Abstract
Objective. To identify the microbiota communities in the vaginal tracts of healthy Mexican women across the pregnancy. Methods. Vaginal swabs were obtained during the prenatal visit of women from all trimesters (n = 64) of healthy pregnant women of Mexico City. DNA was isolated from each sample, and PCR-DGGE and sequencing of 16S rRNA gene fragments were used to identify the bacterial communities. Results. 21 different microorganisms were identified in the vaginal samples. Lactobacillus genus was present in 98% of women studied. Four lactobacilli species were identified in vaginal samples. L. acidophilus was the predominant (78%) followed by L. iners (54%), L. gasseri (20%), and L. delbrueckii (6%). 17 different microorganisms related to bacterial vaginosis conditions were identified. Ureaplasma urealyticum was the predominant (21%) followed by BVAB1 (17%) and Gemella bergeriae (7.8%). Conclusions. Lactobacillus genus predominates in the vaginal samples of Mexican pregnant women associated with different microorganisms related to bacterial vaginosis conditions.
1. Introduction
The healthy human vaginal microbiota in pregnant women plays a pivotal role in reproductive health and disease. The normal biota may prevent colonization of the host by pathogens and the spread of microorganisms related to urogenital infections, including those responsible for bacterial vaginosis. A disturbed vaginal microbiota is primarily associated with preterm labor, preterm rupture of membranes, and an increased risk of maternal and fetal morbidity [1].
Several studies have shown that the natural vaginal microbiota of healthy women of reproductive age is dominated by Lactobacillus spp.
These bacteria play a critical role in preventing the overgrowth of pathogens and pathogenic opportunistic bacteria. The antagonistic effect is mediated by molecules such as hydroxide peroxide, lactic acid, and bacteriocins, which display antibacterial activity against catalase-negative bacteria. H2O2 affects catalase-negative bacteria, but lactic acid and bacteriocins can affect catalase-negative as well as catalase-positive bacteria and Candida albicans specifically those responsible for bacterial vaginosis [2–4].
According to Nugent's classification, a score from 7 and 10 is considered bacterial vaginosis, a clinical condition dominated by the morphological identification of different Gram-negative and -positive bacteria, without evidence of Lactobacillus morphotypes. In contrast, a score from 0 and 3 is considered an undisturbed vaginal microflora dominated by the Lactobacillus genus, identified as the principal Gram-positive rods bacteria [5].
Cultivation-dependent methods have failed to properly characterize vaginal microbiological communities for the following reasons: the culture bias applies to the normal vaginal microbiota as well as to the disturbed vaginal microbiota, the naturally competitive conditions exhibited by microorganisms in vitro can spread into the culture media, the specific or selective media necessary for cultivation of a particular microorganism may be unavailable.
Molecular methods have identified in the vagina of healthy, nonpregnant women the Lactobacillus genus living with a spectrum of bacteria including Gardnerella, Enterococcus, Bifidobacterium, Staphylococcus, Corynebacterium, Streptococcus, Bacterioides, Mycoplasma, Escherichia, Peptostreptococcus, Ureaplasma, Veillonela, and Candida species [6–10]. However, at present the spectrum of bacterial species resident in the vaginal tracts of healthy, pregnant women is not well defined.
In the last decade, molecular techniques based on the analysis of the 16S rRNA gene fragment have allowed the identification of phylogenetically diverse microorganisms living in a precise ecosystem. PCR-denaturing gradient gel electrophoresis (PCR-DGGE) is a rapid and reliable molecular technique that has been applied to characterize the bacterial communities present in different biological niches, including the human vagina, gut, gingival, and skin [11–14].
The aim of present work was to characterize the vaginal bacterial communities present in Mexican women with a Nugent's 0–3 classification by PCR-DGGE and sequencing of 16S rRNA gene fragments in a transversal study.
2. Material and Methods
2.1. Patients and Biological Samples
Healthy, pregnant women without vaginal bleeding, clinical symptoms of vaginal infection, or evidence of Candida colonization were enrolled in the study during routine prenatal examinations at the National Institute of Perinatology, Mexico City. Gestational age was estimated from the last menstrual period and early gestational fetal ultrasonographic measurements. To be eligible, women had to be free of subjective complaints, vaginal bleeding and oral or local antimicrobial therapies within the four weeks prior to enrollment.
During the prenatal care visit, a vaginal sample was taken from 140 women in different weeks of pregnancy in a transversal study. A sterile speculum was inserted into each patient, and a sample from the posterior fornix of the vagina was collected using a Dacron sterile hyssop. Smears were made on microscope slides from vaginal swabs collected from each subject. The slides were Gram-stained and scored by Nugent criteria [5]. A score of 0 to 10 was assigned, considering the relative proportions of large Gram-positive, small Gram-negative, Gram-variable, and curved Gram-variable rods. Only women with a score of 0 to 3 were interpreted as having normal microbiota and were included in the study. The protocol was revised and approved by the internal institutional ethical and academic committee. Signed informed consent was obtained from all participants.
2.2. DNA Extraction
Total DNA of the vaginal samples was extracted using DNAzol reagent (Invitrogen, Carlsbad, Calif, USA), following the specifications provide by the manufacturer. DNA quality was estimated by electrophoresis in 1% agarose gels in TBE buffer (89 mM Tris, pH 8.3; 89 mM boric acid; 2 mM EDTA) and staining with 0.5 μg/mL ethidium bromide. DNA concentrations and A260/A280 were determined spectrophotometrically with a Lambda 1A spectrophotometer (Perkin Elmer, Waltham, Mass, USA). An A260/A280 ratio of 1.8–2.1 was considered acceptable.
