Abstract
The context preexposure facilitation effect (CPFE) is an elaboration of contextual fear conditioning and refers to enhanced contextual conditioning resulting from preexposure to the context prior to a separate, brief context-shock episode. A version of the CPFE developed by Rudy and colleagues in rats has demonstrated greater sensitivity to pre-training hippocampal insult relative to standard contextual fear conditioning preparations. Our aim was to adapt the Rudy CPFE procedures to mice. In Experiment 1 we compared performance of young adult male C57BL6/J mice on two versions of the CPFE. One version – not previously used in mice – adapted methods established by Rudy and colleagues, and the other CPFE task replicated procedures previously established in this mouse strain by Gould and colleagues. In Experiment 2 we compared the effects of pre-training intraperitoneal administration of moderate levels of scopolamine or methylscopolamine on contextual conditioning between mice trained using the Rudy CPFE method and a separate group trained using standard contextual fear procedures. Scopolamine is a muscarinic cholinergic receptor antagonist that impairs hippocampal function. Robust freezing to the conditioning context was observed in mice trained using the Rudy CPFE method (Experiment 1), and greater scopolamine-induced impairments in contextual freezing were observed using this CPFE method relative to mice trained using standard contextual fear procedures (Experiment 2). These findings support use of the Rudy CPFE task as a behavioral assay for hippocampal function in mice.
Keywords: CPFE, context, fear, scopolamine, mouse
1. Introduction
Pavlovian fear conditioning is widely used to explore the neural substrates of learning and memory. In standard fear conditioning paradigms, a rodent receives one or more presentations of a foot shock unconditioned stimulus (US) within 2–3 minutes of initial placement in the training environment. Following this training, robust conditioned fear (e.g.,freezing) is evident in subsequent exposures to the training environment (or “context”). The hippocampal formation is involved in contextual fear conditioning [1–3] though the extent of hippocampal involvement is a matter of debate [4, 5]. Although many studies have reported impairments in conditioned freezing to the context as a result of hippocampal insult [2–3, 6], a number of experiments have failed to replicate these findings [7–11].
One of the factors contributing to these discrepant findings in standard fear conditioning tasks concerns timing of the hippocampal damage relative to training [12]. Anterograde hippocampal lesions (damage prior to training) have failed to produce robust deficits in contextual fear conditioning in some cases [7–11, 13, 14], whereas retrograde hippocampal lesions (damage incurred between training and testing) have yielded more consistent impairments [2, 7, 8, 14–16]. Under normal conditions animals employ a ‘hippocampal’ strategy in which the conditioning context is represented as a unique configuration, separate from the individual sensory components (e.g., texture; brightness; odor) that comprise it. If the hippocampus is compromised at training (anterograde damage), however, alternative strategies are available to support fear memories to the context [7, 17, 18]. Modifications to standard fear conditioning preparations are therefore necessary to reliably link hippocampal functions with learned behaviors in situations in which there is anterograde hippocampal insult (e.g., disease models).
The context preexposure facilitation effect (CPFE) is an elaboration of standard fear conditioning preparations and refers to enhanced contextual conditioning resulting from preexposure to the context prior to a separate, brief context-shock episode [18, 19]. CPFE tasks typically involve three distinct phases of training, each separated by a 24 hour interval: (1) preexposure to the context, (2) shock delivery shortly – or immediately – following placement into the chamber, and (3) context test. Rudy and colleagues have developed a version of the CPFE paradigm in rats involving multiple context exposures at Phase 1 using a distinct black ice bucket for transport [18, 20, 21]. A “conjunctive representation” (or gestalt) of the context is formed as a consequence of preexposure [12, 17, 18], and the distinct transport cue contributes to the activation of this representation during Phase 2 training (immediate shock) via a process termed “pattern completion” [20, 22]. The formation of a unique representation from a combination of elements (conjunctive representations) and activation of the entire representation through exposure to a subset of features (pattern completion) are concepts central to many theories of hippocampal functioning [23–26]. Empirical support for these concepts has been demonstrated in CPFE studies from the Rudy laboratory [21, 22]. Importantly, robust impairments in contextual fear conditioning are observed following anterograde hippocampal damage using Rudy’s CPFE design [11, 21] but not when using a standard contextual fear preparation [11].
Variants of the CPFE have been demonstrated in mice [27–33], though none have replicated the methods employed by Rudy and colleagues. Recent advances in the development of transgenic mice with disruptions in hippocampal functioning [34] has encouraged the identification of behavioral assays sensitive to pre-training hippocampal dysfunction in mice. Given the established sensitivity of Rudy’s CPFE task (in rats) to anterograde hippocampal insult, a novel mouse model using Rudy’s CPFE paradigm may be the ideal assay to link pre-existing hippocampal dysfunction with impairments in associative learning and memory. Experiment 1 in the present study is a replication of procedures employed by Rudy and colleagues [11] using C57BL6 mice. Performance in this novel mouse CPFE model is compared to a group replicating CPFE procedures established by Gould and colleagues using C57BL6 mice [32]. The second experiment is a comparison of the effects of pre-training scopolamine administration on contextual fear conditioning in this novel mouse (Rudy method) CPFE task relative to a standard contextual fear task. Scopolamine is a muscarinic cholinergic receptor antagonist that disrupts hippocampal functioning and produces robust impairments in hippocampal-dependent learning and memory tasks, including contextual fear conditioning [35–39]. Based on previous findings comparing standard and CPFE contextual fear in rats [11], we predict that pre-training administration of scopolamine at doses lower than those shown to impair contextual freezing in mice trained in a standard fear conditioning preparation (0.05 and 0.1 mg/kg; see [39]) will be sufficient to impair contextual fear conditioning in mice trained using Rudy’s CPFE method. Findings of greater scopolamine-induced impairment in contextual freezing in mice trained using the Rudy CPFE procedure relative to mice trained using a standard contextual fear preparation would encourage future use of the Rudy CPFE method in studies assessing functional hippocampal integrity in mouse models.
2. Materials and Methods
2.1. Animals
Subjects were 110 male, C57BL/6 mice (Jackson Laboratories: Experiment 1, n = 32; Experiment 2, n = 78) aged 6–10 weeks at training. Mice were group-housed in standard polycarbonate cages and had ad libitum access to food and water, with room lighting timed for a 12:12-hr light-dark cycle. Mice were acclimated to the colony for at least 1 week before the start of experimentation. All research methods were approved by Temple University’s Institutional Animal Care and Use Committee.
2.2. Apparatus and Materials
Med Associates Near Infrared Video Fear Conditioning System for Mouse (MED-VFC-NIR-M) as described in Anagnostaras et al. [41] was used for fear conditioning in the present study. This system has demonstrated reliability in video coding of common dependent measures of fear (e.g., freezing; activity levels), thus allowing experimenters to avoid labor-intensive post hoc coding of fear behavior. Use of this system has the further advantage of avoiding potential biases in situations in which experimenters coding fear behavior are not blind to group or treatment conditions. Parameters used in the present study replicate those that yielded maximum reliability with experimenter coding of freezing in the Anagnostaras et al. [41] studies.
