, 121.2, 120.5, 119.2, 116.9, 102.9, 101.3, 81.6, 79.7, 60.9, 59.7, 46.4, 35.9, 27.1: HRMS: [M+H]+ [C29H28N2O8+H]+, calcd: 533.1924, found: 533.1926.
2.3. Cell lines and reagents
CEM (lymphoma), A549 (lung), PC-3 (prostate), MIA PaCa-2 (pancreatic), MCF-7 and MDA-MB-231 (breast) cancer cells were purchased from zATCC. PC3, A549, MDA-MB-231 and CEM were cultured in RPMI-1640 medium supplemented with 10% Fetal Bovine Serum (FBS) and 1% penicillin/streptomycin. MIA PaCa-2 and MCF-7 cells were cultured in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin. Primary human dermal fibroblasts (HDF) from the dermis of normal human neonatal foreskin were obtained from the Dermatology Department, Emory University. MTT dye (Thiazolyl Blue Tetrazolium Bromide) dimethyl sulfoxide (DMSO), propidium iodide and RNase were purchased from Sigma (St. Louis, MO). Cells were cultured at 37°C with 5% CO2.
2.3.1. Tubulin purification and polymerization assay
Microtubule proteins (MTP) consisting of ~70% tubulin and ~30% microtubule-associated proteins (MAPs) was isolated from bovine brain by three cycles of temperature-dependent polymerization and depolymerization. MAP-free tubulin (> 99% pure) was purified from MTP by phosphocellulose chromatography [31]. Purified tubulin was drop-frozen in liquid nitrogen, and stored at −80°C until use.
The rate and extent of tubulin polymerization was monitored using a light scattering assay at 350 nm as described previously [32]. Briefly, phosphocellulose-purified MAP-free tubulin (12–15 µM) was incubated with each compound at 0°C for 10 min in PEM buffer (80 mM PIPES, 3 mM MgCl2, and 1 mM EGTA, pH 6.8) in a 96-well format. Following the addition of 1 mM GTP, assembly of tubulin was initiated by transferring the sample containing plate to Spectra Max Plus multi-well plate reader (Molecular Devices, USA). which was temperature pre-adjusted at 35°C.
2.3.2. Cytotoxicity assay
MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) assay [16] was employed to evaluate the proliferative capacity of cells. Essentially, MTT is a colorimetric assay, which utilizes the colorless tetrazolium dye and converts it into a colored formazan salt, which can be quantified by measuring absorbance at 570 nm. Briefly, a 96-well format was used to seed 100 µl medium containing cells at a density of 5 × 103 cells per well. After 24 h of incubation, cells were treated with gradient concentration of the test compounds, which were dissolved in DMSO. The final concentration of DMSO in the culture medium was maintained at 0.1%. After 48 h of drug incubation, the spent medium was removed and the wells were washed twice with PBS. 100 µl of fresh medium and 10 µl of MTT (5mg/ml in PBS) was added to the wells and cells were incubated at 37°C in dark for 4 h. The formazan product was dissolved by adding 100 µl of 100% DMSO after removing the medium from each well. The absorbance was measured at 570 nm using a Spectra Max Plus multi-well plate reader (Molecular Devices, USA).
2.3.3. Cell-cycle analysis
Flow-cytometric evaluation of the cell-cycle status was performed as described previously [14–17]. Briefly, control and drug treated cells were centrifuged, washed with ice-cold PBS, and fixed in 70% ethanol. Tubes containing the cell pellets were stored at 4°C for at least 24 h. Cells were then centrifuged at 1000 g for 10 min and the supernatant was discarded. The pellets were washed twice with 5 ml of PBS and then stained with 0.5 ml of propidium iodide (0.1% in 0.6% Triton-X in PBS) and 0.5 ml of RNase A (2 mg/ml) for 45 min in dark. Samples were then analyzed on a BD FACSCanto II flow-cytometer (BD Biosciences, Sparks, MD).
2.3.4. Immunoblot analysis
Western blots were performed as described earlier [33]. Briefly, proteins were resolved by polyacrylamide gel-electrophoresis and transferred onto polyvinylidene difluoride membranes (Millipore). The membranes were blocked in Tris-buffered saline containing 0.05% Tween-20 and 5% fat-free dry milk and incubated first with primary antibodies against cleaved-PARP (Cell Signaling Inc., Beverly, MA) and survivin (Santa Cruz Biotechnology Inc., Santa Cruz, CA) and then with horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology Inc., Santa Cruz, CA). β-actin was from Sigma (St. Louis, MO). Specific proteins were visualized with enhanced chemiluminescence detection reagent according to the manufacturer’s instructions (Pierce Biotechnology Inc., Rockford, IL).
2.3.5. Caspase 3/7 activity assay
Control or lysates of PC-3 cells treated with 25 µM noscapine derivatives were tested for caspase-3-like activity using Ac-DEVD-7-amino-4-trifluoromethyl-coumarin, which detects the activities of caspase-3 and caspase-7 according to manufacturer’s protocol (Calbiochem, San Diego, CA). The results were evaluated using VictorTM X5 multilabel reader (PerkinElmer, Inc., MA) and expressed as relative fluorescence units.
3. Results and Discussion
3.1. In silico modeling studies
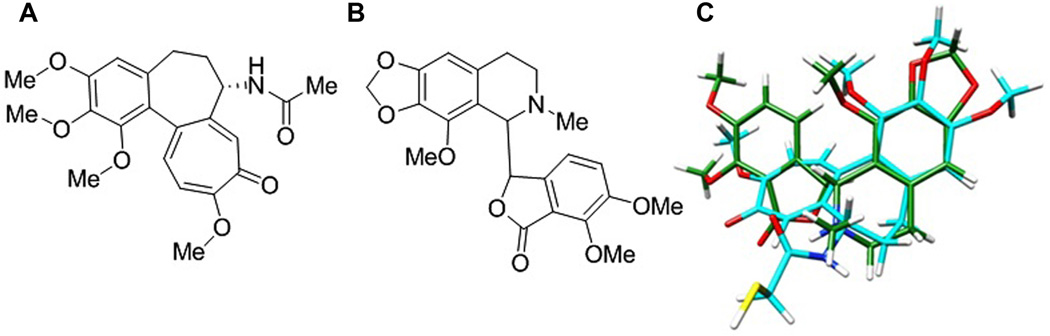
Noscapine was discovered through a semi-rational structural screen of known microtubule poisons such as colchicine, podophyllotoxin, and MTC [2-methoxy-5-(2,3,4-trimethoxyphenyl)-2,4,6-cycloheptatrien-1-one], all of which are believed to bind to the same region of the cellular target, tubulin [3]. The identification of noscapine was based on its structural resemblance with these drugs, such as a hydrophobic trimethoxyphenyl group and other hydrophobic domains (like a lactone, tropolone, or other aromatic rings) as well as small hydrophilic groups (like hydroxyl and amino groups) [3]. Since the 3.5 Å crystal structure of tubulin in complex with colchicine [27] clearly shows the binding conformation of the drug, we first examined the structural similarity of noscapine (Fig. 2B) to colchicine (Fig. 2A) using a flexible ligand-superpositioning program [28]. The overlap of two structures (Fig. 2C) yielded a geometric complementarity score of 72.75%, implying a strong structural similarity.
Fig. 2.
