Non-technical summary
What triggers a realignment of sensations, e.g. a stimulus that is perceived as non-painful in intact skin, but evokes pain in sunburned skin, is yet to be ascertained. This phenomenon is clinically termed allodynia. We show that gentle tactile stimulation (vibration and brushing) of the hairy skin can exacerbate the underlying muscle pain (allodynia) evoked by infusion of hypertonic saline into the tibialis anterior muscle. This effect is dependent upon a low-threshold, mechanosensitive class of nerve fibres in the hairy skin known as C-tactile (CT) fibres. Knowledge of the role of CT fibres in allodynia increases our understanding of the mechanisms that underlie sensory-perceptual abnormalities – a common manifestation of clinical-pain states and neurological disorders.
Abstract
Abstract
We recently showed a contribution of low-threshold cutaneous mechanoreceptors to vibration-evoked changes in the perception of muscle pain. Neutral-touch stimulation (vibration) of the hairy skin during underlying muscle pain evoked an overall increase in pain intensity, i.e. allodynia. This effect appeared to be dependent upon cutaneous afferents, as allodynia was abolished by intradermal anaesthesia. However, it remains unclear whether allodynia results from activation of a single class of cutaneous afferents or the convergence of inputs from multiple classes. Intriguingly, no existing human study has examined the contribution of C-tactile (CT) afferents to allodynia. Detailed psychophysical observations were made in 29 healthy subjects (18 males and 11 females). Sustained muscle pain was induced by infusing hypertonic saline (HS: 5%) into tibialis anterior muscle (TA). Sinusoidal vibration (200 Hz–200 μm) was applied to the hairy skin overlying TA. Pain ratings were recorded using a visual analogue scale (VAS). In order to evaluate the role of myelinated and unmyelinated cutaneous afferents in the expression of vibration-evoked allodynia, compression block of the sciatic nerve, and low-dose intradermal anaesthesia (Xylocaine 0.25%) were used, respectively. In addition, the modulation of muscle pain by gentle brushing (1.0 and 3.0 cm s−1) – known to excite CT fibres – was examined. Brushing stimuli were applied to the hairy skin with all fibres intact and following the blockade of myelinated afferents. During tonic muscle pain (VAS 4–6), vibration evoked a significant and reproducible increase in muscle pain (allodynia) that persisted following compression of myelinated afferents. During compression block, the sense of vibration was abolished, but the vibration-evoked allodynia persisted. In contrast, selective anaesthesia of unmyelinated cutaneous afferents abolished the allodynia, whereas the percept of vibration remained unaffected. Furthermore, allodynia was preserved in the adjacent non-anaesthetized skin. Conformingly, gentle brushing produced allodynia (at both brushing speeds) that persisted during the blockade of myelinated afferents. Prior to the induction and following cessation of muscle pain, all subjects reported vibration and brushing as non-painful (VAS = 0). These results demonstrate that CT fibres in hairy skin mediate allodynia, and that CT-mediated inputs have a pluripotent central effect.
Introduction
Within the somatosensory system, a sensory relay is often described in terms of a ‘labelled line’ that links primary afferents with higher-order neurons in the primary somatosensory cortex via modality-specific spinal pathways. It is widely accepted that discriminative touch is mediated exclusively by large-diameter sensory fibres, whereas painful sensations are mediated by small-diameter fibres. Consistent with this view, selective microstimulation of a single large-diameter myelinated afferent in awake human subjects evokes a fundamental, innocuous (non-painful) sensation that has the quality of pressure, flutter or vibration according to the type of primary afferent excited (Ochoa & Torebjork, 1983; Vallbo et al. 1984; Macefield et al. 1990). Furthermore, the vibrotactile modalities are abolished in patients with large-fibre neuropathy (Olausson et al. 2002) and healthy subjects following blockade of large-diameter axons (Mackenzie et al. 1975; Dellon, 1980). By contrast, activation of small-diameter nociceptors in skin evokes sensations that are distinctly painful – sharp-stabbing for Group III fibres (fast pain) and burning for Group IV fibres (slow pain) – whereas activation of either fibre class in muscle produces sensations that are frequently described as dull and aching in quality and difficult to localize (Torebjork et al. 1984a,b; Ochoa & Torebjork, 1989). In addition to cutaneous nociceptors, which have high mechanical thresholds, there is another class of unmyelinated (C) fibre that has low mechanical thresholds. The existence of low-threshold unmyelinated afferents, termed C-mechanoreceptors, which respond to light touch of the skin, was documented long ago in the hairy skin of the cat and monkey (Zotterman, 1939; Maruhashi et al. 1952; Douglas & Ritchie, 1957; Bessou et al. 1971). Although some investigators had suggested that C low-threshold mechanoreceptors (CLTMs) are vestigial (Kumazawa & Perl, 1977), recent studies have reported a class of unmyelinated fibres in the human hairy skin, known as C-tactile (CT) fibres, that responds to innocuous mechanical stimulation (Johansson et al. 1988; Nordin, 1990; Vallbo et al. 1993).
The response properties of CT fibres have been described using a limited range of stimuli – most notably slowly moving, low-force, mechanical stimuli such as finger stroking and soft brushing (Nordin, 1990; Vallbo et al. 1993, 1999; Loken et al. 2009). Likewise, our limited understanding of the contribution of CT fibres to perception warrants further exploration, as our current understanding of their role is based largely on observations in two subjects with large-fibre sensory neuropathy. In these patients the perceptual responses to light mechanical stimuli varied from trial to trial, but on occasion patients reported a faint sense of pleasantness. It is this latter observation, together with the results of neuroimaging studies that have demonstrated that CT-mediated inputs project onto the insular cortex, which has underpinned the proposition of a CT-mediated emotional touch system (Olausson et al. 2002; Cole et al. 2006; McGlone et al. 2007; Olausson et al. 2008). Intriguingly, in healthy subjects gentle brushing – known to elicit CT fibre responses – can evoke a neutral or even unpleasant sensation at the lowest brushing velocities (Loken et al. 2009), suggesting that gentle tactile stimulation can elicit opposing aspects of touch, i.e. predilection and aversion. A contribution of CT fibres to unpleasant touch has been suggested by recent work showing the activation of superficial dorsal horn neurons by gentle brushing of skin (Andrew, 2010; Craig, 2010). Similarly, these fibres have been implicated in touch hypersensitivity after injury in mice (Seal et al. 2009).
