Summary
Recent genome-wide association studies (GWAS) identified genetic loci associated with pigmentation, nevi, and skin cancer. We performed a review and meta-analysis of GWAS results, grouping them into four categories: (1) loci associated with pigmentation (hair, eye and/or skin color), cutaneous UV-response (sun sensitivity and/or freckling), and skin cancer; (2) loci associated with nevi and melanoma; (3) loci associated with pigmentation and/or cutaneous UV-response, but not skin cancer; and (4) loci distinctly associated with skin cancer, mostly basal cell carcinoma (BCC), but not pigmentation or cutaneous UV-response. These findings suggest at least two pathways for melanoma development (via pigmentation and via nevi), and two pathways for BCC development (via pigmentation and independent of pigmentation). However, further work is necessary to separate the association with skin cancer from the association with pigmentation. As with any GWAS, the identified loci may not include the causal variants and need confirmation by direct genome sequencing.
Keywords: GWAS (genome-wide association study), pigmentation, skin cancer, melanoma, basal cell carcinoma, nevi, meta-analysis
Introduction
An important goal in improving our understanding of skin cancer is to identify mechanisms accounting for increased inherited susceptibility. Basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) are the most common types of skin cancer, and melanoma accounts for the most skin cancer deaths. Several high-penetrance loci for melanoma and basal cell carcinoma have been identified in melanoma-prone families and in families with nevoid basal cell carcinoma syndrome (NBCCS), respectively, including cyclin-dependent kinase inhibitor 2A (CDKN2A coding for p16 and p14ARF) and cyclin-dependent kinase 4 (CDK4) for melanoma and the human homolog of the Drosophila segment polarity gene patched (PTCH1) for BCC. Other high-penetrance loci have been suggested for melanoma on chromosomes 1p22 and 1p36, but the causal genes are yet unidentified (Bale et al., 1989; Gillanders et al., 2003; and Hussein et al., 2003). CDKN2A alterations account for approximately 20%-40% of melanomas occurring in families with three or more affected individuals (Kefford et al., 1999 and Goldstein et al., 2006), and CDK4 and PTCH1-related NBCCS only account for a small percentage of melanomas (Goldstein et al., 2006) and BCCs (Epstein 2008), respectively. Thus, environmental and other genetic factors likely account for the remaining risk.
The remaining genetic risks may be due to low penetrance susceptibility genes, such as the melanocortin-1 receptor (MC1R) gene. First recognized as a gene affecting pigmentation in animals, MC1R variants are associated with pigmentary phenotypes in humans as well, including red hair, pale skin, freckling, and sun sensitivity (reviewed in Rees 2003 and Rees 2004). These phenotypes are known to be associated with skin cancer risk, and MC1R variants are indeed associated with melanoma, BCC, and SCC; interestingly, the risk associated with melanoma remains significant after adjustment for pigmentation, suggesting that MC1R contributes to melanoma risk beyond the pigmentary phenotype or that residual confounding from pigmentation cannot be completely ruled out (Bastiaens et al., 2001; Box et al., 2001; Kennedy et al., 2001; Landi et al., 2005; Liboutet et al., 2006; Matichard et al., 2004; Palmer et al., 2000; Raimondi et al., 2008; Stratigos et al., 2006; Valverde et al., 1996, reviewed in Gerstenblith et al., 2007 and Rees 2006).
Subsequent studies examined other pigmentation genes. Animal studies identified associations with pigmentation for the agouti signaling protein (ASIP) and solute carrier family 24, member 5 (SLC24A5) genes; candidate gene studies in humans confirmed these associations with pigmentation (Graf et al., 2005; Kanetsky et al., 2002; Lamason et al., 2005; and Norton et al., 2007). Studies of the four types of oculocutaneous albinism, disorders of melanin synthesis characterized by light skin, hair, and eye pigmentation among other features, have identified additional pigmentation genes, including tyrosinase (TYR), tyrosinase-related protein 1 (TYRP1), oculocutaneous albinism type 2 gene (OCA2, previously called P gene), and solute carrier family 45, member 2 (SLC45A2, previously called membrane-associated transporter protein, MATP) (reviewed in Tomita and Suzuki, 2004). Patients with certain types of oculocutaneous albinism experience sun sensitivity and are at increased risk of skin cancer, particularly nonmelanoma skin cancers; melanomas, often amelanotic, are much less commonly reported (Luande et al. 1985; Perry and Silverberg, 2001; Terenziani et al. 2003; and Yakubu and Mabogunje, 1993). In population-based studies using candidate-gene approaches, an SLC45A2 variant was associated with dark hair, dark skin, and protection from melanoma (Fernandez et al., 2008; Graf et al., 2005; Guedj et al., 2008; and Nakayama et al., 2002). OCA2 variants were associated with melanoma in other studies (Duffy, et al., 2010 and Jannot et al., 2005), while ASIP was found to modify melanoma risk in the presence of MC1R variants (Landi et al., 2005).
Genome-wide association studies (GWAS) were conducted to possibly identify other genomic loci associated with pigmentation and skin cancer. GWAS can identify common, low-penetrance susceptibility loci without prior hypotheses on the role of specific genes. We performed a review and meta-analysis of summary results from GWAS and replication studies examining skin cancers and their major risk factors, including pigmentation, cutaneous UV-response (including sun sensitivity and/or freckling), and nevi. Using large populations with mostly Caucasian individuals, GWAS confirmed associations already known, such as MC1R with pigmentation and skin cancer, and also identified chromosomal regions previously not known to be associated with pigmentation and/or skin cancer. By including data from multiple studies in one analysis, we can better capture loci that have significant associations with pigmentation traits and susceptibility to melanoma and non-melanoma skin cancers distinctly and in combination. This allows further understanding of the complex interactions of pigmentation, nevi, and skin cancer.
Results
We identified twelve studies that performed genome-wide analysis examining pigmentation traits, cutaneous UV-response, nevi, melanoma, basal cell carcinoma, and squamous cell carcinoma in human populations during the time from the first reports in 2007 through November 1, 2009 (Tables Ia and Ib) (Bishop et al., 2009; Brown et al., 2008; Falchi et al., 2009; Han et al., 2008; Kayser et al., 2008; Nan et al., 2009b; Rafnar et al., 2009; Stacey et al., 2008; Stacey et al., 2009; Stokowski et al., 2007; Sulem et al., 2007; and Sulem et al., 2008). All studied Caucasian individuals except one that examined a South Asian population (Stokowski et al., 2007). In addition to searching for novel susceptibility loci, several of these studies also investigated the associations of previously identified single nucleotide polymorphisms (SNPs) with additional phenotypes (Bishop et al., 2009; Stacey et al., 2009; and Sulem et al., 2008). We also report data from four studies that did not conduct novel GWAS, but expanded or replicated findings from GWAS (Duffy et al., 2010 [epub August 2009]; Gathany et al., 2009; Gudbjartsson et al., 2008; and Nan et al., 2009a). A description of these studies is reported in Table Ic. An overview of the SNPs significantly associated with pigmentation traits including hair, eye, and skin color identified in GWAS and replicated within the same studies are presented in Table IIa. SNPs significantly associated with BCC or SCC identified in GWAS and replicated in the same or another study are presented in Table IIb. We also present detailed data for each significant SNP identified or genotyped in GWAS for hair color, eye color, skin color, sun sensitivity, freckling, nevi, and melanoma and replicated in at least one additional sample population from any study (Supplemental Tables Ia-g). SNPs with significant novel associations identified in the GWAS or replication studies are also included even if found in only one sample population; novel associations are shown in bold.
Table Ia.
Pigmentation GWAS
| Study reference | Study population and description | Initial GWAS | Replication | GWAS platform |
|---|---|---|---|---|
| Stokowski et al. 2007 | GWA pooling study for skin color. [1] Top ~2% SNPs were individually genotyped in original population and an independent replication sample. |
737 Individuals of South Asian descent |
231 Individuals of South Asian descent | DNA pooling using 266,851 log- range PCRs for 1,620,742 SNPs followed by individual genotyping |
| Sulem et al. 2007 | GWAS for variants associated with hair and eye color, sun sensitivity, and freckling. Replication performed in an independent sample from Iceland and a second Dutch population. [2] |
2986 Icelandic individuals | (1) 2718 Icelandic individuals (2) 1214 Dutch individuals |
Illumina HumanHap300 |
| Kayser et al. 2008 | Three independent GWAS of iris color. Replication performed in two independent samples. All groups with ancestry from the Netherlands. |
(1) 192 Distant Dutch relatives [3] (2) 733 Dutch relatives [3] (3) 481 Unrelated Dutch individuals |
(1) 2217 Dutch relatives [3] (2) 6056 Unrelated Dutch individuals |
(1) Affymetrix 500K Array Set (2) 250K Nsp Array from Affymetrix 500K Array Set (3) Illumina HumanHap 300 |
| Han et al. 2008 | GWAS of natural hair color in U.S. women. Replication performed in four additional studies from the U.S. and Australia with data on hair color and other pigmentation phenotypes. |
2287 U.S. women of European ancestry [4] |
(1) 870 U.S. women controls (ctls) from a skin cancer case-ctl study [4] (2) 3750 U.S. women from a diabetes case-ctl study [4] (3) 2405 U.S. men from a diabetes case- ctl study [4] (4) 1440 Parents of twins from a family- based Australian study [4] |
Illumina HumanHap550 |
| Nan et al. 2009b | GWAS of tanning response after UV exposure. Replication performed in three additional U.S. studies. |
2287 U.S. women of European ancestry |
1-3 Same as above (Han et al., 2008) | Illumina HumanHap550 |
| Sulem et al. 2008 | Expanded GWAS from Sulem et al. (2007) in Icelandic individuals. Eight GWAS: 3 for eye color (blue vs. green, blue vs. brown, and blue vs. non-blue); 2 for hair color (red vs. non-red and blond vs. brown); and 3 for skin pigmentation traits (sun sensitivity vs. no sun sensitivity, freckling vs. no freckling, and sun sensitivity + presence of freckling “burning and freckling” vs. no “burning and freckling). [2] |
5130 Icelandic individuals | (1) 2116 Icelandic individuals (2) 1214 Dutch individuals |
Illumina HumanHap300 |
Skin reflectance measurements were obtained using a Minolta chromameter (high reflectance = light skin).
Results from Sulem et al. (2008) are shown in Tables IIa, IIb, and Supplementary Tables Ia-g instead of results from this study because data from Sulem et al. (2007) were included in the larger Sulem et al. (2008) study.
Relatives are all living descendants and spouses of 22 couples who lived in a southwest region of the Netherlands with at least six children in the 19th century. Distant relatives are ≥ to 5 generations apart.
Sample numbers vary across the SNPs tested in the populations. The largest sample numbers are presented in this table.
Table Ib.