A. Colchicine. B. Noscapine C. Flexible ligand superpositioning of noscapine (green carbons) on DAMA-colchicine (cyan carbons) from the colchicine-tubulin complex as determined by Ravelli et al [27]. Superpositioning was calculated based on best-fit geometry using the Schrodinger Phase Flexible Ligand Superpositioning program [28]. Noscapine was found to share a similarity of 72.75% to the DAMA-colchicine conformation used.
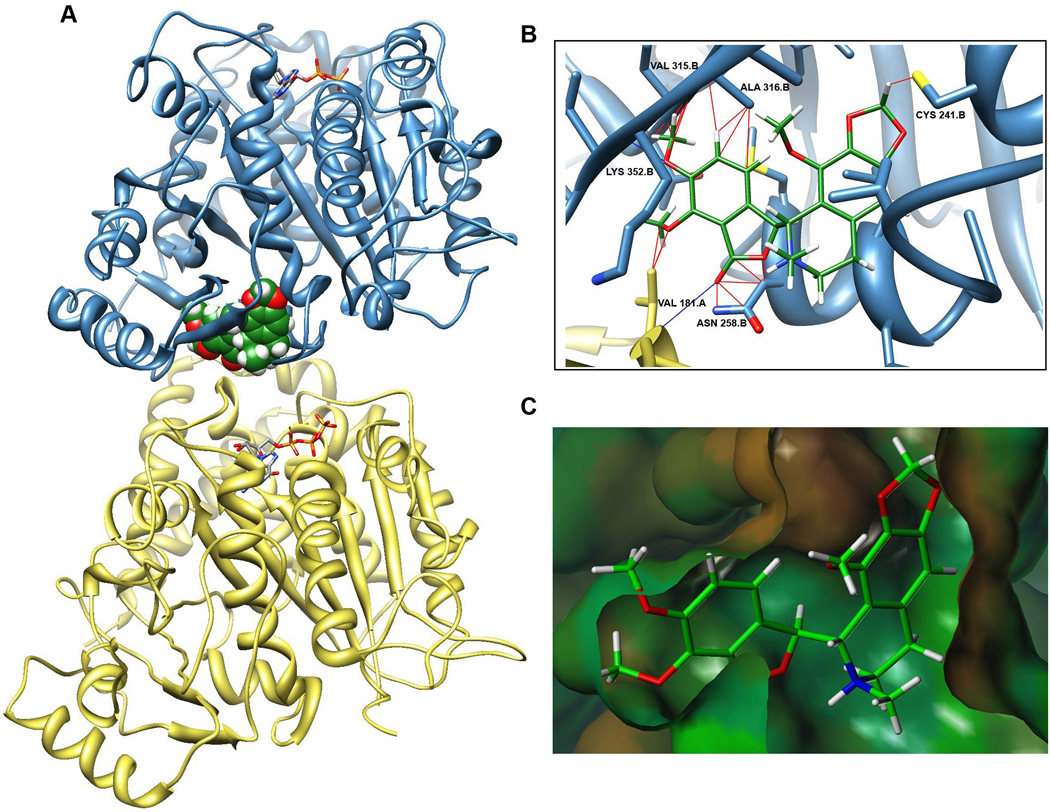
It is becoming recognizable that traditional docking approaches have several limitations when used with dynamic proteins such as tubulin [34]. Thus, in order to model the binding of noscapine to tubulin, we utilized the space-coordinates of colchicine in its docked state to superimpose noscapine into the colchicine-binding domain of 1SA0 PDB structure (Fig 3A). Our modeling data show noscapine in the same position as colchicine, within the β-subunit near the intradimer interface (Fig. 3A). However, the methoxy group at position-7 showed multiple clashes with the valine residue 315 in the β-subunit (Fig 3B,C). This steric strain is perhaps relieved upon O-demethylation at position-7 to yield a 7-hydroxy-compound. This may possibly explain the significantly increased activity of O-demethylated analogs that have been reported [25–26].
Fig. 3.
Model construction predicting noscapine bound in tubulin heterodimer. A. Noscapine (green) is shown as a space-filling Vander Waals model within the tubulin heterodimer (β-subunit in blue, α-subunit in yellow) in the colchicine-binding domain near the intra-dimer interface and the non-exchangeable GTP-site on α-tubulin. B. Close-up of noscapine in the pocket reveals steric clashes (red lines) and H-bonds (blue lines) as predicted by UCSF Chimera software [29]. C. Hydrophobic surface of binding pocket created using Tripos Sybyl shows steric clash at 7-position of noscapine.
Given that replacing the methoxy group at position-7 with a smaller, hydroxyl group increased efficacy by decreasing steric hindrance, we rationalized that substituting an increasingly large-sized group at this position should negatively impact the biological activity. In order to validate this predictive model, we synthesized noscapine analogs by derivatizing position-7 on the benzofuranone ring system of noscapine with larger functional groups.
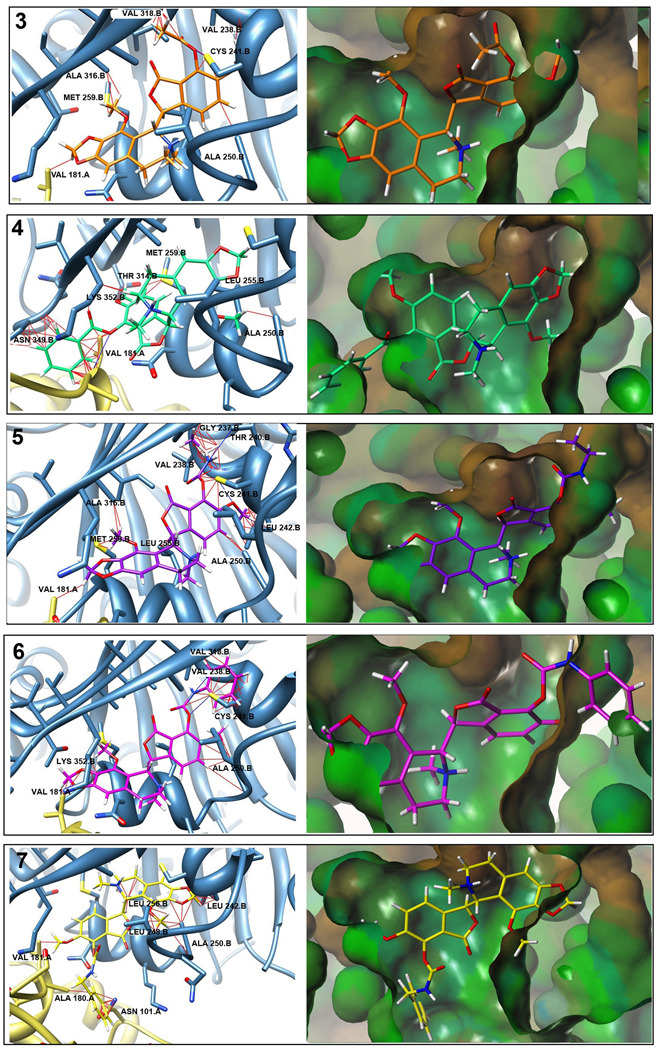
When superimposed onto the colchicine-binding domain of our tubulin docking model, these analogs showed an increase in the number and magnitude of steric clashes at position-7 with an increase in the size of the substituted subgroup (Fig. 4, Table 1). This could lead to altered binding interactions between the functional group at position-7 and the amino acid residues of the protein which would impact the affinity of drug binding to the target, and thus influence drug efficacy. These alterations would thus impact the biological anticancer activity in cellular systems.
