Non-technical summary
MicroRNA (miRNA) molecules are essential intracellular mediators of numerous biological processes including angiogenesis, inflammation, and mitochondrial metabolism. Recently, it has been shown that miRNAs are secreted into the bloodstream and that circulating miRNAs (c-miRNAs) may serve important endocrine functions. This study examined plasma profiles of specific c-miRNAs in healthy competitive athletes at rest and during exhaustive exercise testing, before and after a 90 day period of exercise training. In this setting, we observed four distinct patterns of c-miRNA response to exercise: (1) c-miRNAs up-regulated by acute exhaustive exercise before and after sustained exercise training, (2) c-miRNAs responsive to acute exhaustive exercise before but not after sustained exercise training, (3) c-miRNAs responsive only to sustained exercise training, and (4) non-responsive c-miRNAs. These findings set the stage for further work aimed at defining the role of c-miRNAs as fitness biomarkers and physiological mediators of exercise-induced cardiovascular adaptation.
Abstract
Abstract
MicroRNAs (miRNAs) are intracellular mediators of essential biological functions. Recently, plasma-based ‘circulating’ miRNAs (c-miRNAs) have been shown to control cellular processes, but the c-miRNA response to human exercise remains unknown. We sought to determine whether c-miRNAs are dynamically regulated in response to acute exhaustive cycling exercise and sustained rowing exercise training using a longitudinal, repeated measures study design. Specifically, c-miRNAs involved in angiogenesis (miR-20a, miR-210, miR-221, miR-222, miR-328), inflammation (miR-21, miR-146a), skeletal and cardiac muscle contractility (miR-21, miR-133a), and hypoxia/ischaemia adaptation (miR-21, miR-146a, and miR-210) were measured at rest and immediately following acute exhaustive cycling exercise in competitive male rowers (n = 10, age = 19.1 ± 0.6 years) before and after a 90 day period of rowing training. Distinct patterns of c-miRNA response to exercise were observed and adhered to four major profiles: (1) c-miRNA up-regulated by acute exercise before and after sustained training (miR-146a and miR-222), (2) c-miRNA responsive to acute exercise before but not after sustained training (miR-21 and miR-221), (3) c-miRNA responsive only to sustained training (miR-20a), and (4) non-responsive c-miRNA (miR-133a, miR-210, miR-328). Linear correlations were observed between peak exercise levels of miR-146a and  (r = 0.63, P = 0.003) and between changes in resting miR-20a and changes in
(r = 0.63, P = 0.003) and between changes in resting miR-20a and changes in 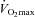 (pre-training vs. post-training, r = 0.73; P = 0.02). Although future work is required, these results suggest the potential value of c-miRNAs as exercise biomarkers and their possible roles as physiological mediators of exercise-induced cardiovascular adaptation.
(pre-training vs. post-training, r = 0.73; P = 0.02). Although future work is required, these results suggest the potential value of c-miRNAs as exercise biomarkers and their possible roles as physiological mediators of exercise-induced cardiovascular adaptation.
Introduction
Exercise training induces numerous cardiovascular changes including mitochondrial synthesis (Kiessling et al. 1973), myocardial remodelling (Baggish et al. 2008), and angiogenesis (Gute et al. 1996). Although such adaptations and their attendant impact on exercise capacity and health outcomes have been well documented, the cellular and molecular mechanisms leading to these changes remain incompletely understood.
Recently, microRNA molecules (miRNAs) have been identified as essential intracellular mediators of processes inherent in exercise adaptation including angiogenesis (Zhang, 2010), inflammation (Davidson-Moncada et al. 2010), mitochondrial metabolism (Chan et al. 2009; Dang, 2010), cardiac/skeletal muscle contractile force generation, and tissue hypertrophy (Williams et al. 2009; Davidsen et al. 2011). miRNAs are conserved, non-protein coding RNA molecules which can be regulated in a transcriptional or post-transcriptional fashion (Ambros, 2004). miRNAs alter cellular function by binding the 3′ untranslated regions (3′ UTR) of messenger RNA (mRNA) transcripts and thereby repress translation and/or degrade mRNA (van Rooij & Olson, 2007). It has recently been discovered that miRNAs are stably secreted into the bloodstream at rest (Mitchell et al. 2008) and in response to tissue injury and other pathological conditions (Laterza et al. 2009; Fichtlscherer et al. 2010; Heneghan et al. 2010). These plasma-based, ‘circulating’ miRNAs (c-miRNAs) may be protected from degradation by several complementary mechanisms including inclusion in phospholipid bilayer-encapsulated vesicles (Valadi et al. 2007) or via the formation of RNA-binding protein complexes (Gupta et al. 2010). Recently, c-miRNAs have been implicated in the control of important cellular functions in the context of disease (Kosaka et al. 2010b; Pegtel et al. 2010; Zhang et al. 2010). It is postulated that c-miRNAs mediate such changes either through signalling induction or direct uptake and intracellular target gene engagement upon encountering putative recipient tissue (Gupta et al. 2010).
While c-miRNAs have been studied as biomarkers of various diseases, data defining c-miRNA behaviour in the settings of acute exercise bouts and sustained exercise training in healthy humans are lacking. Identification of c-miRNAs specifically regulated by exercise could reveal unique biomarkers of exercise physiology and would lend significant insight into the molecular control of exercise adaptation. We therefore sought to define the plasma profiles of specific c-miRNAs with previously established roles in major adaptive processes linked to exercise training. Specifically, we assessed c-miRNA concentrations in healthy competitive athletes at rest and during acute exhaustive exercise testing, before and after a 90 day period of aerobic exercise training.
Methods
Ethical approval and study participants
Student athletes participating in competitive athletics affiliated with the Harvard University Department of Athletics took part in this study. Individuals were considered eligible if they were >18 years old and were recruited members of the men's competitive rowing programme. Written informed consent was obtained from all participants. Ethical approval for this study conformed to the standards of the Declaration of Helsinki. The Harvard University institutional review board and the Partners Human Research Committee approved the protocol before study initiation.
General study design
We utilized a prospective, longitudinal and repeated measures study design to examine c-miRNA profiles in human endurance athletes. Cardiopulmonary exercise testing, consisting of cycle ergometry to maximal exertion, was performed before and after 90 days of sustained aerobic rowing exercise training and was coupled with c-miRNA measurements.