Nevi, Melanoma, and Non-melanoma Skin Cancer GWAS
| Study reference |
Study population and description | Initial GWAS | Replication | GWAS platform | Adjustment for known skin cancer risk factors |
||
|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | ||||
| Brown et al. 2008 | GWA pooling study performed in a melanoma population-based case-control sample of European descendants from Australia. Replication performed in: (1) same population as the initial GWAS, (2) melanoma cases and controls independently sampled from the same population, and (3) melanoma cases and controls from a population-based study of melanoma diagnosed before age 40 in Australians of European descent. [[1],[2]] |
864 Melanoma cases from Australia (pooled) |
864 Controls from Australia (pooled) |
Melanoma cases from: (1) 789 Australia (2) 725 Australia (3) 505 Australia |
Controls from: (1) 854 Australia (2) 797 Australia (3) 454 Australia |
Illumina HumanHap 550 |
Not done |
| Stacey et al. 2008 | GWAS performed in a basal cell carcinoma (BCC) case-control study in Iceland. Replication performed in additional BCC case- control studies in (1) Iceland and (2) Eastern Europe. [3] Significant loci were also examined for association in melanoma cases and controls from Iceland, Sweden, and Spain (3) as well as for association with pigmentation traits in 5130 Icelandic controls from Sulem et al. (2007) and Sulem et al. (2008). [[4]-[7]] |
930 BCC cases from Iceland |
33117 Controls from Iceland |
BCC cases from: (1) 703 Iceland (2) 513 Eastern Europe Melanoma cases from: (1) 565 Iceland (2) 1062 Sweden (3) 277 Spain |
Controls for BCC study from: (1) 2329 Iceland (2) 515 Eastern Europe Controls for melanoma study from: (1) 32061 Iceland (2) 538 Sweden (3) 1292 Spain |
Illumina HumanHap 300 and HumaCNV 370-duo Bead Arrays |
All variants tested for association with pigmentation, sun sensitivity, and freckling; no associations found so results not adjusted |
| Rafnar et al. 2009 | Follow-up study of Stacey et al. (2008) where GWAS of BCC case-control study was repeated with an increased sample size. Replication of the third strongest signal (rs401681) was performed in additional BCC case-control samples from (1) Iceland and (2) Eastern Europe and (3) a melanoma case-control sample from Iceland, Sweden, and Spain. [[2],[3]] |
1505 BCC cases from Iceland [8] |
28890 Controls from Iceland |
BCC cases from: (1) 744 Iceland [9] (2) 525 Eastern Europe Melanoma cases from: (1) 577 Iceland (2) 1056 Sweden (3) 748 Spain |
Controls for BCC study from: (1) 515 Eastern Europe ctls Controls for melanoma study from: (1) 28890 Iceland ctls (2) 522 Sweden ctls (3) 1427 Spain ctls |
Illumina HumanHap 300 and HumanCNV 370 -duo Bead Arrays |
Not done |
| Stacey et al. 2009 | Follow-up study of Stacey et al. (2008) and Rafnar et al. (2009) where 30 more high-ranking SNPs from the GWAS were investigated by genotyping in additional BCC cases from (1) Iceland and (2) a case-control sample from Eastern Europe controls. SNPs at 3 loci (KRT5, CDKN2A/B, and 7q32) were genotyped in BCC case-control samples from the (3) US and (4) Spain. Two other variants (in TERT-CLPTM1L and SLC45A2) were tested for association with BCC. These 5 loci were examined for associations with squamous cell carcinoma (SCC) (in two populations), melanoma (in six populations), and fair pigmentation traits in 6200 Icelandic individuals. [[5],[7],[10],[11]] This study was included in this table (not Table Ic) because it reported GWAS data that were not previously presented in Stacey et al. (2008) or Rafnar et al. (2009). These data were further combined with genotype data from an additional Icelandic population. |
930 BCC cases from Iceland |
34998 Controls from Iceland [12] |
BCC cases from: (1) 1843 Iceland (2) 528 Eastern Europe (3) 930 U.S. (4) 186 Spain Melanoma cases from: (1) 589 Iceland (2) 749 the Netherlands (3) 1065 Sweden (4) 152 Austria (5) 816 Spain (6) 564 Italy SCC cases from: (1) 438 Iceland (2) 710 US |
Controls for BCC study from: (1) 34998 Iceland [12] (2) 533 Eastern Europe (3) 849 U.S. (4) 1758 Spain Controls for melanoma study from: (1) 34998 Iceland (2) 1831 the Netherlands (3)2631 Sweden (4) 376 Austria (5)1703 Spain (6) 368 Italy Controls for SCC study from: (1) 34998 Iceland (2) 849 U.S. |
Illumina HumanHap 300 and HumanCNV 370 -duo Bead Arrays |
All variants tested for association with pigmentation, sun sensitivity, and freckling; association present only for SLC45A2 variant, but results not adjusted |
| Falchi et al. 2009 | GWAS for nevi performed in adult female twins from the Twins U.K. registry. Replication performed in an independent sample of adolescent twins of European ancestry from the Brisbane Twin Nevus Study [13] and two melanoma case-control studies: (1) melanoma cases and controls of northern European descendants from Australia, and (2)1734 incident melanoma cases + 123 melanoma cases with a positive family history from a Leeds melanoma case-control study of North U.K. and controls from the same study + controls from the Wellcome Trust. [[14],[15]] |
1524 Adult female twins from Australia for nevi |
Melanoma cases from: (1) 1734 Australia (2) 1397 U.K. For nevi: 4107 Adolescent twins from Australia |
Controls for melanoma study from: (1) 1811 Australia (2) 1070 (Leeds)+ 1395 (Wellcome Trust) |
Illumina HumanHap 300 |
Number of nevi was not associated with number of sunburns over lifetime and Fitzpatrick score in either sample, so results not adjusted |
|
| Bishop et al. 2009 | GWAS of melanoma cases from GenoMEL international consortium enriched by family history, multiple primary melanomas, or early onset of disease. Replication performed in two additional populations: (1) melanoma cases and controls from GenoMEL and (2) melanoma cases and controls from a population-based study from Leeds, U.K. Findings from three other GWAS studies (Gudbjartsson et al., 2008, Brown et al., 2008, and Falchi et al., 2009) were also replicated. [[2],[15]] |
1650 “Enriched” melanoma cases from GenoMEL [16] |
4336 Controls from France, U.K., and GenoMEL |
Melanoma cases from: (1) 1149 GenoMEL (“enriched cases”) [16] (2) 1163 U.K. |
Controls for melanoma study from: (1) 964 GenoMEL [16] (2) 903 U.K. |
Illumina HumanHap 300 |
Not done |
The same Australian melanoma case-control population (the Q-MEGA study) was used in Falchi et al. (2009) and Brown et al. (2008).
No information was provided on whether the melanoma lesions were in situ or invasive.
Eastern European sample includes individuals from Hungary, Romania, and Slovakia.
Pigmentation traits examined included eye color, hair color, propensity to freckle, and skin sensitivity to the sun (Fitzpatrick score).
Cases included in situ and invasive melanoma. Icelandic sample excluded mucosal and ocular melanoma; histology types not described for other samples.
Self-reported pigmentation data were used.
Sample numbers vary across the SNPs tested and phenotypes assessed. The largest sample numbers are presented in this table.
480 of these BCC cases were added using in silico genotyping, where known genotypes of relatives are used to provide information on BCC cases not genotyped.
Includes some cases used in the initial GWAS through in silico genotyping.
Pigmentation traits examined included eye color, hair color, skin color, skin sensitivity to the sun (Fitzpatrick score and reaction to acute and chronic sun exposure), and propensity to freckle.
Pigmentation data were obtained from Icelandic samples for all SNPs except one around SLC45A2, where pigmentation data were presented in samples from Iceland, Eastern Europe, Spain (including melanoma cases only), and the U.S.
A total of 34998 Icelandic controls were used for the initial GWAS and replication, though numbers for each were not specified.
Nevus count was evaluated using a similar protocol in initial and replication studies.
Cases included invasive melanoma only.
The same Leeds melanoma case-control population was used in the replications in Falchi et al. (2009) and Bishop et al. (2009).
Melanoma cases and controls from the GenoMEL consortium used in the initial GWAS include individuals of European ancestry from Sweden, Australia, the U.K., the Netherlands, France, Italy, and Spain. Melanoma cases and controls from the GenoMEL consortium used for replication include individuals of European ancestry from Sweden, Australia, the Netherlands, France, Italy, Eastern Europe, and Israel.
Table Ic.
Replication of Findings from Pigmentation and Skin Cancer GWAS
| Study reference |
Study population and description | Pigmentation | Melanoma | BCC | SCC | Adjustment for known skin cancer risk factors |
|||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | Cases | Controls | ||||
| Gudbjartsson et al. 2008 | Replication study of 11 variants at 8 loci recently identified from GWAS on hair, eye, and skin pigmentation (Sulem et al., 2007 and Sulem et al., 2008) and 8 MC1R variants tested for association with melanoma and BCC.[[1]] Variants at 3 of the 8 loci (ASIP, TYR and TYRP1) were further tested in an Eastern European sample of BCC cases and controls. [[2],[3]] |
(1) 810 from Iceland (2) 1033 from Sweden (3) 278 from Spain |
(1) 36723 from Iceland (2) 2650 from Sweden (3) 1297 from Spain |
(1) 1649 from Iceland (2) 514 from Eastern Europe |
(1) 33824 from Iceland (2) 522 from Eastern Europe |
Significant variants were adjusted for hair and eye color, freckling, and sun sensitivity in Icelandic individuals only |
|||
| Duffy et al. 2010 | Replication study of 21 SNPs in 8 pigmentation genes tested for association with pigmentation phenotypes[4] and melanoma in an Australian population-based melanoma case-control study. [5] |
4536 from Australia [4] |
1738 from Australia | 4517 from Australia | All variants adjusted for hair, eye, and skin color and for MC1R genotype |
||||
| Nan et al. 2009a | Study of 15 SNPs in 8 candidate pigmentation genes tested for association with pigmentation and sun sensitivity phenotypes, melanoma, BCC, and SCC in a nested case-control study of U.S. Caucasians. [[5],[6]] |
1673 from U.S. [5] |
218 from U.S. | 870 from U.S. | 300 from U.S. | 870 from U.S. | 285 from U.S. |
870 from U.S. |
All variants adjusted for hair and skin color, tanning ability, MC1R variants, and other skin cancer risk factors[7] |
| Gathany et al. 2009 | Study of SNPs in the IRF4 gene tested for association with pigmentation and sun sensitivity phenotypes[8] and non-Hodgkin lymphoma (NHL) in non-Hispanic Caucasian controls from a multi- center U.S. study. |
1818 from U.S. [8] |
|||||||
Melanoma cases include invasive and in situ melanoma. In Gudbjartsson et al. (2008), melanoma cases from Spain include invasive melanoma only.
