Abstract
Challenges associated with the interference observed between the dengue virus components within early tetravalent live-attenuated vaccines led many groups to explore the development of recombinant subunit based vaccines. Initial efforts in the field were hampered by low yields and/or improper folding, but the use of the Drosophila S2 cell expression system provided a mechanism to overcome these limitations. The truncated dengue envelope proteins (DEN-80E) for all four dengue virus types are expressed in the S2 system at high levels and have been shown to maintain native-like conformation. The DEN-80E proteins are potent immunogens when formulated with a variety of adjuvants, inducing high titer virus neutralizing antibody responses and demonstrating protection in both mouse and non-human primate models. Tetravalent vaccine formulations have shown no evidence of immune interference between the four DEN-80E antigens in preclinical models. Based on the promising preclinical data, the recombinant DEN-80E proteins have now advanced into clinical studies. An overview of the relevant preclinical data for these recombinant proteins is presented in this review.
Introduction
Dengue is the most important vector-borne viral disease in terms of morbidity and mortality with an estimated 2.5 billion people throughout the tropics and subtropics at risk of infection. An estimated 50 million infections with dengue occur worldwide annually, with approximately 2.1 million severe cases, 500,000 cases of dengue hemorrhagic fever (DHF), and 20,000 deaths [1–4]. Disease caused by dengue virus infection ranges from asymptomatic to severe life-threatening disease generally referred to as DHF and dengue shock syndrome (DSS).
Dengue is caused by any one of the four dengue viruses (Family Flaviviridae). While infection with one type of dengue virus is widely believed to provide life-long immunity against that virus type, it does not provide long-term protection against infection from the other dengue virus types. Furthermore, while the more severe forms of the disease occur relatively infrequently (< 5%), the vast majority of DHF cases occur following secondary infection [1, 4, 5]. Although there are several hypotheses to explain the association between DHF and secondary infection, nearly all postulate that the severe disease manifestation includes an immunopathogenic component [6–10]. These hypotheses have led to the common theme underlying all dengue vaccine development: vaccines must be tetravalent, and must induce potent balanced immunity to all four viruses. This approach to vaccine development is widely believed to be the best way to limit both dengue virus-induced disease and to mitigate the risk of exacerbated disease.
Up until the late 1980s work on dengue vaccines focused exclusively on live attenuated viruses produced using classic methods (e.g. passage through foreign substrates such as primary dog kidney cells) [11,12]. The traditional approach of a whole killed vaccine was not pursued until recently, mainly because of the generally low productivity of dengue virus in cell culture. As live attenuated viruses advanced from monovalent to tetravalent trials, the interaction/interference between the viruses emerged as an issue [13]. The concept of interference has continued to plague the development of live attenuated dengue vaccines [13–15]. More recently, the use of molecular biology methods has allowed for the development of attenuated viruses based on defined mutations conferring attenuation (e.g. chimerization with other flaviviruses, deletion of portions of the genomes, etc.). The use of these molecularly defined viral vaccine strains and use of extended dosing schedules has helped reduce the impact of vaccine virus interference (see other articles in this Special Issue).
The application of molecular biological tools to dengue vaccine development also led to the pre-clinical development of a number of alternative vaccine approaches including recombinant subunit vaccines, viral vectored vaccines (adenovirus, pox, alphavirus or measles) and DNA delivery [16–22]. All of the vaccine approaches have their own associated advantages and disadvantages in terms of safety, immunogenicity, and general feasibility in terms of the ability to develop into a commercial product. Viral vectored vaccines and DNA vaccines will be the topics of other articles in this Special Issue and will not be considered here. Recombinant subunit vaccine approaches offer one of the safest alternatives, a means to avoid the issue of viral interference, and the ability to administer a tetravalent formulation on an accelerated schedule. One advantage of an accelerated schedule is that full protective immunity would be induced more quickly, thus avoiding the potential of exacerbated disease due to partial immunity during an extended immunization course. Other advantages of an accelerated schedule include better general compliance, more suitability for travelers and military personnel, easier integration into existing immunization schedules, and the potential for use in an outbreak setting. A balanced tetravalent immune response may also be more easily achieved through simple adjustments of dose for each of the four recombinant proteins, compared to live virus vaccines where the interactions between viruses can be complex and unpredictable. For these reasons several groups launched efforts to develop recombinant subunit based dengue vaccines as described below.
The dengue envelope (E) glycoprotein has been the focus of subunit-vaccine development as it is the primary target of neutralizing antibodies [23–26]. In an effort to express recombinant E proteins several different expression systems have been utilized, including Escherichia coli, baculovirus vectors, yeast, mammalian cells, insect cells and more recently plant based expression systems (recently reviewed in ref. 27 and 28 and summarized in Table 1). Proper folding of the envelope protein is required to preserve the integrity of its neutralizing epitopes and this generally requires the coexpression of the premembrane (prM) protein along with E [29]. The prM protein acts as a chaperone for E facilitating proper folding and trafficking through the secretory pathway [18, 30]. Successful expression also requires that the expression system is capable of the proper processing of the prM and E proteins including disulfide bond formation, proteolytic cleavage and glycosylation.
Table 1.
Expression platforms used for recombinant dengue protein expression
| Strengths | Weaknesses | References | |
|---|---|---|---|
| Eschericia coli |
|
|
31–35 |
| Saccharomyces cerevisciae |
|
|
47 |
| Pichia pastoris |
|
|
42, 43, Clements et al., unpublished data |
| Chinese Hamster Ovary DHFR system |
|
|
Coller et al. unpublished data |
| Vaccinia expression in mammalian cells |
|
|
41 |
| Baculovirus in Sf9 or High Five cells |
|
|
30, 36–40 |
| Stably transformed Drosophila S2 cells |
|
|
54, 55 |
High expression level > 10 mg/L
Moderate expression level > 1 mg/L < 10 mg/L
Low expression level < 1 mg/L
Due to the inability of bacteria to efficiently process membrane-associated glycoproteins, much of the work in E.coli has focused on the expression of subregions of E typically fused to other proteins. Epitope mapping of E and NS1 was done using E. coli expressed TrpE-Dengue fusion proteins [31, 32]. The E. coli system has also been used to produce vaccine candidates consisting of sub domains of DENV2 E fused with the meningococcal P64K protein, the Staphylococcal A protein, or the E. coli Maltose Binding Protein (MBP) [33–35]. These subdomain based vaccines are the subject of another article in this Special Issue.
The baculovirus expression system has been most widely utilized for the expression of E alone or co-expressed with prM [30, 36–40]. The construction of C-terminal truncations of E, which removes the membrane anchor sequence, was demonstrated to improve its secretion, facilitate purification and improve its immunogenicity [37, 38, 41].
Coexpression of prM and E can induce the formation of virus-like particles (VLP's). VLP's are expected to be more antigenically similar to dengue virions since they contain glycosylated prM and E in association with a lipid membrane. Dengue VLPs have been expressed from baculovirus, yeast, mammalian cells and insect cells [39 42–46]. Although VLP's are recognized by monoclonal antibodies specific for different domains of E and can induce neutralizing antibodies in mice and non human primates [44], the responses are only weak to moderate. Low production yields have also hindered their commercial application.
While production challenges have slowed progress in the field, it is our view that recombinant subunits provide a promising approach to the development of a tetravalent dengue vaccine. In order for this approach to be successful, it is imperative that an expression system is used that can produce recombinant E proteins that maintain relevant native-like characteristics with yields high enough to support commercial production. The focus of this review is on the C-terminally truncated E proteins produced utilizing the Drosophila S2 cell expression system. The S2 system has been demonstrated to produce high levels of high quality dengue E antigens that are suitable as vaccine candidates.
