Abstract
The ultimate goal of any dental treatment is the regeneration of lost tissues and alveolar bone. Under the appropriate culture conditions, periosteal cells secrete extracellular matrix and form a membranous structure. The periosteum can be easily harvested from the patient's own oral cavity, where the resulting donor site wound is invisible. Owing to the above reasons, the periosteum offers a rich cell source for bone tissue engineering; hence, the regenerative potential of periosteum is immense. Although the use of periosteum as a regenerative tool has been extensive in general medical field, the regenerative potential of periosteum is highly underestimated in dentistry; therefore, the present paper reviews the current literature related to the regenerative potential of periosteum and gives an insight to the future use of periosteum in dentistry.
1. Introduction
Reconstruction of lost tissues is a long cherished goal in medical field. A lot of research has been done in the past, and still research is going on to explore tools and techniques for regeneration of lost tissues as a result of the disease process. The use of various grafts and recent tissue engineering techniques including stem cell research are testimony to the ever increasing need for most suitable treatment option to replace/repair lost tissues due to various pathologic processes. The use of autogenous periosteum in general medical treatment has been extensive and has shown promising results [1–3]; on the contrary in dentistry, the use of periosteum as a regenerative tool has been limited and highly underrated; therefore, the purpose of this paper is to highlight the current status of use of periosteum in dentistry as well as suggesting its future use in various treatment options related specifically to dental field.
2. Periosteum: What Justifies Its Use?
The periosteum is a highly vascular connective tissue sheath covering the external surface of all the bones except for sites of articulation and muscle attachment (Figure 1) [4]. The periosteum comprises of at least two layers, an inner cellular or cambium layer, and an outer fibrous layer [1]. The inner layer contains numerous osteoblasts and osteoprogenitor cells, and the outer layer is composed of dense collagen fiber, fibroblasts, and their progenitor cells [5]; osteogenic progenitor cells from the periosteal cambium layer may work with osteoblasts in initiating and driving the cell differentiation process of bone repair characterized by the development of the initial fracture callus and subsequent remodeling. Periosteum can be described as an osteoprogenitor cell containing bone envelope, capable of being activated to proliferate by trauma, tumors, and lymphocyte mitogens [6]. Research on the structure of periosteum has shown that it is made up of three discrete zones [7]. Zone 1 has an average thickness of 10–20 um consisting predominantly of osteoblasts representing 90% of cell population, while collagen fibrils comprise 15% of the volume. The majority of cells in zone 2 are fibroblasts, with endothelial cells being most of the remainder. Zone 3 has the highest volume of collagen fibrils and fibroblasts among all the three zones. Fibroblasts take up more than 90% of the cells in zone 3. The morphology of fibroblasts is variable across the three zones (Figure 2).
Figure 1.
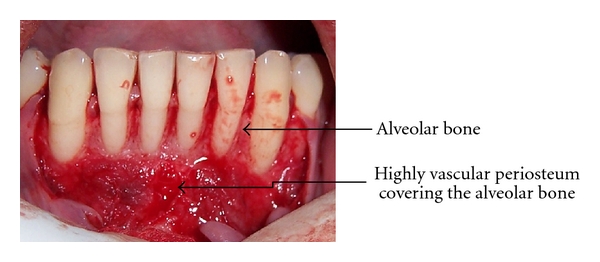
Highly vascular periosteum covering the alveolar bone.
Figure 2.
