Abstract
Preterm birth represents the most significant problem in maternal-child health. The ongoing search to elucidate its underlying causes and pathophysiological mechanisms has identified maternal stress as a variable of interest. Based on emerging models of causation of complex common disorders, we suggest that the effects of maternal stress on risk of preterm birth may, for the most part, vary as a function of context. In this paper we focus on select key issues and questions that highlight the need to develop a better understanding of which particular subgroups of pregnant women, under what circumstances, and at which stage(s) of gestation, may be especially vulnerable to the potentially detrimental effects of maternal stress. Our discussion addresses issues related to the characterization and assessment of maternal stress and candidate biological (maternal-placental-fetal endocrine, immune, vascular and genetic) mechanisms. We propose the adoption of newer approaches (ecological momentary assessment) and a life-course perspective to further our understanding of the contribution of maternal stress to preterm birth.
Keywords: stress, preterm birth, psychosocial, maternal-placental-fetal endocrine, immune, vascular, genetic
1. Introduction
Preterm birth represents the most significant problem in maternal-child health in the United States. It is the leading cause of infant mortality and morbidity; its prevalence in our population is unacceptably high and has not decreased over the last 40 years; and its etiology is unknown in a substantial proportion of cases.1 The ongoing search to better elucidate its underlying causes and pathophysiological mechanisms has identified maternal stress as a variable of interest. The question of the role of stress in preterm birth is, however, complex and challenging for many reasons. First, the basic physiological and pathophysiological mechanisms that underlie the timing of onset of human parturition and preterm birth, respectively, are not yet well understood. Second, the study of stress processes in pregnancy is complicated by the effects that pregnancy-related alterations in maternal physiology produce on central and peripheral systems implicated in the experience of and psychobiological responses to stress. This paper does not purport to comprehensively discuss all the available findings on maternal stress and preterm birth; several reviews and perspectives on this topic have been published and are available elsewhere.2–9 Instead, we focus here on select key issues and questions that we submit warrant further consideration and discussion.
1.1 Rationale for considering a role for maternal stress in preterm birth
The rationale for considering a role for stress in preterm birth derives, in part, from concepts in evolutionary biology and developmental plasticity. The mother and her developing fetus both play an obligatory, active role in the process of human parturition. The length of gestation and timing of onset of mammalian parturition represents a phenotype that emerges as a consequence of the culmination of intrauterine development. Development is a plastic process, wherein a range of different phenotypes can be expressed from a given genotype (contained within the fertilized zygote). The unfolding of all developmental processes across the multi-contoured landscape from genotype to phenotype is context-dependent, wherein the developing embryo/fetus responds to, or is acted upon by, conditions in the internal or external environment. Based on the consideration that key environmental conditions that have shaped evolutionary selection and developmental plasticity include not only variation in energy substrate availability (i.e., nutrition) but also challenges that have the potential to impact the structural or functional integrity and survival of the organism (i.e., stress), it is likely and plausible that prenatal stress represents an important aspect of the intrauterine environment that would be expected to influence many, if not all, developmental outcomes.10 There is, in fact, some evidence that preterm birth may be a maternal adaptation to limit the energetic costs of a pregnancy in the face of adverse conditions, or a fetal adaptation to an unfavorable intrauterine environment when other facultative measures have been unsuccessful.11 Moreover, we submit the application of a prenatal stress and stress biology framework offers an excellent model system for the study of intrauterine development and associated developmental, birth and subsequent health-related phenotypes because it is increasingly apparent that the developing fetus acquires and incorporates information about the nature of its environment in part via the same biological systems that in an already-developed individual mediate adaptation and central and peripheral responses to endogenous and exogenous stress (e.g., the maternal-placental-fetal neuroendocrine and immune systems12).
From the perspective of the maternal compartment, another compelling rationale for considering a role for maternal stress as a contributor to preterm birth derives from the effort to elucidate and better understand the underlying reason(s) for the well-documented, persistent and large socioeconomic and racial/ethnic disparities in the population distribution of preterm birth. Many of the factors that disproportionally affect socially disadvantaged individuals, such as prenatal care, diet/nutrition, and health-related behaviors, have been shown to play a limited role in accounting for these disparities.13–16 The search for alternate explanations has led to the hypothesis that high levels of maternal stress may, in part, independently, or in combination with other factors, explain these disparities, because the experience of social disadvantage and minority racial/ethnic status is characterized by higher levels of psychosocial stress and lack of resources, and because stress and stress-related biological processes have been implicated in a wide array of adverse reproductive and other health outcomes.13, 17
2. Psychosocial stress and preterm birth: a brief overview
The belief that a mother’s emotional or psychological state during pregnancy may influence the development of her fetus has existed since ancient times across all cultures. Empirical studies examining the effects of prenatal psychosocial stress first appeared in the literature in the mid 1950s. Much of the earlier work in this area was, however, limited by major conceptual and methodological problems. Over the last two decades or so, larger, better-designed studies have begun to present a somewhat more consistent set of findings. A rapidly growing body of empirical evidence, based on these prospective, population-based studies in pregnant women of different socio-demographic, racial/ethnic and national backgrounds, now provides support for the premise that women experiencing high levels of psychosocial stress during pregnancy are at significantly increased risk for shortened gestation and preterm delivery, even after accounting for the effects of other established socio-demographic, biophysical, biomedical, and behavioral risk factors.2, 4, 6, 9, 18–20 Although the tremendous heterogeneity in study designs, study populations, methodology and measures makes comparisons of effect size difficult to interpret across studies, it appears that pregnant women reporting “high” levels of psychosocial stress are, on average, at approximately 25–60% increased risk for preterm birth compared to women reporting “low” levels of stress.
Based on these findings, several researchers and public health experts are now increasingly highlighting the need for a new, integrative research and clinical practice agenda, with an emphasis on biopsychosocial processes, including the impact of maternal stress, to address the problem of preterm birth.21, 22 In fact, recent guidelines for obstetric practice issued by the American College of Obstetricians and Gynecologists recommend performing psychosocial screening at least once during each trimester of pregnancy.21 However, before specific clinical guidelines and interventions can be developed for primary, secondary and tertiary prevention we suggest there are some critical issues that warrant further consideration, discussed below.
3. Issues and Questions
At the population level it appears that maternal stress represents a significant risk factor for shortened gestation and increased risk of preterm birth, and that the magnitude of the effect size may be similar to that of many other established socio-demographic, obstetric and behavioral risk factors. However, it also is clear that in any individual pregnancy the specificity and sensitivity of maternal stress as a predictor of adverse birth outcomes is, at best, modest. Some but certainly not all women who experience high levels of stress during pregnancy proceed to deliver preterm, and the challenge of identifying which particular women (or subgroups of women), in what circumstances (context), and at which stage(s) of gestation, are particularly susceptible to the potentially detrimental effects of prenatal stress has yet to be satisfactorily addressed. We suggest it is critical to address these issues before the present research findings can be translated into a public health and clinical framework. Many of these issues have been previously discussed; we attempt here to highlight and expand on some specific aspects related to study design and methodology, stressor-specificity, outcome-specificity, the nature of the relationship, potential interactions with other individual-level or contextual-level factors, and critical periods of susceptibility.
3.1 Study design and methodology
The same study design and methodology issues applicable for rigorous epidemiological, genetic and clinical studies of putative risk factors for preterm birth remain applicable for studies of maternal psychosocial stress. The importance of a prospective design (to minimize ascertainment bias), appropriate study population (to ensure adequate variability in the distribution of the exposures and outcomes of interest), well-defined and characterized measures of the birth phenotype (accurate dating of pregnancy and ascertainment of whether the birth was preceded by spontaneous labor or membranes rupture, or whether it was induced or followed elective c-section), comprehensive assessment of psychosocial stress and related constructs, and measurement of covariates and potential confounding variables, is emphasized.
The importance of accurate dating of pregnancy using last menstrual period (LMP) in combination with early ultrasound and standardization across studies remains a critical issue.23 Another related issue pertains to the use of the conventional <37 completed weeks gestation cutoff for defining preterm birth. Recognizing this cutoff is somewhat arbitrary. Some recent studies have reported that the effects of length of gestation on infant morbidity and mortality risk extend continuously across the normal range of pregnancy duration, instead of merely being a function of preterm birth.24, 25 Thus, in order to assess potential effects across the entire distribution instead of at only one tail of the distribution, the value of including measures of gestational length along a continuum (quantitative trait) in addition to the conventional, clinical categorical classification is emphasized.
The majority of human studies of the effects of prenatal stress necessarily employ a correlational design. The caveat that correlation cannot establish causality is well recognized. Obviously, it is not ethically possible to randomly assign humans to high stress exposure, and studies of interventions to lower stress may not provide the same information as those that would induce additional stress. While the use of experimental manipulations in animal models confers many benefits, one of their major limitations, particularly in the area of preterm birth research, is the considerable inter-species variation in physiological processes underlying parturition and in the developmental time line (discussed in greater length below). Thus, the use of well-designed prospective studies in representative populations with serial, longitudinal assessments to determine the nature and strength of the association of naturally-occurring variation in stress with subsequent birth outcomes after measuring and statistically adjusting for effects of other established sociodemographic, behavioral and environmental risk factors can go a long way to provide the best possible evidence that either supports or refutes an underlying causal model (see, e.g., the considerations about association and causation first articulated by Bradford Hill in his seminal 1965 paper26, and elaborated subsequently by others27–29).
Finally, the importance of good clinical phenotyping of preterm births is emphasized. Preterm birth is a heterogeneous entity in terms of the extent to which the birth is preterm (mild, moderate, or severe), as well as in terms of the precipitating events (induced or elective preterm birth, or spontaneous preterm birth following either preterm labor or preterm rupture of membranes). It is, therefore, important to recognize and examine the possibility that the contribution of maternal stress may vary between these different categories of preterm birth. For example, maternal stress would be hypothesized to have its largest marginal (main) effects on near-term spontaneous births, followed by near-term indicated births. In contrast, an important conditional (moderator) effect of maternal stress (e.g., interaction with intrauterine infection) would be hypothesized for earlier (moderate to severe) preterm births.
3.2 Characterization and assessment of maternal stress
A question that frequently comes up with respect to the putative effects of maternal stress on preterm birth risk concerns the elucidation of the aspects or domains of maternal stress that may be particularly salient. Although there is no universally accepted definition of psychosocial stress, it is clear that stress is not a unidimensional construct, but rather “a person-environment interaction,” in which there is a perceived discrepancy between environmental demands and the individual’s psychological, social or biological resources.30 This transactional view of the stress construct calls for the identification of stressful stimuli, subjects’ appraisal of these stimuli, and their emotional responses. As described in a recent review,4 most human studies of prenatal stress have used measures of major life event stress (e.g. death of a family member), catastrophic community-wide disasters (e.g. earthquakes, acts of terrorism), chronic stress, daily hassles, perceived stress, symptoms of depression or general anxiety, and pregnancy-specific anxiety. Although it is difficult to draw firm conclusions, it appears from this and other reviews that stressors such as major negative life events and catastrophes confer greater risk for preterm birth than less severe chronic stressful exposures and depressive symptoms (see, e.g.31–33). Moreover, measures of stress that focus on pregnancy-related concerns (pregnancy-specific anxiety) seem to be stronger predictors of preterm birth risk than measures of general anxiety.34–38 Our review of pregnancy-specific psychosocial measures36, 37, 39–49 suggests the major constructs that encompass pregnancy-specific stress relate to (a) affect about being pregnant (positive and negative), (b) pregnancy-specific concerns or anxiety about the health of the unborn baby, labor and delivery, and body size/image, (c) pregnancy-specific support (emotional, enacted), (d) pregnancy-related symptoms, and (e) attitudes towards the current pregnancy (intendedness, wantedness).
The distinction between various components or dimensions of psychological stress as discrete entities may be somewhat arbitrary. Different components of psychological stress are not randomly distributed; they tend to co-occur with one another. An acute circumstance such as a stressful life event (e.g., death of a family member) produces chronic psychological distress that could vary considerably across individuals in the nature, intensity and duration of its psychological and physiological consequences. Moreover, in some instances, acute psychological stressors may represent the culmination of a preceding period of increased and chronic psychological distress (e.g., if the death of the family member followed a period of long hospitalization). Chronic stress may, in part, reflect external events, and may, in part, reflect more persistent psychological attributes of the individual that are minimally related to external events (see discussion by DiPietro and colleagues50).
A substantial body of previous research has identified certain key dimensions of psychological stress that may be particularly salient in terms of their potential for producing unfavorable psychological and physiological consequences, especially the dimensions of predictability and controllability (see, e.g., the classical studies by Weiss,51, 52 reviewed by Sapolsky53 and Koolhaas and colleagues54). In addition to these dimensions we suggest there may be yet another dimension of stressful experience that warrants further consideration – the dimension of lability, or variability, or changeability, over time. In considering the cumulative effects of psychological stress, we hypothesize that the number and magnitude of psychological “ups” and “downs” experienced by the individual over the given time period of interest will produce an impact on the likelihood of stress-related health outcomes that is either independent of, or interacts with, the overall mean level of stress over that particular time period. This concept is akin to that of the negative consequences of poor glycemic control, such that among a group of individuals with the same average level of hyperglycemia, those individuals whose blood sugar levels fluctuate a great deal around the mean (number and magnitude of highs and lows) are at greater risk for hyperglycemia-related complications than those who maintain a more consistent or stable level of hyperglycemia. We note that the common approach to assessment of psychological stress using self-report retrospective recall measures does not lend itself well to the assessment of the dimensions of predictability or lability of stress over time.
Other psychological factors that may influence the relationship between maternal stress and preterm birth include maternal psychopathology (e.g. development of posttraumatic stress disorder after a severe stressor),55 personality traits, social support, and coping processes. Moreover, stress may not entirely be an individual-level phenomenon, but may also be linked to the individual’s social-structural context (e.g., experience of racism,56, 57 neighborhood characteristics58–60). Thus, the construct of prenatal psychosocial stress and the social-structural context within which stress is experienced by the individual are areas that clearly need further refinement in our effort to better understand the role of prenatal stress in preterm birth.
Another issue that sometimes comes up from broad and over-generalized assertions that “stress is bad for you and your baby” is the concern that we may inadvertently be contributing to yet another reason for increasing anxiety and worry among pregnant women – the worry about being worried! We believe this is a reasonable and justifiable concern and caution against over-interpretation or over-generalization of findings in this area of research. We do note that although some studies have reported a protective or beneficial effect of mild of moderate stress during pregnancy on certain child neurodevelopmental outcomes (see, e.g.50, 61 -- which, parenthetically, is consistent with the expectation of a fetal adaptation to accelerate development in the context of unfavorable circumstances), to the best of our knowledge there are no studies to date that have found a protective or beneficial effect of maternal stress on the risk of adverse birth outcomes such as preterm birth.
We submit that two key limitations to current approaches for the characterization and assessment of maternal stress in pregnancy relate to the a) problem of retrospective recall measures, and b) the lack of attention to the issue of psychobiological stress reactivity, discussed below.
3.2.1 The problem of retrospective recall measures
With the exception of studies of exposure to natural disasters, wars, or acts of terrorism, human studies to date in the stress and pregnancy outcomes area (including studies that employ a prospective design) have relied almost exclusively on self-report, retrospective-recall measures of stress. Respondents are typically asked to rate, on average, how stressed or depressed or anxious they have felt over the past week or month or since the beginning of their pregnancy. These traditional, self-report recall measures are prone to numerous systematic biases that undermine their validity. These measures rely on autobiographical memory (i.e., in time and place), as opposed to semantic memory (i.e., independent of time and place), and they rely on the respondents’ ability to first integrate and then summarize states and events across the reporting period. Autobiographical memory requires the use of certain heuristic strategies for recall or reconstruction that introduce not only random error but also systematic biases (see, e.g.62, 63). A key example of a biasing cognitive heuristic is the “availability heuristic” – more salient events that are easily “available” in memory, are given greater weight. Furthermore, emotional states and arousal at the time of encoding and recall can bias past memories. Also, the recency effect bias refers to better recall of more recent experience. In addition, most psychosocial and behavioral measures involve global assessment of events and/or states over time. Self-reports of global assessments, as opposed to reports of specific-event recall, are known to be particularly susceptible to all these biases, because they involve not only recall but also integration and summarization of these states and events across the reporting period. Thus, because of all these biases associated with the use of retrospective recall measures of psychosocial stress and related constructs in the context of this area of research, it is difficult to establish whether the modest effect sizes observed in the literature are a function of “true” weak or small effects of prenatal stress on birth outcomes, or of deficiency in measurement procedures.