Fig. 4.
Predicted noscapinoid binding positions on tubulin. Panels on left show predicted binding position of noscapinoids based upon their superpositioning on DAMA-colchicine from colchicine-tubulin crystal structure and then overlay into the protein [27]. Potential steric clashes (red lines) and H-bonds (blue lines) as predicted by UCSF Chimera are shown. Larger substitutions at the 7-position show increased steric hindrances versus those of noscapine. Panels on right show noscapinoids in the hydrophobic surface of the colchicine binding pocket created using Tripos Sybyl. Steric clashes can be seen in portions of the molecules shown outside of the pocket.
Table 1.
Clashes and H-Bonds
| Compound | Substituent | Average Clashes |
Average Overlap |
Average H- Bonds |
|---|---|---|---|---|
| 3 | Acetyl | 32.8 | 1.017 | 2.6 |
| 4 | Benzoyl | 52.6 | 1.131 | 2.2 |
| 5 | Ethylcarbamato | 62.6 | 1.091 | 2.0 |
| 6 | Phenylcarbamato | 61.0 | 1.163 | 2.4 |
| 7 | Benzylcarbamato | 56.2 | 1.159 | 0.8 |
3.2. Chemical synthesis of 7-position benzofuranone noscapine analogs
Sodium azide and sodium iodide in dimethylformamide (DMF) was selectively used to cleave the methyl group at position-7 of benzofuranone ring. As a result, we optimized an efficient method to prepare compound 2 (7-hydroxy noscapine) that excluded the use of Grignard reagent and simplified the work-up procedure to obtain the reaction product. Briefly, noscapine was dissolved in anhydrous DMF along with sodium azide and sodium iodide and the mixture was stirred at 140°C for 4 h. The mixture was condensed under reduced pressure and the residue was extracted in ethylacetate followed by washing with water and brine to remove excess salt. This synthetic method, offered a simple, economic and easy work-up procedure compared to the one reported by Anderson et al [25]. The 7-hydroxy noscapine, 2 thus obtained, served as a scaffold to synthesize various C-7-modified analogs of noscapine.
Two strategies were followed for the synthesis of 7-substituted noscapine derivatives as depicted in Scheme 1. Starting from the key intermediate 2, in the first strategy, we performed acylation reactions using acetic anhydride and benzoyl chloride in the presence of a base to prepare compounds 3 and 4. Compound 3 is the 7-acetyl derivative, which, in contrast to the almost inert original methoxy derivative, has more polarized carbonyl functionality. Compound 4, a benzoyl derivative, was prepared to compare the effect of alkyl to aryl function in the same molecule.
Scheme 1.
Synthesis of benzofuranone derivatives of noscapine 2–7.
It is well recognized that carbamate esters [30] are used to mask free phenolic groups in cytotoxic cancer drugs [35]. Thus, in the second strategy, a series of carbamate esters of the key intermediate, 2 were synthesized using readily available ethyl, phenyl and benzyl isocyanates. These compounds were prepared by the reaction of phenol derivative 2 with various isocyanates in the presence of DMAP (4-N,N’-dimethylamino pyridine) in anhydrous dichloromethane. The partial hydrolysis of isocyanates led to the formation of urea impurities thus increasing the complexity of the purification process. Purification was accomplished by using repetitive flash chromatography. Another challenge was the almost similar Rf of product and corresponding starting materials on standard silica-coated TLC plates. We resolved this problem by performing mass-spectrometric analysis of the reaction mixture before the work-up step. Clearly, the mass data also confirmed the presence of urea impurities in the reaction mixture.
3.3. Biochemical characterization of novel benzofuranone analogs
3.3.1. Benzofuranone ring substituted noscapine analogs inhibit tubulin polymerization in vitro
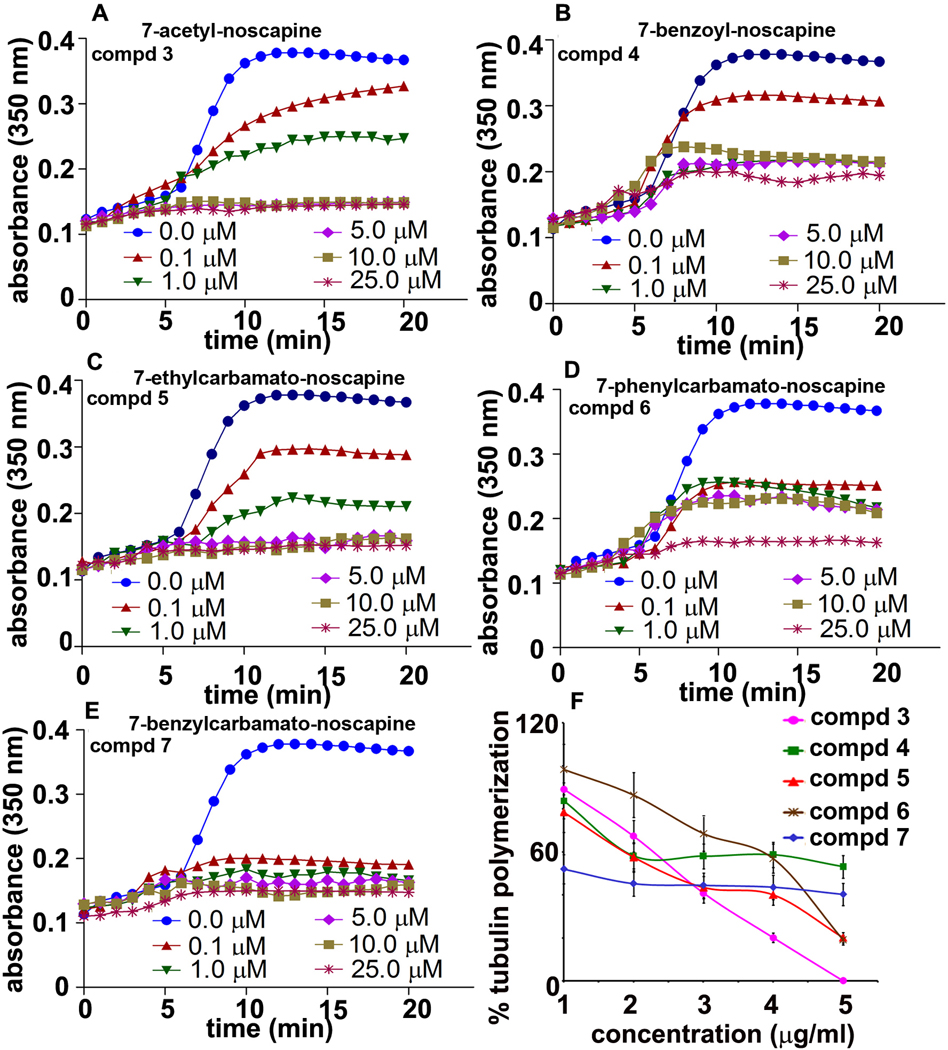
To determine the anti-tubulin activity of these benzofuranone ring substituted noscapine analogs, their effects on tubulin polymerization were examined in vitro. The effects of various concentrations of all five noscapine analogs on the polymerization of tubulin into microtubules are shown in Fig. 5. All five analogs inhibited the light scattering signal in a concentration-dependent manner, indicating that these benzofuranone ring substituted noscapine analogs can bind to tubulin and inhibit microtubule assembly.