Two identical conditioning chambers (32 cm wide, 25 cm high, 25 cm deep; part number VFC-008) located in the same room were used, with each chamber consisting of clear polycarbonate (top, front), white acrylic (back), and stainless steel (sides, shock grids, pan below grids). Each grid floor consisted of 36 rods, and each rod was 2 mm in diameter. Conditioning chambers were each stationed within separate white sound-attenuating boxes (63.5 cm wide, 35.5 cm high, 76 cm deep; NIR-022MD). An overhead LED-based light source (NIR-100) provided visible broad spectrum white light (450–650 nm) and near infrared (NIR) light (940 nm). Background noise (65 db) was provided by internal ventilation fans. Video images of fear training were recorded at a frame rate of 30 frames per second using a video camera (VID-CAM-MONO-2A) secured to the inner door of the sound-attenuating box. Data collection was controlled by Med Associates software (Video Freeze) running on a Windows computer.
2.3. Behavioral Procedures
Procedures common to both experiments will be described first, followed by procedures specific to each experiment. To determine mouse movement, a calibration procedure is implemented for all training and testing sessions in which a reference video image is taken prior to placing the mouse in the chamber. This reference image is compared against activity following placement of the mouse in the chamber, and this comparison determines the Motion Index (see [41] for further details of the calibration procedure).
Prior to the start of training, all mice were given 2–3 days to acclimate to standard polycarbonate temporary housing or transport cages. Following transport from the housing colony, all mice were given 45–60 min to acclimate to the laboratory area in which behavioral training occurred. The same experimenter (KB or DC) handled mice throughout all phases of training. One set of mice in each experiment was trained using behavioral procedures identical to those used for rats as outlined by Rudy and colleagues [11]. These mice – designated as Rudy Experimental (n = 8) – were transported two at a time from their home cage to the conditioning chamber (approx 10 – 15 s transport time) in a black ice bucket with the lid secured so that that mice could not see where they were being taken. All transport between the temporary housing cage and the conditioning context for these mice occurred in these black transport buckets. During day 1 (Phase 1) of training, mice were given 5 min to explore the novel context (70% alcohol odor in black transport bucket and in conditioning chamber) and then transported back to their temporary housing cage for 40 sec before getting transported back to the conditioning chamber where they remained for 40 sec. This 40 sec alternating temporary housing cage/40 sec conditioning chamber exposure was repeated four times. The purpose of these multiple exposures was to establish features of the transport bucket as retrieval cues that could activate representation of the context [11, 18, 20, 21] – there was no shock delivery during Phase 1 of training. Twenty-four hours later (Phase 2) the mice were transported from their temporary housing cage to the same conditioning context explored in Phase 1. Immediately after entry into the context, mice received a single, 2 sec, 0.75 mA shock through the grid floors. Mice were removed immediately after shock termination and transported to their home cage. Phase 3 training occurred twenty-four hours later, consisting of exposure to the same conditioning context experienced in Phases 1 and 2 for 6 min (no shock deliveries during Phase 3). At Phase 4 of training (twenty-four hours after Phase 3), mice were returned to the same chamber as in Phases 1–3 (6 min), but various features of the context were altered so as to make it as different as possible from previous exposures. Tactile (smooth acrylic white insert – ENV-005-GFCW – covering the grid floor, and a white acrylic curved insert covering the back and 2 side walls), olfactory (5% white vinegar odor in chamber and in transport bucket), visual (house light off), and auditory (70 db white noise) stimuli were altered for Phase 4. The purpose of this was to determine whether fear responses exhibited at Phase 3 primarily reflected conditioning to the pre-exposure context or were influenced by generalized fear not specific to the pre-exposure context.
2.3.1. Experiment 1
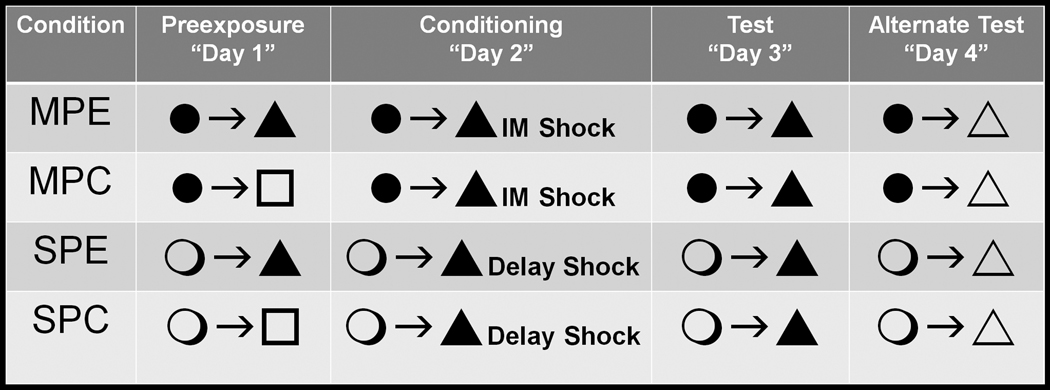
Four behavioral groups of normal male C57BL/6 mice were trained in Experiment 1. In addition to the aforementioned Rudy Experimental subjects, a control group (Rudy Control, n = 8) was trained in an identical manner to that of the Rudy Experimental group during Phases 2–4. These subjects differed only at Phase 1 of training, in which subjects were exposed to a novel polycarbonate cage located in a room adjacent to the experimental equipment. Exposures to this novel cage were of the same duration and pattern (5 min initially, followed by five, 40 sec exposures with repeated transport between the novel environment and temporary housing cages) as that of the conditioning context for the Rudy Experimental group. The remaining groups were trained under conditions identical to those described in Kenney and Gould [32]. Subjects in the experimental group – Gould Experimental (n = 8) – were transported in a standard polycarbonate cage to the conditioning context for a single 10 min pre-exposure session (Phase 1). At Phase 2 these mice were again transported to the conditioning chamber, and 5 sec after placement in the chamber received a single shock identical to that experienced by the Rudy subjects (2 sec, 0.75 mA). Mice were removed from the chamber 60 sec following shock termination. With the exception of the different transport container used between ‘Rudy’ and ‘Gould’ subjects, phases 3 and 4 for the Gould Experimental subjects were identical to that of the Rudy subjects (6 min conditioning context exposures with an altered context at Phase 4). The fourth behavioral group used in Experiment 1 – Gould control (n = 8) – received training identical to that of the Gould Experimental group at phases 2–4. These subjects differed only in Phase 1 training, during which they were exposed to a novel polycarbonate cage (located in a room adjacent to the behavioral equipment) for 10 minutes. Figure 1 provides a visual representation of the design for Experiment 1.
Figure 1.
Depiction of the experimental design used in Experiment 1. All phases of training are separated by 24 h. Black circles represent the black ice bucket used for transportation in ‘Rudy’ subjects and the open circles represent the clear polycarbonate cage used for transportation in ‘Gould’ subjects. Black triangles represent the experimental chamber (context) used for preexposure in experimental (Exp) subjects and during Phases 2 and 3 in all subjects. All sensory features of the environment (e.g., odor; tactile cues) are the same in conditions identified with a black triangle. In contrast, open triangles represent a context in which these sensory cues differ (used at Phase 4). Open squares represent the clear polycarbonate cage used for preexposure in Rudy and Gould control (Ctl) groups. ‘IM Shock’ refers to shock administration immediately following placement into the chamber at Phase 2 for Rudy groups. ‘Delay Shock’ refers to shock administration 5 sec following placement into the chamber at Phase 2 for Gould groups.