Cloning and Expression of Recombinant Envelope Proteins
Initial efforts by Hawaii Biotech Inc. (HBI) scientists to express the recombinant dengue E proteins focused on yeast expression (Saccharomyces cerevisiae and Pichia pastoris) [47]. Using the C-terminal truncations first identified by Men et al., [41] soluble forms of the E ectodomain were expressed in the yeast expression systems. While high levels of E domain III (EDIII, amino acids 296–395) were expressed in yeast systems and showed modest immunogenicity in mice, the levels of expression of larger E subunits including various C-terminal truncations (e.g. 60% truncated at amino acid 295, 80% truncated at amino acid 395 (80E), and 90% truncated at amino acid 445) were much lower (10–100 fold). Furthermore efforts to produce full-length E co-translationally with prM in an effort to generate VLP's were even less successful.
Based on the low expression levels and modest immunogenicity of the yeast-expressed E proteins, alternative expression systems were explored including evaluation of mammalian expression systems (e.g. Chinese hamster ovary cells with dihydrofolate reductase (DHFR) amplification) and insect cell expression systems. The breakthrough in expression levels came with the evaluation of the Drosophila melanogaster S2 cell expression system originally developed by SmithKline Beecham [48–50] and licensed to Invitrogen (Carlsbad, CA). The system utilizes Schneider 2 (S2) cells that are derived from Drosophila melanogaster embryos [51]. This expression system is based on the generation of stably transformed cell lines that express the protein of interest. Targeting the proteins for secretion helps to assure relevant post-translational modifications are efficiently incorporated and facilitates purification. The use of this system has been shown to express heterologous proteins that maintain native-like biological structure and function [52, 53].
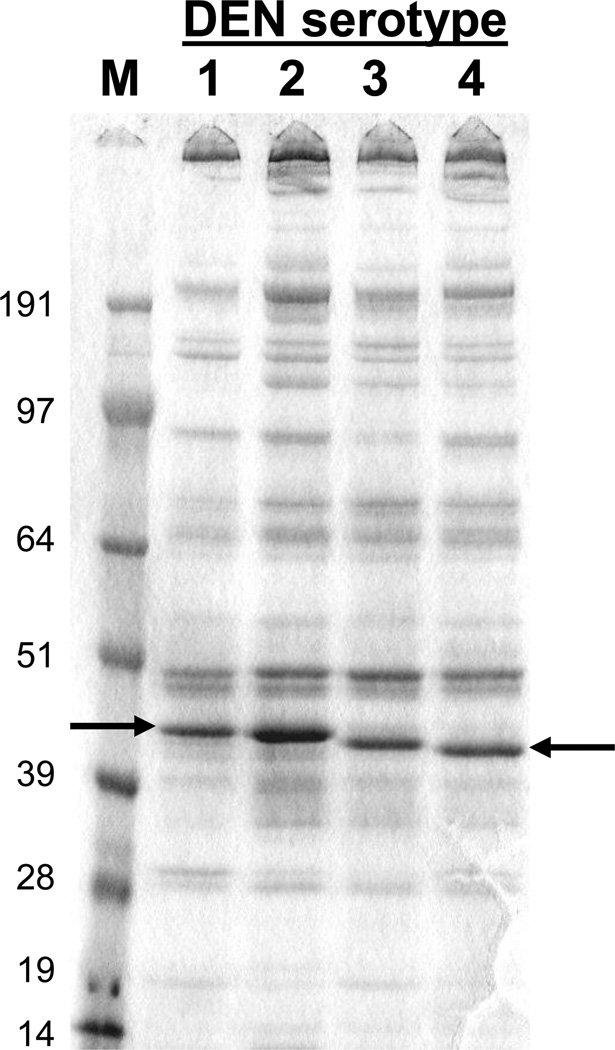
Using the Drosophila S2 cell system, HBI was able to overcome the limitations in expression levels that had challenged the field for years. Focusing on expression of a C-terminally truncated version of E (80E), the cloning of the relevant dengue genes from all four dengue virus types into the S2 cell system led to unprecedented levels of expression and protein quality. Details on the expression constructs have been described [47, 54, 55]. Briefly, dengue sequences encoding the full-length prM protein and 80% of the E protein (80E, truncation at amino acid 395 for DEN1, DEN2 and DEN4, 393 for DEN3) were inserted into the pMttΔXho vector (derived from pMttPA [49]). The 80E truncation removes carboxy-terminal stem region and trans-membrane domain. The dengue gene sequences were derived from the following stains: DENV1 strain 258848, DENV2 strain PR159 S1, DENV3 strain CH53489, and DENV4 strain H241. The expression of the prM-80E sequences in this manner produces a prM-80E polypeptide that is processed by the cells and results in the 80E being secreted into the culture medium. Transformation of Drosophila S2 cells line results in the integration of the expression cassette into the genome to generate stable cell lines. The recombinant proteins are easily detected in the unconcentrated media by Coomassie blue stained SDS-PAGE gels as shown in Figure 1. While the expression levels vary between the dengue types, all DEN-80E proteins are expressed at levels exceeding 10 mg/L and ranging up to approximately 40 mg/L. The high expression levels are illustrated by the prominent band visible in unconcentrated culture supernatant stained with Coomassie blue (Figure 1). It is important that expression levels in this range, or greater, are achieved in order to be able to support cGMP manufacturing. The secreted recombinant DEN-80E subunits are purified from clarified culture medium by immunoaffinity chromatography using conformationally sensitive monoclonal antibodies [54, 55]. The transformed S2 cells are easily adapted to serum free suspension growth which is critical for vaccine manufacture.
Figure 1.
SDS-PAGE Analysis of Secreted Recombinant DEN-80E Products. Ten µl of unconcentrated culture medium from induced S2 cell cultures was separated on a 4–12% SDS-PAGE gel under non-reducing conditions and the proteins stained with Coomassie blue. Size of molecular weight markers in kilodaltons is indicated on the left. Position of DEN-80E bands are indicated by arrows.
The native-like conformation of DEN2-80E and DEN3-80E were subsequently established based on reactivity with conformationally sensitive monoclonal antibodies and the crystal structure determination of these recombinant proteins [56–58]. The DEN-80E structure is very similar to that previously determined for the virally-derived tick-borne encephalitis E ectodomain [59]. The recombinant DEN-80E proteins are glycosylated at both of the N-linked sites encoded by the E protein.
Formulation Evaluations
Soluble recombinant proteins are typically not very immunogenic without an adjuvant [60], so a significant effort was invested to evaluate various candidate vaccine formulations for their ability to induce robust immune responses while still maintaining a safety profile that could be acceptable for humans. Until recently, aluminum salt based adjuvants were the only adjuvants approved for human use. However, over the last 5–10 years a number of vaccine formulations containing novel adjuvants have been approved for human use in various countries around the world. These include adjuvants based on oil-in-water emulsions (e.g. MF59 and AS03 from Novartis and GlaxoSmithKline [GSK] respectively) and adjuvants based on toll-like receptor agonists (e.g. Monophosphoryl lipid A – MPL from GSK) [61]. A number of additional products containing other novel adjuvants are currently undergoing early and late stage clinical development (e.g. ISCOMATRIX™ adjuvant from CSL and AS01 and ASO2 from GSK) and may be licensed for human use in the coming years [62, 63].
The combination of the recombinant DEN-80E antigens with these modern adjuvants may offer advantages in terms of the magnitude, breadth, and durability of the immune response induced compared to classic aluminum salt-based formulations. To that end several potent modern adjuvants, in addition to aluminum salt-based adjuvants, were evaluated in combination with the recombinant DEN-80E antigens in mice and non-human primate studies. Through these studies several formulations were identified which showed potent immunogenicity and efficacy and which can be considered for clinical development. A summary of some of the key studies and their findings is presented below.
Immunogenicity of the Recombinant DEN-80E Subunits in Mice
Immunogenicity of the recombinant DEN-80E antigens was evaluated in inbred and outbred mice with a variety of adjuvant formulations. In an early study utilizing DEN2-80E, the variability of the antibody responses induced by various formulations was noted, Table 2 [55]. In these studies the highest virus neutralizing titers were obtained with formulations containing ISCOMATRIX™ adjuvant or MF75 with threonyl-MDP. A formulation containing Ribi700 adjuvant induced an intermediate virus neutralizing response, while formulations containing aluminum hydroxide, MF59 or Freund's adjuvant induced lower virus neutralizing titers. In comparing the relative ability of the adjuvants to induce virus neutralizing titers when formulated with DEN2-80E, it should be noted that responses following two doses of ISCOMATRIX™ adjuvant administered with 10 µg of antigen were compared with the titers induced by three doses of the other adjuvants formulated with 12.5 or 25 µg of antigen. Despite the lower doses of antigen and fewer immunizations, ISCOMATRIX™ adjuvant formulated with DEN2-80E produced the most potent virus neutralizing antibodies, with a PRNT80 titer of 4000.