Fig. 5.
Inhibition of tubulin assembly by noscapine analogs in vitro. Effects of various analogs on tubulin polymerization were assessed by monitoring the increase in light scattering at 350 nm as described under ‘Materials and Methods’. Each data point was obtained by subtracting the absorbance values of corresponding noscapine analogs in the absence of tubulin. Data are representative of three independent experiments performed in triplicate (p< 0.05).
3.4. Evaluation of antiproliferative activity and anti cell-cycle effects
3.4.1. Benzofuranone ring substituted noscapine analogs display significant antiproliferative activity
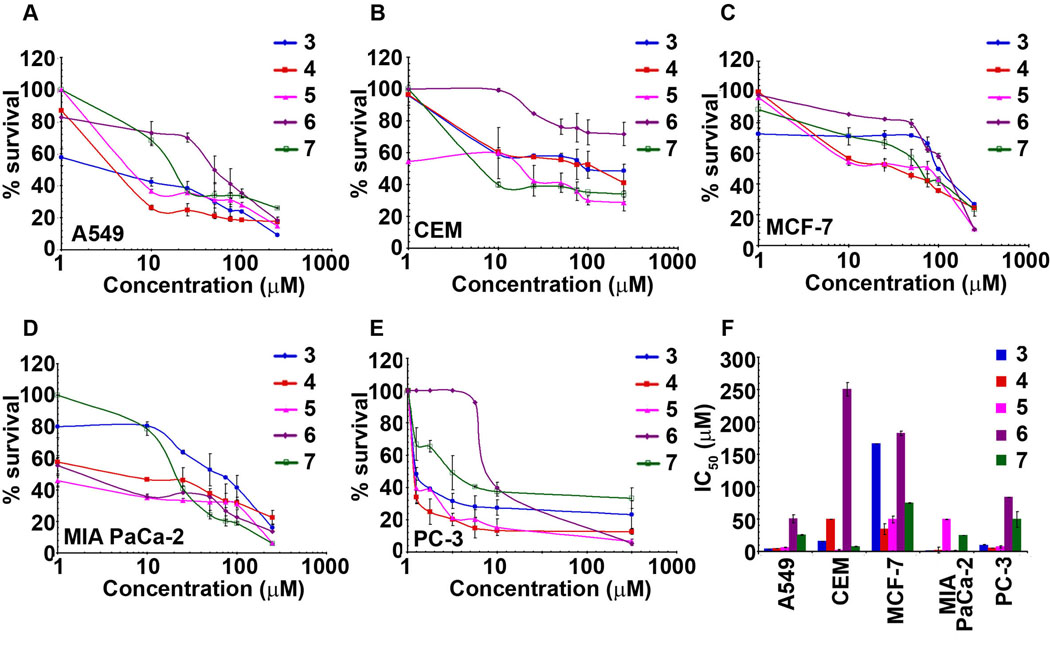
The newly synthesized 7-position analogs of noscapine were tested for their antiproliferative activity in various cancer cells using the MTT assay. Fig. 6 shows line plots of cell survival versus gradient concentrations of various compounds to yield IC50 values of each analog in different cell lines.
Fig. 6.
Cancer cells of varying tissue origin are sensitive to the noscapine analogs. Cells were treated for 48 h with increasing gradient concentrations of various noscapine analogs and the percentage of cell proliferation was measured using MTT assay. A, B, C, D and E represent the sensitivity profile the various cancer cells (A549, CEM, MCF-7, MIA PaCa-2, PC-3) to the five 7-position substituted benzofuranone noscapine analogs. The plot of % cell survival versus noscapine analog concentrations for cancer cells in them. F is a bar-graphical representation of IC50 values of noscapine analogs in various cancer cells used in our study. The values and error bars shown in all the graphs represent average and standard deviations, respectively, of three independent experiments (p <0.05).
The IC50 value (drug concentration at which 50% inhibition of cell proliferation occurs) of these synthesized compounds are presented in Table 2. To appreciate the potency of these novel 7-position substituted benzofurananone noscapine analogs, the IC50 values of the parent molecule noscapine for the various cell lines under study are indicated in Table 2 (bottom-row).
Table 2.
In vitro cytotoxicity (IC50, µM) of noscapine analogs
| Compound | R | Cancer cell line, IC50 (µM) | ||||
|---|---|---|---|---|---|---|
| A549 | CEM | MCF-7 | MIA PaCa-2 | PC-3 | ||
| 3 | -COMe | 3.2 | 15.5 | 166.0 | 1.0 | 9.3 |
| 4 | -COPh | 4.5 | 49.0 | 34.0 | 1.7 | 4.8 |
| 5 | -CONHEt | 5.6 | 1.7 | 49.0 | 49.0 | 6.6 |
| 6 | -CONHPh | 50.0 | 250.0 | 182.0 | 1.0 | 83.2 |
| 7 | -CONHBn | 25.0 | 7.1 | 74.1 | 24.5 | 49.0 |
| Noscapine | 73.0 | 20.0 | 45.0 | 70.0 | 51.0 | |
Among the noscapine analogs tested, compound 3 (7-acetyl-noscapine) was observed to be generally most-effective against most cell lines used in the study (Fig. 6(A,B,D,E)) except for breast cancer cells (Fig. 6C). Pancreatic cancer MIA PaCa-2 cells were particularly sensitive to compounds 3, 4 and 6 as compared (IC50 in the range of 1 to 1.7 µM) to compounds 5 and 7 (IC50 49.0 and 24.5 respectively) (Fig. 6D and Table 2). Lung cancer A549 cells also showed low IC50 for compounds 3, 4 and 5 (Table 2) compared to CEM, lymphoma cells, which were observed to be more sensitive towards compound 5 (Fig. 6B). The IC50 values of the cell lines, A549, CEM, MIA PaCa-2 and PC-3 were within 10 µM (Table 2) for compounds 3, 4 and 5. MCF-7, with higher IC50 values was found to be resistant towards these analogs. Figure 6F is a bar-graph representation depicting a comparison of the IC50 values of five noscapine analogs towards each cancer cell used in the study. As the bulk of the substituent increased in compound 4 (7-benzoyl-noscapine), an increase in IC50s was evident, which was even more pronounced with compound 5 and 6, with a few exceptions. Majority of the cell lines were relatively resistant towards compound 6 (with IC50 values ranging from 50–250 µM, Table 2). The significantly higher IC50 values of compound 6 indicated that the bulk of the substituent plays an important role in determining the biological activity in cellular systems. Compound 7, as observed from Table 2, showed relatively lower IC50 values when compared to compound 6 (Table 2). It is reasonable to speculate that the presence of a CH2 group between the nitrogen atom and aromatic ring system provides flexibility to compound 7, which perhaps relates to its higher activity. Comparison with noscapine demonstrated that 7-position analogs, in particular, compounds 3–5 are significantly better in antiproliferative activity (Table 2). Overall, the differences in the IC50 value of these analogs for the cancer cell lines studied can perhaps be attributed to the bulk of the functional group introduced at position-7 of benzofuranone ring on the parent molecule.