2.3.2. Experiment 2
Two distinct behavioral groups of experimentally naïve mice were used in Experiment 2 – subjects trained in an identical fashion to the Rudy Experimental group (described in Experiment 1), and a group receiving ‘standard’ single-shock contextual fear conditioning using parameters similar to those reported in previous studies in mice [29, 39]. This group – termed Standard Experimental – received three days of training over a four-day span. The major difference between this group and groups trained using variants of the context preexposure facilitation effect (CPFE) is that initial context exposure and shock delivery occurs during the same phase (Phase 1). Subjects in the Standard Experimental group were transported to the conditioning chambers in standard polycarbonate cages. At Phase 1, subjects were exposed to the conditioning chamber for a total of three minutes. A two minute and twenty-eight second baseline period was followed by a 2 sec, 0.75 mA grid floor shock. Mice remained in the chamber for 30 sec following shock termination. Forty-eight hours later (equivalent time period to Phase 3 in CPFE subjects) mice were returned to the conditioning chamber for a 6 min context test. A forty-eight hour interval between initial chamber exposure and the context test was chosen to match the interval between pre-exposure and testing in the CPFE subjects, and this training-testing interval has been shown to produce robust conditioning in normal C57BL/6 mice trained using ‘standard’ contextual freezing parameters [39]. Twenty-four hours after the context test subjects were given another 6 min context test with alterations to the testing environment (altered auditory, olfactory, tactile, and visual cues) identical to those described in Phase 4 of the aforementioned groups.
2.3.2.1. Drugs and administration (Experiment 2)
Scopolamine hydrobromide (Tocris) or methylscopolamine bromide (Sigma) were each dissolved in physiological saline. Methylscopolamine is an analog of scopolamine that does not cross the blood brain barrier. Scopolamine was administered at one of the following concentrations: 0, 0.05, 0.1, or 0.5 mg/kg. Methylscopolamine was administered at 1 mg/kg. One of the concentrations of either scopolamine or methylscopolamine was delivered via intraperitoneal injection 25 minutes prior to the start of Phase 1 training. Injection volumes were 0.01 ml/g body weight.
2.4. Statistical analyses
The dependent measure of interest is percentage of time freezing. Activity levels were assessed as well, but since these measures were highly correlated only the freezing measure was reported for the preexposure and context test phases for brevity. Activity levels were reported for the 2 sec shock administration since this measure is uniquely suited to quantify the unconditioned response (UR) to the shock US. For Experiment 1, repeated measures analysis of variance (ANOVA) included all 4 behavioral groups in analyses of Phases 2–4. Analyses for Phase 1 (pre-exposure) were performed only on subjects in the experimental groups. For Experiment 2, each behavioral group (Rudy Experimental and Standard Experimental) was analyzed separately. Within each group analysis repeated measures ANOVAs included all drug administration conditions. Additional within-group analyses were performed across different phases of training. Main effects of group (Experiment 1) or drug (Experiment 2) were subjected to Tukey’s post-hoc tests. F-values for significant interactive effects were compared to adjusted critical values to identify specific group/drug condition × time (minutes within the context tests) differences. Statistical significance was set to the p < 0.05 level.
3. Results
Our primary goal (Experiment 1) was to establish a novel mouse model of the context preexposure facilitation effect (CPFE) using methods established by Rudy and colleagues for use in rats [11, 18]. This ‘Rudy’ method has demonstrated greater sensitivity to anterograde hippocampal insult relative to standard contextual freezing methods [11]. Performance in this CPFE variant was compared to a CPFE method used by Gould and colleagues shown to produce robust contextual freezing in mice [33; see Figure 1 for an illustration of the design for Experiment 1]. Controls preexposed to an environment different from the training context were used for each method (‘Rudy’ and ‘Gould’ methods). In Experiment 2 contextual freezing in the novel mouse (Rudy) CPFE model was compared to a standard contextual fear task following pre-training systemic administration of scopolamine.
3.1. Experiment 1: Comparison of two CPFE methods in mice
3.1.1. Phases 1 and 2
As expected, freezing levels during Phase 1 (context pre-exposure) were minimal, though the percentage time freezing displayed by the Rudy Experimental subjects (5.84% freezing) was significantly higher than in the Gould Experimental subjects (0.57% freezing), F(1,14) = 18.744, p < 0.002. The enhanced freezing in the Rudy Experimental group appeared to be driven by the multiple transports between the conditioning chamber and the temporary housing. An analysis examining only the first 5 minutes of Phase 1 – prior to the repeated transports in the black ice buckets for the Rudy Experimental group – yielded equivalent levels of freezing between the Rudy (0.55%) and Gould Experimental (0.46%) groups (p > 0.810). Freezing was not assessed during Phase 1 in controls since these subjects could not be monitored with the Video Freeze equipment at this time. Activity levels during the 2 sec shock administration at Phase 2 did not differ between any of the four behavioral groups (p > 0.350).