2.3. PCR-DGGE and Taxonomic Analysis of Vaginal Strains Based on 16S rRNA Gene Fragments Sequences
The diversity of the bacterial communities in each vaginal sample was studied by PCR-DGGE analysis. The V3 variable region of each bacterial 16S rRNA gene fragment was amplified by Muyzer technique [15] using 50 ng of metagenomic DNA from vaginal smears and the primers MAR-1 (5′-CGC CCG CCG CGC GGC GGG CGG GGC GGG GGC ACG GGG CCT ACG GGA GGC AGC AG-3′) and MAR-2 (5′-ATT ACC GCG GCT GCT GG-3′). The PCR consisted of 2.5 μL of 10x PCR buffer (10 mM Tris-HCl, 2.5 mM MgCl2 and 50 mM KCl), 40 pmol of each primer, 0.8 mM of each deoxyribonucleoside triphosphate, 0.5 μL (5 U) of Taq DNA polymerase and 1.5 μL (50 ng) of template DNA solution in a final volume of 25 μL. PCR was carried out for 35 cycles in a thermal gradient cycler (Eppendorf Scientific Inc., Westbury, NY, USA) with a denaturation step of 92°C for 45 s, followed by an annealing step at 55°C for 30 s and an extension step at 72°C for 45 s. A final extension step at 72°C for 7 min was added for all reactions. The expected size of the amplified fragment was 240 bp. In our research group, this PCR-based procedure has been frequently validated using as target bacterial genomic DNA from proteobacteria to sulphate-reducing bacteria and other taxons [16, 17]. DGGE analysis was performed with a D-Code Universal Detection System (Bio-Rad Laboratories, Hercules, Calif, USA). The linear denaturant gradient was attained using a communicating vessel gradient with a 16-cm gel that was 1 mm wide. PCR amplification products (25 μL) were loaded into each well of the gel. Gels were run at 60 V for 16 h and maintained at 60°C in 1x TAE buffer (40 mM Tris, 20 mM acetate, 1 mM EDTA). At the end of the experiment, DNA separated in the DGGE gels was stained with a 1 : 10,000 dilution of reactive Vistra Green (Amersham Biosciences, Piscataway, NJ, USA) diluted in 50 mL of 1x TE buffer, pH 7.5, for 30 min. All visible DGGE bands were excised from gels with a sterile scalpel and placed into single Eppendorf tubes. Gel pieces were washed once in 1x PCR buffer and incubated overnight in 20 μL of the same buffer at 4°C. Five microliters of the buffer solution was used as a template for PCR reamplification. The eubacterial primers without GC clamps and the PCR amplification conditions mentioned above were used for reamplification of each excised band from the DGGE gels. Reamplified bands were purified using the DNA Clean and Concentrator Kit (Zymo Research, Orange, Calif, USA) and sequenced by dideoxy chain termination. All sequences obtained in this work were subjected to a BLAST version 2.2.3 search [18] to assess the taxonomic hierarchy of the sequences and to select the related 16S rDNA bacterial sequences. Multiple alignment analyses with CLUSTAL X [19] were performed using the acquired sequences in this work and the related sequences selected from the NCBI Taxonomy Homepage (TaxBrowser). The identities of the sequences were determined on the basis of the highest percentage (a minimum of 95%) of total nucleotide match in GenBank.
2.4. Statistical Analysis
Patient characteristics, time of vaginal swab collection of enrolled women in the study, and infant-birth characteristics were analyzed by the Kuskal-Wallis one-way ANOVA; P < 0.05 was accepted as a significant difference. Statistical analysis was carried out with Sigma Stat software (Systat Software Inc., San Jose, Calif, USA).
3. Results
3.1. Characteristics of Women Included in the Study
A total of 64 samples from pregnant women with normal vaginal flora according Nugent's score (0–3) were included in the study. Women showed a mean of: 27.5 ± 6.7 years of maternal age; 38.4 ± 1.98 weeks at vaginal delivery; 21.5 ± 9.6 weeks at vaginal swab collection. Obstetric history showed a median of: 2 (1–5; (min-max)) gravities; 0 (1–5) vaginal deliveries; 0 (1–3) abortions; 0 (1–3) caesareans. Infant birth characteristic showed a mean of: 3045 ± 500.16 g weight outcome. Sixteen samples were from first trimester (25%), twenty five were from second trimester (39%), and twenty three were from last trimester (36%). Table 1 shows the information respect to maternal age, obstetric history or infant birth characteristics at delivery of women included in the study rated by trimesters of pregnancy.
Table 1.