3.2.2 Psychobiological stress responsivity
It is well-established that the likelihood of occurrence of a stress-related adverse health outcome is a function of not only the amount of actual or perceived stress exposure over time, but also of that individual’s biological propensity to respond to stress. The concept of biological stress reactivity refers to characteristics of the biological response (e.g., magnitude, duration) to a unit of stress exposure. Evoked physiological responses to behavioral challenge provide reliable and valid measures of an individual’s propensity for biological perturbation during stress, and such measures have proven useful in other health domains in predicting risk of stress-related outcomes (see, e.g.64, 65). We note that overwhelming majority of studies of prenatal stress and birth outcomes have considered only the prenatal stress exposure or experience side of the equation, but have neglected the issue of individual differences in biological stress reactivity in pregnancy. The principle of measuring response to a controlled stimulus certainly is not new; it is, in fact, widely employed in an array of settings, including the diagnostic laboratory (glucose tolerance test, dexamethasone suppression test), the behavioral medicine laboratory (cold pressor test, social evaluation threat (e.g., Trier Social Stress Test66)), and even in clinical obstetrical practice (e.g., pharmacological (glucose), physical (nipple stimulation, sound) or endocrine (oxytocin) challenges). However, only a very small number of studies have used this approach in studies of stress and stress processes in human pregnancy (e.g.67, 68 and see69 for a review). Our use of this approach has led to the findings that there is a progressive attenuation of not only maternal biological but also psychological responses to stress over the course of gestation,67 and that after accounting for the effects of other established risk factors individual differences in the degree (trajectory) of this attenuation is a significant predictor of shortened length of gestation and risk of earlier delivery.70 Thus, we submit that the use of standardized behavioral or psychological probes, using appropriate and ecologically-valid stimuli, to assess individual differences in the responsivity of stress-related maternal or fetal biological systems at various time points over the course of gestation may prove useful in identifying individuals who may be particularly susceptible to the deleterious effects of stress.
3.3 Critical periods of susceptibility
Fetal development is a logarithmic process, with rapid mitosis at early stages and cellular hypertrophy and accumulation of fat, glycogen, and connective tissue later in gestation. It is well-established that there are several sensitive or critical periods in development, and there may be critical periods during pregnancy of altered vulnerability to the effects of prenatal stress. These periods may be related to the times in gestation corresponding to specific developmental events, or to time-specific changes in maternal or fetal physiological responses to stress over the course of gestation.71, 72 Although this premise of susceptible periods is well-supported in the animal literature, a relatively small number of human studies of prenatal stress have incorporated multiple assessments of stress across gestation and tested hypotheses about time-specific effects.
The state of pregnancy is associated with major alterations in maternal biology, including changes in stress-sensitive endocrine, immune and vascular processes and control mechanisms (feedback loops). Furthermore, evidence suggests that the state of pregnancy is associated with alterations in maternal biological responsivity to a stimulus or stressor. Evoked physiological responses to pharmacological and physical challenges have been studied in pregnant women, and these studies generally suggest maternal responses are dampened in pregnancy, and particularly in the later stages of gestation. For example, administration of exogenous corticotrophin-releasing hormone (CRH) in late but not mid pregnancy failed to evoke a significant pituitary or adrenal response,73, 74 and administration of dexamethasone produced less suppression of cortisol production.75 Similarly, autonomic responses (heart rate, blood pressure) to a variety of challenges, including exercise, orthostatic challenge, and the cold pressure test, are attenuated in pregnancy.76–78 Moreover, we have noted that maternal appraisals of psychological stress seem to progressively decrease as pregnancy advances.79–81 We recently conducted a longitudinal study of psychological and biological responses to a standardized laboratory stressor at 3 time points in a population of pregnant and non-pregnant women. Our findings suggest that timing of stressor exposure in pregnancy had a significant impact on the magnitude of the autonomic stress response, such that biological stress responses were greater in earlier compared to later gestation. Furthermore, as pregnancy advanced, the cortisol response to awakening progressively decreased,67 and a larger cortisol awakening response in late pregnancy and reduced attenuation of this response from early to late gestation was significantly associated with shorter gestational length.70
3.3.1 Bidirectional relationship between the maternal and fetal compartments: implications for causality of effects
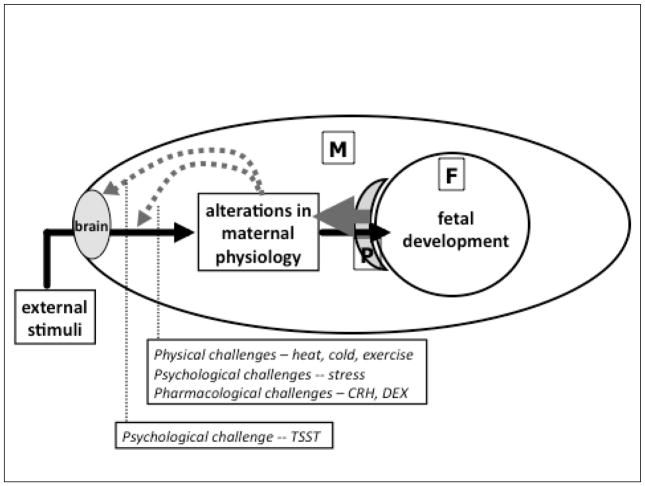
What drives these changes in maternal responses to stress over gestation and hence potentially alters its effects on the developing fetus and birth outcomes? An implicit assumption regarding the direction of causality is that it is unidirectional in nature, in that potentially unfavorable circumstances in a pregnant women’s life that are perceived or appraised by the maternal brain as stressful may then influence maternal physiology, which, in turn, may impact the developing fetus via direct or indirect biological mechanisms. However, the pregnancy-related alterations in maternal physiology are known to originate from the fetal and not maternal compartment (the placenta is an organ of fetal origin). Because these observed variations in maternal physiology (that have consequences for maternal stress responses and fetal susceptibility to maternal stress exposure) originate from, and are sustained by, the developing feto-placental unit over gestation, it gives rise to the intriguing possibility of reciprocal, bi-directional causality. In this scenario, variations in processes that underlie fetal growth, maturation and development result in variations in maternal physiology which, in turn, influence or moderate the effects of maternal stress exposure on the developing fetus, including perhaps, subsequent changes in maternal physiology and fetal susceptibility to maternal stress (see schematic illustration of this concept in Figure 1). The object (fetus) of the influence (maternal stress) is the cause of the process that produces the influence (changes in maternal stress responsivity); this process is dynamic, recursive and bi-directional between the fetal and maternal compartment over the course of gestation.10
FIGURE 1. MATERNAL PHYSIOLOGY AND FETAL DEVELOPMENT: A CASE OF RECIPROCAL DETERMINISM.
External conditions during pregnancy that are appraised by the maternal brain as stressful may result in stress-related alterations in maternal physiology that, in turn, may influence fetal development and birth outcomes. However, the state of pregnancy itself produces progressive changes in maternal physiology that have been shown to alter maternal peripheral (physiological) responses to a variety of physical, psychological and pharmacological challenges, and also maternal central (psychological) responses to a social stressor. These pregnancy-related changes in maternal physiology (that alter maternal psychological and physiological responses to exogenous stressors) originate from the fetal-placental compartment. Hence, the fetus, the object of maternal stress-related physiological perturbations, also is the initiator of alterations in maternal physiology that progressively dampen maternal responses to exogenous (and likely endogenous) challenges – a case of reciprocal determinism.
3.4 The role of context: potential interaction effects of maternal stress
Preterm birth is a multi-factorial outcome. At the individual level, the major risk categories include sociodemographic, historical, biophysical, obstetric, behavioral, psychosocial, genetic and other environmental factors. Studies of the effects of maternal stress and related psychosocial processes on preterm birth generally treat these other risk factors as potential confounding variables and attempt to account (adjust) for their putative effects by either study design (subject selection criteria) or statistical adjustment. However, emerging concepts of causation for complex common disorders, including but not limited to preterm birth, suggest it is not only possible, but in fact probable, that causation does not reside in any single factor or in the additive effects of numerous factors, but lies at the interface between multiple risk factors (interaction, or multiplicative effect).82, 83 We consider here, by way of example and illustration of this critically important concept, two conditions that are important in their own right from the standpoint of preterm birth, and for which there is a high plausibility of interaction effects with maternal stress, namely nutrition and infection.
3.4.1 Prenatal stress x nutrition interactions
As discussed briefly in Section 1 above, the two fundamental processes that are believed to shape evolutionary selection and developmental plasticity are variation in energy substrate availability (nutrition) and challenges that have the potential to impact the structural or functional integrity and survival of the organism (stress). Preterm birth may represent a maternal adaptation to limit the energetic costs of a pregnancy in the face of adverse conditions, or a fetal adaptation to an unfavorable intrauterine environment when other facultative measures have been unsuccessful.11 Maternal nutrition, assessed by indicators of body size such as body-mass index (BMI), nutritional intake or serum measures of nutritional biomarkers, is a well-established risk factor for preterm birth.84, 85 Growing evidence supports the concept of a bi-directional interaction between nutrition and stress, such that the effects of nutrition on health may vary as a function of stress, or that the effects of stress on health may vary as a function of nutritional status. For example, several experimental studies in animals have demonstrated that nutritional manipulations, particularly in the preconception or early pregnancy period, may produce their effects on maternal and fetal outcomes, including preterm birth, via alterations in stress biology (cortisol, inflammatory cytokines).86–94 Conversely, studies in animals and humans of stress induction (by exposure to laboratory-based social stressors or endocrine stress analogues) have demonstrated effects on feeding behavior, food choice (high calorie dense food preference) and the metabolic fate of food in target tissues.95–99 We note, however, that only a small number of studies have examined the relationship between maternal psychosocial stress and diet or nutritional state in pregnancy,100, 101 and we are not aware of any studies that have modeled the interaction effects of maternal stress and nutrition on risk of preterm birth.
3.4.2 Prenatal stress x infection interactions
The effects of intrauterine or systemic infection during pregnancy on preterm birth risk are well-established.84, 102 It is clear, however, that exposure to microbes alone does not inevitably result in infection, and the presence of infection during pregnancy does not inevitably result in preterm birth. Clearly, there are processes that modulate individual differences in susceptibility to acquiring infection and also to the pathophysiological consequences of infection. Maternal stress is an attractive and plausible candidate for increasing the likelihood of developing infection during pregnancy and for potentiating its pathophysiological consequences. We and others have shown that maternal psychosocial stress is associated with an increased risk for reproductive tract infection during pregnancy.103, 104 Moreover, biological stress mediators such as corticotrophin-releasing hormone (CRH) may potentiate inflammation in the context of antigen stimulation (infection).105 The synergistic effects of high psychosocial stress and reproductive tract or intrauterine infection on preterm birth risk have, however, not been examined to date.
4. STRESS PATHWAYS TO PRETERM BIRTH
The influence of psychosocial factors on health outcomes is mediated either directly via physiological pathways, or indirectly via behavioral pathways, or both.19, 106–108 The influence of psychosocial stress in increasing the propensity for engaging in a wide array of unhealthy behaviors, including key behavioral risk factors for preterm birth such as poor diet/nutrition and smoking, is well-established in studies in the general population as well as in pregnant women (see, e.g.37, 109). As discussed above (in Section 3.4), one important issue that has yet to be addressed adequately relates to the possibility of an interaction effect between maternal stress and nutrition, such that the effect of stress on preterm birth risk varies as a function of the nutritional status of the mother, or that the effect of nutritional status on preterm birth risk varies as a function of maternal stress and stress biology during pregnancy.
Biological Pathways to Parturition
Human parturition involves the time- and context-dependent interplay of several systems and signaling molecules within the maternal, placental and fetal tissues. Events leading to parturition start early in pregnancy, occur sequentially, and involve feedback systems. Clinical and experimental evidence broadly support the concept that preterm birth is determined by multiple genetic and environmental factors that reflect the interactions among one or more of several pathophysiological processes, which may ultimately share common biological pathways leading to uterine contractions, cervical changes and rupture of membranes. These pathways include (a) early or excessive activation of the maternal-placental-fetal (MPF) neuroendocrine axis; (b) decidual/chorioamniotic/fetal inflammation caused by ascending genitourinary tract or systemic infection; (c) uteroplacental vascular lesions caused by coagulopathy, hypertension, or abruption/decidual hemorrhage; and (d) pathological distention of the uterus, caused by multiple gestation.1, 34, 110–113 We recognize the possibility that these pathways may not represent all potential routes to preterm birth, and we also note that these pathways are unlikely to be mutually-exclusive and distinct, but rather with substantial overlap and interaction between them.
4.1 The maternal-placental-fetal (MPF) endocrine, immune and vascular systems in mammalian pregnancy: an overview
Pregnancy produces major alterations in neuroendocrine, immune and vascular function, including changes in hormone and cytokine levels and control mechanisms (feedback loops), that are crucial in providing a favorable environment within the uterus and fetal compartment for growth, differentiation and maturation and conveying signals when the fetus is ready for extrauterine life. Starting very early in gestation the placenta, the first fetal organ to develop and function, produces hormones, neuropeptides, growth factors and cytokines, and appears to function in a manner resembling that of compressed hypothalamic-pituitary-target systems.114 Glucocorticoid physiology (cortisol in humans) has received extensive and well-placed consideration as a critical endocrine mediator of fetal development and birth outcomes, with an emphasis on not only hormone production but also hormone action mediated by tissue-specific glucocorticoid receptor expression, sensitivity and affinity, and by maternal-fetal transfer mediated by the activity of the placental 11β-hydroxysteriod dehydrogenase enzyme system (see115 for a recent review). Less well recognized is the potential and perhaps equally important role of the peptide corticotrophin-releasing hormone (CRH). In primates, but not other mammals, the placenta synthesizes and releases CRH in large amounts into the fetal and maternal circulations. In contrast to the inhibitory influence on hypothalamic CRH production, cortisol stimulates placental CRH production,116 and this positive feedback loop results in a progressive amplification of CRH and cortisol production over the course of gestation.117
With respect to the immune axis, one of the major endeavors of pregnancy-related alterations in immune function is to achieve and maintain the optimal balance between tolerating the fetal semi-allograft while not suppressing maternal immune responses to an extent that increases maternal or fetal susceptibility to infection. Thus, a generalized reduction of maternal immune responsiveness occurs during pregnancy, mediated by hormonal changes (e.g., increased levels of progesterone), trophoblast expression of key immunomodulatory molecules, and a progressive switch from a TH1/TH2 balance to a predominantly T-helper 2-type pattern of cytokines.118 A recent study of the longitudinal modulation of the cytokine profile across human pregnancy reported an overall decrease in proinflammatory cytokine trajectories in the innate and adaptive arms of the immune system and an increase in counter-regulatory cytokines as pregnancy progresses.119
Cardiovascular adaptations in pregnancy include anatomic changes, as well as changes in blood volume, cardiac output, and systemic vascular resistance, all of which result in conditions that are normal in pregnancy but would constitute signs of cardiovascular dysfunction in the non-pregnant state.120 The study of cardiovascular changes associated with pregnancy is of relevance, since related disorders (e.g. hypertension, preeclampsia) are one of the major indicators for elective preterm delivery.1, 84
4.2 Interactions between maternal-placental-fetal neuroendocrine, immune-inflammatory and vascular pathways in pregnancy
Although distinct neuroendocrine, immune/inflammatory and vascular pathways have been described, growing evidence suggests that these and other physiological systems involved in pregnancy are highly inter-related, and that they extensively regulate and counter-regulate one another. The potential complexity of the inter-relationships among these physiologic systems is seen when considering the role of infection in the etiology of adverse fetal developmental and birth outcomes. For example, inflammatory cytokines that are produced in response to infection, such as TNF-α, IL-1β and IL-6, can activate components of the MPF-neuroendocrine system that also increase the risk of premature birth.121–124 Conversely, it is also known that HPA hormones such as CRH and cortisol influence the production of cytokines and modulate the inflammatory response to infection.125–127 Central CRH, acting via glucocorticoids and catecholamines, inhibits inflammation, whereas CRH directly secreted by peripheral nerves and mast cells stimulates local inflammation.128 Moreover, it has been postulated that acute and chronic infections may be risk factors for uteroplacental vasculopathies that may be associated with premature birth.111 Impaired nutrient and oxygen exchange associated with uteroplacental vasculopathy may stress the fetus and result in increased production of placental-fetal hormones such as CRH, while placental CRH, in turn, may influence fetal-placental circulation.129 Thus, the relationship of a well-defined risk factor, like prenatal infection, to adverse birth outcomes is likely to involve complex interactions between the endocrine, immune and vascular systems.
