Non-technical summary
Glutamate is a critical excitatory neurotransmitter in the modulation of hypothalamic neuronal activity and neurosecretion from the posterior pituitary. Still, the precise mechanisms and modalities by which it acts remain to be fully elucidated. We show that in addition to mediating conventional fast synaptic transmission, glutamate persistently activates extrasynaptic NMDA receptors, providing a tonic excitatory drive to hypothalamic neurosecretory neurons. We also show that this tonic excitatory modality is influenced by the neighbouring astrocytes, and is enhanced in dehydrated rats. Knowledge on alternative modalities by which glutamate influences hypothalamic neuronal function increases our understanding of general brain mechanisms regulating neurosecretion and bodily homeostasis.
Abstract
Abstract
Despite the long-established presence of glutamate NMDA receptors at extrasynaptic sites (eNMDARs), their functional roles remain poorly understood. Factors influencing the concentration and time course of glutamate in the extrasynaptic space, such as the topography of the neuronal–glial microenvironment, as well as glial glutamate transporters, are expected to affect eNMDAR-mediated signalling strength. In this study, we used in vitro and in vivo electrophysiological recordings to assess the properties, functional relevance and modulation of a persistent excitatory current mediated by activation of eNMDARs in hypothalamic supraoptic nucleus (SON) neurons. We found that ambient glutamate of a non-synaptic origin activates eNMDARs to mediate a persistent excitatory current (termed tonic INMDA), which tonically stimulates neuronal activity. Pharmacological blockade of GLT1 astrocyte glutamate transporters, as well as the gliotoxin α-aminodadipic acid, enhanced tonic INMDA and neuronal activity, supporting an astrocyte regulation of tonic INMDA strength. Dehydration, a physiological challenge known to increase SON firing activity and to induce neuroglial remodelling, including reduced neuronal ensheathment by astrocyte processes, resulted in blunted GLT1 efficacy, enhanced tonic INMDA strength, and increased neuronal activity. Taken together, our studies support the view that glial modulation of tonic INMDA activation contributes to regulation of SON neuronal activity, contributing in turn to neuronal homeostatic responses during a physiological challenge.
Introduction
Conventional fast neurotransimitters such as glutamate are typically thought to mediate temporally and spatially restricted action, via their synaptic release and activation of postsynaptic receptors (Jonas et al., 2004). However, a growing body of evidence suggests that in addition to its classical transient synaptic actions, glutamate in the extracellular space can also evoke a tonic, persistent form of excitation via activation of extrasynaptic NMDA receptors (eNMDARs) (Sah et al. 1989; Dalby & Mody, 2003; Le Meur et al. 2007). Despite this growing evidence, the mechanisms influencing the efficacy of this NMDA-mediated tonic excitatory modality (tonic INMDA), and what its functional relevance is under different physiological conditions, remains poorly understood. In this sense, several studies support synaptic and extrasynaptic NMDARs to be linked to distinct, and even opposing, downstream biological actions (Hardingham et al. 2002; Ivanov et al. 2006; Okamoto et al. 2009), supporting the notion of compartmentalized glutamate signalling mechanism via these two pools of NMDARs.
Differently from synaptic glutamate transmission, the strength of tonic INMDA would depend on factors influencing the concentration and time course of glutamate in the extrasynaptic space. These include the topography of the neuronal–glial microenvironment (Piet et al. 2004a), as well as the activity of glial glutamate transporters, which efficiently remove glutamate from the extracellular space (Rothstein et al. 1996; Rusakov & Kullmann, 1998). Thus, astrocytes appear to be strategically positioned to exert a direct control over extracellular glutamate concentration, influencing in turn tonic INMDA.
The magnocellular neurosecretory system, composed of vasopressin and oxytocin neurons in the supraoptic (SON) and paraventricular hypothalamic nuclei, plays a major role in fluid balance homeostasis, as well as in reproductive function (Silverman & Zimmerman, 1983). Neuronal activity, as well as hormone release in the magnocellular system, is strongly influenced by a compact neuronal–glial microenvironment, in which thin astroglial processes tightly enwrap neurons and their synaptic inputs (Oliet et al. 2001; Piet et al. 2004a). Moreover, a remarkable property of this neuronal–glial microenvironment is its ability to undergo a robust plasticity, including rapid and reversible retraction of astroglial processes, in response to physiological challenges such as dehydration (Theodosis & Poulain, 1999; Hatton, 2004). This remodelling was shown to be necessary for state-dependent adaptation of neurosecretory neuronal firing (Scott & Brown, 2010). Thus, the magnocellular system constitutes an ideal model to study tonic INMDA and its regulation by astrocytes, in conditions where neurons are either tightly or loosely surrounded by astrocytic processes. In this work, we assessed weather tonic INMDA, under the control of glial glutamate transporters, constitutes an important excitatory modality influencing SON neuronal activity. In addition, we evaluated whether the strength of tonic INMDA is altered in dehydration, a condition known to induce state-dependent remodelling of the neuronal–glial microenvironment. Our results show that tonic INMDA, mediated largely by activation of extrasynaptic NMDARs under glial control, efficiently influences SON neuronal activity. Moreover, our results indicate that the strength of tonic INMDA can by dynamically regulated during dehydration, contributing in turn to neuronal homeostatic responses during this physiological challenge.
Methods
Animals
Male and female Wistar rats purchased from Harlan were housed under standardized conditions (12:12 h light–dark cycle, lights on 07.00 h) with food and water available ad libitum. For in vivo experiments, rats were bred in-house by the University of Otago Animal Facility and housed under similar standardized conditions. For dehydration, water was removed for 48 h with free access to dry food. In a subset of animals, plasma osmolarity was measured under urethane anaesthesia (microosmometer Model 3300, Advanced Instruments, Norwood, MA, USA), revealing a significant increase in dehydrated rats (330.1 ± 7.3 vs. 311.0 ± 2.5 mosmol l−1, P < 0.05, n = 8 and 6 for dehydrated and euhydrated rats, respectively). All animal experimentation was in strict compliance with NIH guidelines, and all experimental procedures were carried out in accordance with procedures approved by the Medical College of Georgia Institutional and Animal Care and Use Committee and the University of Otago Animal Ethics Committee.
Drugs
Picrotoxin, d-(–)-2-amino-5-phosphonopentanoic acid (d-AP5), dl-α-aminoadipic acid (αAA) and 4-hydroxyquinoline-2-carboxylic acid (kynurenic acid) were purchased from Sigma-Aldrich (St Louis, MO, USA). Tetrodotoxin (TTX) was purchased from Ascent Scientific (Princeton, NJ, USA). dl-threo-β-Benzyloxyaspartic acid (TBOA), (2S,3S,4R)-2-carboxy-4-isopropyl-3-pyrrolidineacetic acid (DHK, dihydrokainic acid), 2-(4-benzylpiperidino)-1-(4-hydroxyphenyl)-1-propanol hemitartrate (Ifenprodil hemitartrate) were purchased from Tocris Cookson (Bristol, UK). Bafilomycin A1 was purchased from LC Laboratories (Woburn, MA, USA).
Hypothalamic slices
Rats were anaesthetized with pentobarbital (50 mg kg−1), quickly decapitated, and brains dissected out. Coronal slices were cut (300 μm thick) utilizing a vibroslicer (Leica VT1000). An oxygenated ice cold artificial cerebrospinal fluid (ACSF) was used during slicing (containing in mm): 119 NaCl, 2.5 KCl, 1 MgSO4, 26 NaHCO3, 1.25 NaH2PO4, 20 d-glucose, 0.4 ascorbic acid, 2.0 CaCl2 and 2.0 pyruvic acid; pH 7.4; 290–310 mosmol l−1). After sectioning, slices were placed in a holding chamber containing ACSF and kept at room temperature (22°C) until used (see Stern, 2001 for details).