Pregnant women and infant-birth characteristics in each trimester of study.
| First trimester (n = 16) | Second trimester (n = 25) | Third trimester (n = 23) | |
|---|---|---|---|
| Maternal Age (y)* | 28.8 ± 6.0 | 27.4 ± 8.2 | 26.7 ± 5.3 |
| (28; 15–37) | (28; 13–43) | (27; 17–37) | |
|
| |||
| Obstetric history** | |||
| Gravity | 2 (1–5) | 2 (1–5) | 2 (1–5) |
| Vaginal delivery | 1 (0–5) | 0 (0–3) | 0 (0-1) |
|
| |||
| Infant/birth characteristics* | |||
| Gestational age at delivery (wk) | 37.9 ± 1.4 | 38.2 ± 2.5 | 38.9 ± 1.5 |
| (38; 37–40) | (38; 37–41) | (39; 37–42) | |
| Infant weigh outcome at delivery (g) | 3033 ± 393 | 2974 ± 656 | 3132 ± 355 |
| (2955; 2520–3960) | (3110; 2580–3900) | (3130; 2520–3800) | |
|
| |||
| Weeks at vaginal ∗,+ swab collected | 10.1 ± 1.3 | 18.5 ± 2.8 | 32.8 ± 4.2 |
| (10; 7–12) | (18; 13–24) | (33; 27–41) | |
*Data are given in mean ± SD with median and ranges in parenthesis. **Data are given in median with ranges in parenthesis. + P < 0.05; data compared with Kuskal-Wallis one-way ANOVA.
3.2. Identification of Vaginal Microbiota
DGGE-DNA profiles of vaginal samples from 64 women included in the study are show in Figures 1, 2, and 3. Each DNA band in the figures has a number related to Table 2 where the diversity of microbiota identified in vaginal samples and GenBank access number data identification are described.
Figure 1.
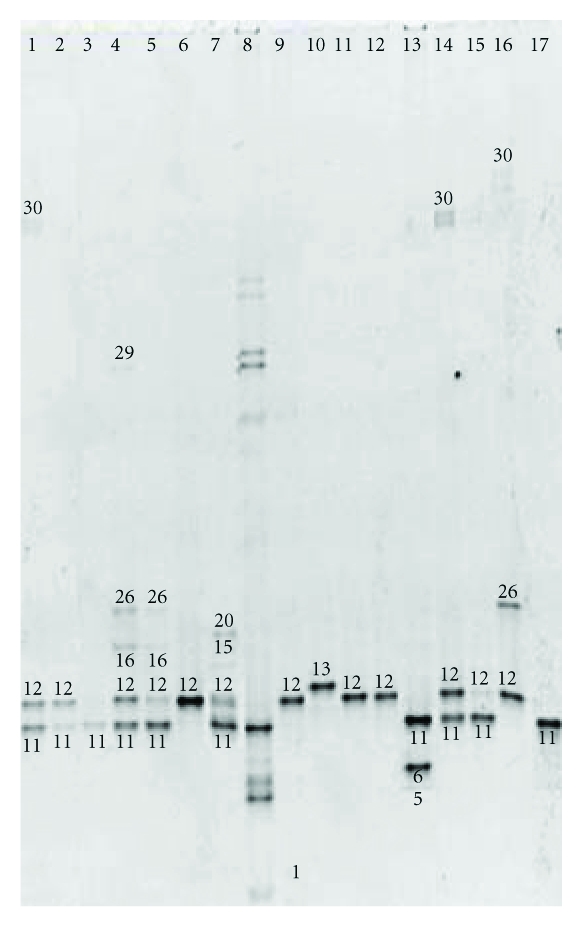
Denaturing gradient gel electrophoresis of vaginal samples from first trimester (n = 16). Lines 1–7 and 9–17 samples. Line 8 internal DNA lab standard. Number of DNA-band in the figure related to Table 2, where the percentage of identification in women studied and GenBank access number data identification are described.
Figure 2.
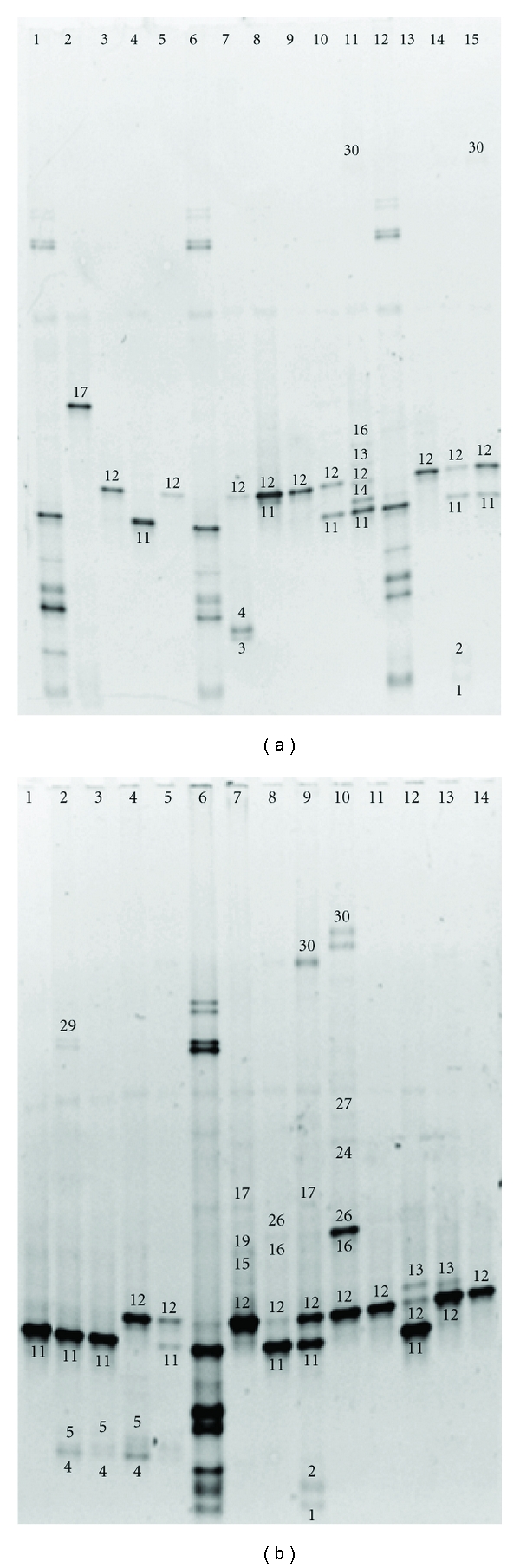
Denaturing gradient gel electrophoresis of vaginal samples from second trimester. Gel A (n = 12); lines 2–5, 7–11, and 13–15 samples. Lines 1,6,12 internal DNA lab-standard. Gel B (n = 13); lines 1–5 and 7–14 samples. Line 6 internal DNA lab-standard. Number of DNA-band in the figure related to Table 2, where the percentage of identification in women studied and GenBank access number data identification are described.