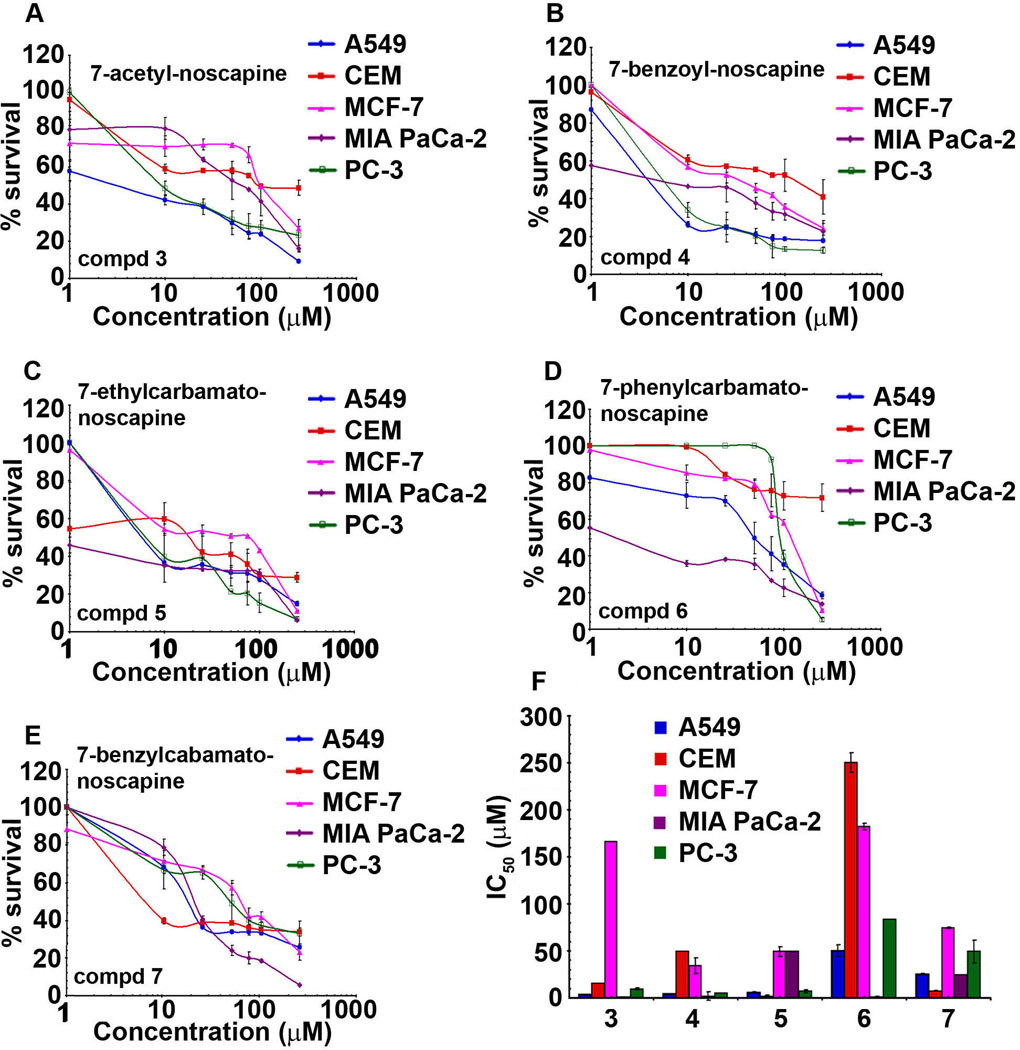
3.4.2. Benzofuranone ring substituted noscapine analogs show significant inter-line variation
The expression level of oncogenes, tumor suppressor and key molecules that regulate apoptosis, drug-resistance and angiogenesis can affect the sensitivity of tumor cells towards any given anticancer agent [36–37]. It is recognizable that anti-tubulin agents like paclitaxel, docetaxel offer superior anti-tumor outcomes in solid malignancies such as breast and ovarian [38–40], while hematological malignancies are best managed by vinca alkaloids (vinblastine, vincristine etc.) [41]. Differential sensitivity of cancer cell lines was observed for each noscapine analog suggesting significant inter-line variations (Fig. 7). Most cancer cells (lung, lymphoma, pancreatic and prostate) significantly responded to compound 3 and displayed much lower IC50 values in the range of 1–15 µM (Fig. 7A). However, a very high IC50 (166.0 µM) was observed for the breast cancer cell line, MCF-7 (Table 2, Fig. 7A), suggesting a high degree of inter-line variability. Compound 4 with a COPh (benzoyl) substituent, although quite effective against lung, pancreatic and prostate cancer cells (IC50 values less than 5 µM) showed resistance towards lymphoma (IC50 49.0 µM) and breast cancer (IC50 34.0 µM) cells (Fig. 7B). On the other hand, compound 5 showed lower activity in CEM (1.7 µM), A549 (5.6 µM) and PC-3 (6.6 µM) (Fig. 7C and Table 2. Interestingly, pancreatic cancer cells were very sensitive to compound 6 (IC50 =1 µM, Fig. 7D). These differences in cellular sensitivities to the same compound may be due to altered expression of β-tubulin isotypes, or point mutations in tubulin resulting in alterations of expression patterns of post-translational modifications of tubulin regulatory proteins, such as stathmin, microtubule associated protein (MAP), tau and MAP4 [42–43]. These changes in microtubule accessory proteins have been well recognized to affect microtubule dynamicity and can perhaps contribute to the development of drug resistance [44].
Fig. 7.
Benzofuranone ring substituted noscapine analogs exhibit significant inter-line variability. A, B, C, D and E are the plots of percent cell survival versus concentration of noscapine analogs for cancer cells from varying tissue origins viz., lung (A549), lymphoma (CEM), breast (MCF-7), pancreas (MIA PaCa-2) and prostate (PC-3) used for determination of IC50 values. F is a bar-graph representation of IC50 values of noscapine analogs in various cancer cells used in our study. The values and error bars shown in the graphs represent average and standard deviations, respectively, of three independent experiments (p <0.05).
Interestingly, compounds 3–7 did not inhibit cell proliferation in normal human dermal fibroblasts (HDFs) even at concentrations as high as 100 µM. As shown in Suppl. Fig. 1, the proliferation capacity of these compounds were compared with the parent compound noscapine and the most well-studied 9-position substituted analog, 9-bromonoscapine (Suppl. Fig.1).