Sample numbers vary across the SNPs tested and phenotypes assessed. The largest sample numbers are presented.
Eastern European sample includes individuals from Hungary, Romania, and Slovakia.
Pigmentation traits examined included eye color, hair color, and skin color. Pigmentation traits examined only in subset of 1438 cases and 3098 controls with 100% Northern European ancestry.
No information was provided on whether the melanoma lesions were in situ or invasive.
Pigmentation traits examined in 803 melanoma, BCC, and SCC cases and 870 controls included hair color, skin color, and tanning ability in childhood and adolescence.
Additional skin cancer risk factors include: family history of skin cancer, number of lifetime severe sunburns that blistered, sunlamp or tanning salon use, cumulative sun exposure while wearing a bathing suit, and geographic regions.
Pigmentation traits examined in 990 NHL cases and 828 controls included eye color, hair color, skin color, tanning (skin reaction to first sun of season), and hours in mid-day sun in the last 10 years.
Table IIa.
SNPs significantly associated with hair color, eye color and skin color
| Chromosomal Locus | Gene Neighborhood |
SNP | Hair Color (p-value) |
Red Hair Color (p-value) |
Eye Color (p-value) |
Skin Color (p-value) |
|---|---|---|---|---|---|---|
| 5p13.3 | SLC45A2 | rs28777 | 1.2×10−17 [1] | |||
| rs16891982 (L374F) |
5.02×10−13 [2] | |||||
| 6p25-p23 | IRF4 | rs12203592 | 1.5×10−137 [1] | 6.1×10−13 [1] | 6.2×10−14 [1] | |
| IRF4/EXOC2 | rs1540771 | 2.1×10−11 [3] | ||||
| EXOC2 | rs6918152 | 6.1×10−8 [1] | ||||
| 9p23 | TYRP1 | rs1408799 | 5.9×10−17 [3] | |||
| 11q13.2 | TPCN2 | rs35264875 | 3.6×10−30 [3] | |||
| rs3829241 | 6.2×10−16 [3] | |||||
| 11q14-21 | TYR | rs1126809 (R402Q) |
8.7×10−17 [3] | |||
| rs1042602 (S192Y) |
6.54×10−11 [2] | |||||
| 12q21.33 | KITLG | rs12821256 | 3.1×10−38 [3] | |||
| 14q32 | SLC24A4 | rs12896399 | 6.0 × 10−62 [1] 1.9 × 10−70 [3] |
6.4×10−39 [3] | ||
| 15q11.2-12 | OCA2 | rs7495174 | 4.0×10−18 [1] | 8.9×10−19 [3] | ||
| rs4778211 | 3.7×10−8 [1] | |||||
| rs7174027 | 9.1 ×10−17 [1] | |||||
| rs11855019 | 1.5×10−24 [1] | |||||
| 15q13.1 | HERC2 | rs1667394 | 2.4 × 10−49 [3] | <10−300 [3] | ||
| 15q13.1 | HERC2 | rs12913832 | 4.2 × 10−103 [1] | |||
| rs7183877 | 5.1 × 10−15 [1] | |||||
| rs11636232 | 1.1 × 10−24 [1] | |||||
| rs8028689 | 9.2 × 10−17 [1] | |||||
| rs8039195 | 6.3×10−42 [[1],[4]] | |||||
| 15q21.1 | SLC24A5 | rs1834640 | 3.39×10−64 [2] | |||
| 16q24 | MC1R | rs1805007 (R151C) |
1.2 × 10−14 [3] | 8.8×10−236 [3] | ||
| rs1805008 (R160W) |
3.9 × 10−16 [3] | 1.4×10−162 [3] | ||||
| CDK10 | rs258322 | 7.2×10−30 [1] | ||||
| ANKRD11 | rs2353033 | 2.8×10−10 [1] | ||||
| DPEP1 | rs164741 | 5.2×10−22 [1] | ||||
| CHMP1A | rs7188458 | 3.8×10−20[1] | ||||
| ZNF276 | rs7204478 | 1.3×10−22 [1] | ||||
| FANCA | rs7195066 | 8.5×10−13 [1] | ||||
| DEF8 | rs8049897 | 1.3×10−18 [1] | ||||
| AFG3L1 | rs4408545 | 1.4×10−19 [1] | ||||
| AFG3L1 | rs4238833 | 8.2×10−25 [1] | ||||
| Unknown | rs4785763 | 1.5×10−30 [1] | ||||
| GAS8 | rs2241039 | 2.4×10−7 [1] | ||||
| PRDM7 | rs7196459 | 2.0×10−17 [1] | ||||
| 20q11.2-12 | ASIP | ASIP Haplotype (rs1015362 and rs4911414) |
2.7 × 10−9 [3] |
Note: SNPs included are those that were significant in the initial GWAS and replicated at least once within the same study. P-values presented are the combined p-values from the initial GWAS and at least one replication within the same study. SNPs in bold are those with novel associations with pigmentation.
For this SNP, the reference allele is assumed based on agreement between Han et al. (2008) and Sulem et al. (2008).
Table IIb.
Skin Cancer: Basal Cell Carcinoma (BCC) and Squamous Cell Carcinoma (SCC)
| BCC | ||||||
|---|---|---|---|---|---|---|
| Chromosomal Locus |
Gene Neighborhood |
SNP | GWAS | Replications within GWAS | Combined (p-value) |
Other replications |
| 1p36 | PADI6 | rs7538876 [A] | OR=1.27 (CI=1.15, 1.41), p=1.9 × 10−6 [1] |
1. OR=1.23 (CI=1.08, 1.40), p=1.6×10−3 [1] 2. OR=1.33 (CI=1.11, 1.59), p =2.1 × 10−3 [1] |
OR=1.28 (CI=1.19, 1.37), p=4.4×10−12 |
|
| 1q42 | RHOU | rs801114[G] | OR=1.32 (CI=1.20, 1.46), p=5.0 × 10−8 [1] |
1. OR=1.23 (CI=1.08, 1.41), p=1.7 × 10−3 [1] 2. OR=1.25 (CI=1.04, 1.50), p =1.5×10−2 [1] |
OR=1.28 (CI=1.19, 1.37), p=5.9×10−12 |
|
| 5p13.3 | SLC45A2 | rs16891982 [G] | OR=1.97 (CI=1.63,2.38) p=1.6×10−12 [[2],[3]] | |||
| rs16891982 [C] | OR=0.61 (CI=0.33-1.11), p=0.10 [4] | |||||
| 5p15.33 |
TERT/
CLPTM1L |
rs2736098 [A] | OR=1.30 (CI=1.18-1.43), p=1.4 ×10−7 [[5],[6]] |
|||
| rs401681 [C] | OR=1.25, p=2.3 × 10−7 [5] | OR=1.20 (CI=1.13, 1.27), p=4.8×10−9 [2] |
||||
| 7q32 | KLF14 | rs157935 [T] | OR=1.23 (CI=1.15, 1.31), p=5.7×10−10 [[2],[7],[8]] |
|||
| 9p21 | CDKN2A/B | rs2151280 [C] | OR=1.19 (CI=1.12, 1.26), p=6.9×10−9 [2] |
|||
| 11q14-21 | TYR |
rs1126809 [A]
(R402Q) |
1. OR=1.14 (CI=1.06, 1.23), p=6.1×10−4 [9] 2. OR=1.04 (CI=0.84-1.27), p=0.74 [4] |
|||
| 12q11-q13 | KRT5 | rs11170164 [A] | OR=1.35 (CI=1.23, 1.50), p=2.1×10−9 [[2],[10]] |
|||
| 16q24 | MC1R | rs1805007 [T] (R151C) |
OR=1.33 (CI=1.17, 1.50), p=6.8×10−6 [[9],[11]] | |||
| 20q11.2-12 | ASIP |
ASIP Haplotype
(rs1015362[G] and rs4911414[T]) |
1. OR=1.09 (CI=0.87, 1.38) [12] |
1. OR=1.35 (CI=1.20, 1.53), p=1.2 ×10−6 [9] 2. OR=1.25 (CI=0.99, 1.58) [12] |
||
| SCC | ||||||
| Chromosome | Gene | SNP | GWAS | Replications within GWAS |
Combined
(p-value) |
Other replications |
| 5p13.3 | SLC45A2 |
rs16891982 [G]
(L374F) |
1. OR=2.71(CI=1.88,3.92) p=1.0×10−7 [2] | |||
Note: SNPs included are those from GWAS that have been replicated either in the same study or additional studies and are statistically significant (p<10−7). We also report SNPs from replication studies if novel significant associations with BCC and/or SCC were identified. In some cases, studies used different reference alleles for the same SNP; the reference alleles are listed to help interpret the direction of association. SNPs in bold are those with novel associations with BCC and/or SCC. GWAS = genome-wide association study. OR = odds ratio. CI = confidence interval. P = p-value. “Combined” = pooled results reported in the original publications. Other replications include studies that further tested the association with these SNPs and BCC and/or SCC.
Stacey et al. (2008) initial GWAS in Icelandic population; replicated in the first phase with an independent Icelandic sample and in the second phase with an Eastern European sample.
In Stacey et al. (2009), the association was not significant in the Icelandic sample.
In Stacey et al. (2009), this SNP was also genotyped, and the association met genome-wide significance for Iceland only but not Iceland and Eastern European populations combined (p = 1.3×10−5); therefore, results shown are for Iceland only. In this study, when the results were adjusted for rs401681[C], rs2736098 [A] was no longer significant.
In Stacey et al. (2009), for U.S. BCC and SCC samples, rs157935 could not be typed, so rs125124 was used as a surrogate SNP. Odds ratios for rs125124 in these samples were included in the analysis here for rs157935.
In Stacey et al. (2009), the effect of this SNP was dependent on paternal origin of the risk allele.
In Gudbjartsson et al. (2008), these variants were identified as being associated with pigmentation and were tested for association with BCC.
In Stacey et al. (2009), the investigation of KRT5 was expanded to include six more exonic polymorphisms common in Europeans that were genotyped in Icelandic, Eastern European and Spain BCC and control samples and were associated with BCC. The SNP rs641615 is in the same linkage disequilibrium block as rs11170164; in a multivariate analysis, the signal from rs641615 remained significant when adjusted for the effect of rs11170164.
Gudbjartsson et al. (2008) data from Icelandic population only. Other non-synonymous MC1R variants (D294H, R160W, D84E, R163Q, I155T, V92M, and V60L) tested were not significantly associated with BCC in the Icelandic sample; however, in a grouped analysis, having any of these variants, including R151C, was significantly associated with BCC (p = 7.5×10−7).
The ASIP haplotype was also examined in Nan et al., 2009a in BCC and SCC cases and controls. P-values for association were not reported.