Anthropometric measurements
Height, weight, medical historical data and resting vital signs were recorded at the time of enrolment. Training volume data during the pre-study period, defined as the 8 weeks before baseline assessment, were collected. To further characterize exercise exposure during the pre-study period, data were recorded about the performance of endurance and strength-building activities. Endurance activity was defined as running, cycling, swimming, rowing, or aerobic machine use at an effort sustainable for >20 min. Strength activity was defined as weight lifting, plyometric exercise and sprint running drills. Training volumes during the pre-study (8 weeks prior to enrolment) and study period were characterized by total number of hours per week and the hours per week dedicated to either endurance or strength activities.
Exercise training exposure
The exercise training study period, further referred to as ‘sustained exercise training’, began at the time of enrolment and lasted for 90 days. Subjects performed organized, team-based rowing training aimed to optimize performance at a 5 km distance that consisted of long duration open water and indoor ergometer sessions (1 to 3 h) at low stroke rates (20 to 24 strokes per minute). Daily data were recorded on the duration and the type of training activities performed during the study period. All participants were questioned confidentially about anabolic steroid use and were excluded if a history of use was elicited. Subjects were excluded from the final data analysis if they undertook any breaks in training of >3 days during the study period.
Measurement of fitness
Acute exhaustive cardiopulmonary exercise testing, further referred to as ‘acute exercise’, was performed before and after the 90 day training period. Testing was completed during the morning hours keeping the exact test time for each participant constant at both study time points. Participants abstained from any physical exercise for >24 h prior to testing and were tested after an overnight dietary fast at both study time points. On the morning of testing, participants were permitted and encouraged to drink water (400–800 mL) but were prohibited from drinking beverages with caloric or electrolyte content. All participants were required to abstain from non-steroidal anti-inflammatory use (including ibuprofen, naproxyn and aspirin) for at least 7 days prior to each study time point.
The exercise test consisted of uninterrupted, incremental cycling exercise using an upright cycle ergometer (CPE 2001, Medical Graphics Corp., St Paul, MN, USA) equipped with an electronically braked ergometer (Warren Collins Corp., Braintree, MA, USA). Cycle ergometry was chosen as the exercise modality to limit the impact of training-induced (rowing) improvements in exercise economy. A tightly fitting mouthpiece and nose clips were in place during exercise to facilitate measurements of gas exchange and ventilation. Pulmonary gas exchange and minute ventilation were measured breath by breath with a commercially available metabolic cart (Medical Graphics Corporation CPX/D). The pneumotachograph was calibrated with a 3 litre syringe at five different flow rates, and the zirconia cell O2 analyser and single-beam CO2 analyser were calibrated with room air and 5% CO2–12% O2 gas. Following a 3 min period of rest to facilitate ventilatory equilibration and a one minute period of unloaded exercise (0 W min−1), workload was increased by 25 W min−1 until volitional exhaustion. Subjects were considered to have reached their peak oxygen consumption ( ) when the following criteria were met: (1) levelling off of oxygen consumption with increasing workload, (2) respiratory exchange ratio values >1.1, and (3) a heart rate of at least 90% of age-predicted maximum.
) when the following criteria were met: (1) levelling off of oxygen consumption with increasing workload, (2) respiratory exchange ratio values >1.1, and (3) a heart rate of at least 90% of age-predicted maximum. 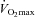 was defined as the highest 15 s average during the final minute of exercise. The ventilatory threshold was determined by the modified V-slope method (Beaver et al. 1986). Blood pressures were determined by auscultation prior to and then at each minute interval during exercise and recovery (5 min). Standard 12-lead electrocardiography was performed at rest and at each minute during exercise and recovery. Continuous heart rate was recorded from lead V1 and continuously displayed on an oscilloscope.
was defined as the highest 15 s average during the final minute of exercise. The ventilatory threshold was determined by the modified V-slope method (Beaver et al. 1986). Blood pressures were determined by auscultation prior to and then at each minute interval during exercise and recovery (5 min). Standard 12-lead electrocardiography was performed at rest and at each minute during exercise and recovery. Continuous heart rate was recorded from lead V1 and continuously displayed on an oscilloscope.
Plasma sampling
Venous blood was collected at three time points during each acute exercise test. A 20-gauge intravenous catheter was placed antiseptically into a dorsal hand vein or a vein in the distal forearm as dictated by favourable anatomy using the Seldinger technique and then secured with tape. The i.v. catheter was flushed with sterile saline to help maintain patency after each blood draw. Ten millilitres of blood was collected in standard anticoagulant (EDTA)-treated vacutainer tubes at baseline (prior to acute exercise testing), immediately post-exercise (within 1 min of completion of exercise testing), and after 1 h of rest following exercise testing. All blood samples were centrifuged at 2000 g for 10 min to pellet cellular elements immediately after each blood draw. To minimize freeze–thaw degradation, the supernatant plasma was then aliquoted and immediately frozen at –80°C.
Selection of candidate microRNA
We selected eight c-miRNAs as representative of those previously implicated in cellular processes underlying exercise adaptation (Fig. 1). Specifically, we chose to study mediators of angiogenesis (miR-20a, Dews et al. 2006; miR-210, Fasanaro et al. 2008; miR-221/miR-222, Poliseno et al. 2006; Kuehbacher et al. 2007; Suárez et al. 2007; and miR-328, Wang et al. 2008); inflammation (miR-21, Urbich et al. 2008; Iliopoulos et al. 2010; and miR-146a, Taganov et al. 2006); skeletal and cardiac muscle function (miR-21, Thum et al. 2008; Cheng et al. 2010; and miR-133a, Chen et al. 2006; Care et al. 2007); and the cellular response to hypoxia (miR-21, Krichevsky & Gabriely, 2009; miR-146a, Chan et al. 2009; and miR-210, Chan & Loscalzo, 2010). Notably, a few of these miRNAs (miR-21, miR-210, and miR-146a) regulate several functions relevant to exercise, identifying them as especially attractive candidates that reflect exercise physiology.
Figure 1.