In a recent pilot study we found that innocuous tactile stimulation (vibration) of hairy skin intensified the underlying muscle pain (allodynia), and that this effect appeared to be dependent upon cutaneous mechanoreceptors as the allodynia was abolished by intradermal anaesthesia (Nagi et al. 2009). In previous studies, allodynia has often been attributed to the activation of large-diameter tactile afferents (Campbell et al. 1988; Treede & Cole, 1993; Wasner et al. 1999; Maihöfner et al. 2003). Consistent with this view, touch-evoked allodynia has been abolished by compression or ischaemic blockade of large-diameter fibres (Gracely et al. 1992; Torebjork et al. 1992; Koltzenburg et al. 1994; Cervero & Laird, 1996). By contrast, other studies have argued for the involvement of small-diameter nociceptive afferents based on the persistence of allodynia following large-fibre blocks (Cline et al. 1989; Price et al. 1992). Moreover, allodynia can be abolished by anaesthesia of small-diameter presumed nociceptors in patients with ongoing pain (Arner et al. 1990; Gracely et al. 1992; Koltzenburg et al. 1994). Whether there is a contribution of CT fibres to allodynia remains untested.
The ambiguity in the literature about the contribution of different fibre classes to allodynia may be attributed in part to the use of a single-compartment model in which innocuous and noxious stimuli are applied to the same or adjacent regions of skin. Such an approach can lead to uncertainty as to whether any change in pain perception reflects peripheral sensitization of nociceptive fibres and/or an altered central convergence of innocuous and noxious inputs. In order to avoid this ambiguity in the present study we used a two-compartment model. Pain was induced in the tibialis anterior muscle (TA) by infusion of hypertonic saline and a neutral tactile stimulus – a low-amplitude (200 μm) vibration (200 Hz) – applied to the overlying skin. The muscle is physically separated from the skin by sheet-like fascia and each is supplied by separate vascular and nerve supplies (O'Rahilly & Muller, 1986; Berry et al. 1995; Salmons, 1995; Gibson et al. 2009). Within the hairy skin it is known that such low-amplitude vibratory stimuli are preferentially encoded by hair follicle afferents at low frequencies (∼5 Hz to 100 Hz) and by Pacinian corpuscle receptors at high frequencies (∼50 Hz to 1000 Hz: Merzenich & Harrington, 1969; Mahns et al. 2006). Although the response properties of CT fibres to vibratory stimulation remain untested, low-threshold mechanical sensitivity has been demonstrated using soft brushing (Vallbo et al. 1999; Olausson et al. 2002; Loken et al. 2009). Furthermore, it has been shown that the perceptual outcome of gentle brushing corresponds best with the CT-fibre responses indicating the ‘preferential’ nature of the resulting stimulus (Loken et al. 2009). The use of innocuous tactile stimuli, i.e. vibration and brushing, coupled with differential nerve blocks, allowed us to disentangle the affective and sensory components of touch and thus quantify the perceptual response of CT fibres in healthy subjects.
Methods
Twenty-nine healthy human subjects (18 males and 11 females) aged 18–38 years, with no reported musculoskeletal disorders, took part in this study. Informed consent was obtained from each subject in writing. All experiments were approved by the UWS Human Research Ethics Committee and conformed to principles of the Declaration of Helsinki. In experimental Series I, we examined the effect of cutaneous vibration on muscle pain following compression of the myelinated afferents (sciatic nerve). A small amount of non-selective local anaesthetic (Xylocaine 2%) was injected intradermally in order to abolish the residual cutaneous input. In experimental Series II, we recorded the modulation of pain by vibration prior to the selective blockade (Xylocaine 0.25%) of unmyelinated cutaneous afferents, within the anaesthetized skin (C fibres blocked) and in the adjacent, non-anaesthetized skin with all fibres intact. In experimental Series III, we used gentle brushing over the hairy skin, with all fibres intact and following compression of myelinated afferents. Subjects sat comfortably on a chair with both legs supported horizontally for the vibration experiments or vertically for the brushing experiments. The anatomical boundaries of TA were identified by palpation during active inversion of the foot and dorsiflexion of the ankle joint (Gibson et al. 2006).
Hypertonic saline-induced muscle pain
A 23 G butterfly cannula was inserted through the skin into TA (∼6 cm distal to the tibial tuberosity and ∼2 cm lateral to the anterior border of tibia) and connected to an infusion pump (model 55–2226, Harvard Apparatus, Holliston, MA, USA) containing hypertonic saline (HS: 5%). The initial infusion rate was set at 200 μl min−1 and once a peak level of pain had developed the infusion rate was adjusted (where needed) in order to maintain a constant pain rating of 4–6 on a visual analogue scale (VAS). The VAS was divided into 10 equal segments within a range of 0 (no pain) to 10 (worst pain). Beyond these initial adjustments, a stable baseline muscle pain was maintained throughout the duration of HS infusion, i.e. ∼15 min, without further adjustments to the infusion rate. A stable baseline pain for at least 2 min was required prior to the concurrent application of tactile stimuli. Subjects reported pain by rotating a calibrated potentiometer, the signal from which was recorded continuously on a computer.
Cutaneous vibration
A circular Perspex (Plexiglas) probe with a rounded 4 mm diameter tip was placed in gentle contact with the skin overlying TA without compressing the underlying structures. Subjects were asked to ensure there was no discomfort around the site of contact (VAS = 0). The probe was positioned perpendicular to the skin surface at a point distal to the muscle belly and the cannula delivering the hypertonic saline, i.e. ∼15 cm distal to the tibial tuberosity and ∼1.5 cm lateral to the anterior border of tibia. The probe was attached to a feedback controlled sinusoidal mechanical stimulator (seeMahns et al. 2006). The frequency (200 Hz) and amplitude (200 μm) parameters of the stimulus were chosen as being unequivocally innocuous (Merzenich & Harrington, 1969; Mahns et al. 2006; Sahai et al. 2006) and devoid of any affective or emotional quale. The sensory neutrality of vibration was confirmed in each subject at the start of the experiment. Vibration was applied before, during and after the HS-induced muscle pain. During muscle pain, at least three consistent, consecutive vibration-evoked increases in muscle pain (allodynia) were required before progressing with the experiment. The period of vibration lasted 30 s and was repeated at 45 s intervals in order to provide sufficient time between trials and avoid desensitization of the activated fibre classes due to repeated stimulation (Iggo, 1960; Bessou et al. 1971). White noise was delivered through headphones to ensure that auditory cues associated with the mechanical stimulator were not evident to the subject (Merzenich & Harrington, 1969).