The significant loci from the GWAS were grouped into four categories: (1) loci associated with pigmentation (hair, eye, and/or skin color), cutaneous UV-response (sun sensitivity and/or freckling), and skin cancer; (2) loci associated with nevi and melanoma; (3) loci associated with pigmentation and/or cutaneous UV-response, but not skin cancer; and (4) loci associated with skin cancers, mostly basal cell carcinoma (BCC), but not pigmentation, or cutaneous UV-response. Table III shows overall associations with pigmentation, sun sensitivity, freckling, nevi, and skin cancer phenotypes for the genes located in the chromosomal regions identified by GWAS and replication studies; both significant and null associations are included for a complete synthesis of all results reported to date.
Table III.
Associations with pigmentation, sun sensitivity, freckling, nevi, and skin cancer for genes located in chromosomal regions identified by GWAS and replication studies
| Chromosomal Locus |
Gene Neighborhood | Pigmentation (Hair Color, Eye Color, Skin Color) |
Sun Sensitivity | Freckling | Nevi (Total Body Nevus Count) |
Melanoma | BCC | SCC |
|---|---|---|---|---|---|---|---|---|
| 1p36 | PADI6 1 | − (H,E) | − | − | − | ++ | − | |
| 1q42 | RHOU 1 | − (H,E) | − | − | − | ++ | − | |
| 5p13.3 | SLC45A2 2,3,14,15 | ++ (H,E,S) | ++ | − | ++ | ++ | ++ | |
| 5p15.33 | TERT/CLPTM1L 2,4 | − (H,E) | − | − | ++ (protective) | ++ | − | |
| 6p25-p23 |
IRF43/between EXOC2 and IRF45,6,13,16 |
++ (H,E,S) | ++ | ++ | − | − | ||
| 7q32 | KLF14 2 | − (H,E) | − | − | − | ++ | − | |
| 9p21 |
CDKN2A/B (adjacent to MTAP)2 |
− (H,E) | − | − | − 17 | ++ | − | |
| 9p21 | MTAP 7 | ++ | ++ | |||||
| 9p23 | TYRP1 5,7,8,15 | +(H);++ (E) | − | − | ++ | − | − | |
| 11q13.2 | TPCN2 5,6 | ++ (H) | − | − | − | − | ||
| 11q14-q21 | TYR 5,6,8-10,13-15 | +(H); ++ (E,S) | ++ | ++ | ++ | ++ | − | |
| 12q13 | KRT5 2 | − (H,E) | − | − | − | ++ | − | |
| 12q21.33 | KITLG 3,5,6,13 | ++ (H); −(E) | − | + | − | − | ||
| 14q32 | SLC24A4 3,5,6,9,13 | ++ (H, E) | ++ | − | − | − | ||
| 15q11.2-12 | OCA2 3,5,6,11,13,14 | ++ (H,E); + (S) | ++ | − | − | − | − | |
| 15q13.1 | HERC2 3,5,6,13 | ++ (H,E,S) | ++ | − | − | − | ||
| 15q21.1 | SLC24A5 10 | + (S) | − | − | − | |||
| 16q24.3 | MC1R 3,5,6,8,13-15 | ++ (H,RH), +(S) | ++ | ++ | ++ | ++ | ||
| 20q11.2-12 | ASIP 5,6,8,12,14,15 | +(H);++ (RH) | ++ | ++ | ++ | ++ | − | |
| 22q13.1 | PLA2G6 7,8 | ++ | ++ |
Note: H = hair color (non-red), E = eye color, S = skin color, RH = red hair color. BCC = basal cell carcinoma. SCC = squamous cell carcinoma. A double plus sign (++) indicates a significant association (p<10−7 for GWAS and p<0.05 for replication studies or lower if multiple SNPs were tested as in Nan et al. 2009a and Duffy et al. 2009) of the locus on the left column with the phenotype or skin cancer type on the top row. A single plus sign (+) in yellow indicates an association (p between 0.01 and 10−7) of the locus on the left column with the phenotype or skin cancer type on the top row. A minus sign (−) in blue indicates a null association (p>0.01) of the locus on the left column with the phenotype or skin cancer type on the top row. A blank cell indicates that that locus on the left column was not tested for an association with the phenotype or skin cancer type on the top row. The colors visually separate the loci groupings: yellow shading indicates loci associated with pigmentation (hair, eye and/or skin color), cutaneous UV-response (sun sensitivity and/or freckling), and skin cancer; purple shading indicates loci associated with nevi and melanoma; green shading indicates loci associated with pigmentation and/or cutaneous-UV response but not skin cancer; and blue shading indicates loci distinctly associated with skin cancer, mostly BCC, but not pigmentation or cutaneous UV-response. Red shading indicates a protective effect, which is opposite that of the effect on BCC.
Although CDKN2A/B was not associated with melanoma in GWAS, CDKN2A is a major melanoma susceptibility gene.
(1) Loci associated with pigmentation, cutaneous UV-response, and skin cancer
GWAS confirmed the known association of SNPs in MC1R, ASIP, TYR, TYRP1, SLC45A2, and OCA2 gene regions with pigmentation factors. SNPs in MC1R were associated with hair color (both red and light hair color), sun sensitivity, and freckling in the GWAS (Table IIa; Supplementary Tables Ia, b, e). In replication studies, Gudbjartsson et al. (2008) confirmed the previously-known associations of melanoma and BCC with MC1R,, and Duffy et al. (2010) confirmed associations with melanoma for two MC1R RHC variants, R151C and R160W (Table IIb; Supplementary Table Ig). The associations were not significant in Duffy et al. (2010), however, after adjustment for pigmentation.
SNPs in ASIP were associated with red hair color, sun sensitivity, and freckling (Table IIa; Supplementary Tables Ib, e). SNPs around MC1R and ASIP gene regions were the only ones significantly associated with red versus non-red hair color. A novel association with melanoma was found in the GWAS for loci around ASIP (Supplementary Table Ig). Replication studies further tested the association of ASIP SNPs with melanoma. In Gudbjartsson et al. (2008) and in a subset of cases and controls with 100% Northern European ancestry in Duffy et al. (2010), ASIP SNPs were associated with melanoma even after adjustment for pigmentation; Gudbjartsson et al. (2008) tested the ASIP haplotype (AH) rs1015362 and rs4911414, and Duffy et al. (2010) tested rs4911442. In Duffy et al. (2010), there was a suggestive pattern of interaction for MC1R and ASIP, as previously observed (Landi et al., 2005). In a third replication study, Nan et al. (2009a), tested the association of melanoma with three ASIP SNPs (rs1015362, rs4911414, and rs6058017) and AH (rs1015362 and rs4911414). The rs1015362 and rs6058017 SNPs and the ASIP haplotype had null associations with melanoma; rs4911414 had a suggestive association with melanoma risk but was not statistically significant.
Whereas Gudbjartsson et al. (2008) identified a novel association for BCC with the ASIP haplotype, Nan et al. (2009a) tested the association of rs1015362, rs4911414, rs6058017, and AH with BCC and SCC (Table IIb) and found that while rs1015362 and AH were not associated, rs4911414 and rs6058017 were nearly significantly associated with risk of BCC (in opposite directions); however, neither was significant after Bonferroni correction for multiple testing. The rs4911414 SNP was associated with SCC and there a suggestive association of rs6058017 with SCC, but these associations were not significant after Bonferroni correction (Nan et al. 2009a).
In GWAS, SNPs in TYR were associated with eye color, skin color, sun sensitivity, and freckling; in addition, a novel association with melanoma was found (Tables IIa; Supplementary Tables Ic-e, g). The association of TYR with melanoma was tested in replication studies. In Gudbjartsson et al. (2008), the TYR SNP rs1126809 (R402Q) was associated with melanoma after adjustment for pigmentation factors. In two other replication studies, however, although the direction of association was the same, this TYR SNP and rs1042602 (S192Y) were not associated with melanoma when adjusted for pigmentation factors (Duffy et al., 2010 and Nan et al., 2009a). In one replication study, the TYR SNP rs1126809 was significantly associated with BCC (Gudbjartsson et al., 2008) (Table IIb), while in Nan et al. (2009a) the association with BCC followed the same direction but was not significant. The TYR SNP rs1042602 was marginally associated with SCC, but the association was further diminished after Bonferroni correction (Nan et al., 2009a).
SNPs in TYRP1 were associated with eye color, and a novel association with melanoma was found in GWAS (Tables IIa; Supplementary Table Ic, g). Three replication studies also tested this association. In Gudbjartsson et al. (2008), the TYRP1 SNP rs1408799 was significantly associated with melanoma risk, even after adjustment for pigmentation factors in the Icelandic sample. In Nan et al. (2009a) and Duffy et al. (2010), the association of the same TYRP1 SNP with melanoma was confirmed, but the association was not statistically significant after Bonferroni correction for multiple comparisons in Nan et al. (2009a) and after adjustment for hair, eye, and skin color in Duffy et al. (2010).
In GWAS, SNPs in SLC45A2 were associated with light hair and skin color and sun sensitivity (Table IIa; Supplementary Tables Ia, d, e). Stacey et al. (2009) confirmed a previously identified association with melanoma. These results were confirmed in Duffy et al. (2010), with three SNPs showing significant associations with melanoma; after adjustment for pigmentation and Bonferroni correction, the SLC45A2 SNP rs16891982 remained significantly associated with melanoma. Nan et al. (2009a) also tested the association of SLC45A2 SNPs and melanoma; one SNP, rs13289, was associated with melanoma risk, but the association was not significant after Bonferroni correction. Stacey et al. (2009) identified novel associations of the SLC45A2 SNP rs16891982 with BCC and SCC (Table IIb; Supplementary Table Ig); in this study, the SLC45A2 SNP rs16891982 was associated with pigmentation factors, but results of the association with skin cancer were not adjusted for pigmentation. A subsequent replication study found no association for three SLC45A2 SNPs with either BCC or SCC (Nan et al., 2009a).
OCA2 SNPs were associated with eye color in GWAS (Table IIa; Supplementary Table 1c), confirming previous studies demonstrating that variation in eye color is linked to the OCA2 region (Duffy et al., 2007). OCA2 SNPs were not associated with melanoma in any GWAS. In a replication study, the OCA2 SNP rs1800407 was associated with melanoma but was not statistically significant after Bonferroni correction for multiple testing (Duffy et al., 2010). Other studies found an association with melanoma for this SNP and other OCA2 SNPs, but these studies were small (Fernandez et al., 2009 and Jannot et al., 2005). In Nan et al. (2009a), this OCA2 SNP was associated with BCC, but the association was not statistically significant after Bonferroni correction.