4.3 Stress biology in human pregnancy
Stress biology refers to the set of biological adaptations in response to challenges or demands that threaten or are perceived to have the potential to threaten the stability of the internal milieu of the organism. The nervous, endocrine, immune and vascular systems play a major role in adaptations to stress. There are no direct neural, vascular or other connections between the mother and her developing fetus -- all communication between the maternal and fetal compartments is mediated via the placenta, an organ of fetal origin. Based on the physiology of stress, parturition and the evidence linking maternal stress to earlier delivery, we have proposed a biobehavioral framework of stress and adverse birth outcomes.12 Substantial evidence in non-pregnant humans and animals demonstrates that stress produces activation of the neuroendocrine system, exaggerated inflammatory responses and alterations in vascular hemodynamics, 130, 131 however, these associations cannot be assumed to also be present in the pregnant state because the above-described changes in endocrine, immune and vascular physiology have consequences for attenuating the responsivity of these systems to stress. Our model proposes a major role for placental CRH in mediating the effects of maternal stress because this system is known to not only play an important role in human parturition, but also because it provides a biologically-plausible link between known environmental risk factors and preterm birth, including in the context of racial/ethnic disparities. For instance, placental production of CRH – a key activator of the hypothalamic-pituitary-adrenal (HPA) axis – has been proposed as an early event that regulates the subsequent cascade of fetal, placental and maternal physiological processes involved in human parturition;112, 132 variations in markers of maternal-placental-fetal neuroendocrine activity, such as placental CRH and estriol concentrations in early to mid gestation, significantly predict subsequent differences in the timing of onset of parturition and risk of preterm birth;133 large racial/ethnic differences have been observed in both the production of MPF hormones and the magnitude of their effect on risk of preterm birth;134, 135 significant racial/ethnic differences have been reported in relative allele frequencies in various regions of the CRH gene;136 Maternal-placental-fetal neuroendocrine activity is altered by obstetric risk conditions and also exposure to environments that reflect adverse social and behavioral conditions;137, 138 and the effects of the infection/inflammation and uteroplacental vasculopathy may be modulated, in part, by the activity of placental CRH and other elements of the maternal-placental-fetal neuroendocrine pathway.17, 132, 139
4.3.1 Prenatal psychosocial stress and maternal-placental-fetal endocrine function
Placental CRH, the key mediator of stress-related maternal-placental-fetal endocrine function in primates, is stress-sensitive. A series of in vitro studies by Petraglia and colleagues140–142 have shown that CRH is released from cultured human placental cells in a dose-response manner in response to all the major biological effectors of stress, including cortisol, inflammatory cytokines, and hypoxia. Other in vivo studies have found significant correlations among maternal pituitary-adrenal stress hormones (ACTH, cortisol) and placental CRH levels.143–145 Moreover, maternal psychosocial stress is significantly correlated with maternal pituitary-adrenal hormone levels, (ACTH, cortisol146–149) that are known to stimulate placental CRH secretion. Some,137, 138, 150 but not all studies,151, 152 also have reported direct associations between maternal psychosocial stress and placental CRH function. In one recent study an association between placental CRH and preterm birth was found only in women with high chronic stress.153 A recent report suggests that a maternal stress signal, elevated cortisol early in pregnancy, is associated with a significant and precocious rise in placental CRH later in pregnancy, and that this effect is associated with risk for preterm delivery.154 Thus, substantial in vitro and in vivo evidence indicates that the placenta detects and responds to a variety of maternal physiological and psychological stress signals in a manner consistent with promoting earlier parturition. We note that one of the challenges in human pregnancy research is the identification of biomarkers that reflect long-term or cumulative stress exposure. In this context, measures of cortisol in hair samples seem to be a promising candidate biomarker -- two recent studies have reported significant associations between measures of maternal psychosocial stress in pregnancy and hair cortisol levels.34, 155
4.3.2 Prenatal psychosocial stress and immune function
Studies have reported that elevated psychosocial stress in pregnant women is associated with higher circulating levels of inflammatory markers like C-reactive protein (CRP) and the pro-inflammatory cytokines IL-1b, IL-6 and TNF-α, with lower circulating levels of the anti-inflammatory cytokine IL-10 and ex vivo endotoxin (LPS)-stimulated levels of IL-1b and IL-6.156–159 Another recent study of pro-inflammatory responses to an in vivo antigen challenge (influenza virus vaccination) in pregnant women reported an association between depressive symptoms and sensitization of the inflammatory cytokine responses.157 Some studies also have reported associations between maternal psychosocial stress and reproductive tract infection (bacterial vaginosis) – a risk factor for preterm birth – in pregnancy.103, 104
4.3.3 Prenatal psychosocial stress and vascular function
Vascular disorders in pregnancy such pregnancy-induced hypertension and preeclampsia are the major indications for elective preterm delivery. Findings suggest that maternal psychosocial stress is significantly associated with increased risk of hypertensive disorders in pregnancy.160–165 In one study, blood pressure responses to a laboratory-based psychosocial stressor in pregnant women predicted the length of gestation and infant birth weight,166 however, this effect was not apparent when blood pressure responses were assessed in non-pregnant state and related to birth outcomes in subsequent pregnancies.152 Reduced aortic blood flow velocity and elevated uteroplacental doppler flow velocity waveform indices have been associated with adverse birth outcomes and impaired neonatal status.68 A few studies have reported that among pregnant women, high anxiety is associated with reduced uterine,167 fetal and umbilical blood flow and pulsatility patterns indicative of fetal hypoxia and compensatory redistribution of blood flow to the fetal brain.168, 169
Placental CRH seems to play an important role in the context of cardiovascular physiology in pregnancy. Several studies have reported an association between CRH and hypertensive disorders.170–172 Furthermore, elevated levels of CRH have been shown to correlate significantly with abnormal uteroplacental flow wave forms.173, 174 Although not conclusive, the reported data support the notion of stress-related vascular pathophysiology contributing to preterm birth.
4.3.4 Multivariable pathway models linking maternal psychosocial stress, biological function and preterm birth
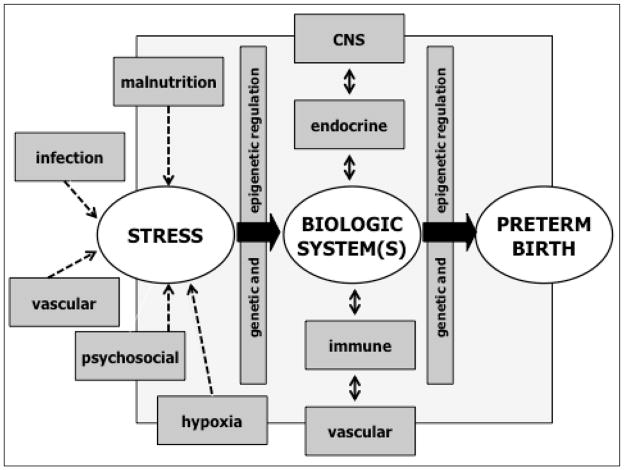
Although the evidence discussed above provides biological plausibility for the concept that psychosocial stress may contribute to preterm birth risk via its effects on several stress-sensitive biological processes implicated in parturition, tests of pathway (meditational) models to demonstrate these linkages have generally been unsuccessful. There are many possible reasons for this difficulty in generating findings to support these hypothesized linkages, including weaknesses in the conceptualization and operationalization of psychosocial stress (discussed in Section 3.2 above), or of the biological processes of interest (single time point biomarkers, biomarkers in systemic circulation as a proxy for local (tissue-specific) biology, biomarkers assessed at basal state as a proxy for biological responses to challenge, etc.), or of the study design (use of cross-sectional assessments to depict biological processes that are characterized by progressive alterations over time/course of gestation). These issues not withstanding, we submit that another major limitation may relate to the over-simplistic manner in which these processes are believed to operate. Consider, for example, a conceptual model frequently evoked in the stress and preterm birth literature, in which variation in maternal psychosocial stress in pregnancy is hypothesized to correlate with variation in maternal cortisol in pregnancy, which, in turn is hypothesized to correlate with variation in gestational age at birth. We know of no instance in biology where there is a simple, one-to-one correspondence between a specific psychological and specific biological state. In the example considered here, cortisol production in vivo is influenced by not only the psychological state of the individual but concurrently by a host of other conditions such as variations in the nutritional milieu, physical activity, infection/inflammation, hypoxia, sleep, chronobiological state, and, in the case of pregnancy, additionally by the stage of gestation. Moreover, the effects of psychological stress on cortisol production likely vary as a consequence of these other conditions (i.e., an interaction, or conditional, or effect-modification model). Second, we know of no instance in biology when perturbation within a particular biological system remains constrained within that system. Regardless of the nature of the initiating stressor (psychological, nutritional, infectious, etc), once a perturbation is produced within the endocrine system, this perturbation will inevitably produce secondary perturbations in the brain, immune, vascular and other endocrine-related systems. The nature and magnitude of these secondary perturbations will then, through negative or positive feedback loops, alter the nature, magnitude and time-course of the initial stress-initiated perturbation (in this case, of the endocrine system). Thus, the effect of stress-related endocrine function on the outcome of interest (preterm birth) will vary as a function of the state of the immune and vascular systems and their feedback effects on the endocrine system (again, an interactional, or conditional, or effect modification model). Therefore, conceptualizations of simple one-to-one correspondence between variation in psychological and biological systems and health outcomes that do not measure and account for the effects of and interactions with other important states or contexts on a particular biological system, or of the impact of secondary perturbations in other closely-linked biological systems on the primary biological system of interest, would be hard pressed to demonstrate evidence to support a pathway or meditational model. It will require the adoption and implementation of a systems biology approach to uncover the complex web of interrelationships and pathways inherent in in vivo human models of complex multi-factorial disorders such as preterm birth (see Figure 2).
FIGURE 2. CONTRIBUTION OF MATERNAL STRESS AND STRESS BIOLOGY TO PRETERM BIRTH: A MATTER OF CONTEXT.
The is no one-to-one correspondence between psychosocial stress and a stress-sensitive biology; the nature, magnitude and duration of the effects of maternal psychosocial stress during pregnancy on any given stress-sensitive biological system are likely altered by the context of other conditions/stressors such as those related to nutrition, infection and hypoxia. Similarly, the nature, magnitude and duration of the effects of a given stress-sensitive biological system in pregnancy on maternal and fetal target systems involved in parturition are likely altered by the secondary perturbations in other closely-related biological systems and their feedback effects on the primary biological system under consideration.
4.3.5 Applicability of animal models for research on prenatal stress and preterm birth
While the use of experimental manipulations using animal models confers many benefits, one of their limitations, particularly in the area of preterm birth research, is the considerable inter-species variation that exists in physiological processes underlying parturition and in the developmental time line. We consider here, for example, the comparative endocrinology of mammalian pregnancy. One of the major challenges in elucidating the endocrinology of human parturition arises from the existence of large inter-species differences in the endocrinology of pregnancy, even among closely-related mammals. In most mammals, the shift over the course of gestation in the balance from a progesterone-dominant to an estrogen-dominant milieu to promote labor is effected by the direct conversion of progesterone to estrogen in the placenta via a mechanism regulated by the fetal hypothalamic-pituitary-adrenal (HPA) axis. Maturation of the fetal HPA axis results in rising concentrations of fetal cortisol, which, in turn, induces placental expression of the enzyme 17α-hydroxylase (P450 cytochrome system) required for the conversion of progesterone to estrogen. However, unlike most other mammals, primates cannot convert progesterone to estrogen because they lack the placental enzyme 17α-hydroxylase required for this purpose. Instead, the primate placenta utilizes another precursor hormone –dehydroepiandrosterone sulfate (DHEA-S) produced by the fetal adrenal zone – to synthesize estrogen (estriol (E3); reviewed in175–177). A second major difference in the endocrinology of pregnancy is that primates are the only mammals that produce the neuropeptide corticotrophin-releasing hormone (CRH) in the placenta.178, 179 However, even among primates, there are large differences in the pattern of production, activity and regulation of placental CRH between New and Old World monkeys and humans. In particular, the baboon exhibits a mid-gestational peak in secretion.180 Studies in other primates also indicate differences in production of the CRH binding protein (CRH-BP179). Great apes (chimpanzees and gorillas) are the only primates where patterns of placental CRH production in pregnancy are similar to those seen in humans, with a clear exponential increase over the entire course of gestation.177
5. GENETIC AND EPIGENETIC CONSIDERATIONS
The ascertainment of genetic contributors to preterm birth is an area of active and intense investigation. Studies in humans of familial aggregation suggest a heritable component for preterm birth. It is clear that the major component of this heritability is polygenic (as opposed to classical Mendelian origin).1 Studies using a candidate gene approach have yielded limited success; they have been difficult to replicate and have explained only a small proportion of the phenotypic variation in preterm birth. Larger studies based on genome wide associations or admixture mapping are currently in progress. These studies, coupled with the elucidation of maternal-fetal gene-gene (epistasis) and gene-environment interactions, will be required for a better understanding of this complex problem (for recent reviews and commentaries see1, 181–187).
With respect to the issue of the contribution of maternal stress to preterm birth, it is very likely that genetic and epigenetic variations will be determined to play an important role in moderating the association between prenatal stress and preterm birth risk via maternal-fetal gene-gene and gene-environment interactions at multiple levels, originating with the likelihood of encountering stressful life circumstances, and culminating in modifying the effects of stress-related biological processes on parturition-related target tissues (see Figure 3). For example, women who are carriers of certain genotypes (high risk alleles in dopamine-related genes) may be more likely to place themselves in stressful life circumstances.188–190 The psychological appraisal of potentially stressful circumstances may be influenced directly by the maternal genotypic variation (e.g., in the serotonin transporter gene191) or indirectly by the fetal genotype (via its effect on alterations in maternal physiology that, in turn, influence maternal psychological appraisals). Next, the ensuing effects of maternal stressful experience on maternal and fetal biology may be moderated by the genetic and epigenetic characteristics of the mother and fetus (e.g., variants in the glucocorticoid receptor gene192), respectively. Finally, the effects of stress-related physiological alterations on target maternal and fetal tissues implicated in parturition may be further influenced by the genetic and epigenetic makeup of the mother and fetus, respectively.
FIGURE 3. MATERNAL-FETAL GENE-ENVIRONMENT INTERACTIONS IN HUMAN PARTURITION.