In vitro electrophysiological recordings
Slices were placed in a submersion style recording chamber, and bathed with solutions (3.0 ml min−1) that were bubbled continuously with a gas mix of 95% O2–5% CO2, and maintained at near physiological temperature (32°C). Thin-walled (1.5 mm o.d., 1.17 mm i.d.) borosilicate glass (G150TF-3, Warner Instruments, Sarasota, FL, USA) was used to pull patch pipettes (3–6 MΩ) on a horizontal Flaming/Brown micropipette puller (P-97, Sutter Instruments, Novato, CA, USA). Whole-cell recordings from visually identified SON neurons (infrared differential interference contrast, IR-DIC) were obtained using a Multiclamp 700A amplifier (Axon Instruments, Union City, CA, USA). The voltage output was digitized at 16-bit resolution, 10 kHz (Digidata 1320A, Axon Instruments, and saved on a computer to be analysed offline. Off-line analysis was performed using pCLAMP9 software (Axon Instruments). Series resistance was periodically monitored throughout the recording. The experiment was discarded if the series resistance was not stable throughout the recording. The GABAA receptor antagonist picrotoxin (300 μm) was used during all experiments, unless otherwise noted.
Voltage-clamp recordings
The internal solution for recordings obtained at a Vholding of –70 mV contained (in mm): 140 potassium gluconate, 0.2 EGTA, 10 Hepes, 10 KCl, 0.9 MgCl2, 4 MgATP, 0.3 NaGTP and 20 phosphocreatine (Na+); pH 7.2–7.3. For voltage-clamp recordings, a low Mg2+ ACSF (20 μm MgSO4,) was used to facilitate measurements of NMDA-mediated currents. In some cases, recordings were also obtained at a Vholding of +40 mV using an internal solution containing (in mm): 135 caesium methanesulphonate, 0.2 EGTA, 10 Hepes, 10 TEA-Cl, 0.9 MgCl2, 4 MgATP, 0.3 NaGTP and 20 sodium phosphocreatine; pH 7.2–7.3. Voltage clamp protocols were run with an output gain of 5 and a Bessel filter of 2 kHz. To determine whether a glutamate-mediated tonic current (tonic IGLU) was present in SON neurons, we assessed changes in holding current (Iholding) and root mean square (RMS) baseline noise levels following bath application of glutamate receptor blockers. The mean tonic IGLU amplitude was calculated as the difference in the holding current measured as the average of a 2 min segment of steady state baseline obtained before and after a 5 min application of the glutamate receptor blockers. The tonic IGLU current density of was determined by dividing the current amplitude by the cell capacitance, obtained by integrating the area under the transient capacitive phase of a 5 mV depolarizing step pulse, in voltage clamp mode. RMS noise was measured using pCLAMP software within seven different 100 ms segments of traces lacking postsynaptic currents (PSCs), and values were averaged. To assess the regulation of tonic IGLU by glutamate transporters, recordings were also obtained in the presence of the glutamate transporter blockers TBOA (100 μm) or DHK (300 μm). Spontaneous glutamate excitatory postsynaptic currents (EPSCs) were recorded at a holding potential of –70 mV, and detection and analysis of was done using Minianalysis software (Synaptosoft, Decatur, GA, USA), as previously described (Park et al. 2007), using a detection threshold of 20 pA. From extracted EPSCs, the mean rise time, peak amplitude, and decay time constant were calculated. Whole-cell capacitance was calculated. Cell capacitance was calculated by integrating the area under the transient capacitive phase of a 5 mV depolarizing step pulse, in the voltage-clamp mode.
Current-clamp recordings
The internal solution for current clamp recordings contained (in mm): 140 potassium gluconate, 0.2 EGTA, 10 Hepes, 10 KCl, 0.9 MgCl2, 4 MgATP, 0.3 NaGTP and 20 sodium phosphocreatine; pH 7.2–7.3. The ACSF for current clamp recordings contained 1 mm MgSO4. Current clamp protocols were run with an output gain of 5 and a Bessel filter of 10 kHz. Changes in firing rate (number of spikes per unit time, Hz) were assessed in the 1 min period before and during bath application of compounds. The mean membrane potential (Vm) within those segments, including only periods lacking action potentials, were also assessed using pCLAMP software.
In vivo electrophysiological recordings
Rats (n = 39) were prepared for extracellular single-unit recording from dialysed SONs as described previously (Ludwig & Leng, 1997). Briefly, rats were anaesthetized by an intraperitoneal injection of urethane (ethyl carbamate; 1.25 g kg−1) and a catheter inserted into the left femoral vein for cholecystokinin (CCK) administration (CCK; 1.25 g kg−1). The pituitary stalk and right SON were exposed through the oral cavity. After removal of the meninges, a U-shaped microdialysis probe (total membrane length 2.0 mm; Spectra/Por RC Hollow fibres, Spectrum Medical Inc., Houston, TX, USA) was bent to position the loop of the membrane flat onto the exposed ventral surface of the brain over the SON. A glass recording microelectrode (15–40 MΩ filled with 0.9% NaCl) was placed in the SON through the centre of the dialysis loop. A side-by-side SNEX-200 stimulating electrode (Science Products GmbH, Hofheim, Germany) was placed on the pituitary stalk to elicit antidromic action potentials. The SON was dialysed with artificial cerebrospinal fluid (dialysate ACSF (dASCF): pH 7.2, composition in mm: 138 NaCl, 3.36 KCl, 9.52 NaHCO3, 0.49 Na2HPO4, 2.16 urea, 1.26 CaCl2, 1.18 MgCl2) at 3 μl min−1 before recording. At the end of the experiment, the rats were killed by intravenous overdose of anaesthetic. SON neuronal activity was recorded onto computer and analysed offline using Spike2 software (Cambridge Electronic Design, Cambridge, UK). SON neurons were characterized as oxytocin neurons on the basis of a transient excitation following i.v. cholecystokinin injection (20 μg kg−1, 0.5 ml kg−1 in 0.9% saline; Sigma) (Brown et al. 1996), or as vasopressin neurons by transient inhibition, or no change in firing rate, following cholecystokinin injection (Sabatier et al. 2004).
Statistical analysis
All values are expressed as means ± SEM. Student's paired t test was used to compare the effects of a drug treatment on tonic IGLU. Between group differences (e.g. euhydrated vs. dehydrated; oxytocin vs. vasopressin) were compared using analysis of variance repeated measures (ANOVA-RM). Where the F ratio was significant, post hoc comparisons were completed using the Bonferroni post hoc test. Differences were considered statistically significant at P < 0.05 and n refers to the number of cells. All statistical analyses were conducted using GraphPad Prism (GraphPad Software, San Diego, CA, USA).
Results
A tonic glutamate-mediated current is present in SON neurosecretory neurons
In order to determine if a persistent, non-inactivating glutamate tonic current (tonic IGLU) is present in magnocellular neurosecretory neurons, whole-cell patch-clamp recordings were obtained from 80 SON neurons in acutely obtained hypothalamic slices. Mean input resistance and series resistance were 1.1 ± 0.1 GΩ and 14.4 ± 27.2 MΩ, respectively. Bath application of the broad-spectrum glutamate receptor blocker kynurenic acid (KYN, 2 mm) induced a sustained inward and outward shift in Iholding at depolarized (+40 mV) and hyperpolarized (−70 mV) holding potentials, respectively (Fig. 1). On average, the KYN-induced shift in Iholding was 31.8 ± 4.8 pA (n = 17 at +40 mV), and −7.4 ± 1.3 pA (n = 9 at –70 mV) (P < 0.0001 in both cases). In addition, KYN significantly reduced RMS at both holding potentials tested: at +40 mV: control = 6.0 ± 1.2 pA; KYN = 3.7 ± 2.1 pA (n = 17, P < 0.0001); at –70 mV: control = 3.6 ± 1.0 pA; KYN = 3.5 ± 1.0 pA (n = 9, P < 0.01). These results suggest that in addition to fast EPSCs, glutamate mediates a persistent tonic excitatory influence on SON neurons.
