Non-technical summary
Cross-fostering of newborn pups to different dams is a method widely used in rodent studies of developmental ‘programming’ to determine whether pregnancy or the suckling period is more important in determining adult characteristics following changes to the maternal environment. We have investigated whether the process of fostering per se influences cardiovascular and metabolic development in mice. Compared with mice reared by their biological mother, fostered mice showed increased appetite, body weight, abdominal fatness and altered blood sugar metabolism. A marked increase in blood pressure was also apparent. This study demonstrates that the process of fostering can lead to profound effects in cardiovascular and metabolic function in otherwise normal mice. The findings have implications both for the interpretation of previous cross-fostering studies in mice and for studies investigating the hypothesis of developmental programming, in which early postnatal manipulation of litters is common practice.
Abstract
Abstract
Cross-fostering is widely used in developmental programming studies to determine the relative contribution of the in utero and suckling periods in establishing the adult offspring phenotype in response to an environmental challenge. We have investigated whether the process of fostering per se influences cardiovascular and metabolic function in adult offspring of C57BL/6J mice in comparison with animals suckled by their biological dams. Cross-fostered (CF) mice demonstrated juvenile onset hyperphagia and significantly higher body weight (from weaning to 12 weeks: male control (CON) vs. CF: P < 0.01, female CON vs. CF: P < 0.001; RM ANOVA) accompanied by increased abdominal adiposity in males only (white adipose tissue mass (mg): CON 280.5 ± 13.4 [mean ± SEM] (n = 7) vs. CF, 549.8 ± 99.3 (n = 8), P < 0.01). Both male and female CF mice demonstrated significantly enhanced glucose tolerance. A marked increase in systolic blood pressure (SBP) was observed in male CF mice (SBP (mmHg), day: CON 100.5 ± 1.4 (n = 6) vs. CF 114.3 ± 0.7 (n = 6), P < 0.001; night: CON 108.0 ± 2.0 (n = 6) vs. CF 123.2 ± 1.1 (n = 6), P < 0.001). Endothelium-dependent relaxation was enhanced in male CF mice, and renal noradrenaline was increased in female CF mice. Concentration of serum triglycerides, cholesterol, insulin and leptin were increased in CF vs. CON. The process of cross-fostering profoundly affects cardiovascular and metabolic phenotype in mice. The findings have implications for the inclusion of appropriate controls in the design of future studies and in the interpretation of previous cross-fostering studies in mice.
Introduction
The method of fostering in which a litter of rodents born to one dam is suckled by another has played an important role in identifying the suckling period as a critical stage of developmental plasticity in response to environmental influences (Cierpial & McCarty, 1987b; Reifsnyder et al. 2000; Ozanne & Hales, 2004; Khan et al. 2005; Kappeler et al. 2009), thereby contributing to the experimental evidence supporting the developmental programming hypothesis. However, very few studies have included appropriate controls to assess whether the process of fostering is itself innocuous. Indeed, isolated reports suggest some effect. In suckling piglets, for example, fostering is associated with a reduced rate of survival (Neal & Irvin, 1991), and in mice with increased body weight and altered emotional behaviour (Bartolomucci et al. 2004; Leussis & Heinrichs, 2009; Lu et al. 2009). Also, in rats changes in nociception and emotional behaviour have been reported after fostering pups to a different dam (Malkesman et al. 2008; Dickinson et al. 2009).
Numerous studies have concluded that modulation of the gestational or post partum environment whether by nutritional, hormonal or behavioural methods gives rise to persistent modification of the adult metabolic and cardiovascular phenotype. In this study therefore we have designed a protocol to address the influence of fostering on these parameters. Further rationale was provided by the limited evidence suggesting that fostering may influence glucose metabolism (Siebel et al. 2008) and the observation that fostering has played an integral role in defining the early life origins of spontaneous hypertension in genetically prone rodents (Cierpial & McCarty, 1987a).
Here we report the effects of cross-fostering on offspring cardiovascular and metabolic function in mice. Offspring body weight, calorific intake, organ weights, serum analytes, activity, heart rate, blood pressure, renal noradrenaline (NA) content, liver triglyceride (TG) content, vascular function and glucose tolerance were assessed. Given the sexual dimorphism frequently observed in models of developmental programming (Grigore et al. 2008; Gabory et al. 2009), we also investigated the potential interaction of sex within the fostering method.
Methods
Ethical approval
All studies were carried out in accordance with the United Kingdom Home Office Regulations and were approved by the King's College London local Ethical Review Procedures Committee.
Animal husbandry
Throughout all protocols, mice were provided with food and water ad libitum and maintained in controlled conditions (12 h light–dark cycle, 25°C). Proven breeder (one previous litter) female C57BL/6J mice (Charles River Laboratories, UK) were fed a standard chow diet (RM1; Special Diets Services, UK). After 6 weeks on the diet, females were mated and maintained on the same diet throughout pregnancy and lactation. In all litters, to obviate any of the reported effects of litter size on metabolic function and to parallel commonly adopted protocols in the developmental programming literature, litters of less than four pups were excluded, and larger litters were reduced to six pups at 48 h post partum, maintaining equal sex ratios where possible. A subgroup of litters was cross-fostered at this time. Control offspring (CON) were suckled by the biological mother (n = 12 litters), whilst fostered offspring (CF) were suckled by a different dam (n = 10 litters). Litter size was not different between groups (CF, 6.2 ± 0.2 versus CON, 5.9 ± 0.4, not significant (NS)) and no litters were lost subsequent to cross-fostering. All offspring were weaned onto standard chow at 21 days of age and cardiovascular and metabolic parameters were assessed at 3 months of age prior to necropsy. Offspring were assigned to procedures randomly. One male and one female per litter were used for; radiotelemetry; myography and collection of organs and serum samples; and GTTs, unless there were less than three pups of a certain sex in the litter, in which case GTTs were performed in animals which were subsequently used for organ collection and myography. All animals were fasted prior to tissue collection.