Candidate miRNAs that regulate cellular processes integral to exercise training and cardiovascular adaptation
RNA extraction
To remove any remaining cellular contents, thawed plasma samples were further centrifuged (15,700 g× 10 min), and plasma supernatant was aliquoted into 100 μl volumes for storage at −80°C or further analysis. Although plasma-based c-miRNAs tend to resist degradation under a variety of harsh environmental conditions (Mitchell et al. 2008), repeated freeze–thaw cycles of plasma samples were nonetheless minimized. To further avoid variability of results due to plasma handling, all samples from a given individual were processed and analysed in a single batch. Specifically, to a standard volume of each plasma supernatant (100 μl), 2 fmol was added of a chemically synthesized miRNA duplex mimic of microRNA-422b (miR-422b) (Ambion/Applied Biosystems, Carlsbad, CA, USA). Given minimal expression of endogenous miR-422b in circulating plasma during various contexts of exercise training as determined by empiric observation (S. Y. Chan, unpublished data, 2010), equivalent levels of exogenously added miR-422b were used for quantitative normalization of c-miRNA plasma levels, as previously described (Mitchell et al. 2008). Total RNA extraction was performed using a MicroRNA Extraction Kit (Benevbio, Mission Viejo, CA, USA). Quantitative efficacy and reproducibility of c-miRNA extraction was confirmed by extraction of known quantities of miR-422b mimic via serial dilutions (S. Y. Chan, unpublished data, 2009).
Quantification of microRNA expression
To quantify levels of select c-miRNA, standard reverse transcription-quantitative (‘real time’) polymerase chain reaction (RT-QPCR) was utilized. This technique was chosen to minimize the inherent variability that can be otherwise associated with current high-throughput miRNA screening techniques. Such variability is particularly relevant when measuring c-miRNAs, which are typically of lower concentration than miRNAs assayed from the intracellular environment (S. Y. Chan, unpublished data, 2009). Specifically, reverse transcription was performed to generate cDNA representing levels of mature c-miRNA molecules (MicroRNA Assay Kit, Applied Biosystems). Owing to the stem loop structures of the c-miRNA primers, only mature c-miRNA molecules were amplified into cDNA. cDNA was amplified via fluorescently labelled Taqman probe and primer sets using an Applied Biosystems 7900HT Fast Real Time PCR device. Fold-change of RNA species was calculated using the formula 2(−ΔΔCt). Specifically, in this formula, Ct represents the ‘real time’ cycle number at which the increase in miRNA probe fluorescence is exponential. As reference control for c-miRNA quantification (ΔCt), Ct values were then subtracted from Ct values obtained from exogenously added miR-422b, as described above. ΔCt values were then compared (ΔΔCt) with each athlete's own resting baseline at the pre-season time point (normalized to fold-change of 1).
Statistical analysis
Subject characteristics and exercise testing data are reported as means ± standard deviation. c-miRNA data are presented in the text as means ± standard error of the mean (SEM). Quantitative measures of c-miRNA levels during exercise are presented in Figure 3 as bar and whisker plots where horizontal lines denote statistical mean, grey boxes denote 25% and 75% percentile confidence intervals, and error bars reflect maximum and minimum values. Paired samples including anthropometric and exercise testing data were compared by Student's t test or Wilcoxon's matched pairs test as appropriate for data distribution. Multiple comparisons (c-miRNA values) were made with a repeated measures ANOVA followed by a Student–Newman–Keuls post hoc test. Correlation analyses were performed using the Spearman's or Pearson's method as appropriate for data distribution. Values of P < 0.05 are considered significant.
Figure 3. Distinct regulatory profiles of specific c-miRNA after acute exhaustive exercise and sustained exercise training.

A–H, for each athlete, baseline c-miRNA levels under resting condition are assigned a fold change of 1, to which measurements obtained during subsequent study time points are compared (i.e. rest vs. 1 min after exhaustive exercise (post-ex) during baseline and post-training stages). In all panels, bar and whisker plots are utilized where horizontal lines denote statistical mean, grey boxes denote 25% and 75% percentile confidence intervals, and error bars reflect maximum and minimum values. Profile 1 denotes c-miRNA that respond to acute exhaustive exercise both before and after sustained training (A and B). Profile 2 denotes c-miRNAs that respond to acute exhaustive exercise before but not after sustained aerobic training (C and D). Profile 3 denotes c-miRNAs that respond to sustained aerobic training but not acute exhaustive exercise (E). Profile 4 denotes c-miRNAs that do not respond to acute or sustained aerobic training (F, G and H). *P < 0.05 compared to baseline resting value, †P < 0.05 compared to post-training resting value, **P marginally greater than 0.05 compared to baseline resting value; NS signifies P > 0.05 compared to baseline resting value.
Results
Baseline and post-training subject characteristics
Of the 11 subjects enrolled, one individual suffered a shoulder injury during the study period necessitating a reduced training load and was excluded from all analysis. Baseline and post-training clinical characteristics for the remaining subjects (n = 10) are shown in Table 1. No subjects had a history of cardiovascular disease or took any prescription medication during the study period.
Table 1.
Clinical characteristics of male participants
| Clinical Parameter | Baseline | Post-training | P-value |
|---|---|---|---|
| Age (years) | 19.1 ± 0.6 | N/A | N/A |
| Height (cm) | 191.2 ± 8.9 | N/A | N/A |
| Body mass (kg) | 90.1 ± 8.6 | 89.3 ± 9.4 | 0.84 |
| Heart rate (beats min−1) | 62 ± 7 | 54 ± 6 | 0.01 |
| Systolic blood pressure (mmHg) | 118 ± 9 | 119 ± 11 | 0.71 |
| Diastolic blood pressure (mmHg) | 64 ± 9 | 54 ± 7 | 0.008 |
Values are mean ± SD
Exercise training and cardiorespiratory fitness
Subjects had performed 5.9 ± 1.2 h week−1 of exercise during the 8 weeks prior to enrolment. During the sustained exercise training study period, exercise training volume increased significantly to 13.1 ± 0.9 h week−1 (P < 0.001) and comprised largely endurance activity (11.9 ± 1.1 h week−1). As anticipated, this training regimen was associated with significant decreases in both resting heart rate and resting diastolic blood pressure. Selected exercise testing parameters at baseline and post-training are shown in Table 2. Peak oxygen consumption ( ) and peak workload increased significantly after sustained exercise training. In addition, oxygen consumption and workload at the ventilatory threshold were increased after exercise testing. There were no significant changes in peak exercise heart rate or blood pressure after training.
