Abstract
Several studies have demonstrated that the auditory system is sensitive to changes in posture, presumably through changes in intracranial pressure (ICP) that in turn alter the intracochlear pressure, which affects the stiffness of the middle-ear system. This observation has led to efforts to develop an ear-canal based noninvasive diagnostic measure for monitoring ICP, which is currently monitored invasively via access through the skull or spine. Here, we demonstrate the effects of postural changes, and presumably ICP changes, on distortion product otoacoustic emissions (DPOAE) magnitude, DPOAE angle, and power reflectance. Measurements were made on 12 normal-hearing subjects in two postural positions: upright at 90 degrees and tilted at −45 degrees to the horizontal. Measurements on each subject were repeated five times across five separate measurement sessions. All three measures showed significant changes (p < 0.001) between upright and tilted for frequencies between 500 and 2000 Hz, and DPOAE angle changes were significant at all measured frequencies (500–4000 Hz). Intrasubject variability, assessed via standard deviations for each subject’s multiple measurements, were generally smaller in the upright position relative to the tilted position.
Keywords: Intracranial pressure, Distortion product otoacoustic emissions, Reflectance
1. Introduction
Noninvasive ear-canal based acoustical measurements have diagnostic potential in the area of neurology to monitor intracranial pressure (ICP) changes. Because the skull is fixed in volume, and its fluid contents are incompressible, changes in cerebral spinal fluid pressure that result from changes in ICP are transmitted to the cochlear fluids. Changes in ICP can be caused by a number of factors, including, head injury, stroke, hydrocephalus, and brain surgery and can lead to worsening brain injury or death by compressing blood vessels supplying the brain or vital brain structures themselves. Current tools used to evaluate ICP objectively (e.g., epidural transducers or intraventricular catheters) are invasive and require direct entry of a probe system through the skull or spine, introducing risks that include infection, intracerebral hemorrhage, and direct brain injury (e.g., Kanter et al., 1985; Maniker et al., 2006; Wolfe and Torbey, 2009; Scheithauer et al., 2009). A noninvasive method for monitoring ICP could eliminate these risks for some patients.
Intracranial pressure changes systematically with postural position (e.g., Chapman et al., 1990). Thus, changing postural position provides a method to induce changes in ICP and study the effects. To this end, it is widely documented that posture affects auditory function, including thresholds, otoacoustic emissions, and middle-ear impedance (for a thorough review see Büki et al., 2000). Thus, the connection between posture and ICP provides a mechanism to study how changes in ICP affect auditory responses and how this relationship might be harnessed to provide a noninvasive means to monitor ICP in some patients.
Wilson (1980) first showed that posture influences otoacoustic emissions, and with this report suggested that the changes might be due to changes in the stiffness of the annular ligament. More recently, a series of publications of both measurements and models from Büki and colleagues demonstrate that low-frequency changes in auditory function with posture are largely a result of changes in middle-ear transmission that result from the changes in ICP associated with changes in posture (Büki et al., 1996, 2000; de Kleine et al., 2000, 2001; Büki et al., 2002). Their measurements and models are generally consistent with the following hypothesis. The auditory system is sensitive to changes in ICP because the cochlear aqueduct connects the cerebral spinal fluid to the cochlear fluid; increases in ICP are transferred to increases in intracochlear pressure, which results in outward static displacements of the compliant oval and round windows. These ICP increases are most likely to be detected as reductions in middle-ear transmission that result from an increased stiffness of the annular ligament, which connects the stapes to the oval window (Büki et al., 2000, 2002; Voss et al., 2006), with the effects of increased stiffness most prominent at frequencies below the middle ear’s resonant frequency (i.e., below about 2000 Hz).
Theoretically, different middle-ear transmission measurements could be used to detect ICP changes, including otoacoustic emissions (Büki et al., 1996; de Kleine et al., 2000; Frank et al., 2000; Büki et al., 2000; de Kleine et al., 2001; Büki et al., 2002; Voss et al., 2006), the cochlear-microphonic potential (Büki et al., 2009), changes in middle-ear impedance (Magnano et al., 1994; Liau, 1999), and other related quantities such as reflectance, and changes in displacement patterns of the tympanic membrane (Marchbanks, 1984), which were later shown to be too variable to monitor ICP (Rosingh et al., 1998; Shimbles et al., 2005). An advantage of evoked otoacoustic emissions is that they are affected by two reductions in middle-ear transmission: one in the forward direction as the stimulus and one in the reverse direction as the emission (Voss and Shera, 2004); a limitation is that the emissions may be weak or absent in individuals with a hearing loss. Thus, the potential for monitoring changes in ICP through concomitant changes in middle-ear transmission should be evaluated using multiple measures, and here we quantify how both distortion product otoacoustic emissions (DPOAEs) and reflectance, which is related to impedance measures (e.g., Keefe et al., 1993; Voss and Allen, 1994), are affected by changes in posture, and presumably ICP changes.
The specific goal of this paper is to present measurements of both DPOAE magnitudes and angles and also power reflectance made at the same time at two extreme postures, presumably resulting in ICP changes. Additionally, these measurements were made multiple times on a given subject so that intra-subject variability of these measures could be assessed.
2. Material and Methods
2.1. Overview
Measurements of DPOAE magnitudes, DPOAE angles, and power reflectance were made to characterize how posture, and presumably intracranial pressure (ICP), affects these three measures. Measurements were made in the supine (upright) position and a position with the subject tilted at −45° relative to the horizontal. Additionally, the intra-subject variability for all three measures is quantified through repeated measurements across five sessions.
2.2. Human Subjects
Measurements are reported from the left ears of 12 normal-hearing healthy subjects (11 females and one male), ages 19 to 42 years, all with a negative history for middle-ear problems, hearing thresholds below 20 dB HL at 500, 1000, 2000, and 4000 Hz, and normal tympanograms. All measurements were made between January 2008 and June 2008. Eight additional subjects were recruited but did not complete five measurement sessions because of the time required or discomfort with being tilted at −45°. Subjects were given an otoscopic examination to ensure no excessive ear wax was present in the ear canal. The measurements were approved by the Smith College Institutional Review Board, and informed consent was obtained from all subjects.
2.3. Acoustic Measurement Equipment
DPOAE magnitudes and angles and reflectance measurements were made with an Etymotic ER-10c probe using software and hardware developed by Mimosa Acoustics (HearID v4.0.13). To maximize the DPOAE magnitude response at the frequency fdp = 2f1 − f2 at the lower frequencies, we fixed f2/f1 = 1.25 and L1 = L2 = 75 dB SPL; DPOAEs were measured at 13 log-spaced frequencies with fdp approximately 500 to 4000 Hz. Response magnitudes were obtained from the discrete Fourier transform of the time-domain average of N responses. The number of responses N varied with noise level, with a maximum N=420. The artifact rejection algorithm with HearID dropped noisy buffers from the averaging; averaging was automatically stopped before N = 420 when the average signal-to-noise ratio of the entire buffer exceeded 15 dB. The noise floor was estimated from a narrow frequency band surrounding the response measured at fdp, and data that fell less than 6 dB above the estimated noise floor were eliminated (Roede et al., 1993). Reflectance and impedance quantities were calculated, as described in the HearID users manual or in Voss and Allen (1994), from pressure measurements made in the ear canal at a level of 75 dB SPL across a broad-band frequency range. Briefly, pressure reflectance R is calculated directly from the impedance, and the pressure reflectance is the complex ratio between the reflected pressure and the incident pressure. The power reflectance ℛ is the square of the magnitude of the pressure reflectance such that ℛ = |R|2, and the power reflectance can be interpreted as the fraction of power reflected in the ear canal and at the tympanic membrane.