Figure 3.
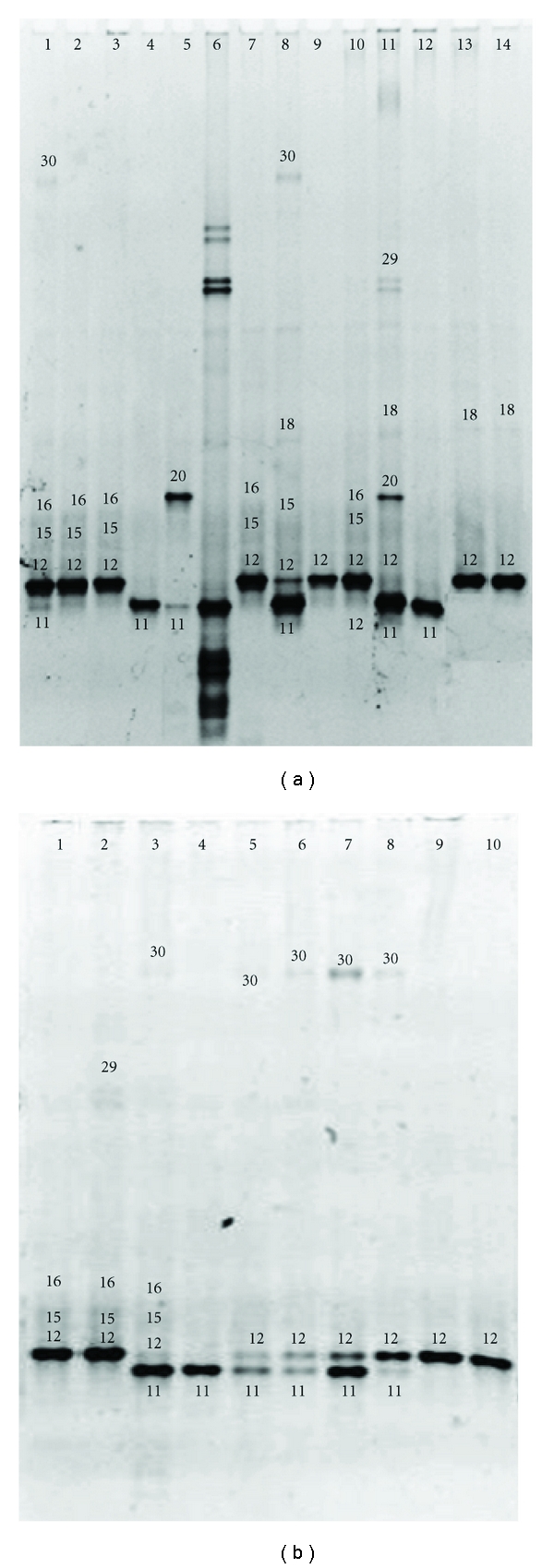
Denaturing gradient gel electrophoresis of vaginal samples from third trimester. Gel A (n = 13); lines 1–5 and 7–14 samples. Line 6 internal DNA lab standard. Gel B (n = 10); lines 1–10 samples. Number of DNA band in the figure related to Table 2, where the percentage of identification in women studied and GenBank access number data identification are described.
Table 2.
Microorganisms identified in the vaginal tract of pregnant healthy women.
| Number of DNA band shows in the figures | Name of microorganism | GenBank access number | Times identified in women (n = 64) | Percentage of women with species identified (%) |
|---|---|---|---|---|
| 12 | Lactobacillus acidophilus | NC_006814 | 50 | 78 |
| 11 | Lactobacillus iners | AY283265 | 35 | 55 |
| 30 | Uncultured Ureaplasma urealyticum | EU644473 | 14 | 22 |
| 16 | Lactobacillus gasseri | NC_008530 | 13 | 20 |
| 15 | BVAB1 | AB034121 | 11 | 17 |
| 26 | Gemella bergeriae | Y13365.1 | 5 | 8 |
| 4 | Gardnerella vaginalis | M58744 | 4 | 6 |
| 13 | Lactobacillus delbrueckii | NC_008529 | 4 | 6 |
| 18 | Leptotrichia amnionii | AY078425 | 4 | 6 |
| 5 | Mobiluncus sp. | EF428974.1 | 4 | 6 |
| 29 | Ureaplasma Urealyticum | AF073455 | 4 | 6 |
| 17 | Peptostreptococcus sp. | AY207059 | 3 | 5 |
| 1 | Uncultured Gardnerella sp. | AY738665.1 | 3 | 5 |
| 20 | Peptoniphilus indolicus | D14147 | 2 | 3 |
| 24 | Anaerococcus vaginalis | AF542229 | 1 | 2 |
| 2 | Atopobium sp. | AY738658.1 | 1 | 2 |
| 6 | Mobiluncus mulieris | AJ427625 | 1 | 2 |
| 3 | Porphiromonas dentalis | X81876.1 | 1 | 2 |
| 14 | Prevotella bivia | L16475 | 1 | 2 |
| 27 | Uncultured Leptotrichia sp. | AY724742.1 | 1 | 2 |
| 19 | Uncultured Peptoniphilus sp. | AY738692.1 | 1 | 2 |
Percentages in the column of “% of women with species identified” were rounded off to whole percents.