) and peak workload increased significantly after sustained exercise training. In addition, oxygen consumption and workload at the ventilatory threshold were increased after exercise testing. There were no significant changes in peak exercise heart rate or blood pressure after training.
Table 2.
Parameters of exercise testing
| Parameter | Baseline | Post-training | P-value |
|---|---|---|---|
| Peak heart rate (beats min−1) | 198 ± 16 | 192 ± 14 | 0.37 |
| Peak systolic blood pressure (mmHg) | 175 ± 12 | 173 ± 16 | 0.74 |
| Peak diastolic blood pressure (mmHg) | 86 ± 8 | 80 ± 10 | 0.08 |
| Peak work load (W) | 360 ± 40 | 440 ± 50 | <0.001 |
| Peak O2 consumption (ml min−1) | 4790 ± 600 | 6070 ± 700 | <0.001 |
| Indexed peak O2 consumption (ml min−1 kg−1) | 52 ± 7 | 66 ± 8 | <0.001 |
| Work load at VT (W) | 224 ± 21 | 259 ± 19 | 0.003 |
| O2 consumption at VT (ml min−1) | 3210 ± 240 | 3610 ± 440 | 0.01 |
| Indexed O2 consumption at VT (ml min−1 kg−1) | 35 ± 3 | 40 ± 5 | 0.01 |
Values are mean ± SD. VT: ventilatory threshold
Baseline: plasma levels of candidate microRNAs are detectable at rest
Based on their known functions in angiogenesis, muscle contractility and hypoxia/ischaemic adaptation, a set of eight c-miRNAs were chosen for comparative analysis (see Methods, Fig. 1). At baseline and under resting conditions, steady-state levels of all selected c-miRNAs were detectable in circulating plasma and displayed a relative range of low (miR-133a, miR-328), medium (miR-146a, miR-221, miR-222, miR-210), and high (miR-20a, miR-21) expression (Fig. 2), which approximates previous studies of healthy, untrained individuals (Mitchell et al. 2008). Upon comparing relative c-miRNA levels among athletes, baseline steady-state levels displayed a modest degree of variability, which is consistent with normal variation previously reported in other human patient cohorts (Fichtlscherer et al. 2010). Importantly, none of the selected c-miRNAs displayed an excessively wide range of variability, thus making unlikely any technical confounders that have been reported in plasma processing or c-miRNA quantification (McDonald et al. 2011). Overall, such consistency of expression at baseline conditions is noteworthy and potentially reflects the intrinsic specificity of regulation of each of these molecules at steady-state, even among c-miRNAs that affect similar functions (Fig. 1). Consequently, we would expect that subsequent change in c-miRNA expression in any individual athlete likely reflects a distinct and relevant regulatory process.
Figure 2. Baseline expression levels of c-miRNAs in plasma.

At baseline resting conditions prior to initiation of the controlled study period, c-miRNA levels in plasma were measured in 10 athletes (n = 10) by RT-QPCR and are displayed as relative levels based on the formula (2−ΔCt× 104). All c-miRNAs chosen for analysis are detectable and display low (miR-133a and miR-328), medium (miR-146a, miR-221, miR-222 and miR-210), or high (miR-20a and miR-21) expression at baseline. Data are presented as statistical means, and error bars show SEM.
Baseline: plasma levels of microRNA are up-regulated after acute exercise
Circulating plasma levels of miR-146a (3.00 ± 0.71-fold change, mean ± SEM), miR-222 (2.46 ± 0.41-fold change), miR-21 (1.89 ± 0.28-fold change), and miR-221 (3.58 ± 0.89-fold change) were significantly up-regulated immediately following peak acute exercise (Fig. 3A–D). In the cases of miR-146a, miR-222 and miR-21, these levels significantly decreased after 1 h of rest following acute exercise (Supplemental Fig. 1), suggesting that these dynamic changes reflect a true training response to exercise. In the case of miR-221, a similar trend of down-regulation was observed. In contrast, miR-20a, miR-328, miR-210 and miR-133a displayed no significant changes in expression immediately following exhaustive exercise (Fig. 3E–H).
Post-training: plasma levels of microRNA are up-regulated at rest following sustained exercise training
After 90 days of sustained exercise training, plasma levels of several c-miRNAs displayed statistically significant elevation at rest as compared with each subject's resting baseline value. These included miR-146a (3.05 ± 0.62-fold change), miR-222 (2.40 ± 0.42-fold change), miR-21 (2.63 ± 0.43-fold change) and miR-221 (5.77 ± 1.93-fold change) (Fig. 3A–D). Notably, these were the same c-miRNAs that increased immediately after acute exercise prior to training. Furthermore, levels of miR-20a displayed a trend towards elevation at rest after sustained exercise training (3.01 ± 0.89-fold change, P = 0.0512) (Fig. 3E). In contrast, levels of miR-328, miR-210 and miR-133a were not significantly different in the post-training resting condition (Fig. 3F–H).
Post-training: differential regulatory patterns of plasma levels of microRNA after acute exercise following sustained exercise training
Acute exercise at the post-season time point induced additional increases of miR-146a and miR-222 in circulating plasma that were significantly higher than those observed in the resting baseline (miR-146a, 7.50 ± 1.48-fold change; miR-222, 4.00 ± 0.52-fold change) and higher than those observed in the resting post-training state (Fig. 3A and B). In contrast, neither miR-21 (3.16 ± 0.70-fold change) nor miR-221 (7.26 ± 1.42-fold change), both of which were increased immediately after acute exercise prior to training and at rest following sustained aerobic exercise training, were significantly further affected by an exhaustive exercise bout following sustained training (Fig. 3C and D). Thus, the inducibility of miR-21 and miR-221 by an acute exercise challenge was blunted by prior sustained exercise training and may suggest a maximal ceiling for extracellular expression of these specific c-miRNAs.
Changes in plasma levels of miR-20a followed an alternative pattern (Fig. 3E). Both before and after sustained exercise training, miR-20a levels were not consistently affected by acute exercise when compared to the correlating resting state. However, after sustained exercise training, levels of miR-20a during the resting state (3.01 ± 0.89-fold change, P = 0.0512) and immediately after exercise (3.14 ± 0.56-fold change, P = 0.0042) were similar and, on average, higher than levels at resting pre-season baseline state. Thus, miR-20a appears to represent a c-miRNA that is up-regulated by sustained exercise training but not affected by acute exercise.