2.4. Measurement Protocol
All measurements were made in a double-walled sound-proof audiometric booth. Subjects were placed on a tilting table (Hangups®II Inversion Table) at two postural positions: upright (90° relative to the horizontal) and tilted (−45° relative to the horizontal). The estimated ICPs of the subjects at these two positions are 0 mmHg at 90° and 22 mmHg (about 30 cm H20 or 293 daPa) at −45° (Chapman et al., 1990; de Kleine et al., 2000; Voss et al., 2006), with some variation from the mean estimates expected. Each subject participated in a total of five measurement sessions across five different days; the duration of time between the first and fifth measurement session ranged from 5 to 34 days, and the time of day which measurements occurred was not controlled. During each session, measurements of DPOAEs and reflectance were made first in the left ear at both upright and tilted positions and second in the right ear at both upright and tilted positions. For each ear, measurements were made in the following order. First, the subject was placed on the tilt table in the upright position. Tympanometry (Earscan, Micro Audiometrics Corp., ES-T) at 226 Hz was used to monitor middle-ear pressure MEP (assumed equal to the tympanic peak pressure TPP). In order to maintain the MEP as close to zero as possible, the subject was asked to swallow; in cases where MEP differed by more than ±25 daPa from zero, subjects were encouraged to continue swallowing until either the MEP was within ±25 daPa of zero or the subject demonstrated an inability to equalize his or her MEP near zero. Once the MEP was documented and as close to zero as possible, the ER-10c’s foam plug was placed in the ear canal and consecutive measurements of DPOAEs and reflectance were made. Next, the subject was tilted to the −45° position. After tilting, emission measurements reach stability (presumably a stable ICP) within 30 seconds (de Kleine et al., 2000), so subjects were tilted for one minute before additional measurements were made. At this position, the MEP sequence described above was repeated, and followed by measurements of DPOAEs and reflectance at the tilted position. Measurements were always made on the left ear first and the right ear second. Once the tilted measurement was completed on the left ear, the subject was raised back to the upright position and measurements commenced on the right ear; there was always a greater than five minute period between when the subject reached the upright position and the DPOAE and reflectance measurements began.
2.5. Inclusion Criteria
Data analysis revealed significant differences on the order of 1 to 3 dB between the DPOAE magnitudes on the left and right sides at about half of the frequencies tested; as a result, we only report measurements made on the left ear, since this was the ear measured first. We do not understand these differences, and one hypothesis is that the five minute period between the subject having been reclined and then upright may not have been long enough for the system to return to its normal state. Büki et al. (2000) showed that when going from a tilted position to upright, the DPOAE phase responses stabilize within several seconds, whereas in some cases the level responses were less stable and did not always reach their pre-tilting levels within five minutes. Thus, at each measurement frequency, a total of 10 measurements of DPOAEs and reflectance were analyzed on each of the left ears from 12 subjects (5 sessions ×1 ear ×2 positions) for a total of 120 measurements across all subjects.
All DPOAE data within 6 dB of the noise floor were removed (Roede et al., 1993). Additionally, some measurements were discarded for artifactually high levels. After all measurements were made, some measurements were observed to have much higher DPOAE magnitudes (> 30 dB SPL) than a typical measurement. These measurements were outliers in both DPOAE magnitudes (much higher than other measurements) and phase (nearly constant phase across most frequencies). These measurements were reproduced in a cavity by making many repeated measurements of DPOAEs in a cavity; most DPOAE measurements were within the noise (low distortion), but occasionally, a high level of distortion was measured in the cavity with characteristics similar to what we observed in our outlier data. It appears that the PC-card used during these measurements could enter an unstable mode. We systematically swapped out pieces of the HearID system and discovered that the distortion only appeared with this particular card. Once the card was retired, no more measurements with high levels of distortion were observed. Of the 120 measurements made on the left ears, we eliminated 12 measurements (10%); one measurement on each of six subjects in the upright position and one measurement on each of six subject in the tilted position. In five of these cases, when one measurement of an upright / tilted combination showed distortion, the other measurement also showed distortion; there were only two exceptions to this generalization.
2.6. Statistical Analyses
The descriptive statistics of means and standard deviations were calculated for cases where three or more data points exist.
A repeated measures regression model was used to determine whether there were significant changes within the DPOAE magnitude and angle data between positions at each of the 13 measurement frequencies. The analysis accounted for clustering within subjects (since DPOAE magnitudes were measured repeatedly on the same subject) using random intercept models (Laird and Ware, 1982; Feldman, 1988; Fitzmaurice et al., 2004). The same analysis was performed on the power reflectance measurements at the same 13 frequencies for which the DPOAEs were measured. This approach is similar to a general linear model that models associations between observations (i.e., multivariate analysis of co-variance, MANCOVA). The models assume that the data are approximately normally distributed and specify a structure for the association within the subjects because the observations are clustered within subjects. The residual errors were assumed to be uncorrelated conditional on a random intercept estimated for each subject. These regression models are attractive because they allow for the incorporation of partially observed subjects under the assumption that missing values depend only on observed quantities [missing at random or MAR in the sense of Little and Rubin (Little and Rubin, 2002)]. Stata version 10.1 was used to fit these models.
To help identify where (measured variable and frequency range) the most information about the change between the upright and tilted positions is likely to be found, we compute p values using resampling, which calculates probabilities directly from the data and does not require an assumption about normality of the data set. The null hypothesis that data collected at the two postural positions are different was tested by calculating p values with a permutation test with 10,000 iterations (Efron and Tibshirani, 1993) and replacement. The test used distances calculated between mean values for the data collected at each of the two postures. Individual p values were calculated for each subject at each frequency for the three quantities: DPOAE magnitudes, DPOAE angles, and power reflectance. Matlab version 7.6 was used for the permutation testing. No adjustments for multiple comparisons were made.
3. Results
3.1. Middle-ear pressures
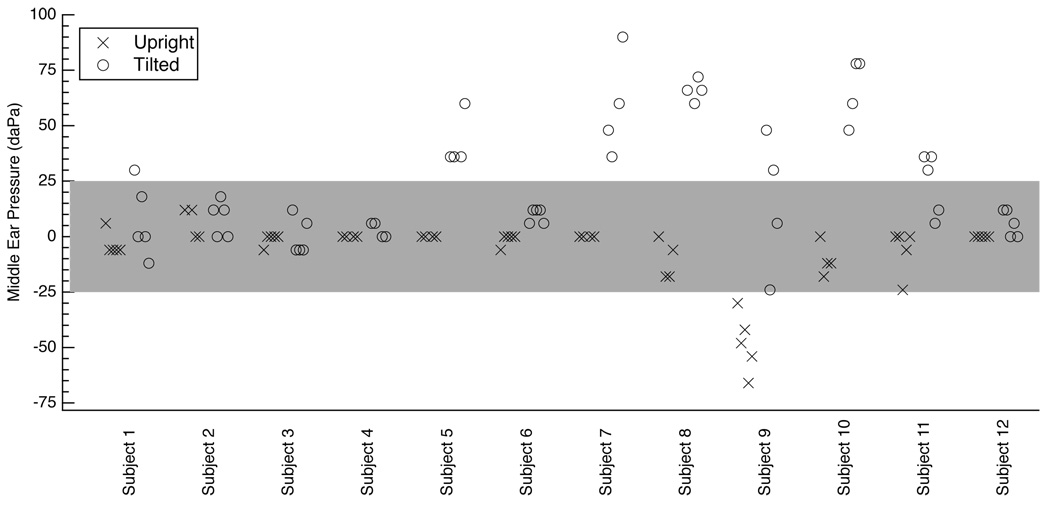
The tympanic peak pressure (TPP), assumed equal to the middle-ear pressure (MEP), was measured before each DPOAE and reflectance measurement session. Figure 1 reports these MEPs at each of the two postural positions. The MEP from 11 of the 12 ears was always within ±25 daPa of zero with the subject in the upright position; the exception was Subject 9, whose MEP ranged from −66 to −30 daPa in the upright position. When the subjects were tilted, 5 of these 11 ears remained within ±25 daPa of zero for all sessions. The seven ears that were not always within ±25 daPa of zero tended to show increases in MEP between the upright position and the tilted position. The average change in MEP when a subject went from upright to tilted was calculated for each subject, and then averaged across all subjects, resulting in an average increase of 32 daPa.
Figure 1.
Middle-ear pressures measured as tympanic peak pressure (TPP) via tympanometry. Measurements are offset slightly on the x-axis in the order they were taken; for a given ear, the upright measurement made first is furthest to the left and the corresponding tilted measurement is also furthest to the left. All ears have four or five middle-ear pressure measurements. Ears with all measurements available have five data points for each position (upright and tilted), and those with DPOAE and reflectance measurement sessions that were determined to be corrupted by high distortion levels are not included here (see methods section 2.6). The tympanometer reports TPP with a resolution of 6 daPa. The gray shaded region indicates TPP within ±25 daPa of zero.