Table 2.
Virus Neutralizing Responses in Mice Immunized with DEN2-80E Formulated with Various Adjuvants
| Antigen | Adjuvant | Geometric Mean PRNT80 Titer |
|---|---|---|
| DEN2-80E | MF59 | 26 |
| DEN2-80E | MF75+ThrMDP | 2297 |
| DEN2-80E | Ribi | 333 |
| DEN2-80E | Alum | 24 |
| DEN2-80E | Freund’s | <10 |
| DEN2-80E | ISCOMATRIX™ Adjuvant | 4000 |
| DEN2-80E | no Adjuvant | 10 |
| PBS | no Adjuvant | <10 |
Evaluation of each of the four recombinant DEN-80E proteins was conducted following formulation with ISCOMATRIX™ adjuvant. Various doses of each DEN-80E were administered to determine the effective immunizing dose for each recombinant protein. All four of the DEN-80E proteins induced a potent virus neutralizing antibody response which reached maximum neutralizing antibody titers (reciprocal PRNT50 titers ranging from 526 to 10,556) at protein doses ranging from 3 to 10 µg of each DEN-80E, Table 3 [55]. While the virus neutralizing titers are not identical for each DEN-80E, it is clear that there is a dose response and that adjustment of the protein dose will impact the virus neutralizing antibody titers induced.
Table 3.
Virus Neutralizing Antibody Titers in Mice Immunized with Various Doses of Recombinant DEN-80E Proteins Formulated with ISCOMATRIX™ Adjuvant
| DEN-80E Antigen Dose |
Geometric Mean PRNT50 Titer | |||
|---|---|---|---|---|
| DENV1 | DENV2 | DENV3 | DENV4 | |
| 30 µg | NT* | NT | 1741 | 728 |
| 10 µg | 1450 | 10,556 | 1550 | 526 |
| 3 µg | 1053 | 8574 | 1908 | 278 |
| 1 µg | 504 | 4595 | 1414 | 144 |
| 0.3 µg | 35 | 2096 | 400 | 28 |
| 0.1 µg | <20 | 590 | NT | NT |
| PBS | <10 | <10 | <10 | <10 |
NT - Not tested
The durability of the immune response in mice was examined with DEN2-80E formulated with selected adjuvants and the virus neutralizing antibody responses were assessed at monthly intervals for 6 months as shown in Table 4 [55]. As with the study comparing various formulations described above, mice were immunized with only 2 doses of vaccine formulated with ISCOMATRIX™ adjuvant at a 4 week interval compared to 3 doses of vaccine containing the other adjuvants (MF75 or Ribi) administered at a 3 week interval. High neutralizing titers of several thousand were elicited following immunization with the all of the formulations tested and the virus neutralizing antibody titers remained at levels of greater than 1000 through 6 months for all but one of the formulations (906 for Ribi at 6 months). At 6 months the mice were given a booster dose to test for a memory immune response for two of the formulations. Virus neutralizing antibody titers were increased for both formulations when tested one month following the 6 month booster immunization [55]. These results demonstrate that DEN2-80E formulated with various adjuvants generated high virus neutralizing antibody responses that persisted for at least 6 months in mice and that a subsequent booster resulted in an anamnestic response indicating that good memory component has been induced.
Table 4.
Duration of Immunity in Mice Immunized with DEN2-80E Formulated with Various Adjuvants
| Vaccine Formulation |
Geometric Mean PRNT80 Titer | ||||||
|---|---|---|---|---|---|---|---|
| Month 1 |
Month 2 |
Month 3 |
Month 4 |
Month 5 |
Month 6 |
Post- Boost |
|
| 10 µg DEN2-80E ISCOMATRIX™ adjuvant* | 6500 | 6062 | 5278 | 3703 | 4924 | 3732 | 12,996 |
| 25 µg DEN2-80E Ribi# | 3177 | 4816 | 3249 | 2276 | 1784 | 906 | 6858 |
| 25 µg DEN2-80E MF75# | 6063 | 6063 | 4756 | 2000 | 1207 | 2000 | NT |
| 25 µg DEN2-80E MF75+ThrMDP# | 8000 | 5278 | 3031 | 3031 | 1320 | 3482 | NT |
NT – not tested, animals did not receive booster dose of vaccine.
Animals were immunized with 2 doses of vaccine administered at a 4 week interval.
Animals were immunized with 3 doses of vaccine at a 3 week interval. Dose 1 included 25 µg of DEN2-80E while the booster doses included 12.5 µg of DEN2-80E.
In order to assess potential immune interference between the four recombinant proteins, the DEN-80E proteins were mixed together in a tetravalent formulation. A mixture consisting of 10 µg of each of the four DEN-80E subunits formulated with ISCOMATRIX™ adjuvant was used to immunize mice. The tetravalent results were compared against results generated from immunization with each individual DEN-80E protein. As shown in Table 5, mice immunized with the tetravalent formulation developed virus neutralizing antibody titers against each dengue virus type similar to the titer obtained in mice immunized with a single DEN-80E type suggesting that immune interference would not be a major issue with this tetravalent recombinant subunit dengue vaccine [55].
Table 5.
Homologous Virus Neutralizing Antibody Titers in Mice Immunized with Monovalent or Tetravalent DEN-80E Proteins Formulated with ISCOMATRIX™ Adjuvant
| Immunogen | Geometric Mean PRNT50 Titer | |||
|---|---|---|---|---|
| DENV1 | DENV2 | DENV3 | DENV4 | |
| Monovalent* | 2759 | 3031 | 381 | 174 |
| Tetravalent+ | 1589 | 2639 | 564 | 159 |
| PBS | <20 | <20 | <20 | <20 |
4 groups of mice were immunized with 10 µg of each of the 4 monovalent 80E subunits. Each group was tested for homologous serotype virus neutralizing antibody titers.
1 group of mice was immunized with a tetravalent mixture containing 10 µg of each of the four 80E subunits. The group was tested individually for virus neutralizing antibody titers for each of the dengue serotypes.
Protection of Mice from Viral Challenge
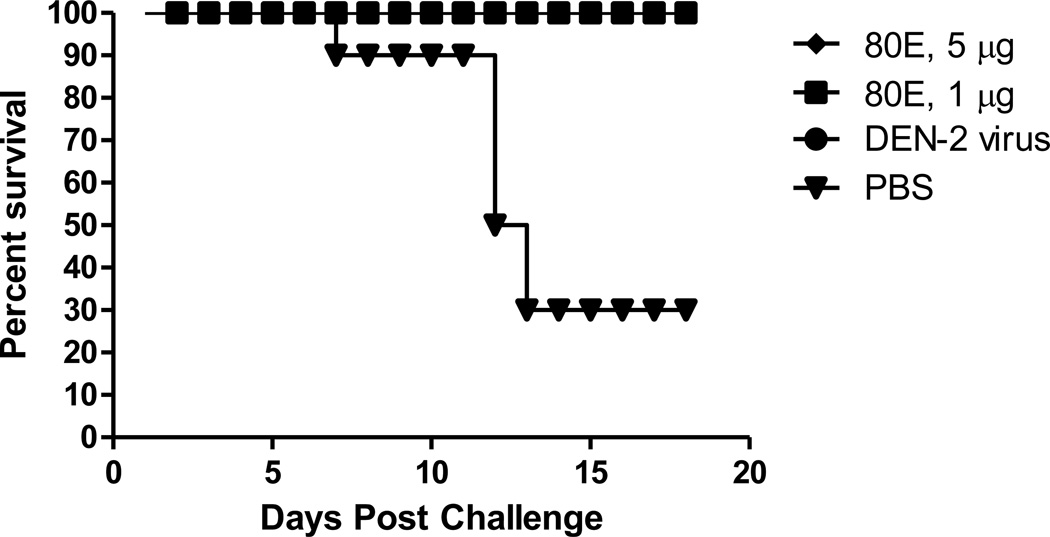
The protective efficacy of the DEN2-80E formulated with ISCOMATRIX™ adjuvant was assessed in a mouse challenge model. Groups of weanling mice were immunized with formulations consisting of 1 and 5 µg doses of DEN2-80E and 2 µg of ISCOMATRIX™ adjuvant and then challenged intracranially with 100 LD50 of mouse-adapted DENV-2 (Figure 2). Complete protection from morbidity and mortality was observed with both doses of recombinant antigen, while 7 of the 10 unimmunized control mice succumbed to viral challenge. These results demonstrate the protective potential of the recombinant DEN-80E antigens [55].