(2) Loci associated with nevi and melanoma
Two loci around the methylthioadenosine phosphorylase (MTAP, near CDKN2A/CDKN2B) and phospholipase A2, group VI (PLA2G6) genes were associated with nevi and melanoma (Supplementary Table If, g). The association between these genes and melanoma was no longer significant after adjustment for nevus count, suggesting that susceptibility loci for nevus count were mediating melanoma risk in this population. Nevus count has been associated with the 9p21 locus containing MTAP and CDKN2A in prior linkage studies (Falchi et al., 2006 and Zhu et al., 2007). GWAS did not confirm variants or loci previously associated with nevi by other methods, such as genome-wide linkage and candidate gene analysis on chromosomes 1, 2, 4, 6, 8, 16, 17 (Zhu et al., 2007), 5q31-32, and 2p24 (Falchi et al., 2006), and OCA2 and myosin VIIA (MYO7A) (Fernandez et al., 2009).
(3) Loci associated with pigmentation and/or cutaneous UV-response
The loci associated with pigmentation and/or cutaneous UV-response but not melanoma or BCC included five genes, namely solute carrier family 24, member 4 (SLC24A4), two-pore segment channel 2 (TPCN2), interferon regulatory factor 4/exocyst complex component 2 (IRF4/EXOC2), kit ligand (KITLG), and hect domain and RCC1-like domain 2 (HERC2) (Table IIa). Three of these, SLC24A4, TPCN2, and IRF4, are novel potential ‘pigmentation genes.’ Loci in and around SLC24A4 were associated with hair and eye color and sun sensitivity (Supplementary Tables Ia, c, e). The association of SLC24A4 SNPs with light hair color was replicated by Duffy et al. (2010), and a new association with blue eye color was reported in that study. SLC24A4 is in the same solute carrier family as the other ‘pigmentation genes’ SLC45A2 and SLC24A5, which are potassium-dependent sodium/calcium exchangers (Lamason et al., 2005 and Sulem et al., 2007). SNPs in SLC24A5 were associated with skin color but were not tested for association with skin cancers in these GWAS (Supplementary Table Id). In a replication study, SNPs around SLC24A5 were not associated with melanoma, BCC, or SCC (Nan et al. 2009a). In GWAS, SNPs around TPCN2 were associated with hair color only (Supplementary Table Ia). Interestingly, TPCN2 encodes a protein also involved in calcium transport (Sulem et al., 2008). The relationship between solute transport and pigmentation, however, is not well-defined.
Unlike other ‘pigmentation genes’ that associate with light (or dark) pigmentation traits uniformly (i.e. light hair, light eye, light skin color and poor tanning), the IRF4 minor variant rs12203592 [T] was associated with dark hair color but light eye and skin color and sun sensitivity in GWAS (Table IIa; Supplementary Tables Ia, c-e). The locus on chromosome 6p25.3 near IRF4 (between IRF4 and EXOC2 (exocyst complex component 2)) was also associated with freckling in another GWAS (Supplementary Table Ie). Two studies further tested these associations (Duffy et al., 2010 and Gathany et al., 2009). Both studies replicated the significant association with dark hair color; Duffy et al. (2010) also replicated the significant associations with light eye and skin color for IRF4, but these associations were not significant in Gathany et al. (2009) perhaps due to the smaller sample size (Supplementary Tables Ia, c, d). A meta-analysis for the association of the IRF4 SNP rs12203592 with hair color confirmed this finding (Supplementary Table Ia). This observation suggests that for this locus, the mechanism of pigmentation control for hair color may differ from that for eye and skin color. Gudbjartsson et al. (2008) found that the association of melanoma and BCC with the chromosome 6p25.3 locus was not significant. In Duffy et al. (2010), the IRF4 SNP rs12203592 was nearly significantly associated with melanoma in the Northern European subset but was not significant after adjustment for pigmentation (Supplementary Table Ig). The IRF4 gene encodes a B cell proliferation/differentiation protein and is a member of the interferon regulatory factor family of transcription factors, which are involved in regulating gene expression in response to interferon and other cytokines. IRF4 is also called MUM1 and was used in one study to detect melanocytic lesions (nevi and melanoma) pathologically (Sundram et al., 2003). In another study, it was found to stain hematolymphoid neoplasms and melanomas but not breast, prostate, or GI tumors (Natkunam et al., 2001).
In GWAS, SNPs around KITLG loci were associated with hair color only (Supplementary Table Ia). Prior to GWAS, KITLG was known to have a role in pigmentation. KITLG functions in melanogenesis and was found to affect pigmentation in animal models (Hultman et al., 2007; reviewed in Wehrle-Haller, 2003). The association of KITLG polymorphisms with pigmentation in humans was confirmed in a study of skin color (Miller et al., 2007). Furthermore, gain of function mutations in KITLG were recently found to cause Familial Progressive Hyperpigmentation, an autosomal dominant syndrome characterized by hyperpigmented patches of the skin that expand and increase with age (Wang et al., 2009).
SNPs in HERC2, a gene located close to OCA2, were associated with hair, eye, and skin color and sun sensitivity (Supplementary Tables Ia, c-e). Different SNPs from the same locus were also associated with eye color in a candidate gene study (Eiberg et al., 2008). Previously, OCA2 variants were thought to be the primary determinants of blue eye color; however, the HERC2 SNP rs12913832 predicted eye color significantly better than any OCA2 haplotype (Sturm et al., 2008). Variants in HERC2 are thought to lead to a decrease in expression of the adjacent OCA2 gene, especially within iris melanocytes (Sturm et al, 2008). Duffy et al. (2010) found no association with melanoma for HERC2.
(4) Loci associated with skin cancer only
Four novel loci identified from GWAS were distinctly associated with BCC and one with BCC and melanoma, but not with pigmentation, sun sensitivity, freckling, or other skin cancers. The loci associated with BCC only are located around the following genes: peptidylarginine deiminase, type VI (PADI6), ras homolog gene family, member u (RHOU), kruppel-like factor 14 (KLF14), and keratin 5 (KRT5) (Table IIb). The mechanisms by which these loci increase the risk for BCC are unknown, but these findings suggest a pathway or pathways independent of pigmentation and sun sensitivity. Cyclin-dependent kinase inhibitor 2A/B (CDKN2A/B) was associated with BCC but not melanoma in GWAS. However, CDKN2A is a high penetrance melanoma susceptibility gene, as discussed previously, and in some families with CDKN2A mutations, there is an increased risk of pancreatic cancer, breast cancer, and neural tumors in addition to melanoma (Bahuau et al., 1998; Borg et al., 2000; Goldstein et al., 2006; Hewitt et al., 2002; Petronzelli et al., 2001; Randerson-Moor et al., 2001; and Rizos et al., 2001).
In GWAS, a SNP around telomerase reverse transcriptase-CLPTM1-like protein (TERT-CLPTM1L) was also associated with an increased risk of BCC and a protective effect on melanoma. The TERT-CLPTM1L locus is particularly interesting in that it is associated with multiple malignancies, including glioma and cancer of the lung, urinary bladder, prostate, cervix, and pancreas (Landi et al., 2009; Petersen et al., 2010; Rafnar et al., 2009; Shete et al., 2009; and Wang et al., 2008). While this locus is associated with increased risk of these malignancies, it is protective for melanoma in cases from Iceland, Sweden, and Spain (Rafnar et al., 2008 and Stacey et al., 2009) (Supplementary Table Ig). Variants in this locus are hypothesized to affect telomere length (Rafnar et al., 2009); in one study, short telomeres were associated with an increased risk of BCC while long telomeres were associated with an increased risk of melanoma (Han et al., 2009). Further exploration of the functional correlates of this locus is required to understand the responsible mechanisms for these associations.
Meta-analysis
We performed a meta-analysis across the GWAS and replication studies to refine previous associations and potentially identify new loci for a given phenotype. When phenotypes were defined uniformly across the GWAS, as for red hair color and melanoma, the results for SNPs identified in more than one GWAS and/or replication study were combined in a meta-analysis (Supplementary Tables Ib, g). Although few new SNPs reached genome-wide significance in the meta-analysis and previous associations were refined, no new loci emerged as significant in the meta-analysis for either phenotype. Forest plots for the melanoma meta-analysis are shown for SNPs with significant associations in at least three studies in Figure 1. The meta-analysis was limited by several factors. First, the phenotypic definitions varied across studies. For example, non-red hair color, eye color, skin color, and sun sensitivity were defined differently across GWAS, limiting our ability to combine results for these phenotypes. Second, manuscripts reported only major GWAS results, often limited to statistically significant associations, precluding the possibility of combining results that could have reached significance in a meta-analysis. Thus, using the available published data and collaboration with the primary investigators of each study, we were able to combine results only for red hair color and melanoma.
Figure 1.
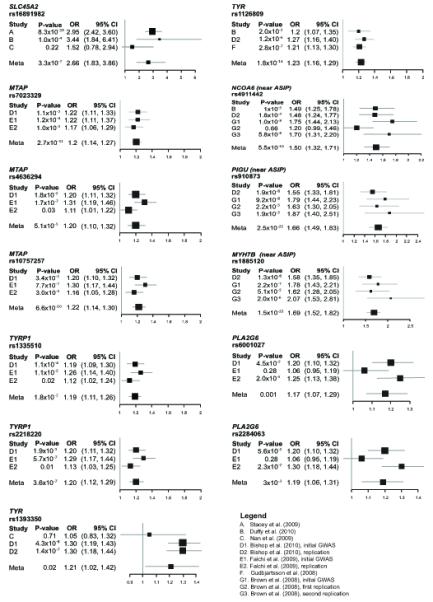
Association between SNPs and melanoma risk from meta-analysis of GWAS and replication studies. SNPs were included if reported in at least three studies. Fixed effects meta-analyses are used for rs7023329, rs1126809, rs910873, and rs1885120 SNPs, whose results showed no heterogeneity across studies; for the remaining SNPs, results based on random effects model are shown. Black squares indicate the odds ratios (OR), with the size of the square inversely proportional to its variance. Horizontal lines represent 95% confidence intervals (CI). All heterogeneity test results and estimates based on both fixed and random effects models for all SNPs shown here as well as meta-analysis results of SNPs based on two studies only are reported in Supplemental Table Ig.
In the meta-analysis for red hair color (Supplementary Table Ib), the strongest signals for red versus non-red hair color were attributed to loci on chromosome 16 near MC1R, particularly the well-known MC1R ‘red hair color’ (RHC) alleles (R151C, R160W, and D294H) known to result in diminished function of the protein (reviewed in Garcia-Borron et al., 2005). The melanoma meta-analysis also includes replication studies, which tested SNPs identified in previous GWAS for associations with melanoma. In the melanoma meta-analysis, there is some redundancy of the study populations for several SNPs as noted in Supplementary Table Ig, which could not be quantified. For example, both Bishop et al. (2009) and Falchi et al. (2009) replicated GWAS findings in samples from the Leeds melanoma case-control study; since Falchi et al. (2009) used more Leeds samples, we excluded the replication data from Bishop et al. (2009) in the meta-analysis calculations. Also, Brown et al. (2008) and Bishop et al. (2009) used samples from the Q-MEGA study, and the redundancy could not be corrected; therefore, for SNPs with data from these studies, the meta-analysis p-values may be slightly reduced. For example, SNPs around ASIP had the strongest associations with melanoma (Figure 1), but the p-values could be affected by the inclusion of the same subjects in some of the studies contributing to the meta-analysis.