Serum and organ collection and analysis
Offspring were killed by exposure to a rising concentration of carbon dioxide following an overnight fast. Serum (from blood obtained by cardiac puncture) and tissue were collected, snap frozen and stored at –80°C. Serum glucose, triglycerides and total cholesterol were determined with an autoanalyser (Synchron LX20, Beckman Coulter, Uppsala, The Netherlands), using Beckman Coulter kits. Mouse insulin (Mercodia, Uppsala, Sweden) and leptin (Biovendor, Brno, Czech Republic) were determined by enzyme-linked immunosorbent assay. For measurement of renal noradrenaline content, kidneys were homogenised in 0.01 mol l−1 HCl in the presence of EDTA (1 mmol l−1) and sodium metabisulphite (4 mmol l−1) for prevention of catecholamine degradation. Homogenates were centrifuged at 4°C and noradrenaline concentration in the supernatant was determined by enzyme immunoassay according to the manufacturer's protocol. (ALPCO Diagnostics, Salem, NH, USA). Whole liver tissue triglyceride content was determined using an adaptation of the Folch Method (Folch et al. 1957) and enzymatic colorimetric assay (UNIMATE 5 TRIG, Roche BC1, Roche Diagnostics, Burgess Hill, West Sussex, UK).
Radiotelemetry
Blood pressure (systolic and diastolic), heart rate and locomotor activity were measured in conscious, unrestrained animals employing radiotelemetry. Implantation of the radio-telemetry probe catheter (TA11PA-C10, O.D 0.4 mm, Data Sciences International Inc., St Paul, MN, USA) into the aortic arch via the left carotid artery was performed under general anaesthesia (medetomidine as Domitor, Orion Corp., Espoo, Finland, 0.5 mg kg−1, i.p. and ketamine; 75 mg kg−1, i.p. as Vetalar, Pfizer, Sandwich, Kent, UK, in PBS). Pre- and post-surgical analgesia was provided and maintained for 24 h post-surgery (Buprenorphine, 0.1 mg kg−1s.c.). Anaesthesia was reversed via subcutaneous administration (0.005 ml (g body weight)−1) of a 1:25 dilution of atipamezole hydrochloride in PBS. Recordings were made over one 24 h period (7 days post-surgery) by scheduled sampling for 10 s every 5 min (Dataquest LabPRO Acquisition System, version 3.01, Data Sciences International). Mice were subsequently exposed to a brief 20 min restraint stress, and cardiovascular responses recorded. Mice were then returned to their home cage, and measurements continued for 120 min.
Vascular function
Concentration-dependent constriction to NA and relaxation to ACh and nitric oxide (NO) was assessed in second order mesenteric arteries mounted in physiological saline solution (PSS) on a wire myograph (Multi Myograph, model 610M, Danish Myo Technology, Aarhus, Denmark) as previously described (Samuelsson et al. 2008).
Intraperitoneal glucose tolerance test
Blood from the tail vein was used to determine baseline blood glucose concentration, measured by glucometer (GlucoMen Sensor, A. Menarini Diagnostics, Winnersh-Wokingham, UK) following and overnight fast. Blood glucose concentration was then measured 15, 30, 60, 90 and 120 min subsequent to an intraperitoneal injection of a glucose bolus (1 g (kg body weight)−1).
Statistical analysis
Where possible, one male and one female from each litter were studied. n refers to the number of litters in each group. Data are expressed as means ± SEM. Data from control (CON) and cross-fostered (CF) offspring were compared using Student's unpaired t test or repeated measures ANOVA. When considering differences at multiple time points, Bonferroni post hoc tests were applied following ANOVA. Statistical significance was assumed at the 5% level. Area under the curve for the glucose tolerance tests was calculated using GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA, USA) employing using the trapezoid rule. Radiotelemetry data were further analysed using generalised estimating equations in SPSS. The effects of fostering and sex were analysed, then interactions between these two factors were examined. Subject effect was defined as number of litters and the model allowed for clustering within the litter where one male and one female from each litter were analysed together. Power calculations for the primary outcome of blood pressure required a minimum sample size of six animals per group to detect a 10% mean difference in SBP with 80% power, at the 5% significance level.
Results
Body weight
Male and female CF mice were heavier than the control (CON) over the time period studied (male CON vs. CF: P < 0.01, female CON vs. CF: P < 0.001; RM ANOVA, Fig. 1A and C). Bonferroni post hoc tests to compare weights at each time point showed that CF were consistently heavier than CON from 9 weeks of age onwards, following an earlier increase in female, but not male CF between weeks 4 and 7 (Fig. 1A and C). Pup weights over the suckling period were not different between the groups (Fig. 1E).
Figure 1. Body weights and calorific intakes.
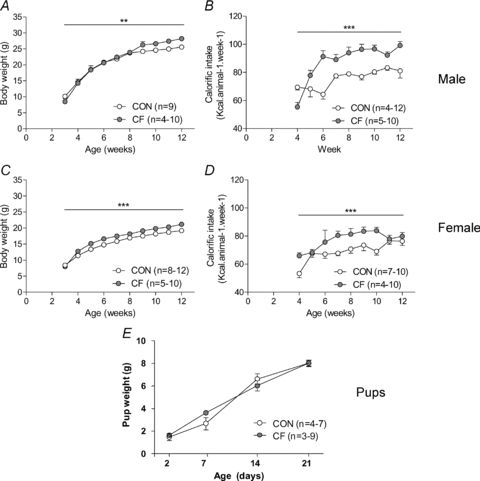
Body weights (A and C and E) and calorific intake (B and D) of male (A and B) and female (C and D) and combined male and female pups (E) for control (CON) and cross-fostered (CF) offspring. *P < 0.05, **P < 0.01, ***P < 0.001 vs. CON, RM ANOVA.
Calorific intake
Both male and female CF mice increased their calorific intake compared to CON over the 3 month time period studied (male CON vs. CF: P < 0.001, female CON vs. CF: P < 0.001; RM ANOVA, Fig. 1B and D).
Organ weights
Abdominal white adipose tissue (WAT, combined abdominal fat pads) mass was increased in male but not female CF mice compared to CON (Table 1). Offspring heart weight was not affected by fostering (Table 1). Fostering was also associated with increased liver weight in male and female CF vs. CON, whilst muscle mass was decreased in male and female CF vs. CON (Table 1).