BLAST analysis of DNA sequences obtained from DGGE excised gel bands from 64 women correspond to 21 different bacterial species. Lactobacillus genus was detected in 63 of 64 women included in the study, only in one woman (1.5%) was not possible identified any species of Lactobacillus genus, solely Peptostreptococcus sp. was identified in that woman (Figure 2(a), lane 2).
The Lactobacillus members were grouped into four species, with L. acidophilus being the most abundant (78.12%), followed by L. iners (54.68%), L. gasseri (20.31%), and L. delbrueckii (6.25%). L. delbrueckii was the most exiguous species in the vaginal tract, given that it was detected only in four samples (Table 2).
43% of women were colonized by one, two, or three different Lactobacillus species. 10.9% were colonized by one Lactobacillus species plus 1 or 2 different microorganism species. 31% of women were colonized by two Lactobacillus species plus 1, 2, 3, or 4 different microorganism species. 10.9% of women were colonized by three Lactobacillus species plus 1 and 2 different microorganism species. 1.5% of women (one woman) were colonized by 4 Lactobacillus species plus 1 different microorganism species (Figure 4).
Figure 4.
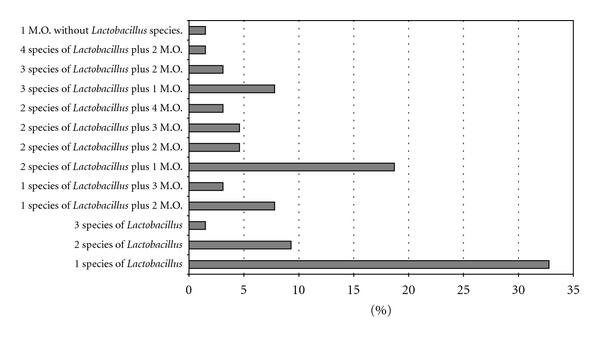
Profile of microorganisms identified in vaginal tract of women studied (n = 64). M.O. = microorganism (any microorganism different of Lactobacilli genus).
Taking into account the total number of 21 microorganism species identified (correspond to 163 bands amplified and sequenced from 64 women), 102 bands corresponded to the Lactobacillus genus (62.5%), 14 bands for uncultured Ureaplasma urealyticum (8.5%), 11 bands for BVAB1 (6.7%), and 5 bands for Gemella bergireae (3.0%). With respect to the remaining 31 bands they corresponded to ten bacteria which account for approximately 20% of the total microorganisms identified in the vaginal tract with individual values between 1% to 6%.
Two microorganism (Porphiromonas dentalis and Mobiluncus mulieris), seven (Atopobium sp., Gardnerella vaginalis, Prevotella bivia, Peptostreptococcus sp., uncultured Peptoniphilus sp., Anaerococcus vaginalis, and uncultured Leptotrichia sp.), and one (Leptotrichia amnionii) were identified for first, second, and third trimesters, respectively (Figure 5).
Figure 5.
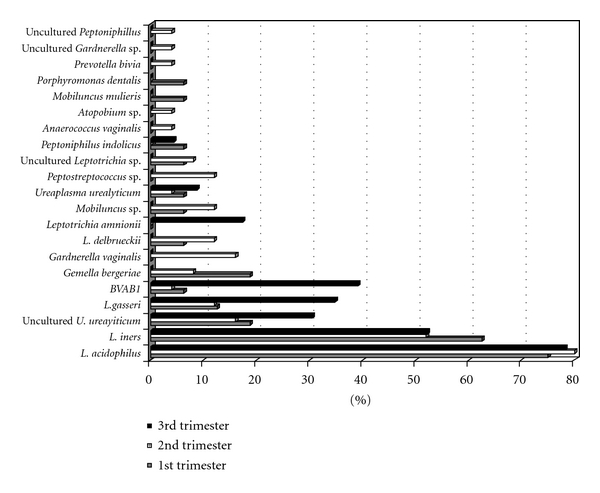
Distribution of 21 different microorganisms identified in vaginal tract of women studied (n = 64) by trimester of pregnancy.
The major diversity of microorganism species was detected in vaginal samples from women enrolled in the second trimester, as samples from this stage contained sixteen bacteria from the total number of species identified. Also, twelve and eighth microorganisms were recognized molecularly in vaginal samples from women enrolled in the first and third trimesters, respectively (Figure 5).
4. Discussion
Human vaginal flora plays a profound role in reproductive health. Nevertheless, given the current limitations in our diagnostic abilities, it is naive to assume that we know all microorganisms present the vaginal tract in healthy and unhealthy pregnancies; the present paper aims to attend to this concern.