Discussion
Genome-wide association studies of pigmentation, sun sensitivity, and skin cancer phenotypes have identified new loci associated with these phenotypes, some of which are shared and some of which are distinct. There are two main subsets of SNPs associated with melanoma risk, one associated with melanoma and pigmentation and one associated with melanoma and nevus count. SNPs in these subsets did not overlap, suggesting that there may be unique pathways to melanoma development. There also appears to be at least two pathways to BCC development: a pigmentation-dependent pathway and a pigmentation-independent pathway. Interestingly, all of the SNPs associated with both BCC and melanoma, except the one around TERT-CLPTM1L, are also associated with pigmentation, including SLC45A2, TYR, MC1R, and ASIP, suggesting that BCC and melanoma share a common pathway to development via pigmentation. An alternative possibility is that these SNPs are associated with pigmentation traits that predispose to skin cancer (melanoma and BCC) but that they do not themselves mechanistically lead to the development of skin cancer. A major limitation for the GWAS reviewed in this manuscript is that all but two skin cancer GWAS (Stacey et al., 2008 and Stacey et al., 2009) did not examine or adjust for skin cancer risk factors when reporting risk loci. Replication studies that adjusted for pigmentation did not consistently confirm associations for some SNPs with melanoma and BCC, including TYRP1, TYR, and ASIP (Duffy et al., 2010 and Nan et al., 2009a). Thus, future studies of these genes should focus on whether subjects carrying these SNPs are at increased risk of melanoma and/or BCC risk through pigmentation alone or through pathways independent of pigmentation.
In the GWAS of nevus count, it did appear that melanoma risk was mediated by nevi, since the association between SNPs and melanoma risk was diminished following adjustment for nevus count (Falchi et al., 2009). In this study, the number of nevi was not associated with frequency of sunburns or skin type; however, other pigmentation factors were not tested for association with nevus count. Similarly, SNPs found associated with nevus count were not tested for association with pigmentation or cutaneous UV-response. Therefore, we cannot definitively conclude that the association between nevus count and melanoma is independent of pigmentation or cutaneous UV-response. In future studies, pigmentation factors and other risk factors for melanoma should be fully accounted for so as to conclusively determine whether the genetic associations with skin cancer are direct or are mediated by pigmentation, nevi, or both. Further studies of the SNPs associated with BCC but not pigmentation may uncover novel pathways for BCC development. Finally, SNPs associated with pigmentation but not other phenotypes in these GWAS should be further examined and tested for skin cancer associations in additional populations.
GWAS have been a powerful tool for identifying novel loci associated with pigmentation and skin cancer phenotypes. Interestingly, many novel associations were found when significant loci identified in one GWAS were replicated for a different phenotype. It was difficult to perform a meta-analysis given varying phenotypic definitions of pigmentation and sun sensitivity traits, suggesting that future studies should employ consistent definitions and categorizations, when possible, to allow for data harmonization.
GWAS do have two major intrinsic limitations; first, only a relatively small number of SNPs are contained on the chip used for genotyping, leaving large numbers of SNPs untested for associations. Second, the identified loci may not be the causal variants, so associated SNPs are not necessarily causally related to the examined phenotype. Studies with MC1R demonstrate these limitations. The MC1R RHC alleles are not on the common platforms that were used for these GWAS. As noted for GWAS of red hair color, in all GWAS reviewed here, a signal on chromosome 16 near MC1R prompted further genotyping of the RHC alleles and multivariable analyses demonstrating that the signals were due to RHC alleles (Han et al., 2008; Nan et al., 2009b; Sulem et al., 2007; and Sulem et al., 2008). Interestingly, some of the MC1R SNPs identified in GWAS were not in linkage disequilibrium with the RHC alleles, suggesting that they acted independently; however, in multivariable analyses, only the association with RHC alleles remained significant. Therefore, GWAS results must be interpreted with caution: significant GWAS findings do not necessarily identify the causal variants or SNPs in linkage disequilibrium with the causal variants. Supporting this cautionary note is a recent study showing that the most significant SNP from a GWAS of a well-studied disease, sickle cell anemia, was located 9kb from the known causal variant; furthermore, these authors suggest that rare causal mutations may create ‘synthetic’ associations in GWAS that are credited to common variants (Dickson et al., 2010). This signifies the importance of prior knowledge of risk factors (like MC1R for melanoma), even in an apparent unbiased, or agnostic, approach and indicates that other tools such as direct genomic sequencing examining rarer variants should be considered in future studies.
In conclusion, although there are limitations, GWAS have provided important information regarding loci associated with pigmentation phenotypes and skin cancer. These data can guide future studies using sequencing and other techniques to identify causal variants, and generate hypotheses regarding biological mechanisms and functional consequences of the identified variants.
Methods
Data collection
Using the terms “genome wide melanoma,” “genome wide sun sensitivity,” “genome wide pigmentation,” genome wide nevus,” “genome wide nevi,” “genome wide basal cell carcinoma,” “genome wide squamous cell carcinoma,” and “genome wide skin cancer,” we conducted a PubMed literature search and identified 12 studies that performed genome-wide analyses examining pigmentation and cutaneous UV-response traits, nevi, melanoma, basal cell carcinoma, and squamous cell carcinoma of the skin in human populations during the time from the first study (Stokowski et al. 2007) through November 1, 2009. We also identified four studies that expanded upon or replicated findings from GWAS within the same time frame. We reported associations that were significant at a GWAS level corresponding to a p-value<1×10−7 and replicated in at least one other sample. In studies replicating GWAS findings, a p-value<0.05 was considered significant unless otherwise specified in the study. Given different phenotypic definitions, a meta-analysis was only possible for red hair color and melanoma phenotypes.
Statistical methods for meta-analysis
For each given SNP associated with red hair color and/or melanoma, a meta-analysis was performed to combine odds ratios for a reference allele weighted by the estimation certainty under a fixed effects model. In all of the studies except those using pooling, the test statistics were corrected for population stratification using principal component analysis or multidimensional scaling. For all studies reviewed in this manuscript, with the exception of Gudbjartsson et al. (2008), Rafnar et al. (2009), Stacey et al. (2008), Stacey et al. (2009), Sulem et al. (2007), and Sulem et al. (2008), there was no strong evidence of cryptic relatedness or substructure based on genome control (GC) values; hence, we have used the original odds ratios (ORs) and p-values in the meta-analysis. The studies performed by deCODE Genetics (Gudbjartsson et al., 2008; Rafnar et al., 2009; Stacey et al., 2008; Stacey et al., 2009; Sulem et al., 2007; and Sulem et al., 2008) reported p-values adjusted by GC values because of strong evidence of cryptic relatedness and substructure. We have used the reported, GC-corrected test results in the meta-analysis.
Many papers reported ORs, confidence interval (CIs) of ORs, and p-values. In some cases, studies used different reference alleles for the same SNP; the reference alleles are listed in the tables to help interpret the direction of association. Where original studies reported different alleles, the reference alleles used for the meta-analyses are specified in bold in the “Meta-analysis” column of the Tables (Supplementary Tables Ib, g). The natural weights used for combining ORs are their standard deviations (SDs), which were derived from the reported CIs. The approach is appropriate if the p-values were derived from Wald-tests based on ORs and their SDs. However, in our situation, the p-values in some papers were computed based on the likelihood ratio statistics (LRT) (Rafnar et al., 2009; Stacey et al., 2008; Stacey et al., 2009; and Stokowski et al., 2007). The LRT produces much more accurate p-values than the Wald test statistic for studies with a very high proportion of controls. In this case, the meta-analysis based on ORs produces incorrect p-values. Instead, we performed a meta-analysis by combining the reported p-values in four steps:
We converted the p-value pi in study i into a normal quantile Zi, with |Zi|=Φ−1(1-pi/2) and the sign determined by the direction of the tested allele. Here, Φ is the cumulative density function of the standard normal distribution.
We computed the positive weight as wi =|log(ORi)|/|zi|.
We computed the Z test statistic for the meta-analysis: The two-sided p-value for the meta-analysis is calculated as p = 2Φ(-|Z|).
- We computed the OR for the meta-analysis as
.
The meta-analysis plots reporting the association between SNPs and melanoma risk (Figure 1) were obtained using the R statistical package “rmeta.”
Heterogeneity and random effects meta-analysis
We computed Cochran’s Q statistic (Cochran, 1954) and I2 statistic (Higgins and Thompson, 2002) to quantify the heterogeneity effect across studies. Under the null hypothesis of no heterogeneity, the Cochran’s Q statistic follows a χ2 distribution with N-1 degree of freedom, where N is the number of studies to be combined. For SNPs with suggestive evidence of heterogeneity (I2>0%), we performed a meta-analysis under a random effects model (Higgins and Thompson, 2002). As expected, the p-values under the fixed effects model and the random effects model are very different when there is strong evidence of heterogeneity.
Meta-analysis limitations
Our analysis included only reported results for most of the reviewed SNPs. For a given SNP reported here, one or more GWAS may have failed to report the test results because the SNP was not genotyped, because the association between the SNP and the disease had been previously demonstrated to be significant, or because the SNP was not among the most significant in that study. For the latter two cases, potential reporting bias could be present in this analysis. However, for the SNPs reported in this manuscript, since existing data have provided very strong evidence of association, the potential reporting bias is unlikely to change the conclusion. Another limitation is that not all studies included in the meta-analysis reported data on all of the same SNPs; thus, we could only combine the SNPs that were reported in more than one study. For example, there were six studies that performed melanoma GWAS (Bishop et al., 2009; Brown et al., 2008; Falchi et al., 2009; Rafnar et al., 2009; Stacey et al., 2008; and Stacey et al., 2009), and four replication studies (Duffy et al. 2009; Gathany et al. 2009; Gudbjartsson et al. 2008; and Nan et al. 2009a); however, for most SNPs, we could combine data across only a few of the studies since these studies did not report data on all of the same SNPs. Only SNPs with novel and/or significant associations for melanoma are included in Supplementary Table Ig, and only SNPs reported in at least three studies are included in Figure 1. For the red hair color meta-analysis, only two GWAS examined this phenotype and were thus included, Sulem et al. (2008) and Han et al. (2008). The combined results across the two studies are reported (Supplementary Table Ib). Both studies reported many of the same SNPs. However, due to the fact that for some SNPs only p-values were reported, the direction of the association could not be inferred; therefore, the meta-analysis for red hair color was possible for most but not all reported significant SNPs.