3.1.2. Phases 3 and 4
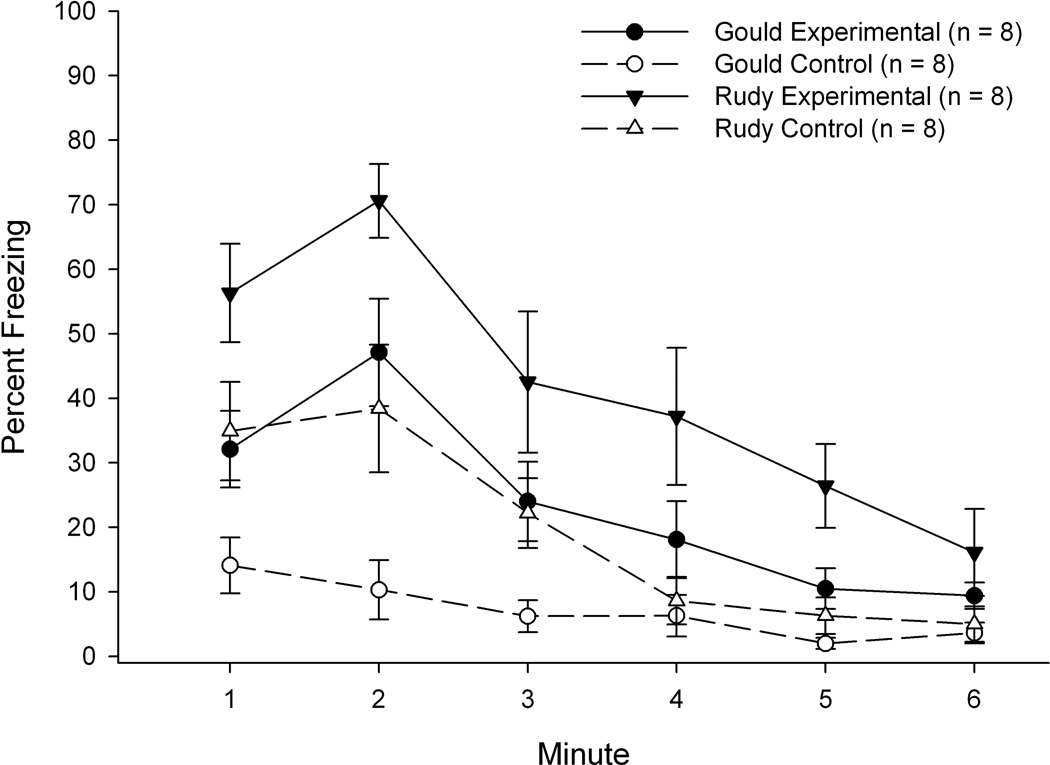
A 4 (Group) × 6 (Minute) repeated measures ANOVA on Phase 3 (context test) freezing revealed significant main effects of group, F(3,28) = 11.695, p < 0.001, and minute, F(5,140) = 29.664, p < 0.001, as well as a significant interaction of Group × Minute: F(15,140) = 2.286, p < 0.007 (see Figure 2). Tukey’s post-hoc tests on the group main effect revealed significant differences in freezing percentage between the following groups: Rudy Experimental > all other groups (ps < 0.025), and Gould Experimental > Gould Controls (p < 0.045). The two control groups were not significantly different in percentage of time freezing at Phase 3. Post-hoc analyses on the Group × Minute interaction revealed significantly greater freezing levels in both experimental groups relative to their respective controls during the 2nd minute of the Phase 3 context test. Additionally, The Rudy Experimental group froze significantly more than the Rudy control group during minutes 4–5 of Phase 3. The main effect of minute reflected a significant reduction in freezing levels over the latter portion of Phase 3, as analyses of freezing levels using adjusted critical values revealed significantly higher levels of freezing during the first minute of the context test relative to minutes 4–6, significantly higher levels during the second minute relative to minutes 3–6, and significantly higher levels during the third minute relative to minutes 5–6.
Figure 2.
Mean percentage of freezing during each of the six minutes of the context test (Phase 3) for the four groups used in Experiment 1. Bars depict standard error of the mean.
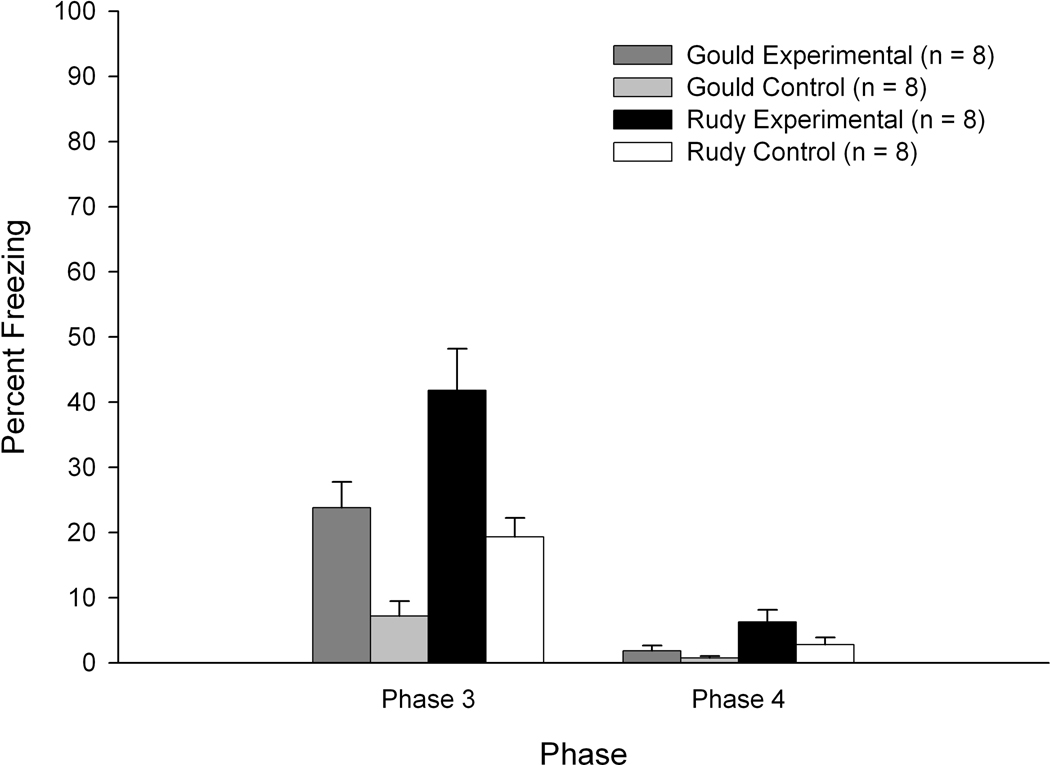
Freezing levels at Phase 4 (altered context test) were relatively low. ANOVA revealed a significant main effect of group, F(3,28) = 4.246, p < 0.015. Post-hoc tests of this analysis revealed significantly greater freezing levels in Rudy Experimental subjects relative to Gould Control subjects (p < 0.012) and a marginally significant effect indicating greater freezing levels at Phase 4 in Rudy Experimental subjects relative to Gould Experimental subjects (p < 0.056). Of primary interest was the within-group comparison of Phase 3 versus Phase 4 freezing levels. As expected, freezing levels in the altered context encountered at Phase 4 were significantly lower than freezing displayed at Phase 3 for most groups. With the exception of the Gould Control group, freezing levels for each group at Phase 3 were significantly higher than their freezing at Phase 4 (all Fs > 18.36; see Figure 3).
Figure 3.
Mean percentage of freezing as a function of training phase for the four groups used in Experiment 1. Bars depict standard error of the mean.
3.1.3. Summary of findings: Experiment 1
Consistent with findings using rats as experimental subjects, applying CPFE methods established by Rudy and colleagues to a mouse model produces robust freezing to the conditioning chamber. Furthermore, the low levels of freezing in the same chamber with altered contextual cues at Phase 4 (e.g., different odor; different tactile and auditory cues) indicate that the high levels of freezing at Phase 3 are driven primarily by memories specific to the preexposure environment. However, Phase 3 freezing levels for Rudy Control subjects were higher than expected, suggesting that some of the Phase 3 freezing in Rudy Experimental subjects may be influenced by factors beyond the pre-exposure context (e.g., the black transport bucket; various contributing factors to this enhanced freezing in Rudy Control subjects will be explored in the Discussion). Additionally, findings in the Gould Experimental and Control subjects replicate those reported in Kenney and Gould [32].