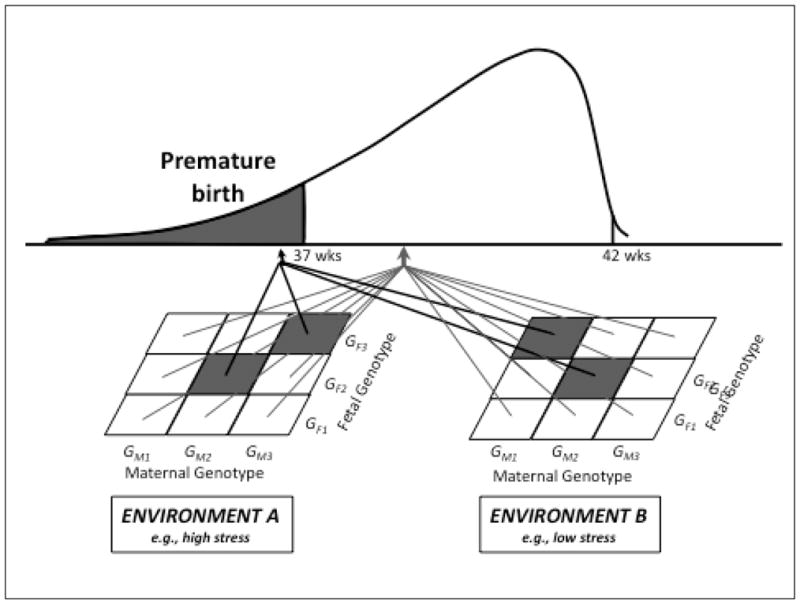
Combinations of genes (epistasis), rather than single genes or single gene variants, likely produce effects on complex health and disease risk outcomes. For birth outcomes such as preterm birth, combinations of genes of the mother as well as the fetus need to be considered (maternal-fetal genetic interactions). The effects of certain maternal-fetal genetic combinations may vary as a function of environmental context (e.g., maternal stress level; gene-environment interactions).
To date, only a small number of studies have systematically addressed the issue of a genetic predisposition for susceptibility to psychosocial stress and related psychobiological states. For example, we and others have described that certain poylmorphisms in the promotor region of the gene encoding the glucocorticoid receptor are associated with changes in the regulation of the hypothalamus-pituitary-adrenal (HPA) axis at different levels including basal level, feedback regulation, and response following a psychosocial stressor (summarized in192). Because several genes that code for proteins involved in the regulation of the stress response also are involved in the physiology of pregnancy and parturition (e.g., CRH, cortisol, IL-6, etc.), individual differences in genetic variation may be another factor underlying susceptibility in terms of the potentially adverse effects of maternal stress on pregnancy outcomes. The participation of placental CRH as a central molecule in regulating various aspects of pregnancy, fetal development and birth outcomes has been discussed in the preceding sections. Thus, DNA sequence variations in the CRH gene, the CRH receptor genes, the glucocorticoid receptor gene, and other genes encoding key enzymes and binding proteins in their biosynthetic pathways may have important implications in this context.
Genetic and epigenetic mechanisms have been proposed to explain the observed racial/ethnic disparities in preterm birth, particularly with respect to the hypothesized contribution of prenatal stress. As discussed earlier, the observed racial/ethnic disparity in preterm birth is commonly assumed to reflect the burden of adverse societal conditions associated with minority racial/ethnic status in the U.S. Prenatal stress is a plausible mediator of the effects of race/ethnicity on preterm birth via one or both of two possibilities: greater cumulative exposure to stress, and greater vulnerability to the effects of stress (arising from differences in psychobiological responses to stress). The characterization of racial/ethnic differences in DNA sequence or epigenetic variation in genes associated with the stress response will prove particularly informative in this regard. For instance, we and others have previously reported significant racial/ethnic differences in stress-related hormonal states in human pregnancy.17, 135 These racial/ethnic differences in neuroendocrine function in pregnancy may, in turn, reflect one or more of three possibilities: First, that particular genetic variations associated with pathophysiology are more frequent in specific racial/ethnic populations. Second, that there are no differences in the frequency of particular genetic variations across population subgroups, however, they are phenotypically expressed only under certain environmental conditions or exposures associated with particular racial/ethnic populations (e.g., high psychosocial stress, reproductive tract infection, social or cultural behavioral practices). And third, that there are no differences in the frequency of particular genetic variations across population subgroups, however, specific gene regions are preferentially expressed or silenced by epigenetic modifications that occurred during sensitive or critical periods of the mother’s own development under environmental conditions associated with particular racial/ethnic populations (e.g., intrauterine exposure to high stress or infection193). One of our on-going projects is in the process of evaluating these possibilities by determining whether the population structure and functional significance of maternal-fetal genetic variation and gene-environment interactions vary as a function of race/ethnicity.
6. EMERGING CONCEPTS AND ADVANCES: ECOLOGICAL MOMENTARY ASSESSMENT (EMA) OF STRESS AND BIOBEHAVIORAL PROCESSES IN HUMAN PREGNANCY
In the preceding sections we have discussed some key issues regarding limitations of traditional approaches in the assessment of maternal psychosocial stress and related constructs, characterization of psychobiological stress responses in pregnancy, and evaluation of context-specific effects in the link between prenatal stress, stress-related biological processes and preterm birth. We propose here that recent technological and methodological advances now afford the opportunity to address many of these issues and limitations through the application of the ecological momentary assessment (EMA) approach to the study of maternal stress and stress biology in human pregnancy. EMA methods emphasize the longitudinal, repeated collection of information about respondents’ momentary, or current, state, affect, experience, behavior and biology in real time.194 These methods serve to minimize biases inherent in retrospective recall approaches because immediate reports of a respondent’s current state or activity do not require retrieval from memory and other processing and are accordingly far less subject to distortions and biases. In addition to obtaining more accurate summary estimates over time, this methodology allows for the computation of indices of other potentially important dimensions such as variability (lability), context-specificity, and dynamic measures of temporal linkages between psychosocial states and biological processes. EMA methods provide greater ecological validity than laboratory-based measures because they are collected in naturalistic settings as respondents go about their normal, day-to-day activities. While individual differences in reactivity to a controlled stimulus in a laboratory setting seem to be relatively stable cross tasks and time, associations between reactivity in the laboratory and in naturalistic settings have generally been only moderate195, 196 because laboratory measures are not obtained at comparable conditions of behavior state and activity in natural, everyday settings.197 Because aggregation and assessment at multiple time points increases the range of stimuli sampled and reduces measurement error, EMA results are more reliable, and variations in values across settings and situations can be assessed.63, 198–201 EMA techniques also require that assessments be timed to ensure they span a wide range of times and locations, therefore tapping at the respondent’s full repertoire of states and behaviors. Last, repeated observations of states and responses not only allow the assessment of within-subjects variation (to reliably compute measures of variability or lability of psychological and physiological states over time), but also measure how a target variable covaries with situational antecedents.62 We suggest that EMA approaches can be used to compute measures of biological stress reactivity that reflect the degree of biological perturbation produced by a unit change in psychological stress (using a within-subject specific criterion), after accounting for the effects of other important influences on biology, including time of day, diet, physical activity, and sleep. We note, for example, that some of our recent findings suggest that ecological momentary assessment (EMA)-derived measures of maternal psychosocial stress are better predictors cortisol in pregnancy that traditional measures, and that EMA-derived measures of cortisol during pregnancy are better predictors of birth outcomes (length of gestation) than cortisol assessed in the clinical setting.70, 202 For these reasons we suggest that the use of EMA–based measures of real-time, ambulatory, repeated sampling of psychological, behavioral and biological states during pregnancy holds promise to address critical questions and gain new, more precise information about the magnitude and context-specificity of the effects of maternal psychosocial stress and biological stress reactivity on pregnancy and birth outcomes.
7. A LIFE-COURSE PERSPECTIVE
We submit it may be important to adopt a life-course perspective in considering the issue of the contribution of maternal stress to preterm birth. The life-course perspective conceptualizes reproductive and birth outcomes as the product of not only the nine months of pregnancy, but of the entire life course of the mother from her own conception onward (or even before her own conception), and leading up to the index pregnancy.203, 204 The suggestion that maternal health prior to pregnancy may have important bearing on pregnancy outcomes is not new,205 but a growing body of evidence is beginning to shed light on the mechanisms by which life-course factors, including stress, might influence birth outcomes such as preterm birth. Two broad mechanisms have been postulated: early programming, and cumulative pathways.
The early programming model suggests that a stimulus or insult (e.g. stress), operating at a critical or sensitive period, can result in a permanent or long-term change in the structure or function of the organism that becomes manifest in health and disease risk in later life.206–208 For example, animal as well as human studies have shown that exposure to maternal stress during pregnancy and in early childhood can program the stress reactivity of her offspring, and that this effect persists well into adulthood.209–211 This process may be mediated by epigenetic alterations in the glucocorticoid receptor gene in the developing brain.212, 213 Exposure to stress hormones during sensitive periods of immune maturation in early life may also influence immune programming, leading to altered immune responses in later life.214 In a series of recent reports on a population of young adults exposed specifically during intrauterine life to high maternal psychosocial stress, we found that prenatal stress exposure was associated with significant dysregulation of metabolic, immune, endocrine and cognitive function consistent with increased risk for obesity, insulin resistance and diabetes, allergy and atopic disorders, and dementia.215–218
Animal studies support the plausibility that such alterations may be transmitted across generations by non-genomic mechanisms.219 Thus, hypothetically, preterm birth may result from not only maternal stress, but stress of the grandmother during her pregnancy which may program the mother’s endocrine and immune stress responses in utero; programmed stress hyper-reactivity could put the mother at greater risk for preterm delivery when she herself becomes pregnant.203
The cumulative pathways model suggests that throughout the life-course exposures and insults gradually accumulate through episodes of illness or injury, adverse environmental conditions and risky behaviors, leading to declines in health and function over time.220 Such risk accumulation creates wear and tear, or “allostatic load”, on the body’s regulatory processes that are central to the maintenance of allostasis.221 Allostatic load refers to the cumulative physiological toll resulting from chronic over- or under-activity of allostatic systems. This may result from overexposure to stress hormones, as often occurs in chronic and repeated stress. Over-exposure to glucocorticoids can adversely impact an individual’s central nervous system, immune system and metabolism. For example, studies have found that animals and humans subjected to chronic and repeated stress exhibit elevated basal cortisol levels and dysregulated hypothalamic-pituitary-adrenal (HPA) response to natural or experimental stressors.222, 223 This HPA dysregulation may reflect the loss of feedback inhibition via down-regulation of glucocorticoid receptors in the brain.222 Similarly, chronically elevated levels of cortisol may also lead to not only relative immune suppression, but also immune-inflammatory dysregulation due to the loss of counter-regulation by the HPA axis.224 Thus, HPA and immune-inflammatory dysregulation are two of several possible pathways by which cumulative stress over the life-course may lead to increased vulnerability to preterm birth as a consequence of stress or infection in pregnancy.
The life-course perspective suggests that vulnerability to preterm birth may be traced to not only stress exposure during pregnancy, but also to host stress responses (e.g. psychobiological autonomic, endocrine and immune/inflammatory stress reactivity) that has been patterned by early programming and cumulative pathways mechanisms over the life-course. Some evidence supports these concepts of the developmental origins of preterm birth, or of the link between allostatic load and preterm birth. Among pregnant women, a history of exposure to early life trauma or abuse has been associated with not only an increased risk of preterm birth225 but also with a higher likelihood of depression,226 reproductive tract infection,227 altered cortisol activity,228 and engaging in preterm birth-related risky behaviors.229 A recent report provides evidence of a three-way interaction in African-American women between lifetime experience of racism, depressive symptoms, and stress in pregnancy on preterm birth risk.230 In another study, non-pregnant women with previous history of infection-related preterm birth have been shown to exhibit higher antigen-stimulated proinflammatory cytokine (TNF) levels; however this study was unable to determine whether the pro-inflammatory trait preceded or followed the occurrence of a preterm birth.231 Thus, the life-course perspective suggests a need to expand the time horizon for preterm birth research, to assess precursors (including stress) at points more distant temporally than has been examined in most previous research, and to understand not only disease pathways but also pre-disease pathways, defined as the “early and long-term biological, behavioral, psychological and social precursors to disease”.232 The life-course perspective also suggests the need for a new prevention paradigm in preterm birth, one that emphasizes not only risk reduction during pregnancy, but also health promotion and optimization of women’s health before conception and over the life course. Accordingly, studies are currently underway to test the effects of preconceptional health interventions on pregnancy and birth outcomes (see, e.g.233–235).
The life course perspective also offers another way to look at the issue of racial/ethnic disparities in preterm birth. As discussed earlier, the cause of these persisting racial disparities remains largely unexplained. Most extant studies have focused on differential exposures to risk and protective factors during pregnancy, such as genetic or socioeconomic differences, maternal behaviors, prenatal care utilization, psychosocial stress, and perinatal infections. These factors during pregnancy, however, may not adequately account for the racial gap in PTB.22 Disparities in birth outcomes (including preterm birth) may represent the consequences of not only differential exposures during pregnancy, but more important differential cumulation of protective and risk factors over the life course.203 The life-course perspective offers an explanatory model for how chronic stressors, such as lifelong experience of racism, “get under the skin” to affect the physiology of the pregnant mother and developmental biology of the child from conception onward.236 It provides the biological basis for the “weathering hypothesis”– that the effects of social inequality on the health of populations may compound with age, leading to growing gaps in health status through young and middle adulthood that can go on to affect fetal health.237
8. SUMMARY AND CONCLUSION
The question of the contribution of maternal stress to preterm birth is a challenging issue. Concepts in evolutionary biology and developmental plasticity support a rationale for considering a role for maternal stress in preterm birth. Evidence from population-based epidemiological and clinical studies suggests that after accounting for the effects of other established socio-demographic, obstetric and behavioral risk factors, women reporting higher levels of psychological stress during pregnancy are at significantly increased risk of preterm birth. However, at the individual level, the specificity and sensitivity of maternal stress as a predictor of preterm birth risk is, at best, modest. In order to translate population-level findings to public health and clinical practice applications it is critical to identify which subgroups of women, in what circumstances, and at which stage(s) of gestation, may be particularly susceptible to the potentially detrimental effects of high prenatal stress. This will require progress on three major fronts. First, the limitations in current approaches to characterize and assess maternal stress need to be addressed, including the problems inherent in retrospective recall measures of stress and the failure to consider the role of individual differences in maternal psychobiological stress responsivity. Second, the possibility needs to be addressed that the effects of maternal stress, for the most part, may be conditional or context-dependent. We suggest that maternal nutrition and infection are candidate processes of considerable interest in this context. Third, it is critical to arrive at a better understanding of the biological pathways by which maternal stress may impact human parturition. We suggest that maternal-placental-fetal neuroendocrine, immune/inflammatory and vascular processes are attractive candidate pathways because they are responsive to stress and they participate in the physiology of parturition. Multivariable conditional models will be required to elucidate the context-dependent effects of maternal stress during pregnancy on these candidate biological systems and on their interactive effects on parturition. Studies of stress-related genetic and epigenetic processes that incorporate the maternal as well as fetal genomes and consider gene-gene (epistasis)and gene-environment interactions are emphasized.
We contend that recent technological and methodological advances now afford the opportunity to address many of these limitations through the adoption of the ecological momentary assessment (EMA) approach to the study of maternal stress and stress biology in human pregnancy. EMA methods emphasize the longitudinal, repeated collection of information about momentary or current states, affects, experiences, behaviors and biology in real time and in naturalistic settings. Finally, we espouse a life-course perspective that conceptualizes reproductive and birth outcomes as the product of not only circumstances and events during gestation but over the entire life course of the mother beginning at the time of her own conception (or before) and leading up to the index pregnancy.
We end by noting that large, national multi-center studies are currently underway of the association of genetic and environmental determinants in early development with maternal and child health outcomes including, but not limited to, preterm birth (e.g., the U.S. National Children’s Study238 and the NICHD Study of Preterm Birth in Nulliparous Women (nuMoM2b study)). We expect that findings from these studies will go a long way in informing our efforts to arrive at a better understanding of the contribution of maternal stress to preterm birth.