Supplementary Material
Acknowledgements
This study was supported by the Intramural Research Program of NIH, National Cancer Institute, Division of Cancer Epidemiology and Genetics. The authors thank Drs. David Duffy, Hongmei Nan, Jiali Han, Mario Falchi, and Patrick Sulem for sharing data that allowed meta-analysis and refined associations for specific loci, and Barbara Rogers, William Wheeler, and Sara De Matteis for help with the graphical items.
References
- Bastiaens MT, ter Huurne JA, Kielich C, Gruis NA, Westendorp RG, Vermeer BJ, Bavinck JN. Melanocortin-1 receptor gene variants determine the risk of nonmelanoma skin cancer independently of fair skin and red hair. Am. J. Hum. Genet. 2001;68:884–894. doi: 10.1086/319500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahuau M, Vidaud D, Jenkins RB, et al. Germ-line deletion involving the INK4 locus in familial proneness to melanoma and nervous system tumors. Cancer Res. 1998;58:2298–303. [PubMed] [Google Scholar]
- Borg A, Sandberg T, Nilsson K, Johannsson O, Klinker M, Måsbäck A, Westerdahl J, Olsson H, Ingvar C. High frequency of multiple melanomas and breast and pancreas carcinomas in CDKN2A mutation-positive melanoma families. J. Natl. Cancer Inst. 2000;92:1260–1266. doi: 10.1093/jnci/92.15.1260. [DOI] [PubMed] [Google Scholar]
- Bale SJ, Dracopoli NC, Tucker MA, Clark WH, Jr, Fraser MC, Stanger BZ, Green P, Donis-Keller H, Housman DE, Greene MH. Mapping the gene for hereditary cutaneous malignant melanoma-dysplastic nevus to chromosome 1p. N. Eng. J. Med. 1989;320:1367–1372. doi: 10.1056/NEJM198905253202102. [DOI] [PubMed] [Google Scholar]
- Bishop DT, Demenais F, Iles MM, et al. Genome-wide association study identifies three loci associated with melanoma risk. Nat. Genet. 2009;41:920–925. doi: 10.1038/ng.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Box NF, Duffy DL, Irving RE, Russell A, Chen W, Griffyths LR, Parsons PG, Green AC, Sturm RA. Melanocortin-1 receptor genotype is a risk factor for basal and squamous cell carcinoma. J. Invest. Dermatol. 2001;116:224–229. doi: 10.1046/j.1523-1747.2001.01224.x. [DOI] [PubMed] [Google Scholar]
- Brown KM, MacGregor S, Montgomery GW, et al. Common sequence variants on 20q11.22 confer melanoma susceptibility. Nat. Genet. 2008;40:838–840. doi: 10.1038/ng.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- Dickson SP, Wang K, Krantz I, Hakonarson H, Goldstein DB. Rare variants create synthetic genome-wide associations. PLoS Biol. 2010;8(1):e1000294. doi: 10.1371/journal.pbio.1000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy DL, Montgomery GW, Chen W, Zhao ZZ, Le L, James MR, Hayward NK, Martin NG, Sturm RA. A three-single-nucleotide polymorphism haplotype in intron 1 of OCA2 explains most human eye-color variation. Am. J. Hum. Genet. 2007;80:241–252. doi: 10.1086/510885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy DL, Zhao ZZ, Sturm RA, Hayward NK, Martin NG, Montgomery GW. Multiple pigmentation gene polymorphisms account for a substantial proportion of risk of cutaneous malignant melanoma. J. Invest. Dermatol. 2010;130:520–528. doi: 10.1038/jid.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiberg H, Troelsen J, Nielsen M, Mikkelsen A, Megel-From J, Kjaer KW, Hansen L. Blue eye color in humans may be caused by a perfectly associated founder mutation in a regulatory element located within the HERC2 gene inhibiting OCA2 expression. Hum. Genet. 2008;123:177–187. doi: 10.1007/s00439-007-0460-x. [DOI] [PubMed] [Google Scholar]
- Epstein EH. Basal cell carcinomas: attack of the hedgehog. Nat. Rev. Cancer. 2008;8:743–754. doi: 10.1038/nrc2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falchi M, Spector TD, Perks U, Kato BS, Bataille V. Genome-wide search for nevus density shows linkage to two melanoma loci on chromosome 9 and identifies a new QTL on 5q31 in an adult twin cohort. Hum. Mol. Genet. 2006;15:2975–2979. doi: 10.1093/hmg/ddl227. [DOI] [PubMed] [Google Scholar]
- Falchi M, Bataille V, Hayward NK, et al. Genome-wide association study identifies variants at 9p21 and 22q13 associated with development of cutaneous nevi. Nat. Genet. 2009;41:915–918. doi: 10.1038/ng.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez LP, Milne RL, Pita G, Avilés JA, Lázaro P, Benítez J, Ribas G. SLC45A2: a novel malignant melanoma-associated gene. Hum. Mutat. 2008;29:1161–1167. doi: 10.1002/humu.20804. [DOI] [PubMed] [Google Scholar]
- Fernandez LP, Milne RL, Pita G, et al. Pigmentation-related genes and their implication in malignant melanoma susceptibility. Exp Dermatol. 2009;18:634–642. doi: 10.1111/j.1600-0625.2009.00846.x. [DOI] [PubMed] [Google Scholar]
- García-Borrón JC, Sánchez-Laorden BL, Jiménez-Cervantes C. Melanocortin-1 receptor structure and functional regulation. Pigment Cell Res. 2005;18:393–410. doi: 10.1111/j.1600-0749.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- Gathany AH, Hartge P, Davis S, Cerhan JR, Severson RK, Cozen W, Rothman N, Chanock SJ, Wang SS. Relationship between interferon regulatory factor 4 genetic polymorphisms, measures of sun sensitivity and risk for non-Hodgkin lymphoma. Cancer Causes Control. 2009 doi: 10.1007/s10552-009-9348-5. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstenblith MR, Goldstein AM, Fargnoli MC, Peris K, Landi MT. Comprehensive evaluation of allele frequency differences of MC1R variants across populations. Hum. Mutat. 2007;28:495–505. doi: 10.1002/humu.20476. [DOI] [PubMed] [Google Scholar]
- Gillanders E, Juo SH, Holland EA, et al. Localization of a novel melanoma susceptibility locus to 1p22. Am. J. Hum. Genet. 2003;73:301–313. doi: 10.1086/377140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein AM, Chan M, Harland M, et al. High-risk melanoma susceptibility genes and pancreatic cancer, neural system tumors, and uveal melanoma across GenoMEL. Cancer Res. 2006;66:9818–9828. doi: 10.1158/0008-5472.CAN-06-0494. [DOI] [PubMed] [Google Scholar]
- Graf J, Hodgson R, van Daal A. Single nucleotide polymorphisms in the MATP gene are associated with normal human pigmentation variation. Hum. Mutat. 2005;25:278–284. doi: 10.1002/humu.20143. [DOI] [PubMed] [Google Scholar]
- Gudbjartsson DF, Sulem P, Stacey SN, et al. ASIP and TYR pigmentation variants associate with cutaneous melanoma and basal cell carcinoma. Nat. Genet. 2008;40:886–891. doi: 10.1038/ng.161. [DOI] [PubMed] [Google Scholar]
- Guedj M, Bourillon A, Combadières C, et al. Variants of the MATP/SLC45A2 gene are protective for melanoma in the French population. Hum. Mutat. 2008;29:1154–1160. doi: 10.1002/humu.20823. [DOI] [PubMed] [Google Scholar]
- Han J, Kraft P, Nan H, et al. A Genome-wide association study identifies novel alleles associated with hair color and skin pigmentation. PLoS Genet. 2008;4:e1000074. doi: 10.1371/journal.pgen.1000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Qureshi AA, Prescott J, Guo Q, Ye L, Hunter DJ, De Vivo I. A prospective study of telomere length and the risk of skin cancer. J. Invest. Dermatol. 2009;129:415–421. doi: 10.1038/jid.2008.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt C, Lee Wu C, Evans G, Howell A, Elles RG, Jordan R, Sloan P, Read AP, Thakker N. Germline mutation of ARF in a melanoma kindred. Hum. Mol. Genet. 2002;11:1273–1279. doi: 10.1093/hmg/11.11.1273. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statist. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Hultman KA, Bahary N, Zon LI, Johnson SL. Gene Duplication of the zebrafish kit ligand and partitioning of melanocyte development functions to kit ligand a. PLoS Genet. 2007;3:e17. doi: 10.1371/journal.pgen.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein MR, Roggero E, Tuthill RJ, et al. Identification of novel deletion loci at 1p36 and 9p22-21 in melanocytic dysplastic nevi and cutaneous malignant melanomas. Arch. Dermatol. 2003;139:816–817. doi: 10.1001/archderm.139.6.816. [DOI] [PubMed] [Google Scholar]
- Jannot AS, Meziani R, Bertrand G, et al. Allele variations in the OCA2 gene (pink-eyed dilution locus) are associated with genetic susceptibility to melanoma. Eur. J. Hum. Genet. 2005;13:913–920. doi: 10.1038/sj.ejhg.5201415. [DOI] [PubMed] [Google Scholar]
- Kanetsky PA, Swoyer J, Panossian S, Holmes R, Guerry D, Rebbeck TR. A polymorphism in the agouti signaling protein gene is associated with human pigmentation. Am. J. Hum. Genet. 2002;70:770–775. doi: 10.1086/339076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser M, Liu F, Janssens JW, et al. Three genome-wide association studies and a linkage analysis identify HERC2 as a human iris color gene. Am. J. Hum. Genet. 2008;82:411–423. doi: 10.1016/j.ajhg.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefford RF, Newton Bishop JA, Bergman W, Tucker MA. Counseling and DNA testing for individuals perceived to be genetically predisposed to melanoma: A consensus statement of the Melanoma Genetics Consortium. J. Clin. Oncol. 1999;17:3245–3251. doi: 10.1200/JCO.1999.17.10.3245. [DOI] [PubMed] [Google Scholar]
- Kennedy C, ter Huurne J, Berkhout M, Gruis N, Bastiaens M, Bergman W, Willemze R, Bavinck JN. Melanocortin 1 receptor (MC1R) gene variants are associated with an increased risk for cutaneous melanoma which is largely independent of skin type and hair color. J. Invest Dermatol. 2001;117:294–300. doi: 10.1046/j.0022-202x.2001.01421.x. [DOI] [PubMed] [Google Scholar]
- Lamason RL, Mohideen MA, Mest JR, et al. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science. 2005;310:1782–1786. doi: 10.1126/science.1116238. [DOI] [PubMed] [Google Scholar]