Series Ia. Compression of myelinated afferents
In 14 subjects, compression block of the sciatic nerve was used to evaluate the contribution of myelinated afferents to the expression of allodynia. A metal bar was placed just distal to the ischial tuberosity to apply compression to the sciatic nerve. Commencement of the HS infusion was timed to coincide with the preferential blockade of large- and small-diameter, myelinated afferents (Laursen et al. 1999). In the intervening period (45 s) between consecutive vibration trains, selective sensory stimuli were applied to test the progression of nerve block. Myelinated blockade was confirmed by the loss of perception of vibration (20 and 200 Hz; 200 μm; 30 s duration), innocuous cold (∼15°C, brass rod in contact with the skin for 5 s) and pinprick (applied to the skin using a sterile hypodermic needle) stimuli. The integrity of C-fibre inputs was confirmed by the preservation of warm sensibility (∼40°C, brass rod in contact with the skin for 5 s: Mackenzie et al. 1975; Weerakkody et al. 2003). To verify the effectiveness of sciatic nerve compression, somatosensory sensibility was compared with skin regions on the medial aspect of the experimental leg, which is innervated by the femoral nerve (Berry et al. 1995), and the contralateral leg. When performing these tasks, subjects were shielded from visual and auditory cues.
Series Ib. Anaesthesia of residual cutaneous afferents
After recording vibration-evoked effects during the myelinated-fibre block, 0.2–0.4 ml of local anaesthetic (Xylocaine 2%) was injected intradermally into a 2–3 cm area surrounding the vibration probe. This small intradermal injection effectively blocked the residual inputs arising from skin without affecting the muscle nociceptors that mediate HS-induced pain (Arner et al. 1990; Mahns et al. 2006). The blockade of C afferents – the only class of fibres intact during compression of the myelinated axons – in the anaesthetized skin was verified by the abolition of warm sensibility. VAS responses to vibration were recorded within the anaesthetized skin (all fibres blocked) and in the adjacent non-anaesthetized skin (C afferents intact) within the innervation territory of the sciatic nerve to determine whether the pre-anaesthetic vibration-evoked response was preserved.
Series II. Anaesthesia of unmyelinated (C) afferents
This was carried out on a total of 10 subjects, including four subjects who had previously participated in the experiments involving compression of the sciatic nerve. Low-dose intradermal anaesthesia was used to test the contribution of unmyelinated cutaneous afferents to the expression of allodynia. After recording vibration-evoked responses during muscle pain, 0.2 ml of low-dose anaesthetic (Xylocaine 0.25%) was injected intradermally, producing an anaesthetized region of 2–3 cm in diameter around the vibration probe. This low-dose intradermal injection preferentially blocked C afferents (Torebjörk & Hallin, 1973; Mackenzie et al. 1975), verified by loss of perception to innocuous warm stimuli (∼40°C, brass rod). In cases where the warm sensibility was not entirely abolished, an additional injection (0.2 ml) of low-dose anaesthetic was given. The preservation of perceptual responses to movements of hairs, cutaneous vibration (20 and 200 Hz) and cold (∼15°C, brass rod: Mackenzie et al. 1975; Mahns et al. 2006) stimuli was taken as evidence that myelinated afferents were intact. Following confirmation of unmyelinated cutaneous block, vibration-evoked effects were retested within the anaesthetized skin (C afferents blocked) and in the adjacent non-anaesthetized skin with a full complement of sensory fibres.
Series III. Gentle brushing
This was carried out on 11 subjects, including two subjects who had previously participated in experimental Series I and II. A paintbrush (0.7 cm thick and ∼7.5 cm wide) of goat's hair (3.0 cm long) was swept bi-directionally, perpendicular to the skin surface along the anterolateral aspect of leg. The stimulus brush was moved at speeds of 1.0 cm s−1 or 3.0 cm s−1 through a stroke of 10.0 cm plus a 1.0 cm turnaround at each end. The brushing motion was produced using a linear motor on a 3D Gantry system (Baldor Australia Pty Ltd, Seven Hills, NSW, Australia) that was under feedback of a PMAC motion controller (Delta Tau Data Systems, Inc., Chatsworth, CA, USA). These brushing speeds were chosen as CT fibres respond particularly well to these stimuli, which are perceived as pleasant (Loken et al. 2009). The non-noxious nature of the brushing was confirmed in each subject at the start of the experiment. Brushing stimuli were delivered before, during and after the HS-induced muscle pain – with all fibre classes intact and following blockade of myelinated afferents. During muscle pain, VAS responses to brushing were recorded for each of the two brushing speeds. Akin to vibratory stimulation, the period of brushing lasted 30 s and was repeated at 45 s intervals. White noise was delivered through headphones to ensure that auditory cues associated with the brushing system were not provided (Merzenich & Harrington, 1969).
Statistical analysis
Data are presented as means ± standard error of the mean (±SEM). In each individual, the responses to vibration or brushing were expressed as a percentage of the HS-induced muscle pain (Base) observed immediately preceding the superimposition of tactile stimuli (Figs 2–5). Each touch-evoked change in muscle pain was treated as an independent, sequential event. Significant changes were detected using one-way analysis of variance (ANOVA; Zar, 1984). Where significant differences were indicated (P < 0.05), individual groups were compared using a Newman–Keuls multiple comparison test. All statistical comparisons were made using Prism 5 (GraphPad Software Inc., La Jolla, CA, USA).
Figure 2. Vibration-evoked allodynia is not mediated by myelinated tactile afferents.
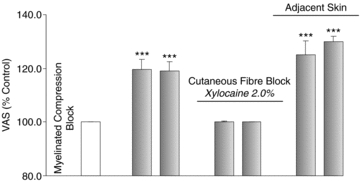
Mean vibration-evoked responses (±SEM; n = 10; grey) were expressed as a percentage of the baseline muscle pain (unshaded). Cutaneous vibration evoked a significant increase in HS-induced muscle pain (allodynia) during compression of myelinated afferents. Allodynia was abolished upon anaesthesia of the residual cutaneous input (C afferents). In the adjacent, non-anaesthetized skin (C afferents intact) within the innervation territory of blocked myelinated afferents, allodynia was preserved. ***P < 0.001.
Figure 5. Allodynia evoked by gentle brushing in absence of myelinated afferents.
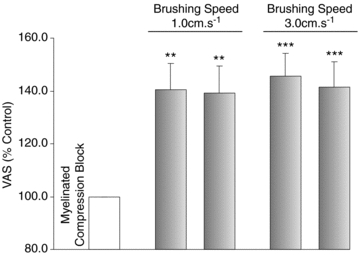
Mean brush-evoked responses (±SEM; n = 5; grey) were expressed as a percentage of the baseline muscle pain (unshaded). Gentle brushing at speeds of 1 cm s−1 and 3 cm s−1 evoked a significant increase in HS-induced muscle pain (allodynia) during compression of myelinated afferents. The expression of allodynia was comparable between the two brushing speeds. Note that scaling is different in this figure. **P < 0.01; ***P < 0.001.