There were a total of 54 pressure measurements in the upright position and 54 measurements in the tilted position (excluding those that correspond to high distortion measurements, see methods section 2.6); 53 of these measurements are common to measurements made consecutively in the upright and tilted position, resulting in the ability to calculate 53 changes in pressure between the upright and tilted position. If we define ΔMEP as ΔMEP ≡ MEPtilted − MEPupright, then of the 53 measurements of ΔMEP, 31 cases have −6 ≤ ΔMEP ≤ 25 daPa, 8 cases have 25 < ΔMEP < 50 daPa, 7 cases have 50 < ΔMEP < 75 daPa, and 7 cases have 75 < ΔMEP ≤ 90 daPa.
3.2. Upright and Tilted Measurements
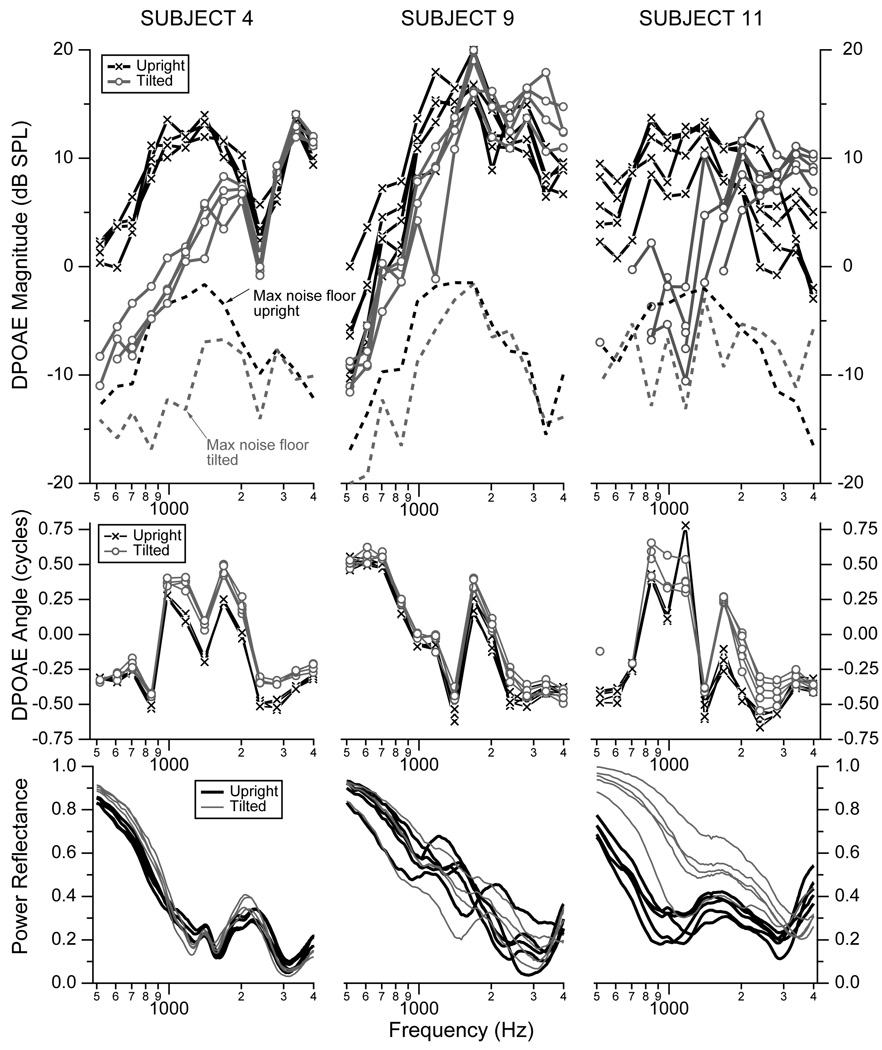
Distortion product otoacoustic emissions (DPOAE) and reflectance were measured on the left ears from 12 subjects in both the upright and tilted positions. Figure 2 shows an example of these measurements from Subjects 4, 9, and 11. Subjects 4 and 11 were chosen at random from the 12 subjects, using the “rand” function in Matlab, and subject 9 was chosen as the ear that showed some of the smaller changes in DPOAE measures with tilting and had the least repeatable reflectance measures. The upper row of Fig. 2 plots the DPOAE magnitudes for each ear with the subject in the upright position (black) and the tilted position (gray), the middle row plots the corresponding DPOAE angles, and the lower row plots the power reflectances. The results from these example subjects have several features in common with the results from the other nine subjects (not shown). In general, tilting the subject reduced the DPOAE magnitudes the most at the lower frequencies, with reductions in magnitude up to 1500 to 2000 Hz. Above 2000 Hz, the changes in DPOAE magnitude between upright and tilted were generally less than 5 dB.
Figure 2.
DPOAE magnitudes (upper row), DPOAE angles (middle row) and power reflectance (lower row) from the left ears of Subject 4 (left column), Subject 9 (middle column), and Subject 11 (right column). Measurements plotted in black correspond to the upright position, and those plotted in gray correspond to the subject tilted at −45° to the horizontal with an estimated ICP on the order of 22 mm Hg (about 30 cm H20 or 293 daPa). The maximum noise floor measured for each subject at each frequency across all measurement sessions is plotted in dashed lines on the magnitude plots. Noise levels are generally higher at low frequencies for upright postures (black dashed lines) because the measurements were stopped once the signal-to-noise level reached 15 dB. All DPOAE data with a magnitude within 6 dB of the noise floor were assumed to be corrupted by noise and were not plotted. The DPOAE magnitudes and angles are plotted as a function of the frequency f2.
The DPOAE angles showed systematic changes between the upright and tilted conditions, but unlike the DPOAE magnitudes, the changes in angle are larger for frequencies above about 1000 Hz. For most frequencies the tilted position led to increases in the angle, which varied from a small fraction of a cycle to more than a quarter of a cycle. The change in angle depends on both frequency and the specific ear. This systematic change in angle between the upright and tilted positions documented here for the example subjects is consistent with the measurements on all other ears. In some of the other ears, the change in angle was a reduction in angle instead of an increase in angle when tilted; however, the repeatable and steady change between the two positions occurred across all ears.
The power reflectance generally increases with tilting for the lower frequencies, with smaller changes above 1000 to 2000 Hz. While some ears show repeatable power reflectance from session-to-session (e.g., Subject 4), others show both more intrasubject variability across sessions that include either clear separation between upright and tilted measurements (e.g., Subject 11) or some overlap between the upright and tilted measurements (e.g., Subject 9 is one of three ears that fit this latter category).
3.3. Changes in DPOAE and reflectance measurements with changes in posture
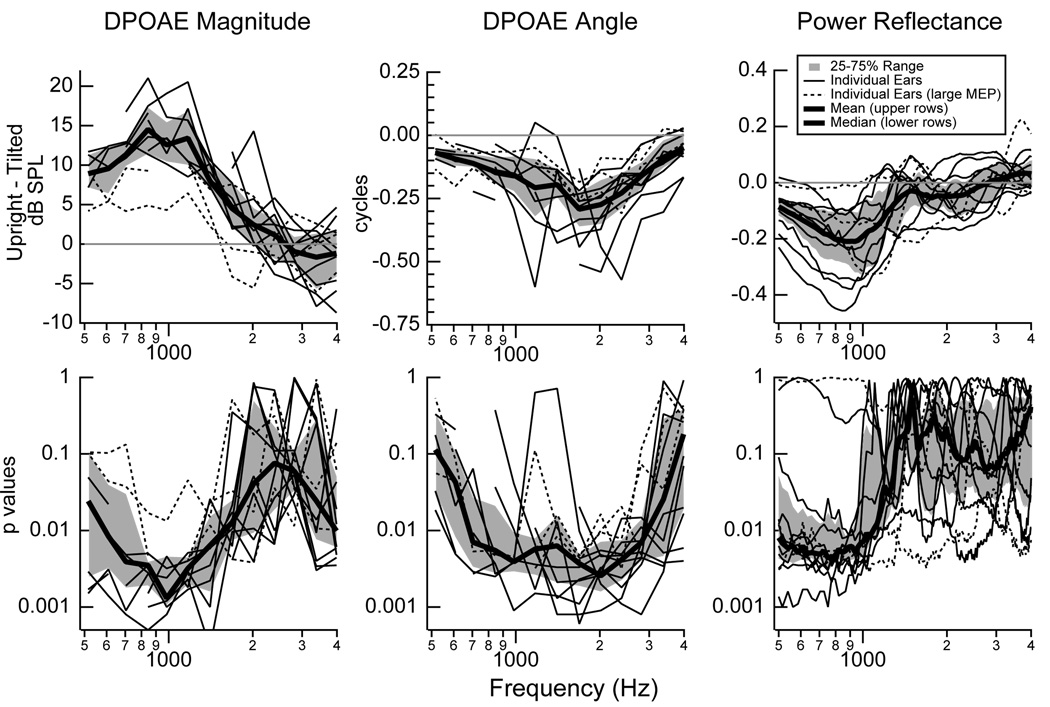
For each measurement session on each ear, the difference between the DPOAE magnitude, DPOAE angle, and power reflectance in the upright and tilted positions was calculated, and the mean difference for each ear is plotted (Fig. 3 upper row). The upper-left plot shows that the group mean of the individual mean DPOAE magnitude differences increases from about 10 dB at 500 Hz to 13 dB at 1000 Hz, decreases to 7 dB at 1400 Hz, and then decreases to nearly zero above 2000 Hz. All measurements on all ears show this general pattern of larger low-frequency differences and small to nearly no differences above about 2000 Hz. Thus, DPOAE magnitudes are systematically reduced at frequencies below 1500 Hz when a subject is tilted.