Lactobacilli genus was present in vaginal samples from all pregnant women studied except for one woman of second trimester, who showed Peptostreptococcus sp., as the only identified microorganism. On the other hand, a very similar distribution with respect to the Lactobacillus species was observed in the remaining women. L. acidophilus was the most abundant microorganism (78%), followed by L. iners (54%), L. gasseri (20%), and L. delbrueckii (6%).
Very few studies have been published with respect to the vaginal microbiota in healthy and unhealthy pregnancies. In 2007, Kiss et al. studied 126 healthy, pregnant Swedish women (Nugent score 0–3) between 11 to 14 gestational weeks and applied a species-specific PCR technique on vaginal samples. The author identified the presence of eight different lactobacilli species, with L. gasseri (26.4%), L. crispatus (23.6%), L. jensenii (19.4%), and L. rhamnosus (9.7%) being the most abundantly observed species [20]. However, the author did not detect L. acidophilus, the lactobacilli species most frequently detected in our study. Likewise, in 2007, Tamrakar et al. [21] studied 98 healthy, pregnant Japanese women between 5 to 36 gestational weeks (mean of 23 weeks) and applied a species-specific PCR technique for fourteen Lactobacilli species on vaginal samples. Four lactobacilli, L. crispatus (61.2%), L. jensenii (29.6%), L. gasseri (33.7%), and L. iners (39.8%), showed the highest prevalence in the vaginal samples. L. delbrueckii was not detected, and L. acidophilus was not included as a target of the study.
Our results agree with the two authors mentioned above in that the four discussed Lactobacilli species were the most abundant microorganisms observed across all trimesters of pregnancy. However, we cannot recognize L. crispatus and L. jensenii which were identified in the previous mentioned manuscripts and are the most predominant species worldwide reported.
On this respect, using the same conditions and PCR procedures described in Material and Methods section, we confirmed that the primers can amplify these species from a cultured strain (data not shown) and produce DGGE fragments with the expected molecular size, which can be distinguished from those of other species after sequence the DGGE fragments and apply the bioinformatic analyses. This evidence demonstrates that the PCR-DGGE strategy is proper to recognize Lactobacillus spp. DNA target.
On the other hand, the absence of L. crispatus and L. jensenii in Mexican population samples is a surprising data that must be confirmed. However, an independent study reveals that both species were not frequently isolated from Mexican population (Castro-Escarpulli G., personal communication). Also, culture independent studies have not detected L. jensenii [22], and relevant differences in the composition of vaginal microbial communities, particularly Lactobacillus spp., have been found in healthy Caucasian and black women [23].
Although few data have been reported in this area, vaginal Lactobacillus spp. distributions can vary across specific groups, perhaps as a consequence of ethnic conditions, food intake, behavior, habits, and customs [24–27]; evidently, more information must be accumulated.
Molecular studies performed with nonpregnant healthy women have shown a limited Lactobacillus diversity in the vagina that is restricted to three to seven species, with the specific distribution of lactobacilli species being dependent on the group of women studied [28, 29].
On the other hand, a different distribution of microorganisms related to bacterial vaginosis conditions [30–32] were characterized in the vaginal tract of women studied. Several manuscripts, where molecular techniques were applied to evaluate disturbed vaginal tract conditions, have shown a wide distribution of vaginosis-associated bacteria (VAB), with a clear decrease in the number and/or abundance of protective lactobacilli species [33–35]. Our data showed a wide distribution of VAB in vaginal samples of women studied; however, clinical data and morphological vaginal characterization of smears by Nugent's criteria were compatible with healthy vaginal tract. Despite any experiment was done to evaluated the protective effect of lactobacilli group in the women studied, we think that Lactobacillus species confer protection against the overgrowth of potentially pathogenic bacteria by means of the release of metabolic products such as H2O2, lactic acid, and bacteriocins as have been described previously [2–4], which maintain the status of normal vaginal microbiota inhibiting the colonization and spreading of local or transit pathogens.
In our results, the pattern of distribution of lactobacilli species was very constant among studied women, since 33% of women showed L. acidophilus, followed by the pair of L. acidophilus plus L. iners (8%) and the triad of L. acidophilus plus L. gasseri (2%) as the microorganisms only identified in vaginal samples. This pattern of distribution of Lactobacillus species was the same even though vaginosis-associated bacteria were detected in vaginal samples, since women with L. acidophilus plus 2 and 3 VAB account 9%, the L. acidophilus/L. iners plus 2–4 VAB account 29% and the triad L. acidophilus/L. iners/L. gasseri plus 1 and 2 VAB account 10%. This data support the idea of that a specific group of Lactobacillus species in vaginal tract of women prevents the spread of microorganisms potentially capable to cause urogenital infections, including those responsible for bacterial vaginosis.
In this respect, a manuscript published by Verstraelen et al. [36] demonstrated in a prospective study of pregnant women that the presence of specific lactobacilli species in the vaginal tract of healthy women is a pivotal or protective factor for the conversion to abnormal microbiota evaluated by Gram stained smears. The presence of L. crispatus alone in the vaginal tract of healthy women or accompanied with other lactobacilli species as L. jensenii, L. gasseri and L. iners confers a protector effect (RR 0.2; 95% CI 0.05–0.89) to development an abnormal vaginal microbiota, against the presence of L. gasseri/iners who account an increased risk (RR 10.41; 95% CI 1.39–78.12) for the conversion to abnormal vaginal microbiota.