Figure 1. Glutamate-mediated tonic current (tonic IGLU) in magnocellular SON neurons.
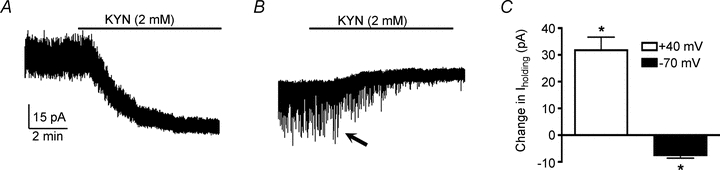
A, the broad-spectrum glutamate receptor blocker KYN (2 mm) induced an inward shift in Iholding and reduced RMS when neurons were held at +40 mV. B, an opposite change in Iholding was observed when neurons were held at −70 mV. C, group data showing mean changes in Iholding evoked by KYN at each membrane potential. Note that KYN also blocked fast, inward EPSCs (arrow). *P < 0.0001 vs. baseline, paired t test. All recordings were obtained in a low Mg2+ ACSF (Methods).
Tonic IGLU is predominantly mediated by activation of NMDA receptors
Studies in other neuronal types have shown that tonic IGLU is mostly mediated by activation of NR2B subunit-containing NMDARs of a putative extrasynaptic location (Tovar & Westbrook, 1999; Dalby & Mody, 2003; Le Meur et al. 2007). Thus, we aimed to determine the contribution of NMDARs to tonic IGLU in SON neurons. As summarized in Fig. 2, and at a Vholding of +40 mV, bath application of the NR2B NMDAR subunit selective blocker ifenprodil (10 μm) (Williams, 1993) induced a significant change in Iholding of 40.32 ± 18.6 pA and decreased RMS from 5.9 ± 0.6 pA to 5.1 ± 0.4 (n = 8, P < 0.03 in both cases). Subsequent addition of the non-selective NMDA receptor antagonist d-AP5 (100 μm) evoked an additional change in Iholding of 24.9 ± 6.9 pA (i.e. the total mean NMDA-mediated current was 65.2 ± 18.6 pA) and decreased RMS by an additional 0.8 ± 0.2 pA (14.0 ± 4.2%; n = 8, P < 0.05 in both cases). Finally, complete blockade of glutamate receptors with the subsequent addition of KYN induced an additional change in Iholding of 1.3 ± 1.2 pA and decreased RMS by an additional 0.15 ± 0.1 pA (3.9 ± 2.8%; n = 8), differences that did not reach, however, statistical significance (P < 0.3 in both cases). No differences between magnocellular neurosecretory cells (MNCs) recorded from male (n = 5) and female (n = 3) rats were observed (not shown). When results were expressed as a percentage of the total KYN-sensitive outward current, our results indicate that 97.7 ± 2.3% (n = 8) of the tonic IGLU was mediated by activation of NMDARs, a proportion of which (55.2 ± 8.8%, n = 8) was sensitive to the NR2B subunit selective blocker ifenprodil. Taken together, these results indicate that NMDARs mediate tonic IGLU in SON neurons (thus termed tonic INMDA).
Figure 2. NR2B-containing NMDA receptors contribute to tonic IGLU in SON neurons.
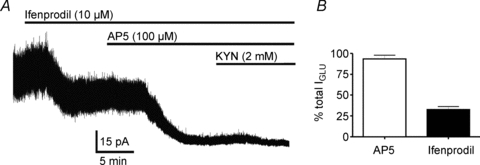
A, representative example showing changes in Iholding (+40 mV) following the sequential application of ifenprodil (10 μm), AP5 (100 μm) and KYN (2 mm). B, group data showing mean percentage changes of the total tonic IGLU (i.e. baseline – KYN) evoked by AP5 and ifenprodil (n = 5).
Tonic INMDA is controlled by the activity of glial glutamate transporters
Previous studies have shown that the ability of ambient glutamate to activate extrasynaptic NMDARs is tightly controlled by the activity of glutamate transporters, mainly those located in glia (Jabaudon et al. 1999; Cavelier & Attwell, 2005; Le Meur et al. 2007; Milnerwood et al. 2010). Based on this, and given that SON neurons under basal conditions are tightly enwrapped by astroglial processes (Theodosis & Poulain, 1999; Oliet et al. 2001), we tested whether the magnitude of tonic INMDA in SON neurons was dependent on glutamate transporter activity. Bath application of the glial transporter GLT1 selective inhibitor dihydrokainate (DHK, 300 μm) induced an inward shift in Iholding of 25.5 ± 6.2 pA (n = 10, P < 0.01), and increased RMS by 2.2 ± 0.5 pA (n = 10 P < 0.0001). DHK effects were prevented when NMDARs were blocked by AP5 (100 μm) previous to DHK application (ΔIholding 3.1 ± 4.4 pA n = 4, P < 0.5). No differences in DHK effects between MNCs recorded from male (n = 6) and female (n = 4) rats were observed (not shown). Conversely, preincubation of slices with the AMPA receptor blocker 6,7-dinitro-quinoxaline-2,3-dione (DNQX; 10 μm) did not affect the magnitude of the DHK-induced inward current (ΔIholding 27.6 ± 3.9 pA n = 4, P < 0.5).
Bath-applied DHK induced membrane depolarization (Δ 16.0 ± 5.2 mV; n = 5, P < 0.05) and triggered firing activity in the recorded SON neurons (from a silent baseline to 4.1 ± 2.5 Hz), effects that were blocked by KYN (n = 5, Fig. 3Aa–c). Similar, though larger, effects were observed with the broad-spectrum glutamate transporter blocker TBOA (100 μm): an inward shift in Iholding of 137.7 ± 14.3 pA (n = 9, P < 0.001), an increased RMS of 3.2 ± 0.9 pA (n = 9, P < 0.0001), and membrane depolarization (Δ 23.5 ± 3.2 mV; n = 6, P < 0.001); and it also triggered firing activity in the recorded neurons (from a silent baseline to 5.6 ± 1.6 Hz).
Figure 3. Glial glutamate transporters regulate the magnitude of tonic IGLU.
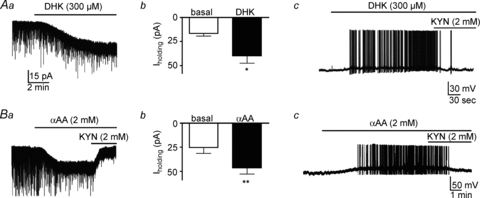
Aa, bath application of the glial-selective glutamate transporter blocker dihydrokainate (DHK, 300 μm) induced an inward shift in Iholding. Ab, group data showing a significant DHK-mediated increase in Iholding (n = 10). Ac, example showing a DHK-mediated membrane depolarization and firing discharge in an SON neuron. Ba, bath application of the selective gliotoxin α-aminoadipic acid (αAA, 2 mm) induced a similar inward shift in Iholding as that evoked by DHK. Bb, group data showing a significant αAA-mediated increase in Iholding (n = 7). Bc, example showing an αAA-mediated membrane depolarization and firing discharge in an SON neuron. Note that the DHK and αAA effects were blocked by KYN. *P < 0.001 and **P < 0.0001 vs. basal, paired t test. All recordings shown obtained at a holding Vm of −70 mV.