Figure 3.
Mean changes (upper row) and the corresponding p values (lower row) for each individual ear in DPOAE magnitudes (left), DPOAE angles (middle), and power reflectance (right) between the upright and tilted positions. The DPOAE magnitudes and angles and corresponding p values are plotted as a function of the frequency f2. UPPER ROW: The difference between the upright and tilted quantity was calculated for each measurement. For each subject, the mean of the differences was calculated and plotted in either thin solid lines (9 ears) or dashed lines (3 ears). The dashed lines correspond to three ears that include 11 of the 14 largest changes in middle-ear pressure (|ΔMEP| > 50daPa). The group means calculated from the individual’s means plotted on each graph is indicated by a thick black line, and the 25 to 75% range of all individual means is indicated by the regions shaded gray. For all cases, means were only calculated at frequencies where three or more data points exist. LOWER ROW: Computed p values to test the hypothesis that data collected at the two postural positions are different. Individual p values were calculated for each subject at each frequency. Thin solid and dashed lines (pressure outliers described above) represent p values for individual ears, the region shaded gray is the range for 25 to 75% of the ears, and the thick black line is the median p value at each frequency. Values were computed with a permutation test with 10,000 iterations and replacement.
The middle plot (Fig. 3) shows that the group mean of the individual mean DPOAE angle differences is systematically different from zero. For most subjects at most frequencies, the angle difference is on the order of −0.10 to −0.25 cycles. Thus, DPOAE angles appear to be systematically changed from normal at all frequencies in the 500 to 4000 Hz range when a subject is tilted.
The right plot shows that the group mean of the individual mean power reflectance differences is systematically different from zero at lower frequencies; below 1500 Hz, most ears show mean changes ranging from −0.05 to −0.25. Above 1500 Hz, the changes are smaller and are both positive and negative, with a mean that hovers near zero. In summary, changes in reflectance measures between the upright and tilted positions appear to be systematically different from zero at frequencies below 1000 to 1500 Hz.
Figure 3 (lower row) plots p values computed for each individual subject to test whether or not the data collected at the two positions (upright and tilted) are different. The DPOAE magnitudes show their strongest changes at the lowest frequencies, with a median p value below 0.01 for frequencies from 600 to about 1500 Hz. Above about 2000 Hz, the DPOAE magnitudes become more similar between the two conditions, and the p values associated with DPOAE magnitudes measured at the two positions increase above 0.05. In contrast to the DPOAE magnitudes, the DPOAE angles have a median p value below 0.01 from 600 to 3000 Hz; above 3000 Hz the 25 to 75% range approaches 0.5, but below 3000 Hz the p values associated with all DPOAE angles are generally below 0.05. The power reflectances show their smallest p values at the lowest frequencies. Below about 1000 Hz, the median p values associated with changes in power reflectance are below p = 0.01, and above 1000 Hz the median p value exceeds 0.05.
The dashed lines in all of the plots of Fig. 3 correspond to three ears that include 11 of the 14 largest changes in middle-ear pressure (|ΔMEP| > 50daPa). There is no evidence that the results from these ears with larger ΔMEP changes differ systematically from ears with smaller changes in ΔMEP.
The repeated measures regression model results are shown in Table 1. This model includes all data taken on all ears and predicts significant changes between positions for all three measurements at the p < 0.001 level up to 2016 Hz. The DPOAE magnitudes difference between upright and tilted increases with frequency up to a maximum of 14.3 dB at 844 Hz and then decreases with increasing frequency at most of the measured frequencies. There is not a systematic trend with frequency in the DPOAE angle difference, but the value itself ranges from −0.08 to −0.22 cycles for the 516 to 2016 Hz frequency range. The effect of frequency on the power reflectance difference is similar to that for the DPOAE magnitudes; the difference increases with frequency to a maximum of 0.21 at 844 Hz and then generally decreases with frequency to 0.05 at 2016 Hz. Above 2000 Hz, the DPOAE angle continues to be a significant predictor at the p < 0.001 level up to the highest frequency measured of 3984 Hz. The DPOAE magnitudes and power reflectance are significant at some frequencies above 2000 Hz.
Table 1.
Differences in the predicted least-squares means of DPOAE magnitudes (dB), DPOAE angles (cycles), and power reflectance ℛ between the upright (90°) and tilted (−45°) positions.
| Frequency (Hz) |
DPOAE Mag (dB) |
DPOAE angle (cycles) |
ℛ |
|---|---|---|---|
| 516 | *** 8.2 | ***-0.08 | ***0.09 |
| 609 | *** 9.3 | ***-0.11 | ***0.13 |
| 703 | ***10.7 | ***-0.11 | ***0.17 |
| 844 | ***14.3 | ***-0.15 | ***0.21 |
| 984 | ***12.9 | ***-0.19 | ***0.20 |
| 1172 | ***13.3 | ***-0.10 | ***0.13 |
| 1406 | *** 8.6 | ***-0.16 | ***0.04 |
| 1688 | *** 4.4 | ***-0.22 | ***0.05 |
| 2016 | *** 2.3 | ***-0.20 | ***0.05 |
| 2391 | 0.9 | ***-0.15 | ***0.04 |
| 2813 | *-1.1 | ***-0.16 | 0.01 |
| 3375 | ***-1.8 | ***-0.09 | *-0.02 |
| 3984 | **-1.4 | ***-0.05 | **-0.03 |
The asterisks indicates significance at the p < 0.001 level (***),
p < 0.01 level (**),
p < 0.05 level (*).