Results
Pain induced by intramuscular infusion of hypertonic saline and concurrent tactile stimulation
In 22 of the 25 subjects where hypertonic saline was infused, a constant level of pain within a VAS range of 4–6 was reported. On average, it took about 3–5 min to attain a stable baseline pain that persisted for the duration of HS infusion. Data from the remaining three subjects were discarded because a constant background level of pain could not be obtained. Prior to the induction of muscle pain, all subjects reported that cutaneous vibration and brushing were not painful (VAS = 0) when applied to the hairy skin. Infusion of hypertonic saline into the TA evoked sensations of a dull ache that radiated distally from the injection site and in 12 subjects extended (referred) beyond the ankle. In the presence of sustained muscle pain, cutaneous vibration and gentle brushing evoked an increase in muscle pain (allodynia). This allodynia was reproducible over time and disappeared upon cessation of the underlying muscle pain. At this point, the vibratory/brushing stimuli were once again described as being non-painful (VAS = 0). Raw data from one subject and mean data of two consecutive vibration-evoked responses are shown in Fig. 1. Gentle brushing evoked a cognate expression of allodynia.
Figure 1. Effect of vibration before, during and after hypertonic saline-induced muscle pain.
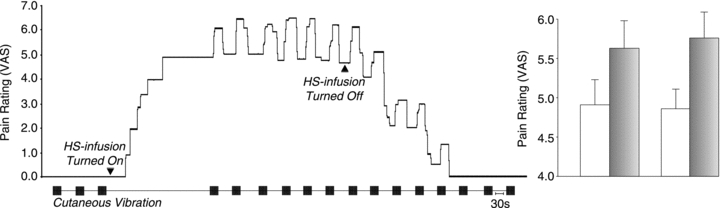
VAS recordings over time of a typical subject (left) and the mean data of successive vibration-evoked responses (±SEM; n = 7; grey: allodynia; unshaded: baseline muscle pain) are shown. Cutaneous vibration (200 Hz–200 μm) evoked a reproducible increase in muscle pain throughout the sustained level of background pain (VAS 4–6), as well as during the decay phase following cessation of hypertonic saline (HS) infusion. Vibration-evoked allodynia ebbed before the onset of subsequent vibration trains. Prior to the initiation and on termination of HS-induced muscle pain, vibration was reported as non-painful (VAS = 0).
Series Ia. Vibration-evoked allodynia persists during compression of myelinated afferents
Compression block of the sciatic nerve was used to test whether allodynia can be evoked in the absence of myelinated afferents. In 4 of the 14 subjects, effective blockade of myelinated afferents could not be achieved, presumably because the metal bar had been placed incorrectly. Hence, hypertonic saline was not used in these subjects. During compression, the time taken to fully block the perceptual responses associated with myelinated afferents varied from ∼30 to 45 min across subjects. The blockade of large- and small-diameter, myelinated afferents was confirmed in each subject by the loss of perception of vibratory, innocuous cold and pinprick stimuli. The integrity of C-fibre inputs was confirmed by the preservation of warm sensibility. The mean data (±SEM) demonstrated that muscle pain increased significantly during vibration (allodynia), was reproducible over time and ebbed before the onset of subsequent periods of vibration (base: 100%; vibration: 119.7 ± 3.8%, 119.1 ± 3.5%; P < 0.001; n = 10). Apart from the vibration-evoked allodynia, there was no percept of vibration during the compression block. No changes in the intensity of the underlying muscle pain (base) or the magnitude of vibration-evoked allodynia were observed (P > 0.05) in the responses to consecutive vibration trains. Mean data (% control) of successive vibration-evoked responses in duplicate sets are shown in Fig. 2.
Series Ib. Vibration-evoked allodynia was abolished by anaesthesia of residual cutaneous afferents
Following compression block, intradermal anaesthetic was used to abolish all residual inputs from the skin. The effectiveness of the block was verified by the abolition of warm sensibility. The baseline pain remained unaffected, confirming that muscle nociceptor afferents were not affected. Following cutaneous anaesthesia, vibration had no effect on the underlying muscle pain, i.e. the allodynia was abolished (base: 100%; vibration: 100.2 ± 0.2%, 100.0 ± 0.0%; P > 0.05; n = 10). In those subjects in whom vibration was applied in the adjacent non-anaesthetized skin (C fibres intact) – within the innervation territory of sciatic afferents – vibration evoked a significant increase (allodynia) in the baseline HS-pain, i.e. allodynia was preserved (base: 100%; vibration: 125.1 ± 5.2%, 129.9 ± 2.0%; P < 0.001; n = 7). Thus, allodynia was evoked regardless of the blockade of myelinated afferents, but was abolished by the inactivation of unmyelinated cutaneous afferents. Conversely, in the adjacent (non-anaesthetized) skin with intact C afferents, allodynia was preserved (ANOVA: F = 21.12, P < 0.0001; Fig. 2).
Series II. Vibration-evoked allodynia mediated by C-tactile afferents
Low-dose intradermal anaesthetic (Xylocaine 0.25%) was used to test whether allodynia persists following the inactivation of unmyelinated fibres. Preferential blockade of C afferents was confirmed by the abolition of warm sensibility and a preserved appreciation of vibration and cold stimuli. The use of intradermal anaesthetic had no perceptible effect on the intensity of baseline muscle pain. Prior to intradermal anaesthesia (all fibres intact), the mean data (±SEM) demonstrate that muscle pain significantly increased during vibration (base: 100%; vibration: 115.0 ± 3.6%, 118.9 ± 4.8%; P < 0.01; n = 7). No changes in the intensity of muscle pain (base) or the magnitude of vibration-evoked allodynia were observed (P > 0.05) in successive vibration trains. Moreover, the vibration-evoked allodynia disappeared before the onset of subsequent vibration trains. Following the blockade of unmyelinated cutaneous afferents, vibration had no effect on the underlying muscle pain, i.e. the allodynia was abolished (base: 100%; vibration: 103.3 ± 3.2%, 100.0 ± 4.9%; P > 0.05; n = 6). In one of the seven subjects, intradermal anaesthetic was not administered due to the emergence of an unstable baseline. Furthermore, in two of the remaining six subjects, an additional injection of low-dose anaesthetic (0.2 ml) was required in order to abolish the warm sensibility. In contrast to the blockade of myelinated afferents where allodynia was preserved but the percept of vibration was abolished, the blockade of unmyelinated cutaneous afferents abolished allodynia while the sense of vibration was unaffected. In the adjacent non-anaesthetized skin, vibration evoked a significant increase in the underlying muscle pain, i.e. the allodynia was preserved (base: 100%; vibration: 122.9 ± 5.8%, 119.0 ± 4.7%; P < 0.001; n = 6). Thus, allodynia was evoked prior to anaesthesia of unmyelinated afferents or in the adjacent skin with intact cutaneous afferents, whereas allodynia was abolished within the anaesthetized skin (ANOVA: F = 8.399, P < 0.0001). Mean data (% control) of sequent vibration-evoked responses in duplicate sets are illustrated in Fig. 3.