Taken together, these data demonstrate distinct and specific patterns of regulation. These can be categorized into four major profiles: (1) responsive to acute exercise both before and after sustained exercise training (miR-146a, Fig. 3A; and miR-222, Fig. 3B), (2) responsive to acute exercise before but not after sustained exercise training (miR-21, Fig. 3C; and miR-221, Fig. 3D), (3) responsive to sustained exercise training but not acute exercise (miR-20a, Fig. 3E), and (4) non-responsive (miR-328, miR-210, miR-133a, Fig. 3F–H).
Linear correlation between the expression of c-miRNA and peak oxygen consumption
To explore the feasibility of using c-miRNAs as biomarkers of exercise capacity and adaptation, the association of specific changes in c-miRNA was examined in relation to peak oxygen consumption ( ). In particular, miR-146a displayed directionally consistent increases during acute exercise in nearly every participant before (9 of 10 athletes) and in every participant after (10 of 10 athletes) sustained aerobic training (Fig. 4A). We therefore assessed the relationship between absolute peak exercise miR-146a level and peak oxygen consumption (
). In particular, miR-146a displayed directionally consistent increases during acute exercise in nearly every participant before (9 of 10 athletes) and in every participant after (10 of 10 athletes) sustained aerobic training (Fig. 4A). We therefore assessed the relationship between absolute peak exercise miR-146a level and peak oxygen consumption ( ) and observed a significant linear correlation (r = 0.63, P = 0.003; Fig. 4B). Thus, miR-146a may represent a quantitative, plasma-based marker of cardiorespiratory fitness and peak exercise capacity. Similarly, resting state levels of miR-20a increased in a directionally consistent manner in most participants (7 of 10 athletes) after sustained aerobic training as compared to pre-training resting levels (Fig. 4C). The strong linear correlation between change in resting miR-20a and change in
) and observed a significant linear correlation (r = 0.63, P = 0.003; Fig. 4B). Thus, miR-146a may represent a quantitative, plasma-based marker of cardiorespiratory fitness and peak exercise capacity. Similarly, resting state levels of miR-20a increased in a directionally consistent manner in most participants (7 of 10 athletes) after sustained aerobic training as compared to pre-training resting levels (Fig. 4C). The strong linear correlation between change in resting miR-20a and change in 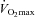 (r = 0.73, P = 0.02; Fig. 4D) suggests a potential role for this c-miRNA as a marker of training-induced changes in cardiorespiratory fitness and exercise adaptation.
(r = 0.73, P = 0.02; Fig. 4D) suggests a potential role for this c-miRNA as a marker of training-induced changes in cardiorespiratory fitness and exercise adaptation.
Figure 4. Alterations in specific c-miRNAs directly correlate with changes in peak oxygen consumption.

For each athlete, baseline c-miRNA levels under resting condition are assigned a fold change of 1, to which measurements obtained during subsequent study time points are compared. Scatter plots display circulating levels of miR-146a (A) and miR-20a (C) for each participant at 4 study time points (i.e. rest vs. 1 min post-exercise (post-ex) during baseline and post-training stages). A direct correlation (r = correlation coefficient) is observed between peak exercise levels of miR-146a (baseline and post-training) and peak oxygen consumption,  (baseline and post-training) (B). A direct correlation is also observed between changes in resting levels of miR-20a (baseline vs. post-training, %Δ in miR-20a) and changes in peak oxygen consumption (baseline vs. post-training, %Δ in
(baseline and post-training) (B). A direct correlation is also observed between changes in resting levels of miR-20a (baseline vs. post-training, %Δ in miR-20a) and changes in peak oxygen consumption (baseline vs. post-training, %Δ in 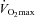 ) (D).
) (D).
Discussion
We report the induction of unique signatures of c-miRNAs in response to acute exhaustive exercise and sustained aerobic exercise training. Using a prospective, longitudinal study design with repeated quantitative assessment of exercise capacity and specific c-miRNA, we have demonstrated that certain plasma-based c-miRNAs are significantly up-regulated in the setting of volitional human exercise. Our data indicate that c-miRNA induction is a heterogeneous process in which changes in individual c-miRNAs conform to several specific profiles in response to: (1) acute exercise only, (2) sustained exercise training only, and (3) acute and sustained exercise training. In turn, such regulation of specific c-miRNAs (i.e. miR-146a and miR-20a) appears to quantitatively correlate with peak exercise capacity and cardiorespiratory fitness. These observations suggest a potential role for c-miRNAs as biomarkers of exercise physiology and provide novel insight into the potential regulatory role of c-miRNA in the myriad physiological processes that accompany exercise.
To date, little is known about the regulation and function of c-miRNAs during aerobic exercise. Prior studies examining skeletal muscle in mice (Safdar et al. 2009; Aoi et al. 2010) and in humans before and after maximal aerobic (Nielsen et al. 2010) or resistance exercise training (Davidsen et al. 2011) demonstrated exercise-dependent up-regulation of intramuscular miRNAs. Another recent study documented changes in neutrophil-specific miRNAs after aerobic exertion in non-trained individuals (Radom-Aizik et al. 2010) suggesting alterations of gene expression in inflammatory cell in response to exercise (Wessner et al. 2010). Results from the present study are unique in several ways. First, to our knowledge, this represents the sentinel report documenting significant changes in circulating miRNAs in response to exercise. Second, our data demonstrate that these changes may occur in response either to a single acute bout of exhaustive exercise or to sustained exercise training. Thus, these molecules appear integrally linked to both the initial stresses and the longer term adaptations that are characteristic of exercise. Finally, given the unique patterns of regulation that are described (Fig. 3), our data reveal an intricate specificity in the c-miRNA response to exercise. In correlation, prior studies have demonstrated that the expression of a number of validated and predicted gene targets of these miRNAs are down-regulated after acute exercise. For example, in the case of miR-221 and miR-222, decreased expression of their direct target, the cell cycle regulator p27/KIP1, has been observed in skeletal muscle after acute resistance exercise (Roberts et al. 2010) and carries potentially critical implications for angiogenesis. Similarly, decreased skeletal muscle expression of the miR-21-specific targets PTEN and PDCD4 has been reported in rodent studies of acute exercise (Liu et al. 2010), with potentially important effects on muscle hypertrophy. In the case of miR-20a, expression of its target, the cell cycle regulator p21/WAF1, is also affected by resistance exercise (Bickel et al. 2005; Drummond et al. 2008), but the exact nature of this regulation is not clear. Given our observation of an increase of miR-20a only after sustained training (Fig. 3), it would be interesting if expression of p21/WAF1 correspondingly decreased, thus revealing a potential molecular pathway for adaptive responses seen in endurance training. Finally, while less is known regarding the molecular changes associated with inflammatory cells during exercise, certain predicted targets of miR-146a (Lewis et al. 2005) relevant to the inflammatory response such as CD80 (Lancaster et al. 2005) and GLUT3 (Stuart et al. 2001) are similarly down-regulated during acute exercise. In aggregate, our findings lend support to the notion of deliberate roles of each c-miRNA to coordinate the physiological adaptation to exercise and thus may be key factors for the integration of system-wide adaptation.