Table 1.
Offspring organ weights
| Organ | Sex | CON | CF |
|---|---|---|---|
| WAT (mg) | ♂ | 280.5 ± 13.38 (n = 7) | 549.8 ± 99.28*↑ (n = 8) |
| ♀ | 256.4 ± 22.92 (n = 9) | 358.6 ± 58.31 (n = 8) | |
| Heart (mg) | ♂ | 184.5 ± 10.49 (n = 7) | 156.6 ± 12.93 (n = 8) |
| ♀ | 149.2 ± 5.32 (n = 9) | 132.9 ± 6.14 (n = 8) | |
| Liver (mg) | ♂ | 843.5 ± 53.99 (n = 6) | 1235 ± 51.61***↑ (n = 8) |
| ♀ | 804.1 ± 18.95 (n = 9) | 940.9 ± 40.32**↑ (n = 8) | |
| Muscle (mg) | ♂ | 149.3 ± 2.06 (n = 6) | 103.3 ± 6.73***↓ (n = 7) |
| ♀ | 125.1 ± 4.22 (n = 6) | 101.0 ± 2.60***↓ (n = 8) |
Body weights and organ weights of male and female control (CON) and cross-fostered (CF) offspring at 3 months of age. Data expressed as means ± SEM. WAT, white adipose tissue (inguinal fat pad); Muscle, tibialis anterior.
P < 0.05
P < 0.01
P < 0.001 vs. non-cross-fostered offspring, Student's unpaired t test.
Serum analysis
Fostering was associated with increased serum triglyceride, cholesterol and insulin concentrations in both males and females compared to controls (Fig. 2A and B). No effect of fostering on fasting serum glucose was observed. As anticipated, the higher values for WAT mass were associated with a higher leptin concentration which was elevated approximately 2-fold in male but not female fostered mice compared to controls.
Figure 2. Serum analytes at 3 months of age.

Concentrations of serum analytes from control (CON) and cross-fostered (CF) offspring at 3 months of age. Data expressed as means ± SEM. *P < 0.05, **P < 0.01 vs. CON, Student's unpaired t test. Astrix indicate 10 times and 100 times concentration
Locomotor activity, heart rate and blood pressure
No differences in activity were observed between CF and CON in either males (Fig. 3A and B) or females (Fig. 3C and D). No interaction was observed between fostering status and sex; analysis of the combined data continued to show no significant differences in activity.
Figure 3. Activity and heart rate at 3 months of age.
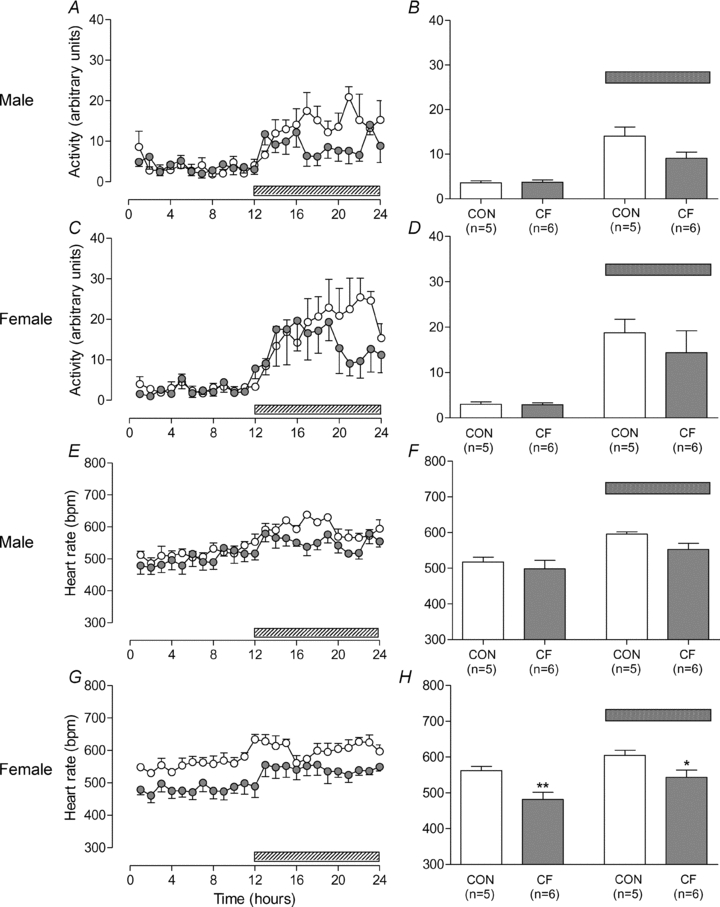
Hourly averages measured over 24 h (A, C, E, G) and overall averages (B, D, F, H) for day and night in male (A, B, E, F) and female (C, D, G, H) control (CON) and cross-fostered (CF) offspring at 3 months of age. Data expressed as means ± SEM. *P < 0.05, **P < 0.01 vs. CON, unpaired t test (B, D, F, H). Shaded bar represents night-time period.
No effect of fostering on heart rate was observed in male offspring (Fig. 3E and F); however heart rate was reduced in female CF vs. CON during both the day and night (Fig. 3G and H). No interaction was observed between fostering status and sex. Analysis of the combined data showed a significant overall decrease in heart rate during both the day (P = 0.003) and the night (P < 0.001).
Systolic blood pressure (SBP) was markedly increased in male CF compared to CON during both the day and night (Fig. 4E and B). No effect of fostering on female SBP was observed (Fig. 4C and D). A significant (P < 0.001) interaction was observed between fostering status and sex.
Figure 4. Systolic blood pressure at 3 months of age.