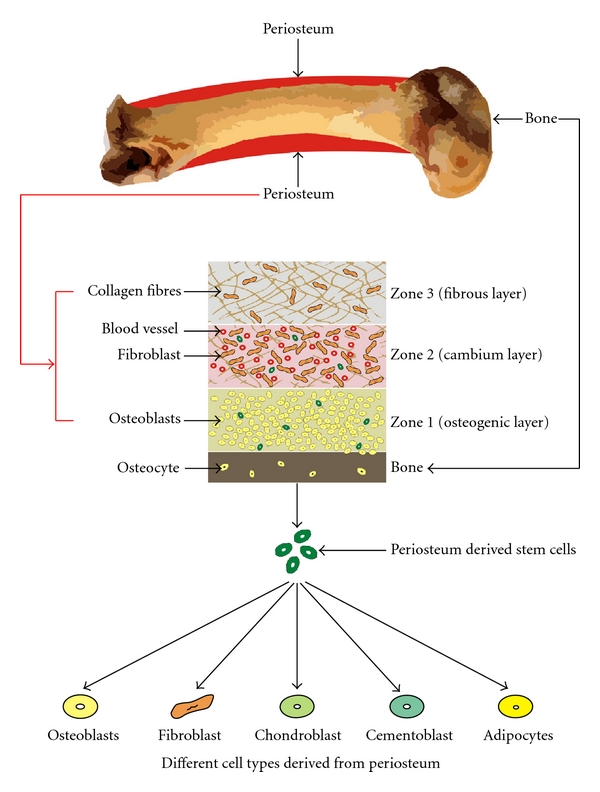
The three different Zones of periosteum; Zone 1 has an average thickness of 10–20 um consisting predominantly of osteoblasts; the majority of cells in Zone 2 are fibroblasts, with endothelial cells being most of the remainder. Zone 3 has the highest volume of collagen fibrils among all the three zones. The bottom of the figure shows regenerative capacity of the periosteum to form different cell types.
The structure of periosteum varies with age. In infants and children it is thicker, more vascular, active, and loosely attached as compared to adults where it is thinner, less active, and firmly adherent [8]. In all age groups, the cells of the periosteum retain the ability to differentiate into fibroblasts, osteoblasts, chondrocytes, adipocytes, and skeletal myocytes. The tissues produced by these cells include cementum with periodontal ligament fibers and bone. The periosteum has a rich vascular plexus and is regarded as the “umbilical cord of bone” [9]. The vasculature system of the periosteum was first studied in detail by Zucman and later by Eyre-Brook [10].Bourke's studies showed that the capillaries supplying blood to bone reside within the cortex linking the medullary and periosteal vessels; a recent study has even shown that periosteal cells release vascular endothelial growth factor [11] which promote revascularization during wound healing. Recently, studies have reported the existence of osteogenic progenitors, similar to mesenchymal stem cells (MSCs), in the periosteum [12, 13]. Under the appropriate culture conditions, periosteal cells secrete extracellular matrix and form a membranous structure [14]. The periosteum can be easily harvested from the patient's own oral cavity, where the resulting donor site wound is invisible. Owing to the above reasons, the periosteum offers a rich cell source for bone tissue engineering; hence, the regenerative potential of periosteum is immense.
3. Periosteum as a Tool in Medicine and Dentistry
Developing bone substitutes for bone defect repair has inspired orthopedic surgeons, bone biologists, bioengineering researchers to work together in order to design and develop the promising products for clinical applications. Duhame in the year 1742 can be considered the first investigator to study the osteogenic potential of periosteum and published his findings in the article “Sur le Development et la Crueded Os des Animaux” [15]. A century later, another French surgeon, Ollier, discovered that the transplanted periosteum could induce de novo bone formation. One of the earliest experimental studies to demonstrate osteogenic potential of periosteum was that of Urist and McLean who reported that periosteum produced bone when transplanted to the anterior chamber of the eye of the rat [16]. Skoog subsequently introduced the use of periosteal flaps for closure of maxillary cleft defects in humans; he reported the presence of new bone in cleft defects within 3–6 months following surgery [17]. Since then, surgeons have reported the successful use of maxillary periosteal flaps [18, 19] as well as periosteal grafts from the tibia or rib. Melcher observed that new bone is laid down in parietal bone defects of rats and was deposited by periosteum that had not been previously elevated or disturbed in any other way [20], while other investigators have suggested that the contact between the periosteal flap or graft and the underlying bone is crucial to stimulation of osteogenesis [21, 22]. More recently, the osteogenic/chondrogenic capacity of periosteum and related mechanisms have been confirmed, and the underlying biology is better understood through a number of studies [23–40].