To further test the role of astrocytes in influencing tonic INMDA and SON neuronal excitability, the effect of the gliotoxin dl-2-α-aminodipic acid (αAA) (Xu et al. 2005) was tested. Bath application of 2 mmαAA, a concentration previously shown to evoke gliotoxicity in cultured astrocytes (Brown & Kretzschmar, 1998), induced an inward shift in Iholding of −21.2 ± 8.0 pA, and increased RMS by 0.3 ± 0.1 pA (n = 7, P < 0.0001 in both cases) (Fig. 3Ba and b), effects that were significantly diminished by KYN. Finally, similarly to DHK, αAA induced membrane depolarization (Δ 14.6 ± 1.0 mV; n = 6, P < 0.0001) and triggered firing discharge in the recorded SON neurons (from a silent baseline to 2.9 ± 0.5 Hz; n = 6, Fig. 3Bc). Taken together, these results suggest that glial glutamate transporters influence ambient glutamate levels and the degree of NMDAR activation, modulating in turn SON neuronal excitability.
Tonic INMDA is mediated by the activation of extrasynaptic glutamate receptors
To determine whether extrasynaptic NMDARs contribute to tonic INMDA in SON neurons, we tested the effects of memantine (MEM, 10–30 μm), an NMDAR antagonist that preferentially targets extrasynaptic receptors (Lipton, 2006; Leveille et al. 2008; Papadia et al. 2008; Okamoto et al. 2009). Bath application of MEM per se induced an outward shift in Iholding (Δ 14.3 ± 3.1 pA, n = 3, P < 0.05). Moreover, the DHK-induced change in Iholding was largely blocked by MEM, while subsequent addition of AP5 failed to induce any additional change (n = 7, F = 7.6, P < 0.01, one way ANOVA). Similar results were observed when RMS was measured (n = 7, F = 7.1, P < 0.002, one way ANOVA). The results are summarized in Fig. 4A and B. On the other hand, MEM failed to affect the properties of spontaneous synaptic EPSCs recorded in the low Mg2+-containing ACSF (Table 1).
Figure 4. Tonic IGLU under glial control is mediated by activation of extrasynaptic NMDARs.
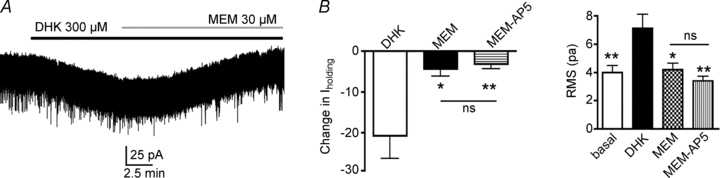
A, representative example showing memantine (MEM) block of dihydrokainate (DHK, 300 μm)-induced inward shift in Iholding. B, group data (n = 7) showing MEM effects on the DHK-induced shift in Iholding (left) and RMS (right). Note that further addition of AP5 failed to induce an additional effect on the DHK-induced current. *P < 0.05 and **P < 0.01 vs. DHK, Bonferroni's post hoc test. NS, non-significant difference. All recordings shown obtained at a holding Vm of −70 mV.
Table 1.
Memantine failed to affect basic properties of spontaneous glutamate EPSCs recorded in a low-Mg2+ ACSF (n = 7)
| Frequency (Hz) | Amplitude (pA) | Decay τ (ms) | Area (pA ms) | |
|---|---|---|---|---|
| Control ACSF | 2.6 ± 0.7 | −28.0 ± 1.9 | 5.8 ± 1.0 | −170.9 ± 63.9 |
| Memantine (10–30 μm) | 2.4 ± 0.7 | −26.5 ± 2.0 | 5.4 ± 0.8 | −152.5 ± 65.1 |
Synaptically released glutamate does not contribute to tonic INMDA
To determine whether glutamate of a synaptic source contributed to tonic INMDA, we assessed the effects of bafilomycin A1 (BafA1), an inhibitor of the vacuolar H+-ATPase, a pump that establishes the proton gradient across vesicular membrane, driving transmitter uptake into synaptic vesicles (Drose & Altendorf, 1997). For these experiments, slices obtained from the same animal were preincubated in either ACSF or in ACSF containing BafA1 (4 μm, 2.5 h). Cell capacitance was not affected by treatment with BafA1 (not shown). As shown in Fig. 5A, spontaneous postsynaptic currents (EPSCs) were almost completely abolished in slices preincubated with BafA1. In this condition, both DHK (n = 12) and TBOA (n = 9) still induced a significant inward shift in Iholding (P < 0.001 and P < 0.05, respectively), effects that were not different from those observed in control slices incubated in control ACSF (P > 0.5, Fig. 5B and C). Moreover, basal RMS measured in BafA1 treated slices was not significantly different from controls (Controls: 3.6 ± 1.1 pA, BafA1: 3.3 ± 0.7 pA, n = 10 and 12 in control and BafA1, respectively, P = 0.4), suggesting that basal tonic INMDA was not affected by BafA1 treatment. Finally, we tested whether an increase in the frequency of ongoing synaptic EPSCs affected INMDA. To this end, slices were exposed to the K+ channel blocker 4-aminopyridine (4-AP, 100 μm), an approach known to increase neurotransmitter release (Buckle & Haas, 1982). As we previously reported for GABA IPSCs in SON neurons (Park et al. 2007), 4-AP robustly increased EPSC frequency (basal: 1.0 ± 0.3 Hz; 4-AP: 20.2 ± 5.9 Hz, P < 0.02, n = 6) and also increased EPSC amplitude (basal: 39.3 ± 8.3 Hz; 4-AP: 45.1 ± 10.2 Hz, P < 0.05, n = 6). On the other hand, no changes in Iholding (Δ−1.4 ± 1.0 pA, P > 0.9) or RMS (Δ 0.1 ± 0.1 pA) were evoked by 4-AP.
Figure 5. Glutamate of a synaptic origin does not contribute to tonic INMDA.
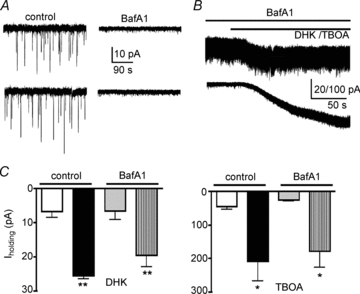
A, representative examples showing the presence and absence of glutamate EPSCs in slices preincubated in control ACSF (left) or in bafilomycin A1 (BafA1, 4 μm, right), respectively. B, representative examples of inward shifts in Iholding induced by DHK (300 μm, upper trace) and TBOA (100 μm, lower trace), in slices preincubated in the presence of BafA1 (4 μm). C, group data showing changes in Iholding induced by DHK (left) and TBOA (right) in control ACSF and in the presence of BafA1. *P < 0.05 and **P < 0.001 vs. respective basal, paired t test. All recordings shown obtained at a holding Vm of −70 mV.
Diminished glial GLT1 efficacy contributes to enhanced tonic INMDA magnitude and increased firing activity during a dehydration challenge
To further address the functional relevance of tonic INMDA and its regulation by astrocytes, we evaluated whether the magnitude and properties of tonic INMDA were altered during dehydration, a condition known to strongly affect the neuronal–glial microenvironment in the SON, resulting in diminished astroglial ensheathment of neuronal profiles (Hatton et al. 1984; Tweedle & Hatton, 1984; Chapman et al. 1986; Tasker et al. 2002). To this end, a group of rats were subjected to a 48 h water deprivation (48WD) challenge, or maintained with free access to water (euhydrated, EU). Firstly, we found basal RMS to be larger in neurons recorded from 48WD rats (EU = 3.37 ± 0.2 pA n = 11; 48WD = 3.93 ± 0.15 pA n = 19 (P < 0.05). Secondly, KYN induced a significantly larger shift in Iholding in 48WD rats, when compared to EU rats (EU = 5.9 ± 1.3 pA n = 8; 48WD = 16.8 ± 2.7 pA n = 8 (P < 0.01). Despite a tendency for a larger cell capacitance in neurones recorded from 48WD rats (EU: 24.4 ± 1.2 pF; 48WD: 27.9 ± 2.1 pF, P > 0.1), the current density change induced by KYN was still significantly larger in 48WD rats (EU: 0.22 ± 0.04 pA pF−1; DEH: 0.59 ± 0.1 pA pF−1, P < 0.01).