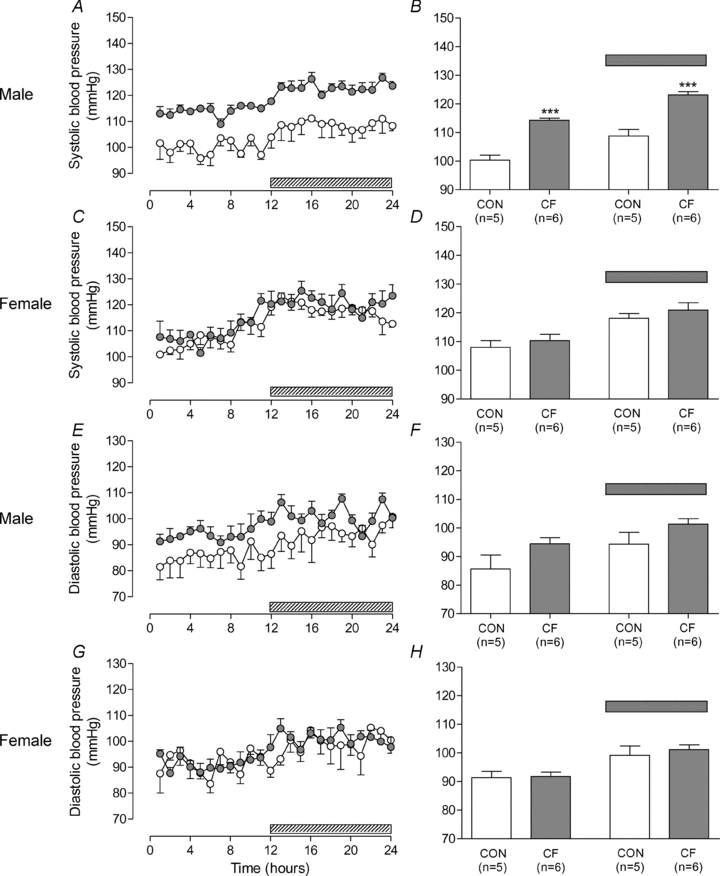
Hourly averages measured over 24 h (A, C, E, G) and overall averages (B, D, F, H) for day and night in male (A, B, E, F) and female (C, D, G, H) control (CON) and cross-fostered (CF) offspring at 3 months of age. Data expressed as means ± SEM. ***P < 0.001 vs. CON, unpaired t test (B, D, F, H). Shaded bar represents night-time period.
No differences in diastolic blood pressure between CF and CON were observed in offspring of either sex (Fig. 4E–H). No interaction was observed between fostering status and sex. Analysis of the combined data showed no significant differences in diastolic blood pressure. Mean arterial pressure (MAP) was significantly increased in the male offspring (day-time MAP (mmHg) CF, 109.2 ± 5.0 versus CON, 93.9 ± 1.9, P < 0.05, n = 5-6; night-time MAP (mmHg) CF, 119.8 ± 3.9 versus CON, 101.0 ± 3.1, P < 0.01, n = 5-6).
Cardiovascular reactivity to stress
Responses to restraint stress are shown in Fig. 5A and B. Male CF mice showed a significantly higher SBP response to restraint (RM ANOVA, P < 0.05) compared to control. SBP remained elevated in male CF mice over the 120 min recovery period (RM ANOVA, P < 0.05) relative to controls, indicating an exaggerated and prolonged stress response in CF mice. Female CF mice showed no difference in SBP reactivity to restrain stress.
Figure 5. Cardiovascular stress reactivity and basal renal noradrenaline content.
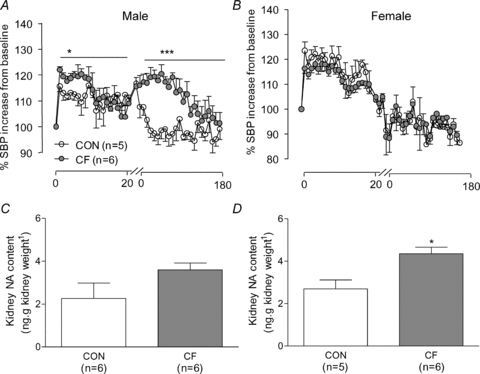
Systolic blood pressure reactivity to a 20 min restraint stress in male (A) and female (B) control (CON) and cross-fostered (CF) offspring at 3 months of age. Data expressed as means ± SEM. *P < 0.05 vs. CON, RM ANOVA. †P < 0.05, ††P < 0.01, ††P < 0.001 vs. CON. Kidney noradrenaline (NA) content in male (C) and female (D) control (CON) and cross-fostered (CF) offspring at 3 months of age. Data expressed as means ± SEM. *P < 0.05, ***P < 0.001 vs. CON, Student's unpaired t test.
Renal noradrenaline
A significant increase in renal noradrenaline content was observed in female (Fig. 5D) CF compared to CON, but not in male CF mice (Fig. 5C and D).
Liver triglycerides
A significant increase in liver triglyceride content was observed in female CF compared to CON (Liver triglyceride content (mmol l−1 g−1) female, CON 4.8 ± 0.9, n = 3 vs. 30.2 ± 2.9, n = 5, P < 0.001).
Vascular function
Normalised vessel diameter was significantly greater in male and female CF compared to CON (Vessel diameter (microns) (mean ± SEM) male CON: 193 ± 15, n = 5 vs. CF: 248 ± 6, n = 7, P < 0.05; Student's unpaired t test). To control for variation in vessel size, contractile responses were expressed as the percentage of the maximum response to a 5 μm solution of NA in potassium-substituted PSS. In males and females, no significant effect of fostering on response to NA was observed across the concentration range (Fig. 6A and B; NS, RM ANOVA) or when comparing maximum responses (Vmax) or sensitivity (pEC50).
Figure 6. Vascular function at 3 months of age.