The use of periosteum in dentistry is not new. Various research papers have been published explaining the osteogenic potential of human periosteal grafts [41, 42]. The use of periosteum as a GTR has been suggested by many studies [43–46], although long-term results are still awaited to establish the regular and the most effective use of periosteal grafts as barrier membranes. The need for a graft, which has its own blood supply, which can be harvested adjacent to the recession defect in sufficient amounts without requiring any second surgical site and has a potential of promoting the regeneration of lost periodontal tissue is a long-felt need. The adult human periosteum is highly vascular and is known to contain fibroblasts and their progenitor cells, osteoblasts and their progenitor cells, and stem cells. In all the age groups, the cells of the periosteum retain the ability to differentiate into fibroblasts, osteoblasts, chondrocytes, adipocytes, and skeletal myocytes. The tissues produced by these cells include cementum with periodontal ligament fibers and bone; in addition the presence of periosteum adjacent to the gingival recession defects in sufficient amounts make it a suitable graft. Recent papers published have shown promising results with the use of periosteum in the treatment of gingival recession defects (Figure 3) [47, 48]; moreover, with the advancement in tissue engineering techniques the periosteal derived stem cells have been grown effectively to reconstruct lost tissues. Periosteum-derived progenitor cells may serve as an optimal cell source for tissue engineering based on their accessibility, ability to proliferate rapidly, and capability to differentiate into multiple mesenchymal lineages. The periosteum is a specialized connective tissue that forms a fibrovascular membrane covering all bone surfaces except for that of articular cartilage, muscle, and tendon insertions and sesamoid bones. Cells residing within the periosteum may be excised from any number of surgically accessible bone surfaces; in addition, when properly stimulated, the periosteum has the potential to serve as a bioreactor supporting a dramatic increase in the progenitor cell population over the course of a few days. Further, once the cells are removed from the periosteum, they have the potential to proliferate at much higher rates than bone marrow, cortical bone, or trabecular bone-derived progenitor cells [49].
Figure 3.
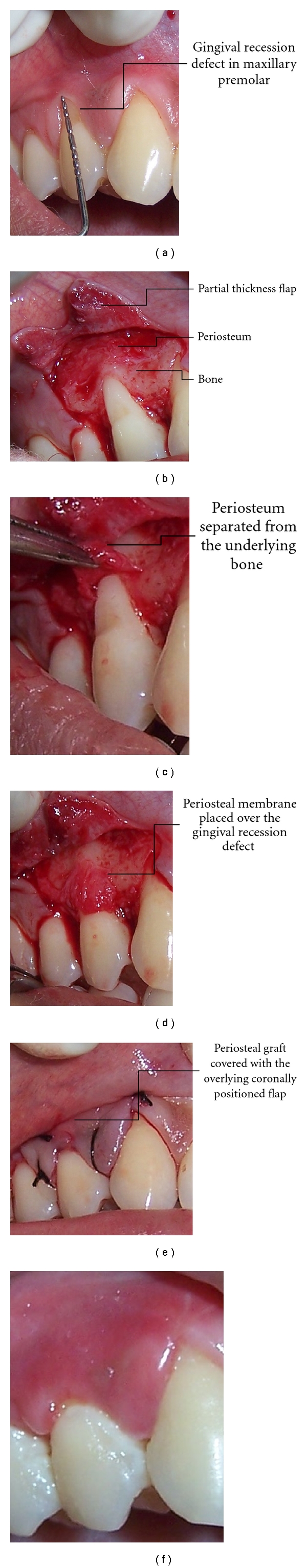
The use of periosteum for the treatment of gingival recession defect. (a) Clinical photograph showing gingival recession defect in relation to the maxillary first right premolar. (b) A partial thickness flap lifted to expose the underlying periosteum covering the alveolar bone. (c) The periosteum which is separated from the underlying bone. (d) The periosteum is used as a pedicle graft for covering the recession defect. (e) The periosteal graft is covered with the overlying coronally advanced flap which is sutured using 4–0 silk suture. (f) Satisfactory treatment outcome.