3.4.3. Benzofuranone ring substituted noscapine analogs cause cell cycle arrest followed by apoptosis
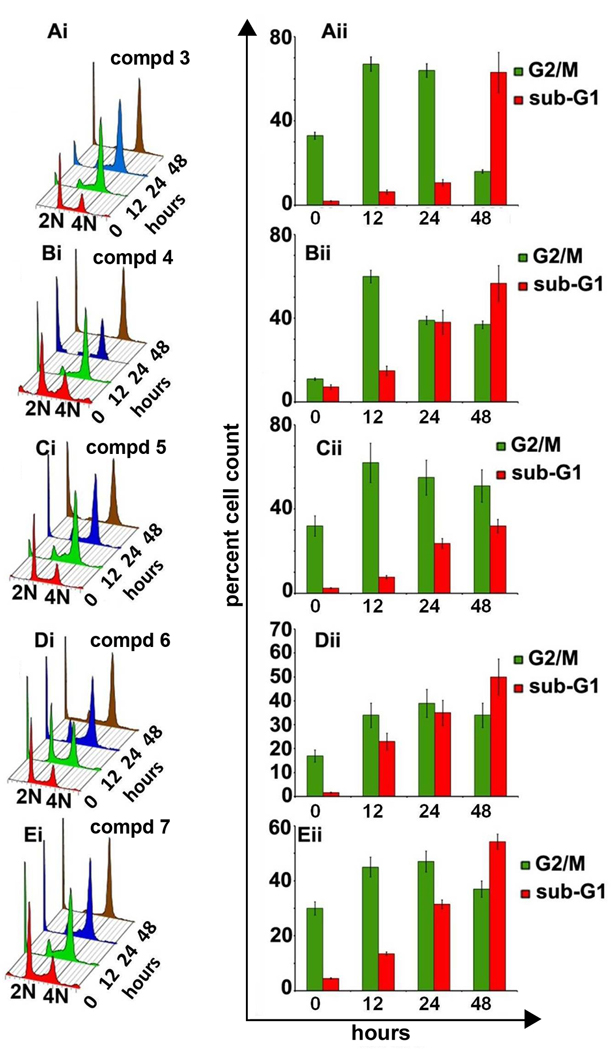
To gain further insights into the precise mechanisms responsible for inhibition of cellular proliferation, we next examined the effect of benzofuranone ring substituted analogs on cell cycle distributing profiles of breast cancer MDA-MB-231 cells. The effect of compounds 3–7 on mitotic index (percent G2/M cells) and apoptotic index (percent sub-G1 cells) as a function of time in MDA-MB-231 cells was studied using fluorescence activated cell sorting (FACS) analysis. All 5 compounds were used at 25 µM concentration over 48 h of treatment. Fig. 8 (Ai–Ei) shows cell-cycle profiles upon treatment with compounds 3–7 over various time points (0,12,24,48 h) in a three-dimensional disposition. While 2N and 4N DNA complements are representative of G1 and G2/M populations, respectively, sub-G1 hypodiploid population is usually suggestive of fragmented DNA and is a hallmark of apoptosis.
Fig. 8.
Panels Ai to Ei depicts cell-cycle distribution of MDA-MB-231 cells in a three-dimensional disposition as determined by flow-cytometry at different time point upon treatment with 25 µM concentration of compounds 3–7. Results are representative of three experiments done in triplicate. The right panels (Aii to Eii) show bar-graph representation of the percent G2/M and sub-G1 populations at different time points for compounds 3–7. Values and error bars shown in the graph represent mean and standard deviation, respectively, of three independent experiments performed in triplicate.
Treatment of MDA-MB-231 cells with compounds 3–7 showed significant accumulation of cells in G2/M phase until 24 h of drug exposure (Fig. 8 Aii–Eii). The G2/M population however started to decline beyond 24 h and thereafter a concomitant increase of sub-G1 population was observed until 48 h. In case of compounds 3 and 5, as is evident from the bar graph; the G2/M population increased considerably at 12 and 24 h (Fig. 8Aii,Cii). However, at 48 h the G2/M population decreased significantly perhaps leading to apoptotic cell death in case of compound 3 (Fig. Aii). Even after 48 h, compound 5 treated cells mostly remained arrested in G2/M phase and the apoptotic index was lower compared to compound 3. Compounds 4, 6 and 7 also show similar pattern of G2/M arrest over 24 h of treatment followed by a decline in the percent G2/M cells and emergence of a hypodiploid population, indicative of apoptosis (Fig. Bii,Dii,Eii).
Although staining of DNA with propidium iodide is extremely useful to have an overview of the percent cell population in various cell-cycle phases, however it has its limitations. It cannot dissect out the differences between G2 and M phases as both have 4N DNA amounts. Thus to explore further, we chose a mitosis specific marker MPM-2, to distinctly identify which phase the cells get stuck in. Our data revealed that all five noscapine analogs appeared to induce strong G2 arrest starting as early as 12 h (~65%) continuing till 24 h. In case of compound 5, the arrest was maintained until 48 h. Cell population corresponding to the G2/M peak was clearly negative for MPM2, suggesting that the cells accumulated in G2 phase for a long time before succumbing to apoptosis (Suppl. Fig. 2).
In addition, compounds 3–7 induced apoptotic cell death in PC-3 cells which was associated with decreased expression of the anti-apoptotic protein survivin (Suppl. Fig.3A), an enhanced caspase-3 activity (Suppl. Fig.3B), and cleavage of PARP (Suppl. Fig.3A).
4. Discussion
Noscapine and its analogs, collectively referred to as the noscapinoid family, typify a novel class of microtubule-modulating agents that evade the ‘harsher’ side-effects of currently-available tubulin-binding chemotherapeutics by preserving the total polymer mass of tubulin [33]. This novel class of non-toxic microtubule-modulating agents is based upon the parent molecule, noscapine, a relatively innocuous, non-sedative, isoquinoline alkaloid from opium, known for its antitussive properties for decades. Unlike the two major classes of tubulin-binding drugs, which either overpolymerize and bundle microtubules (taxanes) or depolymerize them and form paracrystals (vincas), noscapine and its analog do not exert gross affects on the microtubular ultrastructure [33]. Thus, noscapine and its analogs thus do not impair crucial microtubule functions and thus cause minimal toxicity, if any, and are best characterized as ‘kinder and gentler’ microtubule-modulating agents [24]. Since for clinical significance, the therapeutic efficacy is based upon the potency and selectivity (non-toxicity to normal cells), noscapine derivatives can potentially be exploited for therapeutic usage individually or in combination with existing toxic anti-microtubule drugs.
In silico molecular modeling efforts predicted the rational design of novel second generation noscapine analogs substituted at position-7 of the benzofuranone ring system, presented in this study. Although each synthesized analog showed cytotoxicity activity within a narrow range for most cell lines, significant inter cell line variations were found to exist, in that a particular compound exhibited differential sensitivity in cell lines from varying tissue origin. These differences are not easy to comprehend, and can perhaps be attributable to the presence of varying tubulin isotype expression and mutations in the tubulin gene in different cell types [45–47]. It is also likely that altered expression of survival and drug resistance mechanisms in cell lines from different tissue types dictate cellular sensitivities [48].
Successful chemotherapy relies on the strategic induction of robust apoptosis in cancer cells while sparing normal cells. It is noteworthy that these benzofuranone noscapine analogs did not affect the viability of normal human fibroblasts at concentrations as high as 100 µM. Essentially, chemotherapeutic agents induce cell death by arresting cell cycle progression, upregulating the expression of pro-apoptotic molecules while downregulating survival signaling players that encumber apoptosis. The rate and extent to which cell lines from various tissue types respond to a particular test compound essentially depends on the status of death-resisting anti-apoptosis molecules, as well as death-favoring pro-apoptotic molecules in that cell type and how these molecules are affected upon drug administration. For example, survivin, an antiapoptotic protein of the inhibitor of apoptosis family that blocks apoptosis by inhibiting caspases [49] has been shown to a player in dictating cellular sensitivity to 9-bromonoscapine [15]. Our data show that these compounds were able to alter survivin levels as part of their anti-proliferative and pro-apoptotic program. Examining the expression levels of survivin upon treatment with these compounds caused a decline in survivin, a property shared in common with 9-bromonoscapine [15]. Even though a definite relationship of the sensitivity of various cancer cells to these analogs is cell-type dependent, it is apparent that tubulin presents a potential target for these compounds. In addition, these second generation benzofuranone ring substituted noscapine analogs have been shown to spare the normal human dermal fibroblasts up to a concentration as high as 100 µM. These data perhaps demonstrate that the 7-position benzofuranone analogs may prove to be significantly better than the founding compound, noscapine.