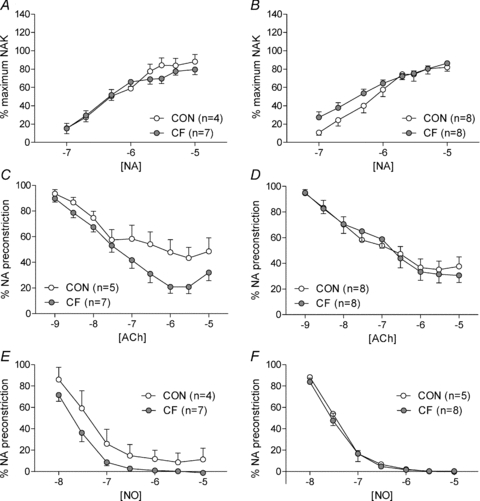
Contractile responses to noradrenaline (NA; A and B), endothelium-dependent relaxation to acetylcholine (ACh; C and D) and endothelium-independent relaxation to nitric oxide (NO; E and F) of small mesenteric arteries from male (A, C, E) and female (B, D, F) control (CON) and cross-fostered (CF) offspring at 3 months of age. Data expressed as means ± SEM.
In male offspring fostering did not significantly affect the response to ACh across the concentration range (Fig. 6C; NS, RM ANOVA) or the sensitivity to ACh (pEC50); however, the maximum response to ACh was increased in CF compared to CON (Vmax to ACh (% initial pre-constriction) (mean ± SEM) CON: 39.1 ± 8.3, n = 5 vs. CF: 18.1 ± 4.17, n = 7, P < 0.05; Student's unpaired t test). Responses to ACh were similar in female CF and CON (Fig. 6D).
Fostering did not result in any differences in the response to nitric oxide (NO) in males or females (Fig. 6E and F).
Offspring glucose tolerance
Fostering was associated with significantly enhanced glucose tolerance, in male and female CF mice (Fig. 7) as evidenced by a reduced area under the glucose curve (Fig. 7C and D, P < 0.001, RM ANOVA), a lower peak in the glucose curve and an improved recovery phase (Fig. 7A and B).
Figure 7. Glucose tolerance tests at 3 months of age.
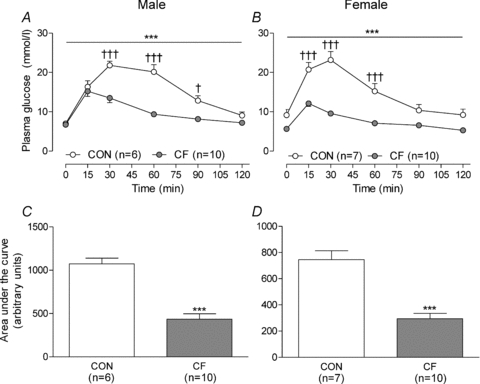
Plasma glucose measured over 2 h following an intraperitoneal injection (1 g kg−1) of glucose (A and B) and area under the glucose curve (C and D) in male (A and C) and female (B and D) control (CON) and cross-fostered (CF) offspring at 3 months of age. Data expressed as means ± SEM. ***P < 0.001 vs. CON, RM AMOVA (A and B) or unpaired t test (C and D). †P < 0.05, †††P < 0.001 vs. CON, Bonferroni post hoc test following RM ANOVA (A and B).
Discussion
This study demonstrates that the process of cross-fostering in mice can effect changes in offspring cardiovascular and metabolic function in adulthood. The results also emphasise the importance of the suckling period as a critical stage of developmental plasticity. The influence of fostering was profound, leading to changes in most of the parameters studied, with important sex differences.
Fostering appears to programme a ‘thrifty’ phenotype, particularly in the males, all elements of which would tend towards energy conservation. Thus food intake was increased and glucose tolerance enhanced. As would be anticipated, the mass of white adipose tissue and of the liver both increased.
Fostering-induced hyperphagia and adiposity have not previously been reported and are likely to contribute to the increase in body weight observed in this and potentially in a previous report demonstrating increased body weight in cross-fostered Swiss CD1 adult mice (Bartolomucci et al. 2004).
Fostering led to a marked increase in systolic blood pressure in male offspring. Few studies have reported the measurement of blood pressure in fostering protocols in mice. In a recent report, in which pups were redistributed between dams to yield large and small litters, hypertension was associated with small litter rearing. Variable degrees of fostering between groups, on the basis of the present data, could have influenced cardiovascular function (Kappeler et al. 2009). Others have shown that fostering offspring of the spontaneously hypertensive rat (SHR) or the Dahl hypertension-sensitive rat to a normotensive dam significantly reduces adulthood blood pressure (Cierpial & McCarty, 1987a, 1991; Di Nicolantonio, 1987; Cierpial et al. 1988; Murphy & McCarty, 1989). This would not imply that fostering itself led to an increase in blood pressure. However, the converse, i.e. suckling pups of normotensive dams to genetically hypertensive dams, did not lead to an increase in blood pressure, suggesting that the response to suckling by the normotensive dam was genetically determined in SHRs (Cierpial et al. 1988; McCarty et al. 1992). Importantly, appropriate within-group fostering controls were not performed in these studies.
Increases in renal sympathetic nerve activity, as measured indirectly by an increase in renal NA content, have been associated with the developmental programming of hypertension (Alexander et al. 2005; Samuelsson et al. 2010). Since increased basal renal NA content was only observed in fostered female mice, mechanisms other than a sympatho-excitatory effect on the kidney are more likely to contribute to basal hypertension in fostered males. The exaggerated and prolonged cardiovascular stress responses to restraint in male CF mice may in part be explained by a resetting of the HPA axis, or an enhanced sympathetically mediated response to an acute stressful stimulus, which is implicated in the developmental programming of hypertension (for review see Seckl & Meaney, 2004; McMillen et al. 2008; Cottrell & Seckl, 2009). Endothelial dysfunction did not appear to contribute to the observed hypertension in fostered male mice. However, without confirmation in other vascular beds a role for the endothelium cannot be definitively excluded (Docherty et al. 2001). Enhanced endothelium-dependent relaxation in mesenteric vessels may represent a response to the persistent hyperphagia, since feeding is associated with a decrease in vascular resistance, leading to increased mesenteric blood flow (Sit & Chou, 1984). This is consistent with the observed increase in normalised mesenteric vessel diameter in the fostered animals. The increase in blood pressure in fostered male mice in the absence of a fall in heart rate may be indicative of altered baroreceptor function, although this was not formally investigated in the current protocol.