Finally, while DHK increased tonic INMDA in EU rats by 23.8 ± 2.4 pA (n = 10), this effect was significantly diminished in 48WD rats (10.4 ± 1.1 pA n = 12, P < 0.01 vs. EU rats, Fig. 6A). Similar results were observed when data were expressed as current density (EU: 0.71 ± 0.1 pA pF−1; 48WD: 0.4 ± 0.05 pA pF−1, P < 0.01). These results support a larger basal tonic INMDA in 48WD rats, likely to be due to a diminished efficacy of glial GLT1 transporters in this condition.
Figure 6. Diminished glial glutamate transporter regulation of tonic IGLU in SON neurons during a dehydration challenge.
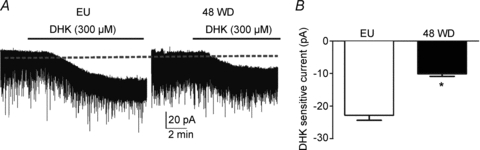
A, example of dihydrokainate (DHK)-induced inward shifts in Iholding in euhydrated (EU) and 48 h water deprived (48WD) rats. Note the diminished inward shift and the larger baseline noise level (RSM) in the 48WD rat. B, group data showing a significantly smaller DHK-sensitive current in 48WD rats compared to EU rats (n = 12 and 10, respectively). *P < 0.01 vs. EU rats.
Tonic INMDA contributes to increased SON firing activity during a dehydration challenge
Finally, to assess whether the blunted astroglial regulation of tonic IGLU resulted in an increased SON firing discharge during dehydration, and to extend the functional relevance of tonic INMDA to the whole system level, extracellular single-unit recordings were obtained from 71 SON neurons from 39 urethane-anaesthetized rats (see Fig. 7). A two-way ANOVA showed that, similar to previous observations (Wakerley et al. 1978; Scott et al. 2009), the mean spontaneous firing rate of SON neurons was dependent on the hydration status of the animal (F = 10.3, P < 0.01). However, no effects of neuronal phenotye (i.e. oxytocin and vasopressin) (F = 0.1, P = 0.7) were observed. Thus, oxytocin and vasopressin neurons were grouped together for subsequent analyses. Overall, the firing activity of 36 SON neurons recorded from 48WD rats (7.3 ± 0.9 spikes s−1) was significantly augmented, when compared to 35 SON neurons recorded from EU rats (4.7 ± 0.5 spikes s−1; P < 0.02, unpaired t test). We then tested the effects of intra-SON microdialysis administration of DHK (1 mm for 45–60 min) on SON firing rate. In EU rats, DHK induced a progressive and significant increase in the firing rate of SON neurons (F = 2.5, n = 16, P < 0.05, one way repeated measures ANOVA), reaching the maximum effect (47.0 ± 18.6% change from baseline) within 40–50 min after start of DHK infusion. Conversely, intra-SON DHK failed to increase SON firing activity in 48WD rats (F = 1.9, n = 14, P > 0.05 one way repeated measures ANOVA).
Figure 7. Diminished glial glutamate transporter regulation of in vivo SON firing activity during a dehydration challenge.
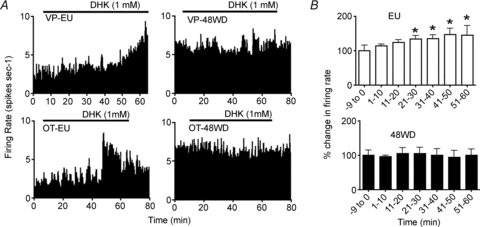
A, ratemeter records (in 10 s bins) of the activity of vasopressin (upper panels) and oxytocin (lower panels) neurons in euhydrated (EU) and 48 h water deprived (48WD) rats. Intra-SON microdialysis administration of DHK (1 mm in the dialysis probe, continuous lines; note that it is estimated that intra-SON drug concentrations are approximately 1000-fold lower than the intra-probe concentrations, Ludwig & Leng, 1997) increased the firing rate of neurons in euhydrated rats, but not in 48WD rats. B, group data showing mean percentage changes in firing rate of SON neurons as a function of time (in 10 min bins) during intra-SON microdialysis administration of DHK, recorded from euhydrated rats (n = 16) and 48 WD rats (n = 14) (*P < 0.05 vs. baseline (−9 to 0 min), Student–Newman–Keuls post hoc test).
When analysed together, we found a strong negative correlation between spontaneous (pre-DHK) firing rate and the DHK-induced change in firing rate (Pearson's product correlation coefficient, c = −0.40; P = 0.03 n = 29). Hence, slower firing neurons were more excited by DHK than faster firing neurons. Taken together, these results indicate that glial uptake of ambient glutamate restrains the activity of SON neurons under basal conditions, and that a reduction of glial glutamate uptake contributes to the increased activity of SON neurons during a dehydration challenge.
Discussion
In this paper we provide evidence for the presence of a persistent glutamate excitatory modality in SON neurons, termed tonic INMDA. We aimed in this work to elucidate mechanisms influencing the magnitude of tonic INMDA, to asses the functional relevance of tonic INMDA under different physiological conditions, and to determine whether it can undergo state-dependent changes in its strength. Overall, our studies suggest that tonic activation of extrasynaptic NMDARs under glial control contributes to regulation of SON neuronal firing activity. Moreover, we found the strength of tonic INMDA to increase during dehydration, a condition in which the local neuronal–glial microenvironment undergoes remodelling, contributing in turn to neuronal homeostatic responses during this physiological challenge.
Ambient glutamate of a non-synaptic origin tonically activates extrasynaptic NMDARs
Bath application of non-selective or NMDA-selective glutamate receptor blockers induced a sustained outward shift in Iholding (INMDA). This was accompanied by a decrease in background noise (RMS), consistent with the block of stochastic openings of NMDA receptor channels (Rosenmund et al. 1995; Chen et al. 1999). Thus, under basal conditions, ambient glutamate persistently activates NMDARs to mediate a sustained excitatory drive in SON neurons. We found INMDA to be largely blocked by memantine, an NMDAR antagonist that preferentially targets extrasynaptic receptors (Lipton, 2006; Leveille et al. 2008; Papadia et al. 2008; Okamoto et al. 2009). Moreover, a large proportion of INMDA was mediated by NR2B-containing receptors, known to have higher affinity and low desensitization rates for glutamate, and to be preferentially located extrasynaptically (Tovar & Westbrook, 1999; Kim et al. 2005). Taken together, these results suggest that tonic INMDA in SON neurons is likely to be mediated by NR2B-containing, extrasynaptically located NMDARs, a finding that is in agreement with previous reports in other neuronal types (Sah et al. 1989; Dalby & Mody, 2003; Le Meur et al. 2007; Milnerwood et al. 2010).