In addition to their robust proliferation aptitude, it is well established that periosteum-derived progenitor cells have the potential to differentiate into both bone and cartilage. Further, their potential for regenerating both bone and cartilage constructs is superior to that of adipose-derived progenitor cells and comparable with that of bone marrow-derived mesenchymal stem cells. A recent study by De Bari et al. indicates that periosteal progenitor cells are able to differentiate not only into bone and cartilage cells but also into adipocyte and skeletal myocyte cells [50]. There is a growing requirement for dentists to regenerate alveolar bone as a regenerative therapy for periodontitis and in implant dentistry. Concerning the donor site, it is easier for general dentists to harvest periosteum than marrow stromal cells, because they can access the mandibular periosteum during routine oral surgery [51]; also the regenerative potential of periosteum has been effectively used in “osteodistraction” which has the benefit of simultaneously increasing the bone length and the volume of surrounding tissues. Although distraction technology has been used mainly in the field of orthopedics, early results in humans indicated that the process can be applied to correct deformities of the jaw. These techniques are now utilized extensively by maxillofacial surgeons for the correction of micrognathia, midface, and fronto-orbital hypoplasia in patients with craniofacial deformities [52].
4. Conclusion
The use of periosteum can revolutionize the success of various dental treatments which require either bone or soft tissue regeneration; particularly the future use of periosteum must be explored in periodontal and implant surgical procedures. Although the regenerative potential of periosteum has been proved by numerous studies, till date the use of periosteum-derived grafts has still not become a standard tool in the armamentarium of dental surgeons, and it may still need some time, and further research before the full regenerative potential of periosteum is utilized.
Acknowledgments
I would like to acknowledge the help of Mr. Vinay Chandla, VanChum, The Mall, Shimla for his technical support. I also thank my wife Dr. Poonam mahajan for her support in editing this manuscript.
References
- 1.Finley JM, Acland RD, Wood MB. Revascularized periosteal grafts. A new method to produce functional new bone without bone grafting. Plastic and Reconstructive Surgery. 1978;61(1):1–6. doi: 10.1097/00006534-197801000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Reynders P, Becker J, Broos P. The osteogenic potential of free periosteal autografts in tibial fractures with severe soft tissue damage: an experimental study. Acta Orthopaedica Belgica. 1998;64(2):184–192. [PubMed] [Google Scholar]
- 3.Reynders P, Becker J, Broos P. Osteogenic ability of freeperiosteal autografts in tibial fracture with severe soft tissue damage. Journal Of Orthopaedic Trauma. 1999;13:121–128. doi: 10.1097/00005131-199902000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Provenza DV, Seibel W. Basic Tissues, Oral Histology Inheritance and Development. 2nd edition. Lea and Feibger; 1986. [Google Scholar]
- 5.Orban BJ, Bhaskar SN. Orbans Oral Histology and Embryology. 11th edition 2002. [Google Scholar]
- 6.Tran Van PT, Vignery A, Baron R. Cellular kinetics of the bone remodeling sequence in the rat. Anatomical Record. 1982;202(4):445–451. doi: 10.1002/ar.1092020403. [DOI] [PubMed] [Google Scholar]