Figure 2.
Survival of Mice Immunized with two different doses of DEN2-80E formulated with ISCOMATRIX™ adjuvant and Challenged with Live DEN2 Virus.
Immunogenicity and Protective Efficacy of the Recombinant DEN-80E Proteins in Non-Human Primates
In a series of immunogenicity and efficacy studies conducted in non-human primates, the ability of monovalent and tetravalent formulations to induce virus neutralizing antibody and protect from viral challenge was evaluated. The rhesus monkey model is not a disease model since the monkeys do not develop dengue-like illness. However, the monkeys do develop a measurable viremia and prevention of viremia is generally taken as an indicator of protective efficacy. Since viremia levels in humans generally correlate with disease severity [5, 64], and the level of viremia developed in monkeys is generally lower than that typically seen in humans who develop disease (e.g. Putnak et al 2005, [54]), it is reasonable to assume that the ability to prevent significant viremia in monkeys will indicate an ability to prevent viremia and therefore disease in humans.
The studies conducted included both monovalent and tetravalent formulations and included the evaluation of several different adjuvants. In one study [54], DEN2-80E was evaluated in formulations containing adjuvants developed by GSK and referred to as the AS series of adjuvants. The adjuvants evaluated in this study included aluminum hydroxide alone (Alum), AS04 (MPL + either of two different aluminum salts – AS04-OH aluminum hydroxide and AS04-PO aluminum phosphate), AS05 (MPL + aluminum hydroxide + the saponin QS21), and AS08 (aluminum hydroxide + QS21). In two other studies formulations with ISCOMATRIX™ adjuvant were evaluated [55]. One focused on DEN2-80E alone and the other focused on tetravalent formulations containing all four DEN-80E proteins plus non-structural protein 1 (NS1) from dengue virus type 2 (DEN2-NS1). The results of each study are summarized in the following paragraphs and demonstrate the potent immunogenicity and protective efficacy of the recombinant DEN-80E proteins formulated with various adjuvants when tested in non-human primates.
Immunogenicity and Efficacy of DEN2-80E formulated with AS adjuvants in rhesus macaques
In this study the efficacy of purified DEN2-80E with several of the GSK (AS) adjuvants were evaluated. Groups of three animals were immunized with two doses of each vaccine formulation or saline placebo on day 0 and day 90. The primary immunization was administered by the subcutaneous route and dose 2 was administered by the intramuscular route. Measureable virus neutralizing antibodies were detected post dose 2 in all animals receiving the DEN2-80E regardless of vaccine formulation or antigen dose, Table 6. The highest geometric mean titer (GMT) of neutralizing antibody after the second dose was observed in the group that received 5 µg of DEN2-80E in AS08 (GMT=7,723), followed by groups that received 5 µg of DEN2-80E in AS05 (GMT=4,660), 1 µg of DEN2-80E in AS05 (GMT=3,222), and 20 µg of DEN2-80E in AS04-OH (GMT=2,671). For recipients of the DEN2-80E formulations, the GMT of neutralizing antibody peaked following dose 2 and then declined at various rates, but all animals still had measurable antibody at 2 months following dose 2 and prior to the virus challenge, Table 6.
Table 6.
Immunogenicity and Efficacy of DEN2-80E Formulated with Several AS Adjuvants in Rhesus Macaques
| Vaccine Formulation* |
Animal ID | PRNT50 Titer Post Dose 2 |
PRNT50 Titer Day of Challenge |
Viremia by Cell Culture (pfu/mL serum) |
|---|---|---|---|---|
| 5 µg DEN2-80E + aluminum hydroxide | GTF | 660 | 1,300 | 3.6 |
| GPE | 680 | 110 | 8.2 | |
| FVF | 345 | 60 | 10.5 | |
| 5 µg DEN2-80E + AS04-OH | GTP | 1100 | 270 | 1.7 |
| GCE | 790 | 100 | 9.2 | |
| JEJ | 570 | 200 | 0 | |
| 20 µg DEN2-80E + AS04- OH | KBV | 4200 | 450 | 0 |
| GKF | 1900 | 400 | 0 | |
| FXX | 2400 | 880 | 0 | |
| 5 µg DEN2-80E + AS04-PO | GPH | 360 | 100 | 1.7 |
| EKB | 250 | 60 | 13.4 | |
| GVV | 490 | 250 | 0 | |
| 1 µg DEN2-80E + AS05 | DBP | 1400 | 1,200 | 0 |
| GEV | 4500 | 730 | 0 | |
| FKT | 5300 | 700 | 0 | |
| 5 µg DEN2-80E + AS05 | GPT | 4700 | 650 | 0 |
| GBA | 5700 | 2,500 | 0 | |
| EEE | 3200 | 750 | 0 | |
| 5 µg DEN2-80E + AS08 | JFE | 8400 | 740 | 1.7 |
| FGC | 9600 | 5,300 | 0 | |
| HPH | 5800 | 1,200 | 0 | |
| Saline | JEH | <10 | <10 | 7.6 |
| GKB | <10 | <10 | 8.7 | |
| GWP | <10 | <10 | 10.10 | |
Animals were immunized with 2 doses of vaccine administered on day 0 and day 90 by the subcutaneous and intramuscular routes respectively.
The animals were challenged subcutaneously with approximately 104 plaque forming units (pfu) of the near wild-type strain of DENV2 S16803 at two months post dose 2. The challenge strain is heterologous relative to the vaccine formulation (PR159 S1 strain) and therefore represents a stringent challenge. The level of viremia was determined for 12 consecutive days after challenge. Viremia was measured using virus isolation on C6/36 mosquito cells (to screen for positive samples) followed by direct plaque assay on Vero cells to determine actual virus titer in the serum (Table 6). All three saline control animals developed viremia starting 4 to 5 days after challenge and lasted through day 8. In contrast, several formulations including the 20 µg dose of DEN2-80E + AS04, and both the 1 and 5 µg dose of DEN2-80E + AS05 had no viremia detectable by plaque assay. In all cases there was an anamnestic antibody response to challenge consistent with limited viral replication and strong memory. High virus neutralizing antibody titers (e.g. >200) were generally associated with protection from viremia as measured by plaque assay, but interestingly a few animals developed detectable viremia despite the presence of high titer virus neutralizing antibody (e.g animal GTF). This highlights the complexity of assessing protection for dengue and a limitation of the model with no disease endpoint. Non-replicating vaccine formulations, and in particular formulations containing aluminum salts, are generally administered by the intramuscular route so the potential impact of administration of the first vaccine dose in this study by the subcutaneous route is not known. Therefore, it is possible that responses were less than ideal under these conditions and the potential for even higher antibody titers and efficacy with these aluminum salt-based formulations may have been seen with intramuscular vaccination for both doses.