Our studies suggest that the source of glutamate activating extrasynaptic NMDARs, at least under basal conditions, is of a non-synaptic origin. Firstly, under conditions in which vesicular synaptic exocytotic release was blocked (i.e. slices incubated in BafA1), basal tonic INMDA magnitude, and that observed in the presence of glutamate transporter blockers, was largely unaffected. Moreover, drastically increasing the ongoing frequency of glutamate EPSCs also failed to alter INMDA magnitude. These results also suggest then that summation of slow EPSCs generated by remote synaptic inputs is not a factor contributing to tonic INMDA. Similar results supporting a non-synaptic contribution to extrasynaptic NMDAR activation have been reported elsewhere (Le Meur et al. 2007). However, we cannot rule out that spillover of synaptic glutamate during conditions of strong synchronous afferent activity could activate extrasynaptically located glutamate receptors in SON neurons (Asztely et al. 1997; Semyanov & Kullmann, 2000; Diamond, 2001; Lozovaya et al. 2004).
Astrocytes influence INMDA magnitude and its effect on neuronal firing activity
Glutamate transporters, particularly the astrocytic GLT1 and GLAST isoforms, constitute the major mechanism for glutamate clearance and maintenance of low ambient glutamate levels in the CNS (Rothstein et al. 1996). Our results showing that selective blockade of GLT1 increased the strength of tonic INMDA to induce and/or augment neuronal firing discharge indicate that glial GLT1 efficiently influences ambient glutamate levels, and consequently, the degree of extrasynaptic NMDAR activation, and SON neuronal excitability. While experiments with the gliotoxin selective compound dl-2-α-aminodipic acid (αAA) (Olney et al. 1971; Huck et al. 1984a; Bridges et al. 1992; Pow, 2001; Xu et al. 2005) are also in line with this notion, results should be taken more cautiously, given that its mechanism of action is still debated (Huck et al. 1984b; Pow, 2001). It is also important to take into consideration that the non-selective transporter blocker TBOA induced larger effects than those evoked by the GLT1-selective compound DHK, suggesting that in addition to GLT1, other glutamate transporters (e.g. EAAT1/3) may also contribute to the regulation of ambient glutamate levels in the SON.
A prominent role for astroglial regulation of ambient glutamate levels in the SON has been highlighted in previous studies, which showed that blockade of GLT1 increased the tonic activation of presynaptic mGluRs in the SON (Oliet et al. 2001; Boudaba et al. 2003). Thus, taken together, those studies and our present results support the presence of multiple modalities and targets (i.e. presynaptic mGluRs and extrasynaptic NMDARs) by which ambient glutamate under astrocytic control can tonically influence neuronal activity in the magnocellular neurosecretory system. While tonic activation of presynaptic mGluRs regulates homo- and heterosynaptic efficacy, as well as signal-to-noise ratio (Oliet et al. 2001; Boudaba et al. 2003; Piet et al. 2004b), it is likely that tonic activation of extrasynaptic NMDARs more globally affects the overall activity and gain of the magnocellular system. The relative contribution and functional relevance of each of these modalities remains to be more thoroughly investigated.
Blunted GLT1 efficacy and enhanced tonic INMDA magnitude contribute to increased SON activity during a dehydration challenge
Given the unique SON neuronal–glial microenvironment, and its ability to dynamically change in response to physiological challenges (Theodosis & Poulain, 1999; Hatton, 2004), we investigated whether the strength of extrasynaptic tonic INMDA was affected in a condition known to induce activity-dependent remodelling in the local neuronal–glial microenvironment. To test for this possibility, we used a challenge of dehydration, which similarly to lactation, results in a rapid and reversible retraction of astroglial processes, leading to diminished astrocytic coverage of synaptic and neuronal surfaces in the SON (Tweedle & Hatton, 1984; Miyata et al. 1994; Theodosis & Poulain, 1999; Tasker, 2002). In dehydrated rats, we found basal INMDA to be enhanced when compared to euhydrated controls. Conversely, the enhancement of tonic INMDA following glial GLT1 blockade was blunted in slices from dehydrated rats. We obtained similar results in whole animal studies. As previously reported (Wakerley et al. 1978; Scott et al. 2009), basal firing activity of both oxytocin and vasopressin neurons was elevated in dehydrated rats, and a blunted increased firing activity following glial GLT1 blockade was observed in this group when compared to euhydrated controls. Taken together, these results support an enhanced tonic INMDA strength in SON neurons in dehydrated rats, and suggest that this is due to a diminished ability of glial GLT1 to buffer ambient glutamate levels, causing in turn a more robust activation of extrasynaptic NMDARs and increased neuronal activity. Potential mechanisms underlying dehydration blunted GLT1 efficacy and enhanced tonic INMDA include physical retraction of GLT1-containing glial processes during this challenge (Tweedle & Hatton, 1984; Miyata et al. 1994; Theodosis & Poulain, 1999; Tasker, 2002), as well as diminished expression of GLT1 (Boudaba et al. 2003).
The fact that tonic INMDA strength in SON neurons is controlled in a state-dependent manner has significant physiological implications. Hormonal secretion from the posterior pituitary is dependent on the degree and pattern of SON neuronal activity (Wakerley et al. 1978; Poulain & Wakerley, 1982). Thus, in conditions such as dehydration and lactation, SON neurons increase their firing discharge in order to cope with the increased hormonal demand. Structural and functional remodelling of the magnocellular system has been shown to be critical for such homeostatic response, and numerous underlying mechanisms have been proposed, including increased density and activity of synaptic inputs (El Majdoubi et al. 1997; Stern et al. 2000; Di & Tasker, 2004), altered intrinsic membrane properties (Stern & Armstrong, 1996; Teruyama & Armstrong, 2005) and dendritic remodelling (Tweedle et al. 1993; Stern & Armstrong, 1998). Results from the present study indicate that state-dependent astrocyte regulation of extrasynaptic NMDA-mediated excitation constitutes an additional factor contributing to neuronal homeostatic responses during dehydration.
Finally, we recently reported that SON and PVN astrocytes also modulate ambient GABA levels and activation of an extrasynaptic GABAA-mediated inhibitory tone (Park et al. 2006, 2009). Thus, SON astrocytes appear to be strategically positioned to influence the balance of excitatory and inhibitory extrasynaptic tonic modalities in SON neurons. Whether individual astrocytes influence both glutamate and GABA extrasynaptic levels, and whether a crosstalk between these two mechanisms occurs in SON astrocytes, as recently shown in hippocampal astrocytes (Heja et al. 2009), will be the focus of future studies.
Acknowledgments
Supported by NIH R01 HL085767 (J.E.S.) and the New Zealand Lottery Health Board (C.H.B.).
Glossary
Abbreviations
- eNMDAR
extrasynaptic NMDA receptor
- MNCs
magnocellular neurosecretory cells
- RMS
root mean square
- SON
supraoptic nucleus
Author contributions
T.F.M. and N.K. participated in the collection analysis and interpretation of in vitro electrophysiology experiments, which were completed at the Medical College of Georgia. J.E.S. was involved in the conception and design of in vitro experiments, and in the drafting of the article. C.H.B. and V.S. designed the in vivo electrophysiology experiments, which T.F.M., N.J. and V.S. completed at the University of Otago; this data was analysed by N.J. under CHB's direction. All authors approved the final version of the manuscript submitted for publication.