- 7.Squier CA, Ghoneim S, Kremenak CR. Ultrastructure of the periosteum from membrane bone. Journal of Anatomy. 1990;171:233–239. [PMC free article] [PubMed] [Google Scholar]
- 8.Fan W, Crawford R, Xiao Y. Structural and cellular differences between metaphyseal and diphyseal periosteumin different aged rats. Bone. 2008;42(1):81–89. doi: 10.1016/j.bone.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 9.Chanavaz M. The periosteum: the umbilical cord of bone. Quantification of the blood supply of cortical bone of periosteal origin. Revue de Stomatologie et de Chirurgie Maxillo-Faciale. 1995;96(4):262–267. [PubMed] [Google Scholar]
- 10.Eyre-Brook AL. The periosteum: its function reassessed. Clinical Orthopaedics and Related Research. 1984;189:300–307. [PubMed] [Google Scholar]
- 11.Bourke HE, Sandison A, Hughes SPF, Reichert ILH. Vascular Endothelial Growth Factor (VEGF) in human periosteum normal expression and response to fracture. Journal of Bone and Joint Surgery. 2003;85:p. 4. [Google Scholar]
- 12.Tenenbaum HC, Heersche JNM. Dexamethasone stimulates osteogenesis in chick periosteum in vitro. Endocrinology. 1985;117(5):2211–2217. doi: 10.1210/endo-117-5-2211. [DOI] [PubMed] [Google Scholar]
- 13.Zohar R, Jaro S, Christopher A, McCulloch G. Characterization of stromal progenitor cells enriched by flow cytometry. Blood. 1997;90(9):3471–3481. [PubMed] [Google Scholar]
- 14.Mizuno H, Hata KI, Kojima K, Bonassar LJ, Vacanti CA, Ueda M. A novel approach to regenerating periodontal tissue by grafting autologous cultured periosteum. Tissue Engineering. 2006;12(5):1227–1235. doi: 10.1089/ten.2006.12.1227. [DOI] [PubMed] [Google Scholar]
- 15.Hutmacher DW, Sittinger M. Periosteal cells in bone tissue engineering. Tissue Engineering. 2003;9(1, supplement):S45–S64. doi: 10.1089/10763270360696978. [DOI] [PubMed] [Google Scholar]
- 16.Urist MR, McLean FC. Osteogenetic potency and new-bone formation by induction in transplants to the anterior chamber of the eye. The Journal of Bone and Joint Surgery. 1952;34(2):443–476. [PubMed] [Google Scholar]
- 17.Skoog T. The use of periosteal flaps in the repair of clefts of the primary palate. The Cleft Palate Journal. 1985;2:332–339. [PubMed] [Google Scholar]
- 18.O’Brien BM. The maxillary periosteal flap in primary palate surgery. Australian and New Zealand Journal of Surgery. 1970;40(1):65–70. doi: 10.1111/j.1445-2197.1970.tb04029.x. [DOI] [PubMed] [Google Scholar]
- 19.Rintala AE, Ranta R. Periosteal flaps and grafts in primary cleft repair: a follow-up study. Plastic and Reconstructive Surgery. 1989;83(1):17–22. doi: 10.1097/00006534-198901000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Melcher AH. Role of the periosteum in repair of wounds of the parietal bone of the rat. Archives of Oral Biology. 1969;14(9):1101–1109. doi: 10.1016/0003-9969(69)90079-x. [DOI] [PubMed] [Google Scholar]
- 21.Cestero HJ, Salyer KE. Regenerative potential of bone and periosteum. Surgical Forum. 1975;26:555–556. [PubMed] [Google Scholar]
- 22.Rauch F, Travers R, Glorieux FH. Intracortical remodeling during human bone development-A histomorphometric study. Bone. 2007;40(2):274–280. doi: 10.1016/j.bone.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Seeman E. Periosteal bone formation—a neglected determinant of bone strength. New England Journal of Medicine. 2003;349(4):320–323. doi: 10.1056/NEJMp038101. [DOI] [PubMed] [Google Scholar]
- 24.Emans PJ, Surtel DAM, Frings EJJ, Bulstra SK, Kuijer R. In vivo generation of cartilage from periosteum. Tissue Engineering. 2005;11(3-4):369–377. doi: 10.1089/ten.2005.11.369. [DOI] [PubMed] [Google Scholar]