Figure 3. Vibration-evoked allodynia is mediated by C-tactile afferents in hairy skin.
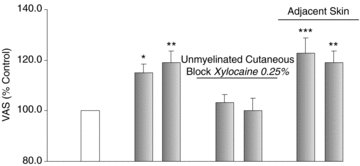
Mean vibration-evoked responses (±SEM; n = 7; grey) were expressed as a percentage of the baseline muscle pain (unshaded). Cutaneous vibration evoked a significant increase in HS-induced muscle pain (allodynia). Allodynia was abolished upon anaesthesia of C afferents in the skin (unmyelinated cutaneous block). In the adjacent, non-anaesthetized skin with a full complement of sensory fibres, allodynia was preserved. *P < 0.05; **P < 0.01; ***P < 0.001.
Series III. Brush-evoked allodynia mediated by C-tactile afferents
Gentle brushing was used to confirm the role of CT fibres in allodynia, given the documented capacity of brushing stimuli for generating a particularly profound response in CT fibres (Loken et al. 2009). Prior to the induction of muscle pain, all subjects described brushing as non-painful (VAS = 0) and devoid of negative affective qualities. By contrast, during muscle pain the mean data (±SEM) demonstrate that brushing at 1 cm s−1 and 3 cm s−1 evoked a significant and reproducible increase in the pain intensity that abated before the onset of subsequent periods of brushing (base: 100%; brushing at 1.0 cm s−1: 127.0 ± 4.1, 126.8 ± 6.0; brushing at 3.0 cm s−1: 125.2 ± 5.2, 126.7 ± 5.5; ANOVA: F = 14.52, P < 0.0001; n = 9). This effect was not dependent upon intact myelinated fibre inputs, as brush-evoked allodynia was preserved, or amplified, following compression block of myelinated afferents (base: 100%; brushing at 1.0 cm s−1: 140.5 ± 9.9, 139.3 ± 10.2; brushing at 3.0 cm s−1: 145.7 ± 8.7, 141.6 ± 9.3; ANOVA: F = 11.06, P < 0.0001; n = 5). No changes in the intensity of muscle pain (base) or the magnitude of brush-evoked allodynia were observed (P > 0.05) in subsequent periods of brushing. Furthermore, the expression of allodynia was comparable between the two brushing speeds. Thus, a gentle tactile stimulus capable of activating CT fibres, in the presence of a background nociceptive input, triggered a crossover between innocuous-touch and noxious-touch, i.e. allodynia. Mean data (% control) of two successive brush-evoked responses at each of the brushing speeds are shown in Figs 4 and 5.
Figure 4. Allodynia evoked by brushing stimuli that elicit C-tactile fibre responses.
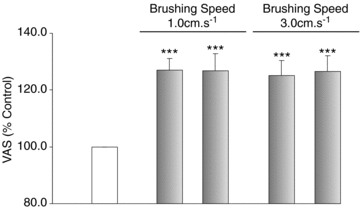
Mean brush-evoked responses (±SEM; n = 9; grey) were expressed as a percentage of the baseline muscle pain (unshaded). Gentle brushing at speeds of 1 cm s−1 and 3 cm s−1 evoked a significant increase in HS-induced muscle pain (allodynia). The expression of allodynia was comparable between the two brushing speeds. Prior to the induction and on cessation of muscle pain, brushing was reported as innocuous. ***P < 0.001.
Discussion
We have shown that innocuous cutaneous vibration can increase the intensity of underlying muscle pain, induced by intramuscular infusion of hypertonic saline, and that this effect (i) persists during compression blockade of myelinated fibres but (ii) is abolished by selective anaesthesia of unmyelinated cutaneous afferents. Thus, vibration-evoked allodynia is evidently dependent upon intact C fibre inputs from the skin, and that these C-fibres have a low mechanical threshold (they responded to 200 μm vibration). The abolition of allodynia was not associated with a decline in the muscle pain or a generalized decline in the capacity of vibration to evoke allodynia, as the vibration-evoked increases in pain were preserved in the adjacent non-anaesthetized skin. Vibration was described as non-painful by all subjects prior to the induction, and following cessation, of muscle pain. Our observations clearly implicate the mechanically sensitive C-tactile (CT) fibres in mediating this vibration-evoked allodynia. In contrast to earlier work, our psychophysical data indicate that the mechanical sensitivity of CT fibres need not be limited to slowly moving stimuli, as allodynia was evoked by vibration following blockade of myelinated afferents. Although the responsiveness of CT fibres to vibratory stimuli remains untested, recent studies have revealed a broader range of response properties, such as delayed acceleration (Vallbo et al. 1999), that may account for the vibrotactile responsiveness shown in this study. To our knowledge, this is the first study that has demonstrated a distinct function attributable to C-tactile fibres in human subjects with a full complement of sensory fibres.
Much of the recent work has focused on CT fibres in the context of an emotional touch system, largely based on the variable reports of neutral or pleasant responses to gentle brushing in two patients with loss of large-fibre function (Olausson et al. 2002, 2008; Cole et al. 2006; McGlone et al. 2007). However, brush stroking is not a CT-specific stimulus and is known to also activate large-diameter tactile afferents (Krämer et al. 2007; Loken et al. 2009; Andrew, 2010). Using the same data presented by Loken et al. (2009) an alternative explanation can be advanced, namely that C-fibre and large-diameter afferents are activated in parallel during brush stroking, with a sense of pleasantness emerging when large-diameter responsiveness exceeds that of C-fibres. Conversely, in their study neutral or unpleasant (negative pleasant) scores were reported at the lowest brushing velocities, where CT responsiveness may well exceed the activation of large-diameter tactile afferents, suggesting that the role C-tactile fibres need not be confined to pleasant touch. Likewise, it is intriguing that the reported absence of CT fibres in glabrous skin does not preclude our capacity to perceive the pleasantness of affective touch stimuli such as brush-stroking or velvet-fabric (Rolls et al. 2003; Krämer et al. 2007). In our study, brushing stimulation – at reportedly pleasant speeds – evoked allodynia during muscle pain. Thus, it is the concurrent activation of muscle nociceptors during hypertonic saline infusion and cutaneous mechanoreceptors during brushing (and vibratory) stimulation that leads to the allodynia. The use of differential nerve blocks to avoid the co-activation of multiple fibre classes during tactile stimulation – an ambiguity that has plagued earlier studies – confirms the role of CT fibres in mediating allodynia. Hence, it is the complement of active sensory fibres, rather than the activation of a single class of afferents, which determines the perceptual outcome of activating CT fibres. Our observations are indeed consistent with a role of CT fibres in emotional touch, in particular the crossover between neutral-touch and painful-touch (allodynia). Furthermore, the abolition of vibratory sense during myelinated-compression block argues against the role of CT fibres in discriminative touch. Whether they can play a more circumscribed role in the detection of punctuate stimuli warrants further investigation, given the report that patients with large-fibre sensory neuropathy are able to detect weak monofilaments on the hairy skin (Cole et al. 2006).