Acknowledgments
The preparation of this manuscript was supported, in part, by US PHS (NIH) grants RO1 HD-060628 and PO1 HD-047609 to PDW, RO1 HD-065825 to SE, and RO1 MH-091351 to CB.
Footnotes
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFRENCES
- 1.Muglia LJ, Katz M. The enigma of spontaneous preterm birth. N Engl J Med. 2010 Feb 11;362(6):529–535. doi: 10.1056/NEJMra0904308. [DOI] [PubMed] [Google Scholar]
- 2.Beydoun H, Saftlas AF. Physical and mental health outcomes of prenatal maternal stress in human and animal studies: a review of recent evidence. Paediatr Perinat Epidemiol. 2008 Sep;22(5):438–466. doi: 10.1111/j.1365-3016.2008.00951.x. [DOI] [PubMed] [Google Scholar]
- 3.Chambliss LR. Intimate partner violence and its implication for pregnancy. Clin Obstet Gynecol. 2008 Jun;51(2):385–397. doi: 10.1097/GRF.0b013e31816f29ce. [DOI] [PubMed] [Google Scholar]
- 4.Dunkel Schetter C. Psychological science on pregnancy: stress processes, biopsychosocial models, and emerging research issues. Annu Rev Psychol. 2011;62:531–558. doi: 10.1146/annurev.psych.031809.130727. [DOI] [PubMed] [Google Scholar]
- 5.Hodnett ED, Fredericks S, Weston J. Support during pregnancy for women at increased risk of low birthweight babies. Cochrane Database Syst Rev. (6):CD000198. doi: 10.1002/14651858.CD000198. [DOI] [PubMed] [Google Scholar]
- 6.Paarlberg KM, Vingerhoets AJ, Passchier J, Dekker GA, Van Geijn HP. Psychosocial factors and pregnancy outcome: a review with emphasis on methodological issues. J Psychosom Res. 1995 Jul;39(5):563–595. doi: 10.1016/0022-3999(95)00018-6. [DOI] [PubMed] [Google Scholar]
- 7.Shah PS, Zao J, Ali S. Maternal Marital Status and Birth Outcomes: A Systematic Review and Meta-Analyses. Matern Child Health J. Aug 6; doi: 10.1007/s10995-010-0654-z. [DOI] [PubMed] [Google Scholar]
- 8.Hobel CJ, Goldstein A, Barrett ES. Psychosocial stress and pregnancy outcome. Clin Obstet Gynecol. 2008 Jun;51(2):333–348. doi: 10.1097/GRF.0b013e31816f2709. [DOI] [PubMed] [Google Scholar]
- 9.Alder J, Fink N, Bitzer J, Hosli I, Holzgreve W. Depression and anxiety during pregnancy: a risk factor for obstetric, fetal and neonatal outcome? A critical review of the literature. J Matern Fetal Neonatal Med. 2007 Mar;20(3):189–209. doi: 10.1080/14767050701209560. [DOI] [PubMed] [Google Scholar]
- 10.Entringer S, Buss C, Wadhwa PD. Prenatal stress and developmental programming of human health and disease risk: concepts and integration of empirical findings. Curr Opin Endocrinol Diabetes Obes. 2010 Dec;17(6):507–516. doi: 10.1097/MED.0b013e3283405921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pike IL. Maternal stress and fetal responses: evolutionary perspectives on preterm delivery. Am J Hum Biol. 2005 Jan-Feb;17(1):55–65. doi: 10.1002/ajhb.20093. [DOI] [PubMed] [Google Scholar]
- 12.Wadhwa PD. Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology. 2005 Sep;30(8):724–743. doi: 10.1016/j.psyneuen.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Giscombe CL, Lobel M. Explaining disproportionately high rates of adverse birth outcomes among African Americans: the impact of stress, racism, and related factors in pregnancy. Psychol Bull. 2005 Sep;131(5):662–683. doi: 10.1037/0033-2909.131.5.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGrady GA, Sung JF, Rowley DL, Hogue CJ. Preterm delivery and low birth weight among first-born infants of black and white college graduates. Am J Epidemiol. 1992 Aug 1;136(3):266–276. doi: 10.1093/oxfordjournals.aje.a116492. [DOI] [PubMed] [Google Scholar]
- 15.Schoendorf KC, Hogue CJ, Kleinman JC, Rowley D. Mortality among infants of black as compared with white college-educated parents. N Engl J Med. 1992 Jun 4;326(23):1522–1526. doi: 10.1056/NEJM199206043262303. [DOI] [PubMed] [Google Scholar]
- 16.Ventura SJ, Martin JA, Curtin SC, Mathews TJ, Park MM. Births: final data for 1998. Natl Vital Stat Rep. 2000 Mar 28;48(3):1–100. [PubMed] [Google Scholar]
- 17.Wadhwa PD, Culhane JF, Rauh V, et al. Stress, infection and preterm birth: a biobehavioural perspective. Paediatr Perinat Epidemiol. 2001 Jul;15 (Suppl 2):17–29. doi: 10.1046/j.1365-3016.2001.00005.x. [DOI] [PubMed] [Google Scholar]
- 18.IOM. Preterm Birth: Causes, Consequences, and Prevention. The National Academy of Science Press; 2007. [PubMed] [Google Scholar]
- 19.Adler NE, Rehkopf DH. U.S. disparities in health: descriptions, causes, and mechanisms. Annu Rev Public Health. 2008;29:235–252. doi: 10.1146/annurev.publhealth.29.020907.090852. [DOI] [PubMed] [Google Scholar]
- 20.Shah PS, Shah J. Maternal exposure to domestic violence and pregnancy and birth outcomes: a systematic review and meta-analyses. J Womens Health (Larchmt) Nov;19(11):2017–2031. doi: 10.1089/jwh.2010.2051. [DOI] [PubMed] [Google Scholar]
- 21.ACOG. ACOG Committee Opinion No. 343: Psychosocial risk factors: perinatal screening and intervention. Obstet Gynecol. 2006 Aug;108(2):469–477. doi: 10.1097/00006250-200608000-00046. [DOI] [PubMed] [Google Scholar]
- 22.Institute of Medicine. Preterm birth: Causes, consequences, and prevention. Washington DC: The National Academies Press; 2007. Committee on Understanding Premature Birth and Assuring Healthy Outcomes, Board on Health Sciences Policy. [Google Scholar]
- 23.Lynch CD, Zhang J. The research implications of the selection of a gestational age estimation method. Paediatr Perinat Epidemiol. 2007 Sep;21 (Suppl 2):86–96. doi: 10.1111/j.1365-3016.2007.00865.x. [DOI] [PubMed] [Google Scholar]
- 24.Tita AT, Landon MB, Spong CY, et al. Timing of elective repeat cesarean delivery at term and neonatal outcomes. N Engl J Med. 2009 Jan 8;360(2):111–120. doi: 10.1056/NEJMoa0803267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, Kramer MS. Variations in Mortality and Morbidity by Gestational Age among Infants Born at Term. J Pediatr. 2008 Oct 23; doi: 10.1016/j.jpeds.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Hill AB. The environment and disease: association or causation? Proceedings of the Royal Society of Medicine. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoefler M. The Bradford Hill considerations on causality: a counterfactual perspective. Emerging Themes in Epidemiology. 2005;2(11) doi: 10.1186/1742-7622-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phillips CV, Goodman KJ. The missed lessons of Sir Austin Bradford Hill. Epidemiol Perspect Innov. 2004 Oct 4;1(1):3. doi: 10.1186/1742-5573-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ward AC. The role of causal criteria in causal inferences: Bradford Hill’s “aspects of association”. Epidemiol Perspect Innov. 2009;6:2. doi: 10.1186/1742-5573-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lazarus RS, Folkman S. Stress, Appraisal, and Coping. New York: Springer; 1984. [Google Scholar]
- 31.Zhu P, Tao F, Hao J, Sun Y, Jiang X. Prenatal life events stress: implications for preterm birth and infant birthweight. Am J Obstet Gynecol. Jul;203(1):34, e31–38. doi: 10.1016/j.ajog.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 32.Khashan AS, McNamee R, Abel KM, et al. Rates of preterm birth following antenatal maternal exposure to severe life events: a population-based cohort study. Hum Reprod. 2009 Feb;24(2):429–437. doi: 10.1093/humrep/den418. [DOI] [PubMed] [Google Scholar]
- 33.Xiong X, Harville EW, Mattison DR, Elkind-Hirsch K, Pridjian G, Buekens P. Exposure to Hurricane Katrina, post-traumatic stress disorder and birth outcomes. Am J Med Sci. 2008 Aug;336(2):111–115. doi: 10.1097/MAJ.0b013e318180f21c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kramer MS, Lydon J, Seguin L, et al. Stress pathways to spontaneous preterm birth: the role of stressors, psychological distress, and stress hormones. Am J Epidemiol. 2009 Jun 1;169(11):1319–1326. doi: 10.1093/aje/kwp061. [DOI] [PubMed] [Google Scholar]
- 35.Roesch SC, Dunkel-Schetter C, Woo G, Hobel C. Modeling the types and timing of stress in pregnancy. Anxiety, Stress & Coping. 2004;17:87–102. [Google Scholar]
- 36.Wadhwa PD, Sandman CA, Porto M, Dunkel-Schetter C, Garite TJ. The association between prenatal stress and infant birth weight and gestational age at birth: a prospective investigation. Am J Obstet Gynecol. 1993 Oct;169(4):858–865. doi: 10.1016/0002-9378(93)90016-c. [DOI] [PubMed] [Google Scholar]
- 37.Lobel M, Cannella DL, Graham JE, DeVincent C, Schneider J, Meyer BA. Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health Psychol. 2008 Sep;27(5):604–615. doi: 10.1037/a0013242. [DOI] [PubMed] [Google Scholar]
- 38.Dole N, Savitz DA, Hertz-Picciotto I, Siega-Riz AM, McMahon MJ, Buekens P. Maternal stress and preterm birth. Am J Epidemiol. 2003 Jan 1;157(1):14–24. doi: 10.1093/aje/kwf176. [DOI] [PubMed] [Google Scholar]
- 39.Collins NL, Dunkel-Schetter C, Lobel M, Scrimshaw SC. Social support in pregnancy: psychosocial correlates of birth outcomes and postpartum depression. J Pers Soc Psychol. 1993 Dec;65(6):1243–1258. doi: 10.1037//0022-3514.65.6.1243. [DOI] [PubMed] [Google Scholar]
- 40.Curry MA, Burton D, Fields J. The Prenatal Psychosocial Profile: a research and clinical tool. Res Nurs Health. 1998 Jun;21(3):211–219. doi: 10.1002/(sici)1098-240x(199806)21:3<211::aid-nur4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 41.DiPietro JA, Christensen AL, Costigan KA. The pregnancy experience scale-brief version. J Psychosom Obstet Gynaecol. 2008 Dec;29(4):262–267. doi: 10.1080/01674820802546220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DiPietro JA, Ghera MM, Costigan K, Hawkins M. Measuring the ups and downs of pregnancy stress. J Psychosom Obstet Gynaecol. 2004 Sep-Dec;25(3–4):189–201. doi: 10.1080/01674820400017830. [DOI] [PubMed] [Google Scholar]
- 43.Huizink AC, Mulder EJ, Robles de Medina PG, Visser GH, Buitelaar JK. Is pregnancy anxiety a distinctive syndrome? Early Hum Dev. 2004 Sep;79(2):81–91. doi: 10.1016/j.earlhumdev.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 44.Rini CK, Dunkel-Schetter C, Wadhwa PD, Sandman CA. Psychological adaptation and birth outcomes: the role of personal resources, stress, and sociocultural context in pregnancy. Health Psychol. 1999 Jul;18(4):333–345. doi: 10.1037//0278-6133.18.4.333. [DOI] [PubMed] [Google Scholar]
- 45.Sable MR, Wilkinson DS. Impact of perceived stress, major life events and pregnancy attitudes on low birth weight. Fam Plann Perspect. 2000 Nov-Dec;32(6):288–294. [PubMed] [Google Scholar]
- 46.Speizer IS, Santelli JS, Afable-Munsuz A, Kendall C. Measuring factors underlying intendedness of women’s first and later pregnancies. Perspect Sex Reprod Health. 2004 Sep-Oct;36(5):198–205. doi: 10.1363/psrh.36.198.04. [DOI] [PubMed] [Google Scholar]
- 47.Yali AM, Lobel M. Coping and distress in pregnancy: an investigation of medically high risk women. J Psychosom Obstet Gynaecol. 1999 Mar;20(1):39–52. doi: 10.3109/01674829909075575. [DOI] [PubMed] [Google Scholar]
- 48.Zambrana RE, Dunkel-Schetter C, Collins NL, Scrimshaw SC. Mediators of ethnic-associated differences in infant birth weight. J Urban Health. 1999 Mar;76(1):102–116. doi: 10.1007/BF02344465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alderdice F, Lynn F. Factor structure of the Prenatal Distress Questionnaire. Midwifery. Sep 9; doi: 10.1016/j.midw.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 50.Dipietro JA, Novak MF, Costigan KA, Atella LD, Reusing SP. Maternal psychological distress during pregnancy in relation to child development at age two. Child Dev. 2006 May-Jun;77(3):573–587. doi: 10.1111/j.1467-8624.2006.00891.x. [DOI] [PubMed] [Google Scholar]
- 51.Weiss JM. Somatic effects of predictable and unpredictable shock. Psychosom Med. 1970 Jul-Aug;32(4):397–408. doi: 10.1097/00006842-197007000-00008. [DOI] [PubMed] [Google Scholar]
- 52.Weiss JM. Effects of coping behavior in different warning signal conditions on stress pathology in rats. J Comp Physiol Psychol. 1971 Oct;77(1):1–13. doi: 10.1037/h0031583. [DOI] [PubMed] [Google Scholar]
- 53.Sapolsky R. Why zebras don’t get ulcers: a guide to stress, stress-related diseases, and coping. New York: W.H. Freeman & Co; 1994. pp. 1–19.pp. 283–285. [Google Scholar]
- 54.Koolhaas JM, de Boer SF, Buwalda B, van Reenen K. Individual variation in coping with stress: a multidimensional approach of ultimate and proximate mechanisms. Brain Behav Evol. 2007;70(4):218–226. doi: 10.1159/000105485. [DOI] [PubMed] [Google Scholar]
- 55.Lipkind HS, Curry AE, Huynh M, Thorpe LE, Matte T. Birth outcomes among offspring of women exposed to the September 11, 2001, terrorist attacks. Obstet Gynecol. Oct;116(4):917–925. doi: 10.1097/AOG.0b013e3181f2f6a2. [DOI] [PubMed] [Google Scholar]
- 56.Kramer MR, Hogue CR. What causes racial disparities in very preterm birth? A biosocial perspective. Epidemiol Rev. 2009;31:84–98. doi: 10.1093/ajerev/mxp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dominguez TP. Race, racism, and racial disparities in adverse birth outcomes. Clin Obstet Gynecol. 2008 Jun;51(2):360–370. doi: 10.1097/GRF.0b013e31816f28de. [DOI] [PubMed] [Google Scholar]
- 58.Collins JW, Jr, David RJ. Urban violence and African-American pregnancy outcome: an ecologic study. Ethn Dis. 1997 Autumn;7(3):184–190. [PubMed] [Google Scholar]
- 59.O’Campo P, Burke JG, Culhane J, et al. Neighborhood deprivation and preterm birth among non-Hispanic Black and White women in eight geographic areas in the United States. Am J Epidemiol. 2008 Jan 15;167(2):155–163. doi: 10.1093/aje/kwm277. [DOI] [PubMed] [Google Scholar]
- 60.Culhane JF, Elo IT. Neighborhood context and reproductive health. Am J Obstet Gynecol. 2005 May;192(5 Suppl):S22–29. doi: 10.1016/j.ajog.2005.01.071. [DOI] [PubMed] [Google Scholar]
- 61.Davis EP, Sandman CA. The timing of prental exposure to maternal cortisolo and psychosocial stress Is associated with human infant cognitive development. Child Development. 2010;81(1):131–148. doi: 10.1111/j.1467-8624.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gorin AA, Stone AA. Recall biases and cognitive errors in retrospective self-reports: A call for momentary assessments. In: Baum T, Revenson, Singer J, editors. Handbook of health psychology. Mahwah, NJ: Lawrence Erlbaum; 2001. pp. 405–413. [Google Scholar]