Mechanistic conclusions regarding the regulation of c-miRNAs by exercise are beyond the scope of this study but deserve mention. The rapid up-regulation of c-miRNAs in the setting of acute exercise (i.e. release of plasma c-miRNA after 10–15 min of incremental exercise) is unlikely to be explained by de novo c-miRNA transcription. More plausible mechanisms may include post-transcriptional processing of pre-existing, but inactive, premature miRNA forms (previously described for miR-21; Davis et al. 2008) or rapid increases in the cellular secretion or excretion of specific intracellular miRNA. In contrast, up-regulation after chronic exercise training may rely upon modulation of either transcriptional or post-transcriptional processing of intracellular miRNA. Non-specific release of miRNA from peripheral muscle tissue may also contribute to increases in c-miRNA levels, as strenuous and/or eccentric aerobic exercise can acutely cause microscopic damage to muscle cells (Clarkson, 1997). However, the contribution of tissue damage to c-miRNA levels does not appear to be significant in the present study, given that levels of miR-133a, a muscle-specific miRNA that is up-regulated by exercise in the intracellular space (Nielsen et al. 2010), remain stable with both acute exercise and sustained aerobic exercise training. Finally, although we did not directly measure plasma volume, it is unlikely that plasma volume contraction (haemoconcentration during acute exhaustive exercise) or plasma volume expansion (haemodilution during sustained exercise training) contributed significantly to our findings for two reasons. First, the heterogeneous behaviour across c-miRNA molecules suggests that simple shifts in plasma volumes cannot be explanatory. Second, we observed changes in c-miRNA levels of a magnitude that far exceed the typical changes in plasma volume associated with acute exhaustive exercise (Convertino et al. 1981) and chronic endurance training (Convertino et al. 1980).
The exact cellular sources of exercise-induced c-miRNAs remain similarly unclear. A variety of tissue types relevant to exercise (skeletal, myocardial and smooth muscle; vascular endothelium; and plasma-based platelets and leukocytes) can release c-miRNA into the extracellular space, including the plasma (Kosaka et al. 2010a). Based on prior work examining tissues that are particularly active in modulating the functions of c-miRNAs in plasma (Zhang et al. 2010), vascular endothelium and blood-borne cells represent attractive candidates as sources of exercise-induced c-miRNAs. Specifically, vascular endothelial cells are capable of releasing high levels of some of the specific c-miRNAs that we observe in the present study (i.e. miR-21, miR-146a, miR-221 and miR-222) (S. Y. Chan, unpublished observations, 2010). In correlation, many of these c-miRNAs have been shown to be important mediators of angiogenesis in skeletal and cardiac muscle, which is a key aspect of exercise adaptation.
Findings from this study set the stage for important areas of future work. First, c-miRNAs show promise as biomarkers capable of defining the magnitude of stress and adaptation caused by exercise. Subsequent studies are needed to explore this concept through examining c-miRNAs across diverse populations exposed to exercise stimuli of variable type, duration and intensity. Expanding such analysis to additional c-miRNAs is also important to better define the complete c-miRNA signature relevant to exercise and differentiate these changes from those seen in human disease conditions that involve similar c-miRNA molecules (Kosaka et al. 2010a). Second, characterization of structural and functional tissue adaptation coupled to simultaneous c-miRNA profiling will be necessary to clarify the biological activity and importance of c-miRNAs in the setting of exercise. It is plausible that c-miRNAs are focally released and transported to distant tissue, thus allowing for a novel endocrine function that can integrate the physiological response to exercise at the level of the whole organism. Third, and perhaps most intriguingly, c-miRNAs may ultimately serve as therapeutic targets, tailored to improve exercise capacity in patients with specific pathophysiological conditions, such as arterial insufficiency in which targeted angiogenesis may have important results.
Limitations of this study should be acknowledged. First, our exercise training stimulus involved team-based rowing training which was determined by team coaches and not prescribed by the investigators. Thus, the volume or intensity of the stimulus could not be controlled. However, great care was taken to document training volume and to perform quantitative assessment of exercise capacity before and after training, as an indication of changes in fitness. Second, to maximize the precision by which this exercise protocol was implemented, the sample size of this pilot study was limited to 10 participants. Future inclusion of additional subjects and additional training protocols in less trained subjects are planned. Nonetheless, despite the small sample size, the use of a longitudinal study design with repeated measures allowed each individual to serve as his own control and afforded substantial rigor in our interpretation of these novel trends in c-miRNA expression. Third, to maximize the accuracy of this initial ‘proof-of-concept’ study, careful quantitative analysis was restricted to a subset of eight relevant c-miRNAs rather than to attempt to utilize more comprehensive, but perhaps less reliable, high-throughput screening of all c-miRNAs. In light of these data, additional c-miRNAs are being studied to obtain a more complete and likely more complex profile of regulation. Finally, the current study examines young, healthy endurance athletes with significant previous exercise exposure. Informed conclusions regarding the impact of exercise on c-miRNAs cannot be extrapolated to untrained, sedentary, or diseased populations at this time.
In summary, we report unique and dynamic alterations in the levels of c-miRNAs following acute exhaustive exercise before and after sustained aerobic exercise training. Future studies will be required to define their potential use as biomarkers of exercise training and to better understand their mechanistic role in the control physiological exercise adaptation.