3.2. Experiment 2: Effects of scopolamine on CPFE and standard contextual fear paradigms
3.2.1. CPFE method (Rudy Experimental): Phases 1 and 2
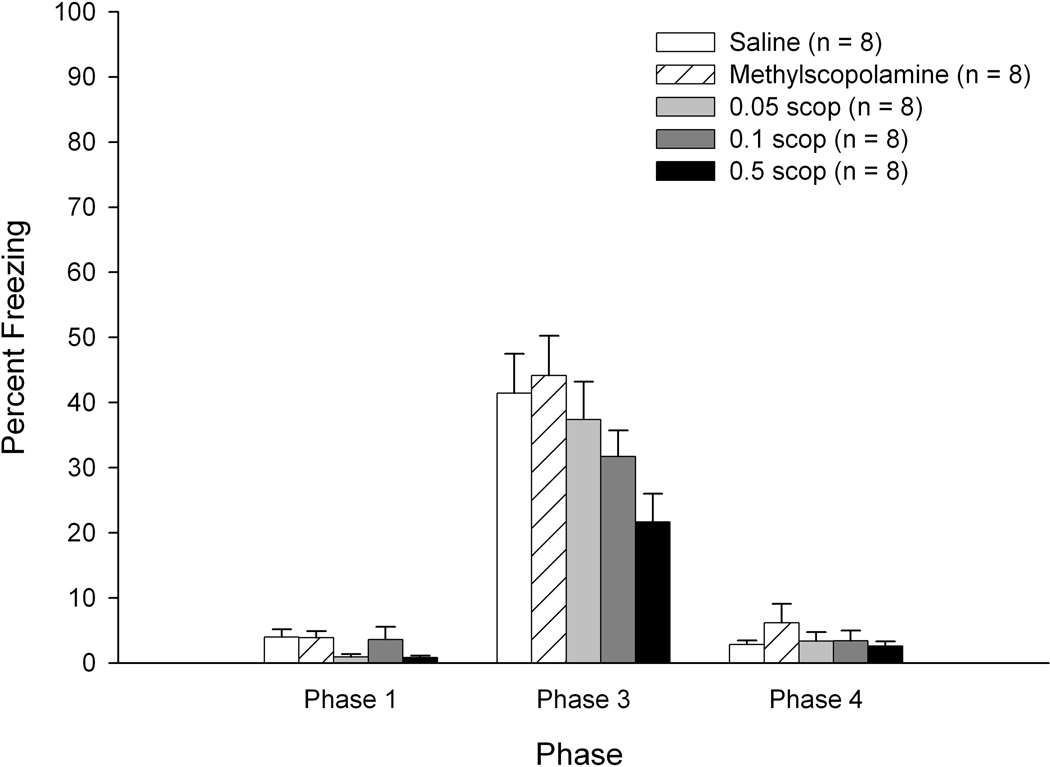
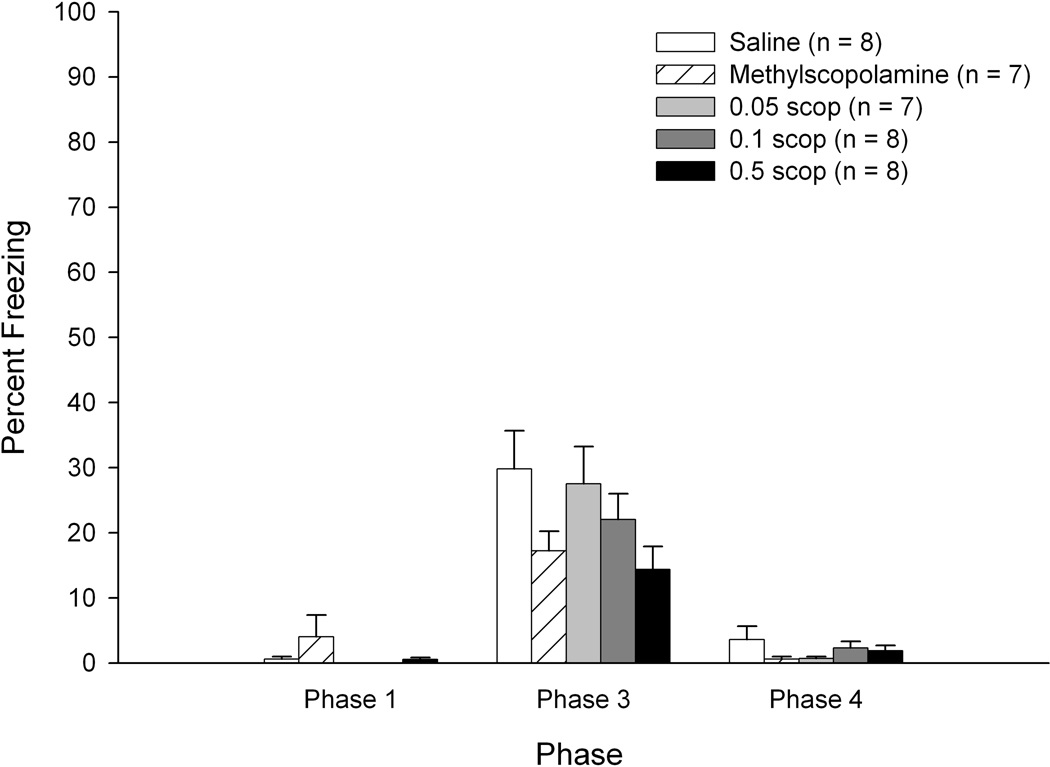
There were no significant differences as a function of drug dosage on Phase 1 freezing percentage (less than 4 percent freezing for all groups – see Figure 4, left side) or on Phase 2 shock activity levels (ps > 0.1) for the Rudy Experimental subjects. Irrespective of drug treatment condition, freezing levels increased during the latter 40 second Phase 1 chamber exposures (main effect of exposure: F(4,140) = 3.820, p < 0.007). Similar to findings in Experiment 1, these data suggest that repeated transports in the black transport buckets contribute to enhanced fear responses.
Figure 4.
Mean percentage of freezing as a function of drug and dosage during Phases 1, 3, and 4 of training for subjects trained using Rudy CPFE procedures in Experiment 2. Bars depict standard error of the mean.
3.2.2. CPFE method (Rudy Experimental): Phases 3 and 4
A 5 (Drug/Dose) × 6 (Minute) repeated measures ANOVA on Phase 3 (context test) freezing levels in the Rudy Experimental subjects revealed significant main effects of drug/dose, F(4,35) = 2.824, p < 0.040, and minute, F(5,175) = 64.922, p < 0.001. Tukey’s post-hoc tests of the drug/dose main effect revealed significantly lower levels of freezing in the high dose scopolamine group (0.5 mg/kg) relative to the methylscopolamine group (p < 0.038) and a marginally significant effect when comparing the high-dose scopolamine group with saline controls (p < 0.089; see Figure 4, middle). The remaining drug/dose contrasts failed to achieve statistical significance. For the main effect of minute, as in Experiment 1 freezing levels declined significantly after the first 2 minutes of Phase 3. Analyses of freezing data using adjusted critical values revealed significantly higher levels of freezing during the first two minutes relative to minutes 3–6 (minutes 1 and 2 did not differ statistically). Additionally, there was significantly greater freezing during the 3rd minute relative to minutes 4–6; minutes 4–6 did not differ significantly.
An ANOVA of Phase 4 (altered context) total freezing levels revealed no significant effect of drug/dose (p > 0.57). Within-group (drug/dosage) comparisons of Phase 3 versus Phase 4 freezing levels revealed higher levels of freezing at Phase 3 for all treatment groups (all Fs > 16; see Figure 4).