Immunogenicity and Efficacy of DEN2-80E formulated with ISCOMATRIX™ adjuvant in rhesus macaques
In this study the ability of the DEN2-80E formulated with ISCOMATRIX™ adjuvant to protect monkeys from virus challenge was tested. Animals were immunized with different doses of purified DEN2-80E antigen formulated with ISCOMATRIX™ adjuvant or aluminum hydroxide adjuvant. The animals were immunized by the subcutaneous route at days 0, 34 and 97 and then challenged subcutaneously with a near wild-type DENV-2 (strain S16803) approximately one month after the last dose (challenge strain heterologous to vaccine strain). Virus neutralizing antibodies measured over the course of the immunization schedule and following virus challenge are presented in Table 7. All DEN2-80E formulations elicited virus neutralizing antibodies by two weeks post dose 2. Peak titers post dose 3 were in the range of 660 to >1280 and do not appear to be dose dependent.
Table 7.
Virus Neutralizing Antibody in Sera from Rhesus Monkeys Immunized with Recombinant DEN2-80E ISCOMATRIX™ Adjuvant Formulations
| Vaccine Formulation# |
Animal ID |
Virus Neutralizing Antibody Titers (PRNT50) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 0 |
Day 34 |
Day 49 |
Day 69 |
Day 97 |
Day 113 |
Day 132 |
Day 159 |
||
| 100 µg DEN2-80E + ISCOMATRIX™ adjuvant | G617 | <10 | 10 | 180 | 460 | 230 | 920 | 400 | >12,800 |
| B7487 | <10 | 110 | 480 | 480 | 600 | >640 | >640 | >12,800 | |
| 25 µg 80E + ISCOMATRIX™ adjuvant | F477 | <10 | <10 | 300 | 230 | 450 | 660 | 470 | >12,800 |
| I613 | <10 | 90 | 3000 | 1000 | 1100 | >1280 | >1280 | >12,800 | |
| 5 µg 80E + ISCOMATRIX™ adjuvant | I619 | <10 | 50 | 1600 | 590 | 1900 | >1280 | 1200 | >12,800 |
| H7J | <10 | 10 | 620 | 760 | 530 | >1280 | >640 | >12,800 | |
| 100 µg DEN2-80E + Aluminum hydroxide | F485 | <10 | 60 | 650 | 1200 | 250 | 770 | 600 | >12,800 |
| Placebo | N637 | NT* | NT | NT | NT | NT | NT | <10 | 860 |
| N670 | NT | NT | NT | NT | NT | NT | <10 | 1600 | |
| N816 | NT | NT | NT | NT | NT | NT | <10 | 990 | |
NT - not tested
Animals were immunized with 3 doses of vaccine administered by the subcutaneous route on days 0, 34, and 97.
The protective efficacy results of the vaccine formulations as determined by the level of viremia are shown in Table 8. The unimmunized controls developed detectable viremia for an average of 9 days. In contrast, all the animals vaccinated with the DEN2-80E formulations exhibited some degree of reduction in viremia levels which in all cases was statistically significant compared to the control group (p <0.0001, 0.0219, and 0.0496, for the low, mid, and high dose groups respectively). Interestingly, there was no detectable viremia in animals that received the lowest dose (5 µg) of DEN2-80E, while animals immunized with higher doses of vaccine of 25 µg or 100 µg showed some breakthrough viremia. Since the numbers of animals are small it is not clear if this represents a reproducible result or not. However, follow on experiments conducted in mice suggested that lower antigen doses administered in a formulation containing ISCOMATRIX™ adjuvant tended to have a more Th1-type profile compared to higher antigen doses (Coller et al., unpublished data) which could be consistent with better protective efficacy. As with the previous study, vaccinated animals exhibited strong anamnestic responses following challenge. In this study the subcutaneous route was used for all immunizations. Based on published data [65] it appears that the intramuscular route is the preferred route for clinical testing of vaccine formulations containing ISCOMATRIX™ adjuvant, so like the previous study where aluminum-salt based formulations were administered partly by the subcutaneous route, this study may represent a less than ideal test of the capabilities of the formulation.
Table 8.
Viremia in Vaccinated Rhesus Monkeys After Challenge with Live DEN2 Virus
| ID | Vaccine | Viremia on Day: | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| G617 | 100 µg DEN2-80E ISCOMATRIX™ adjuvant | 01 | 0 | 0 | 0 | 0 | 0 | +2 | + | 0 | 0 | 0 | 0 |
| B7487 | 100 µg DEN2-80E ISCOMATRIX™ adjuvant | 0 | 0 | 0 | 0 | 0 | + | + | + | 0 | 0 | 0 | 0 |
| F477 | 25 µg DEN2-80E ISCOMATRIX™ adjuvant | 0 | 0 | 0 | + | + | + | + | + | 0 | 0 | 0 | 0 |
| I613 | 25 µg DEN2-80E ISCOMATRIX™ adjuvant | 0 | 0 | 0 | 0 | 0 | + | 0 | 0 | 0 | 0 | 0 | 0 |
| I619 | 5 µg DEN2-80E ISCOMATRIX™ adjuvant | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H7J | 5 µg DEN2-80E ISCOMATRIX™ adjuvant | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| F485 | 100 µg DEN2- 80E/Alum | 0 | 0 | 0 | 0 | 0 | 0 | + | + | + | + | 0 | 0 |
| 517Z | PIV/Alum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| N637 | none | 0 | + | + | + | + | + | + | + | + | 0 | 0 | + |
| N670 | none | 0 | + | + | F3 | + | + | + | + | + | + | + | 0 |
| N816 | none | 0 | + | + | + | F | + | + | 0 | + | + | 0 | 0 |
0 - no virus plaques
+ - > 5 virus plaques
F - 1–5 virus plaques
Immunogenicity and Protective Efficacy of Tetravalent Formulations in Monkeys
In a small pilot study the safety and immunogenicity of a tetravalent DEN-80E vaccine formulation was assessed. In this study two groups with two monkeys each were immunized with different doses (1 µg or 5 µg each) of tetravalent DEN-80E formulated with ISCOMATRIX™ adjuvant. Both tetravalent formulations included DEN2-NS1. Animals were vaccinated by the intramuscular route at 0, 1, 2, and 3 months. Virus neutralizing antibodies measured over the course of the immunization schedule are presented in Table 9. All vaccinated animals developed virus neutralizing antibodies to each of the four dengue types, although the antibody titers varied among animals.
Table 9.
Virus Neutralizing Antibody Responses Induced in Rhesus Macaques Following Vaccination with a Tetravalent Recombinant Subunit Vaccine Formulation
| Group/ Vaccine* |
Animal ID |
Dengue Serotype for PRNT Testing |
Day 0 (Dose 1) |
Day 28 (Dose 2) |
Day 67 (Dose 3) |
Day 102 (Dose 4) |
Day 130 |
Day 275 (Challenge) |
Day 293 |
|---|---|---|---|---|---|---|---|---|---|
| 1 µg each tetravalent DEN-80E + 0.1 µg NS1 + ISCOMATRIX™ Adjuvant | AA37 | DENV-1 | <10 | <10 | 68 | 290 | 510 | 72 | 161 |
| DENV-2 | <10 | <10 | 112 | 194 | 604 | 232 | 135 | ||
| DENV-3 | <10 | <10 | 29 | 136 | 127 | 33 | 61 | ||
| DENV-4 | <10 | <10 | 47 | 83 | 300 | 89 | 107 | ||
| FTH | DENV-1 | <10 | <10 | 232 | 1230 | 1361 | 231 | 711 | |
| DENV-2 | <10 | <10 | 105 | 887 | 1969 | 293 | 548 | ||
| DENV-3 | <10 | <10 | <20 | 231 | 305 | 90 | 747 | ||
| DENV-4 | <10 | <10 | 39 | 313 | 894 | 105 | 812 | ||
| 5 µg each tetravalent DEN-80E + 0.5 µg NS1 + ISCOMATRIX ™ Adjuvant | T206 | DENV-1 | <10 | <10 | 34 | 88 | 193 | 20 | 1300 |
| DENV-2 | <10 | <10 | 53 | 363 | 602 | NA | 630 | ||
| DENV-3 | <10 | <10 | <10 | 75 | 147 | 136 | 6845 | ||
| DENV-4 | <10 | <10 | <10 | 32 | 59 | 17 | 5958 | ||
| AJ14 | DENV-1 | <10 | <10 | 82 | 420 | 326 | 117 | 700 | |
| DENV-2 | <10 | <10 | 340 | 1048 | 2536 | 200 | 9500 | ||
| DENV-3 | <10 | <10 | 157 | 206 | 201 | 355 | 4020 | ||
| DENV-4 | <10 | <10 | 92 | 170 | 282 | 237 | 1889 | ||
NA – no valid result available
Animals were immunized with 4 doses of vaccine administered at monthly intervals by the intramuscular route.