- 63.Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- 64.Barnett PA, Spence JD, Manuck SB, Jennings JR. Psychological stress and the progression of carotid artery disease. J Hypertens. 1997 Jan;15(1):49–55. doi: 10.1097/00004872-199715010-00004. [DOI] [PubMed] [Google Scholar]
- 65.al’Absi M, Bongard S. Neuroendocrine and behavioral mechanisms mediating the relationship between anger expression and cardiovascular risk: assessment considerations and improvements. J Behav Med. 2006 Dec;29(6):573–591. doi: 10.1007/s10865-006-9077-0. [DOI] [PubMed] [Google Scholar]
- 66.Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 67.Entringer S, Buss C, Shirtcliff EA, et al. Attenuation of maternal psychophysiological stress responses and the maternal cortisol awakening response over the course of human pregnancy. Stress. 2010 May;13(3):258–268. doi: 10.3109/10253890903349501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCubbin JA, Lawson EJ, Cox S, Sherman JJ, Norton JA, Read JA. Prenatal maternal blood pressure response to stress predicts birth weight and gestational age: a preliminary study. Am J Obstet Gynecol. 1996 Sep;175(3 Pt 1):706–712. doi: 10.1053/ob.1996.v175.a74286. [DOI] [PubMed] [Google Scholar]
- 69.de Weerth C, Buitelaar JK. Physiological stress reactivity in human pregnancy--a review. Neurosci Biobehav Rev. 2005 Apr;29(2):295–312. doi: 10.1016/j.neubiorev.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 70.Buss C, Entringer S, Reyes JF, et al. The maternal cortisol awakening response in human pregnancy is associated with the length of gestation. Am J Obstet Gynecol. 2009 Aug 27;201(4):398.e391–398. doi: 10.1016/j.ajog.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 71.Brunton PJ. Resetting the dynamic range of hypothalamic-pituitary-adrenal axis stress responses through pregnancy. J Neuroendocrinol. Nov;22(11):1198–1213. doi: 10.1111/j.1365-2826.2010.02067.x. [DOI] [PubMed] [Google Scholar]
- 72.Douglas AJ. Baby on board: do responses to stress in the maternal brain mediate adverse pregnancy outcome? Front Neuroendocrinol. Jul;31(3):359–376. doi: 10.1016/j.yfrne.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 73.Schulte HM, Weisner D, Allolio B. The corticotrophin releasing hormone test in late pregnancy: lack of adrenocorticotrophin and cortisol response. Clin Endocrinol (Oxf) 1990 Jul;33(1):99–106. doi: 10.1111/j.1365-2265.1990.tb00470.x. [DOI] [PubMed] [Google Scholar]
- 74.Sasaki A, Shinkawa O, Yoshinaga K. Placental corticotropin-releasing hormone may be a stimulator of maternal pituitary adrenocorticotropic hormone secretion in humans. J Clin Invest. 1989 Dec;84(6):1997–2001. doi: 10.1172/JCI114390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Odagiri E, Ishiwatari N, Abe Y, et al. Hypercortisolism and the resistance to dexamethasone suppression during gestation. Endocrinol Jpn. 1988 Oct;35(5):685–690. doi: 10.1507/endocrj1954.35.685. [DOI] [PubMed] [Google Scholar]
- 76.Ekholm EM, Erkkola RU. Autonomic cardiovascular control in pregnancy. Eur J Obstet Gynecol Reprod Biol. 1996 Jan;64(1):29–36. doi: 10.1016/0301-2115(95)02255-4. [DOI] [PubMed] [Google Scholar]
- 77.de Weerth C, Buitelaar JK. Cortisol awakening response in pregnant women. Psychoneuroendocrinology. 2005 Oct;30(9):902–907. doi: 10.1016/j.psyneuen.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 78.Wolfe LA, Weissgerber TL. Clinical physiology of exercise in pregnancy: a literature review. J Obstet Gynaecol Can. 2003 Jun;25(6):473–483. doi: 10.1016/s1701-2163(16)30309-7. [DOI] [PubMed] [Google Scholar]
- 79.Glynn LM, Wadhwa PD, Dunkel-Schetter C, Chicz-Demet A, Sandman CA. When stress happens matters: effects of earthquake timing on stress responsivity in pregnancy. Am J Obstet Gynecol. 2001 Mar;184(4):637–642. doi: 10.1067/mob.2001.111066. [DOI] [PubMed] [Google Scholar]
- 80.Glynn LM, Schetter CD, Wadhwa PD, Sandman CA. Pregnancy affects appraisal of negative life events. J Psychosom Res. 2004 Jan;56(1):47–52. doi: 10.1016/S0022-3999(03)00133-8. [DOI] [PubMed] [Google Scholar]
- 81.Glynn LM, Schetter CD, Hobel CJ, Sandman CA. Pattern of perceived stress and anxiety in pregnancy predicts preterm birth. Health Psychol. 2008 Jan;27(1):43–51. doi: 10.1037/0278-6133.27.1.43. [DOI] [PubMed] [Google Scholar]
- 82.Clayton DG. Prediction and interaction in complex disease genetics: experience in type 1 diabetes. PLoS Genet. 2009 Jul;5(7):e1000540. doi: 10.1371/journal.pgen.1000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hunter DJ. Gene-environment interactions in human diseases. Nat Rev Genet. 2005 Apr;6(4):287–298. doi: 10.1038/nrg1578. [DOI] [PubMed] [Google Scholar]
- 84.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008 Jan 5;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Torloni MR, Betran AP, Daher S, et al. Maternal BMI and preterm birth: a systematic review of the literature with meta-analysis. J Matern Fetal Neonatal Med. 2009 Nov;22(11):957–970. doi: 10.3109/14767050903042561. [DOI] [PubMed] [Google Scholar]
- 86.Bloomfield FH, Oliver MH, Hawkins P, et al. Periconceptional undernutrition in sheep accelerates maturation of the fetal hypothalamic-pituitary-adrenal axis in late gestation. Endocrinology. 2004 Sep;145(9):4278–4285. doi: 10.1210/en.2004-0424. [DOI] [PubMed] [Google Scholar]
- 87.Chadio SE, Kotsampasi B, Papadomichelakis G, et al. Impact of maternal undernutrition on the hypothalamic-pituitary-adrenal axis responsiveness in sheep at different ages postnatal. J Endocrinol. 2007 Mar;192(3):495–503. doi: 10.1677/JOE-06-0172. [DOI] [PubMed] [Google Scholar]
- 88.Bispham J, Gopalakrishnan GS, Dandrea J, et al. Maternal endocrine adaptation throughout pregnancy to nutritional manipulation: consequences for maternal plasma leptin and cortisol and the programming of fetal adipose tissue development. Endocrinology. 2003 Aug;144(8):3575–3585. doi: 10.1210/en.2003-0320. [DOI] [PubMed] [Google Scholar]
- 89.Ford SP, Zhang L, Zhu M, et al. Maternal obesity accelerates fetal pancreatic beta-cell but not alpha-cell development in sheep: prenatal consequences. Am J Physiol Regul Integr Comp Physiol. 2009 Sep;297(3):R835–843. doi: 10.1152/ajpregu.00072.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lingas R, Dean F, Matthews SG. Maternal nutrient restriction (48 h) modifies brain corticosteroid receptor expression and endocrine function in the fetal guinea pig. Brain Res. 1999 Nov 6;846(2):236–242. doi: 10.1016/s0006-8993(99)02058-2. [DOI] [PubMed] [Google Scholar]
- 91.Dwyer CM, Stickland NC. The effects of maternal undernutrition on maternal and fetal serum insulin-like growth factors, thyroid hormones and cortisol in the guinea pig. J Dev Physiol. 1992 Dec;18(6):303–313. [PubMed] [Google Scholar]
- 92.Shen Q, Li ZQ, Sun Y, et al. The role of pro-inflammatory factors in mediating the effects on the fetus of prenatal undernutrition: implications for schizophrenia. Schizophr Res. 2008 Feb;99(1–3):48–55. doi: 10.1016/j.schres.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 93.Tamashiro KL, Terrillion CE, Hyun J, Koenig JI, Moran TH. Prenatal stress or high-fat diet increases susceptibility to diet-induced obesity in rat offspring. Diabetes. 2009 May;58(5):1116–1125. doi: 10.2337/db08-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gonzalez-Bono E, Rohleder N, Hellhammer DH, Salvador A, Kirschbaum C. Glucose but not protein or fat load amplifies the cortisol response to psychosocial stress. Horm Behav. 2002 May;41(3):328–333. doi: 10.1006/hbeh.2002.1766. [DOI] [PubMed] [Google Scholar]
- 95.Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001 Jan;26(1):37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- 96.Hitze B, Hubold C, van Dyken R, et al. How the selfish brain organizes its supply and demand. Front Neuroenergetics. 2010;2:7. doi: 10.3389/fnene.2010.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.George SA, Khan S, Briggs H, Abelson JL. CRH-stimulated cortisol release and food intake in healthy, non-obese adults. Psychoneuroendocrinology. 2010 May;35(4):607–612. doi: 10.1016/j.psyneuen.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tataranni PA, Larson DE, Snitker S, Young JB, Flatt JP, Ravussin E. Effects of glucocorticoids on energy metabolism and food intake in humans. Am J Physiol. 1996 Aug;271(2 Pt 1):E317–325. doi: 10.1152/ajpendo.1996.271.2.E317. [DOI] [PubMed] [Google Scholar]
- 99.la Fleur SE, Akana SF, Manalo SL, Dallman MF. Interaction between corticosterone and insulin in obesity: regulation of lard intake and fat stores. Endocrinology. 2004 May;145(5):2174–2185. doi: 10.1210/en.2003-1359. [DOI] [PubMed] [Google Scholar]
- 100.Hurley KM, Caulfield LE, Sacco LM, Costigan KA, Dipietro JA. Psychosocial influences in dietary patterns during pregnancy. J Am Diet Assoc. 2005 Jun;105(6):963–966. doi: 10.1016/j.jada.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 101.Han YS, Ha EH, Park HS, Kim YJ, Lee SS. Relationships between pregnancy outcomes, biochemical markers and pre–pregnancy body mass index. Int J Obes (Lond) 2010 Aug 31; doi: 10.1038/ijo.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev. 2007 Dec;65(12 Pt 2):S194–202. doi: 10.1111/j.1753-4887.2007.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 103.Culhane JF, Rauh V, McCollum KF, Elo IT, Hogan V. Exposure to chronic stress and ethnic differences in rates of bacterial vaginosis among pregnant women. Am J Obstet Gynecol. 2002 Nov;187(5):1272–1276. doi: 10.1067/mob.2002.127311. [DOI] [PubMed] [Google Scholar]
- 104.Culhane JF, Rauh V, McCollum KF, Hogan VK, Agnew K, Wadhwa PD. Maternal stress is associated with bacterial vaginosis in human pregnancy. Matern Child Health J. 2001 Jun;5(2):127–134. doi: 10.1023/a:1011305300690. [DOI] [PubMed] [Google Scholar]
- 105.Agelaki S, Tsatsanis C, Gravanis A, Margioris AN. Corticotropin-releasing hormone augments proinflammatory cytokine production from macrophages in vitro and in lipopolysaccharide-induced endotoxin shock in mice. Infect Immun. 2002 Nov;70(11):6068–6074. doi: 10.1128/IAI.70.11.6068-6074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kristenson M, Eriksen HR, Sluiter JK, Starke D, Ursin H. Psychobiological mechanisms of socioeconomic differences in health. Soc Sci Med. 2004 Apr;58(8):1511–1522. doi: 10.1016/S0277-9536(03)00353-8. [DOI] [PubMed] [Google Scholar]
- 107.McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008 Apr 7;583(2–3):174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schneiderman N, Ironson G, Siegel SD. Stress and health: psychological, behavioral, and biological determinants. Annu Rev Clin Psychol. 2005;1:607–628. doi: 10.1146/annurev.clinpsy.1.102803.144141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Weisman CS, Hillemeier MM, Chase GA, et al. Preconceptional health: risks of adverse pregnancy outcomes by reproductive life stage in the Central Pennsylvania Women’s Health Study (CePAWHS) Womens Health Issues. 2006 Jul-Aug;16(4):216–224. doi: 10.1016/j.whi.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 110.Petraglia F, Imperatore A, Challis JR. Neuroendocrine mechanisms in pregnancy and parturition. Endocr Rev. Dec;31(6):783–816. doi: 10.1210/er.2009-0019. [DOI] [PubMed] [Google Scholar]
- 111.Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. Bjog. 2006 Dec;113 (Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Smith R. Parturition. N Engl J Med. 2007 Jan 18;356(3):271–283. doi: 10.1056/NEJMra061360. [DOI] [PubMed] [Google Scholar]
- 113.Kalantaridou SN, Zoumakis E, Makrigiannakis A, Lavasidis LG, Vrekoussis T, Chrousos GP. Corticotropin-releasing hormone, stress and human reproduction: an update. J Reprod Immunol. May;85(1):33–39. doi: 10.1016/j.jri.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 114.Yen SCEopICR, Resnick R., editors. Maternal-Fetal Medicine: Principles and practice. Philadelphia, PA: WB Saunders; 1994. [Google Scholar]; Creasy RK, Resnick R, editors. Maternal-Fetal Medicine: Principles and practice. Philadelphia, PA: WB Saunders; 1994. Endocrinology of pregnancy. [Google Scholar]
- 115.Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav. 2010 Jun 19; doi: 10.1016/j.yhbeh.2010.06.007. early online. [DOI] [PubMed] [Google Scholar]
- 116.Cheng YH, Nicholson RC, King B, Chan EC, Fitter JT, Smith R. Corticotropin-releasing hormone gene expression in primary placental cells is modulated by cyclic adenosine 3’,5’-monophosphate. J Clin Endocrinol Metab. 2000 Mar;85(3):1239–1244. doi: 10.1210/jcem.85.3.6420. [DOI] [PubMed] [Google Scholar]
- 117.Lowry PJ. Corticotropin-releasing factor and its binding protein in human plasma. Ciba Found Symp. 1993;172:108–115. discussion 115–128. [PubMed] [Google Scholar]
- 118.Weetman AP. Immunity, thyroid function and pregnancy: molecular mechanisms. Nat Rev Endocrinol. 2010 Jun;6(6):311–318. doi: 10.1038/nrendo.2010.46. [DOI] [PubMed] [Google Scholar]
- 119.Denney JM, Nelson EL, Wadhwa PD, et al. Longitudinal modulation of immune system cytokine profile during pregnancy. Cytokine. Feb;53(2):170–177. doi: 10.1016/j.cyto.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Abbas AE, Brodie B, Dixon S, et al. Incidence and prognostic impact of gastrointestinal bleeding after percutaneous coronary intervention for acute myocardial infarction. Am J Cardiol. 2005 Jul 15;96(2):173–176. doi: 10.1016/j.amjcard.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 121.Romero R, Gomez R, Ghezzi F, et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol. 1998 Jul;179(1):186–193. doi: 10.1016/s0002-9378(98)70271-6. [DOI] [PubMed] [Google Scholar]
- 122.Athayde N, Edwin SS, Romero R, et al. A role for matrix metalloproteinase-9 in spontaneous rupture of the fetal membranes. Am J Obstet Gynecol. 1998 Nov;179(5):1248–1253. doi: 10.1016/s0002-9378(98)70141-3. [DOI] [PubMed] [Google Scholar]
- 123.Yoon BH, Romero R, Jun JK, et al. An increase in fetal plasma cortisol but not dehydroepiandrosterone sulfate is followed by the onset of preterm labor in patients with preterm premature rupture of the membranes. Am J Obstet Gynecol. 1998 Nov;179(5):1107–1114. doi: 10.1016/s0002-9378(98)70114-0. [DOI] [PubMed] [Google Scholar]