The contribution of large-diameter tactile afferents and small-diameter nociceptors to allodynia has been extensively studied (Campbell et al. 1988; Cline et al. 1989; Price et al. 1992; Torebjork et al. 1992; Koltzenburg et al. 1994; Maihöfner et al. 2003). However, the role of CT fibres in mediating allodynia is a novel observation. The contribution of C-tactile afferents may have been obscured due to the use a single-compartment model, in which innocuous and noxious stimuli are applied to the same or adjacent regions of skin. As noted in the Introduction, such an approach can lead to uncertainty as to whether any change in pain perception reflects peripheral sensitization of nociceptive fibres or an altered central convergence of tactile and nociceptive inputs. In this study such ambiguities were avoided by adopting a two-compartment approach; however it remains to be tested whether CT-mediated allodynia is limited to heterologous structures such as skin and muscle or can be produced by inputs arising from cutaneous nociceptors and CT fibres.
Studies in experimental animals have shown that CT input can activate ‘modality-ambiguous’ or wide dynamic range lamina I neurons (Keller et al. 2007; Andrew, 2010). Furthermore, nociceptive lamina I neurons display increased responsiveness during CT input following peripheral nerve injury (Keller et al. 2007). These observations provide a possible pathway by which mechanoreceptive impulses in CT fibres converge with inputs from muscle nociceptors and transmit to the higher centres. Although musculosomatic convergence has been demonstrated in experimental animals (Foreman et al. 1979), this is the first study to unravel the role of CT fibres in altered central integration of cross-modality inputs thereby eliciting allodynia. CT-related processing is often described as occurring below a conscious level, which is consistent with a lack of distinct quality of sensation (Olausson et al. 2010). Whether activation of CT afferents is associated with a unique set of qualia needs to be systematically studied using affective- (positive and negative) and neutral-touch stimuli in protocols that do not limit the resulting sensation to the persistence of background pain.
What triggers a crossover between pain and pleasure during affective- or neutral-touch processing is yet to be ascertained. Neuroimaging studies have shown differential representation of pleasant and painful tactile stimuli in certain areas of the brain involved in emotional processing (insular, orbitofrontal and anterior cingulate cortices: Olausson et al. 2002; Rolls et al. 2003). However, cortical activation evoked by a neutral tactile stimulus predominantly activates the discriminative-cognitive areas, the primary and secondary somatosensory cortices. It has been argued that the tactile allodynia experienced following administration of sumatriptan may be explained by an induced deficit in affective (pleasant) processing (Krämer et al. 2007). In contrast, our results strongly indicate that CT activation can mediate a crossover to the affective component of touch, i.e. neutral to painful.
The qualia of touch may have evolved mainly in a social context to create a useful construct of the world, e.g. to predict whether the intent behind another's action was benign or sinister; synthesized with the sense of ‘self’, these inputs subserve reflective self-awareness that characterizes humans as immensely social creatures (Ramachandran, 2004). Individuals with autism suffer from sensory-perceptual abnormalities such as hypersensitivity or defensiveness to touch stimuli (e.g. vibration), which in part at least contributes to social withdrawal (Baranek, 1999; Cascio et al. 2008). These observations in individuals with autism are consistent with our experimental results in healthy subjects, suggesting a role of CT afferents in behavioural responses such as sensory-avoiding. Clearly, the contribution of these seemingly primitive tactile fibres to the emotional touch system has immense implications for understanding self-regulating mechanisms that at least in part determine social behaviours.
Acknowledgments
This research was supported by the National Health and Medical Research Council of Australia.
Glossary
Abbreviations
- CT
C-tactile
- HS
hypertonic saline
- TA
tibialis anterior muscle
- VAS
visual analogue scale
Author contributions
All experiments were performed at the School of Medicine, University of Western Sydney. The contribution of each author to this study is as follows. Conception and design, or analysis and interpretation of data: all authors contributed; drafting the article or revising it critically for important intellectual content: all authors contributed; final approval of the version to be published: all authors approved.