The glucose tolerance tests indicate improved glucose tolerance in young fostered offspring. In a rat model of prenatal fetal growth restriction achieved by bilateral uterine artery ligation, fostering the pups to a dam with normal uterine artery blood flow also led to improved glucose tolerance and first phase insulin secretion (Siebel et al. 2008). The decreased areas under the glucose curves observed in the present study together with markedly elevated fasting plasma insulin, suggests a similar enhancement in insulin profiles. Hyperinsulinaemia is one of the earliest metabolic markers of obesity and type 2 diabetes and is a key feature of developmental programming models of prenatal undernutrition in which the offspring phenotype is characterised by hyperphagia, hypertension, obesity, hyperleptinaemia and insulin hypersecretion in the progression to frank diabetes (Vickers et al. 2000, 2001). Maturation of both β cell metabolism and elements of the secretory apparatus are established in early postnatal life (Roberts et al. 2001) and may therefore be influenced by fostering.
The imposition of specific phenotypic traits through fostering must arise either as a consequence of an altered postnatal maternal environment, in which case the programming vector must be either milk-borne or behavioural in nature, or alternatively the neonate is capable of sensing and mounting a physiological response to the change in its maternal environmental. We did not measure maternal food intake during the suckling period, but pup weights were similar throughout the suckling period suggesting normal lactation and pup growth trajectory. Moreover, cross-fostering occurred at 48 h after delivery between dams at the same stage of lactation thereby maintaining a similar plane of nutrition and growth. This is in agreement with the study by Lu et al. (2009), in which no differences in pup weight were observed between control and ‘in-fostered’ offspring during the suckling period. No pup rejection was observed in the cross-fostered group, but the potential role of altered maternal grooming and nursing behaviour following fostering warrants further investigation (Maccari et al. 1995; Barbazanges et al. 1996; Curley et al. 2010). The possibility that fostering causes changes in maternal behaviour which may programme offspring phenotype through epigenetic modification of genes (Weaver et al. 2004; Meaney & Szyf, 2005) is further supported by a recent study in fostered mice (Hager et al. 2009). These authors identified 10 quantitative trait loci in a genome-wide scan which were dependent on fostering, highlighting the considerable plasticity of genomic imprinting during the postnatal period.
Notwithstanding changes in maternal grooming behaviour, fostering may induce a stress response in the mother that is transferable to pups via corticosterone in the maternal milk (Angelucci et al. 1985; Catalani et al. 1993). Stress-induced increase in maternal plasma corticosterone is associated with altered anxiety traits in adult male offspring, and also in cross-fostered male offspring (Bartolomucci et al. 2004; Moles et al. 2004, 2008). Similar to the observed phenotype in fostered mice, early postnatal exposure to exogenous glucocotricods in rodents also leads to increased body weight in adulthood (Gonzalez et al. 1990; Loizzo et al. 2006) and increased hepatic triglyceride content (Liu et al. 2007). A detailed profiling of the maternal and neonatal stress axes in response to fostering is beyond the scope of this study, but extensive temporal profiling of glucocorticoids would be required to establish the role of the hypothalamic–pituitary–adrenal (HPA) axis.
The male phenotype resulting from fostering was exaggerated compared with that of females, particularly in relation to adiposity and blood pressure. A study of neonatal maternal separation in rat pups has also reported exaggerated HPA-mediated blood pressure responses preferentially in male offspring (Genest et al. 2004). Bartolomucci et al. (2004) reported that fostering was linked with reduced preputial gland weight and relative testis weight in adult male offspring, which could result in a reduction in testosterone (Gustafasson & Pousette, 1975), a risk factor for obesity and metabolic syndrome (Traish et al. 2009). The role of sex steroids in the sexual dimorphism observed in the fostered animals warrants further investigation.
Conclusions
Cross-fostering has been widely used in the field of ‘developmental programming’ but few, if any, of these studies have adequately controlled for the potential inherent effects of fostering. Results of the current study suggest that reports in mice should be interpreted with some caution. In addition to the potential implications for fostering studies, our findings may also have wider implications for other animal models in which early postnatal manipulation of litter size is common. Further investigation of the mechanisms by which fostering exerts effects on adult phenotype could lead to improvements in the technique or to the development of alternative methods.
Acknowledgments
This work was supported by grants from the British Heart Foundation, EARNEST and Tommy's the Baby Charity. We would also like to thank Mr Jahm Persaud, Biochemistry Department, Royal Free Hospital, for help with biochemical analyses.
Glossary
Abbreviations
- ACh
acetylcholine
- CF
cross-fostered
- NA
noradrenaline
- NO
nitric oxide
- SBP
systolic blood pressure
- TG
triglyceride
- WAT
white adipose tissue
Author contributions
Experiments were performed by P.A.M, A.M.S. and J.P. Concept and experimental design by L.P. and P.D.T. P.A.M. and P.D.T. prepared the manuscript. All authors approved the final version for publication. The work was performed in the Maternal & Fetal Research laboratories at King's College London and Centre for Hepatology, Royal Free Hospital, London.