3.2.3. Standard contextual fear method: Phase 1
There were no significant differences as a function of drug dosage on freezing percentage during the first 2 minutes and 28 seconds of Phase 1 (preexposure period prior to shock delivery; see Figure 5, left side) or on activity levels during the 2 sec shock delivery at Phase 1 (ps > 0.28) for the Standard Experimental subjects.
Figure 5.
Mean percentage of freezing as a function of drug and dosage during Phases 1, 3, and 4 of training for subjects trained using standard fear conditioning procedures in Experiment 2. Bars depict standard error of the mean.
3.2.4. Standard contextual fear method: Phases 3 and 4
A 5 (Drug/Dose) × 6 (Minute) repeated measures ANOVA on Phase 3 freezing levels in the Standard Experimental subjects revealed a significant main effect of minute, F(5,165) = 20.815, p < 0.001. There were no significant effects involving drug/dosage (p > 0.1; see Figure 5, middle). There was significantly more freezing during each of the first 3 minutes of Phase 3 relative to each of the last 3 minutes of Phase 3 (Fs > 9). The lack of statistical significance involving drug/dosage held even when removing the seemingly aberrant methylscopolamine group from analyses and when examining only the first 3 minutes of Phase 3 (ps > 0.1).
As expected, ANOVA of Phase 4 total freezing levels revealed no significant effect of drug/dose (p > 0.334). Within-group (drug/dosage) comparisons of Phase 3 versus Phase 4 freezing levels revealed higher levels of freezing at Phase 3 for all treatment groups (all Fs > 10; see Figure 5).
3.2.5. Summary of findings: Experiment 2
Mice trained using the Rudy CPFE method showed greater scopolamine-induced impairments in contextual freezing compared to mice trained using a standard contextual fear paradigm. Though the Rudy CPFE mice displayed scopolamine-induced impairments, the predicted dose-response effects were not observed, as significant effects on conditioned freezing to the preexposure context were present only for the high-dose group (0.5 mg/kg). In the ‘standard’ group, lower freezing levels were observed in the high dose scopolamine groups relative to saline controls, but this difference failed to achieve statistical significance. An unexpected finding in this group was the low levels of freezing in subjects administered methylscopolamine, a muscarinic receptor antagonist shown to have negligible CNS effects. Factors contributing to this result will be explored further in the Discussion.
4. Discussion and conclusions
The present study is the first to employ the Rudy laboratory CPFE procedures in mice. Contextual freezing in Rudy CPFE groups was robust and greater than freezing levels in mice trained using CPFE procedures previously established in mice [32]. In Experiment 2 mice trained using the Rudy CPFE method showed greater scopolamine-induced impairment in contextual freezing relative to mice trained using a standard contextual fear paradigm. Given the established sensitivity of this CPFE procedure to pre-training hippocampal insult in rats [11, 21], these findings encourage future use of this novel mouse model in studies investigating the functional consequences of pre-existing hippocampal insult. Unlike the Rudy CPFE task, standard contextual fear preparations do not require pattern completion processes unique to the hippocampal system [12]. The Rudy CPFE task therefore provides a more sensitive measure of hippocampal integrity than standard contextual fear tasks. Contextual freezing levels in experimental mice trained using the Rudy CPFE method were similar to those reported by Rudy and colleagues using rats [18]. The most pronounced difference between our results and results from the Rudy laboratory concerns performance of control groups. CPFE control mice (Experiment 1) exhibited greater levels of freezing during the context test (20%; Figure 3) than control CPFE rats (approximately 10%; [21, 22]). Expected low levels of contextual freezing in controls can be attributed to the “immediate shock freezing deficit” [19, 41] whereby negligible levels of post-shock freezing are observed when shock is delivered immediately after placement in the training environment (also see [42]). According to Fanselow [19], sufficient context exposure prior to shock is necessary to form associations between the context and the shock US. Possible contributions to elevated contextual freezing in our controls – findings that are not in accord with the immediate shock hypothesis - are discussed below.
The interval between initial chamber exposure and shock delivery (1–2 sec, at Phase 2 immediate shock) in Rudy controls would be expected to yield negligible contextual freezing since these subjects were not preexposed to this context [18, 19, 28, 29, 43]. Elevated freezing at the context test (Phase 3) in Rudy control mice may therefore reflect contributions beyond the training/shock context. The possibility of (1) generalization between the polycarbonate cage used for preexposure (Phase 1) and the conditioning context, and/or (2) conditioning due to high shock intensity (see [45]) must be considered, though minimal freezing during the context test in Gould controls (Experiment 1) also preexposed to the polycarbonate cage and receiving the same shock (2 sec, 0.75 mA) suggests otherwise. Procedures specific to Rudy’s CPFE methodology (designed for rats) appear to be a more likely source of elevated freezing in control mice. Indeed, evidence for non-specific fear associated with transport was observed at Phase 1, as freezing increased with successive transports in Rudy subjects. To reduce these contributions to Phase 3 freezing modifications pertaining to transport may be considered, including use of a transport different from our black ice bucket, fewer transports at Phase 1, and/or use of a novel transport at the Phase 3 context test to reduce “priming” resulting from exposure to transport cues associated with Phases 1 and 2 (see [21] for use of a transport at the context test different from the transport used at the preexposure and shock phases).
Since the same transport was used at all phases of training, the hypothesis that fear associated with transport cues is driving much of the freezing at Phase 3 would predict comparable levels of freezing between the Phase 3 and 4 context tests in controls. Reduced freezing at Phase 4 (altered context test) in Rudy controls provides some argument against this proposal. Furthermore, unpublished findings suggest that this does not reflect an extinction-like effect, as reexposure to the original (Phase 3) test context 24 hours after the altered context test revealed elevated freezing in Rudy controls relative to their Phase 4 freezing levels. These findings indicate that cues specific to the immediate shock context contribute to elevated freezing in controls. If Phase 3 freezing in Rudy control mice is influenced by unexpected context-shock associations formed during the immediate shock session, the most logical modification may be to administer Phase 2 in a different context. Rudy et al. [21] demonstrated that robust contextual conditioning occurs when the immediate shock occurs in a context different from the preexposure and test context since a memory of the preexposure context during the shock phase is essential for forming context-shock associations [18]. If this applies to the mouse model, this adjustment may reduce non-specific contributions to contextual freezing.
Findings of significant scopolamine-induced contextual freezing impairments in Rudy CPFE subjects but not in mice trained using a standard fear conditioning preparation (Experiment 2) provide support for use of this mouse CPFE model in studies assessing functional consequences of pre-training hippocampal insult. The hippocampus receives extensive cholinergic input, and deficits resulting from muscarinic cholinergic antagonism are similar to effects produced by direct hippocampal insult [3, 35]. Since scopolamine was administered systemically, these outcomes cannot be attributed solely to disruptions in hippocampal functioning, though previous findings suggest that systemic scopolamine – particularly at the moderate doses used in the present study - does not significantly disrupt other neural regions essential for fear conditioning (e.g., amygdala; see [36]). Both direct hippocampal insult and scopolamine exposure have been linked with heightened motor activity, a condition that has potential to interfere with interpretations of freezing and activity level data [4, 36]. However, the absence of drug effects on Phase 1 behavior as well as unimpaired contextual freezing in methylscopolamine-exposed CPFE subjects suggests that scopolamine-induced deficits represent impaired learning instead of a performance deficit (but see below for possible contributions of scopolamine/methylscopolamine on US processing).