3.4. Intra-subject variability of measurements
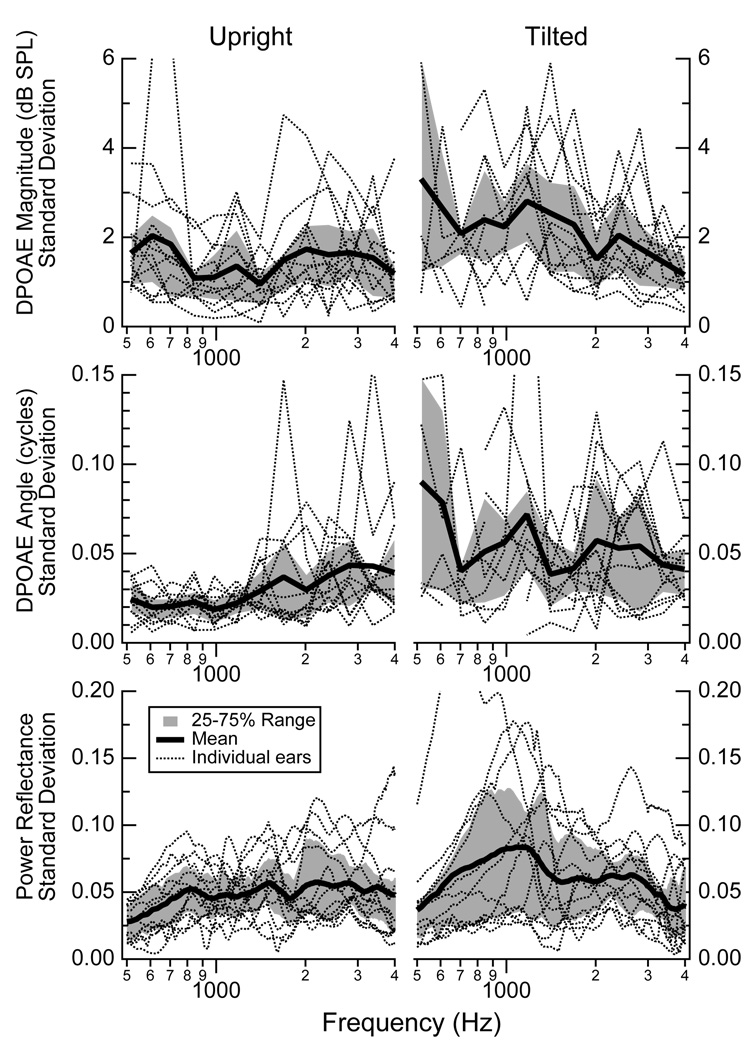
Multiple measurements of DPOAE magnitudes, DPOAE angles, and reflectance were made on each subject. Figure 4 plots the standard deviations of the multiple measurements for each individual ear for each condition. In the upright position, DPOAE magnitudes have standard deviations between about 1 and 2 dB SPL (25 to 75% range) for the frequency range of 500 to 4000 Hz (Fig. 4, upper left). The tilted condition (Fig. 4, upper middle) has a somewhat larger 25 to 75% range of about 1.5 to 3 dB SPL for frequencies below 2000 Hz, with a similar range to the upright condition above 2000 Hz. The standard deviations for the DPOAE angles (Fig. 4 middle row) are smaller in the upright position than tilted, with a 25 to 75% range generally within about 0.01 to 0.04 cycles for upright and about 0.02 to 0.06 cycles when tilted. The standard deviations for the power reflectance (Fig. 4, lower row) have means that hover near 0.05 for both conditions, with a 25 to 75% range that is smaller for the upright position than for the tilted one. For frequencies below about 2000 Hz, the tilted subjects have a larger range of standard deviations that approach 0.10 for some frequencies.
Figure 4.
Standard deviations calculated for DPOAE magnitudes (upper row), angles (middle row), and power reflectance (lower row) for upright (left column) and tilted (right column) postures. For each subject and each posture, a standard deviation was calculated at all frequencies where three or more data points existed from repeated measurements. Reasons for less than three include the elimination of data affected by either distortion or noise. The individual standard deviations from separate ears are plotted with dotted lines; the mean standard deviation for each condition at each frequency is indicated by the thick black line; and the 25 to 75% range of data is indicated by the region shaded gray. The DPOAE magnitudes and angles are plotted as a function of the frequency f2.
4. Discussion
4.1. Summary of Results
DPOAE magnitudes, DPOAE angles, and power reflectance all showed systematic changes with posture, and presumably with ICP. The significance of the changes were assessed with two methods: p values computed via (1) a resampling numerical approach for each subject (Fig. 3) and (2) a repeated measures regression model that combined all subjects (Table 1). In both tests, all three measures showed significant changes at multiple frequencies. We note that changes at the lowest frequencies in DPOAE magnitudes might be artifactually smaller than their actual values; the elimination of data within the noise floor potentially removes the smaller DPOAE magnitudes, leaving a subset of data for which the upright and tilted DPOAE magnitudes are potentially closer together than those that were removed.
The resampling approach focused on individual differences within a subject, and the population results were reported along with the median p values (Fig. 3, lower row). With the resampling approach, the DPOAE magnitudes had the strongest separation between upright and tilted positions: for frequencies below 1500 Hz the DPOAE magnitudes resulted in the smallest p values of all measurements for the range of 500 < f2 < 1500. Considering all three measures, DPOAE angles showed consistent separation between the postures across the largest frequency range, with the majority of measurements having p values below 0.05 from 500 < f2 < 3000. The power reflectance showed systematic changes that are statistically significant with p < 0.05 only for the lowest frequencies: below about 1000 Hz the median and the majority of measurements have p < 0.05, however, just above 1000 Hz, the number of ears for which the p value exceeds 0.05 increases to more than half. Thus, within the individual measurements, reflectance measures appear to provide meaningful information regarding posture, and presumably ICP, for frequencies below about 1000 Hz.
The overall population based effects of tilting the subject were assessed via the repeated measures regression model, which combined all data across all 12 ears (Table 1). With this larger data set, the differences between upright and tilted appear stronger than with the resampling approach that involves individual ears. All three measures showed significant changes between upright and tilted at the p < 0.001 level for frequencies between 516 and 2016 Hz. At the higher frequencies (2391 to 3984 Hz), most of the measures continued to show significant differences, including the DPOAE angles at all frequencies.
Multiple measurements on the same ear, repeated during different sessions on different days, provide data regarding the variability of DPOAE magnitudes, DPOAE angles, and reflectance. We report standard deviations as a measure of repeatability of these measurements (Fig. 4). In general, the DPOAE magnitude standard deviations in the upright position appear lower than or comparable to other reports in the literature (Franklin et al., 1992; Roede et al., 1993; Zhao and Stephens, 1999; Beattie et al., 2003; Wagner et al., 2008). Here, the standard deviations in the tilted position are generally larger than those in the upright position but are still comparable to standard deviations reported in the literature. Similarly, the standard deviations for the DPOAE angles and reflectance measures are somewhat smaller for the upright position, as compared to the tilted position.
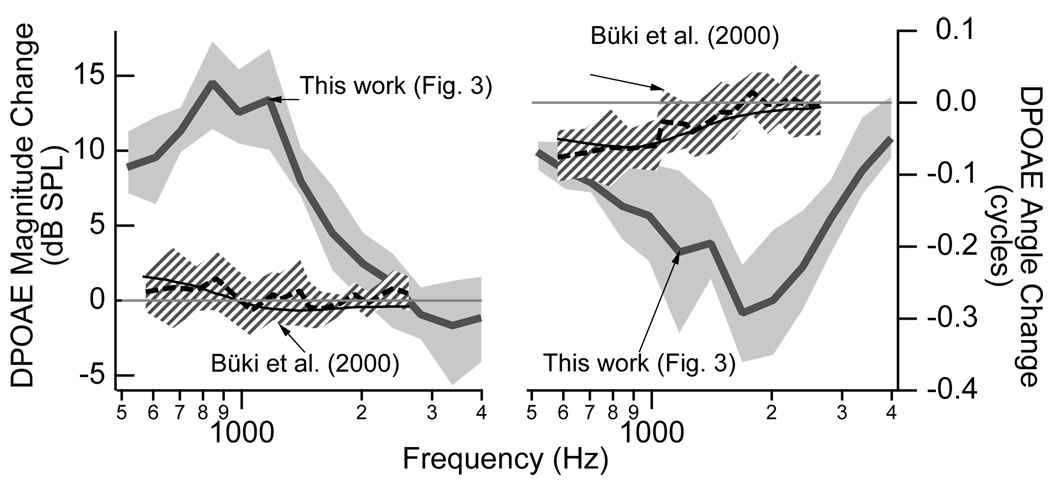
Figure 5 compares the measurements of DPOAE magnitudes and angles made here to those made by Büki et al. (2000). The changes in both magnitude and angle were substantially larger in our study. There are at least two major differences between these sets of measurements. First, our subjects were tilted to −45° as compared with −30° for the Büki et al. (2000) subjects, which presumably corresponds to a larger posture-induced ICP change in this work. Second, our DPOAE stimulus levels were equal at 75 dB SPL whereas the Büki et al. (1996) stimulus levels were 60 dB SPL at the f2 frequency and 70 dB SPL at the f1 frequency; our stimulus conditions may have provided a larger signal-to-noise ratio at the lower frequencies that potentially allowed measurements with larger differences to be included in the analysis and not discarded due to proximity to the noise floor. Additionally, we included all data within 6 dB of the noise floor while Büki et al. (2000) required a signal-to-noise ratio of 12 dB. Büki et al. (2000) also included results from a lumped-element model that assumed posture (and changes in ICP) affected the stiffness of the annular ligament. The model prediction fits the general pattern of the results, and future work might explore if it is possible for this model to fit the results we present with a larger postural change. If it is not possible for this model to match these larger changes in DPOAE magnitudes and angles that we measured here, then one interpretation might be that larger ICP values influence both the middle ear (via the compliant structures) as well as the nonlinear behavior of the outer hair cells. For example, as the annular ligament and round window stretch toward their maximum outward positions, then it is possible that changes in static pressure within the cochlea could influence outer hair cell function.