- 124.Yoon BH, Romero R, Park JS, et al. Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol. 1998 Nov;179(5):1254–1260. doi: 10.1016/s0002-9378(98)70142-5. [DOI] [PubMed] [Google Scholar]
- 125.Chrousos GP. Stressors, stress, and neuroendocrine integration of the adaptive response. The 1997 Hans Selye Memorial Lecture. Ann N Y Acad Sci. 1998 Jun 30;851:311–335. doi: 10.1111/j.1749-6632.1998.tb09006.x. [DOI] [PubMed] [Google Scholar]
- 126.Haddad JJ, Saade NE, Safieh-Garabedian B. Cytokines and neuro-immune-endocrine interactions: a role for the hypothalamic-pituitary-adrenal revolving axis. J Neuroimmunol. 2002 Dec;133(1–2):1–19. doi: 10.1016/s0165-5728(02)00357-0. [DOI] [PubMed] [Google Scholar]
- 127.McEwen BS, Biron CA, Brunson KW, et al. The role of adrenocorticoids as modulators of immune function in health and disease: neural, endocrine and immune interactions. Brain Res Brain Res Rev. 1997 Feb;23(1–2):79–133. doi: 10.1016/s0165-0173(96)00012-4. [DOI] [PubMed] [Google Scholar]
- 128.Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002 Oct;53(4):865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 129.Clifton VL, Wallace EM, Smith R. Short-term effects of glucocorticoids in the human fetal-placental circulation in vitro. J Clin Endocrinol Metab. 2002 Jun;87(6):2838–2842. doi: 10.1210/jcem.87.6.8541. [DOI] [PubMed] [Google Scholar]
- 130.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. Jama. 1992 Mar 4;267(9):1244–1252. [PubMed] [Google Scholar]
- 131.Elenkov IJ, Chrousos GP. Stress Hormones, Th1/Th2 patterns, Pro/Anti-inflammatory Cytokines and Susceptibility to Disease. Trends Endocrinol Metab. 1999 Nov;10(9):359–368. doi: 10.1016/s1043-2760(99)00188-5. [DOI] [PubMed] [Google Scholar]
- 132.Smith R, Mesiano S, McGrath S. Hormone trajectories leading to human birth. Regul Pept. 2002 Oct 15;108(2–3):159–164. doi: 10.1016/s0167-0115(02)00105-2. [DOI] [PubMed] [Google Scholar]
- 133.McLean M, Bisits A, Davies J, Woods R, Lowry P, Smith R. A placental clock controlling the length of human pregnancy. Nat Med. 1995 May;1(5):460–463. doi: 10.1038/nm0595-460. [DOI] [PubMed] [Google Scholar]
- 134.Holzman C, Jetton J, Siler-Khodr T, Fisher R, Rip T. Second trimester corticotropin-releasing hormone levels in relation to preterm delivery and ethnicity. Obstet Gynecol. 2001 May;97(5 Pt 1):657–663. doi: 10.1016/s0029-7844(00)01209-6. [DOI] [PubMed] [Google Scholar]
- 135.Glynn LM, Schetter CD, Chicz-DeMet A, Hobel CJ, Sandman CA. Ethnic differences in adrenocorticotropic hormone, cortisol and corticotropin-releasing hormone during pregnancy. Peptides. 2007 Jun;28(6):1155–1161. doi: 10.1016/j.peptides.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 136.Shimmin LC, Natarajan S, Ibarguen H, et al. Corticotropin releasing hormone (CRH) gene variation: comprehensive resequencing for variant and molecular haplotype discovery in monosomic hybrid cell lines. DNA Seq. 2007 Dec;18(6):434–444. doi: 10.1080/10425170701388719. [DOI] [PubMed] [Google Scholar]
- 137.Hobel CJ, Dunkel-Schetter C, Roesch SC, Castro LC, Arora CP. Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks’ gestation in pregnancies ending in preterm delivery. Am J Obstet Gynecol. 1999 Jan;180(1 Pt 3):S257–263. doi: 10.1016/s0002-9378(99)70712-x. [DOI] [PubMed] [Google Scholar]
- 138.Erickson K, Thorsen P, Chrousos G, et al. Preterm birth: associated neuroendocrine, medical, and behavioral risk factors. J Clin Endocrinol Metab. 2001 Jun;86(6):2544–2552. doi: 10.1210/jcem.86.6.7607. [DOI] [PubMed] [Google Scholar]
- 139.Wadhwa PD, Glynn L, Hobel CJ, et al. Behavioral perinatology: biobehavioral processes in human fetal development. Regul Pept. 2002 Oct 15;108(2–3):149–157. doi: 10.1016/s0167-0115(02)00102-7. [DOI] [PubMed] [Google Scholar]
- 140.Petraglia F, Calza L, Garuti GC, Giardino L, De Ramundo BM, Angioni S. New aspects of placental endocrinology. J Endocrinol Invest. 1990 Apr;13(4):353–371. doi: 10.1007/BF03349579. [DOI] [PubMed] [Google Scholar]
- 141.Petraglia F, Sawchenko PE, Rivier J, Vale W. Evidence for local stimulation of ACTH secretion by corticotropin-releasing factor in human placenta. Nature. 1987 Aug 20–26;328(6132):717–719. doi: 10.1038/328717a0. [DOI] [PubMed] [Google Scholar]
- 142.Petraglia F, Sutton S, Vale W. Neurotransmitters and peptides modulate the release of immunoreactive corticotropin-releasing factor from cultured human placental cells. Am J Obstet Gynecol. 1989 Jan;160(1):247–251. doi: 10.1016/0002-9378(89)90130-0. [DOI] [PubMed] [Google Scholar]
- 143.Chan EC, Smith R, Lewin T, et al. Plasma corticotropin-releasing hormone, beta-endorphin and cortisol inter-relationships during human pregnancy. Acta Endocrinol (Copenh) 1993 Apr;128(4):339–344. doi: 10.1530/acta.0.1280339. [DOI] [PubMed] [Google Scholar]
- 144.Goland RS, Conwell IM, Warren WB, Wardlaw SL. Placental corticotropin-releasing hormone and pituitary-adrenal function during pregnancy. Neuroendocrinology. 1992 Nov;56(5):742–749. doi: 10.1159/000126302. [DOI] [PubMed] [Google Scholar]
- 145.Wadhwa PD, Sandman CA, Chicz-DeMet A, Porto M. Placental CRH modulates maternal pituitary adrenal function in human pregnancy. Ann N Y Acad Sci. 1997 Apr 24;814:276–281. doi: 10.1111/j.1749-6632.1997.tb46163.x. [DOI] [PubMed] [Google Scholar]
- 146.Wadhwa PD, Dunkel-Schetter C, Chicz-DeMet A, Porto M, Sandman CA. Prenatal psychosocial factors and the neuroendocrine axis in human pregnancy. Psychosom Med. 1996;58(5):432–446. doi: 10.1097/00006842-199609000-00006. [DOI] [PubMed] [Google Scholar]
- 147.Valladares E, Pena R, Ellsberg M, Persson LA, Hogberg U. Neuroendocrine response to violence during pregnancy--impact on duration of pregnancy and fetal growth. Acta Obstet Gynecol Scand. 2009;88(7):818–823. doi: 10.1080/00016340903015321. [DOI] [PubMed] [Google Scholar]
- 148.Kivlighan KT, DiPietro JA, Costigan KA, Laudenslager ML. Diurnal rhythm of cortisol during late pregnancy: associations with maternal psychological well-being and fetal growth. Psychoneuroendocrinology. 2008 Oct;33(9):1225–1235. doi: 10.1016/j.psyneuen.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Evans LM, Myers MM, Monk C. Pregnant women’s cortisol is elevated with anxiety and depression - but only when comorbid. Arch Womens Ment Health. 2008 Jul;11(3):239–248. doi: 10.1007/s00737-008-0019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Latendresse G, Ruiz RJ. Maternal coping style and perceived adequacy of income predict CRH levels at 14–20 weeks of gestation. Biol Res Nurs. Oct;12(2):125–136. doi: 10.1177/1099800410377111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Petraglia F, Hatch MC, Lapinski R, et al. Lack of effect of psychosocial stress on maternal corticotrophin-releasing factor and catecholamine levels at 28 weeks’ gestation. J Soc Gynecol Investig. 2001 Mar-Apr;8(2):83–88. [PubMed] [Google Scholar]
- 152.Harville EW, Savitz DA, Dole N, Herring AH, Thorp JM. Stress questionnaires and stress biomarkers during pregnancy. J Womens Health (Larchmt) 2009 Sep;18(9):1425–1433. doi: 10.1089/jwh.2008.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Guendelman S, Kosa JL, Pearl M, Graham S, Kharrazi M. Exploring the relationship of second-trimester corticotropin releasing hormone, chronic stress and preterm delivery. J Matern Fetal Neonatal Med. 2008 Nov;21(11):788–795. doi: 10.1080/14767050802379031. [DOI] [PubMed] [Google Scholar]
- 154.Sandman CA, Glynn L, Schetter CD, et al. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides. 2006 Jun;27(6):1457–1463. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 155.Kalra S, Einarson A, Karaskov T, Van Uum S, Koren G. The relationship between stress and hair cortisol in healthy pregnant women. Clin Invest Med. 2007;30(2):E103–107. doi: 10.25011/cim.v30i2.986. [DOI] [PubMed] [Google Scholar]
- 156.Coussons-Read ME, Okun ML, Schmitt MP, Giese S. Prenatal stress alters cytokine levels in a manner that may endanger human pregnancy. Psychosom Med. 2005 Jul-Aug;67(4):625–631. doi: 10.1097/01.psy.0000170331.74960.ad. [DOI] [PubMed] [Google Scholar]
- 157.Christian LM, Franco A, Glaser R, Iams JD. Depressive symptoms are associated with elevated serum proinflammatory cytokines among pregnant women. Brain Behav Immun. 2009 Mar 1;23(6):750–754. doi: 10.1016/j.bbi.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Paul K, Boutain D, Agnew K, Thomas J, Hitti J. The relationship between racial identity, income, stress and C-reactive protein among parous women: implications for preterm birth disparity research. J Natl Med Assoc. 2008 May;100(5):540–546. doi: 10.1016/s0027-9684(15)31300-6. [DOI] [PubMed] [Google Scholar]
- 159.Coussons-Read ME, Okun ML, Nettles CD. Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain Behav Immun. 2007 Mar;21(3):343–350. doi: 10.1016/j.bbi.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 160.Klonoff-Cohen HS, Cross JL, Pieper CF. Job stress and preeclampsia. Epidemiology. 1996 May;7(3):245–249. doi: 10.1097/00001648-199605000-00005. [DOI] [PubMed] [Google Scholar]
- 161.Marcoux S, Berube S, Brisson C, Mondor M. Job strain and pregnancy-induced hypertension. Epidemiology. 1999 Jul;10(4):376–382. doi: 10.1097/00001648-199907000-00004. [DOI] [PubMed] [Google Scholar]
- 162.Landsbergis PA, Hatch MC. Psychosocial work stress and pregnancy-induced hypertension. Epidemiology. 1996 Jul;7(4):346–351. doi: 10.1097/00001648-199607000-00002. [DOI] [PubMed] [Google Scholar]
- 163.Wergeland E, Strand K. Working conditions and prevalence of pre-eclampsia, Norway 1989. Int J Gynaecol Obstet. 1997 Aug;58(2):189–196. doi: 10.1016/s0020-7292(97)00083-0. [DOI] [PubMed] [Google Scholar]
- 164.Qiu C, Williams MA, Calderon-Margalit R, Cripe SM, Sorensen TK. Preeclampsia risk in relation to maternal mood and anxiety disorders diagnosed before or during early pregnancy. Am J Hypertens. 2009 Apr;22(4):397–402. doi: 10.1038/ajh.2008.366. [DOI] [PubMed] [Google Scholar]
- 165.Leeners B, Neumaier-Wagner P, Kuse S, Stiller R, Rath W. Emotional stress and the risk to develop hypertensive diseases in pregnancy. Hypertens Pregnancy. 2007;26(2):211–226. doi: 10.1080/10641950701274870. [DOI] [PubMed] [Google Scholar]
- 166.Chang Q, Natelson BH, Ottenweller JE, Conway RS. Stress triggers different pathophysiological mechanisms in younger and older cardiomyopathic hamsters. Cardiovasc Res. 1995 Dec;30(6):985–991. [PubMed] [Google Scholar]
- 167.Vythilingum B, Geerts L, Fincham D, et al. Association between antenatal distress and uterine artery pulsatility index. Arch Womens Ment Health. Aug 13;4:359–364. doi: 10.1007/s00737-010-0144-8. [DOI] [PubMed] [Google Scholar]
- 168.Huneke B, Ude C. Uteroplacental and fetal arterial Ultrasound Doppler Flow Velocity measurements in unselected pregnancies as a screening test at 32 to 34 gestational weeks. Z Geburtshilfe Neonatol. 2002 Apr;206(2):57–64. doi: 10.1055/s-2002-30138. [DOI] [PubMed] [Google Scholar]
- 169.Sjostrom K, Valentin L, Thelin T, Marsal K. Maternal anxiety in late pregnancy: effect on fetal movements and fetal heart rate. Early Hum Dev. 2002 Apr;67(1–2):87–100. doi: 10.1016/s0378-3782(01)00256-0. [DOI] [PubMed] [Google Scholar]
- 170.Teixeira JM, Fisk NM, Glover V. Association between maternal anxiety in pregnancy and increased uterine artery resistance index: cohort based study. Bmj. 1999 Jan 16;318(7177):153–157. doi: 10.1136/bmj.318.7177.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jeske W, Soszynski P, Lukaszewicz E, et al. Enhancement of plasma corticotropin-releasing hormone in pregnancy-induced hypertension. Acta Endocrinol (Copenh) 1990 Jun;122(6):711–714. doi: 10.1530/acta.0.1220711. [DOI] [PubMed] [Google Scholar]
- 172.Perkins AV, Linton EA, Eben F, Simpson J, Wolfe CD, Redman CW. Corticotrophin-releasing hormone and corticotrophin-releasing hormone binding protein in normal and pre-eclamptic human pregnancies. Br J Obstet Gynaecol. 1995 Feb;102(2):118–122. doi: 10.1111/j.1471-0528.1995.tb09063.x. [DOI] [PubMed] [Google Scholar]
- 173.Warren WB, Gurewitsch ED, Goland RS. Corticotropin-releasing hormone and pituitary-adrenal hormones in pregnancies complicated by chronic hypertension. Am J Obstet Gynecol. 1995 Feb;172(2 Pt 1):661–666. doi: 10.1016/0002-9378(95)90589-8. [DOI] [PubMed] [Google Scholar]
- 174.Harville EW, Savitz DA, Dole N, Herring AH, Thorp JM, Light KC. Stress and placental resistance measured by Doppler ultrasound in early and mid-pregnancy. Ultrasound Obstet Gynecol. 2008 Jul;32(1):23–30. doi: 10.1002/uog.5344. [DOI] [PubMed] [Google Scholar]
- 175.Jaffe RB. Role of human fetal adrenal gland in the initiation of parturition. In: Smith R, editor. The Endocrinology of Parturition. Newcastle, Australia: Karger; 2001. [Google Scholar]
- 176.Mesiano S. Roles of estrogen and progesterone in human parturiton. In: Smith R, editor. The Endocrinology of Parturition. Newcastle, Australia: Karger; 2001. [DOI] [PubMed] [Google Scholar]
- 177.Smith R. The timing of birth. Sci Am. 1999 Mar;280(3):68–75. doi: 10.1038/scientificamerican0399-68. [DOI] [PubMed] [Google Scholar]
- 178.Robinson BG, Emanuel RL, Frim DM, Majzoub JA. Glucocorticoid stimulates expression of corticotrophin-releasing hormone gene in human placenta. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5244–5248. doi: 10.1073/pnas.85.14.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bowman ME, Lopata A, Jaffe RB, Golos TG, Wickings J, Smith R. Corticotropin-releasing hormone-binding protein in primates. Am J Primatol. 2001 Mar;53(3):123–130. doi: 10.1002/1098-2345(200103)53:3<123::AID-AJP3>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 180.Smith R, Chan EC, Bowman ME, Harewood WJ, Phippard AF. Corticotropin-releasing hormone in baboon pregnancy. J Clin Endocrinol Metab. 1993 Apr;76(4):1063–1068. doi: 10.1210/jcem.76.4.8473382. [DOI] [PubMed] [Google Scholar]
- 181.Chaudhari BP, Plunkett J, Ratajczak CK, Shen TT, DeFranco EA, Muglia LJ. The genetics of birth timing: insights into a fundamental component of human development. Clin Genet. 2008 Dec;74(6):493–501. doi: 10.1111/j.1399-0004.2008.01124.x. [DOI] [PubMed] [Google Scholar]