Acknowledgments
We thank J. Loscalzo (fruitful discussions); S. K. Chan (critical reading of the manuscript); and S. Tribuna (administrative assistance). This work was supported by the American Society of Echocardiography and the American Heart Association grant 09FTF2220328 (A.L.B) and by the American Heart Association (grant 0825906D), NIH (K08), and the Pulmonary Hypertension Association (S.Y.C.). The authors have no conflicts of interest to report.
Glossary
Abbreviations
- c-miRNA
circulating microRNA
- miRNA
microRNA
Author contributions
The prescribed exercise protocol was performed and patient samples were obtained in the laboratory of Dr. Aaron Baggish. RNA extraction and miRNA analyses were performed in the laboratory of Dr. Stephen Chan. Individual author contributions were as follows: conception and design of the experiments (A.L.B. and S.Y.C.); collection, analysis and interpretation of data (A.L.B., S.Y.C., A.H., R.B.W., G.D.L., D.S., F.W., T.J.W.); drafting the article and revising it critically for important intellectual content (A.L.B. and S.Y.C.). All authors approved the final version.
Supplementary material
References
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Aoi W, Naito Y, Mizushima K, Takanami Y, Kawai Y, Ichikawa H, Yoshikawa T. The microRNA miR-696 regulates PGC-1α in mouse skeletal muscle in response to physical activity. Am J Physiol Endocrinol Metab. 2010;298:E799–806. doi: 10.1152/ajpendo.00448.2009. [DOI] [PubMed] [Google Scholar]
- Baggish AL, Wang F, Weiner RB, Elinoff JM, Tournoux F, Boland A, Picard MH, Hutter AM, Jr, Wood MJ. Training-specific changes in cardiac structure and function: a prospective and longitudinal assessment of competitive athletes. J Appl Physiol. 2008;104:1121–1128. doi: 10.1152/japplphysiol.01170.2007. [DOI] [PubMed] [Google Scholar]
- Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol. 1986;60:2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- Bickel CS, Slade J, Mahoney E, Haddad F, Dudley GA, Adams GR. Time course of molecular responses of human skeletal muscle to acute bouts of resistance exercise. J Appl Physiol. 2005;98:482–488. doi: 10.1152/japplphysiol.00895.2004. [DOI] [PubMed] [Google Scholar]
- Care A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, Elia L, Latronico MV, Hoydal M, Autore C, Russo MA, Dorn GW, 2nd, Ellingsen O, Ruiz-Lozano P, Peterson KL, Croce CM, Peschle C, Condorelli G. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007;13:613–618. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- Chan SY, Loscalzo J. MicroRNA-210: A unique and pleiotropic hypoxamir. Cell Cycle. 2010;9:1072–1083. doi: 10.4161/cc.9.6.11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SY, Zhang YY, Hemann C, Mahoney CE, Zweier JL, Loscalzo J. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab. 2009;10:273–284. doi: 10.1016/j.cmet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL, Wang DZ. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38:228–233. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Zhu P, Yang J, Liu X, Dong S, Wang X, Chun B, Zhuang J, Zhang C. Ischaemic preconditioning-regulated miR-21 protects heart against ischaemia/reperfusion injury via anti-apoptosis through its target PDCD4. Cardiovasc Res. 2010;87:431–439. doi: 10.1093/cvr/cvq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson PM. Eccentric exercise and muscle damage. Int J Sports Med. 1997;18(Suppl 4):S314–317. doi: 10.1055/s-2007-972741. [DOI] [PubMed] [Google Scholar]
- Convertino VA, Brock PJ, Keil LC, Bernauer EM, Greenleaf JE. Exercise training-induced hypervolemia: role of plasma albumin, renin, and vasopressin. J Appl Physiol. 1980;48:665–669. doi: 10.1152/jappl.1980.48.4.665. [DOI] [PubMed] [Google Scholar]
- Convertino VA, Keil LC, Bernauer EM, Greenleaf JE. Plasma volume, osmolality, vasopressin, and renin activity during graded exercise in man. J Appl Physiol. 1981;50:123–128. doi: 10.1152/jappl.1981.50.1.123. [DOI] [PubMed] [Google Scholar]
- Dang CV. Rethinking the Warburg effect with Myc micromanaging glutamine metabolism. Cancer Res. 2010;70:859–862. doi: 10.1158/0008-5472.CAN-09-3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidsen PK, Gallagher IJ, Hartman JW, Tarnopolsky MA, Dela F, Helge JW, Timmons JA, Phillips SM. High responders to resistance exercise training demonstrate differential regulation of skeletal muscle microRNA expression. J Appl Physiol. 2011;110:309–317. doi: 10.1152/japplphysiol.00901.2010. [DOI] [PubMed] [Google Scholar]
- Davidson-Moncada J, Papavasiliou FN, Tam W. MicroRNAs of the immune system: roles in inflammation and cancer. Ann N Y Acad Sci. 2010;1183:183–194. doi: 10.1111/j.1749-6632.2009.05121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454:56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dews M, Homayouni A, Yu D, Murphy D, Sevignani C, Wentzel E, Furth EE, Lee WM, Enders GH, Mendell JT, Thomas-Tikhonenko A. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat Genet. 2006;38:1060–1065. doi: 10.1038/ng1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MJ, Fujita S, Abe T, Dreyer HC, Volpi E, Rasmussen BB. Human muscle gene expression following resistance exercise and blood flow restriction. Med Sci Sports Exerc. 2008;40:691–698. doi: 10.1249/MSS.0b013e318160ff84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasanaro P, D'Alessandra Y, Di Stefano V, Melchionna R, Romani S, Pompilio G, Capogrossi M, Martelli F. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem. 2008;283:15878–15883. doi: 10.1074/jbc.M800731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Roxe T, Muller-Ardogan M, Bonauer A, Zeiher AM, Dimmeler S. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107:677–684. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- Gupta SK, Bang C, Thum T. Circulating microRNAs as biomarkers and potential paracrine mediators of cardiovascular disease. Circ Cardiovasc Genet. 2010;3:484–488. doi: 10.1161/CIRCGENETICS.110.958363. [DOI] [PubMed] [Google Scholar]
- Gute D, Fraga C, Laughlin MH, Amann JF. Regional changes in capillary supply in skeletal muscle of high-intensity endurance-trained rats. J Appl Physiol. 1996;81:619–626. doi: 10.1152/jappl.1996.81.2.619. [DOI] [PubMed] [Google Scholar]