Figure 5.
Comparison of changes (upright minus tilted) in DPOAE magnitudes (left) and angles (right) between the work presented here in Fig. 3 and that presented by Büki et al. (2000). The postural changes were from upright to −45° in this work and from upright to −30° in Büki et al. (2000). The mean data from Büki et al. (2000) is represented by the dashed black line with the hashed area indicating ±1 standard deviation, and the solid black line represents a model prediction. As presented in Fig. 3, the gray line indicates the mean changes from the work here with the 25–75% range shaded in gray.
4.2. Effects of middle-ear static pressure
Changes in middle-ear static pressure present a complication to the use of middle-ear transmission to monitor changes in ICP. It is widely recognized that static pressure differences across the tympanic membrane, with either positive or negative middle-ear pressures, affect middle-ear function and thus DPOAEs (e.g., Huttenbrink, 1988; Hauser et al., 1993; Osterhammel et al., 1993; Plinkert et al., 1994; Sun and Shaver, 2009). Specifically, for low frequency DPOAEs, the DPOAE magnitude has been shown to decrease with nonzero middle-ear pressures (e.g., Huttenbrink, 1988; Hauser et al., 1993; Osterhammel et al., 1993; Plinkert et al., 1994; Sun and Shaver, 2009). Sun and Shaver (2009) provide the most systematic data related to the effect of middle-ear pressure on DPOAE magnitude, showing that middle-ear pressures of −40 to −65 daPa lead to low-frequency reductions in DPOAE magnitudes of 4 to 6 dB, and as middle-ear pressures decrease below −65 daPa, the DPOAE magnitudes are reduced further at low frequencies. Thus, while it is likely that pressure changes that occur with posture changes affect the DPOAEs, the magnitude of the low-frequency DPOAE changes induced by postural changes here (8–14 dB) is larger than than that induced by pressure changes of −40 to −65 daPa.
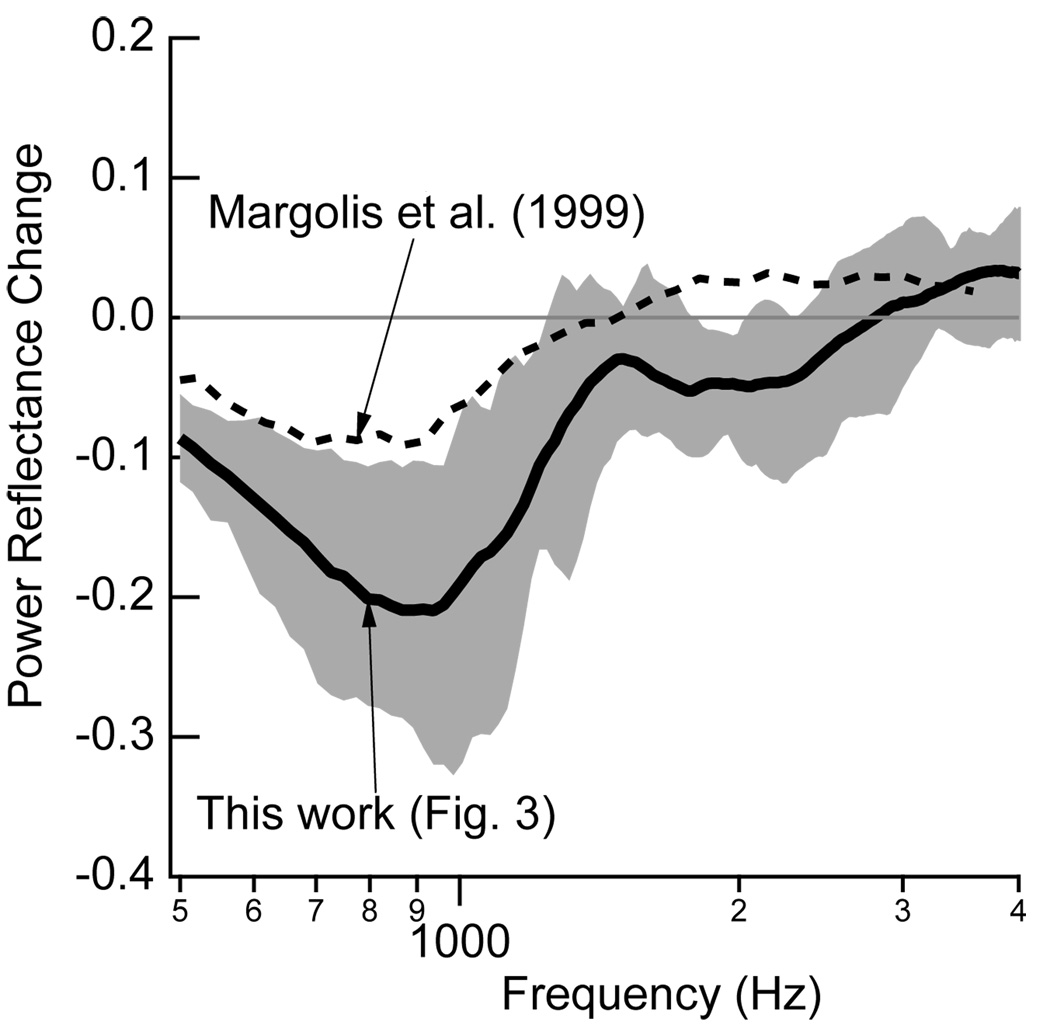
Static pressure differences across the tympanic membrane also influence reflectance measures (e.g., Keefe and Levi, 1996; Margolis et al., 1999; Feeney et al., 2003; Liu et al., 2008). Figure 7 of Margolis et al. (1999) compares reflectance measurements at ambient ear-canal pressures to those measured at a compensated ear-canal pressure equal to TPP. Their range of ambient pressures is −42 daPa to 14 dPa, which, while not identical, is comparable to many of our subjects (Fig. 1). Figure 6 compares the difference between the energy reflectance for these compensated and ambient ear-canal pressures to the changes in energy reflectance measured here with postural changes. It appears that at the lower frequencies the postural changes have substantially larger effects on energy reflectance than small middle-ear pressures. We note that Liu et al. (2008) also reported changes in energy reflectance between ambient and compensated ear-canal pressures, and these changes appear comparable to the changes reported here in Fig. 3; however, Liu et al. (2008) also question whether or not they had valid measurements of ear-canal pressure under the ambient condition.
Figure 6.
Comparison of the effects of postural changes and ear-canal static pressures on energy reflectance. The difference in energy reflectance between the upright and tilted −45° postures reported in Fig. 3 are represented by the mean measurement plotted in solid black and the 25–75% range (shaded gray). Margolis et al. (1999) report the means of measurements of reflectance made at ambient ear-canal pressure and at an ear-canal pressure compensated to match the tympanic peak pressure measured via tympanometry. These reflectance measurements were extracted from Fig. 7 of Margolis et al. (1999), converted to energy reflectance, and the difference between the two conditions (compensated minus ambient) is plotted in the dashed line.
In the measurement protocol here, the subjects went from an upright to a tilted position, and this change in position generally led to increases in middle-ear pressure (Fig. 1). Averaged across subjects, the middle ear pressure increased by 32 daPa as a result of tilting to −45°. This result is similar to the findings of Büki et al. (1996) who report an average increase of 36 daPa when posture was changed from upright to −30° relative to the horizontal. Consistent with the results here, Büki et al. (1996) report a large standard deviation across subjects for the change in middle-ear pressure with posture. These results are consistent with the measurements of Liau (1999) who also reported an increase in tympanic peak pressure via tympanometry with posture changes in 12 of 17 tested ears. A difference between our measurements and the Büki et al. (1996) measurements is that it appears that the Büki et al. (1996) subjects were not asked to swallow upon being tilted. The similarity of the results suggests that the swallowing may have had little or no effect.
We hypothesize that as a subject is tilted, and the ICP increases, the stapes equilibrium position shifts so that the stapes is pushed into the middle ear by the increased ICP and corresponding increased intracochlear pressure. This shift in the stapes has at least two effects: (1) the volume of the middle-ear air space is reduced, which leads to an increase in the pressure within the air space, and (2) the stiffness of the annular ligament is changed (presumably increased) as the stapes is pushed out of its normal equilibrium position. In our protocol, subjects were asked to swallow multiple times after being tilted, so that any increase in middle-ear pressure would be eliminated via opening of the Eustachian tube. We note that the request for the subjects to swallow might be an imperfect control, as swallowing induces contraction of the tensor tympani muscle, which in turn could theoretically have an effect on the middle ear or inner ear pressure (e.g., Salt, 2007).