- Landi MT, Kanetsky PA, Tsang S, Gold B, Munroe D, Rebbeck T, Swoyer J, Ter-Minassian M, Hedayati M, Grossman L, et al. MC1R, ASIP, and DNA repair in sporadic and familial melanoma in a Mediterranean population. J. Natl. Cancer Inst. 2005;97:998–1007. doi: 10.1093/jnci/dji176. [DOI] [PubMed] [Google Scholar]
- Landi MT, Chatterjee N, Yu K, et al. A genome-wide association study of lung cancer identifies a region of chromosome 5p15 associated with risk of adenocarcinoma. Am. J. Hum. Genet. 2009;85:679–691. doi: 10.1016/j.ajhg.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liboutet M, Portela M, Delestaing G, Vilmer C, Dupin N, Gorin I, Saiag P, Lebbe C, Kerob D, Dubertret L, et al. MC1R and PTCH Gene Polymorphism in French Patients with Basal Cell Carcinomas. J. Invest Dermatol. 2006;126:1510–1517. doi: 10.1038/sj.jid.5700263. [DOI] [PubMed] [Google Scholar]
- Luande J, Henschke CI, Mohammed N. The Tanzanian human albino skin. Natural history. Cancer. 1985;55:1823–1828. doi: 10.1002/1097-0142(19850415)55:8<1823::aid-cncr2820550830>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Matichard E, Verpillat P, Meziani R, Gerard B, Descamps V, Legroux E, Burnouf M, Bertrand G, Bouscarat F, Archimbaud A, et al. Melanocortin 1 receptor (MC1R) gene variants may increase the risk of melanoma in France independently of clinical risk factors and UV exposure. J. Med. Genet. 2004;41:e13. doi: 10.1136/jmg.2003.011536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CT, Beleza S, Pollen AA, Schluter D, Kittles RA, Shriver MD, Kingsley DM. cis-Regulatory changes in Kit ligand expression and parallel evolution of pigmentation in sticklebacks and humans. Cell. 2007;131:1179–1189. doi: 10.1016/j.cell.2007.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K, Fukamachi S, Kimura H, Koda Y, Soemantri A, Ishida T. Distinctive distribution of AIM1 polymorphism among major human populations with different skin color. J. Hum. Genet. 2002;47:92–94. doi: 10.1007/s100380200007. [DOI] [PubMed] [Google Scholar]
- Nan H, Kraft P, Hunter DJ, Han J. Genetic variants in pigmentation genes, pigmentary phentoypes, and risk of skin cancer in Caucasians. Int. J. Cancer. 2009a;125:909–917. doi: 10.1002/ijc.24327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan H, Kraft P, Qureshi AA, et al. Genome-wide association study of tanning phenotype in a population of European ancestry. J. Invest. Dermatol. 2009b;129:2250–2257. doi: 10.1038/jid.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natkunam Y, Warnke RA, Montgomery K, Flini B, van De Rijn M. Analysis of MUM1/IRF4 protein expression using tissue microarrays and immunohistochemistry. Mod. Pathol. 2001;14:686–694. doi: 10.1038/modpathol.3880373. [DOI] [PubMed] [Google Scholar]
- Norton HL, Kittles RA, Parra E, McKeigue P, Mao X, Cheng K, Canfield VA, Bradley DG, McEvoy B, Shriver MD. Genetic evidence for the convergent evolution of light skin in Europeans and East Asians. Mol. Bio. Evol. 2007;24:710–722. doi: 10.1093/molbev/msl203. [DOI] [PubMed] [Google Scholar]
- Palmer JS, Duffy DL, Box NF, Aitken JF, O’Gorman LE, Green AC, Hayward NK, Martin NG, Sturm RA. Melanocortin-1 receptor polymorphisms and risk of melanoma: is the association explained solely by pigmentation phenotype? Am. J. Hum. Genet. 2000;66:176–186. doi: 10.1086/302711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry PK, Silverberg NB. Cutaneous malignancy in albinism. Cutis. 2001;67:427–430. [PubMed] [Google Scholar]
- Petersen GM, Amundadottir L, Fuchs CS, et al. A genome-wide association study identifies pancreatic cancer susceptibility loci on chromosomes 13q22.1, 1q32.1 and 5p15.33. Nat. Genet. 2010;42:224–228. doi: 10.1038/ng.522. Epub 2010 Jan 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronzelli F, Sollima D, Coppola G, Martini-Neri ME, Neri G, Genuardi M. CDKN2A germline splicing mutation affecting both P16INK4 and P14ARF RNA processing in a melanoma/neurofibroma kindred. Genes Chromosomes Cancer. 2001;31:398–401. doi: 10.1002/gcc.1159. [DOI] [PubMed] [Google Scholar]
- Rafnar T, Sulem P, Stacey SN, et al. Sequence variants at the TER-CLPTM1L locus associate with many cancer types. Nat. Genet. 2009;41:221–227. doi: 10.1038/ng.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondi S, Sera F, Gandini S, Iodice S, Caini S, Maisonneuve P, Fargnoli MC. MC1R variants, melanoma and red hair color phenotype: a meta-analysis. Int. J. Cancer. 2008;122:2753–2760. doi: 10.1002/ijc.23396. [DOI] [PubMed] [Google Scholar]
- Randerson-Moor JA, Harland M, Williams S, et al. A germline deletion of p14ARF but not CDKN2A in a melanoma-neural system tumor syndrome family. Hum. Mol. Genet. 2001;10:55–62. doi: 10.1093/hmg/10.1.55. [DOI] [PubMed] [Google Scholar]
- Rees JL. Genetics of hair and skin color. Annu. Rev. Genet. 2003;37:67–90. doi: 10.1146/annurev.genet.37.110801.143233. [DOI] [PubMed] [Google Scholar]
- Rees JL. The genetics of sun sensitivity in humans. Am. J. Hum. Genet. 2004;75:739–751. doi: 10.1086/425285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees J. Plenty new under the sun. J. Invest. Dermatol. 2006;126:1691–1692. doi: 10.1038/sj.jid.5700345. [DOI] [PubMed] [Google Scholar]
- Rizos H, Puig S, Badenas C, Malvehy J, Darmanian AP, Jiménez L, Milà M, Kefford RF. A melanoma-associated germline mutation in exon 1ß inactivates p14ARF. Oncogene. 2001;20:5543–5547. doi: 10.1038/sj.onc.1204728. [DOI] [PubMed] [Google Scholar]
- Shete S, Hosking FJ, Robertson LB, et al. Genome-wide association study identifies five susceptibility loci for glioma. Nat. Genet. 2009;41:899–904. doi: 10.1038/ng.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey SN, Gudbjartsson DF, Sulem P, et al. Common variants on 1p36 and 1q42 are associated with cutaneous basal cell carcinoma but not with melanoma or pigmentation traits. Nat. Genet. 2008;40:1313–1318. doi: 10.1038/ng.234. [DOI] [PubMed] [Google Scholar]
- Stacey SN, Sulem P, Masson G, et al. New common variants affecting susceptibility to basal cell carcinoma. Nat. Genet. 2009;41:909–914. doi: 10.1038/ng.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokowski RP, Pant PVK, Dadd T, et al. A genomewide association study of skin pigmentation in a South Asian population. Am. J. Hum. Genet. 2007;81:1119–1132. doi: 10.1086/522235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratigos AJ, Dimisianos G, Nikolaou V, Poulou M, Sypsa V, Stefanaki I, Papadopoulos O, Polydorou D, Plaka M, Christofidou E, et al. Melanocortin Receptor-1 Gene Polymorphisms and the Risk of Cutaneous Melanoma in a Low-Risk Southern European Population. J. Invest Dermatol. 2006;126:1842–1849. doi: 10.1038/sj.jid.5700292. [DOI] [PubMed] [Google Scholar]
- Sturm RA, Duffy DL, Zhao ZZ, Leite FP, Stark MS, Hayward NK, Martin NG, Montgomery GW. A single SNP in an evolutionary conserved region within intron 86 of the HERC2 gene determines human blue-brown eye color. Am. J. Hum. Genet. 2008;82:424–431. doi: 10.1016/j.ajhg.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulem P, Gudbjartsson DF, Stacey SN, et al. Genetic determinants of hair, eye and skin pigmentation in Europeans. Nat. Genet. 2007;39:1443–1452. doi: 10.1038/ng.2007.13. [DOI] [PubMed] [Google Scholar]
- Sulem P, Gudbjartsson DF, Stacey SN, et al. Two newly identified genetic determinants of pigmentation in Europeans. Nat. Genet. 2008;40:835–837. doi: 10.1038/ng.160. [DOI] [PubMed] [Google Scholar]
- Sundram U, Harvell JD, Rouse RV, Natkunam Y. Expression of the B-cell proliferation marker MUM1 by melanocytic lesions and comparison with s100, gp1 (HMB45), and MelanA. Mod. Pathol. 2003;16:802–810. doi: 10.1097/01.MP.0000081726.49886.CF. [DOI] [PubMed] [Google Scholar]
- Terenziani M, Spreafico F, Serra A, Podda M, Cereda S, Belli F. Amelanotic melanoma in a child with oculocutaneous albinism. Med. Pediatr. Oncol. 2003;41:179–180. doi: 10.1002/mpo.10317. [DOI] [PubMed] [Google Scholar]
- Tomita Y, Suzuki T. Genetics of pigmentary disorders. Am. J. Med. Genet. C. Semin. Med. Genet. 2004;131C:75–81. doi: 10.1002/ajmg.c.30036. [DOI] [PubMed] [Google Scholar]
- Valverde P, Healy E, Sikkink S, Haldane F, Thody AJ, Carothers A, Jackson IJ, Rees JL. The Asp84Glu variant of the melanocortin 1 receptor (MC1R) is associated with melanoma. Hum. Mol. Genet. 1996;5:1663–1666. doi: 10.1093/hmg/5.10.1663. [DOI] [PubMed] [Google Scholar]
- Wang Y, Broderick P, Webb E, et al. Common 5p15.33 and 6p21.33 variants influence lung cancer risk. Nat. Genet. 2008;40:1407–1409. doi: 10.1038/ng.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZQ, Si L, Tang Q, et al. Gain-of-function mutation of KIT ligand on melanin synthesis causes familial progressive hyperpigmenation. Am. J. Hum. Genet. 2009;84:672–677. doi: 10.1016/j.ajhg.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrle-Haller B. The role of Kit-ligand in melanocyte development and epidermal homeostasis. Pigment. Cell Res. 2003;16:287–296. doi: 10.1034/j.1600-0749.2003.00055.x. [DOI] [PubMed] [Google Scholar]
- Yakubu A, Mabogunje OA. Skin cancer in African albinos. Acta. Oncol. 1993;32:621–622. doi: 10.3109/02841869309092440. [DOI] [PubMed] [Google Scholar]
- Zhu G, Montgomery GW, James MR, Trent JM, Hayward NK, Martin NG, Duffy DL. A genome-wide scan for naevus count: linkage to CDKN2A and to other chromosome regions. Eur. J. Hum. Genet. 2007;15:94–102. doi: 10.1038/sj.ejhg.5201729. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