The data present herein showed a characteristic pattern of Lactobacillus species in healthy women even when different vaginosis-associated bacteria were detected in vaginal samples. Although the study design of the present paper and the Verstraelen is different, the comparison of Lactobacillus species found in our paper and the Lactobacillus species reported by the author in the vaginal tract of healthy women (Grade I) of first trimester, showed a similar distribution respect to the number of Lactobacillus species detected, since the author reported 67% of the women colonized by one species of lactobacilli, 24.7% by two species and 6.5% by three and four species.
The paper present herein adds information respect to the Lactobacillus genus that resides in the vaginal tract of Hispanic women. In this area, improved knowledge of normal microbiological species present in the vaginal tracts of healthy, pregnant women in a particular population could aid in the development of specific probiotic and prebiotic therapies as well as prophylactic alternatives to help patients avoid vaginosis-associated deleterious fetomaternal outcomes.
5. Conclusions
Twenty one different bacteria species were detected in vaginal samples from healthy women. The Lactobacillus genus was detected in 63 of 64 women included in the study. The lactobacilli members were grouped into four species, with L. acidophilus being the most abundant (78.12%) followed by L. iners (54.68%), L. gasseri (20.31%), and L. delbrueckii (6.25%). Seventeen different microorganisms related to disturbed or bacterial vaginosis conditions were identified in the vaginal tract of pregnant women, with dissimilar distributions among studied women. Uncultured U. realyticum was the most abundant microorganism (21%) followed by BVAB1 (17%) and Gemella bergirae (7.8%). Fourteen remain microorganisms showed prevalence between 1 to 6%.
References
- 1.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. The Lancet. 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vallor AC, Antonio MAD, Hawes SE, Hillier SL. Factors associated with acquisition of, or persistent colonization by, vaginal Lactobacilli: role of hydrogen peroxide production. Journal of Infectious Diseases. 2001;184(11):1431–1436. doi: 10.1086/324445. [DOI] [PubMed] [Google Scholar]
- 3.Kaewsrichan J, Peeyananjarassri K, Kongprasertkit J. Selection and identification of anaerobic lactobacilli producing inhibitory compounds against vaginal pathogens. FEMS Immunology and Medical Microbiology. 2006;48(1):75–83. doi: 10.1111/j.1574-695X.2006.00124.x. [DOI] [PubMed] [Google Scholar]
- 4.Dover SE, Aroutcheva AA, Faro S, Chikindas ML. Natural antimicrobials and their role in vaginal health: a short review. International Journal of Probiotics and Prebiotics. 2008;3(4):219–230. [PMC free article] [PubMed] [Google Scholar]
- 5.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. Journal of Clinical Microbiology. 1991;29(2):297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou X, Bent SJ, Schneider MG, Davis CC, Islam MR, Forney LJ. Characterization of vaginal microbial communities in adult healthy women using cultivation-independent methods. Microbiology. 2004;150(8):2565–2573. doi: 10.1099/mic.0.26905-0. [DOI] [PubMed] [Google Scholar]
- 7.Hill JE, Goh SH, Money DM, et al. Characterization of vaginal microflora of healthy, nonpregnant women by chaperonin-60 sequence-based methods. American Journal of Obstetrics and Gynecology. 2005;193(3):682–692. doi: 10.1016/j.ajog.2005.02.094. [DOI] [PubMed] [Google Scholar]
- 8.Hyman RW, Fukushima M, Diamond L, Kumm J, Giudice LC, Davis RW. Microbes on the human vaginal epithelium. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(22):7952–7957. doi: 10.1073/pnas.0503236102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vitali B, Pugliese C, Biagi E, et al. Dynamics of vaginal bacterial communities in women developing bacterial vaginosis, candidiasis, or no infection, analyzed by PCR-denaturing gradient gel electrophoresis and real-time PCR. Applied and Environmental Microbiology. 2007;73(18):5731–5741. doi: 10.1128/AEM.01251-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nam H, Whang K, Lee Y. Analysis of vaginal lactic acid producing bacteria in healthy women. Journal of Microbiology. 2007;45(6):515–520. [PubMed] [Google Scholar]
- 11.Oakley BB, Fiedler TL, Marrazzo JM, Fredricks DN. Diversity of human vaginal bacterial communities and associations with clinically defined bacterial vaginosis. Applied and Environmental Microbiology. 2008;74(15):4898–4909. doi: 10.1128/AEM.02884-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vitali B, Ndagijimana M, Cruciani F, et al. Impact of a synbiotic food on the gut microbial ecology and metabolic profiles. BMC Microbiology. 2010;10, article 4 doi: 10.1186/1471-2180-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ledder RG, Gilbert P, Huws SA, et al. Molecular analysis of the subgingival microbiota in health and disease. Applied and Environmental Microbiology. 2007;73(2):516–523. doi: 10.1128/AEM.01419-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grice EA, Kong HH, Renaud G, et al. A diversity profile of the human skin microbiota. Genome Research. 2008;18(7):1043–1050. doi: 10.1101/gr.075549.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muyzer G, de Waal EC, Uitterlinden AG. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Applied and Environmental Microbiology. 1993;59(3):695–700. doi: 10.1128/aem.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neria-González I, Wang ET, Ramírez F, Romero JM, Hernández-Rodríguez C. Characterization of bacterial community associated to biofilms of corroded oil pipelines from the southeast of Mexico. Anaerobe. 2006;12(3):122–133. doi: 10.1016/j.anaerobe.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Zermeño-Eguia JA, Jan-Roblero J, de La Serna JZ-D, de León AV-P, Hernández-Rodríguez C. Degradation of polychlorinated biphenyl (PCB) by a consortium obtained from a contaminated soil composed of Brevibacterium, Pandoraea and Ochrobactrum . World Journal of Microbiology and Biotechnology. 2009;25(1):165–170. [Google Scholar]