Our goal in designing the standard fear conditioning task (Experiment 2) was to follow procedures used in Rudy CPFE mice (e.g., single, unsignaled shock) such that the preexposure and immediate shock phases unique to the CPFE design were the salient differences between tasks. Using C57 male mice at an age similar to those used in the present study, Wiltgen et al. [29] demonstrated robust contextual conditioning in a standard fear preparation following intervals between initial context placement and shock (2 sec, 0.75 mA) of 60–180 sec. We employed these parameters, setting the context placement-shock interval at 148 sec since it matches the timing of the first shock delivery in the Feiro and Gould [39] study. The major differences between ours and the Feiro and Gould design are that (1) mice in the Feiro and Gould study received two shocks at training, with shock intensity at 0.57 mA, (2) shocks in the Feiro and Gould study were each preceded by a 30 sec white noise CS, and (3) total chamber exposure at training in Feiro and Gould’s [39] study (over 300 sec) exceeded that in used in our standard fear group (180 sec). These procedural differences may account for significant impairments in contextual fear conditioning resulting from moderate pre-training scopolamine (0.5 mg/kg) in the Feiro and Gould [39] study but not in the present study. Increasing the duration of context exposure at training in our ‘standard’ group may allow for the most appropriate comparison with both the Feiro and Gould design and with Rudy CPFE mice (500 sec of context exposure at training) and perhaps promote greater sensitivity to pre-training systemic scopolamine exposure.
Findings of reduced contextual freezing in methylscopolamine-exposed mice trained in the standard contextual fear paradigm were unexpected (Figure 5). Methylscopolamine is an analog of scopolamine that does not cross the blood brain barrier and is typically used as a control for peripheral contributions of muscarinic cholinergic antagonism. Since methylscopolamine had no effect on contextual freezing in the Rudy CPFE group, procedural differences between our standard and CPFE tasks may account for this dissociation. In contrast to CPFE subjects given injections on day 1 and receiving shock on the second day of training, mice in the ‘standard’ group were under the influence of methylscopolamine during shock administration (Phase 1). This raises the possibility that drug-induced attenuation in shock sensitivity contributed to these unexpected findings. Though this provides a feasible explanation for decreased contextual freezing in methylscopolamine-exposed subjects trained in the standard but not in the CPFE paradigm, it does not readily explain (non-significantly) lower levels of Phase 3 freezing in methylscopolamine-exposed ‘standard’ subjects relative to scopolamine-exposed ‘standard’ subjects. However, the dose of methylscopolamine used (1 mg/kg) was twice as high as our highest scopolamine dose (0.5 mg/kg). It may be that the 1 mg/kg dose elicited a decrease in (peripheral) pain threshold not reached at the lower doses of scopolamine. The absence of group differences in activity levels during shock administration in Experiment 2 does not support this proposal (see [36] for corresponding null effects of scopolamine or methylscopolamine on activity during the US in rats), but previous fear conditioning findings in C57 mice using a 1 mg/kg dose of scopolamine or methylscopolamine revealed evidence for decreased sensitivity to a footshock US based on other dependent measures associated with pain sensitivity (flinch and vocalization; [38]). Multiple dependent measures may therefore be necessary to assess the extent to which scopolamine and methylscopolamine-induced effects reflect altered US processing.
The present findings support use of this mouse CPFE model as an assay for hippocampal function. The rapid development of transgenic mice impaired in some aspect(s) of normal hippocampal functioning has made identification of tasks sensitive to (pre-training) hippocampal insult a priority. Standard fear conditioning paradigms are inadequate for linking hippocampal dysfunction with behavior (e.g., impaired contextual fear conditioning) since non-hippocampal strategies are readily employed when the hippocampus is compromised prior to training. The CPFE method established by Rudy and colleagues, on the other hand, requires an intact hippocampal formation and may therefore be ideal for identifying functional impairments resulting from hippocampal dysfunction in transgenic mouse models.
Research Highlights.
Alternative strategies can be employed in contextual fear conditioning (CFC) but not in the context preexposure facilitation effect (CPFE) when the hippocampus is compromised prior to training.
In Expt 1 we extend CPFE methods from rats to mice comparing 2 CPFE techniques.
In Expt 2, sensitivity of CPFE and CFC was assessed with scopolamine.
CPFE requires an intact hippocampus and is a useful paradigm for identifying functional impairments in transgenic mouse models.
Acknowledgements
This research was supported by grants from the National Institute on Aging, 1 R01 AG021925 and 1 R01 AG023742 to DSW-P.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Selden NRW, Everitt BJ, Jarrard LE, Robbins Complementary roles for the amygdala and hippocampus in aversive conditioning to explicit and contextual cues. Neuroscience. 1991;42:335–350. doi: 10.1016/0306-4522(91)90379-3. [DOI] [PubMed] [Google Scholar]
- 2.Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 3.Philips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 4.Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Maren S. Pavlovian fear conditioning as a behavioral assay for hippocampus and amygdala function: cautions and caveats. Eur J Neurosci. 2008;28:1661–1666. doi: 10.1111/j.1460-9568.2008.06485.x. [DOI] [PubMed] [Google Scholar]
- 6.Young SL, Bohenek DL, Fanselow MS. NMDA processes mediate anterograde amnesia of contextual fear conditioning induced by hippocampal damage: immunization against amnesia by context preexposure. Behav Neurosci. 1994;108:19–29. doi: 10.1037//0735-7044.108.1.19. [DOI] [PubMed] [Google Scholar]
- 7.Maren SM, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav Brain Res. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- 8.Frankland PW, Cestari V, Filipkowski RK, McDonald RJ, Silva AJ. The dorsal hippocampus is essential for context discrimination but not for contextual conditioning. Behav Neurosci. 1998;112:863–874. doi: 10.1037//0735-7044.112.4.863. [DOI] [PubMed] [Google Scholar]
- 9.Cho YH, Friedman E, Silva AJ. Ibotenate lesions of the hippocampus impair spatial learning but not contextual fear conditioning in mice. Behav Brain Res. 1999;98:77–87. doi: 10.1016/s0166-4328(98)00054-0. [DOI] [PubMed] [Google Scholar]
- 10.Richmond MA, Yee BK, Pouzet B, Veenman L, Rawlins JNP, Feldon J, et al. Dissociating context and space within the hippocampus: effects of complete, dorsal, and ventral excitotoxic hippocampal lesions on conditioned freezing and spatial learning. Behav Neurosci. 1999;113:1189–1203. doi: 10.1037/0735-7044.113.6.1189. [DOI] [PubMed] [Google Scholar]