References
- Alexander BT, Hendon AE, Ferril G, Dwyer TM. Renal denervation abolishes hypertension in low-birth-weight offspring from pregnant rats with reduced uterine perfusion. Hypertension. 2005;45:754–758. doi: 10.1161/01.HYP.0000153319.20340.2a. [DOI] [PubMed] [Google Scholar]
- Angelucci L, Patacchioli FR, Scaccianoce S, Di Sciullo A, Cardillo A, Maccari S. A model for later-life effects of perinatal drug exposure: maternal hormone mediation. Neurobehav Toxicol Teratol. 1985;7:511–517. [PubMed] [Google Scholar]
- Barbazanges A, Vallee M, Mayo W, Day J, Simon H, Le Moal M, Maccari S. Early and later adoptions have different long-term effects on male rat offspring. J Neurosci. 1996;16:7783–7790. doi: 10.1523/JNEUROSCI.16-23-07783.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomucci A, Gioiosa L, Chirieleison A, Ceresini G, Parmigiani S, Palanza P. Cross fostering in mice: behavioral and physiological carry-over effects in adulthood. Genes Brain Behav. 2004;3:115–122. doi: 10.1111/j.1601-183x.2003.00059.x. [DOI] [PubMed] [Google Scholar]
- Catalani A, Marinelli M, Scaccianoce S, Nicolai R, Muscolo LA, Porcu A, Koranyi L, Piazza PV, Angelucci L. Progeny of mothers drinking corticosterone during lactation has lower stress-induced corticosterone secretion and better cognitive performance. Brain Res. 1993;624:209–215. doi: 10.1016/0006-8993(93)90079-3. [DOI] [PubMed] [Google Scholar]
- Cierpial MA, Konarska M, McCarty R. Maternal effects on the development of spontaneous hypertension. Health Psychol. 1988;7:125–135. doi: 10.1037//0278-6133.7.2.125. [DOI] [PubMed] [Google Scholar]
- Cierpial MA, McCarty R. Hypertension in SHR rats: contribution of maternal environment. Am J Physiol Heart Circ Physiol. 1987a;253:H980–H984. doi: 10.1152/ajpheart.1987.253.4.H980. [DOI] [PubMed] [Google Scholar]
- Cierpial MA, McCarty R. Preweanling behavioral development in spontaneously hypertensive, borderline hypertensive, and Wistar-Kyoto normotensive rats. Dev Psychobiol. 1987b;20:57–69. doi: 10.1002/dev.420200109. [DOI] [PubMed] [Google Scholar]
- Cierpial MA, McCarty R. Adult blood pressure reduction in spontaneously hypertensive rats reared by normotensive Sprague-Dawley mothers. Behav Neural Biol. 1991;56:262–270. doi: 10.1016/0163-1047(91)90409-j. [DOI] [PubMed] [Google Scholar]
- Cottrell EC, Seckl JR. Prenatal stress, glucocorticoids and the programming of adult disease. Front Behav Neurosci. 2009;3:19. doi: 10.3389/neuro.08.019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley JP, Rock V, Moynihan AM, Bateson P, Keverne EB, Champagne FA. Developmental shifts in the behavioral phenotypes of inbred mice: the role of postnatal and juvenile social experiences. Behav Genet. 2010;40:220–232. doi: 10.1007/s10519-010-9334-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nicolantonio R. Blood pressure and salt appetite of cross-suckled spontaneously hypertensive and normotensive rats. J Hypertens. 1987;5:413–416. [PubMed] [Google Scholar]
- Dickinson AL, Leach MC, Flecknell PA. Influence of early neonatal experience on nociceptive responses and analgesic effects in rats. Lab Anim. 2009;43:11–16. doi: 10.1258/la.2007.007078. [DOI] [PubMed] [Google Scholar]
- Docherty CC, Kalmar-Nagy J, Engelen M, Koenen SV, Nijland M, Kuc RE, Davenport AP, Nathanielsz PW. Effect of in vivo fetal infusion of dexamethasone at 0.75 GA on fetal ovine resistance artery responses to ET–1. Am J Physiol Regul Integr Comp Physiol. 2001;281:R261–R268. doi: 10.1152/ajpregu.2001.281.1.R261. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Gabory A, Attig L, Junien C. Sexual dimorphism in environmental epigenetic programming. Mol Cell Endocrinol. 2009;304:8–18. doi: 10.1016/j.mce.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Genest SE, Gulemetova R, Laforest S, Drolet G, Kinkead R. Neonatal maternal separation and sex-specific plasticity of the hypoxic ventilatory response in awake rat. J Physiol. 2004;554:543–557. doi: 10.1113/jphysiol.2003.052894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez AS, Rodriguez Echandia EL, Cabrera R, Foscolo MR, Fracchia LN. Neonatal chronic stress induces subsensitivity to chronic stress in adult rats. I. Effects on forced swim behavior and endocrine responses. Physiol Behav. 1990;47:735–741. doi: 10.1016/0031-9384(90)90087-k. [DOI] [PubMed] [Google Scholar]
- Grigore D, Ojeda NB, Alexander BT. Sex differences in the fetal programming of hypertension. Gend Med. 2008;5(Suppl A):S121–S132. doi: 10.1016/j.genm.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafasson J, Pousette K. Demonstration and partial characterization of cytosol receptors for testosterone. Biochemistry. 1975;14:3094–3101. doi: 10.1021/bi00685a009. [DOI] [PubMed] [Google Scholar]
- Hager R, Cheverud JM, Wolf JB. Change in maternal environment induced by cross-fostering alters genetic and epigenetic effects on complex traits in mice. Proc Biol Sci. 2009;276:2949–2954. doi: 10.1098/rspb.2009.0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappeler L, De Magalhaes Filho C, Leneuve P, Xu J, Brunel N, Chatziantoniou C, Le Bouc Y, Holzenberger M. Early postnatal nutrition determines somatotropic function in mice. Endocrinology. 2009;150:314–323. doi: 10.1210/en.2008-0981. [DOI] [PubMed] [Google Scholar]
- Khan IY, Dekou V, Douglas G, Jensen R, Hanson MA, Poston L, Taylor PD. A high-fat diet during rat pregnancy or suckling induces cardiovascular dysfunction in adult offspring. Am J Physiol Regul Integr Comp Physiol. 2005;288:R127–133. doi: 10.1152/ajpregu.00354.2004. [DOI] [PubMed] [Google Scholar]