References
- Asztely F, Erdemli G, Kullmann DM. Extrasynaptic glutamate spillover in the hippocampus: dependence on temperature and the role of active glutamate uptake. Neuron. 1997;18:281–293. doi: 10.1016/s0896-6273(00)80268-8. [DOI] [PubMed] [Google Scholar]
- Boudaba C, Linn DM, Halmos KC, Tasker JG. Increased tonic activation of presynaptic metabotropic glutamate receptors in the rat supraoptic nucleus following chronic dehydration. J Physiol. 2003;551:815–823. doi: 10.1113/jphysiol.2003.042739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RJ, Hatalski CG, Shim SN, Cummings BJ, Vijayan V, Kundi A, Cotman CW. Gliotoxic actions of excitatory amino acids. Neuropharmacology. 1992;31:899–907. doi: 10.1016/0028-3908(92)90128-c. [DOI] [PubMed] [Google Scholar]
- Brown CH, Munro G, Murphy NP, Leng G, Russell JA. Activation of oxytocin neurones by systemic cholecystokinin is unchanged by morphine dependence or withdrawal excitation in the rat. J Physiol. 1996;496:787–794. doi: 10.1113/jphysiol.1996.sp021727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DR, Kretzschmar HA. The glio-toxic mechanism of α-aminoadipic acid on cultured astrocytes. J Neurocytol. 1998;27:109–118. doi: 10.1023/a:1006947322342. [DOI] [PubMed] [Google Scholar]
- Buckle PJ, Haas HL. Enhancement of synaptic transmission by 4-aminopyridine in hippocampal slices of the rat. J Physiol. 1982;326:109–122. doi: 10.1113/jphysiol.1982.sp014180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavelier P, Attwell D. Tonic release of glutamate by a DIDS-sensitive mechanism in rat hippocampal slices. J Physiol. 2005;564:397–410. doi: 10.1113/jphysiol.2004.082131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman DB, Theodosis DT, Montagnese C, Poulain DA, Morris JF. Osmotic stimulation causes structural plasticity of neurone-glia relationships of the oxytocin but not vasopressin secreting neurones in the hypothalamic supraoptic nucleus. Neuroscience. 1986;17:679–686. doi: 10.1016/0306-4522(86)90039-4. [DOI] [PubMed] [Google Scholar]
- Chen N, Luo T, Raymond LA. Subtype-dependence of NMDA receptor channel open probability. J Neurosci. 1999;19:6844–6854. doi: 10.1523/JNEUROSCI.19-16-06844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby NO, Mody I. Activation of NMDA receptors in rat dentate gyrus granule cells by spontaneous and evoked transmitter release. J Neurophysiol. 2003;90:786–797. doi: 10.1152/jn.00118.2003. [DOI] [PubMed] [Google Scholar]
- Di S, Tasker JG. Dehydration-induced synaptic plasticity in magnocellular neurons of the hypothalamic supraoptic nucleus. Endocrinology. 2004;145:5141–5149. doi: 10.1210/en.2004-0702. [DOI] [PubMed] [Google Scholar]
- Diamond JS. Neuronal glutamate transporters limit activation of NMDA receptors by neurotransmitter spillover on CA1 pyramidal cells. J Neurosci. 2001;21:8328–8338. doi: 10.1523/JNEUROSCI.21-21-08328.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drose S, Altendorf K. Bafilomycins and concanamycins as inhibitors of V-ATPases and P-ATPases. J Exp Biol. 1997;200:1–8. doi: 10.1242/jeb.200.1.1. [DOI] [PubMed] [Google Scholar]
- El Majdoubi M, Poulain DA, Theodosis DT. Lactation-induced plasticity in the supraoptic nucleus augments axodendritic and axosomatic GABAergic and glutamatergic synapses: an ultrastructural analysis using the disector method. Neuroscience. 1997;80:1137–1147. doi: 10.1016/s0306-4522(97)00193-0. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Hatton GI. Dynamic neuronal-glial interactions: an overview 20 years later. Peptides. 2004;25:403–411. doi: 10.1016/j.peptides.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Hatton GI, Perlmutter LS, Salm AK, Tweedle CD. Dynamic neuronal-glial interactions in hypothalamus and pituitary: implications for control of hormone synthesis and release. Peptides. 1984;5(Suppl 1):121–138. doi: 10.1016/0196-9781(84)90271-7. [DOI] [PubMed] [Google Scholar]
- Heja L, Barabas P, Nyitrai G, Kekesi KA, Lasztoczi B, Toke O, Tarkanyi G, Madsen K, Schousboe A, Dobolyi A, Palkovits M, Kardos J. Glutamate uptake triggers transporter-mediated GABA release from astrocytes. PLoS One. 2009;4:e7153. doi: 10.1371/journal.pone.0007153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huck S, Grass F, Hatten ME. Gliotoxic effects of α-aminoadipic acid on monolayer cultures of dissociated postnatal mouse cerebellum. Neuroscience. 1984a;12:783–791. doi: 10.1016/0306-4522(84)90170-2. [DOI] [PubMed] [Google Scholar]
- Huck S, Grass F, Hortnagl H. The glutamate analogue α-aminoadipic acid is taken up by astrocytes before exerting its gliotoxic effect in vitro. J Neurosci. 1984b;4:2650–2657. doi: 10.1523/JNEUROSCI.04-10-02650.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A, Pellegrino C, Rama S, Dumalska I, Salyha Y, Ben-Ari Y, Medina I. Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons. J Physiol. 2006;572:789–798. doi: 10.1113/jphysiol.2006.105510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabaudon D, Shimamoto K, Yasuda-Kamatani Y, Scanziani M, Gähwiler BH, Gerber U. Inhibition of uptake unmasks rapid extracellular turnover of glutamate of nonvesicular origin. Proc Natl Acad Sci U S A. 1999;96:8733–8738. doi: 10.1073/pnas.96.15.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P, Bischofberger J, Fricker D, Miles R. Interneuron Diversity series: Fast in, fast out – temporal and spatial signal processing in hippocampal interneurons. Trends Neurosci. 2004;27:30–40. doi: 10.1016/j.tins.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Dunah AW, Wang YT, Sheng M. Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signalling and AMPA receptor trafficking. Neuron. 2005;46:745–760. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Le Meur K, Galante M, Angulo MC, Audinat E. Tonic activation of NMDA receptors by ambient glutamate of non-synaptic origin in the rat hippocampus. J Physiol. 2007;580:373–383. doi: 10.1113/jphysiol.2006.123570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveille F, El Gaamouch F, Gouix E, Lecocq M, Lobner D, Nicole O, Buisson A. Neuronal viability is controlled by a functional relation between synaptic and extrasynaptic NMDA receptors. FASEB J. 2008;22:4258–4271. doi: 10.1096/fj.08-107268. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat Rev Drug Discov. 2006;5:160–170. doi: 10.1038/nrd1958. [DOI] [PubMed] [Google Scholar]
- Lozovaya NA, Grebenyuk SE, Tsintsadze T, Feng B, Monaghan DT, Krishtal OA. Extrasynaptic NR2B and NR2D subunits of NMDA receptors shape ‘superslow’ afterburst EPSC in rat hippocampus. J Physiol. 2004;558:451–463. doi: 10.1113/jphysiol.2004.063792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Autoinhibition of supraoptic nucleus vasopressin neurons in vivo: a combined retrodialysis/electrophysiological study in rats. Eur J Neurosci. 1997;9:2532–2540. doi: 10.1111/j.1460-9568.1997.tb01682.x. [DOI] [PubMed] [Google Scholar]
- Milnerwood AJ, Gladding CM, Pouladi MA, Kaufman AM, Hines RM, Boyd JD, Ko RW, Vasuta OC, Graham RK, Hayden MR, Murphy TH, Raymond LA. Early increase in extrasynaptic NMDA receptor signalling and expression contributes to phenotype onset in Huntington's disease mice. Neuron. 2010;65:178–190. doi: 10.1016/j.neuron.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Miyata S, Nakashima T, Kiyohara T. Structural dynamics of neural plasticity in the supraoptic nucleus of the rat hypothalamus during dehydration and rehydration. Brain Res Bull. 1994;34:169–175. doi: 10.1016/0361-9230(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Okamoto S, Pouladi MA, Talantova M, Yao D, Xia P, Ehrnhoefer DE, Zaidi R, Clemente A, Kaul M, Graham RK, Zhang D, Vincent Chen HS, Tong G, Hayden MR, Lipton SA. Balance between synaptic versus extrasynaptic NMDA receptor activity influences inclusions and neurotoxicity of mutant huntingtin. Nat Med. 2009;15:1407–1413. doi: 10.1038/nm.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliet SH, Piet R, Poulain DA. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science. 2001;292:923–926. doi: 10.1126/science.1059162. [DOI] [PubMed] [Google Scholar]