- 25.Eyckmans J, Luyten FP. Species specificity of ectopic bone formation using periosteum-derived mesenchymal progenitor cells. Tissue Engineering. 2006;12(8):2203–2213. doi: 10.1089/ten.2006.12.2203. [DOI] [PubMed] [Google Scholar]
- 26.Hadjiargyrou M, Ahrens W, Rubin CT. Temporal expression of the chondrogenic and angiogenic growth factor CYR61 during fracture repair. Journal of Bone and Mineral Research. 2000;15(6):1014–1023. doi: 10.1359/jbmr.2000.15.6.1014. [DOI] [PubMed] [Google Scholar]
- 27.Romana M, Masqelet A. Vascularized periosteum associated with cancellous bone graft: An experimental study. Plastic and Reconstructive Surgery. 1990;85:587–592. doi: 10.1097/00006534-199004000-00014. [DOI] [PubMed] [Google Scholar]
- 28.Iwasaki M, Nakahara H, Nakata K, Nakase T, Kimura T, Ono K. Regulation of proliferation and osteochondrogenic differentiation of periosteum-derived cells by transforming growth factor-β and basic fibroblast growth factor. Journal of Bone and Joint Surgery. 1995;77(4):543–554. doi: 10.2106/00004623-199504000-00007. [DOI] [PubMed] [Google Scholar]
- 29.O’Driscoll SW. Articular cartilage regeneration using periosteum. Clinical Orthopaedics and Related Research. 1999;(367):S186–S203. doi: 10.1097/00003086-199910001-00020. [DOI] [PubMed] [Google Scholar]
- 30.Sakata Y, Ueno T, Kagawa T, et al. Osteogenic potential of cultured human periosteum-derived cells—a pilot study of human cell transplantation into a rat calvarial defect model. Journal of Cranio-Maxillofacial Surgery. 2006;34(8):461–465. doi: 10.1016/j.jcms.2006.07.861. [DOI] [PubMed] [Google Scholar]
- 31.Schantz JT, Hutmacher DW, Ng KW, Khor HL, Lim TC, Teoh SH. Evaluation of a tissue-engineered membrane-cell construct for guided bone regeneration. International Journal of Oral and Maxillofacial Implants. 2002;17(2):161–174. [PubMed] [Google Scholar]
- 32.Ueno T, Kagawa T, Fukunaga J, Mizukawa N, Sugahara T, Yamamoto T. Evaluation of osteogenic/chondrogenic cellular proliferation and differentiation in the xenogeneic periosteal graft. Annals of Plastic Surgery. 2002;48(5):539–545. doi: 10.1097/00000637-200205000-00016. [DOI] [PubMed] [Google Scholar]
- 33.Wiesmann HP, Nazer N, Klatt C, Szuwart T, Meyer U. Bone tissue engineering by primary osteoblast like cells in a monolayer system and 3-dimensional collagen gel. Journal of Oral and Maxillofacial Surgery. 2003;61(12):1455–1462. doi: 10.1016/j.joms.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Xie C, Lin ASP, et al. Periosteal progenitor cell fate in segmental cortical bone graft transplantations: implications for functional tissue engineering. Journal of Bone and Mineral Research. 2005;20(12):2124–2137. doi: 10.1359/JBMR.050806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simon TM, Van Sickle DC, Kunishima DH, Jackson DW. Cambium cell stimulation from surgical release of the periosteum. Journal of Orthopaedic Research. 2003;21(3):470–480. doi: 10.1016/S0736-0266(02)00206-1. [DOI] [PubMed] [Google Scholar]
- 36.Ueno T, Kagawa T, Fukunaga J, et al. Regeneration of the mandibular head from grafted periosteum. Annals of Plastic Surgery. 2003;51(1):77–83. doi: 10.1097/01.SAP.0000054180.78960.15. [DOI] [PubMed] [Google Scholar]
- 37.Ng AMH, Saim AB, Tan KK, et al. Comparison of bioengineered human bone construct from four sources of osteogenic cells. Journal of Orthopaedic Science. 2005;10(2):192–199. doi: 10.1007/s00776-004-0884-2. [DOI] [PubMed] [Google Scholar]
- 38.Hammarstrom L. Enamel matrix, cementum development and regeneration. Journal of Clinical Periodontology. 1997;24:658–668. doi: 10.1111/j.1600-051x.1997.tb00247.x. [DOI] [PubMed] [Google Scholar]