- Leussis MP, Heinrichs SC. Quality of rearing guides expression of behavioral and neural seizure phenotypes in El mice. Brain Res. 2009;1260:84–93. doi: 10.1016/j.brainres.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Liu Y, Havinga R, Bloks VW, Baller JF, van der Leij FR, Reijngoud DJ, Sauer PJ, Kuipers F. Postnatal treatment with dexamethasone perturbs hepatic and cardiac energy metabolism and is associated with a sustained atherogenic plasma lipid profile in suckling rats. Pediatr Res. 2007;61:165–170. doi: 10.1203/pdr.0b013e31802d89ff. [DOI] [PubMed] [Google Scholar]
- Loizzo A, Loizzo S, Galietta G, Caiola S, Spampinato S, Campana G, Seghieri G, Ghirlanda G, Franconi F. Overweight and metabolic and hormonal parameter disruption are induced in adult male mice by manipulations during lactation period. Pediatr Res. 2006;59:111–115. doi: 10.1203/01.pdr.0000190575.12965.ce. [DOI] [PubMed] [Google Scholar]
- Lu L, Mamiya T, Lu P, Niwa M, Mouri A, Zou LB, Nagai T, Hiramatsu M, Nabeshima T. The long-lasting effects of cross-fostering on the emotional behavior in ICR mice. Behav Brain Res. 2009;198:172–178. doi: 10.1016/j.bbr.2008.10.031. [DOI] [PubMed] [Google Scholar]
- Maccari S, Piazza PV, Kabbaj M, Barbazanges A, Simon H, Le Moal M. Adoption reverses the long-term impairment in glucocorticoid feedback induced by prenatal stress. J Neurosci. 1995;15:110–116. doi: 10.1523/JNEUROSCI.15-01-00110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkesman O, Lavi-Avnon Y, Maayan R, Weizman A. A cross-fostering study in a genetic animal model of depression: maternal behavior and depression-like symptoms. Pharmacol Biochem Behav. 2008;91:1–8. doi: 10.1016/j.pbb.2008.06.004. [DOI] [PubMed] [Google Scholar]
- McCarty R, Cierpial MA, Murphy CA, Lee JH, Fields-Okotcha C. Maternal involvement in the development of cardiovascular phenotype. Experientia. 1992;48:315–322. doi: 10.1007/BF01923425. [DOI] [PubMed] [Google Scholar]
- McMillen IC, MacLaughlin SM, Muhlhausler BS, Gentili S, Duffield JL, Morrison JL. Developmental origins of adult health and disease: the role of periconceptional and foetal nutrition. Basic Clin Pharmacol Toxicol. 2008;102:82–89. doi: 10.1111/j.1742-7843.2007.00188.x. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci. 2005;7:103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moles A, Rizzi R, D'Amato FR. Postnatal stress in mice: does ‘stressing’ the mother have the same effect as ‘stressing’ the pups? Dev Psychobiol. 2004;44:230–237. doi: 10.1002/dev.20008. [DOI] [PubMed] [Google Scholar]
- Moles A, Sarli C, Bartolomucci A, D'Amato FR. Interaction with stressed mothers affects corticosterone levels in pups after reunion and impairs the response to dexamethasone in adult mice. Psychoneuroendocrinology. 2008;33:462–470. doi: 10.1016/j.psyneuen.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Murphy CA, McCarty R. Maternal environment and development of high blood pressure in Dahl hypertensive rats. Am J Physiol. 1989;257:H1396–H1401. doi: 10.1152/ajpheart.1989.257.5.H1396. [DOI] [PubMed] [Google Scholar]
- Neal SM, Irvin KM. The effects of crossfostering pigs on survival and growth. J Anim Sci. 1991;69:41–46. doi: 10.2527/1991.69141x. [DOI] [PubMed] [Google Scholar]
- Ozanne SE, Hales CN. Lifespan: catch-up growth and obesity in male mice. Nature. 2004;427:411–412. doi: 10.1038/427411b. [DOI] [PubMed] [Google Scholar]
- Reifsnyder PC, Churchill G, Leiter EH. Maternal environment and genotype interact to establish diabesity in mice. Genome Res. 2000;10:1568–1578. doi: 10.1101/gr.147000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C, Roberts GA, Lobner K, Bearzatto M, Clark A, Bonifacio E, Christie MR. Expression of the protein tyrosine phosphatase-like protein IA-2 during pancreatic islet development. J Histochem Cytochem. 2001;49:767–776. doi: 10.1177/002215540104900610. [DOI] [PubMed] [Google Scholar]
- Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EH, Piersma AH, Ozanne SE, Twinn DF, Remacle C, Rowlerson A, Poston L, Taylor PD. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension. 2008;51:383–392. doi: 10.1161/HYPERTENSIONAHA.107.101477. [DOI] [PubMed] [Google Scholar]
- Samuelsson AM, Morris A, Igosheva N, Kirk SL, Pombo JM, Coen CW, Poston L, Taylor PD. Evidence for sympathetic origins of hypertension in juvenile offspring of obese rats. Hypertension. 2010;55:76–82. doi: 10.1161/HYPERTENSIONAHA.109.139402. [DOI] [PubMed] [Google Scholar]
- Seckl JR, Meaney MJ. Glucocorticoid programming. Ann N Y Acad Sci. 2004;1032:63–84. doi: 10.1196/annals.1314.006. [DOI] [PubMed] [Google Scholar]
- Siebel AL, Mibus A, De Blasio MJ, Westcott KT, Morris MJ, Prior L, Owens JA, Wlodek ME. Improved lactational nutrition and postnatal growth ameliorates impairment of glucose tolerance by uteroplacental insufficiency in male rat offspring. Endocrinology. 2008;149:3067–3076. doi: 10.1210/en.2008-0128. [DOI] [PubMed] [Google Scholar]
- Sit SP, Chou CC. Time course of jejunal blood flow, O2 uptake, and O2 extraction during nutrient absorption. Am J Physiol Heart Circ Physiol. 1984;247:H395–H402. doi: 10.1152/ajpheart.1984.247.3.H395. [DOI] [PubMed] [Google Scholar]
- Traish AM, Guay A, Feeley R, Saad F. The dark side of testosterone deficiency: I. Metabolic syndrome and erectile dysfunction. J Androl. 2009;30:10–22. doi: 10.2164/jandrol.108.005215. [DOI] [PubMed] [Google Scholar]
- Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab. 2000;279:E83–E87. doi: 10.1152/ajpendo.2000.279.1.E83. [DOI] [PubMed] [Google Scholar]
- Vickers MH, Reddy S, Ikenasio BA, Breier BH. Dysregulation of the adipoinsular axis – a mechanism for the pathogenesis of hyperleptinemia and adipogenic diabetes induced by fetal programming. J Endocrinol. 2001;170:323–332. doi: 10.1677/joe.0.1700323. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]