- Heneghan HM, Miller N, Kerin MJ. MiRNAs as biomarkers and therapeutic targets in cancer. Curr Opin Pharmacol. 2010;10:543–550. doi: 10.1016/j.coph.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell. 2010;39:493–506. doi: 10.1016/j.molcel.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling KH, Pilstrom L, Karlsson J, Piehl K. Mitochondrial volume in skeletal muscle from young and old physically untrained and trained healthy men and from alcoholics. Clin Sci. 1973;44:547–554. doi: 10.1042/cs0440547. [DOI] [PubMed] [Google Scholar]
- Kosaka N, Iguchi H, Ochiya T. Circulating microRNA in body fluid: a new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 2010a;101:2087–2092. doi: 10.1111/j.1349-7006.2010.01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010b;285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krichevsky AM, Gabriely G. miR-21: a small multi-faceted RNA. J Cell Mol Med. 2009;13:39–53. doi: 10.1111/j.1582-4934.2008.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehbacher A, Urbich C, Zeiher A, Dimmeler S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res. 2007;101:59–68. doi: 10.1161/CIRCRESAHA.107.153916. [DOI] [PubMed] [Google Scholar]
- Lancaster GI, Khan Q, Drysdale P, Wallace F, Jeukendrup AE, Drayson MT, Gleeson M. The physiological regulation of toll-like receptor expression and function in humans. J Physiol. 2005;563:945–955. doi: 10.1113/jphysiol.2004.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laterza OF, Lim L, Garrett-Engele PW, Vlasakova K, Muniappa N, Tanaka WK, Johnson JM, Sina JF, Fare TL, Sistare FD, Glaab WE. Plasma MicroRNAs as sensitive and specific biomarkers of tissue injury. Clin Chem. 2009;55:1977–1983. doi: 10.1373/clinchem.2009.131797. [DOI] [PubMed] [Google Scholar]
- Lewis B, Burge C, Bartel D. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Liu G, Keeler BE, Zhukareva V, Houle JD. Cycling exercise affects the expression of apoptosis-associated microRNAs after spinal cord injury in rats. Exp Neurol. 2010;226:200–206. doi: 10.1016/j.expneurol.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JS, Milosevic D, Reddi HV, Grebe SK, Algeciras-Schimnich A. Analysis of circulating microRNA: preanalytical and analytical challenges. Clin Chem. 2011 doi: 10.1373/clinchem.2010.157198. [DOI] [PubMed] [Google Scholar]
- Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S, Scheele C, Yfanti C, Akerstrom T, Nielsen AR, Pedersen BK, Laye M. Muscle specific microRNAs are regulated by endurance exercise in human skeletal muscle. J Physiol. 2010;588:4029–4037. doi: 10.1113/jphysiol.2010.189860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, de Gruijl TD, Wurdinger T, Middeldorp JM. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A. 2010;107:6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliseno L, Tuccoli A, Mariani L, Evangelista M, Citti L, Woods K, Mercatanti A, Hammond S, Rainaldi G. MicroRNAs modulate the angiogenic properties of HUVECs. Blood. 2006;108:3068–3071. doi: 10.1182/blood-2006-01-012369. [DOI] [PubMed] [Google Scholar]
- Radom-Aizik S, Zaldivar F, Jr, Oliver S, Galassetti P, Cooper DM. Evidence for microRNA involvement in exercise-associated neutrophil gene expression changes. J Appl Physiol. 2010;109:252–261. doi: 10.1152/japplphysiol.01291.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MD, Dalbo VJ, Hassell SE, Brown R, Kerksick CM. Effects of preexercise feeding on markers of satellite cell activation. Med Sci Sports Exerc. 2010;42:1861–1869. doi: 10.1249/MSS.0b013e3181da8a29. [DOI] [PubMed] [Google Scholar]
- Safdar A, Abadi A, Akhtar M, Hettinga BP, Tarnopolsky MA. miRNA in the regulation of skeletal muscle adaptation to acute endurance exercise in C57Bl/6J male mice. PloS One. 2009;4:e5610. doi: 10.1371/journal.pone.0005610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart CA, Wen G, Williamson ME, Jiang J, Gilkison CR, Blackwell SJ, Nagamani M, Ferrando AA. Altered GLUT1 and GLUT3 gene expression and subcellular redistribution of GLUT4: protein in muscle from patients with acanthosis nigricans and severe insulin resistance. Metabolism. 2001;50:771–777. doi: 10.1053/meta.2001.24202. [DOI] [PubMed] [Google Scholar]
- Suárez Y, Fernández-Hernando C, Pober J, Sessa W. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res. 2007;100:1164–1173. doi: 10.1161/01.RES.0000265065.26744.17. [DOI] [PubMed] [Google Scholar]
- Taganov K, Boldin M, Chang K, Baltimore D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signalling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc Res. 2008;79:581–588. doi: 10.1093/cvr/cvn156. [DOI] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- van Rooij E, Olson E. MicroRNAs: powerful new regulators of heart disease and provocative therapeutic targets. J Clin Invest. 2007;117:2369–2376. doi: 10.1172/JCI33099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CH, Lee DY, Deng Z, Jeyapalan Z, Lee SC, Kahai S, Lu WY, Zhang Y, Yang BB. MicroRNA miR-328 regulates zonation morphogenesis by targeting CD44 expression. PloS One. 2008;3:e2420. doi: 10.1371/journal.pone.0002420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessner B, Gryadunov-Masutti L, Tschan H, Bachl N, Roth E. Is there a role for microRNAs in exercise immunology? A synopsis of current literature and future developments. Exerc Immunol Rev. 2010;16:22–39. [PubMed] [Google Scholar]
- Williams AH, Liu N, van Rooij E, Olson EN. MicroRNA control of muscle development and disease. Curr Opin Cell Biol. 2009;21:461–469. doi: 10.1016/j.ceb.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C( MicroRNAs in vascular biology and vascular disease. J Cardiovasc Transl Res. 2010;3:235–240. doi: 10.1007/s12265-010-9164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu D, Chen X, Li J, Li L, Bian Z, Sun F, Lu J, Yin Y, Cai X, Sun Q, Wang K, Ba Y, Wang Q, Wang D, Yang J, Liu P, Xu T, Yan Q, Zhang J, Zen K, Zhang CY. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell. 2010;39:133–144. doi: 10.1016/j.molcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