- 39.Nakahara H, Bruder SP, Goldberg VM, Caplan AI. In vivo osteochondrogenic potential of cultured cells derived from the periosteum. Clinical Orthopaedics and Related Research. 1990;(259):223–232. [PubMed] [Google Scholar]
- 40.Nakahara H, Bruder SP, Haynesworth SE, et al. Bone and parallels in fibroblast and periosteal cell differentiation cartilage formation in diffusion chambers by subcultured cells derived from the periosteum. Bone. 1990;11:p. 181. doi: 10.1016/8756-3282(90)90212-h. [DOI] [PubMed] [Google Scholar]
- 41.Taba Jr. M, Jin Q, Sugai JV, Giannobile WV. Current concepts in periodontal bioengineering. Orthodontics and Craniofacial Research. 2005;8:292–302. doi: 10.1111/j.1601-6343.2005.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tobon-Arroyave SI, Dominguez-Mejia JS, Florez-Moreno GA. Periosteal grafts as barriers in periradicular surgery: report of two cases. International Endodontic Journal. 2004;37(9):632–642. doi: 10.1111/j.1365-2591.2004.00855.x. [DOI] [PubMed] [Google Scholar]
- 43.Lekovic V, Kenney EB, Carranza FA, Martignoni M. The use of autogenous periosteal grafts as barriers for the treatment of Class II furcation involvements in lower molars. Journal of Periodontology. 1991;62(12):775–780. doi: 10.1902/jop.1991.62.12.775. [DOI] [PubMed] [Google Scholar]
- 44.Lekovic V, Klokkevold PR, Camargo PM, Kenney EB, Nedic M, Weinlaender M. Evaluation of periosteal membranes and coronally positioned flaps in the treatment of Class II furcation defects: a comparative clinical study in humans. Journal of Periodontology. 1998;69(9):1050–1055. doi: 10.1902/jop.1998.69.9.1050. [DOI] [PubMed] [Google Scholar]
- 45.Kwan SK, Lekovic V, Camargo PM, et al. The use of autogenous periosteal grafts as barriers for the treatment of intrabony defects in humans. Journal of Periodontology. 1998;69(11):1203–1209. doi: 10.1902/jop.1998.69.11.1203. [DOI] [PubMed] [Google Scholar]
- 46.Gamal AY, Mailhot JM. A novel marginal periosteal pedicle graft as an autogenous guided tissue membrane for the treatment of intrabony periodontal defects. Journal of the International Academy of Periodontology. 2008;10(4):106–117. [PubMed] [Google Scholar]
- 47.Mahajan A. Treatment of multiple gingival recession defects using periosteal pedicle graft: a case series. Journal of Periodontology. 2010;81(10):1426–1431. doi: 10.1902/jop.2010.100134. [DOI] [PubMed] [Google Scholar]
- 48.Mahajan A. Periosteal pedicle graft for the treatment of gingival recession defects: a novel technique. Australian Dental Journal. 2009;54(3):250–254. doi: 10.1111/j.1834-7819.2009.01128.x. [DOI] [PubMed] [Google Scholar]
- 49.Arnsdorf EJ, Jones LM, Carter DR, Jacobs CR. The periosteum as a cellular source for functional tissue engineering. Tissue Engineering. 2009;15(9):2637–2642. doi: 10.1089/ten.tea.2008.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Bari C, Dell’Accio F, Vanlauwe J, et al. Mesenchymal multipotency of adult human periosteal cells demonstrated by single-cell lineage analysis. Arthritis and Rheumatism. 2006;54(4):1209–1221. doi: 10.1002/art.21753. [DOI] [PubMed] [Google Scholar]
- 51.Agata H, Asahina I, Yamazaki Y, et al. Effective bone engineering with periosteum-derived cells. Journal of Dental Research. 2007;86(1):79–83. doi: 10.1177/154405910708600113. [DOI] [PubMed] [Google Scholar]
- 52.Chin M, Toth BA. Distraction osteogenesis in maxillofacial surgery using internal devices: review of five cases. Journal of Oral and Maxillofacial Surgery. 1996;54(1):45–53. doi: 10.1016/s0278-2391(96)90303-1. [DOI] [PubMed] [Google Scholar]