To assess the protective efficacy of the tetravalent DEN-80E vaccine formulation the vaccinated animals and unvaccinated controls were challenged with DENV2 or DENV-4 (one animal each from each group) five months after the last dose. The challenge results are presented in Table 10. The DENV2 control animal developed viremia which lasted for four days and the DENV4 control animal developed viremia lasting for five days. In contrast, 3 out of 4 immunized animals did not have any detectable viremia as measured by cell culture. One of the animals (T206) that received the 5 µg dose of the tetravalent formulation did exhibit some breakthrough viremia. This animal had an anti-DENV-4 virus neutralizing titer of 14 one month before and 17 at the time of DENV-4 challenge consistent with the trend noted in a previous study that higher virus neutralizing antibody titers are generally associated with protection [54]. These data demonstrated that properly formulated tetravalent vaccines based on recombinant DEN-80E subunit proteins can induce virus neutralizing antibodies and protective immunity against more than one dengue virus type.
Table 10.
Live Virus Challenge of Monkeys after Tetravalent Immunization.
| Group | Animal ID |
PRNT50 for each serotype one month before challenge |
Challenge serotype assigned randomly |
Viremia (positive days) |
|---|---|---|---|---|
| 1 µg each tetravalent DEN-80E + 0.1 µg NS1 + ISCOMATRIX™ Adjuvant | AA37 | DEN1: 63 | ||
| DEN2: 139 | DEN4 | None | ||
| DEN3: 59 | ||||
| DEN4: 61 | ||||
| FTH | DEN1: 242 | |||
| DEN2: 309 | DEN2 | None | ||
| DEN3: 64 | ||||
| DEN4: 181 | ||||
| 5 µg each tetravalent DEN-80E + 0.5 µg NS1 + ISCOMATRIX™ Adjuvant | T206 | DEN1: 49 | ||
| DEN2: 39 | DEN4 | Days 4, 5, 6, 7 | ||
| DEN3: 192 | ||||
| DEN4: 14 | ||||
| AJ14 | DEN1: 51 | |||
| DEN2: 530 | DEN2 | None | ||
| DEN3: 270 | ||||
| DEN4: 148 | ||||
| Naive controls | B34Z | <10 for each serotype | DEN4 | Days 6, 7, 8, 9, 10 |
| B08Z | <10 for each serotype | DEN2 | Days 7, 8, 9, 10 | |
Summary and Conclusions
Challenges associated with interference between component dengue viruses within early tetravalent live-attenuated vaccines led researchers at Hawaii Biotech to target development of a recombinant subunit envelope-based vaccine. Though initial efforts in the field at expressing dengue envelope subunits were stymied by low yields and/or improper folding, the use of the Drosophila S2 cell expression system provided the means to overcome these limitations. The DEN-80E recombinant subunit proteins for all four dengue virus types are expressed at high levels and have been shown to maintain native-like conformation. When formulated with a variety of adjuvants the antigens are potent immunogens and induce high titer virus neutralizing antibody responses. Furthermore, the antigens have been shown to protect against viral challenge in both mouse and non-human primate models. Tetravalent vaccine formulations have also been evaluated in preclinical models with no evidence of immune interference or competition between the four DEN-80E antigens being observed. These proof of concept preclinical studies led to the advancement of a monovalent DEN1-80E vaccine candidate into clinical testing. The phase 1 trial was a double-blind, placebo-controlled safety study conducted in 16 healthy adult, flavivirus-naïve individuals. Two dose levels of DEN1-80E (10 and 50 µg) both adjuvanted with aluminum hydroxide were evaluated. Three intramuscular injections of each vaccine dose level were administered to subjects at 4 week intervals. The study is now complete and data unblinding and publication is expected in the near future. Preparations for clinical testing of a tetravalent formulation are now ongoing following the transfer of the HBI program to Merck.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, et al. Dengue: a continuing global threat. Nat Rev Microbiol. 2010;8(12 Suppl):S7–S16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitehorn J, Farrar J. Dengue. Br Med Bull. 2010;95:161–173. doi: 10.1093/bmb/ldq019. [DOI] [PubMed] [Google Scholar]
- 3.Tapia-Conyer R, Méndez-Galván JF, Gallardo-Rincón H. The growing burden of dengue in Latin America. J Clin Virol. 2009;(46 Suppl 2):S3–S6. doi: 10.1016/S1386-6532(09)70286-0. [DOI] [PubMed] [Google Scholar]
- 4.Halstead SB. Dengue. Lancet. 2007;370:1644–1652. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 5.Srikiatkhachorn A, Green S. Markers of dengue disease severity. Curr Top Microbiol Immunol. 2010;338:67–82. doi: 10.1007/978-3-642-02215-9_6. [DOI] [PubMed] [Google Scholar]
- 6.Balsitis SJ, Williams KL, Lachica R, Flores D, Kyle JL, Mehlhop E, et al. Lethal antibody enhancement of dengue disease in mice is prevented by Fc modification. PLoS Pathogens. 2010;6:e1000790. doi: 10.1371/journal.ppat.1000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halstead SB. Antibodies determine virulence in dengue. Ann NY Acad Sci. 2009;1171 Suppl 1:E48–E56. doi: 10.1111/j.1749-6632.2009.05052.x. [DOI] [PubMed] [Google Scholar]
- 8.Mathew A, Rothman AL. Understanding the contribution of cellular immunity to dengue disease pathogenesis. Immunol Rev. 2008;225:300–313. doi: 10.1111/j.1600-065X.2008.00678.x. [DOI] [PubMed] [Google Scholar]
- 9.Noisakran S, Chokephaibulkit K, Songprakhon P, Onlamoon N, Hsiao H-M, Villinger F, et al. A re-evaluation of the mechanisms leading to dengue hemorrhagic fever. Ann NY Acad Sci. 2009;1171:E24–E35. doi: 10.1111/j.1749-6632.2009.05050.x. [DOI] [PubMed] [Google Scholar]
- 10.Mongkolsapaya J, Dejnirattisai W, Xu X-N, Vasanawathana S, Tangthawornchaikul N, Chairunsri A, et al. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nature Medicine. 2003;9:921–927. doi: 10.1038/nm887. [DOI] [PubMed] [Google Scholar]
- 11.Sun W, Edelman R, Kanesa-Thasan N, Eckels KH, Putnak JR, King AD, et al. Vaccination of human volunteers with monovalent and tetravalent live-attenuated dengue vaccine candidates. Am J Trop Med Hyg. 2003;69(6 Suppl):24–31. doi: 10.4269/ajtmh.2003.69.6_suppl.0690024. [DOI] [PubMed] [Google Scholar]
- 12.Blok J, McWilliam SM, Butler HC, Gibbs AJ, Weiller G, Herring BL, et al. Comparison of a dengue-2 virus and its candidate vaccine derivative: sequence relationships with the flaviviruses and other viruses. Virology. 1992;187:573–590. doi: 10.1016/0042-6822(92)90460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edelman R, Wasserman SS, Bodison SA, Putnak RJ, Eckels KH, Tang D, et al. Phase I trial of 16 formulations of a tetravalent live-attenuated dengue vaccine. Am J Trop Med Hyg. 2003;69(6 Suppl):48–60. doi: 10.4269/ajtmh.2003.69.48. [DOI] [PubMed] [Google Scholar]