In summary, we have provided simplest methods for the direct, and regioselective chemical manipulation of noscapine at position-7 that yielded the benzofuranone analogs in high quantitative yields. Taken together, like noscapine and its first generation analogs, these second generation analogs indicate a great potential for further preclinical evaluation for their therapeutic efficacy in animals models. Based upon in silico cues and rational drug-design, it is our ultimate goal to design, synthesize and develop a new class of novel ‘kinder and gentler’ noscapine analogs that will target variant tubulins and thereby help reduce the adverse side effects associated with their use. Owing to the minimal toxicity of noscapine analogs, we envision that combination regimens of more toxic drugs with noscapinoids may present an opportunity to reduce the dose-level of the toxic drugs to improve disease-free survival and enhance the quality of life.
Supplementary Material
Acknowledgements
This work was supported by grants to RA from the Department of Defense and the National Cancer Institute at the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 2.Zhou J, Giannakakou P. Targeting microtubules for cancer chemotherapy. Curr Med Chem Anticancer Agents. 2005;5:65–71. doi: 10.2174/1568011053352569. [DOI] [PubMed] [Google Scholar]
- 3.Ye K, Ke Y, Keshava N, Shanks J, Kapp JA, Tekmal RR, et al. Opium alkaloid noscapine is an antitumor agent that arrests metaphase and induces apoptosis in dividing cells. Proc Natl Acad Sci U S A. 1998;95:1601–1606. doi: 10.1073/pnas.95.4.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Checchi PM, Nettles JH, Zhou J, Snyder JP, Joshi HC. Microtubule-interacting drugs for cancer treatment. Trends Pharmacol Sci. 2003;24:361–365. doi: 10.1016/S0165-6147(03)00161-5. [DOI] [PubMed] [Google Scholar]
- 5.Ke Y, Ye K, Grossniklaus HE, Archer DR, Joshi HC, Kapp JA. Noscapine inhibits tumor growth with little toxicity to normal tissues or inhibition of immune responses. Cancer Immunol Immunother. 2000;49:217–225. doi: 10.1007/s002620000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sung B, Ahn KS, Aggarwal BB. Noscapine, a benzylisoquinoline alkaloid, sensitizes leukemic cells to chemotherapeutic agents and cytokines by modulating the NF-kappaB signaling pathway. Cancer Res. 2010;70:3259–3268. doi: 10.1158/0008-5472.CAN-09-4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landen JW, Lang R, McMahon SJ, Rusan NM, Yvon AM, Adams AW, et al. Noscapine alters microtubule dynamics in living cells and inhibits the progression of melanoma. Cancer Res. 2002;62:4109–4114. [PubMed] [Google Scholar]
- 8.Zhou J, Gupta K, Yao J, Ye K, Panda D, Giannakakou P, et al. Paclitaxel-resistant human ovarian cancer cells undergo c-Jun NH2-terminal kinase-mediated apoptosis in response to noscapine. J Biol Chem. 2002;277:39777–39785. doi: 10.1074/jbc.M203927200. [DOI] [PubMed] [Google Scholar]
- 9.Landen JW, Hau V, Wang M, Davis T, Ciliax B, Wainer BH, et al. Noscapine crosses the blood-brain barrier and inhibits glioblastoma growth. Clin Cancer Res. 2004;10:5187–5201. doi: 10.1158/1078-0432.CCR-04-0360. [DOI] [PubMed] [Google Scholar]
- 10.Aneja R, Lopus M, Zhou J, Vangapandu SN, Ghaleb A, Yao J, et al. Rational design of the microtubule-targeting anti-breast cancer drug EM015. Cancer Res. 2006;66:3782–3791. doi: 10.1158/0008-5472.CAN-05-2962. [DOI] [PubMed] [Google Scholar]
- 11.Jackson T, Chougule MB, Ichite N, Patlolla RR, Singh M. Antitumor activity of noscapine in human non-small cell lung cancer xenograft model. Cancer Chemother Pharmacol. 2008;63:117–126. doi: 10.1007/s00280-008-0720-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aneja R, Ghaleb AM, Zhou J, Yang VW, Joshi HC. p53 and p21 determine the sensitivity of noscapine-induced apoptosis in colon cancer cells. Cancer Res. 2007;67:3862–3870. doi: 10.1158/0008-5472.CAN-06-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aneja R, Vangapandu SN, Lopus M, Chandra R, Panda D, Joshi HC. Development of a novel nitro-derivative of noscapine for the potential treatment of drug-resistant ovarian cancer and T-cell lymphoma. Mol Pharmacol. 2006;69:1801–1809. doi: 10.1124/mol.105.021899. [DOI] [PubMed] [Google Scholar]
- 14.Aneja R, Zhou J, Zhou B, Chandra R, Joshi HC. Treatment of hormone-refractory breast cancer: apoptosis and regression of human tumors implanted in mice. Mol Cancer Ther. 2006;5:2366–2377. doi: 10.1158/1535-7163.MCT-06-0205. [DOI] [PubMed] [Google Scholar]
- 15.Karna P, Sharp SM, Yates C, Prakash S, Aneja R. EM011 activates a survivin-dependent apoptotic program in human non-small cell lung cancer cells. Mol Cancer. 2009;8:93. doi: 10.1186/1476-4598-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aneja R, Miyagi T, Karna P, Ezell T, Shukla D, Vij Gupta M, et al. A novel microtubule-modulating agent induces mitochondrially driven caspase-dependent apoptosis via mitotic checkpoint activation in human prostate cancer cells. Eur J Cancer. 2010;46:1668–1678. doi: 10.1016/j.ejca.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aneja R, Asress S, Dhiman N, Awasthi A, Rida PC, Arora SK, et al. Non-toxic melanoma therapy by a novel tubulin-binding agent. Int J Cancer. 2010;126:256–265. doi: 10.1002/ijc.24765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aneja R, Dhiman N, Idnani J, Awasthi A, Arora SK, Chandra R, et al. Preclinical pharmacokinetics and bioavailability of noscapine, a tubulin-binding anticancer agent. Cancer Chemother Pharmacol. 2007;60:831–839. doi: 10.1007/s00280-007-0430-y. [DOI] [PubMed] [Google Scholar]
- 19.Aneja R, Vangapandu SN, Lopus M, Viswesarappa VG, Dhiman N, Verma A, et al. Synthesis of microtubule-interfering halogenated noscapine analogs that perturb mitosis in cancer cells followed by cell death. Biochem Pharmacol. 2006;72:415–426. doi: 10.1016/j.bcp.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Aneja R, Vangapandu SN, Joshi HC. Synthesis and biological evaluation of a cyclic ether fluorinated noscapine analog. Bioorg Med Chem. 2006;14:8352–8358. doi: 10.1016/j.bmc.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Zhou J, Gupta K, Aggarwal S, Aneja R, Chandra R, Panda D, et al. Brominated derivatives of noscapine are potent microtubule-interfering agents that perturb mitosis and inhibit cell proliferation. Mol Pharmacol. 2003;63:799–807. doi: 10.1124/mol.63.4.799. [DOI] [PubMed] [Google Scholar]