- 11.Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. J Neurosci. 2004;24:2431–2439. doi: 10.1523/JNEUROSCI.1598-03.2004. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudy JW. Context representations, context functions, and the parahippocampal-hippocampal system. Learn Mem. 2009;16:573–585. doi: 10.1101/lm.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Philips RG, LeDoux JE. Lesions of the dorsal hippocampal formation interfere with background but not foreground contextual fear conditioning. Learn Mem. 1994;1:34–44. [PubMed] [Google Scholar]
- 14.Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, Fanselow MS. Context fear learning in the absence of the hippocampus. J Neurosci. 2006;26:5484–5491. doi: 10.1523/JNEUROSCI.2685-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anagnostaras SG, Maren S, Fanselow MS. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: within-subjects examination. J Neurosci. 1999;19:1106–1114. doi: 10.1523/JNEUROSCI.19-03-01106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anagnostaras SG, Josselyn SA, Frankland PW, Silva AJ. Computer-assisted behavioral assessment of Pavlovian fear conditioning in mice. Learn Mem. 2000;7:58–72. doi: 10.1101/lm.7.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res. 2000;110:73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- 18.Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: insights from a two-process model. Neurosci Biobehav Rev. 2004;28:675–685. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Fanselow MS. Factors governing one-trial contextual conditioning. Anim Learn Behav. 1990;18:264–270. [Google Scholar]
- 20.Rudy JW, O’Reilly RC. Conjunctive representations, the hippocampus, and contextual fear conditioning. Cogn Aff Behav Neurosci. 2001;1:66–82. doi: 10.3758/cabn.1.1.66. [DOI] [PubMed] [Google Scholar]
- 21.Rudy JW, Barrientos RM, O’Reilly RC. Hippocampal formation supports conditioning to memory of a context. Behav Neurosci. 2002;116:530–538. doi: 10.1037//0735-7044.116.4.530. [DOI] [PubMed] [Google Scholar]
- 22.Rudy JW, O’Reilly RC. Contextual fear conditioning, conjunctive representations, pattern completion, and the hippocampus. Behav Neurosci. 1999;113:867–880. doi: 10.1037//0735-7044.113.5.867. [DOI] [PubMed] [Google Scholar]
- 23.McNaughton BL, Morris RG. Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends Neurosci. 1987;10:408–415. [Google Scholar]
- 24.Sutherland RJ, Rudy JW. Configural association theory: the role of the hippocampal formation in learning, memory, and amnesia. Psychobiol. 1989;17:129–144. [Google Scholar]
- 25.Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 26.O’Reilly RC, McLelland JL. Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus. 1994;4:661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- 27.Paylor R, Tracy R, Wehner J, Rudy JW. DBA/2 and C57BL/6 mice differ in contextual fear but not auditory fear conditioning. Behav Neurosci. 1994;108:810–817. doi: 10.1037//0735-7044.108.4.810. [DOI] [PubMed] [Google Scholar]
- 28.Lattal KM, Abel T. An immediate-shock freezing deficit with discrete cues: a possible role for unconditioned stimulus processing mechanisms. J Exp Psychol Anim B. 2001;27:394–406. [PubMed] [Google Scholar]
- 29.Wiltgen BJ, Sanders MJ, Behne NS, Fanselow MS. Sex differences, context preexposure, and the immediate shock deficit in Pavlovian context conditioning with mice. Behav Neurosci. 2001;115:26–32. doi: 10.1037/0735-7044.115.1.26. [DOI] [PubMed] [Google Scholar]
- 30.Frankland PW, Josselyn SA, Anagnostaras SG, Kogan JH, Takahashi E, Silva AJ. Consolidation of CS and US representations in associative fear conditioning. Hippocampus. 2004;14:557–569. doi: 10.1002/hipo.10208. [DOI] [PubMed] [Google Scholar]
- 31.McHugh TJ, Tonegawa S. Spatial exploration is required for the formation of contextual fear memory. Behav Neurosci. 2007;121:335–339. doi: 10.1037/0735-7044.121.2.335. [DOI] [PubMed] [Google Scholar]
- 32.Kenney JW, Gould TJ. Nicotine enhances context learning but not context-shock associative learning. Behav Neurosci. 2008;122:1158–1165. doi: 10.1037/a0012807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goeldner C, Reiss D, Wichmann J, Kieffer BL, Ouagazzal A-M. Activation of nociceptin opioid peptide (NOP) receptor impairs contextual fear learning in mice through glutamatergic mechanisms. Neurobiol Learn Mem. 2009;91:393–401. doi: 10.1016/j.nlm.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knobloch M, Farinelli M, Konietzko U, Nitsch RM, Mansuy IM. Aβ Oligomer-mediated long-term potentiation impairment involves protein phosphatase 1-dependent mechanisms. J Neurosci. 2007;27:7648–7653. doi: 10.1523/JNEUROSCI.0395-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anagnostaras SG, Maren S, Fanselow MS. Scopolamine selectively disrupts the acquisition of contextual fear conditioning in rats. Neurobiol Learn Mem. 1995;64:191–194. doi: 10.1006/nlme.1995.0001. [DOI] [PubMed] [Google Scholar]
- 36.Anagnostaras SG, Maren S, Sage JR, Goodrich S, Fanselow MS. Scopolamine and Pavlovian fear conditioning in rats: dose-effect analysis. Neuropsychopharmacol. 1999;21:731–744. doi: 10.1016/S0893-133X(99)00083-4. [DOI] [PubMed] [Google Scholar]
- 37.Rudy JW. Scopolamine administered before and after training impairs both contextual and auditory-cue fear conditioning. Neurobiol Learn Mem. 1996;65:73–81. doi: 10.1006/nlme.1996.0008. [DOI] [PubMed] [Google Scholar]
- 38.DeLorey TM, Lin RC, McBrady B, He X, Cook JM, Lameh J, et al. Influence of benzodiazepine binding site ligands on fear-conditioned contextual memory. Eur J Pharmacol. 2001;426:45–54. doi: 10.1016/s0014-2999(01)01199-2. [DOI] [PubMed] [Google Scholar]
- 39.Feiro O, Gould TJ. The interactive effects of nicotinic and muscarinic cholinergic receptor inhibition on fear conditioning in young and agedC57BL/6 mice. Pharmacol Biochem Be. 2005;80:251–262. doi: 10.1016/j.pbb.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 40.Anagnostaras SG, Wood SC, Shuman T, Cai DJ, LeDuc AD, Zum KR, et al. Automated assessment of Pavlovian conditioned freezing and shock reactivity in mice using the VideoFreeze system. Front Behav Neurosci. 2010;4:1–11. doi: 10.3389/fnbeh.2010.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fanselow MS. Associative vs topographical accounts of the immediate shock-freezing deficit in rats: implications for the response selection rules governing species-specific defensive reactions. Learn Motiv. 1986;17:16–39. [Google Scholar]
- 42.Blanchard RJ, Fukunaga KK, Blanchard CD. Environmental control of defensive reactions to footshock. B Psychonomic Soc. 1976;8:129–130. [Google Scholar]
- 43.Kiernan MJ, Westbrook RF. Effects of exposure to a to-be-shocked environment upon the rat’s freezing response: evidence for facilitation, latent inhibition, and perceptual learning. Q J Exp Psychol-B. 1993;46:271–288. doi: 10.1080/14640749308401089. [DOI] [PubMed] [Google Scholar]