- 14.Kitchener S, Nissen M, Nasveld P, Forrat R, Yoksan S, Lang J, et al. Immunogenicity and safety of two live-attenuated tetravalent dengue vaccine formulations in healthy Australian adults. Vaccine. 2006;24:1238–1241. doi: 10.1016/j.vaccine.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 15.Sun W, Cunningham D, Wasserman SS, Perry J, Putnak JR, Eckels KH, et al. Phase 2 clinical trial of three formulations of tetravalent live-attenuated dengue vaccine in flavivirus-naïve adults. Human Vaccines. 2009;5:33–40. doi: 10.4161/hv.5.1.6348. [DOI] [PubMed] [Google Scholar]
- 16.Jaiswal S, Khanna N, Swaminathan S. Replication-defective adenoviral vaccine vector for the induction of immune responses to dengue virus type 2. J Virol. 2003;77:12907–12913. doi: 10.1128/JVI.77.23.12907-12913.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raviprakash K, Wang D, Ewing D, Holman DH, Block K, Woraratanadharm J, et al. A tetravalent dengue vaccine based on a complex adenovirus vector provides significant protection in rhesus monkeys against all four serotypes of dengue virus. J Virol. 2008;82:6927–6934. doi: 10.1128/JVI.02724-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fonseca BAL, Pincus S, Shope RE, Paoletti E, Mason PW. Recombinant vaccinia viruses co-expressing dengue-1 glycoproteins prM and E induce neutralizing antibodies in mice. Vaccine. 1994;12:279–285. doi: 10.1016/0264-410x(94)90206-2. [DOI] [PubMed] [Google Scholar]
- 19.White LJ, Parsons MM, Whitmore AC, Williams BM, de Silva A, Johnston RE. An immunogenic and protective alphavirus replicon particle-based dengue vaccine overcomes maternal antibody interference in weanling mice. J Virol. 2007;81:10329–10339. doi: 10.1128/JVI.00512-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Paula SO, Lima DM, deOliveira Franca RF, Gomes-Ruiz AC, da Fonseca BA. A DNA vaccine candidate expressing dengue-3 virus prM and E proteins elicits neutralizing antibodies and protects mice against lethal challenge. Arch Virol. 2008;153:2215–2223. doi: 10.1007/s00705-008-0250-3. [DOI] [PubMed] [Google Scholar]
- 21.Brandlera S, Ruffiea C, Najburga V, Frenkielb M-P, Bedouelleb H, Desprèsb P, et al. Pediatric measles vaccine expressing a dengue tetravalent antigen elicits neutralizing antibodies against all four dengue viruses. Vaccine. 2010;28:6730–6739. doi: 10.1016/j.vaccine.2010.07.073. [DOI] [PubMed] [Google Scholar]
- 21.Kochel T, Wu SJ, Raviprakash K, Hobart P, Hoffman S, Porter K, et al. Inoculation of plasmids expressing the dengue-2 envelope gene elicit neutralizing antibodies in mice. Vaccine. 1997;15:547–552. doi: 10.1016/s0264-410x(97)00215-6. [DOI] [PubMed] [Google Scholar]
- 22.Raviprakash K, Kochel TJ, Ewing D, Simmons M, Phillips I, Hayes CG, et al. Immunogenicity of dengue virus type 1 DNA vaccines expressing truncated and full length envelope protein. Vaccine. 2000;18:2426–2434. doi: 10.1016/s0264-410x(99)00570-8. [DOI] [PubMed] [Google Scholar]
- 23.Trent DW. Antigenic characterization of flavivirus structural proteins separated by isoelectric focusing. J Virol. 1977;22:608–618. doi: 10.1128/jvi.22.3.608-618.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henchal EA, Gentry MK, McCown JM, Brandt WE. Dengue virus-specific and flavivirus group determinants identified with monoclonal antibodies by indirect immunofluorescence. Am J Trop Med Hyg. 1982;31:830–836. doi: 10.4269/ajtmh.1982.31.830. [DOI] [PubMed] [Google Scholar]
- 25.Roehrig JT, Bolin RA, Kelly RG. Monoclonal antibody mapping of the envelope glycoprotein of the dengue 2 virus, Jamaica. Virology. 1998;246:317–328. doi: 10.1006/viro.1998.9200. [DOI] [PubMed] [Google Scholar]
- 26.Beltramello M, Williams KL, Simmons CP, Macagno A, Simonelli L, Quyen NTH, et al. The human immune response to dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host & Microbe. 2010;8:271–283. doi: 10.1016/j.chom.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durbin AP, Whitehead SS. Dengue vaccine candidates in development. In: Rothman AL, editor. Dengue Virus, Current Topics in Microbiology and Immunology. pp. 129–143. [DOI] [PubMed] [Google Scholar]
- 28.Yap Y-K, Smith DR. Strategies for the plant-based expression of dengue subunit vaccines. Biotechnol Appl Biochem. 2010;57:47–53. doi: 10.1042/BA20100248. [DOI] [PubMed] [Google Scholar]
- 29.Allison SL, Stadler K, Mandl CW, Kunz C, Heinz FX. Synthesis and secretion of recombinant tick-borne encephalitis virus protein E in soluble and particulate form. J Virol. 1995;69:5816–5820. doi: 10.1128/jvi.69.9.5816-5820.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feighny R, Burrous J, Putnak R. Dengue type-2 virus envelope protein made using recombinant baculovirus protects mice against virus challenge. Am J Trop Med Hyg. 1994;50:322–328. doi: 10.4269/ajtmh.1994.50.322. [DOI] [PubMed] [Google Scholar]
- 31.Mason PW, Zugal MU, Semproni AR, Fournier MJ, Mason TL. The antigenic structure of dengue type 1 virus envelope and NS1 proteins expressed in Escherichia coli. J Gen Virol. 1990;71:2107–2114. doi: 10.1099/0022-1317-71-9-2107. [DOI] [PubMed] [Google Scholar]
- 32.Megret F, Hugnot JP, Falconar A, Gentry MK, Morens DM, Murray JM, et al. Use of recombinant fusion proteins and monoclonal antibodies to define linear and discontinuous antigenic sites on the dengue virus envelope glycoprotein. Virology. 1992;187:480–491. doi: 10.1016/0042-6822(92)90450-4. [DOI] [PubMed] [Google Scholar]
- 33.Hermida L, Rodriguez R, Lazo L, Silva R, Zulueta A, Chinea G, et al. A dengue-2 Envelope fragment inserted within the structure of the P64k meningococcal protein carrier enables a functional immune response against the virus in mice. J Virol Methods. 2004;115:41–49. doi: 10.1016/j.jviromet.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 34.Simmons CP, Farrar J. Changing patterns of dengue epidemiology and implications for clinical management and vaccines. PLoS Med. 2009;9:e1000129. doi: 10.1371/journal.pmed.1000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srivastava AK, Putnak JR, Warren RL, Hoke CH., Jr Mice immunized with a dengue type 2 virus E and NS1 fusion protein made in Escherichia coli are protected against lethal dengue virus infection. Vaccine. 1995;13:1251–1258. doi: 10.1016/0264-410x(94)00059-v. [DOI] [PubMed] [Google Scholar]
- 36.Bielefeldt-Ohmann H, Beasley DW, Fitzpatrick DR, Aaskov JG. Analysis of a recombinant dengue-2 virus–dengue-3 virus hybrid envelope protein expressed in a secretory baculovirus system. J Gen Virol. 1997;78:2723–2733. doi: 10.1099/0022-1317-78-11-2723. [DOI] [PubMed] [Google Scholar]
- 37.Delenda C, Staropoli I, Frenkiel MP, Cabanie L, Deubel V. Analysis of C-terminally truncated dengue 2 and dengue 3 virus envelope glycoproteins: processing in insect cells and immunogenic properties in mice. J Gen Virol. 1994;75:1569–1578. doi: 10.1099/0022-1317-75-7-1569. [DOI] [PubMed] [Google Scholar]
- 38.Deubel V, Bordier M, Megret F, Gentry MK, Schlesinger JJ, Girard M. Processing, secretion, and immunoreactivity of carboxy terminally truncated dengue-2 virus envelope proteins expressed in insect cells by recombinant baculoviruses. Virology. 1991;180:442–447. doi: 10.1016/0042-6822(91)90055-g. [DOI] [PubMed] [Google Scholar]