We assessed middle-ear pressure via the TPP from tympanometry, which reports the ear-canal static pressure for which the admittance is a maximum; this assumption that TPP is equivalent to middle-ear pressure when the stapes is not beginning in its equilibrium position at ambient pressure has not been tested. Thus, our measured changes in middle-ear pressure between the upright and tilted position could occur (1) because the subject was unable to equalize his or her middle-ear pressure via the Eustachian tube and a true pressure differential exists or (2) the measure of TPP could be inaccurate in some subjects when the stapes is not in its equilibrium position at an ambient middle-ear pressure.
In our data set of 54 sequences (from 12 ears) of measurements with the subject first upright and then tilted, the change in middle-ear pressure between the two positions was between −25 and +6 daPa for 31 of the 54 measurements; thus, in 57% of our cases the middle-ear pressure changes were minimal. An additional 8 cases had changes between −25 and −50 daPa (15%), and 14 cases had changes between −50 and −90 daPa (26%). The reasons for these larger changes might be one of the two reasons hypothesized above: either the subject was unable to equilibrate his or her pressure or the measure of TPP is an inaccurate measure of middle-ear pressure. Nonetheless, the changes in pressure we estimate are generally small. As a further test of the effect of changes in middle-ear pressure, we highlight the three ears that include 11 of the 14 largest pressure differences measured via TPP. As shown in Fig. 3, these ears are not collectively outliers in the measures of mean changes (between upright and tilted) or p values for any of the measured quantities (DPOAE magnitudes, DPOAE angles, and power reflectance).
4.3. Clinical application of monitoring ICP with ear-canal based measurements
Changes in middle-ear transmission appear to offer potential for a noninvasive method to monitor ICP changes. Systematic changes in both DPOAEs and reflectance measures occur with posture changes (and presumably increases in ICP); in normal-hearing patients, a combination of DPOAE and reflectance measures are a candidate for noninvasive detection of changes in ICP. DPOAE measures require a functioning inner ear, while reflectance measures require a normal middle ear.
The method of detecting changes in middle-ear transmission as an indicator for changes in ICP will not lead to numerical estimates for ICP, but instead will provide a metric for detecting changes in ICP. This method therefore requires at least one and preferably a series of baseline measurements from each patient in a particular state. In the long term, the DPOAE and reflectance measurement system might be designed with a built-in tympanometric measurement system that could monitor and compensate for middle-ear pressure, using a compensation procedure similar to the one proposed by Sun and Shaver (2009) or used by Margolis et al. (1999) and Liu et al. (2008). Situations where this method to monitor ICP might be particularly useful include long-term monitoring of patients with potential for changes in ICP (e.g., hydrocephalus, brain tumors), post-surgical monitoring of ICP, and monitoring of ambulatory patients during transport to medical facilities (e.g., traumatic injuries such as acquired via traffic accidents or in the military field).
4.4. Unanswered Questions
The development of a metric that uses changes in middle-ear transmission to detect changes in ICP requires more work. Future work might explore methods to combine (1) the information from DPOAE magnitudes and angles and reflectance measures into a single metric and (2) a magnitude and angle analysis in the complex plane to determine how points affected by noise might be identified in ways different than imposing an elimination of points with DPOAE magnitudes within 6 dB of the noise floor.
A second area of unanswered questions relates to how middle-ear transmission and / or otoacoustic emissions might be affected by changes in the intralabyrinthine pressure that purportedly occur with Ménière’s disease. Since both Ménière’s disease and changes in intracranial pressure appear to alter the intralabyrinthine pressure, work in these areas might, in the long run, provide synergistic information for the goals of monitoring either intracranial pressure changes or Ménière’s disease with noninvasive ear-canal based measurements. As an example, Mom et al. (2009) measured click-evoked otoacoustic emissions on both normal-hearing subjects and Ménière’s disease subjects to study the effects of glycerol and body tilt on the measurements. While the results are not directly comparable to the work presented here (different type of emission and subjects tilted to zero degrees and not −45°), the results are consistent with the work here in that both the magnitude and phase of the click-evoked otoacoustic emissions were systematically altered with posture.
Acknowledgments
This work was supported by a CAREER award from the National Science Foundation (SEV) and grant R01 DC003687 (CAS) from the NIDCD, National Institutes of Health. We also thank our volunteer subjects and three helpful Hearing Research anonymous reviewers.
Abbreviations
- DPOAE
distortion product otoacoustic emissions
- ICP
intracranial pressure
- TPP
tympanic peak pressure
- MEP
middle ear pressure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Susan E. Voss, Email: svoss@smith.edu, Picker Engineering Program, Smith College, Northampton, MA, USA, 51 College Lane, Northampton, MA 01063, USA.
Modupe F. Adegoke, Email: madegoke@smith.edu, Picker Engineering Program, Smith College, Northampton, MA, USA.
Nicholas J. Horton, Email: nhorton@smith.edu, Department of Mathematics and Statistics, Smith College, Northampton, MA, USA.
Kevin N. Sheth, Email: kshethmd@gmail.com, Division of Stroke & Neuro-Critical Care, Department of Neurology, University of Maryland School of Medicine, Baltimore, MD, USA.
Jonathan Rosand, Email: rosand@chgr.mgh.harvard.edu, Vascular & Critical Care Neurology, Center for Human Genetic Research, Massachusetts General Hospital, Boston, MA, USA.
Christopher A. Shera, Email: shera@epl.meei.harvard.edu, Eaton Peabody Laboratory of Auditory Physiology, Massachusetts Eye & Ear Infirmary, 241 Charles St. Boston, MA, USA.
References
- Beattie RC, Kenworthy OT, Luna CA. Immediate and short-term reliability of distortion-product otoacoustic emissions. Int. J. Audiol. 2003;42:348–354. doi: 10.3109/14992020309101328. [DOI] [PubMed] [Google Scholar]
- Büki B, Avan P, Lemaire JJ, Dordain M, Chazal J, Ribari O. Otoacoustic emissions: A new tool for monitoring intracranial pressure changes through stapes displacements. Hear. Res. 1996;94:125–139. doi: 10.1016/0378-5955(96)00015-9. [DOI] [PubMed] [Google Scholar]
- Büki B, Chomicki A, Dordain M, Lemaire JJ, Wit HP, Chazal J, Avan P. Middle-ear influence on otoacoustic emissions. II: contributions of posture and intracranial pressure. Hear. Res. 2000;140:202–211. doi: 10.1016/s0378-5955(99)00202-6. [DOI] [PubMed] [Google Scholar]
- Büki B, de Kleine E, Wit HP, Avan P. Detection of intracochlear and intracranial pressure changes with otoacoustic emissions: A gerbil model. Hear. Res. 2002;167:180–191. doi: 10.1016/s0378-5955(02)00392-1. [DOI] [PubMed] [Google Scholar]
- Büki B, Giraudet F, Avan P. Non-invasive measurements of intralabyrinthine pressure changes by electrocochleography and otoacoustic emissions. Hear. Res. 2009;251:51–59. doi: 10.1016/j.heares.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Chapman PH, Cosman ER, Arnold MA. The relationship between ventricular fluid pressure and body position in normal subjects and subjects with shunts: A telemetric study. Neurosurgery. 1990;26:181–189. doi: 10.1097/00006123-199002000-00001. [DOI] [PubMed] [Google Scholar]