- 18.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of Molecular Biology. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 19.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research. 1997;25(24):4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiss H, Kögler B, Petricevic L, et al. Vaginal Lactobacillus microbiota of healthy women in the late first trimester of pregnancy. BJOG: An International Journal of Obstetrics and Gynaecology. 2007;114(11):1402–1407. doi: 10.1111/j.1471-0528.2007.01412.x. [DOI] [PubMed] [Google Scholar]
- 21.Tamrakar R, Yamada T, Furuta I, et al. Association between Lactobacillus species and bacterial vaginosis-related bacteria, and bacterial vaginosis scores in pregnant Japanese women. BMC Infectious Diseases. 2007;7, article 128 doi: 10.1186/1471-2334-7-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anukam KC, Osazuwa EO, Ahonkhai I, Reid G. Lactobacillus vaginal microbiota of women attending a reproductive health care service in Benin city, Nigeria. Sexually Transmitted Diseases. 2006;33(1):59–62. doi: 10.1097/01.olq.0000175367.15559.c4. [DOI] [PubMed] [Google Scholar]
- 23.Zhou X, Brown CJ, Abdo Z, et al. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. ISME Journal. 2007;1(2):121–133. doi: 10.1038/ismej.2007.12. [DOI] [PubMed] [Google Scholar]
- 24.Brown CJ, Wong M, Davis CC, Kanti A, Zhou X, Forney LJ. Preliminary characterization of the normal microbiota of the human vulva using cultivation-independent methods. Journal of Medical Microbiology. 2007;56(2):271–276. doi: 10.1099/jmm.0.46607-0. [DOI] [PubMed] [Google Scholar]
- 25.Thies FL, König W, König B. Rapid characterization of the normal and disturbed vaginal microbiota by application of 16S rRNA gene terminal RFLP fingerprinting. Journal of Medical Microbiology. 2007;56(6):755–761. doi: 10.1099/jmm.0.46562-0. [DOI] [PubMed] [Google Scholar]
- 26.Spear GT, Sikaroodi M, Zariffard MR, Landay AL, French AL, Gillevet PM. Comparison of the diversity of the vaginal microbiota in HIV-infected and HIV-uninfected women with or without bacterial vaginosis. Journal of Infectious Diseases. 2008;198(8):1131–1140. doi: 10.1086/591942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(supplement 1):4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pavlova SI, Kilic AO, Kilic SS, et al. Genetic diversity of vaginal lactobacilli from women in different countries based on 16S rRNA gene sequences. Journal of Applied Microbiology. 2002;92(3):451–459. doi: 10.1046/j.1365-2672.2002.01547.x. [DOI] [PubMed] [Google Scholar]
- 29.Jin L, Tao L, Pavlova SI, et al. Species diversity and relative abundance of vaginal lactic acid bacteria from women in Uganda and Korea. Journal of Applied Microbiology. 2007;102(4):1107–1115. doi: 10.1111/j.1365-2672.2006.03147.x. [DOI] [PubMed] [Google Scholar]
- 30.Verhelst R, Verstraelen H, Claeys G, et al. Cloning of 16S rRNA genes amplified from normal and disturbed vaginal microflora suggests a strong association between Atopobium vaginae, Gardnerella vaginalis and bacterial vaginosis. BMC Microbiology. 2004;4, article 16 doi: 10.1186/1471-2180-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. The New England Journal of Medicine. 2005;353(18):1899–1911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 32.Fredricks DN, Fiedler TL, Thomas KK, Oakley BB, Marrazzo JM. Targeted PCR for detection of vaginal bacteria associated with bacterial vaginosis. Journal of Clinical Microbiology. 2007;45(10):3270–3276. doi: 10.1128/JCM.01272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilks M, Wiggins R, Whiley A, et al. Identification and H2O2 production of vaginal lactobacilli from pregnant women at high risk of preterm birth and relation with outcome. Journal of Clinical Microbiology. 2004;42(2):713–717. doi: 10.1128/JCM.42.2.713-717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Backer E, Verhelst R, Verstraelen H, et al. Quantitative determination by real-time PCR of four vaginal Lactobacillus species, Gardnerella vaginalis and Atopobium vaginae indicates an inverse relationship between L. gasseri and L. iners . BMC Microbiology. 2007;7, article 115 doi: 10.1186/1471-2180-7-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fredricks DN, Fiedler TL, Thomas KK, Mitchell CM, Marrazzo JM. Changes in vaginal bacterial concentrations with intravaginal metronidazole therapy for bacterial vaginosis as assessed by quantitative PCR. Journal of Clinical Microbiology. 2009;47(3):721–726. doi: 10.1128/JCM.01384-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verstraelen H, Verhelst R, Claeys G, De Backer E, Temmerman M, Vaneechoutte M. Longitudinal analysis of the vaginal microflora in pregnancy suggests that L. crispatus promotes the stability of the normal vaginal microflora and that L. gasseri and/or L. iners are more conducive to the occurrence of abnormal vaginal microflora. BMC Microbiology. 2009;9, article 116 doi: 10.1186/1471-2180-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]