- Olney JW, Ho OL, Rhee V. Cytotoxic effects of acidic and sulphur containing amino acids on the infant mouse central nervous system. Exp Brain Res. 1971;14:61–76. doi: 10.1007/BF00234911. [DOI] [PubMed] [Google Scholar]
- Papadia S, Soriano FX, Leveille F, Martel MA, Dakin KA, Hansen HH, Kaindl A, Sifringer M, Fowler J, Stefovska V, McKenzie G, Craigon M, Corriveau R, Ghazal P, Horsburgh K, Yankner BA, Wyllie DJ, Ikonomidou C, Hardingham GE. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci. 2008;11:476–487. doi: 10.1038/nn2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JB, Jo JY, Zheng H, Patel KP, Stern JE. Regulation of tonic GABA inhibitory function, presympathetic neuronal activity and sympathetic outflow from the paraventricular nucleus by astroglial GABA transporters. J Physiol. 2009;587:4645–4660. doi: 10.1113/jphysiol.2009.173435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JB, Skalska S, Son S, Stern JE. Dual GABAA receptor-mediated inhibition in rat presympathetic paraventricular nucleus neurons. J Physiol. 2007;582:539–551. doi: 10.1113/jphysiol.2007.133223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JB, Skalska S, Stern JE. Characterization of a novel tonic γ-aminobutyric acidA receptor-mediated inhibition in magnocellular neurosecretory neurons and its modulation by glia. Endocrinology. 2006;147:3746–3760. doi: 10.1210/en.2006-0218. [DOI] [PubMed] [Google Scholar]
- Piet R, Poulain DA, Oliet SHR. Contribution of astrocytes to synaptic transmission in the rat supraoptic nucleus. Neurochem Int. 2004a;45:251–257. doi: 10.1016/j.neuint.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Piet R, Vargova L, Sykova E, Poulain DA, Oliet SH. Physiological contribution of the astrocytic environment of neurons to intersynaptic crosstalk. Proc Natl Acad Sci U S A. 2004b;101:2151–2155. doi: 10.1073/pnas.0308408100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain DA, Wakerley JB. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience. 1982;7:773–808. doi: 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- Pow DV. Visualising the activity of the cystine-glutamate antiporter in glial cells using antibodies to aminoadipic acid, a selectively transported substrate. Glia. 2001;34:27–38. doi: 10.1002/glia.1037. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Feltz A, Westbrook GL. Synaptic NMDA receptor channels have a low open probability. J Neurosci. 1995;15:2788–2795. doi: 10.1523/JNEUROSCI.15-04-02788.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Rusakov DA, Kullmann DM. Extrasynaptic glutamate diffusion in the hippocampus: ultrastructural constraints, uptake, and receptor activation. J Neurosci. 1998;18:3158–3170. doi: 10.1523/JNEUROSCI.18-09-03158.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier N, Brown CH, Ludwig M, Leng G. Phasic spike patterning in rat supraoptic neurones in vivo and in vitro. J Physiol. 2004;558:161–180. doi: 10.1113/jphysiol.2004.063982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Hestrin S, Nicoll R. Tonic activation of NMDA receptors by ambient glutamate enhances excitability of neurons. Science. 1989;246:815–818. doi: 10.1126/science.2573153. [DOI] [PubMed] [Google Scholar]
- Scott V, Bishop VR, Leng G, Brown CH. Dehydration-induced modulation of κ-opioid inhibition of vasopressin neurone activity. J Physiol. 2009;587:5679–5689. doi: 10.1113/jphysiol.2009.180232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott V, Brown CH. State-dependent plasticity in vasopressin neurones: dehydration-induced changes in activity patterning. J Neuroendocrinol. 2010;22:343–354. doi: 10.1111/j.1365-2826.2010.01961.x. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Kullmann DM. Modulation of GABAergic signalling among interneurons by metabotropic glutamate receptors. Neuron. 2000;25:663–672. doi: 10.1016/s0896-6273(00)81068-5. [DOI] [PubMed] [Google Scholar]
- Silverman AJ, Zimmerman EA. Magnocellular neurosecretory system. Annu Rev Neurosci. 1983;6:357–380. doi: 10.1146/annurev.ne.06.030183.002041. [DOI] [PubMed] [Google Scholar]
- Stern JE. Electrophysiological and morphological properties of pre-autonomic neurones in the rat hypothalamic paraventricular nucleus. J Physiol. 2001;537:161–177. doi: 10.1111/j.1469-7793.2001.0161k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE, Armstrong WE. Changes in the electrical properties of supraoptic nucleus oxytocin and vasopressin neurons during lactation. J Neurosci. 1996;16:4861–4871. doi: 10.1523/JNEUROSCI.16-16-04861.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE, Armstrong WE. Reorganization of the dendritic trees of oxytocin and vasopressin neurons of the rat supraoptic nucleus during lactation. J Neurosci. 1998;18:841–853. doi: 10.1523/JNEUROSCI.18-03-00841.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE, Hestrin S, Armstrong WE. Enhanced neurotransmitter release at glutamatergic synapses on oxytocin neurones during lactation in the rat. J Physiol. 2000;526:109–114. doi: 10.1111/j.1469-7793.2000.t01-1-00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasker Functional synaptic plasticity in hypothalamic magnocellular neurons. Prog Brain Research. 2002;139:113–119. doi: 10.1016/s0079-6123(02)39011-3. [DOI] [PubMed] [Google Scholar]
- Tasker JG, Di S, Boudaba C. Functional synaptic plasticity in hypothalamic magnocellular neurons. Prog Brain Res. 2002;139:113–119. doi: 10.1016/s0079-6123(02)39011-3. [DOI] [PubMed] [Google Scholar]
- Teruyama R, Armstrong WE. Enhancement of calcium-dependent afterpotentials in oxytocin neurons of the rat supraoptic nucleus during lactation. J Physiol. 2005;566:505–518. doi: 10.1113/jphysiol.2005.085985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodosis DT, Poulain DA. Contribution of astrocytes to activity-dependent structural plasticity in the adult brain. Adv Exp Med Biol. 1999;468:175–182. doi: 10.1007/978-1-4615-4685-6_14. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedle CD, Hatton GI. Synapse formation and disappearance in adult rat supraoptic nucleus during different hydration states. Brain Res. 1984;309:373–376. doi: 10.1016/0006-8993(84)90607-3. [DOI] [PubMed] [Google Scholar]
- Tweedle CD, Smithson KG, Hatton GI. Rapid synaptic changes and bundling in the supraoptic dendritic zone of the perfused rat brain. Exp Neurol. 1993;124:200–207. doi: 10.1006/exnr.1993.1190. [DOI] [PubMed] [Google Scholar]
- Wakerley JB, Poulain DA, Brown D. Comparison of firing patterns in oxytocin- and vasopressin-releasing neurones during progressive dehydration. Brain Res. 1978;148:425–440. doi: 10.1016/0006-8993(78)90730-8. [DOI] [PubMed] [Google Scholar]
- Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol. 1993;44:851–859. [PubMed] [Google Scholar]
- Xu HL, Ye S, Baughman VL, Feinstein DL, Pelligrino DA. The role of the glia limitans in ADP-induced pial arteriolar relaxation in intact and ovariectomized female rats. Am J Physiol Heart Circ Physiol. 2005;288:H382–388. doi: 10.1152/ajpheart.00727.2004. [DOI] [PubMed] [Google Scholar]