- 39.Kelly EP, Greene JJ, King AD, Innis BL. Purified dengue 2 virus envelope glycoprotein aggregates produced by baculovirus are immunogenic in mice. Vaccine. 2000;18:2549–2559. doi: 10.1016/s0264-410x(00)00032-3. [DOI] [PubMed] [Google Scholar]
- 40.Putnak R, Feighny R, Burrous J, Cochran M, Hackett C, Smith G, et al. Dengue-1 virus envelope glycoprotein gene expressed in recombinant baculovirus elicits virus-neutralizing antibody in mice and protects them from virus challenge. Am J Trop Med Hyg. 1991;45:159–167. doi: 10.4269/ajtmh.1991.45.159. [DOI] [PubMed] [Google Scholar]
- 41.Men RH, Bray M, Lai CJ. Carboxy-terminally truncated dengue virus envelope glycoproteins expressed on the cell surface and secreted extracellularly exhibit increased immunogenicity in mice. J Virol. 1991;65:1400–1407. doi: 10.1128/jvi.65.3.1400-1407.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sugrue RJ, Fu J, Howe J, Chan YC. Expression of the dengue virus structural proteins in Pichia pastoris leads to the generation of virus-like particles. J Gen Virol. 1997;78:1861–1866. doi: 10.1099/0022-1317-78-8-1861. [DOI] [PubMed] [Google Scholar]
- 43.Liu W, Jiang H, Zhou J, Yang X, Tang Y, Fang D, et al. Recombinant dengue virus-like particles from Pichia pastoris: efficient production and immunological properties. Virus Genes. 2010;40:53–59. doi: 10.1007/s11262-009-0418-2. [DOI] [PubMed] [Google Scholar]
- 44.Konishi E, Fujii A. Dengue type 2 virus subviral extracellular particles produced by a stably transfected mammalian cell line and their evaluation for a subunit vaccine. Vaccine. 2002;20:1058–1067. doi: 10.1016/s0264-410x(01)00446-7. [DOI] [PubMed] [Google Scholar]
- 45.Chang GJJ, Hunt AR, Holmes DA, Springfield T, Chiueh TS, Roehrig JT, et al. Enhancing biosynthesis and secretion of premembrane and envelope proteins by the chimeric plasmid of dengue virus type 2 and Japanese encephalitis virus. Virology. 2003;306:170–180. doi: 10.1016/s0042-6822(02)00028-4. [DOI] [PubMed] [Google Scholar]
- 46.Kuwahara M, Konishi E. Evaluation of extracellular subviral particles of dengue virus type 2 and japanese encephalitis virus produced by Spodoptera frugiperda cells for use as vaccine and diagnostic antigens. Clin Vac Immunol. 2010;17:1560–1566. doi: 10.1128/CVI.00087-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ivy J, Nakano E, Clements D. Methods of preparing carboxy-terminally truncated recombinant flavivirus envelope glycoproteins employing drosophila malanogaster expression systems. 6136561. US patent. 2000
- 48.van der Straten A, Johansen H, Rosenberg M, Sweet RW. Introduction and constitutive expression of gene products in cultured drosophila cells using hygromycin B selection. Meth Mol Cell Bio. 1989;1:1–8. [Google Scholar]
- 49.Culp JS, Johansen H, Hellmig B, Beck J, Matthews TJ, Delers A, et al. Regulated expression allows high level production and secretion of HIV-1 gp120 envelope glycoprotein in Drosophila Schneider cells. Bio/Technology. 1991;9:173–177. doi: 10.1038/nbt0291-173. [DOI] [PubMed] [Google Scholar]
- 50.Kirkpatrick RB, Shatzman A. Gene Expression Systems using nature for the art of expression. Chapter 11. Academic Press; 1999. Drosophila S2 system for heterologous gene expression; pp. 289–329. [Google Scholar]
- 51.Schneider I. Cell lines derived from late embryonic stages of Drosophila melanogaster. J Embryol Exp Morph. 1972;27:353–365. [PubMed] [Google Scholar]
- 52.Li B, Tsing S, Kosaka AH, Nguyen B, Osen EG, Bach C, et al. Expression of human dopamine β-hydroxylase in Drosophila Schneider 2 cells. Biochem J. 1996;313:57–64. doi: 10.1042/bj3130057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Incardona JP, Rosenberry TL. Replacement of the glycoinositol phospholipid anchor of Drosophila acetylcholinesterase with a transmembrane domain does not alter sorting in neurons and epithelia but results in behavioral defects. Mol Biol Cell. 1996;7:613–630. doi: 10.1091/mbc.7.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Putnak JR, Coller B-A, Voss D, Vaughn DW, Clements D, Houng HS, et al. Recombinant subunit protein and inactivated virus vaccines for dengue type-2 formulated with new adjuvants induce high-titered virus-neutralizing antibodies and confer protection against virus challenge in rhesus macaques. Vaccine. 2005;23:4442–4452. [Google Scholar]
- 55.Clements DE, Coller BA, Lieberman MM, Ogata S, Wang G, Harada KE, et al. Development of a recombinant tetravalent dengue virus vaccine: immunogenicity and efficacy studies in mice and monkeys. Vaccine. 2010;28:2705–2715. doi: 10.1016/j.vaccine.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Modis Y, Ogata S, Clements D, Harrison SC. Structure of the dengue virus envelope protein after membrane fusion. Nature. 2004;427:313–319. doi: 10.1038/nature02165. [DOI] [PubMed] [Google Scholar]
- 57.Modis Y, Ogata S, Clements D, Harrison SC. Variable surface epitopes in the crystal structure of dengue virus type 3 envelope glycoprotein. J Virol. 2005;79:1223–1231. doi: 10.1128/JVI.79.2.1223-1231.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y, Zhang W, Ogata S, Clements D, Strauss JH, Baker TS, et al. Conformational changes of the flavivirus E glycoprotein. Structure. 2004;12:1607–1618. doi: 10.1016/j.str.2004.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rey FA, Heinz FX, Mandl C, Kunz C, Harrison SC. The envelope glycoprotein from tick-borne encephalitis virus at 2 A resolution. Nature. 1995;375:291–298. doi: 10.1038/375291a0. [DOI] [PubMed] [Google Scholar]
- 60.Guy B. The perfect mix: recent progress in adjuvant research. Nat Rev Microbiol. 2007;5:505–517. doi: 10.1038/nrmicro1681. [DOI] [PubMed] [Google Scholar]
- 61.McKeage K, Romanowski B. AS04-adjuvanted human papillomavirus (HPV) types 16 and 18 vaccine (Cervarix®): a review of its use in the prevention of premalignant cervical lesions and cervical cancer causally related to certain oncogenic HPV types. Drugs. 2011;71:465–488. doi: 10.2165/11206820-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 62.McKenzie A, Watt M, Gittleson C. ISCOMATRIX™ vaccines: Safety in human clinical studies. Hum Vaccin. 2010;6:237–246. doi: 10.4161/hv.6.3.10754. [DOI] [PubMed] [Google Scholar]
- 63.Garçon N, Van Mechelen M. Recent clinical experience with vaccines using MPL- and QS-21-containing Adjuvant Systems. Expert Rev Vaccines. 2011;10:471–486. doi: 10.1586/erv.11.29. [DOI] [PubMed] [Google Scholar]
- 64.Libraty DH, Endy TP, Houng HS, Green S, Kalayanarooj S, Suntayakorn S, Chansiriwongs W, Vaughn DW, Nisalak A, Ennis FA, Rothman AL. Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infections. J Infect Dis. 2002;185:1213–1221. doi: 10.1086/340365. [DOI] [PubMed] [Google Scholar]
- 65.Drane D, Gittleson C, Boyle J, Maraskovsky E. ISCOMATRIX adjuvant for prophylactic and therapeutic vaccines. Expert Rev Vaccines. 2007;6:761–772. doi: 10.1586/14760584.6.5.761. [DOI] [PubMed] [Google Scholar]