- 182.Himes KP, Simhan HN. Genetic susceptibility to infection-mediated preterm birth. Infect Dis Clin North Am. 2008 Dec;22(4):741–753. vii. doi: 10.1016/j.idc.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 183.Anum EA, Springel EH, Shriver MD, Strauss JF., 3rd Genetic contributions to disparities in preterm birth. Pediatr Res. 2009 Jan;65(1):1–9. doi: 10.1203/PDR.0b013e31818912e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Plunkett J, Muglia LJ. Genetic contributions to preterm birth: implications from epidemiological and genetic association studies. Ann Med. 2008;40(3):167–195. doi: 10.1080/07853890701806181. [DOI] [PubMed] [Google Scholar]
- 185.Pennell CE, Jacobsson B, Williams SM, et al. Genetic epidemiologic studies of preterm birth: guidelines for research. Am J Obstet Gynecol. 2007 Feb;196(2):107–118. doi: 10.1016/j.ajog.2006.03.109. [DOI] [PubMed] [Google Scholar]
- 186.Crider KS, Whitehead N, Buus RM. Genetic variation associated with preterm birth: a HuGE review. Genet Med. 2005 Nov-Dec;7(9):593–604. doi: 10.1097/01.gim.0000187223.69947.db. [DOI] [PubMed] [Google Scholar]
- 187.Menon R, Fortunato SJ, Thorsen P, Williams S. Genetic associations in preterm birth: a primer of marker selection, study design, and data analysis. J Soc Gynecol Investig. 2006 Dec;13(8):531–541. doi: 10.1016/j.jsgi.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 188.Derringer J, Krueger RF, Dick DM, et al. Predicting sensation seeking from dopamine genes. A candidate-system approach. Psychol Sci. Sep;21(9):1282–1290. doi: 10.1177/0956797610380699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hamidovic A, Dlugos A, Skol A, Palmer AA, de Wit H. Evaluation of genetic variability in the dopamine receptor D2 in relation to behavioral inhibition and impulsivity/sensation seeking: an exploratory study with d-amphetamine in healthy participants. Exp Clin Psychopharmacol. 2009 Dec;17(6):374–383. doi: 10.1037/a0017840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Adriani W, Boyer F, Gioiosa L, Macri S, Dreyer JL, Laviola G. Increased impulsive behavior and risk proneness following lentivirus-mediated dopamine transporter over-expression in rats’ nucleus accumbens. Neuroscience. 2009 Mar 3;159(1):47–58. doi: 10.1016/j.neuroscience.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 191.Szily E, Bowen J, Unoka Z, Simon L, Keri S. Emotion appraisal is modulated by the genetic polymorphism of the serotonin transporter. J Neural Transm. 2008 Jun;115(6):819–822. doi: 10.1007/s00702-008-0029-4. [DOI] [PubMed] [Google Scholar]
- 192.Derijk RH, de Kloet ER. Corticosteroid receptor polymorphisms: determinants of vulnerability and resilience. Eur J Pharmacol. 2008 Apr 7;583(2–3):303–311. doi: 10.1016/j.ejphar.2007.11.072. [DOI] [PubMed] [Google Scholar]
- 193.Burris HH, Collins JW., Jr Race and preterm birth--the case for epigenetic inquiry. Ethn Dis. Summer;20(3):296–299. [PubMed] [Google Scholar]
- 194.Stone AA, Shiffman S. Ecological momentary assessment (EMA) in behavioral medicine. Annals of Behavioral Medicine Society of Behavioral Medicine. 1994;16:199–202. [Google Scholar]
- 195.Walker SP, Permezel M, Brennecke SP, Ugoni AM, Higgins JR. Blood pressure in late pregnancy and work outside the home. Obstet Gynecol. 2001 Mar;97(3):361–365. doi: 10.1016/s0029-7844(00)01166-2. [DOI] [PubMed] [Google Scholar]
- 196.Kamarck TW, Muldoon MF, Shiffman S, Sutton-Tyrrell K, Gwaltney C, Janicki DL. Experiences of demand and control in daily life as correlates of subclinical carotid atherosclerosis in a healthy older sample. Health Psychol. 2004 Jan;23(1):24–32. doi: 10.1037/0278-6133.23.1.24. [DOI] [PubMed] [Google Scholar]
- 197.van Doornen LJ, van Blokland RW. The relationship between cardiovascular and catecholamine reactions to laboratory and real-life stress. Psychophysiology. 1992 Mar;29(2):173–181. doi: 10.1111/j.1469-8986.1992.tb01682.x. [DOI] [PubMed] [Google Scholar]
- 198.Kamarck TW, Debski TT, Manuck SB. Enhancing the laboratory-to-life generalizability of cardiovascular reactivity using multiple occasions of measurement. Psychophysiology. 2000 Jul;37(4):533–542. [PubMed] [Google Scholar]
- 199.Kamarck TW, Muldoon MF, Shiffman SS, Sutton-Tyrrell K. Experiences of demand and control during daily life are predictors of carotid atherosclerotic progression among healthy men. Health Psychol. 2007 May;26(3):324–332. doi: 10.1037/0278-6133.26.3.324. [DOI] [PubMed] [Google Scholar]
- 200.Schwartz JE, Warren K, Pickering TG. Mood, location and physical position as predictors of ambulatory blood pressure and heart rate: Application of a multi-level random effects model. Annals of Behavioral Medicine Society of Behavioral Medicine. 1994;16:210–220. [Google Scholar]
- 201.Shiffman S. Real-time self-report of momentary states in the natural environment: Computerized ecological momentary assessment. In: Stone AA, Turkkan JJ, Bachrach CA, et al., editors. The Science of Self-Report: Implications for Research and Practice. Mahwah, NJ: Lawrence Erlbaum Associates; 2000. [Google Scholar]
- 202.Entringer S, Buss C, Andersen J, Chicz-DeMet A, Wadhwa E. Ecological momentary assessment (EMA) of maternal cortisol profiles over a multiple-day period predict the length of human gestation. Psychosomatic Medicine. doi: 10.1097/PSY.0b013e31821fbf9a. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lu MC, Halfon N. Racial and ethnic disparities in birth outcomes: a life-course perspective. Matern Child Health J. 2003 Mar;7(1):13–30. doi: 10.1023/a:1022537516969. [DOI] [PubMed] [Google Scholar]
- 204.Misra DP, Guyer B, Allston A. Integrated perinatal health framework. A multiple determinants model with a life span approach. Am J Prev Med. 2003 Jul;25(1):65–75. doi: 10.1016/s0749-3797(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 205.Kermack WO, McKendrick AG, McKinlay PL. Death-rates in Great Britain and Sweden: Expression of Specific Mortality Rates as Products of Two Factors, and some Consequences thereof. J Hyg (Lond) 1934 Dec;34(4):433–457. doi: 10.1017/s0022172400043230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008 Jul 3;359(1):61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Swanson JM, Entringer S, Buss C, Wadhwa PD. Developmental origins of health and disease: environmental exposures. Semin Reprod Med. 2009 Sep;27(5):391–402. doi: 10.1055/s-0029-1237427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med. 2009 Sep;27(5):358–368. doi: 10.1055/s-0029-1237424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Seckl JR. Physiologic programming of the fetus. Clin Perinatol. 1998 Dec;25(4):939–962. vii. [PubMed] [Google Scholar]
- 210.Hertzman C. The biological embedding of early experience and its effects on health in adulthood. Ann N Y Acad Sci. 1999;896:85–95. doi: 10.1111/j.1749-6632.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- 211.Suomi SJ. Early determinants of behaviour: evidence from primate studies. Br Med Bull. 1997 Jan;53(1):170–184. doi: 10.1093/oxfordjournals.bmb.a011598. [DOI] [PubMed] [Google Scholar]
- 212.Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci. 2005;7(2):103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Weaver IC, Champagne FA, Brown SE, et al. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci. 2005 Nov 23;25(47):11045–11054. doi: 10.1523/JNEUROSCI.3652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Coe LC. Psychosocial factors and psychoneuroimmunology within a lifespan perspective. In: Keating DP, Hertzman C, editors. Developmental health and the wealth of nations: social, biological and educational dynamics. New York: Guilford Stress; 1999. pp. 201–219. [Google Scholar]
- 215.Entringer S, Buss C, Kumsta R, Hellhammer DH, Wadhwa PD, Wust S. Prenatal psychosocial stress exposure is associated with subsequent working memory performance in young women. Behav Neurosci. 2009 Aug;123(4):886–893. doi: 10.1037/a0016265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Entringer S, Kumsta R, Hellhammer DH, Wadhwa PD, Wust S. Prenatal exposure to maternal psychosocial stress and HPA axis regulation in young adults. Horm Behav. 2009 Feb;55(2):292–298. doi: 10.1016/j.yhbeh.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 217.Entringer S, Kumsta R, Nelson EL, Hellhammer DH, Wadhwa PD, Wust S. Influence of prenatal psychosocial stress on cytokine production in adult women. Dev Psychobiol. 2008 Aug 5;50(6):579–587. doi: 10.1002/dev.20316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Entringer S, Wust S, Kumsta R, et al. Prenatal psychosocial stress exposure is associated with insulin resistance in young adults. Am J Obstet Gynecol. 2008 Nov;199(5):498.e491–497. doi: 10.1016/j.ajog.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Champagne F, Meaney MJ. Like mother, like daughter: evidence for non-genomic transmission of parental behavior and stress responsivity. Prog Brain Res. 2001;133:287–302. doi: 10.1016/s0079-6123(01)33022-4. [DOI] [PubMed] [Google Scholar]
- 220.Power C, Matthews S. Origins of health inequalities in a national population sample. Lancet. 1997 Nov 29;350(9091):1584–1589. doi: 10.1016/S0140-6736(97)07474-6. [DOI] [PubMed] [Google Scholar]
- 221.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998 Jan 15;338(3):171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 222.Sapolsky RM. Social subordinance as a marker of hypercortisolism. Some unexpected subtleties. Ann N Y Acad Sci. 1995 Dec 29;771:626–639. doi: 10.1111/j.1749-6632.1995.tb44715.x. [DOI] [PubMed] [Google Scholar]
- 223.Kristenson M, Kucinskiene Z, Bergdahl B, Calkauskas H, Urmonas V, Orth-Gomer K. Increased psychosocial strain in Lithuanian versus Swedish men: the LiVicordia study. Psychosom Med. 1998 May-Jun;60(3):277–282. doi: 10.1097/00006842-199805000-00011. [DOI] [PubMed] [Google Scholar]
- 224.Chrousos GP. The stress response and immune function: clinical implications. The 1999 Novera H. Spector Lecture. Ann N Y Acad Sci. 2000;917:38–67. doi: 10.1111/j.1749-6632.2000.tb05371.x. [DOI] [PubMed] [Google Scholar]
- 225.Noll JG, Schulkin J, Trickett PK, Susman EJ, Breech L, Putnam FW. Differential pathways to preterm delivery for sexually abused and comparison women. J Pediatr Psychol. 2007 Nov-Dec;32(10):1238–1248. doi: 10.1093/jpepsy/jsm046. [DOI] [PubMed] [Google Scholar]
- 226.Chung EK, Mathew L, Elo IT, Coyne JC, Culhane JF. Depressive symptoms in disadvantaged women receiving prenatal care: the influence of adverse and positive childhood experiences. Ambul Pediatr. 2008 Mar-Apr;8(2):109–116. doi: 10.1016/j.ambp.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 227.Cammack AL, Buss C, Entringer S, HOGUE CJ, HOBEL CJ, WADHWA PD. The association between early life adversity and bacterial vaginosis during pregnancy. American Journal of Obstetrics and Gynecology. doi: 10.1016/j.ajog.2011.01.054. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Shea AK, Streiner DL, Fleming A, Kamath MV, Broad K, Steiner M. The effect of depression, anxiety and early life trauma on the cortisol awakening response during pregnancy: preliminary results. Psychoneuroendocrinology. 2007 Sep-Nov;32(8–10):1013–1020. doi: 10.1016/j.psyneuen.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 229.Chung EK, Nurmohamed L, Mathew L, Elo IT, Coyne JC, Culhane JF. Risky health behaviors among mothers-to-be: the impact of adverse childhood experiences. Acad Pediatr. Jul-Aug;10(4):245–251. doi: 10.1016/j.acap.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Misra D, Strobino D, Trabert B. Effects of social and psychosocial factors on risk of preterm birth in black women. Paediatr Perinat Epidemiol. Nov;24(6):546–554. doi: 10.1111/j.1365-3016.2010.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Amory JH, Hitti J, Lawler R, Eschenbach DA. Increased tumor necrosis factor-alpha production after lipopolysaccharide stimulation of whole blood in patients with previous preterm delivery complicated by intra-amniotic infection or inflammation. Am J Obstet Gynecol. 2001 Nov;185(5):1064–1067. doi: 10.1067/mob.2001.117637. [DOI] [PubMed] [Google Scholar]
- 232.National Research Council (U.S.) New horizons in health: An integrative approach. Washington DC: National Academy Press; 2001. Committee on Future Directions for Behavioral and Social Sciences Research at the National Institute of Health. [PubMed] [Google Scholar]
- 233.Downs DS, Feinberg M, Hillemeier MM, et al. Design of the Central Pennsylvania Women’s Health Study (CePAWHS) strong healthy women intervention: improving preconceptional health. Matern Child Health J. 2009 Jan;13(1):18–28. doi: 10.1007/s10995-008-0323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Weisman CS, Hillemeier MM, Downs DS, Chuang CH, Dyer AM. Preconception predictors of weight gain during pregnancy: prospective findings from the Central Pennsylvania Women’s Health Study. Womens Health Issues. Mar-Apr;20(2):126–132. doi: 10.1016/j.whi.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Webb DA, Coyne JC, Goldenberg RL, et al. Recruitment and retention of women in a large randomized control trial to reduce repeat preterm births: the Philadelphia Collaborative Preterm Prevention Project. BMC Med Res Methodol. 10:88. doi: 10.1186/1471-2288-10-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Collins JW, Jr, David RJ, Handler A, Wall S, Andes S. Very low birthweight in African American infants: the role of maternal exposure to interpersonal racial discrimination. Am J Public Health. 2004 Dec;94(12):2132–2138. doi: 10.2105/ajph.94.12.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Geronimus AT. Black/white differences in the relationship of maternal age to birthweight: a population-based test of the weathering hypothesis. Soc Sci Med. 1996 Feb;42(4):589–597. doi: 10.1016/0277-9536(95)00159-x. [DOI] [PubMed] [Google Scholar]
- 238.Landrigan PJ, Trasande L, Thorpe LE, et al. The National Children’s Study: a 21-year prospective study of 100,000 American children. Pediatrics. 2006 Nov;118(5):2173–2186. doi: 10.1542/peds.2006-0360. [DOI] [PubMed] [Google Scholar]