- 22.Aneja R, Zhou J, Vangapandu SN, Zhou B, Chandra R, Joshi HC. Drug-resistant T-lymphoid tumors undergo apoptosis selectively in response to an antimicrotubule agent, EM011. Blood. 2006;107:2486–2492. doi: 10.1182/blood-2005-08-3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aneja R, Liu M, Yates C, Gao J, Dong X, Zhou B, et al. Multidrug resistance-associated protein-overexpressing teniposide-resistant human lymphomas undergo apoptosis by a tubulin-binding agent. Cancer Res. 2008;68:1495–1503. doi: 10.1158/0008-5472.CAN-07-1874. [DOI] [PubMed] [Google Scholar]
- 24.Heidemann S. Microtubules, leukemia, and cough syrup. Blood. 2006;107:2216-a-7. [Google Scholar]
- 25.Anderson JT, Ting AE, Boozer S, Brunden KR, Crumrine C, Danzig J, et al. Identification of novel and improved antimitotic agents derived from noscapine. J Med Chem. 2005;48:7096–7098. doi: 10.1021/jm050674q. [DOI] [PubMed] [Google Scholar]
- 26.Anderson JT, Ting AE, Boozer S, Brunden KR, Danzig J, Dent T, et al. Discovery of S-phase arresting agents derived from noscapine. J Med Chem. 2005;48:2756–2758. doi: 10.1021/jm0494220. [DOI] [PubMed] [Google Scholar]
- 27.Ravelli RB, Gigant B, Curmi PA, Jourdain I, Lachkar S, Sobel A, et al. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature. 2004;428:198–202. doi: 10.1038/nature02393. [DOI] [PubMed] [Google Scholar]
- 28.Dixon SL, Smondyrev AM, Knoll EH, Rao SN, Shaw DE, Friesner RA. PHASE: a new engine for pharmacophore perception, 3D QSAR model development, and 3D database screening: 1. Methodology and preliminary results. J Comput Aided Mol Des. 2006;20:647–671. doi: 10.1007/s10822-006-9087-6. [DOI] [PubMed] [Google Scholar]
- 29.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, et al. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 30.Bergenheim AT, Henriksson R. Pharmacokinetics and pharmacodynamics of estramustine phosphate. Clin Pharmacokinet. 1998;34:163–172. doi: 10.2165/00003088-199834020-00004. [DOI] [PubMed] [Google Scholar]
- 31.Miller HP, Wilson L. Preparation of microtubule protein and purified tubulin from bovine brain by cycles of assembly and disassembly and phosphocellulose chromatography. Methods Cell Biol. 2010;95:3–15. doi: 10.1016/S0091-679X(10)95001-2. [DOI] [PubMed] [Google Scholar]
- 32.Gaskin F, Cantor CR, Shelanski ML. Turbidimetric studies of the in vitro assembly and disassembly of porcine neurotubules. J Mol Biol. 1974;89:737–755. doi: 10.1016/0022-2836(74)90048-5. [DOI] [PubMed] [Google Scholar]
- 33.Karna P, Rida PC, Pannu V, Gupta KK, Dalton WB, Joshi H, et al. A novel microtubule-modulating noscapinoid triggers apoptosis by inducing spindle multipolarity via centrosome amplification and declustering. Cell Death Differ. 2011;18:632–644. doi: 10.1038/cdd.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warren GL, Andrews CW, Capelli AM, Clarke B, LaLonde J, Lambert MH, et al. A critical assessment of docking programs and scoring functions. J Med Chem. 2006;49:5912–5931. doi: 10.1021/jm050362n. [DOI] [PubMed] [Google Scholar]
- 35.Ray S, Chaturvedi D. Application of organic carbamates in drug design. Part 1: anticancer agents - recent reports. Drugs of the Future. 2004;29 [Google Scholar]
- 36.Efferth T, Sauerbrey A, Olbrich A, Gebhart E, Rauch P, Weber HO, et al. Molecular modes of action of artesunate in tumor cell lines. Mol Pharmacol. 2003;64:382–394. doi: 10.1124/mol.64.2.382. [DOI] [PubMed] [Google Scholar]
- 37.Anfosso L, Efferth T, Albini A, Pfeffer U. Microarray expression profiles of angiogenesis-related genes predict tumor cell response to artemisinins. Pharmacogenomics J. 2006;6:269–278. doi: 10.1038/sj.tpj.6500371. [DOI] [PubMed] [Google Scholar]
- 38.Perez EA. Paclitaxel in Breast Cancer. Oncologist. 1998;3:373–389. [PubMed] [Google Scholar]
- 39.Katsumata N. Docetaxel: an alternative taxane in ovarian cancer. Br J Cancer. 2003;89 Suppl 3:S9–S15. doi: 10.1038/sj.bjc.6601495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crown J, O'Leary M, Ooi WS. Docetaxel and paclitaxel in the treatment of breast cancer: a review of clinical experience. Oncologist. 2004;9 Suppl 2:24–32. doi: 10.1634/theoncologist.9-suppl_2-24. [DOI] [PubMed] [Google Scholar]
- 41.Johnson IS, Armstrong JG, Gorman M, Burnett JP., Jr The Vinca Alkaloids: A New Class of Oncolytic Agents. Cancer Res. 1963;23:1390–1427. [PubMed] [Google Scholar]
- 42.Burkhart CA, Kavallaris M, Band Horwitz S. The role of beta-tubulin isotypes in resistance to antimitotic drugs. Biochim Biophys Acta. 2001;1471:O1–O9. doi: 10.1016/s0304-419x(00)00022-6. [DOI] [PubMed] [Google Scholar]
- 43.Cabral F, Abraham I, Gottesman MM. Revertants of a Chinese hamster ovary cell mutant with an altered beta-tubulin: evidence that the altered tubulin confers both colcemid resistance and temperature sensitivity on the cell. Mol Cell Biol. 1982;2:720–729. doi: 10.1128/mcb.2.6.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu B, Staren ED, Iwamura T, Appert HE, Howard JM. Mechanisms of taxotere-related drug resistance in pancreatic carcinoma. J Surg Res. 2001;99:179–186. doi: 10.1006/jsre.2001.6126. [DOI] [PubMed] [Google Scholar]
- 45.Cowan NJ, Dudley L. Tubulin isotypes and the multigene tubulin families. Int Rev Cytol. 1983;85:147–173. doi: 10.1016/s0074-7696(08)62372-4. [DOI] [PubMed] [Google Scholar]
- 46.Kavallaris M, Kuo DY, Burkhart CA, Regl DL, Norris MD, Haber M, et al. Taxol-resistant epithelial ovarian tumors are associated with altered expression of specific beta-tubulin isotypes. J Clin Invest. 1997;100:1282–1293. doi: 10.1172/JCI119642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huzil JT, Chen K, Kurgan L, Tuszynski JA. The roles of beta-tubulin mutations and isotype expression in acquired drug resistance. Cancer Inform. 2007;3:159–181. [PMC free article] [PubMed] [Google Scholar]
- 48.Kavallaris M, Tait AS, Walsh BJ, He L, Horwitz SB, Norris MD, et al. Multiple microtubule alterations are associated with Vinca alkaloid resistance in human leukemia cells. Cancer Res. 2001;61:5803–5809. [PubMed] [Google Scholar]
- 49.Cheung CH, Cheng L, Chang KY, Chen HH, Chang JY. Investigations of survivin: the past, present and future. Front Biosci. 2011;16:952–961. doi: 10.2741/3728. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.