- de Kleine E, Wit HP, Avan P, Van Dijk P. The behavior of evoked otoacoustic emissions during and after postural changes. J. Acoust. Soc. Am. 2001;110:973–980. doi: 10.1121/1.1381025. [DOI] [PubMed] [Google Scholar]
- de Kleine E, Wit HP, Van Dijk P, Avan P. The behavior of spontaneous otoacoustic emissions during and after postural changes. J. Acoust. Soc. Am. 2000;107:3308–3316. doi: 10.1121/1.429403. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Chapman & Hall; 1993. [Google Scholar]
- Feeney MP, Grant IL, Marryott LP. Wideband energy reflectance measurements in adults with middle-ear disorders. J. Speech Lang. Hear. R. 2003;46:901–911. doi: 10.1044/1092-4388(2003/070). [DOI] [PubMed] [Google Scholar]
- Feldman HA. Families of lines: Random effects in linear regression analysis. J. Appl. Physiol. 1988;64:1721–1732. doi: 10.1152/jappl.1988.64.4.1721. [DOI] [PubMed] [Google Scholar]
- Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. New York: John Wiley & Sons; 2004. [Google Scholar]
- Franklin DJ, McCoy MJ, Martin GK, Lonsbury-Martin BL. Test/retest reliability of distortion-product and transiently evoked otoacoustic emissions. Ear Hear. 1992;13 doi: 10.1097/00003446-199212000-00008. 417-129. [DOI] [PubMed] [Google Scholar]
- Frank A, Alexiou C, Hulin P, Janssen T, Arnold W, Trappe AE. Non-invasive measurement of intracranial pressure changes by otoacoustic emissions (OAEs) – A report of preliminary data. Zentralbl Neurochir. 2000;61:177–180. doi: 10.1055/s-2000-15597. [DOI] [PubMed] [Google Scholar]
- Hauser R, Probst R, Harris FP. Effects of atmospheric pressure variation on spontaneous, transiently evoked, and distortion product otoacoustic emissions in normal human ears. Hear. Res. 1993;69:133–145. doi: 10.1016/0378-5955(93)90101-6. [DOI] [PubMed] [Google Scholar]
- Huttenbrink KB. The mechanics of the middle ear at static air pressures. Acta Otolaryngol. 1988 Suppl 451:1–35. doi: 10.3109/00016488809099007. [DOI] [PubMed] [Google Scholar]
- Kanter RK, Weiner LB, Patti AM, Robson LK. Infectious complications and duration of intracranial pressure monitoring. Crit Care Med. 1985:837–839. doi: 10.1097/00003246-198510000-00012. [DOI] [PubMed] [Google Scholar]
- Keefe DH, Bulen JC, Arehart KH, Burns EM. Ear-canal impedance and reflection coefficient in human infants and adults. J. Acoust. Soc. Am. 1993;94(5):2617–2638. doi: 10.1121/1.407347. [DOI] [PubMed] [Google Scholar]
- Keefe DH, Levi EC. Maturation of the middle and external ears: Acoustic power-based responses and reflectance tympanometry. Ear Hear. 1996;17:361–373. doi: 10.1097/00003446-199610000-00002. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Liau J. Ph.D. thesis. Stanford University; 1999. Non-invasive methods to monitor intracranial pressure: An exploratory study. [Google Scholar]
- Little RJA, Rubin DB. Statistical Analysis With Missing Data, 2nd edition. 2nd Edition. New York: John Wiley & Sons; 2002. [Google Scholar]
- Liu Y-W, Sanford CA, Ellison JC, Fitzpatrick DF, Gorga MP, Keefe DH. Wideband absorbance tympanometry using pressure sweeps: System development and results on adults with normal hearing. J. Acoust. Soc. Am. 2008:3708–3719. doi: 10.1121/1.3001712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnano M, Albera R, Lacilla M, Gabini A, Naddeo M, Bruno D. Impedance measurement as a noninvasive technique for the monitoring of intracranial pressure variations. Audiology. 1994;33:237–243. doi: 10.3109/00206099409071883. [DOI] [PubMed] [Google Scholar]
- Maniker AH, Vaynman AY, Karimi RJ, Sabit AO, Holland B. Hemorrhagic complications of external ventricular drainage. Neurosurgery. 2006:419–424. doi: 10.1227/01.NEU.0000222817.99752.E6. [DOI] [PubMed] [Google Scholar]
- Marchbanks RJ. Measurement of tympanic membrane displacement arising from aural cardiovascular activity, swallowing, and intraaural muscle reflex. Acta Otolaryngol. 1984;98:119–129. doi: 10.3109/00016488409107543. [DOI] [PubMed] [Google Scholar]
- Margolis RH, Saly GL, Keefe DH. Wideband reflectance tympanometry in normal adults. J. Acoust. Soc. Am. 1999:265–280. doi: 10.1121/1.427055. [DOI] [PubMed] [Google Scholar]
- Mom T, Gilain L, Avan P. Effects of glycerol intake and body tilt on otoacoustic emissions reflect labyrinthine pressure changes in Meni´ere’s disease. Hear. Res. 2009;250:38–45. doi: 10.1016/j.heares.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Osterhammel PA, Nielsen LH, Rasmussen AN. Distortion product otoacoustic emissions: The influence of the middle ear transmission. Scand. Audiol. 1993;30:111116. doi: 10.3109/01050399309046026. [DOI] [PubMed] [Google Scholar]
- Plinkert PK, Bootz F, Voβieck T. Influence of static middle ear pressure on transiently evoked otoacoustic emissions and distortion products. Eur. Arch. Otorhinolaryngol. 1994;251:95–99. doi: 10.1007/BF00179900. [DOI] [PubMed] [Google Scholar]
- Roede J, Harris FP, Probst R, Xu L. Repeatability of distortion product otoacoustic emissions in normally hearing humans. Audiology. 1993;32:273–281. doi: 10.3109/00206099309072943. [DOI] [PubMed] [Google Scholar]
- Rosingh HJ, Wit HP, Albers FWJ. Limitations of the MMS-10 tympanic displacement analyser. Audiology. 1998;37:1–6. doi: 10.3109/00206099809072956. [DOI] [PubMed] [Google Scholar]
- Salt AN. Letter to the Editor: The endolymphatic sinus is a possible detector of endolymph volume status. Hear. Res. 2007;224:117–118. doi: 10.1016/j.heares.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheithauer S, Bürgel U, Ryang YM, Haase G, Schiefer J, Koch S, Häfner H, Lemmen S. Prospective surveillance of drain associated meningitis / ventriculitis in a neurosurgery and neurological intensive care unit. J Neurol Neurosurg Psychiatry. 2009:1381–1385. doi: 10.1136/jnnp.2008.165357. [DOI] [PubMed] [Google Scholar]
- Shimbles S, Dodd C, Mendelow AD, Chambers IR. Clinical comparison of tympanic membrane displacement with invasive intracranial pressure measurements. Physiol. Meas. 2005;26:1085–1092. doi: 10.1088/0967-3334/26/6/017. [DOI] [PubMed] [Google Scholar]
- Sun X-M, Shaver MD. Effects of negative middle ear pressure on distortion product otoacoustic emissions and application of a compensation procedure in humans. Ear Hear. 2009;22:191–202. doi: 10.1097/AUD.0b013e31819769e1. [DOI] [PubMed] [Google Scholar]
- Voss SE, Allen JB. Measurement of acoustic impedance and reflectance in the human ear canal. J. Acoust. Soc. Am. 1994;95:372–384. doi: 10.1121/1.408329. [DOI] [PubMed] [Google Scholar]
- Voss SE, Horton NJ, Tabucchi THP, Folowosele F, Shera CA. Posture-induced changes in distortion-product otoacoustic emissions and the potential for noninvasive monitoring of changes in intracranial pressure. Neurocrit. Care. 2006;4:251–257. doi: 10.1385/NCC:4:3:251. [DOI] [PubMed] [Google Scholar]
- Voss SE, Shera CA. Simultaneous measurement of middle-ear input impedance and forward/reverse transmission in cat. J. Acoust. Soc. Am. 2004;116:2187–2198. doi: 10.1121/1.1785832. [DOI] [PubMed] [Google Scholar]
- Wagner W, Heppelmann G, Vonthein R, Zenner HP. Test-retest repeatability of distortion product otoacoustic emissions. Ear Hear. 2008;29:378–391. doi: 10.1097/AUD.0b013e31816906e7. [DOI] [PubMed] [Google Scholar]
- Wilson JP. Evidence for a cochlear origin for acoustic re-emissions, threshold fine-structure and tonal tinnitus. Hear. Res. 1980:233–252. doi: 10.1016/0378-5955(80)90060-x. [DOI] [PubMed] [Google Scholar]
- Wolfe TJ, Torbey MT. Management of intracranial pressure. Curr Neurol Neurosci Rep. 2009:477–485. doi: 10.1007/s11910-009-0070-1. [DOI] [PubMed] [Google Scholar]
- Zhao F, Stephens D. Test-retest variability of distortion-product otoacoustic emissions in human ears with normal hearing. Scand. Audiol. 1999;28:171–178. doi: 10.1080/010503999424743. [DOI] [PubMed] [Google Scholar]