References
- Andrew D. Quantitative characterization of low-threshold mechanoreceptor inputs to lamina I spinoparabrachial neurons in the rat. J Physiol. 2010;588:117–124. doi: 10.1113/jphysiol.2009.181511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner S, Lindblom U, Meyerson B, Molander C. Prolonged relief of neuralgia after regional anesthetic blocks. A call for further experimental and systematic clinical studies. Pain. 1990;43:287–297. doi: 10.1016/0304-3959(90)90026-A. [DOI] [PubMed] [Google Scholar]
- Baranek GT. Autism during infancy: a retrospective video analysis of sensory-motor and social behaviors at 9–12 months of age. J Autism Dev Disord. 1999;29:213–224. doi: 10.1023/a:1023080005650. [DOI] [PubMed] [Google Scholar]
- Berry M, Bannister LH, Standring SM. Nervous system. In: Williams PL, editor. Gray's Anatomy: The Anatomical Basis of Medicine and Surgery. 38th edn. London: Churchill Livingstone; 1995. pp. 901–1397. [Google Scholar]
- Bessou P, Burgess PR, Perl ER, Taylor CB. Dynamic properties of mechanoreceptors with unmyelinated (C) fibers. J Neurophysiol. 1971;34:116–131. doi: 10.1152/jn.1971.34.1.116. [DOI] [PubMed] [Google Scholar]
- Campbell J, Raja S, Meyer R, Mackinnon S. Myelinated afferents signal the hyperalgesia associated with nerve injury. Pain. 1988;32:89–94. doi: 10.1016/0304-3959(88)90027-9. [DOI] [PubMed] [Google Scholar]
- Cascio C, McGlone F, Folger S, Tannan V, Baranek G, Pelphrey K, Essick G. Tactile perception in adults with autism: a multidimensional psychophysical study. J Autism Dev Disord. 2008;38:127–137. doi: 10.1007/s10803-007-0370-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervero F, Laird J. Mechanisms of touch-evoked pain (allodynia): a new model. Pain. 1996;68:13–23. doi: 10.1016/S0304-3959(96)03165-X. [DOI] [PubMed] [Google Scholar]
- Cline M, Ochoa J, Torebjork H. Chronic hyperalgesia and skin warming caused by sensitized C nociceptors. Brain. 1989;112:621–647. doi: 10.1093/brain/112.3.621. [DOI] [PubMed] [Google Scholar]
- Cole J, Bushnell MC, McGlone F, Elam M, Lamarre Y, Vallbo A, Olausson H. Unmyelinated tactile afferents underpin detection of low-force monofilaments. Muscle Nerve. 2006;34:105–107. doi: 10.1002/mus.20534. [DOI] [PubMed] [Google Scholar]
- Craig AD. Why a soft touch can hurt. J Physiol. 2010;588:13. doi: 10.1113/jphysiol.2009.185116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellon AL. Clinical use of vibratory stimuli to evaluate peripheral nerve injury and compression neuropathy. Plast Reconstr Surg. 1980;65:466–476. doi: 10.1097/00006534-198004000-00011. [DOI] [PubMed] [Google Scholar]
- Douglas WW, Ritchie JM. Non-medullated fibres in the saphenous nerve which signal touch. J Physiol. 1957;139:385–399. doi: 10.1113/jphysiol.1957.sp005899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman R, Schmidt R, Willis W. Effects of mechanical and chemical stimulation of fine muscle afferents upon primate spinothalamic tract cells. J Physiol. 1979;286:215–231. doi: 10.1113/jphysiol.1979.sp012615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W, Arendt-Nielsen L, Graven-Nielsen T. Delayed onset muscle soreness at tendon–bone junction and muscle tissue is associated with facilitated referred pain. Exp Brain Res. 2006;174:351–360. doi: 10.1007/s00221-006-0466-y. [DOI] [PubMed] [Google Scholar]
- Gibson W, Arendt-Nielsen L, Taguchi T, Mizumura K, Graven-Nielsen T. Increased pain from muscle fascia following eccentric exercise: animal and human findings. Exp Brain Res. 2009;194:299–308. doi: 10.1007/s00221-008-1699-8. [DOI] [PubMed] [Google Scholar]
- Gracely R, Lynch S, Bennett G. Painful neuropathy: altered central processing maintained dynamically by peripheral input. Pain. 1992;51:175–194. doi: 10.1016/0304-3959(92)90259-E. [DOI] [PubMed] [Google Scholar]
- Iggo A. Cutaneous mechanoreceptors with afferent C fibres. J Physiol. 1960;152:337–353. doi: 10.1113/jphysiol.1960.sp006491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson R, Trulsson M, Olsson K, Westberg K. Mechanoreceptor activity from the human face and oral mucosa. Exp Brain Res. 1988;72:204–208. doi: 10.1007/BF00248518. [DOI] [PubMed] [Google Scholar]
- Keller AF, Beggs S, Salter M, De Koninck Y. Transformation of the output of spinal lamina I neurons after nerve injury and microglia stimulation underlying neuropathic pain. Molecular Pain. 2007;3:27. doi: 10.1186/1744-8069-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltzenburg M, Torebjork H, Wahren L. Nociceptor modulated central sensitization causes mechanical hyperalgesia in acute chemogenic and chronic neuropathic pain. Brain. 1994;117:579–591. doi: 10.1093/brain/117.3.579. [DOI] [PubMed] [Google Scholar]
- Krämer HH, Lundblad L, Birklein F, Linde M, Karlsson T, Elam M, Olausson H. Activation of the cortical pain network by soft tactile stimulation after injection of sumatriptan. Pain. 2007;133:72–78. doi: 10.1016/j.pain.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Kumazawa T, Perl ER. Primate cutaneous sensory units with unmyelinated (C) afferent fibers. J Neurophysiol. 1977;40:1325–1338. doi: 10.1152/jn.1977.40.6.1325. [DOI] [PubMed] [Google Scholar]
- Laursen RJ, Graven-Nielsen T, Jensen TS, Arendt-Nielsen L. The effect of compression and regional anaesthetic block on referred pain intensity in humans. Pain. 1999;80:257–263. doi: 10.1016/s0304-3959(98)00214-0. [DOI] [PubMed] [Google Scholar]
- Loken LS, Wessberg J, Morrison I, McGlone F, Olausson H. Coding of pleasant touch by unmyelinated afferents in humans. Nat Neurosci. 2009;12:547–548. doi: 10.1038/nn.2312. [DOI] [PubMed] [Google Scholar]
- Macefield G, Gandevia SC, Burke D. Perceptual responses to microstimulation of single afferents innervating joints, muscles and skin of the human hand. J Physiol. 1990;429:113–129. doi: 10.1113/jphysiol.1990.sp018247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie RA, Burke D, Skuse NF, Lethlean AK. Fibre function and perception during cutaneous nerve block. J Neurol Neurosurg Psychiatry. 1975;38:865–873. doi: 10.1136/jnnp.38.9.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahns D, Perkins N, Sahai V, Robinson L, Rowe M. Vibrotactile frequency discrimination in human hairy skin. J Neurophysiol. 2006;95:1442–1450. doi: 10.1152/jn.00483.2005. [DOI] [PubMed] [Google Scholar]
- Maihöfner C, Neundörfer B, Stefan H, Handwerker H. Cortical processing of brush-evoked allodynia. Neuroreport. 2003;14:785–789. doi: 10.1097/00001756-200305060-00002. [DOI] [PubMed] [Google Scholar]
- Maruhashi J, Mizuguchi K, Tasaki I. Action currents in single afferent nerve fibres elicited by stimulation of the skin of the toad and the cat. J Physiol. 1952;117:129–151. doi: 10.1113/jphysiol.1952.sp004736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlone F, Vallbo AB, Olausson H, Loken L, Wessberg J. Discriminative touch and emotional touch. Can J Exp Psychol. 2007;61:173–183. doi: 10.1037/cjep2007019. [DOI] [PubMed] [Google Scholar]
- Merzenich M, Harrington T. The sense of flutter-vibration evoked by stimulation of the hairy skin of primates: comparison of human sensory capacity with the responses of mechanoreceptive afferents innervating the hairy skin of monkeys. Exp Brain Res. 1969;9:236–260. doi: 10.1007/BF00234457. [DOI] [PubMed] [Google Scholar]
- Nagi SS, Rubin TK, Macefield VG, Mahns DA. Differential contribution of tactile afferents to allodynia and analgesia. Proc Austral Neurosci Soc. 2009;29:1–146. [Google Scholar]
- Nordin M. Low-threshold mechanoreceptive and nociceptive units with unmyelinated (C) fibres in the human supraorbital nerve. J Physiol. 1990;426:229–240. doi: 10.1113/jphysiol.1990.sp018135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rahilly R, Muller F. Gardner-Gray-O'Rahilly Anatomy. Philadelphia: W. B. Saunders company; 1986. [Google Scholar]
- Ochoa J, Torebjork E. Sensations evoked by intraneural microstimulation of single mechanoreceptor units innervating the human hand. J Physiol. 1983;342:633–654. doi: 10.1113/jphysiol.1983.sp014873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa J, Torebjork E. Sensations evoked by intraneural microstimulation of C nociceptor fibres in human skin nerves. J Physiol. 1989;415:583–599. doi: 10.1113/jphysiol.1989.sp017737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olausson H, Cole J, Rylander K, McGlone F, Lamarre Y, Wallin B, Krämer H, Wessberg J, Elam M, Bushnell M, Vallbo Å. Functional role of unmyelinated tactile afferents in human hairy skin: sympathetic response and perceptual localization. Exp Brain Res. 2008;184:135–140. doi: 10.1007/s00221-007-1175-x. [DOI] [PubMed] [Google Scholar]
- Olausson H, Lamarre Y, Backlund H, Morin C, Wallin BG, Starck G, Ekholm S, Strigo I, Worsley K, Vallbo AB, Bushnell MC. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat Neurosci. 2002;5:900–904. doi: 10.1038/nn896. [DOI] [PubMed] [Google Scholar]
- Olausson H, Wessberg J, Morrison I, McGlone F, Vallbo Å. The neurophysiology of unmyelinated tactile afferents. Neurosci Biobehav Rev. 2010;34:185–191. doi: 10.1016/j.neubiorev.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Price D, Long S, Huitt C. Sensory testing of pathophysiological mechanisms of pain in patients with reflex sympathetic dystrophy. Pain. 1992;49:163–173. doi: 10.1016/0304-3959(92)90139-3. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS. A Brief Tour of Human Consciousness: From Impostor Poodles to Purple Numbers. New York: Pi Press; 2004. [Google Scholar]
- Rolls ET, O'Doherty J, Kringelbach ML, Francis S, Bowtell R, McGlone F. Representations of pleasant and painful touch in the human orbitofrontal and cingulate cortices. Cereb Cortex. 2003;13:308–317. doi: 10.1093/cercor/13.3.308. [DOI] [PubMed] [Google Scholar]
- Sahai V, Mahns D, Robinson L, Perkins N, Coleman G, Rowe M. Processing of vibrotactile inputs from hairy skin by neurons of the dorsal column nuclei in the cat. J Neurophysiol. 2006;95:1451–1464. doi: 10.1152/jn.00485.2005. [DOI] [PubMed] [Google Scholar]
- Salmons S. Muscle. In: Williams PL, editor. Gray's Anatomy: The Anatomical Basis of Medicine and Surgery. 38th edn. London: Churchill Livingstone; 1995. pp. 737–900. [Google Scholar]
- Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, Edwards RH. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature. 2009;462:651–655. doi: 10.1038/nature08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torebjörk HE, Hallin RG. Perceptual changes accompanying controlled preferential blocking of A and C fibre responses in intact human skin nerves. Exp Brain Res. 1973;16:321–332. doi: 10.1007/BF00233334. [DOI] [PubMed] [Google Scholar]
- Torebjork H, Lundberg L, LaMotte R. Central changes in processing of mechanoreceptive input in capsaicin-induced secondary hyperalgesia in humans. J Physiol. 1992;448:765–780. doi: 10.1113/jphysiol.1992.sp019069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torebjork HE, Ochoa JL, Schady W. Referred pain from intraneural stimulation of muscle fascicles in the median nerve. Pain. 1984a;18:145–156. doi: 10.1016/0304-3959(84)90882-0. [DOI] [PubMed] [Google Scholar]
- Torebjork HE, Schady W, Ochoa J. Sensory correlates of somatic afferent fibre activation. Hum Neurobiol. 1984b;3:15–20. [PubMed] [Google Scholar]
- Treede R, Cole J. Dissociated secondary hyperalgesia in a subject with a large-fibre sensory neuropathy. Pain. 1993;53:169–174. doi: 10.1016/0304-3959(93)90077-3. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Olausson H, Wessberg J. Unmyelinated afferents constitute a second system coding tactile stimuli of the human hairy skin. J Neurophysiol. 1999;81:2753–2763. doi: 10.1152/jn.1999.81.6.2753. [DOI] [PubMed] [Google Scholar]
- Vallbo A, Olausson H, Wessberg J, Norrsell U. A system of unmyelinated afferents for innocuous mechanoreception in the human skin. Brain Research. 1993;628:301–304. doi: 10.1016/0006-8993(93)90968-s. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Olsson KA, Westberg KG, Clark FJ. Microstimulation of single tactile afferents from the human handSensory attributes related to unit type and properties of receptive fields. Brain. 1984;107:727–749. doi: 10.1093/brain/107.3.727. [DOI] [PubMed] [Google Scholar]
- Wasner G, Baron R, Janig W. Dynamic mechanical allodynia in humans is not mediated by a central presynaptic interaction of A beta-mechanoreceptive and nociceptive C-afferents. Pain. 1999;79:113–119. doi: 10.1016/s0304-3959(98)00159-6. [DOI] [PubMed] [Google Scholar]
- Weerakkody NS, Percival P, Hickey MW, Morgan DL, Gregory JE, Canny BJ, Proske U. Effects of local pressure and vibration on muscle pain from eccentric exercise and hypertonic saline. Pain. 2003;105:425–435. doi: 10.1016/S0304-3959(03)00257-4. [DOI] [PubMed] [Google Scholar]
- Zar J. Biostatistical Analysis. New Jersey: Prentice-Hall International; 1984. [Google Scholar]
- Zotterman Y. Touch, pain and tickling: an electro-physiological investigation on cutaneous sensory nerves. J Physiol. 1939;95:1–28. doi: 10.1113/jphysiol.1939.sp003707. [DOI] [PMC free article] [PubMed] [Google Scholar]


